Abstract
No validated biological markers (or biomarkers) currently exist for appropriately selecting patients with cancer for antiangiogenic therapy. Nor are there biomarkers identifying escape pathways that should be targeted after tumors develop resistance to a given antiangiogenic agent. A number of potential systemic, circulating, tissue and imaging biomarkers have emerged from recently completed phase I–III studies. Some of these are measured at baseline (for example VEGF polymorphisms), others are measured during treatment (such as hypertension, MRI-measured Ktrans, circulating angiogenic molecules or collagen IV), and all are mechanistically based. Some of these biomarkers might be pharmacodynamic (for example, increase in circulating VEGF, placental growth factor) while others have potential for predicting clinical benefit or identifying the escape pathways (for example, stromal-cell-derived factor 1α, interleukin-6). Most biomarkers are disease and/or agent specific and all of them need to be validated prospectively. We discuss the current challenges in establishing biomarkers of antiangiogenic therapy, define systemic, circulating, tissue and imaging biomarkers and their advantages and disadvantages, and comment on the future opportunities for validating biomarkers of antiangiogenic therapy.
Introduction
Tumors acquire blood vessels by co-option of neighboring vessels, from sprouting or intussusceptive microvascular growth and by vasculogenesis from endothelial precursor cells.1 In most solid tumors the newly formed vessels are plagued by structural and functional abnormalities owing to the sustained and excessive exposure to angiogenic factors produced by the growing tumor.2 Despite being abnormal, these new vessels allow tumor expansion at early stages of carcinogenesis and progression from in situ lesions to locally invasive, and eventually to metastatic tumors. The hypothesis that tumor progression can be arrested by antiangiogenesis3 has been confirmed experimentally by a large body of evidence over the past three decades. Enhanced survival of patients has yet to be achieved in phase III clinical trials by antiangiogenic agents that only target VEGF. Nevertheless, the addition of bevacizumab (a VEGF-specific blocking antibody) to standard chemotherapies or to interferon therapy (in metastatic renal cell carcinoma [mRCC]), as well as the use of anti-VEGF receptor tyrosine kinase inhibitors (TKIs) with wide spectra of activity, has proven efficacious in multiple advanced cancers such as metastatic colorectal cancer (mCRC), metastatic non-small-cell lung cancer (mNSCLC), metastatic breast cancer, mRCC, hepatocellular carcinoma (HCC) and gastrointestinal stromal tumors (GISTs).4–12 Moreover, bevacizumab has been recently approved for recurrent glioblastoma based on phase II trial data.
These agents have changed the practice of oncology but stimulated important questions: how do these therapies work in patients? Is their mechanism of action in patients the same as originally envisioned for antiangiogenic agents? Is the mechanism the same as demonstrated in animal models? Could the overall survival benefit be increased beyond a few months? Could we successfully use these agents in the adjuvant setting after surgical resection? Why do some patients develop severe toxicities from antiangiogenic therapy? Why is the benefit from antiangiogenic therapies seen only in some patients? How do we preselect these patients, or the most appropriate therapy? Why do tumors stop responding to antiangiogenic therapy? What new pathways should be targeted to optimize the response and prolong the duration of response and survival without increasing toxic effects? How do we tailor these new therapies to individual patients? How do we schedule them with contemporary and future therapeutics? The answers to these fundamental questions are not fully known for the approved antiangiogenic agents, and will be critical in choosing the appropriate agent(s), and to determine their optimum dose and schedule.13–17 We propose that validated pharmacodynamic, prognostic, predictive and surrogate biomarker studies can address these questions (Box 1), and we outline challenges in identifying and validating biomarkers for response, toxicity and resistance to antiangiogenic therapy, and finally, discuss emerging systemic, circulating, tissue and imaging biomarkers.
Box 1 | Why do oncologists and pharmaceutical companies need biomarkers
Systemic chemotherapy has been used by oncologists for decades, but the necessity of biological markers (biomarkers) has become a priority with the emergence of ‘targeted’ agents.
Although the overall survival benefit from combining bevacizumab with chemotherapy or using TKIs as monotherapy is modest, some patients respond better than others. Thus, it is critical to establish predictive markers for selection of patients.
The dose and schedule of antiangiogenic and cytotoxic therapies when used in combination might not be optimal; biomarkers could be useful, therefore, in optimizing the dose and schedule of these agents.
The benefit in response to antiangiogenic therapy is often transient owing to redundancy of the target or mechanisms of acquiring blood vessels; tumors might, therefore, be intrinsically resistant or acquire resistance to a particular agent. Biomarkers of resistance could be useful in identifying new targets.
Antiangiogenic therapies have unique adverse effects and/or can amplify the adverse effects of chemotherapy, and biomarkers could help identify patients at high risk for adverse effects.
Finally, the cost of these agents is becoming an increasingly important consideration. We propose that biomarker validation could benefit the pharmaceutical industry tremendously. Validated biomarkers could reduce attrition of lead compounds as well as improve the safety and efficacy in early and late development phases of experimental agents by stratification of patients. They could help establish optimal agent dosing for each patient, which is likely to be the key to better safety and efficacy profiles. Moreover, biomarkers could prove invaluable for driving future development of molecular therapeutics with novel targets and mechanism(s) of action.
Challenges in identifying biomarkers
An array of antiangiogenic biomarkers have been studied (Box 2), including systemic measurements (for example, changes in systemic blood pressure), genotypic analyses (for example, VEGF or interleukin [IL]-8 polymorphisms), circulating markers (for example, plasma levels of VEGF), tissue markers (tumor microvessel density) and imaging parameters (for example, Ktrans, the volume transfer constants of gadolinium between blood plasma and the extravascular extracellular space measured by MRI; Table 1). Although promising candidates have been identified, important challenges limit their translation into practice.
Box 2 | Definitions of various types of biomarkers
Biomarker: A distinctive biological or biologically derived indicator (as a biochemical metabolite in the body) of a process, event, or condition (as aging, disease, or exposure to a toxic substance) (Webster Medical Dictionary).
Prognostic biomarkers: Biomarkers that provide information about the patients overall cancer outcome, regardless of therapy.16
Predictive biomarkers: Biomarkers that can be used in advance of therapy to estimate response or survival of a specific patient on a specific treatment compared with another treatment.17
Pharmacodynamic biomarkers: Biomarker whose changes after treatment are associated with target modulation by a specific agent.
Surrogate marker: A biomarker intended to substitute for a clinical end point.
Table 1.
Advantages and challenges in measuring biomarkers of antiangiogenic therapy
| Biomarker type | Advantages | Challenges |
|---|---|---|
| Tumor | ||
| Tissue | Allows baseline or serial measurements of highly relevant parameters:
|
Technologically challenging and labor intensive Highly invasive (often difficult to obtain or to access, particularly serially) Heterogeneity within the tumor and sample preparation Samples of primary tumors—obtained at the time of biopsy or surgery—might not accurately reflect the features of recurrent or metastatic disease Requires standardization |
| Imaging | Noninvasive techniques that could serially measure, regardless of tumor type or location:
are constantly being upgraded and can be combined |
Technologically challenging and costly The output is often a composite parameter, which might depend on both blood flow and permeability The high heterogeneity of blood flow in tumors—a determinant for the distribution of chemotherapeutics and oxygen in tumor tissue—cannot yet be evaluated with high spatial resolution Requires standardization |
| Systemic | ||
| Blood/urinary proteins |
Minimally/noninvasive Can serially measure changes in circulating biomarkers Availability of multiple reliable platforms and reagents to measure protein concentration |
Costly Requires standardization |
| Blood cells | Minimally invasive Can serially measure changes in multiple circulating cells (for example, endothelial cells, progenitors, platelets) as potential biomarkers Availability of multiple reliable platforms and reagents to measure cell phenotype and number |
The function of multiple circulating cell types in cancer is poorly characterized Some of the circulating cell populations are present in very low numbers Costly Requires standardization |
| Genotype | Minimally/noninvasive Availability of standardized techniques Relatively inexpensive |
ND |
Abbreviations: IFP, interstitial fluid pressure; MVD, microvascular density; ND, not determined.
The first challenge is establishing adequate criteria of response. This issue is especially problematic when using agents that target stroma, such as antiangiogenic agents. Standard lesion size (defined, by RECIST [Response Evaluation Criteria In Solid Tumors] or WHO criteria) evaluations of response might not optimally assess these agents, particularly when used as monotherapy with agents such as sunitinib or sorafenib in RCC or HCC. Anti-VEGF therapy has primarily cytostatic effects, might prune and normalize the tumor vasculature, and can have substantial systemic effects such as modulation of circulating proangiogenic and proinflammatory cytokines and cells.18–23 These effects might not shrink but rather stabilize the tumor size.24
The success in identifying a predictive biomarker for a drug will require elucidation of its mechanism of action (Supplementary Table 1). For example, detection of over-expression or amplification of human epidermal growth factor receptor 2 (HER2/neu) in breast cancer cells and its use as a predictive biomarker is consistent with the mechanism of action of the anti-Her2 antibody trastuzumab or the EGFR-HER2 TKI lapatinib.25,26 Unfortunately, the mechanisms of action of currently approved antiangiogenic agents are not fully understood.27,28
The anti-VEGF antibody bevacizumab has yet to show improved overall survival as monotherapy in a phase III trial. The mechanisms of action of multitargeted TKIs are even less well understood—they probably target both stromal and cancer cells. Indeed, a number of potential mechanisms by which these different agents work have been hypothesized. Of these mechanisms, ‘vascular normalization’ has the most robust clinical evidence,29 and this mechanism alone might contribute to improved survival in some cancers such as glioblastoma and/or potentially as a sensitizer to cytotoxic therapies in others (for example, rectal carcinoma)18,20,22,30 (Box 3 and Table 2). Thus, the determinants of vascular normalization could serve as candidate biomarkers. Conversely, a candidate biomarker that is associated with improved benefit could provide insight into the mechanism of action or resistance of a drug.
Box 3 | Mechanisms of action of antiangiogenic therapy supported by biomarkers
Biomarker selection has been based on tumor-specific antivascular and normalizing effects as well as systemic effects of these therapies (Table 2). We have shown that bevacizumab decreases microvascular density, increases pericyte coverage and lowers interstitial fluid pressure.22,31,32 All of these pharmacodynamic changes support the vascular normalization hypothesis. As a result of the normalized microenvironment, the proliferation rate of cancer cells remains high or increases, potentially increasing sensitivity of these tumors to chemotherapy and radiation therapy. Apoptosis of cancer cells increases in response to reduced microvascular density. In addition, reports have shown that anti-VEGF therapy might have important systemic effects: it might increase the blood pressure level, could decrease circulating progenitor cell populations, decrease or increase circulating cytokine levels, and increase tumor infiltration of immunosuppressive myeloid cells.28,33–35 Moreover, tumor vascular normalization can lead to increased accumulation in tumors of effector T lymphocytes.36
Imaging studies have also provided pharmacodynamic evidence for both the antivascular and normalization hypotheses. A number of studies have reported decreases in perfusion and vascular permeability using a variety of imaging technologies (Table 5). It is important to note the inability to separate perfusion from permeability in the transport parameters extracted from these studies. Using a more-sophisticated MRI protocol, we found that anti-VEGF therapy can create a window of normalization that lasts at least 1 month in patients with recurrent glioblastoma, characterized by reduced permeability and vessel diameter.18,37 This effect alone might confer survival benefits in these patients.20,69
Finally, fluorodeoxyglucose-PET studies have also shown that the fluorodeoxyglucose delivery and uptake by rectal cancers does not go down after bevacizumab monotherapy despite a decrease in microvessel density and blood flow.22,31,32 This finding provides additional evidence in support of vascular normalization by VEGF blockade.
Table 2.
Characteristics of biomarker selection
| Tumor vascular normalizing effects |
Tumor antivascular effects |
Systemic effects |
|---|---|---|
| Permeability | Microvessel density | Blood pressure |
| Microvessel density | Vascular volume | Bone-marrow-derived cells (circulating or infiltrating the tumors: effector T Iymphocyes, myeloid cells, progenitor cells) |
| Diameter | NA | NA |
| Interstitial fluid pressure | NA | NA |
| Edema | NA | NA |
| Basement membrane thickness | NA | NA |
| Pericyte coverage | NA | NA |
| Penetration of macromolecules | NA | NA |
| Perfusion | NA | NA |
| Oxygenation | NA | NA |
Abbreviation: NA, not applicable.
The second challenge results from the heterogeneous and dynamic nature of cancer. Ideally, treatment outcome would be predicted from a single measurement in the tumor biopsy sample or from the circulation before treatment initiation. Indeed, VEGF polymorphisms in tumor biopsy sample and baseline VEGF plasma levels are candidate biomarkers for bevacizumab combined with chemotherapy for patients with metastatic breast cancer.38,39 By contrast, baseline VEGF is not associated with survival outcomes for mCRC or mNSCLC.40,41 The dynamics of cancer must be recognized: not only might the biology of the primary tumor be different from its metastases, but it might also change with tumor progression and treatment. Thus, a biopsy sample before first treatment might not reflect the biology before subsequent treatment. Finally, there is regional heterogeneity: one part of a tumor may not have the same vascularity or angiogenesis as another part. Thus there is a need for spatially resolved ‘dynamic biomarkers’.
The third challenge is the inability to perform repeated biopsies (that is, before and after antiangiogenic therapy) to assess ‘dynamic biomarkers’. This challenge can be partially addressed by using novel imaging techniques, which can also provide spatial information. Such an approach has shown promise in identifying potential biomarkers for treatment outcome in patients with glioblastoma. In some cases, different types of biomarkers (for example, imaging and circulating) might need to be combined, yielding a ‘composite biomarker’, to make robust predictions.
The fourth challenge in identify biomarkers resides in the inherent design of clinical trials. Human studies are expensive and require expertise in a wide range of areas, and almost all exploratory biomarkers to date have emerged from single-arm trials.42 It is, therefore, difficult to ascertain whether the biomarker is prognostic or predictive. This is problematic for anti-VEGF therapies where VEGF levels in the circulation or tumor biopsy samples have been shown to be prognostic in a number of cancer types.43,44 Even markers identified from randomized trials have emerged from secondary analyses, and require independent validation.
A fifth challenge is the unpredictability of response or toxicity, and resistance by activation of tumor VEGF-independent angiogenic pathways. Thus, biological (mechanism-driven) marker (biomarker) discovery has become a priority for these costly therapies that can be associated with rare but serious adverse effects.
A sixth challenge is to optimize and standardize various biomarkers assays. For example, different approaches are being used to measure vascular imaging parameters or circulating proteins and cells. Each approach gives a different result, which makes it difficult to compare trial results. This is further confounded by the inability of widely used imaging techniques to distinguish antivascular effects from antitumor effects of antiangiogenic agents.45 Thus, overall response rate and/or progression-free survival outcomes on the basis of contrast-enhanced imaging might not reflect a true antitumor response.
When a biomarker has been identified and validated the question for their clinical implementation will arise. Will they be generic for any anti-VEGF drug, any tumor type or stage or combination regimen? Despite these limitations, a number of candidate biomarkers are emerging for antiangiogenic therapy of cancer. Some of these findings are provocative and raise new questions about the efficacy, safety and cost–benefit ratios of these therapies. We discuss the current understanding and the future directions in establishing candidate ‘predictive biomarkers’, ‘toxicity biomarkers’ and ‘VEGF-resistance biomarkers’, and the steps necessary for their future validation.
Biomarkers of response
Pharmacodynamic biomarkers should reflect modulation of a defined biological target. Whether this biological change translates into a clinical benefit in the patient can only be ascertained if the biomarker correlates with the treatment outcome (that is, it is a predictive biomarker). Although several pharmacodynamic biomarkers have emerged, little progress has been made in the validation of prognostic and predictive biomarkers (Tables 3 and 4).
Table 3.
Potential predictive biomarkers of benefit of antiangiogenic therapy
| Cancer type (and study phase) |
Treatment | Study size and time points |
Biomarker findings | Comments and references | |
|---|---|---|---|---|---|
| Antiangiogenic agent |
Cytotoxic agent |
||||
| mNSCLC (II–III) | Bevacizumab | Carboplatin and paclitaxel |
n = 56–166 Pre and post (week 7) |
Baseline sICAM1 is an independent prognostic factor for OS |
Biomarker not specific for bevacizumab41 |
| mBC (III) | Bevacizumab | Paclitaxel |
n = 363 Baseline |
VEGF-2578 AA genotype: superior median OS VEGF-1154 A allele: superior median OS Patients with grade 3/4 hypertension: superior median OS VEGF=634 CC and VEGF-1498 TT genotypes: significantly less grade 3/4 hypertension |
Needs independent validation studies38 |
| mBC (chemorefractory) (II) |
Bevacizumab | Vinorelbine |
n = 56 Baseline |
Lower baseline levels of plasma VEGF were associated with longer TTP |
Not clear if these are prognostic or predictive biomarker candidate on the basis of single-arm study39 |
| Advanced RCC (bevacizumab refractory) (II) |
Sunitinib | No |
n = 61 Pre and post (day 28) |
Lower baseline levels of plasma sVEGFR3 and VEGF-C were associated with longer PFS |
Not clear if these are prognostic or predictive biomarkers on the basis of single-arm study48 |
| mRCC (II) | Vatalanib | No |
n = 10 Pre and post (1 month) |
Patients with stable disease or partial response had a significant decrease in tumor blood flow at 1 month |
Not clear if these are prognostic or predictive biomarkers on the basis of single-arm study81 |
| Advanced HCC (II) | Sunitinib | No |
n = 34 Pre and post (days 14,28, 56, 84 and 112) |
Patients with clinical benefit had significantly greater decreases in Ktrans, IL-6 and soluble c-KIT at day 14 An increase in AFP, soluble c-KIT, SDF1α, sVEGFR1, CPCs or IL-6 at any time-point was associated with rapid progression and/or death |
Not clear if these are prognostic or predictive biomarkers on the basis of single-arm study23 |
| Advanced ovarian cancer (II) |
Bevacizumab | Low-dose carboplatin |
n = 53 Baseline |
IL-8 A-251T polymorphism might be a molecular predictor of response to bevacizumab-based chemotherapy |
Not clear if these are prognostic or predictive biomarkers on the basis of single-arm study62 |
| mCRC (III) | Vatalanib | FOLFOX |
n = 254 (sera) n = 191 (tissue biopsy) Baseline |
Patients with high baseline serum LDH in the vatalanib treatment arm had significantly longer PFS and OS Responses to vatalanib and chemotherapy correlated directly with tissue mRNA levels of VEGFR1, LDHA and Glut1 (in previously untreated patients [CONFIRM1]) and inversely with HIF-1α in the second-line setting (CONFIRM2) |
Both trials failed to meet the prespecified end point and the development of the drug in mCRC was discontinued60,61 |
| NSCLC (II) | Vandetanib | Gemcitabine Docetaxel Paclitaxel and carboplatin |
n = 207 Baseline |
Low baseline VEGF: lower risk of progressive disease when treated with vandetanib vs gefitinib Low baseline VEGF: lower risk of progressive disease when treated with vandetanib and docetaxel vs docetaxel monotherapy |
Not clear if VEGF is a prognostic or a predictive biomarker given the poor anti-VEGFR activity of the agent46 |
| rGBM (II) | Bevacizumab | Irinotecan |
n = 21 Pre and post (week 1/2, 6) |
Both early and later FLT-PET changes: more significant predictors of OS compared with the MRI changes |
The uptake of fluorothymidine by a tumor might be confounded by changes in vascular permeability induced by bevacizumab70 |
| rGBM (II) | Cediranib | No |
n = 31 Pre and post (days 1, 2, 9, 28, 56, 84 and 112) |
Patients with clinical benefit had significantly greater decreases in Ktrans, collagen IV and CECs at day 2 Progression was associated with an increase in SDF1α and bFGF |
Not clear if these are prognostic or predictive biomarker candidate on the basis of single-arm study37 |
Abbreviations: AFP, alphafetoprotein; bFGF, basic fibroblast growth factor; CECs, circulating endothelial cells; CPC, circulating progenitor cell; FLT-PET, fluorothymidine PET; FOLFOX, 5-FU, leucovorin and oxaliplatin; HCC hepatocellular carcinoma; HIF-1α, hypoxia inducible factor 1α; IL, interleukin; LDH, lactate dehydrogenase; mBC, metastatic breast cancer; mCRC, metastatic colorectal cancer; mNSCLC, metastatic non-small-cell lung cancer; OS, overall survival; PFS, progression-free survival; RCC, renal cell carcinoma; rGBM, recurrent glioblastoma; sICAM1, soluble intercellular adhesion molecule 1; SDF1α, stromal derived factor 1 alpha; TTP, time-to-progression.
Table 4.
Biomarkers that have not been predictive of the benefit provided by antiangiogenic therapy
| Type of cancer |
Treatment | Study phase |
Study size and time points |
Biomarker findings | |
|---|---|---|---|---|---|
| Antiangiogenic agent |
Cytotoxic agent |
||||
| rGBM | Cediranib | No | II |
n = 31 Pre and post treatment (days 1, 2, 9, 28, 56, 84 and 112) |
No statistically significant relationship between baseline interleukin 8, Ang1, CPC or their changes after treatment and outcome37 |
| Advanced HCC | Sunitinib | No | II |
n = 34 Pre and post treatment (days 14, 28, 56, 84 and 112) |
No statistically significant relationship between baseline PIGF, bFGF and VEGF-C or their changes after treatment and outcome23 |
| Advanced RC | Bevacizumab | 5-FU EBRT |
II |
n = 32 Pre and post treatment (days 3, 12 after blood volume alone, 33, and 96) |
No statistically significant relationship between baseline interleukin 8, bFGF, CPC or their changes after treatment and outcome22 |
| mCRC | Bevacizumab | IFL | III |
n = 312 Baseline |
No statistically significant relationship between VEGF, thrombospondin-2 or microvessel density and the increase in median overall survival40 |
| mCRC | Bevacizumab | IFL | II–III |
n = 295 Baseline |
No statistically significant relationship between mutations of KRAS, BRAF, or P53 and the increase in median overall survival59 |
| mNSCLC | Bevacizumab | Carboplatin and paclitaxel |
III |
n = 56–166 Pre and post treatment (week 7) |
No statistically significant relationship between baseline VEGF and bFGF or their changes at week 7 and outcome41 |
Abbreviations: bFGF, basic fibroblast growth factor; CPC, circulating progenitor cell; EBRT, external beam radiation therapy; 5-FU, five fluorouracil; HCC hepatocellular carcinoma; IFL, irinotecan, 5-FU and leucovorin; mCRC, metastatic colorectal cancer; mNSCLC, metastatic non-small-cell lung cancer; PIGF, placental growth factor; RC, rectal cancer; RCC, renal cell carcinoma; rGBM, recurrent glioblastoma.
Blood pressure as a biomarker
The most widely used pharmacodynamic biomarker is a systemic effect of most antiangiogenic agents—that is, the increase in blood pressure (hypertension). Hypertension has been observed in patients with cancer treated with anti-VEGF antibodies or TKIs and is clinically manageable in most cases with medication. Two studies have proposed the degree of hypertension as a predictive biomarker of survival in patients with cancer after bevacizumab or axitinib treatment.34,38 This finding should be validated in other large studies.
VEGF as a biomarker
Several parameters measured in the tumor itself or in the blood circulation of patients with cancer might hold pharmacodynamic or predictive biomarker value. Naturally, the most extensively explored biomarker has been VEGF. Associations between outcomes of antiangiogenic therapy with VEGF levels in the circulation has been reported in some phase II studies. In three randomized trials of vandetanib, baseline plasma VEGF levels were correlated directly with progression-free survival in patients with advanced NSCLC, but only in patients with low pre treatment plasma VEGF levels. Likewise, baseline plasma VEGF levels were correlated with time to progression in patients with metastatic breast cancer in a study of bevacizumab with chemotherapy, and also with progression-free survival in patients with HCC treated with sunitinib.23,39,46 By contrast, a randomized study of sorafenib with or without interferon in patients with mRCC reported an inverse correlation between baseline plasma VEGF levels and progression-free survival (Table 3).47 The reason(s) for this contrasting association are unclear.
Many studies have shown a lack of correlation between VEGF levels at baseline and outcome of antiangiogenic therapy. In the pivotal phase III trial of bevacizumab with chemotherapy in patients with mCRC, VEGF expression in primary tumor tissue has not been predictive of outcome (Table 4).40 Similarly, in a phase II/III trial examining bevacizumab and chemotherapy treatment in patients with mNSCLC, a high baseline circulating plasma VEGF level did not predict progression-free survival or overall survival, despite a correlation with improved overall response rate.41 Phase II studies of sunitinib in RCC, bevacizumab combined with chemoradiation in rectal cancer and cediranib in patients with recurrent glioblastoma showed no correlation between VEGF and outcome of therapy.18,22,48
The inconsistencies in these results emphasize the necessity of evaluating the predictive biomarkers in a dynamic manner, that is, before and soon after commencement of antiangiogenic treatment. Intriguingly, the circulating levels of VEGF seem to be significantly elevated after most antiangiogenic therapies targeting this pathway.32 Circulating plasma VEGF has also been shown to increase after therapy with anti-VEGFR TKIs (Table 5).18,21–23,32,39,48–53 In addition, plasma VEGF levels decrease after antiangiogenic treatment is discontinued, which supports its potential pharmacodynamic biomarker value.18,21,23,49,52
Table 5.
Potential pharmacodynamic biomarkers of antiangiogenic therapy
| Biomarkers | Clinical evidence | Challenges and comments |
|---|---|---|
| Tumor | ||
| MRI (Ktrans) | rGBM: drop at days 1, 28, 56, 112 after cediranib Advanced HCC: drop at day 14 after sunitinib Multiple tumors: drop at day 2 after axitinib |
Unclear when is the optimal time of evaluation as pharmacodynamic biomarker after anti-VEGF treatment18,22,23,77–80 |
| MRI (Ki) | Multiple tumors: drop at day 2 after vatalanib | Unclear when is the optimal time of evaluation as pharmacodynamic biomarker after anti-VEGF treatment18,22,23,77–80 |
| MRI (BF, BV) | rGBM: increase after treatment, decrease after treatment interruptions |
Unclear when is the optimal time of evaluation as pharmacodynamic biomarker after anti-VEGF treatment18,22,23,77–80 |
| CT (BF, BV) | Locally advanced rectal cancer: drop at day 12 and 96 after bevacizumab Advanced HCC: drop at day 12 after bevacizumab mRCC: drop at day 2 and week 18 after bevacizumab with interferon |
Unclear when is the optimal time of evaluation as pharmacodynamic biomarker after anti-VEGF treatment18,22,23,77–80 |
| IFP | Locally advanced rectal cancer: drop at day 12 after bevacizumab | Unclear when is the optimal time of evaluation as pharmacodynamic biomarker after anti-VEGF treatment. Measurement limited by lack of accessibility in some tumors22 |
| Systemic | ||
| Plasma VEGF | Bevacizumab alone and with chemoradiation increases plasma VEGF in patients with locally advanced rectal cancer Sunitinib increases plasma VEGF in patients with mRCC Cediranib increases plasma VEGF in patients with rGBM Cediranib increases plasma VEGF in patients with solid tumors Sunitinib increases plasma VEGF in patients with mRCC Sunitinib increases plasma VEGF in patients with GISTs Sunitinib increases plasma VEGF in patients with mCRC Semaxanib with thalidomide increases serum VEGF in patients with metastatic melanoma Sunitinib increases plasma VEGF in patients with mBC Sunitinib increases plasma VEGF in patients with advanced HCC |
Unclear when is the optimal time of evaluation as pharmacodynamic biomarker after anti-VEGF treatment6,18,21,22,23,32,39,49–53,58 |
| Plasma PIGF | Bevacizumab alone and with chemoradiation increases plasma PIGF in patients with locally advanced rectal cancer Sunitinib increases plasma PIGF in patients with mRCC Cediranib increases plasma PIGF in patients with rGBM Cediranib increases plasma PIGF in patients with solid tumors Sunitinib increases plasma PIGF in patients with mRCC Sunitinib increases plasma PIGF in patients with advanced HCC |
Unclear when is the optimal time of evaluation as pharmacodynamic biomarker after anti-VEGF treatment18,21,22,23,32,49,50,52,58 |
| Plasma soluble VEGFRs |
Sunitinib decreases plasma sVEGFR2 in patients with GIST Cediranib decreases plasma sVEGFR2 in patients with rGBM Cediranib decreases plasma sVEGFR2 in patients with solid tumors Sunitinib decreases plasma sVEGFR2 and sVEGFR3 in patients with mRCC Sunitinib decreases plasma sVEGFR2 and sVEGFR3 in patients with mCRC Sunitinib decreases plasma sVEGFR2 and sVEGFR3 in patients with mBC Sunitinib decreases plasma sVEGFR2 and sVEGFR3 in patients with advanced HCC |
Unclear when is the optimal time of evaluation as pharmacodynamic biomarker after anti-VEGF treatment. Bevacizumab does not decrease the plasma VEGFR2 levels6,18,21,22,23,39,49,50,52,53,58 |
| Circulating cells |
Bevacizumab decreases CD31+CD45− and CD34+CD133+ cells in patients with locally advanced rectal cancer Sunitinib decreases the monocytes in patients with GISTs Sunitinib decreases CD34+CD133+ cells in patients with advanced HCC |
Changes are transient and are dependent on the incorporation of cytotoxics in the regimen Unclear what population has pharmacodynamic biomarker value21,23,32 |
Abbreviations: BC, breast cancer; BF, blood flow; BV, blood volume; GIST, gastrointestinal stromal tumors; HCC hepatocellular carcinoma; IFP, interstitial fluid pressure; mBC, metastatic breast cancer; mCRC, metastatic colorectal cancer; mRCC, metastatic renal-cell carcinoma; PIGF, placental growth factor; rGBM, recurrent glioblastoma.
The increased level of VEGF after antiangiogenic treatment raises many questions. In patients treated with bevacizumab, is the VEGF detected by various technologies freely circulating or bound to the drug? Does it emanate from the tumor or host cells? Why does it increase? What does this excess VEGF do? We and others have tried to address these questions. Given that bevacizumab is administered at doses high enough to bind to circulating VEGF, there is a debate whether the circulating VEGF is free or bound to the antibody. Our studies using standard enzyme-linked immunosorbent assays and multiplex arrays suggest that the measured protein is free.22 Moreover, the phenomenon occurs in the context of VEGFR blockade with TKIs. Preclinical data indicate that this increase in VEGF might be induced by hypoxia in tumors as a result of excessive vessel pruning.54 However, in mice lacking tumors, circulating VEGF has also been shown to increase after TKI blockade of VEGF signaling.19 By comparing the expression profiles of cancer and stromal cells in rectal carcinoma biopsy samples taken before and after a single cycle of bevacizumab monotherapy, we have observed that the increased circulating VEGF most likely emanates from stromal cells (L. Xu, unpublished data). Thus, this increase in VEGF is likely to be a host-response to neutralizing such a critical growth factor.
Perhaps a more intriguing question is what does this VEGF do? One study has shown that platelets take up bevacizumab, which neutralizes the VEGF stored within platelet granules.55 Another preclinical study has suggested that VEGF (and other cytokines) released after sunitinib treatment might facilitate the growth of metastases in mice.56 If confirmed in patients, this finding would indicate that treatment discontinuation with these agents should be avoided in patients with tumor progression. However, a recent phase III trial of adjuvant bevacizumab in early-stage CRC failed to meet its efficacy endpoint.57
While the use of circulating VEGF as a biomarker remains unclear, evaluation of the VEGF genotype has emerged as a predictive biomarker candidate from the phase III study of bevacizumab with chemotherapy versus chemotherapy alone in patients with metastatic breast cancer (ECOG 2100 trial). In that study, the VEGF-2578AA genotype was associated with a superior overall survival in the combination arm compared with the alternate genotypes combined,38 and should be tested in future trials of bevacizumab and other anti-VEGF agents. Unfortunately, baseline plasma levels of VEGF were not available in this study, so this polymorphism could not be compared with the circulating levels of VEGF in these patients.
PIGF and soluble VEGF receptors
Circulating levels of PIGF (placental growth factor)—another VEGF family member—also increase in response to anti-VEGF treatment. Thus, plasma PIGF dynamics is now being considered as a potential pharmacodynamic biomarker (Table 5).22,23,37,48,50,52,58 In addition, targeting PIGF is being considered as a novel approach to prevent tumor escape from anti-VEGF therapy.54 It is worth noting that in a study of bevacizumab in rectal carcinoma, increased circulating PIGF levels, as well as VEGF levels, emanate from host cells (L. Xu, unpublished data). Moreover, the extent of increase in PIGF levels in plasma was associated with a better outcome in patients with rectal cancer treated with bevacizumab and chemo-radiotherapy and cediranib monotherapy in patients with recurrent glioblastoma.22,37 The limitation of these single-arm phase II studies is that one cannot distinguish between predictive and prognostic biomarkers. The role of PIGF needs to be further explored in large studies, therefore, both as a target after VEGF blockade and as an early pharmacodynamic marker and predictive biomarker for antiangiogenic therapy. Other pharmacodynamic biomarker candidates seem to be agent-specific. For example, circulating levels of soluble VEGFR2 and VEGFR3 proteins are decreased by TKIs that directly target these receptors (Table 5),18,21,23,39,48–50,52,53 but not by bevacizumab.22,58 The mechanisms by which these changes occur, their biological significance, and predictive biomarker value are not understood.
Other proteins as biomarkers
Exploration of biomarkers other than VEGF members is critical given their known involvement in tumor angiogenesis and vessel maturation.1,2 However, in patients with mCRC treated with bevacizumab and chemotherapy, pretreatment evaluation of biomarkers such as microvascular density, tumor tissue expression of TSP2, P53 and KRAS mutations has not been predictive of efficacy (Table 4).40,59 On the other hand, in previously untreated patients with mCRC responses to vatalanib plus chemotherapy in correlated directly with tissue messenger RNA levels of VEGFR1, LDHA (lactate dehydrogenase A) and Glut1 (CONFIRM1 trial) and inversely with hypoxia-inducible factor 1α (in the second line setting—CONFIRM2 trial; Table 3).60 In addition, patients with high baseline serum lactate dehydrogenase levels had longer progression-free survival and overall survival after treatment with vatalanib and chemoradiation.61 Unfortunately, both trials failed to show benefit in the experimental arm containing vatalanib.61 Baseline soluble intracellular adhesion molecule 1 was an independent prognostic factor of overall survival in patients treated with bevacizumab and chemotherapy or with chemotherapy alone in the phase III trial of bevacizumab in mNSCLC.41
Certain inflammatory cytokines might have potent proangiogenic effects (IL-1β, IL-6, IL-8, stromal-cell-derived factor [SDF]-1α, etc.). A phase II study suggested that the IL-8A-251T polymorphism (associated with increased protein expression) might be a molecular predictor of response to bevacizumab-based chemotherapy in ovarian cancer.62 Finally, in phase II studies, the extent of increase in inflammatory cytokines such as IL-6 in the plasma during treatment was associated with an inferior outcome in patients with rectal and ovarian cancer after bevacizumab and chemoradiation treatment, and an inferior outcome in patients with advanced HCC after sunitinib therapy.22,23,58 In line with these findings, preclinical studies have shown that sunitinib can induce elevation of circulating inflammatory cytokines in mice, which might result in more-aggressive recurrent or metastatic tumors.19,56,63,64
Circulating cells
VEGF and other pathways targeted by certain TKIs (for example, c-KIT by sunitinib) might be important for the proliferation, survival and/or mobilization of certain cell populations into the blood circulation. Several groups have explored blood-circulating cells as potential biomarkers of antiangiogenic therapy (Tables 3 and 5). Indeed, in response to sunitinib, the number of circulating progenitor cells and monocytes can be decreased in patients with HCC and GIST, respectively.21,23 However, TKIs such as cediranib or bevacizumab combined with chemotherapy did not decrease or increase the circulating progenitor cells.65 The reasons for these differing results need to be addressed in preclinical models.
Imaging biomarkers
Several noninvasive, reproducible and quantitative radiological methods are emerging as potential pharmacodynamic biomarkers. For example, changes in dynamic MRI and CT-based tissue vascular measures such as blood flow, blood volume, or permeability have been shown to occur after treatment with bevacizumab or anti-VEGFR TKIs in clinical studies (Table 5). Water self-diffusion is also sensitive to changes in tumors after therapy,66 and might be a predictive marker in patients with glioblastoma treated with chemoradiation.67 Magnetic resonance spectroscopy (MRS) also holds promise as it provides chemically specific information;68 however, exploitation of the ability of this technique in predicting response to antiangiogenic agents is still in early stages of development. It remains unclear how and when these measurements should be performed for each agent, and whether these biomarkers have a predictive value.
Our group reported that the extent of decrease in Ktrans at day 1 after a single dose of cediranib (compared with the pretreatment value) as measured by vascular MRI in a patient with recurrent glioblastoma was associated with improved progression-free survival and overall survival.69 Similarly, the extent of drop in Ktrans at day 14 after sunitinib (compared with the pretreatment value) in advanced HCC was significantly associated with progression-free survival.23 Our data might explain, at least in part, the association between the decrease in tumor fluorothymidine uptake on PET assessment and overall survival in patients with recurrent glioblastoma treated with bevacizumab and irinotecan.70
The decrease in tumor vascular permeability and/or flow, as estimated by Ktrans,71 is consistent with vascular normalization, so we have proposed a composite ‘vascular normalization index’ as a biomarker that is associated with improved outcomes after cediranib treatment. This index integrated dynamics of Ktrans, MRI-measured cerebral blood volume and plasma collagen IV after one dosing of cediranib correlated with both progression-free survival and overall survival in patients with recurrent glioblastoma.69
Other functional biomarkers
Increased interstitial fluid pressure is a hallmark of solid tumors, and is caused by tumor vascular abnormalities.29 Given the potential of antiangiogenic agents to normalize tumor vasculature, this functional tumor parameter is also being explored as a biomarker in clinical studies. Indeed, in a study of bevacizumab in patients with rectal cancer, blockade of VEGF led to a drop in tumor interstitial fluid pressure (Table 5).22 Our group has also observed a decrease in tumor interstitial fluid pressure after bevacizumab treatment in patients with ovarian and metastatic breast cancer (Y. Boucher and R. K. Jain, unpublished data). Unfortunately, these measurements require insertion of a pressure-sensing needle into tumors and certain tumors are not amenable to this measurement. Thus, even if this biomarker can be validated independently, it would be hard to implement it in the clinic. Finally, another approach for predicting outcome has been the development of predictive models or nomogram. One such nomogram was developed for sunitinib in mRCC and included clinical scores as well as serum levels of alkaline phosphatase and lactate dehydrogenase.72
Collectively, these studies show that vascular permeability and perfusion, and circulating VEGF and PIGF should be further investigated as potential generic pharmacodynamic biomarkers for antiangiogenic therapies. Other candidates, such as soluble VEGFRs or circulating progenitor cells should be further evaluated as potential pharmacodynamic biomarkers for specific antiangiogenic agents or specific tumor types (Supplementary Tables 2–5). Prognostic biomarkers will most likely be disease specific and have the potential to aid the clinical management of cancer. Establishment of a predictive biomarker (Figure 1) remains a challenge, as discovery and validation will have to be tailored to the known mechanisms of action of a certain agent in a certain disease, and will probably necessitate standardization of costly, sophisticated protocols. Nonetheless, the benefit to patients—once these predictive biomarkers are established—is clear.
Figure 1.
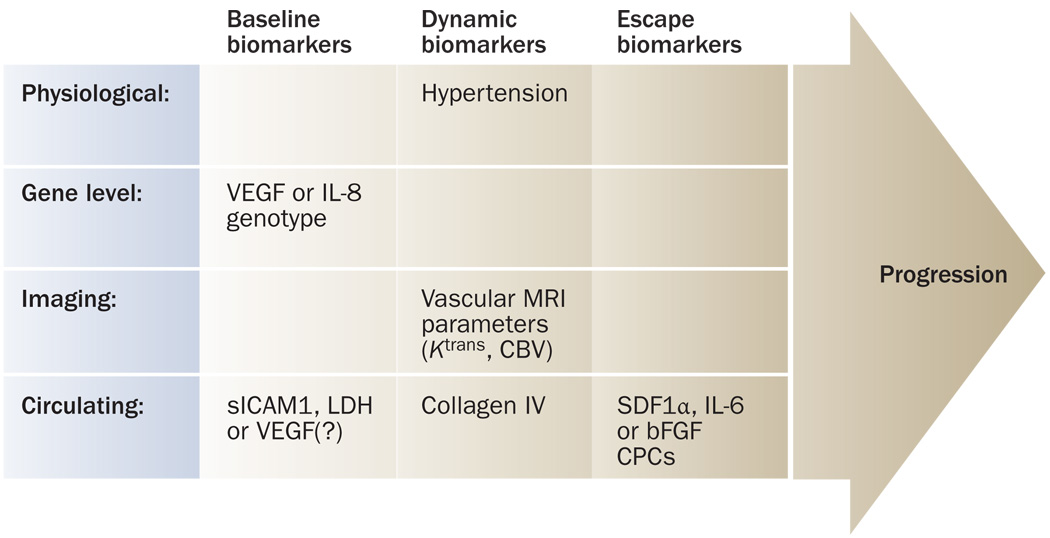
Candidate biomarkers of response and resistance to antiangiogenic therapy. At baseline, the genotype of VEGF and/or IL-8 might associate with outcome of bevacizumab with chemotherapy. Randomized trials will establish if the biomarkers are predictive or prognostic. Among dynamic biomarkers, the extent of hypertension, decrease in Ktrans (in patients with glioblastoma or hepatocellular carcinoma), increase in small (cerebral) blood vessel volume (glioblastoma) and/or increase in circulating collagen IV (glioblastoma) might be predictive of outcome of antiangiogenic therapy. Finally, molecular pathways such as SDF1α (in glioblastoma, hepatocellular carcinoma or renal-cell carcinoma), IL-6 (in hepatocellular carcinoma or renal-cell carcinoma) or bFGF (glioblastoma), and CPCs (in hepatocellular carcinoma) might be associated with resistance to antiangiogenic therapy. Abbreviations: bFGF, basic fibroblast growth factor, CBV, cerebral blood volume; CPC, circulating progenitor cell; IL, interleukin; LDH, lactate dehydrogenase; SDF1α, stromal-cell-derived factor 1α; sICAM1, soluble intracellular adhesion molecule 1.
Toxicity biomarkers
Avoiding serious toxic effects is critical in oncology, as most regimens contain potent cytotoxic drugs. It has been difficult to identify biomarkers of toxicity, primarily because of the low incidence of serious adverse events (for example, hemorrhage, perforations). Retrospective analysis of data from patients with lung cancer treated with chemotherapy plus bevacizumab in a phase III trial showed that tumor cavitation pretreatment might be a potential biomarker of pulmonary hemorrhage.73 Analysis of the VEGF-634 CC and VEGF-1498 TT genotypes in patients with metastatic breast cancer treated with bevacizumab plus chemotherapy showed significant associations with reduced risk of grade 3 or 4 hypertension.38 The clinical benefit in this study, however, was more prevalent in patients who developed grade 3 or 4 hypertension, which raises important questions as to whether this toxic effect should be a dose-limiting one.38 Collectively, these data show that the quest to establish biomarkers of toxicity will be challenging. Identifying the mechanisms underlying serious toxic effects might enable the discovery of such biomarkers.
Resistance biomarkers
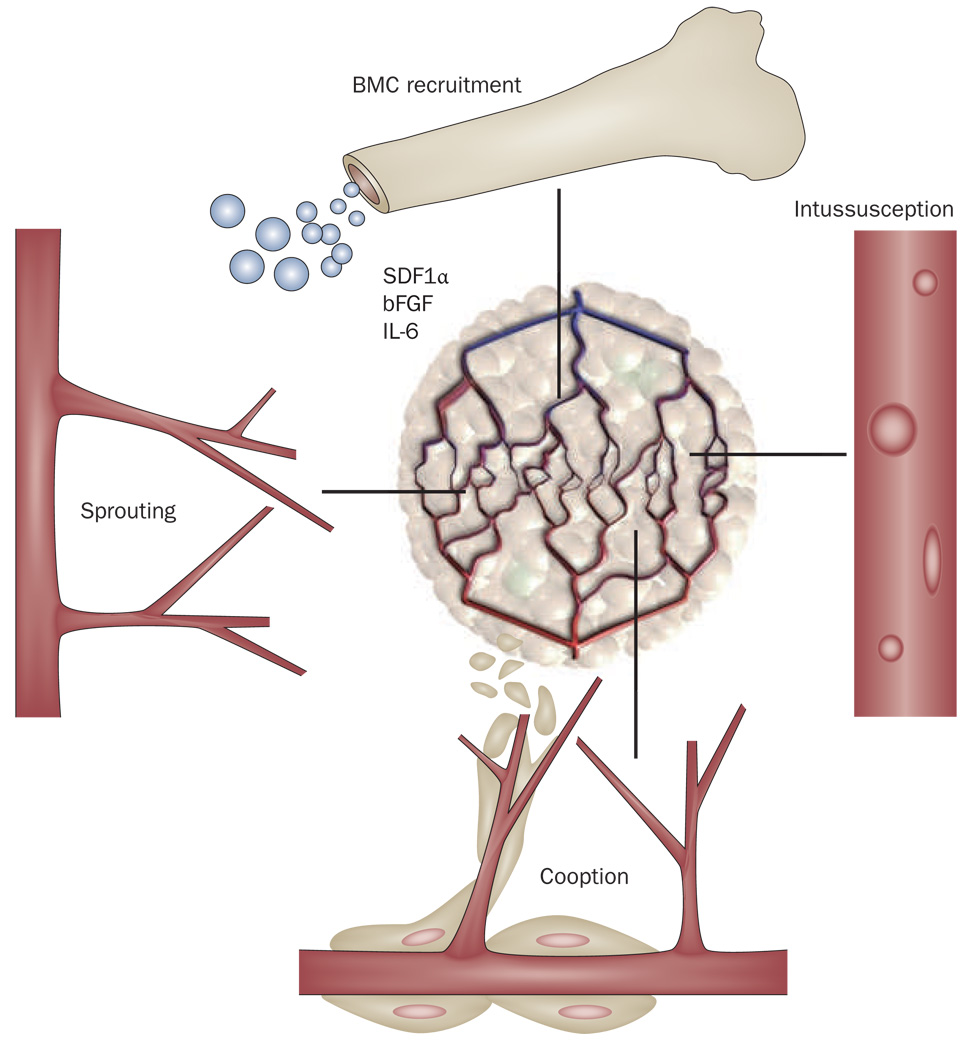
Clinical experience has shown that antivascular effects of antiangiogenic agents are transient, and that tumors remain vascularized, probably by using or activating alternative proangiogenic pathways (Figure 2 and Supplementary Table 6). This hypothesis has been tested and proven in several preclinical studies,20,74–76 and clinical experience confirms that recurrent tumors are often highly vascularized after antiangiogenic therapy. Tumor tissues are difficult to obtain at recurrence after therapy, so most of the evidence has been obtained by studying circulating biomarkers. In phase II studies, our group has found that elevated plasma basic fibroblast growth factor and SDF1α in patients with recurrent glioblastoma receiving cediranib, and elevated plasma SDF1α and IL-6 and circulating progenitor cells in patients with advanced HCC treated with sunitinib, were associated with a poor outcome.23,37 Although these proangiogenic and proinflammatory biomarkers of resistance might not directly help in the clinical management of patients, they may aid in the identification of new targets and ideally, in the future, by allowing design of combinatorial schemes for individualized antiangiogenic therapy.
Figure 2.
Modes of vessel recruitment to tumors that might be involved in tumor escape from antiangiogenic therapy. Abbreviations: bFGF, basic fibroblast growth factor; BMC, bone marrow cell; IL-6, interleukin 6; SDF1α, stromal-cell-derived factor 1α. Adapted with permission from Nature Publishing Group © Carmeliet, P. and Jain, R. K. Nature 407, 249–257 (2000).
Alternative proangiogenic pathways might have a key role in cancer resistance to antiangiogenic therapy. Fortunately, since the interactions between these pathways can be studied preclinically, and because many of these targets can be inhibited with drugs, there is optimism that combinations of antiangiogenic agents or multitargeted antiangiogenic agents will substantially improve the outcomes of this therapy beyond a few months.
Conclusions and future perspectives
With increasing numbers of antiangiogenic agents being approved, or considered for approval, the need for biomarkers is more critical than ever for efficacy, safety, and cost considerations. Preliminary biomarker data are emerging. These data will have to be tested and validated in large, well-designed, prospective clinical trials. Biomarker selection would be greatly supported if we achieved a better understanding of the mechanism of action of these agents in cancer patients. Finally, once the candidate biomarkers are identified, standardized techniques will be required to measure imaging or circulating biomarkers. Although many challenges remain, future validation of biomarkers and their eventual incorporation into clinical practice holds promise for improved cancer treatment with anti angiogenic agents. Now, a collaborative effort between pharmaceutical companies, governmental agencies and private foundations is needed to realize this goal.
Review criteria
Information on clinical trials of antiangiogenic agents (available from the NIH databases) and the publications related to these studies were retrieved from the NIH website (http://www.clinicaltrials.gov), using the search engine on this site. PubMed was searched for studies of antiangiogenic agents using Entrez for articles published before 24 February 2009, including early-release publications. Search terms included “cancer”, “clinical trial”, “biomarker”, “anti-angiogenesis”, “anti-vascular”, “imaging”, and “tyrosine kinase inhibitor”. Full articles were checked for additional material when appropriate. The results of unpublished data conveyed to the authors by personal communication have also been included.
Supplementary Material
Acknowledgments
The authors thank the members of the Steele Lab, especially M. Ancukiewicz, Y. Boucher, E. di Tomaso, and L. Xu and M. Buyse, H. Chen, A. Grothey, C. Hudis, R. Horvitz, and A. Marshall for their helpful comments on this manuscript. The authors’ work is supported by grants from the National Cancer Institute P01-CA80124, P41-RR14075, R01-CA115767, R01-CA85140, R01CA126642, R21-CA99237, R21-CA117079, R01-CA129371, R01CA57683, K24-CA125440, Federal Share/NCI Proton Beam Program Income, M01-RR-01066, Harvard Clinical and Translational Science Center (CTSC) grant; the National Foundation for Cancer Research; the Richard and Nancy Simches Endowment for Brain Tumor Research; the Montesi Family Fund; and MIND Institute.
Footnotes
Competing interests
R. K. Jain declares associations with the following companies: AstraZeneca, Dyax, Millenium, Pfizer, Roche and SynDevRx. C. G. Willett declares associations with the following company: Genentech. A. X. Zhu declares associations with the following companies: Bayer and Genentech. T. T. Batchelor declares associations with the following companies: AstraZeneca, EMD-Serono, Exelixis, Genentech, ImClone Systems, Millenium and Schering-Plough. A. G. Sorensen declares associations with the following companies: AstraZeneca, Exelixis, Genentech, Millenium, Novartis and Schering-Plough. See the article online for full details of the relationships. The other authors declare no competing interests.
Supplementary Information is linked to the online version of the paper at www.nature.com/nrclinonc
References
- 1.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 2.Jain RK. Molecular regulation of vessel maturation. Nat. Med. 2003;9:685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- 3.Folkman J. Tumor angiogenesis: therapeutic implications. N. Engl. J. Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 4.Hurwitz H, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 5.Miller K, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N. Engl. J. Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 6.Rini BI, et al. Bevacizumab plus interferon alfa compared with interferon alfa monotherapy in patients with metastatic renal cell carcinoma: CALGB 90206. J. Clin. Oncol. 2008;26:5422–5328. doi: 10.1200/JCO.2008.16.9847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sandler A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N. Engl. J. Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 8.Escudier B, et al. sorafenib in advanced clear-cell renal-cell carcinoma. N. Engl. J. Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 9.Motzer RJ, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N. Engl. J. Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 10.Llovet JM, et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 11.Giantonio BJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J. Clin. Oncol. 2007;25:1539–1544. doi: 10.1200/JCO.2006.09.6305. [DOI] [PubMed] [Google Scholar]
- 12.Demetri GD, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006;368:1329–1338. doi: 10.1016/S0140-6736(06)69446-4. [DOI] [PubMed] [Google Scholar]
- 13.Hughes B. Novel risk-sharing scheme puts the spotlight on biomarkers. Nat. Rev. Drug Discov. 2007;6:945. [Google Scholar]
- 14.Sinha G. Expensive cancer drugs with modest benefit ignite debate over solutions. J. Natl. Cancer Inst. 2008;100:1347–1349. doi: 10.1093/jnci/djn357. [DOI] [PubMed] [Google Scholar]
- 15.Karapetis CS, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N. Engl. J. Med. 2008;359:1757–1765. doi: 10.1056/NEJMoa0804385. [DOI] [PubMed] [Google Scholar]
- 16.Oldenhuis CN, Oosting SF, Gietema JA, de Vries EG. Prognostic versus predictive value of biomarkers in oncology. Eur. J. Cancer. 2008;44:946–953. doi: 10.1016/j.ejca.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 17.Mcshane LM, et al. Reporting recommendations for tumor marker prognostic studies (REMARK) Nat. Clin. Pract. Oncol. 2005;2:416–422. [PubMed] [Google Scholar]
- 18.Batchelor TT, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11:83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ebos JM, Lee CR, Christensen JG, Mutsaers AJ, Kerbel RS. Multiple circulating proangiogenic factors induced by sunitinib malate are tumor-independent and correlate with antitumor efficacy. Proc. Natl Acad. Sci. USA. 2007;104:17069–17074. doi: 10.1073/pnas.0708148104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamoun WS, et al. Edema control by cediranib, a vascular endothelial growth factor receptor-targeted kinase inhibitor, prolongs survival despite persistent brain tumor growth in mice. J. Clin. Oncol. 2009 doi: 10.1200/JCO.2008.19.9356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Norden-Zfoni A, et al. Blood-based biomarkers of SU11248 activity and clinical outcome in patients with metastatic imatinib-resistant gastrointestinal stromal tumor. Clin. Cancer Res. 2007;13:2643–2650. doi: 10.1158/1078-0432.CCR-06-0919. [DOI] [PubMed] [Google Scholar]
- 22.Willett CG, et al. Efficacy, safety and biomarkers of neoadjuvant bevacizumab, radiation therapy and 5-Fluorouracil in rectal cancer: a multidisciplinary phase II study. J. Clin. Oncol. 2009 doi: 10.1200/JCO.2008.21.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu AX, et al. Efficacy, safety and potential biomarkers of sunitinib monotherapy in advanced hepatocellular carcinoma: A phase II study. J. Clin. Oncol. doi: 10.1200/JCO.2008.20.9908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crabb SJ, et al. Tumor cavitation: impact on objective response evaluation in trials of angiogenesis inhibitors in non-small-cell lung cancer. J. Clin. Oncol. 2009;27:404–410. doi: 10.1200/JCO.2008.16.2545. [DOI] [PubMed] [Google Scholar]
- 25.Hudis CA. Trastuzumab—mechanism of action and use in clinical practice. N. Engl. J. Med. 2007;357:39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- 26.Cameron DA, Stein S. Drug Insight: intracellular inhibitors of HER2–clinical development of lapatinib in breast cancer. Nat. Clin. Pract. Oncol. 2008;5:512–520. doi: 10.1038/ncponc1156. [DOI] [PubMed] [Google Scholar]
- 27.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 28.Jain RK, Duda DG, Clark JW, Loeffler JS. Lessons from phase III clinical trials on anti-VEGF therapy for cancer. Nat. Clin. Pract. Oncol. 2006;3:24–40. doi: 10.1038/ncponc0403. [DOI] [PubMed] [Google Scholar]
- 29.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 30.Winkler F, et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell. 2004;6:553–563. doi: 10.1016/j.ccr.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 31.Willett CG, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat. Med. 2004;10:145–147. doi: 10.1038/nm988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Willett CG, et al. Surrogate markers for antiangiogenic therapy and dose-limiting toxicities for bevacizumab with radiation and chemotherapy: continued experience of a phase I trial in rectal cancer patients. J. Clin. Oncol. 2005;23:8136–8139. doi: 10.1200/JCO.2005.02.5635. [DOI] [PubMed] [Google Scholar]
- 33.Kerbel RS. Tumor angiogenesis. N. Engl. J. Med. 2008;358:2039–2049. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rini BI, et al. Association of diastolic blood pressure (dBP) ≥90 mmHg with overall survival (OS) in patients treated with axitinib (AG-013736) ASCO Meeting Abstracts. 2008;26:3543. [Abstract] [Google Scholar]
- 35.shojaei F, et al. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+Gr1+ myeloid cells. Nat. Biotechnol. 2007;25:911–920. doi: 10.1038/nbt1323. [DOI] [PubMed] [Google Scholar]
- 36.Hamzah J, et al. Vascular normalization in Rgs5-deficient tumours promotes immune destruction. Nature. 2008;453:410–414. doi: 10.1038/nature06868. [DOI] [PubMed] [Google Scholar]
- 37.Batchelor TT, et al. A multidisciplinary phase II study of AZD2171 (cediranib), an oral pan-VEGF receptor tyrosine kinase inhibitor, in patients with recurrent glioblastoma. AACR Meeting Abstracts. 2008 [Abstract] LB-247. [Google Scholar]
- 38.Schneider BP, et al. Association of vascular endothelial growth factor and vascular endothelial growth factor receptor-2 genetic polymorphisms with outcome in a trial of paclitaxel compared with paclitaxel plus bevacizumab in advanced breast cancer: ECOG 2100. J. Clin. Oncol. 2008;26:4672–4678. doi: 10.1200/JCO.2008.16.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burstein HJ, et al. VEGF as a marker for outcome among advanced breast cancer patients receiving anti-VEGF therapy with bevacizumab and vinorelbine chemotherapy. Clin. Cancer Res. 2008;14:7871–7877. doi: 10.1158/1078-0432.CCR-08-0593. [DOI] [PubMed] [Google Scholar]
- 40.Jubb AM, et al. Impact of vascular endothelial growth factor-A expression, thrombospondin-2 expression, and microvessel density on the treatment effect of bevacizumab in metastatic colorectal cancer. J. Clin. Oncol. 2006;24:217–227. doi: 10.1200/JCO.2005.01.5388. [DOI] [PubMed] [Google Scholar]
- 41.Dowlati A, Gray R, Sandler AB, Schiller JH, Johnson DH. Cell adhesion molecules, vascular endothelial growth factor, and basic fibroblast growth factor in patients with non-small cell lung cancer treated with chemotherapy with or without bevacizumab—an Eastern Cooperative Oncology Group Study. Clin. Cancer Res. 2008;14:1407–1412. doi: 10.1158/1078-0432.CCR-07-1154. [DOI] [PubMed] [Google Scholar]
- 42.Jain RK. Lessons from multidisciplinary translational trials on anti-angiogenic therapy of cancer. Nat. Rev. Cancer. 2008;8:309–316. doi: 10.1038/nrc2346. [DOI] [PubMed] [Google Scholar]
- 43.Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat. Rev. Drug Discov. 2004;3:391–400. doi: 10.1038/nrd1381. [DOI] [PubMed] [Google Scholar]
- 44.Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J. Clin. Oncol. 2002;20:4368–4380. doi: 10.1200/JCO.2002.10.088. [DOI] [PubMed] [Google Scholar]
- 45.Sorensen AG, Batchelor TT, Wen PY, Zhang WT, Jain RK. Response criteria for glioma. Nat. Clin. Pract. Oncol. 2008;5:634–644. doi: 10.1038/ncponc1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heymach JV, et al. Baseline VEGF as a potential predictive biomarker of vandetanib clinical benefit in patients with advanced NSCLC. ASCO Meeting Abstracts. 2008;26:8009. [Abstract] [Google Scholar]
- 47.Tannir NM, et al. A randomized phase II trial of sorafenib versus sorafenib plus low-dose interferon-alfa: Clinical results and biomarker analysis. ASCO Meeting Abstracts. 2008;26:5093. [Abstract] [Google Scholar]
- 48.Rini BI, et al. Antitumor activity and biomarker analysis of sunitinib in patients with bevacizumab-refractory metastatic renal cell carcinoma. J. Clin. Oncol. 2008;26:3743–3748. doi: 10.1200/JCO.2007.15.5416. [DOI] [PubMed] [Google Scholar]
- 49.Deprimo SE, et al. Circulating protein biomarkers of pharmacodynamic activity of sunitinib in patients with metastatic renal cell carcinoma: modulation of VEGF and VEGF-related proteins. J. Transl. Med. 2007;5:32. doi: 10.1186/1479-5876-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Drevs J, et al. Phase I clinical study of AZD2171, an oral vascular endothelial growth factor signaling inhibitor, in patients with advanced solid tumors. J. Clin. Oncol. 2007;25:3045–3054. doi: 10.1200/JCO.2006.07.2066. [DOI] [PubMed] [Google Scholar]
- 51.Mita MM, et al. A phase II, pharmacokinetic, and biologic study of semaxanib and thalidomide in patients with metastatic melanoma. Cancer Chemother. Pharmacol. 2007;59:165–174. doi: 10.1007/s00280-006-0255-0. [DOI] [PubMed] [Google Scholar]
- 52.Motzer RJ, et al. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J. Clin. Oncol. 2006;24:16–24. doi: 10.1200/JCO.2005.02.2574. [DOI] [PubMed] [Google Scholar]
- 53.Saltz LB, et al. Phase II trial of sunitinib in patients with metastatic colorectal cancer after failure of standard therapy. J. Clin. Oncol. 2007;25:4793–4799. doi: 10.1200/JCO.2007.12.8637. [DOI] [PubMed] [Google Scholar]
- 54.Fischer C, et al. Anti-PIGF inhibits growth of VEGF(R)-inhibitor-resistant tumors without affecting healthy vessels. Cell. 2007;131:463–475. doi: 10.1016/j.cell.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 55.Verheul HM, et al. Platelets take up the monoclonal antibody bevacizumab. Clin. Cancer Res. 2007;13:5341–5347. doi: 10.1158/1078-0432.CCR-07-0847. [DOI] [PubMed] [Google Scholar]
- 56.Ebos JM, et al. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell. 2009;15:232–239. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Phase III C-08 study of Avastin in early-stage colon cancer does not meet primary endpoint. 2009 [online] http://www.roche.com/media_releases/med-cor-009-04-22.htm.
- 58.Horowitz NS, et al. A multidisciplinary phase II study of bevacizumab combined with oxaliplatin, gemcitabine in women with recurrent mullerian carcinoma. AACR Annual Abstracts. 2008:4484. [Abstract] [Google Scholar]
- 59.Ince WL, et al. Association of k-ras, b-raf, and p53 status with the treatment effect of bevacizumab. J. Natl Cancer Inst. 2005;97:981–989. doi: 10.1093/jnci/dji174. [DOI] [PubMed] [Google Scholar]
- 60.Wilson PM, et al. Use of intratumoral mRNA expression of genes involved in angiogenesis and HIF1 pathway to predict outcome to VEGFR tyrosine kinase inhibitor in patients enrolled in CONFIRM1 and CONFIRM2. ASCO Meeting Abstracts. 2008;26:4002. [Abstract] [Google Scholar]
- 61.Kohne C, et al. Final results of CONFIRM 2: A multinational, randomized, double-blind, phase III study in 2nd line patients with metastatic colorectal cancer receiving FOLFOX4 and PTK787/ZK 222584 (PTK/ZK) or placebo. ASCO Meeting Abstracts. 2007;25:4033. [Abstract] [Google Scholar]
- 62.Schultheis AM, et al. Polymorphisms and clinical outcome in recurrent ovarian cancer treated with cyclophosphamide and bevacizumab. Clin. Cancer Res. 2008;14:7554–7563. doi: 10.1158/1078-0432.CCR-08-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Loges S, Mazzone M, Hohensinner P, Carmeliet P. Silencing or fueling metastasis with VEGF inhibitors: antiangiogenesis revisited. Cancer Cell. 2009;15:167–170. doi: 10.1016/j.ccr.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 64.Paez-Ribes M, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Duda DG, et al. A comparative study of circulating endothelial cells (CECs) and circulating progenitor cells (CPCs) kinetics in four multidisciplinary phase 2 studies of antiangiogenic agents. ASCO Meeting Abstracts. 2008;26:3544. [Abstract] [Google Scholar]
- 66.Patterson DM, Padhani AR, Collins DJ. Technology insight: water diffusion MRI—a potential new biomarker of response to cancer therapy. Nat. Clin. Pract. Oncol. 2008;5:220–233. doi: 10.1038/ncponc1073. [DOI] [PubMed] [Google Scholar]
- 67.Hamstra DA, et al. Functional diffusion map as an early imaging biomarker for high-grade glioma: correlation with conventional radiologic response and overall survival. J. Clin. Oncol. 2008;26:3387–3394. doi: 10.1200/JCO.2007.15.2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sorensen AG. Magnetic resonance as a cancer imaging biomarker. J. Clin. Oncol. 2006;24:3274–3281. doi: 10.1200/JCO.2006.06.6597. [DOI] [PubMed] [Google Scholar]
- 69.Sorensen AG, et al. A “vascular normalization index” as potential mechanistic biomarker to predict survival after a single dose of cediranib in recurrent glioblastoma patients: Insights from a phase II study. Cancer Res. doi: 10.1158/0008-5472.CAN-09-0814. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen W, et al. Predicting treatment response of malignant gliomas to bevacizumab and irinotecan by imaging proliferation with [18F] fluorothymidine positron emission tomography: a pilot study. J. Clin. Oncol. 2007;25:4714–4721. doi: 10.1200/JCO.2006.10.5825. [DOI] [PubMed] [Google Scholar]
- 71.Tofts PS, et al. Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J. Magn. Reson. Imaging. 1999;10:223–232. doi: 10.1002/(sici)1522-2586(199909)10:3<223::aid-jmri2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 72.Motzer RJ, et al. Prognostic nomogram for sunitinib in patients with metastatic renal cell carcinoma. Cancer. 2008;113:1552–1558. doi: 10.1002/cncr.23776. [DOI] [PubMed] [Google Scholar]
- 73.Sandler AB, et al. Retrospective evaluation of the clinical and radiographic risk factors associated with severe pulmonary hemorrhage in first-line advanced, unresectable non-small-cell lung cancer treated with carboplatin and paclitaxel plus bevacizumab. J. Clin. Oncol. 2009;27:1405–1412. doi: 10.1200/JCO.2008.16.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Casanovas O, Hicklin DJ, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell. 2005;8:299–309. doi: 10.1016/j.ccr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 75.Relf M, et al. Expression of the angiogenic factors vascular endothelial cell growth factor, acidic and basic fibroblast growth factor, tumor growth factor beta-1, platelet-derived endothelial cell growth factor, placenta growth factor, and pleiotrophin in human primary breast cancer and its relation to angiogenesis. Cancer Res. 1997;57:963–969. [PubMed] [Google Scholar]
- 76.Yoshiji H, Harris SR, Thorgeirsson UP. Vascular endothelial growth factor is essential for initial but not continued in vivo growth of human breast carcinoma cells. Cancer Res. 1997;57:3924–3928. [PubMed] [Google Scholar]
- 77.Liu G, et al. Dynamic contrast-enhanced magnetic resonance imaging as a pharmacodynamic measure of response after acute dosing of AG-013736, an oral angiogenesis inhibitor, in patients with advanced solid tumors: results from a phase I study. J. Clin. Oncol. 2005;23:5464–5473. doi: 10.1200/JCO.2005.04.143. [DOI] [PubMed] [Google Scholar]
- 78.Thomas AL, et al. Phase I study of the safety, tolerability, pharmacokinetics, and pharmacodynamics of PTK787/ZK 222584 administered twice daily in patients with advanced cancer. J. Clin. Oncol. 2005;23:4162–4171. doi: 10.1200/JCO.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 79.Zhu AX, et al. Early antiangiogenic activity of bevacizumab evaluated by computed tomography perfusion scan in patients with advanced hepatocellular carcinoma. Oncologist. 2008;13:120–125. doi: 10.1634/theoncologist.2007-0174. [DOI] [PubMed] [Google Scholar]
- 80.Yao JC, et al. Targeting vascular endothelial growth factor in advanced carcinoid tumor: a random assignment phase II study of depot octreotide with bevacizumab and pegylated interferon alpha-2b. J. Clin. Oncol. 2008;26:1316–1323. doi: 10.1200/JCO.2007.13.6374. [DOI] [PubMed] [Google Scholar]
- 81.de Bazelaire C, et al. Magnetic resonance imaging-measured blood flow change after antiangiogenic therapy with PTK787/ZK 222584 correlates with clinical outcome in metastatic renal cell carcinoma. Clin. Cancer Res. 2008;14:5548–5554. doi: 10.1158/1078-0432.CCR-08-0417. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.