Abstract
Complexes of specific assembly factors and generic endoplasmic reticulum (ER) chaperones collectively called the major histocompatibility complex (MHC) class I peptide loading complex (PLC) function in the folding and assembly of MHC class I molecules. The glycan binding chaperone calreticulin and partner oxidoreductase ERp57 are important in MHC class I assembly, but the sequence of assembly events and specific interactions involved remain incompletely understood. We show that the recruitments of calreticulin and ERp57 to the PLC are co-dependent and also dependent upon the ERp57 binding site and the glycan of the assembly factor tapasin. Furthermore, the ERp57 binding site and the glycan of tapasin enhance β2m and MHC class I heavy chain recruitment to the PLC, with the ERp57 binding site having the dominant effect. On the other hand, the conserved MHC class I heavy chain glycan played a minor role in calreticulin recruitment into the PLC, but impacted the recruitment of heavy chains into the PLC, and glycan-deficient heavy chains were impaired for tapasin-independent and tapasin-assisted assembly. The conserved MHC class I glycan and tapasin facilitated an early step in the assembly of heavy chain-β2m heterodimers, for which tapasin-ERp57 or tapasin-calreticulin complexes were not required. Together, these studies provide insights into how PLCs are constructed, demonstrate two distinct mechanisms by which PLCs can be stabilized, and suggest the presence of intermediate heavy chain-deficient PLCs.
INTRODUCTION
The MHC class I molecule is a heterotrimer composed of a heavy chain (HC), a light chain (β2-microglobulin or β2m) and an 8–9 residue peptide. MHC class I HC assembly with β2m and peptide is facilitated by a multi-protein peptide loading complex (PLC) composed of the transporter associated with antigen processing (TAP), an assembly factor tapasin, the thiol oxido-reducatse ERp57 and the endoplasmic reticulum (ER) chaperones calreticulin and calnexin (1). TAP is an ATP-binding cassette (ABC) transporter involved in translocation of peptides from the cytosol into the ER. Tapasin acts as a bridge between the PLC components and TAP and facilitates loading of high affinity peptides onto MHC class I molecules. Following loading of high affinity peptides, MHC class I molecules dissociate from the PLC and are transported to the cell surface for recognition by CD8+ T cells (1).
The specific function of each protein in MHC class I assembly, and the sequence of assembly events are not completely understood. MHC class I folding is facilitated by the lectin chaperones calnexin and calreticulin. Calnexin and calreticulin typically interact with monoglucosylated glycans on substrate glycoproteins via a carbohydrate-binding site present within a globular domain (2). Calnexin and calreticulin also interact with their partner ER oxidoreductase ERp57 via an elongated β-stranded hairpin structure called the P-domain (3). An oligosaccharide structure containing three glucose resides (Glc3Man9GlcNAc2) is initially attached to asparagine residues on newly-synthesized glycoproteins. This oligosaccharide structure is modified to a monoglucosylated form by glucosidases I and II, which allows for recognition by calnexin and calreticulin. Following correct folding, the glycoprotein is deglucosylated by glucosidase II, resulting in release from calnexin and calreticulin (4). Previous studies have shown that MHC class I binding to calreticulin and TAP are impacted by blocking glucosidases I and II with the inhibitor castanospermine, or by inducing MHC class I deglycosylation by point mutations of a highly conserved N-linked glycosylation site at asparagine 86 (5–7).
Calreticulin plays a more critical role in MHC class I assembly than calnexin (8, 9), with calreticulin deficiency resulting in reduced cell surface MHC class I (10, 11), enhanced intracellular trafficking rates of MHC class I molecules (10, 11), and reduced steady-state levels of MHC class I heavy chains and tapasin (12). We recently showed that the glycan and ERp57 binding sites of calreticulin are important for its recruitment into the PLC and for the assembly-promoting functions of calreticulin. These studies suggested that calreticulin binding to the glycans of MHC class I or tapasin or both molecules could be involved in the recruitment of calreticulin into the PLC (12). Within the PLC, ERp57 is recruited to tapasin via a disulfide-linked interaction between C57 of ERp57 and C95 of tapasin (13), and mutation of tapasin C95 abrogates tapasin-ERp57 binding in human cells (13–16), and reduces the efficiency of calreticulin recruitment to the PLC (14, 16). Since calreticulin and ERp57 are able to interact independently of other PLC components (3), P domain-dependent binding between calreticulin and ERp57 could serve as a point for the recruitment of calreticulin into the PLC, in addition to a glycan within the PLC (MHC class I and/or tapasin). To better understand the interactions and functions mediated by the glycans of tapasin and MHC class I molecules, in this study, we used tapasin and heavy chain glycan mutants to show that the glycan of tapasin influences the recruitment of calreticulin into the PLC. On the other hand, the conserved MHC class I glycan plays a minor role in calreticulin recruitment into the PLC, but is important for MHC class I heavy chain recruitment into the PLC and for the assembly of MHC class I heavy chain-β2m complexes. These studies provided new insights into intermediate complexes of the MHC class I assembly pathway and suggest that intermediate complexes can function as a platform for peptide exchange.
MATERIALS AND METHODS
Cell lines
A human melanoma cell line M553 (17) (obtained from Dr. Naveen Bangia) and 721.221 (18) (obtained from Dr. Robert DeMars) were grown in RPMI 1640 (Gibco). BOSC cells (obtained from Dr. Kathleen Collins) (19). were grown in DMEM (Gibco). All growth media were supplemented with 10% (v/v) fetal bovine serum (Gibco), 100 μg/ml streptomycin and 100 U/ml of penicillin (Gibco). For 721.221 cells, the media was also supplemented with 1X L-glutamine.
Mice
C57Bl/6 and β2m−/− mice were purchased from The Jackson Laboratory. All mice were maintained in a specific pathogen free mouse facility. The mice were cared for according to the guidelines set by the University of Michigan Committee on Use and Care of Animals (UCUCA).
DNA constructs
The construction of retroviral vectors encoding wild-type and C95A human tapasin has been previously described (16). The N233Q mutants on the wild-type and tapasin (C95A) backgrounds were generated using the QuikChange site-directed mutagenesis kit (Stratagene). The MSCV-puro vector expressing wild-type or C95A tapasin was used as template and mutated using the following oligonucleotides, 5′-GGCCCATGGACCGGACAGGGGACCTTCTGGCTG-3′ and 5′-CAGCCAGAAGGTCCCCTGTCCGGTCCATGGGCC-3′. The retroviral vector encoding influenza hemagglutinin(HA)-tagged HLA-A2 (HA-HLA-A2) was obtained from Dr. Kathleen Collins (20). The N86Q mutation of wild-type HA-HLA-A2 was generated using QuikChange site-directed mutagenesis kit. The MSCV-neo vector expressing wild-type HA-HLA-A2 was used as template and mutated using the following oligonucleotides, 5′-CTGCGCGGCTACTACCAGCAGAGCGAGGCCGGT-3′ and 5′-ACCGGCCTCGCTCTGCTGGTAGTAGCCGCGCAG-3′. HLA-B*3503 was cloned into the pMSCV-neo vector using ligation-independent cloning (LIC) as previously described (12), but using the plasmid pMSCV-neo as the parent vector for construction of a ligation-independent-cloning (LIC) variant. This was accomplished by inserting a unique sequence that can be opened with the restriction enzyme Pme I. LIC overhangs were generated by treatment of the linearized vector with T4 DNA polymerase exonuclease activity. The inserted sequence was constructed by annealing two oligos to generate a fragment with overhangs complimentary to an EcoR I restriction site. The oligo sequences used were 5′-AATTAGAGAGTTTAAACTTCCAC-3′ and 5′-AATTGTGGAAGTTTAAACTCTCT-3′. The lyophilized oligos were resuspended in distilled water at a concentration of 20 μM. Prior to annealing these were phosphorylated using T4 polynucleotide kinase (New England Biolabs). The 20 μl reactions contained 2 μl of 10 mM ATP, 12.5 μl of 20 mM oligo, 2 μl of 10X reaction buffer, 1 μl of enzyme and 2.5 μl of distilled water. Reactions were incubated for 1 h at 37 °C then shifted to 95 °C for an additional 10 min. The two oligo reactions were then mixed together at 95 °C and slowly cooled to 37 °C. A 2.5 μl aliquot was then added to a 20 μl ligation reaction composed of 1 μl of 45 ng/μl EcoR I cut pMSCV-neo, previously treated with calf intestinal phosphatase, 2 μl 10X reaction buffer, and 13.5 μl of distilled water. The reaction was slowly cooled from 37 °C to 16 °C and 1 μl of 1unit/μl T4 DNA ligase (New England Biolabs) was added. Incubation was continued at 16 °C for approximately 16 h. A 2 μl aliquot of the ligation reaction was used to transform 20 μl of competent XL1-Blue cells. These were plated on Luria Broth (LB) agar with ampicillin (Amp) selection and incubated at 37 °C overnight. Colonies were picked and grown in 5 ml of LB-Amp and plasmid DNA was isolated using Qiagen minispin columns. Positives clones were identified by restriction digestion with Pme I and Cla I and confirmed by DNA sequencing. Oligo sequences for insertion of the HLA-B*3503 gene into LIC-pMSCV-neo were: Start – 5′-GGAATTAGAGAGTTTCACCATGCGGGTCACGGC-3′ and Stop – 5′-GAATTGTGGAAG TTTCCTAAGCTGTGAGAGACACATC-3′. The screening oligos used were: Forward–5′-CACCCTAAGCCTCCGCCTCC-3′ and Reverse 5′-AATGTGTGCGAGGCCAGAGGCC-3′.
Viruses and infections
Retroviruses were generated in BOSC cells using the different tapasin constructs in the MSCV-puro vector or different HA-HLA-A2 constructs or untagged HLA-B*3503 in the MSCV-neo vectors using previously described procedures (16, 20). 5.5 μg of retroviral vectors encoding tapasin, HA-HLA-A2, HA-HLA-A2(N86Q), HLA-B*3503 or control empty vectors were mixed with 4 μg pCL-Eco DNA and 0.5 μg VSV-G encoding plasmid, and added to a mixture of Opti-MEM (Invitrogen) and FuGENE 6 (Roche). Following incubation for 20 min at RT, the mixtures were added to BOSC cells that had been grown to 70% confluency in a 10 cm tissue culture dish. Media was changed after 24 h, and after 48 h, supernatants containing retroviruses were harvested, filtered and used to infect M553 or 721.221 cells. Infected cells were selected by treatment with 1 μg/ml puromycin (Sigma) or 1 mg/ml Geneticin (Invitrogen) and maintained in 0.5 μg/ml puromycin or 0.5 mg/ml Geneticin. After verifying HA-HLA-A2 and HA-HLA-A2(N86Q) expression by flow cytometric analyses and by immunoblotting analyses of cell lysates with anti-HA (Covance), M553/A2 cells were transduced with the tapasin-encoding retroviruses, and selected by treatment with 1 μg/ml puromycin (Sigma). 721.221 cells were infected with retroviruses encoding HA-HLA-A2, HA-HLA-A2N86Q or HLA-B*3503 by spin infections, selected and maintained in Geneticin as above.
FACS analysis to assess MHC class I cell surface expression
1×105–106 cells were washed three times with FACS buffer (PBS pH 7.4 containing 1% FBS) and then incubated with W6/32 or anti-HA (Covance) at 1:250 dilutions for 1 h on ice. Following this incubation, the cells were washed three times with FACS buffer and incubated with FITC-conjugated goat anti-mouse IgG (Jackson Laboratories) at 1:250 dilutions for 1 h on ice. Following incubation the cells were washed three times with FACS buffer and analyzed using a FACS-Canto Cytometer. The FACS data was analyzed with WinMDI software (Joe Trotter, The Scripps Institute, Flow Cytometry Core Facility).
Immunoprecipitation analyses
M553 cells expressing different tapasin constructs were treated with 500 IU IFN-γ(PeproTech) for 48 h and processed for anti-TAP1 immunoprecipitation. 721.221 cells were used without IFN-γ treatment. The cells were washed in PBS (pH 7.4.) and lysed in digitonin lysis buffer (1% digitonin (Wako Chemicals USA, Inc.) in PBS containing EDTA-free protease inhibitors [Roche], pH 7.4) for 1 h on ice. The lysates were centrifuged at 4 °C for 30 min to remove cell debris and protein concentration in lysates was determined by a BCA protein assay (Pierce, Thermo Scientific). Equal microgram amounts of cell lysates were incubated with anti-TAP1 antisera (21) (obtained from Dr. M. J. Androlewicz) overnight at 4 °C. For anti-tapasin (Pasta-1, obtained from Dr. Peter Cresswell, Yale University) immunoprecipitations, M553 cells expressing different tapasin constructs were treated with 500 IU IFN-γ as above or not treated with IFN-γ, harvested, washed with PBS (pH 7.4) and then incubated with a membrane permeable cross-linker, Dimethyl 3,3′ dithiobispropionimidate 2HCl (DTBP) in PBS for 1 h at RT. The cross-linker was quenched with 1 M Tris pH 7.4 for 15 min at RT and cells were then washed once with PBS pH 7.4 and lysed and processed as above. Equal microgram amounts of lysates were immunoprecipitated with anti-tapasin (Pasta-1) antibody overnight at 4°C. For BBM.1(22) immunoprecipitation analysis, M553 cells expressing different tapasin constructs were harvested, washed with PBS and then incubated with the cross-linker and processed for immunoprecipitation analysis as above. The 721.221 cells were processed for BBM.1 immunoprecipitation analyses without treatment with cross-linker. Following overnight incubation, the immunoprecipitation samples were centrifuged at 13,000 rpm for 30 min at 4°C to remove non-specific precipitates and immunoprecipitates were isolated with Protein G Sepharose beads (GE Healthcare) for 1–2 h at 4°C. The beads were washed 3–4 times with lysis buffer containing 0.1% digitonin, re-suspended in reducing SDS-PAGE buffer and boiled. Samples were separated on 10–15% SDS PAGE, and transferred to Immobilon membranes (Millipore) for immunoblotting. Membranes were blocked in 5% milk in Tris Buffered Saline (TBS) for 1 h at RT, followed by an overnight incubation with primary antibody in TBS-Tween-20 at 4°C. Membranes were washed for 2 h in TBS-Tween-20, incubated for 45–60 min with secondary antibody, and washed again for 2 h. Chemiluminescence was detected using the GE Healthcare ECL Plus kit. Western blotting analysis was done using mouse anti-β2m (Biolegend) or rabbit anti-β2m antibody (Sigma), mouse anti-TAP1 antibody 148.3 (obtained from Dr. Robert Tampe, Institute of Biochemistry, Goethe University), mouse anti-tapasin antibody (23) (obtained from Dr. Soldano Ferrone, University of Pittsburgh) or rabbit anti-tapasin antisera (generated against an N-terminal peptide of tapasin, obtained from Dr. Ted Hanson, Washington University), mouse anti-ERp57 (Abcam) or rabbit polyclonal anti-ERp57 antibodies (Santa Cruz Biotechnology, Inc.), goat polyclonal anti-calreticulin antibody (Santa Cruz Biotechnology), HC10 (24) and anti-HA (Covance) antibody. The secondary antibodies used were Peroxidase AffiniPure goat anti-mouse or anti-rabbit IgG, Peroxidase AffiniPure bovine anti-goat IgG and Peroxidase AffiniPure mouse anti-rabbit IgG light chain specific (Jackson ImmunoResearch Laboratories, Inc.).
Immunoprecipitations from mouse cells
Splenocytes from wild-type (C57Bl/6) and β2m−/− mice were isolated and the red blood cells were lysed using red cell lysis buffer (Sigma R7757). The remaining splenocytes were lysed in lysis buffer (10 mM Na2HPO4, 10 mM Tris, 130 mM NaCl, 1% digitonin (Wako Chemicals USA, Inc.) and complete EDTA free protease inhibitors [Roche], pH 7.5). Samples were lysed on ice for 1 h, followed by a 30 min centrifugation for removal of cell debris. Lysate supernatants were incubated with or without indicated antibodies for 2 h at 4 °C, followed by centrifugation to remove non-specific precipitates. Following the incubation, the samples were incubated for 1 h with Protein G Sepharose beads (GE Healthcare) and the beads were washed 3 times with 0.1% digitonin lysis buffer. The proteins were separated by SDS-PAGE and analyzed by western blotting using the ECL Plus Kit (GE Healthcare) as above. A rabbit anti-mouse TAP1 serum (obtained from Dr. Ted Hansen, Washington University) was used at 1:15 dilution to immunoisolate murine TAP1 and associated proteins. A polyclonal goat anti-TAP antibody (Santa Cruz Biotechnology, Inc. cat # sc-11465) was used at 1:2000 dilution to detect TAP by immunoblot. To detect mouse calreticulin by immunoblot, a goat antibody specific to the N-terminus of mouse calreticulin (Santa Cruz Biotechnology, Inc. cat # sc-7431) was used at a 1:2000 dilution. Hamster tapasin-specific antibody and rabbit anti-β2m serum were also obtained from Dr. Ted Hansen and used at 1:3000 dilutions to detect murine tapasin and β2m respectively by immunoblotting. A rabbit anti Kb antiserum (EX8) was used to detect heavy chains by immunoblot (1:7500) and was provided by Dr. Jonathan Yewdell. To detect ERp57, a rabbit polyclonal antibody (Santa Cruz Biotechnology, Inc. cat # 28823) was used (1:3000). Secondary antibodies (Jackson ImmunoResearch) were all conjugated to horseradish peroxidase: mouse anti-rabbit (light chain specific), bovine anti-goat, and goat anti-hamster.
Metabolic labeling analysis
Cells expressing HA-HLA-A2 or HA-HLA-A2(N86Q) and tapasin were pulsed with 0.2 mCi [35S]-labeled methionine-cysteine for 10 min and chased in cold media for 0, 30 and 60 min. The cells were then lysed in digitonin lysis buffer (1% digitonin in PBS containing EDTA free protease inhibitors, pH 7.4) for 1 h on ice. The lysates were centrifuged at 4 °C for 30 min to remove cell debris. Proteins in lysates were immunoprecipitated with anti-HA or BBM.1 antibodies for 1 h at 4°C and followed by protein G beads for 1 h at 4°C. The beads were washed 3–4 times with lysis buffer containing 0.1% digitonin, resuspended in reducing SDS-PAGE buffer and boiled. For secondary immunoprecipitations, the primary BBM.1 IP samples were boiled for 5 min in 1X glycoprotein denaturing buffer (New England Biolab) and diluted in 1 ml buffer containing 1% NP-40 (Sigma). The samples were processed for immunoprecipitation with anti-HA antibody as above. The proteins were separated by SDS-PAGE and analyzed by phosphorimaging analyses. M553 cells expressing different tapasin constructs were pulsed with 0.2 mCi [35S]-labeled methionine-cysteine for 10 min and treated with cross-linker before lysis in digitonin lysis buffer as above. Proteins in lysates were processed for primary and secondary immunoprecipitations as above using BBM.1 and heavy chain-specific (HC10) antibody (24).
Statistical analysis
Analyses were performed using the GraphPad Prism software (GraphPad Software, Inc.). The p values were calculated using the Student’s paired or unpaired t tests. p values of < 0.05 were considered statistically significant.
RESULTS
The glycan of tapasin facilitates calreticulin recruitment to the PLC and enhances the functional activity of tapasin
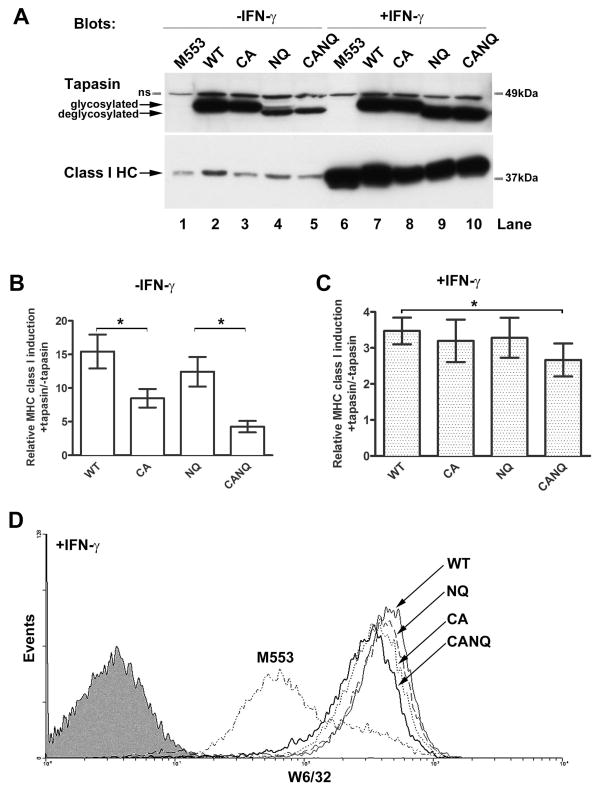
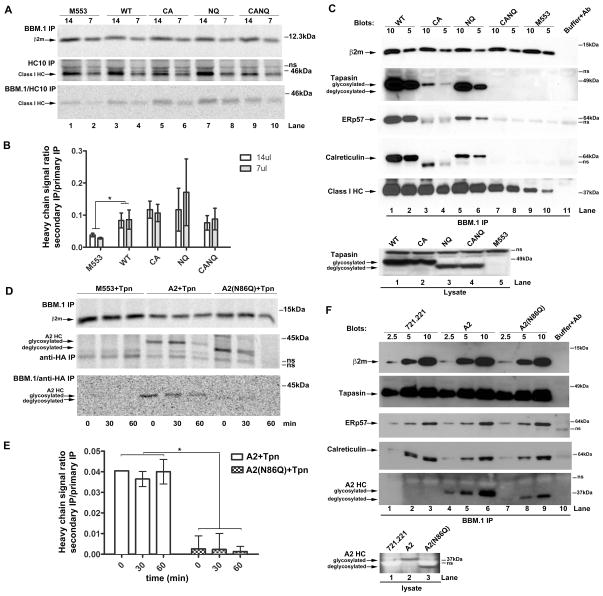
Tapasin deficient M553 cells (17) were used to characterize interactions and functional activities mediated by tapasin, deglycosylated (N233Q) tapasin, the C95A mutant of tapasin that is deficient in ERp57 binding, and a double mutant with altered glycosylation and ERp57 binding sites (tapasin C95A/N233Q, hereafter referred to as tapasin(CANQ)). The de-glycosylated version of tapasin was expressed at lower levels than the glycosylated version (Figure 1A, lanes 4–5 compared to lanes 2–3). However, in interferon gamma (IFN-γ) -treated cells, expression of tapasin(N233Q) and tapasin(CANQ) were restored to levels similar to those of the wild-type protein (Figure 1A, lanes 7–10). The molecular basis for IFN-γ–mediated stabilization of deglycosylated tapasin remain unclear, but this was a consistent result which could relate to IFN-γ–mediated upregulation of TAP and MHC class I, resulting in stabilization of the PLC. As expected based on previous studies (13, 15, 16, 25) tapasin(C95A) had reduced functional activity in inducing MHC class I cell surface expression (Figure 1B). Despite reduced expression, tapasin(N233Q) had higher activity than tapasin(C95A), suggesting that the tapasin(N233Q) mutation, in isolation, did not significantly impair the functional activity of tapasin (Figure 1B). However, in IFN-γ treated cells, in which all MHC-encoded PLC components were significantly up-regulated, tapasin(CANQ) was the only mutant that significantly impacted the functional activity of tapasin (Figure 1C and D).
FIGURE 1. The glycan of tapasin impacts its functional activity.
Tapasin constructs are abbreviated as wild-type (WT), C95A (CA), N233Q (NQ) and C95AN233Q (CANQ). A, Representative immunoblots show expression of tapasin and MHC class I heavy chains in lysates from M553 cells expressing no tapasin or the indicated tapasin constructs following 48 h IFN-γ (500 IU) treatment or no treatment. Rabbit anti-tapasin and MHC class I heavy chain-specific HC10 antibodies respectively were used. Class I HC indicates MHC class I heavy chains, in this and all subsequent blots. Non-specific bands are indicated as ns. B and C, Bar graphs show induction of MHC class I cell surface expression in M553 cells by the indicated tapasin constructs following 48 h IFN-γ treatment (C) or no treatment (B). Y axes show ratios of mean fluorescence intensities (MFI) of MHC class I staining in cells that had been infected with retroviral constructs encoding indicated tapasin constructs relative to the parent uninfected M553 cells (+tapasin/−tapasin MFI ratios). The tapasin(CA) and tapasin(CANQ) mutants were significantly impaired in restoring MHC class I cell surface expression compared to tapasin(WT) and tapasin(NQ) respectively (p =0.0275 and 0.0264 respectively, depicted by *; only these comparisons were made since the proteins were not expression-matched in other cases). In IFN-γ treated cells, only the tapasin(CANQ) mutant was significantly impaired in restoring class I surface expression compared to tapasin(WT) (p = 0.0335, depicted by *). Data are averages of three (B, C) independent FACS analyses. D, Representative histograms show cell surface induction of MHC class I molecules in IFN-γ-treated M553 cells in the presence or absence of the indicated tapasin constructs. Filled histogram shows control staining without primary antibody. MHC class I surface expression was analyzed by flow cytometry using the W6/32 antibody.
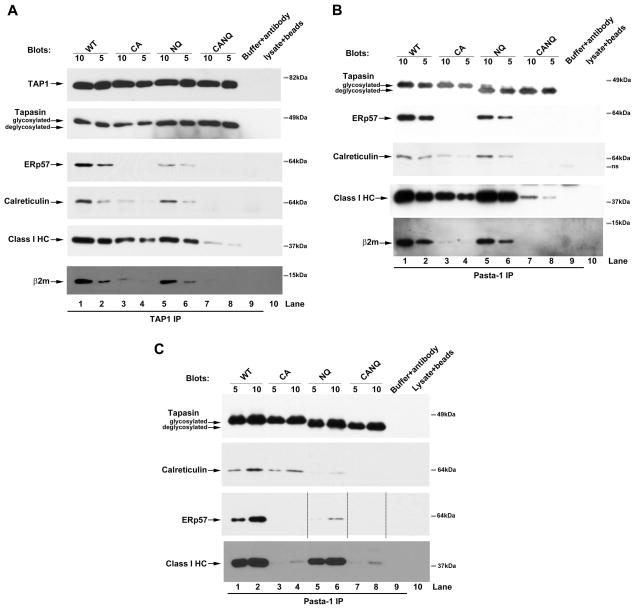
To study interactions of the tapasin mutants with PLC components, M553 cells expressing wild-type tapasin or various tapasin mutants were treated with IFN-γ for 48 h to equalize tapasin expression, and cell lysates were immunoprecipitated with anti-TAP1 (Figure 2A). As expected based on previous studies, ERp57 recruitment to TAP was C95 dependent (Figure 2A, ERp57 blot, lanes 3–4 compared to lane 1–2). Notably however, TAP-ERp57 binding was also reduced in cells expressing tapasin(N233Q) (Figure 2A, ERp57 blot, lanes 5–6 compared to lanes 1–2). Calreticulin interaction with TAP was reduced in cells expressing tapasin(C95A) as previously shown (14, 16). The new result that calreticulin recruitment was slightly impacted in cells expressing tapasin(N233Q) (note the slightly higher level of tapasin present in the context of this mutant), and essentially undetectable in cells expressing tapasin(CANQ) (Figure 2A, CRT blot, lanes 1–8) indicated that tapasin’s glycan and tapasin-associated ERp57 represented the major modes of calreticulin recruitment to the PLC. Similar trends were observed in anti-tapasin immunoprecipitations of proteins from IFN-γ-treated cells (Figure 2B, ERp57 and CRT blots), although an impact of the single tapasin(NQ) mutation on tapasin-calreticulin binding was not visualized. In anti-tapasin immunoprecipitations, efficient observation of PLC components required the use of an alkylating agent or a cell permeable cross-linker such as DTBP prior to cell lysis (16). The former treatment stabilizes disulfide-linked tapasin-ERp57 conjugates and associated PLC components, and the latter treatment stabilizes the same interactions, but using a cross-linking approach (DTBP is a reversible cross-linker). β2m recruitment to TAP was essentially undetectable in the context of the tapasin(CANQ) mutant, reduced by the tapasin(N233Q) mutation and strongly reduced in the context of the tapasin(C95A) mutation (Figure 2A, β2m blot, lanes 1–8). Similarly, while the tapasin(C95A) mutation reduced the efficiency of TAP-MHC class I heavy chain binding in IFN-γ–treated cells (Figures 2A, heavy chain blot, lanes 1–8), the tapasin(CANQ) double mutant induced a marked impairment of heavy chain recruitment to the PLC. Similar results were obtained in the anti-tapasin (Pasta-1) immunoprecipitation analyses (Figure 2B, heavy chain and β2m blots). In summary, in IFN-γ–treated cells, TAP and tapasin binding to calreticulin and heavy chains were abrogated or strongly impacted by the tapasin(CANQ) mutation and reduced by the tapasin(C95A) mutation. Interactions of ERp57 and β2m with TAP and tapasin were strongly tapasin(C95)-dependent and partially impacted by the tapasin(N233Q) single mutant. These findings indicated that the glycan of tapasin and its ERp57 binding site cooperate in the assembly of the PLC.
FIGURE 2. The glycan of tapasin impacts interactions within the PLC.
For these analyses (A and B) M553 cells expressing wild-type or various tapasin mutants were treated with IFN-γ to up-regulate TAP and MHC class I or not treated with IFN-γ (C). A, Immunoblotting analyses of anti-TAP1 immunoprecipitates of the indicated cells. B and C, Immunoblotting analysis of anti-tapasin (Pasta-1) immunoprecipitates of the indicated cells. For Pasta-1 immunoprecipitations, cells were treated with the membrane permeable cross-linker DTBP prior to lysis. Membranes were probed using antibodies specific for indicated proteins. Indicated amounts (5–10μl) of immunoprecipitation samples were used for the analyses. Data are representative of two (B), three (C) or four (A) analyses. The lanes labeled lysate+beads corresponds to lysates incubated with protein G beads (without antibody) and the lanes labeled buffer+antibody correspond to antibody incubated with buffer and protein G beads (no cell lysates were used). Antibody-derived or other non-specific bands are indicated as ns. Dotted lines in panel C, ERp57 blot, indicate lanes that were cut and pasted from same blot in order to preserve the order of presentation of lanes.
Despite reduced expression of the tapasin(N233Q) constructs in the absence of IFN-γ-treatment, Pasta-1 immunoprecipitations recovered comparable amounts of each tapasin protein (Figure 2C, tapasin panel), thus allowing for assessment of effects of the different mutations upon the stabilities of tapasin binding to PLC components in the absence of IFN-γ-treatment. Under this condition, tapasin-calreticulin binding was significantly impacted by the tapasin(N233Q) mutation, and appeared to be more strongly impacted by tapasin(N233Q) compared to the tapasin(C95A) mutation (Figure 2C, CRT blots, lane 5–6 compared to lane 3–4). Tapasin-calreticulin binding was also undetectable in the context of tapasin(CANQ). Additionally, the tapasin-ERp57 interaction was significantly impacted by the tapasin(N233Q) mutation (Figure 2C, ERp57 blots, lane 5–6 compared to 1–2). In the absence of IFN-γ-treatment, a much stronger effect of the tapasin(C95A) mutation upon tapasin-heavy chain binding was apparent compared to IFN-γ-treated cells (Figures 2B and 2C, heavy chain blots, lanes 1–2 compared to 3–4), and β2m binding to tapasin was below the detection threshold in the absence of IFN-γ-treatment, even in the context of wild-type tapasin (data not shown). Overall, the interactions analyses of Figure 2C suggested that tapasin-calreticulin binding was more strongly impacted by tapasin(N233Q) relative to tapasin(C95A) in the absence of IFN-γ (Figure 2C, calreticulin blot), although the opposite result was obtained from IFN-γ-treated cells (Figure 2B, calreticulin blot).
β2m is required for efficient calreticulin and ERp57 recruitment to the PLC
The findings described above indicated that calreticulin recruitment to the PLC was abrogated by mutations of the glycosylation and ERp57 binding sites of tapasin. Previous studies have described that calreticulin recruitment to the PLC is also reduced in β2m-deficient human cells (5, 26). To further investigate requirements for calreticulin and ERp57 recruitment, β2m−/− mouse splenocytes were analyzed for their PLC compositions (Figure 3). Steady-state levels of MHC class I heavy chains are not reduced in the lysates of β2m-deficient cells compared to wild-type cells (Figure 3, lanes 1 and 2, MHC class I heavy chain blot). However, the species that exists is entirely ER-localized as assessed by Endo H digestion, whereas a minority of the heavy chains in wild-type lysates are ER-localized (data not shown). Splenic cell lysates from wild-type C57Bl/6 or β2m−/− mice were immunoprecipitated with anti-TAP1 (Figure 3). As expected based on previous results (27, 28), MHC class I heavy chain recruitment was strongly impacted in cells lacking β2m compared to wild-type cells (Figure 3, class I heavy chain blot, lanes 6, 7). Additionally, TAP-calreticulin association is essentially undetectable in cells lacking β2m compared to wild-type cells as previously shown (5, 26) (Figure 3, CRT blot, lanes 6, 7). Notably, ERp57 recruitment efficiency was reduced in β2m-deficient cells (Figure 3, ERp57 blot, lanes 6, 7). ERp57 is thought to be recruited to the PLC via heterodimeric interactions with tapasin (13, 29). However, it appears that β2m also aids in the recruitment of ERp57 (Figure 3, ERp57 blot), either via direct interactions, or indirectly. MHC class I heavy chains have been shown in some studies to interact directly with ERp57 (30, 31). It is thus also possible that β2m indirectly facilitates recruitment of MHC class I-associated ERp57 by stabilizing a conformation of MHC class I heavy chain that is better able to interact with ERp57.
FIGURE 3. β2m−/− cells are deficient in recruiting calreticulin into the PLC.
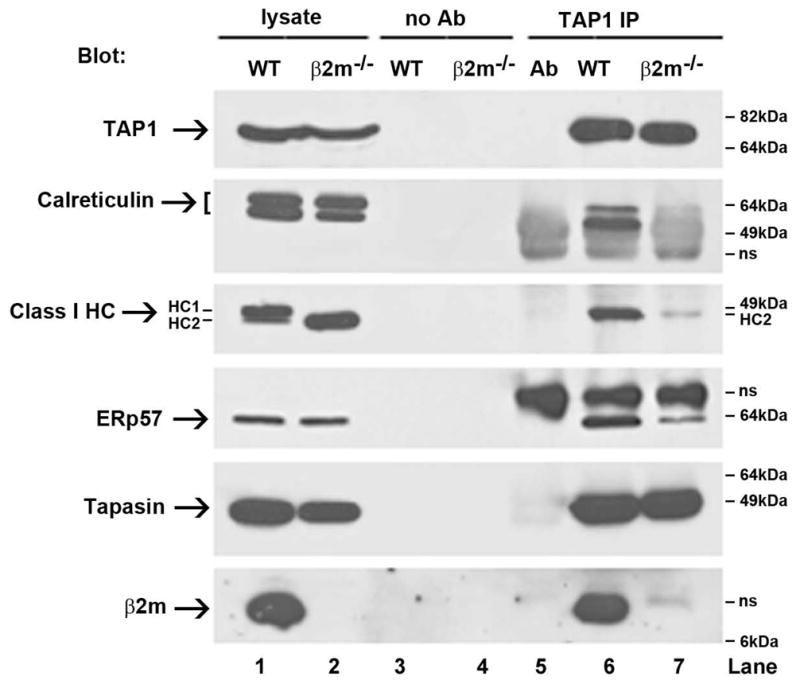
Anti-TAP1 immunoprecipitated proteins (IP) or lysates were analyzed by immunoblotting with the indicated antibodies. Splenic lysates from WT or β2m−/− mice were used for the immunoprecipitations. Data are representative of one analysis for β2m, 2 analyses for class I heavy chain, 2 analyses for ERp57, 3 analyses for tapasin, and 5 analyses for calreticulin. Two bands are observed for calreticulin, likely corresponding to a full-length version and a degradation product. Two species of MHC class I heavy chains are observed in the cell lysates (labeled as HC1 and HC2), likely corresponding to differently glycosylated forms of heavy chains. Only HC2 is detectable in the immunoprecipitations and in β2m−/− cells. No antibody (No Ab) controls were performed by incubating indicated lysates with protein G beads, and Ab indicates signals obtained with antibody alone (without lysates). Antibody-derived or other non-specific bands are indicated as ns.
The conserved glycan of MHC class I heavy chains facilitates heavy chain recruitment into the PLC, and mediates intrinsic and tapasin-assisted assembly
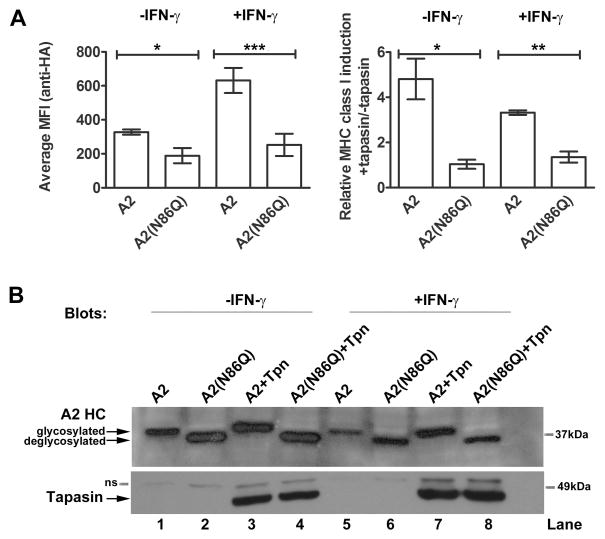
We next investigated the role of the highly-conserved MHC class I heavy chain glycans (N86) in PLC formation and tapasin-assisted assembly. HA-HLA-A2 and deglycosylated HA-HLA-A2(N86Q) were first expressed in M553 cells to assess impacts of heavy chain de-glycosylation on tapasin-assisted assembly. Flow cytometric analysis showed that HA-HLA-A2(N86Q) was impaired in cell surface expression compared to wild-type HA-HLA-A2, particularly in IFN-γ-treated cells (Figure 4A). This was despite the slightly stronger intracellular expression of HA-HLA-A2(N86Q) (Figure 4B, lanes 1, 2, 5 and 6). Whereas treatment of cells expressing HA-HLA-A2 with IFN-γ caused an 1.9-fold induction of cell surface expression, surface expression of HA-HLA-A2(N86Q) was much less significantly impacted by the treatment (Figure 4A, left panel). The HA-HLA-A2(N86Q) mutant was also highly impaired in tapasin-assisted assembly, with no tapasin-mediated induction of cell surface expression observable either in the presence or absence of IFN-γ, in contrast to the wild-type counterpart, whose cell surface expression was strongly-induced by tapasin (Figure 4A, right panel).
FIGURE 4. The conserved MHC class I glycan impacts tapasin-independent and tapasin-assisted MHC class I assembly.
A, Left panel: Bar graphs show mean fluorescence of M553 cells expressing HA-HLA-A2 or HA-HLA-A2(N86Q) as assessed by staining with the anti-HA antibody in untreated and 72 h IFN-γ treated cells (in the absence of tapasin expression). Cell surface expression of HA-HLA-A2(N86Q) was significantly impaired compared to that of HA-HLA-A2 both in IFN-γ treated cells (p = 0.0005; depicted as ***) and in untreated cells (p = 0.0404; depicted as *). Right panel: Tapasin-mediated induction of HA-HLA-A2 or HA-HLA-A2(N86Q) in untreated and 72 h IFN-γ treated M553 cells. Y axis shows ratio of MFI of MHC class I staining in M553 cells expressing tapasin and indicated class I relative to M553 cells expressing indicated class I alone (+tapasin/−tapasin MFI ratios). The differences in cell surface expression between HA-HLA-A2 and HA-HLA-A2(N86Q) were also significant in tapasin-expressing IFN-γ treated (p = 0.0018; depicted as **) or untreated cells (p = 0.0153, depicted as *). Paired t tests were used for the statistical analyses. B, Immunoblotting analyses of indicated cell lysates using antibodies specific for tapasin and HA-HLA-A2 (anti-HA). Data are averages of three (A) independent FACS analysis and the blots (B) are representative of two independent analyses. Non-specific bands are indicated as ns.
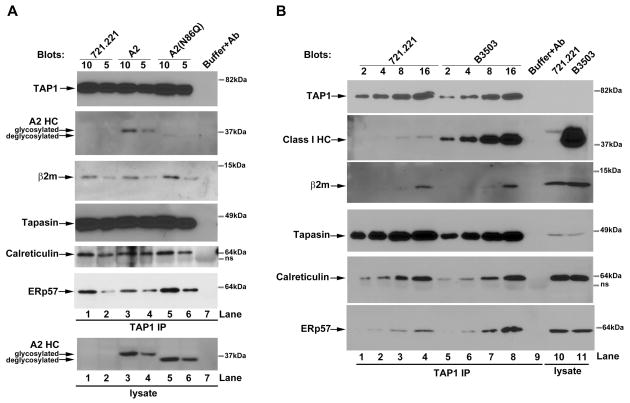
To assess interactions mediated or stabilized by HA-HLA-A2 compared to those mediated by HA-HLA-A2(N86Q), the constructs were expressed in the MHC class I heavy chain-deficient 721.221 cells, which lack HLA-A, -B, and-C expression and which express very low levels of HLA-E and F (18). Cells were lysed and proteins immunoprecipitated with anti-TAP1. As previously reported with other deglycosylated class I molecules (6, 7, 32), HA-HLA-A2(N86Q) showed reduced binding to TAP compared to HA-HLA-A2 (Figure 5A). However, despite impaired recruitment of HA-HLA-A2(N86Q) heavy chains, the HA-HLA-A2(N86Q) mutation had no significant impact on the recruitment of β2m, tapasin, calreticulin and ERp57 into the PLC (Figure 5A, lanes 3–4 compared to lanes 5–6 of β2m, tapasin, calreticulin and ERp57 blots). Slightly more TAP1 was immunoprecipitated from HA-HLA-A2(N86Q) cells compared to HA-HLA-A2 cells, and correspondingly more β2m, tapasin, calreticulin and ERp57 were co-immunoprecipitated with TAP1 (Figure 5A).
FIGURE 5. Peptide loading complexes of MHC class I-deficient 721.221 cells display defective recruitment of de-glycosylated heavy chains and do not significantly alter their compositions under conditions where more HC10-reactive heavy chains are recruited.
A, Upper panel: Immunoblotting analyses of anti-TAP1 immunoprecipitates from 721.221 cells expressing HA-HLA-A2 or HA-HLA-A2(N86Q) using antibodies specific for indicated proteins. Lower panel: Immunoblotting analyses of lysates from 721.221 cells expressing HA-HLA-A2 or HA-HLA-A2(N86Q) using antibody specific for HA-HLA-A2 (anti-HA). B, Immunoblotting analysis of anti-TAP1 immunoprecipitates or lysates from 721.221 cells expressing or lacking exogenous HLA-B*3503 using antibodies specific for indicated proteins. Indicated amounts (5–10μl for A and 2–16μl for B) of immunoprecipitation samples were used for the analyses. Data are representative of two (B) or three (A) independent analyses. The lanes labeled buffer+Ab corresponds to antibody and lysis buffer (no lysates) incubated with protein G beads. Antibody-derived or other non-specific bands are indicated as ns.
The finding that enhanced recruitment of HA-HLA-A2 heavy chains into the PLC did not cause the additional recruitment of β2m, tapasin, calreticulin and ERp57 suggested the possibility that the latter interactions were independent of heavy chain recruitment. This finding was confirmed by over-expression of another MHC class I heavy chain, HLA-B*3503, in 721.221 cells (Figure 5B). In immunoblotting analyses, HLA-B*3503 is efficiently recognized by the heavy chain specific (HC10) antibody (which does not efficiently recognize HA-HLA-A2). Using this antibody, we estimated that there was an eight-fold increase in the recruitment of HC10-reactive heavy chains into the PLC of 721.221-B3503 cells compared to the parent 721 .221 cells (Figure 5B, HC10 blot, compare lanes 5–8 with lanes 1–4). A lower than two-fold increase in ERp57 association with TAP was observed in the context of HLA-B*3503 that had not been observed in the context of HA-HLA-A2 (Figure 5B and 5A). HLA-B*3503-induced ERp57 recruitment to the PLC could be an allele-specific effect or could reflect different levels of expression of HLA-B*3503 compared to HA-HLA-A2 in 721.221 cells. Notably however, the eight-fold enhancement in the recruitment of heavy chains into the PLC did not correlate with significant increases in PLC-associated calreticulin (Figure 5B). Thus, calreticulin recruitment was largely independent of heavy chain recruitment. Furthermore, β2m recruitment was also not strongly linked to that of the heavy chain. Two interpretations of these data are 1) that heavy chains are recruited into pre-assembled PLCs containing β2m, with heterodimer assembly occurring within the PLC or 2) the PLC may function as a platform for peptide and β2m exchange.
Tapasin and the MHC class I glycan facilitate an early step in the formation of β2m-heavy chain heterodimers
To further investigate the possibility that the initial assembly of heterodimeric MHC class I molecules occurs within the PLC, we compared early assembly of β2m-heavy chain complexes in cells expressing different tapasin mutants by metabolic labeling analyses (10 minute pulse). The tapasin(C95A) mutation was a good tool for these analyses, as the tapasin(C95A) mutation strongly impacted the recruitment of β2m into the PLC (Figure 2). Immunoprecipitations were undertaken with BBM.1, an antibody that can recognize both free β2m and heterodimeric forms of β2m (22), followed by a secondary immunoprecipitation with a heavy chain-specific antibody (HC10) (24). Whereas tapasin-deficiency impacted the formation of heavy chain-β2m complexes (relative to cells expressing wild-type tapasin), none of the tapasin mutants displayed a significant impairment in early steps of heterodimer assembly ((Figures 6A (phosphorimaging analyses) and 6B (quantification of phosphorimaging analyses)). Impaired binding of β2m to PLC components in the context of tapasin(C95A) mutants was verified by immunoblotting analyses of BBM.1 immunoprecipitates with various antibodies (Figure 6C). The immunoprecipitation/immunoblotting analyses (Figure 6C) were undertaken without IFN-γ treatment of cells, as were the pulse-chase analyses (Figure 6A). Under these conditions, tapasin(N233Q) and tapasin(CANQ) display reduced expression relative to the wild-type and tapasin(C95A) constructs, as shown in Figure 1 and Figure 6C (tapasin blots, lysates). The tapasin(C95A) mutation strongly reduced or impaired β2m-tapasin, β2m-ERp57 and β2m-calreticulin binding efficiencies, and the tapasin(CANQ) mutation rendered those interactions essentially undetectable (Figure 6C, tapasin, ERp57, and calreticulin blots). Recovery of heavy chains was slightly reduced in cells expressing tapasin(C95A) and more strongly reduced in cells expressing tapasin(CANQ) compared to those expressing wild-type tapasin (Figure 6C, heavy chain blot), consistent with the reductions in functional activities of the CANQ mutants (Figure 1B). These results indicated that the steady-state assembly of heterodimers was sub-optimal in the context of the tapasin(C95A) mutants (Figure 6C), as expected. However, the C95A mutation did not impact the initial assembly efficiency of MHC class I heterodimers (Figure 6B). Thus, whereas the presence of tapasin did significantly influence an early step in heterodimer assembly (Figures 6A and 6B), an intact PLC is not required for efficient early assembly of MHC class I heterodimers. Instead, an intact PLC appears to be required for optimization of heterodimer assembly.
FIGURE 6. Tapasin and the conserved MHC class I glycan impact an early step in the assembly of MHC class I heterodimers.
A, M553 cells expressing indicated tapasin constructs or M553 cells lacking tapasin were pulsed with [35S]-methionine-cysteine, treated with the cross-linker (DTBP) followed by cell lysis and immunoprecipitations. Phosphorimaging analyses show relevant bands from primary immunoprecipitation with anti-β2m (BBM.1; top panel), anti-MHC class I (HC10; middle panel) and secondary immunoprecipitation with HC10 following primary immunoprecipitation with BBM.1 (bottom panel). B, Quantification of A, plotting the ratios of band intensity quantifications (bottom panel/top panel). The Y-axis shows the ratio of intensities of the heavy chain band in the bottom panel relative to the corresponding β2m band intensity in the top panel. Averaged ratios from two independent analyses are presented. Ratios derived from M553 cells were significantly reduced compared those obtained from M553 cells expressing wild-type tapasin (p =0.0179 based on an unpaired t test), whereas the ratios derived from M553 cells expressing mutant tapasins were not significantly reduced. C, Upper panel: Immunoblotting analysis of anti-β2m (BBM.1) immunoprecipitates from M553 cells expressing indicated tapasin constructs using antibodies specific for indicated proteins. Cells were treated with DTBP prior to lysis. Lower panel: Immunoblotting analysis of lysates from M553 cells expressing indicated tapasin constructs using antibody specific for tapasin (Tpn). D, M553 cells expressing tapasin and indicated HA-HLA-A2 constructs were pulsed with [35S]-methionine-cysteine and chased in cold media for indicated time points, followed by cell lyses and immunoprecipitations. Phosphorimaging analyses show primary immunoprecipitation with anti-β2m (BBM.1; top panel) or anti-HA (middle panel) and secondary immunoprecipitation with anti-HA following primary immunoprecipitation with BBM.1 (bottom panel). Samples were processed as indicated in A, but without treatment with DTBP. E, Quantification of D, plotting the ratios of band intensity quantifications (bottom panel/top panel). The Y-axis shows the ratio of intensities of the HA-HLA-A2 or HA-HLA-A2(N86Q) heavy chain band in the bottom panel relative to the corresponding β2m band intensity in the top panel. Ratios are plotted rather than the actual bottom panel HA-HLA-A2 and HA-HLA-A2(N86Q) intensities, in order to normalize for potential differences in the immunoprecipitation efficiencies of the different samples. Significant differences between HA-HLA-A2 and HA-HLA-A2(N86Q) were observed in two of three experiments (p =0.0037 and p =0.0002; depicted as *) based on a paired t test. The differences between HA-HLA-A2 and HA-HLA-A2(N86Q) in a third experiment, although reduced at all three time points for HA-HLA-A2(N86Q) compared to HA-HLA-A2, did not achieve statistical significance. F, Upper panel: Immunoblotting analysis of anti-β2m (BBM.1) immunoprecipitates from 721.221 cells expressing HA-HLA-A2 or HA-HLA-A2(N86Q), using antibodies directed against indicated proteins. Lower panel: Immunoblotting analysis of lysates from 721.221 cells expressing HA-HLA-A2 or HA-HLA-A2(N86Q) using anti-HA antibody. In C and F, the lanes labeled buffer+Ab corresponds to antibody incubated with lysis buffer and protein G beads (no cell lysates were used). Antibody-derived or other non-specific bands are indicated as ns. Indicated amounts (7–14μl for A, 5–10μl for C and 2.5–10μl for F) of immunoprecipitation samples were used for the analyses. Data are representative of two (A, B, C, F) or three (D, E) independent analysis.
The heavy chain glycan mutation strongly impaired the recovery of heterodimers ((Figures 6D (phosphorimaging analyses) and 6E (quantification of phosphorimaging analyses)), indicating that the MHC class I glycan is also critical for the efficient early assembly of MHC class I heterodimers. Additionally, there was a reduction in steady-state level of heterodimer formation in the context of HA-HLA-A2(N86Q) compared to HA-HLA-A2 in the 721.221 cells (Figure 6F), as previously reported for the HLA-B*2705 N86Q glycan mutant, by MHC class I and BBM.1 immunoprecipitation analyses (7). However, no significant effects of HA-HLA-A2 glycan mutation or HA-HLA-A2 expression upon β2m interactions with tapasin, calreticulin or ERp57 were detectable (Figure 6F), again indicating an absence of coupling of β2m binding to TAP, tapasin or calreticulin to the β2m-heavy chain interaction.
DISCUSSION
Several studies have addressed the role of MHC class I glycans in MHC class I folding and assembly (5–7, 33–35) but the role of the glycan of tapasin in the functional activity of tapasin has not been investigated. We previously observed that glycan and ERp57-dependent interactions contribute to calreticulin recruitment to the PLCs of murine fibroblasts (12). The current findings (Figure 2) support the model that tapasin-associated ERp57 and tapasin’s glycan are the relevant sites that mediate calreticulin recruitment. PLCs from cells expressing the tapasin(C95A) mutant showed the presence of calreticulin, heavy chains and β2m, although at reduced levels compared to that those present in the context of wild-type tapasin (Figure 2A, lanes 3–4). Mutation to tapasin(CANQ) further reduced the efficiency of heavy chain recruitment and rendered β2m and calreticulin binding undetectable (Figure 2A, lanes 3–4 compared to 7–8). These findings indicate the presence of PLCs in which MHC class I recruitment to TAP is stabilized by tapasin’s glycan, likely via the binding of calreticulin (Figure 7A). The observation that the tapasin(N233Q) mutation has a stronger impact upon PLC formation in the context of tapasin(C95A) compared to the wild-type tapasin context (Figure 2A and 2B) is important, as it suggests that tapasin’s glycan may in fact be relevant for stabilizing PLCs containing tapasin molecules that are not in heterodimeric association with ERp57.
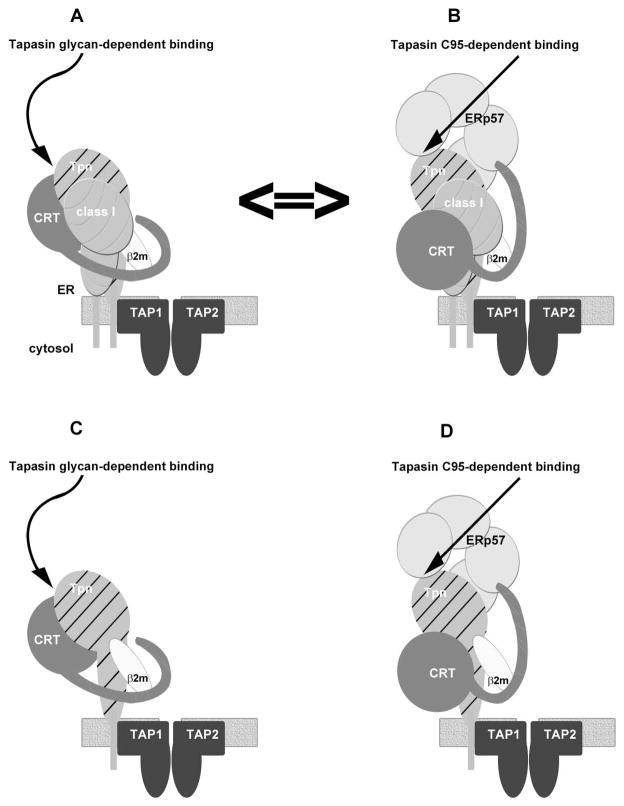
FIGURE 7. Within the PLC, calreticulin equilibrates between tapasin glycan- and tapasin(C95)-associated forms, and intermediate heavy chain-depleted PLCs are detectable.
In A, Tapasin’s glycan contributes to the stabilization of calreticulin, heavy chain and β2m recruitment to tapasin. Such a complex or its sub-complex may facilitate ERp57 recruitment by transient interaction of ERp57 with the tip of calreticulin’s P-domain, thus bringing ERp57 into proximity to tapasin(C95). In B, ERp57 becomes conjugated to tapasin(C95). This is shown to displace calreticulin from tapasin’s glycan, and link calreticulin to the PLC via P domain-mediated interactions with tapasin(C95)-associated ERp57. In this study, recruitments of calreticulin and ERp57 to the PLC are shown to be co-dependent and also dependent upon tapasin(C95) and tapasin’s glycan. In IFN-γ-treated M553 cells, where there is strong upregulation of MHC class I expression, calreticulin recruitment to tapasin was largely tapasin(C95)-dependent. Tapasin’s glycan and tapasin(C95) both enhanced MHC class I recruitment to the PLC, with dominant stability conferred by tapasin(C95). Thus the equilibrium is shifted towards B in IFN-γ-treated M553 cells, under conditions where ERp57 is not limiting. In M553 cells not treated with IFN-γ, conditions under which MHC class I heavy chain and β2m expression levels are low, calreticulin recruitment to tapasin was largely tapasin(N233)-dependent. Thus, the equilibrium is shifted towards A. MHC class I recruitment was nevertheless largely C95-dependent, suggesting that the A=>B transition is favored by the presence of both MHC class I heterodimers and ERp57. Thus, cellular conditions are expected to determine the steady-state prevalence of each complex and their sub-complexes. C and D, In proposed models of the MHC class I assembly pathways based on the data from Figures 5 and 6, intermediate heavy chain-depleted complexes are predicted to form. Sub-optimally assembled MHC class I heterodimers are predicted to associate with intermediate PLC, resulting in β2m exchange and peptide dissociation from incoming heterodimers, and resulting in the assembly of a mature PLC that has incorporated heavy chains. Intermediate heavy chain-depleted complexes are depicted as being tapasin(N233)- and tapasin(C95)-linked (C and D respectively). However, further investigations will be needed to verify each possibility.
The tapasin(N233Q) mutation reduced the extent of ERp57 recruitment to tapasin and TAP compared to wild-type tapasin (Figure 2, ERp57 blots, lanes 1–2 compared to 5–6). Furthermore β2m deficiency, which strongly impaired calreticulin recruitment to the PLC (Figure 3), or calreticulin-deficiency itself, also impacted the recruitment efficiencies of ERp57 into the PLC (data not shown). Calreticulin bound to tapasin’s glycan could transiently initiate ERp57 recruitment via a calreticulin P-domain-ERp57 interaction. This interaction could position ERp57 in appropriate proximity for conjugation to C95 of tapasin. Molecular modeling studies (using calnexin’s structure (2) as a model for calreticulin structure and the tapasin-ERp57 complex structure (36)) suggest that a calreticulin bound to tapasin’s glycan cannot simultaneously contact the ERp57 molecule present within the same tapasin-ERp57 conjugate (Wijeyesakare and Raghavan, unpublished observations). Thus, we postulate that conjugation between C57 of ERp57 and C95 of tapasin may induce a conformational change within the complex depicted in Figure 7A, that disengages calreticulin from tapasin’s glycan, re-positioning calreticulin for binding to tapasin-conjugated ERp57, via a P domain-based interaction (Figure 7B). Thus, calreticulin may equilibrate between conformational states in which its recruitment to the PLC is stabilized by tapasin’s glycan and tapasin-associated ERp57 (Figure 7A ⇔ 7B). In turn, calreticulin could stabilize the recruitment of both ERp57 and MHC class I molecules via cooperative binding interactions.
Within the same cell type, differences were noted in the compositions and functional activities of tapasin complexes in IFN-γ treated cells compared to untreated cells (Figure 2B compared to 2C). Calreticulin recruitment to tapasin was more strongly tapasin(N233)-dependent in cells that were not treated with IFN-γ Figure 2B compared to 2C, calreticulin blots, lanes 1–6). Nonetheless, tapasin(N233)-mediated stabilization of MHC class I recruitment was not readily detectable in the absence of IFN-γ treatment (Figure 2C, MHC class I blot, lanes 3–4 compared to 7–8). Since MHC class I heavy chain and β2m expression levels are both low in M553 cells in the absence of IFN-γ treatment, these findings are consistent with the possibility that binding of MHC class I molecules to tapasin in an interaction stabilized by calreticulin, shifts the 7A ⇔ 7B binding equilibrium towards 7B. Thus, sub-complexes of 7A (for example, those involving just tapasin and calreticulin) may accumulate under conditions where MHC class I expression is low (Figure 2C). Furthermore, complexes of the 7A type might also accumulate under conditions where ERp57 levels become limiting relative to those of tapasin and MHC class I molecules, a scenario possible in IFN-γ-treated cells, when tapasin is under endogenous IFN-γ control. Together, the findings of Figure 2 suggest a coupling between tapasin(N233)-mediated complexes and tapasin(C95)-mediated complexes (Figure 7A ⇔ 7B), and that cellular conditions determine the particular complex that predominates in the steady-state. The data also indicate that, in human cells, tapasin(C95)-mediated complexes are more stabilizing for MHC class I recruitment. Tapasin from species that lack C95 but which have N233, such as Grass carp (Ctenopharyngodon idellus), Zebra fish (Danio rerio) and Atlantic salmon (Salmo salar), may stabilize their interactions with MHC class I molecules solely via the tapasin glycan-dependent mechanism.
Tapasin-MHC class I interactions were generally stronger in IFN-γ-treated cells compared to untreated cells. For example, whereas β2m recruitment to tapasin was readily detectable in IFN-γ-treated cells, this interaction was not detectable in untreated cells even in the context of wild-type tapasin, and heavy chain recruitment was strongly diminished by the tapasin(C95A) mutation alone in cells that were not treated with IFN-γ (Figures 2B and 2C). IFN-γ-treatment markedly enhances steady-state levels of TAP, tapasin, MHC class I heavy chains and β2m, and the increase in these protein concentrations could directly contribute to stronger PLC assembly in IFN-γ-treated M553 cells compared to untreated cells. Somewhat paradoxically, the assembly-promoting functions of tapasin were much less significant in IFN-γ-treated M553 cells than in untreated cells (Figure 1B compared to 1C). However, it is to be noted that under the experimental conditions used in this study, tapasin expression is not under endogenous IFN-γ-control. Thus, tapasin’s activity may become limited (saturated) under conditions in which MHC class I heavy chains or β2m are expressed in stoichiometric excess relative to tapasin. Further investigations are needed to understand whether, in tapasin-sufficient cells, IFN-γ treatments generally induce stoichiometric increments in TAP, tapasin and MHC class I heavy chain components. Regardless of tapasin expression, when TAP activity is high following IFN-γ–treatment, there may be increased probability for tapasin-independent assembly arising from increased peptide availability, resulting in a reduction in the stringency of quality control. Such a scenario may be advantageous to the host in an infectious setting, in order to present repertoires of both optimal and sub-optimal peptide-MHC complexes, and increase the diversity of pathogenic peptides that can be presented.
PLCs of 721.221 cells are able to incorporate β2m, calreticulin and ERp57 under conditions of heavy chain deficiency, and enhanced recruitment of heavy chains into the PLC of 721.221 cells does not result in a parallel increase in the recruitment of β2m, calreticulin or ERp57 (Figure 5). The latter findings are supported by previous studies that have also shown binding of β2m and calreticulin to TAP in the absence of classical heavy chains in 721.221 cells (37, 38), and the lack of significant induction of β2m or calreticulin recruitment into the TAP complex by over-expression of HLA-B27 heavy chains (38). The analyses described here confirm that associations of β2m and calreticulin with the PLC are relatively unaffected by an eight-fold or higher enhancement in heavy chain incorporation. ERp57 incorporation into the PLC was slightly induced by the presence of HLA-B*3503 heavy chains. While it is possible that non-classical MHC class I heavy chains facilitate β2m, calreticulin and ERp57 recruitment, non-classical heavy chains are estimated to be present at low levels (≤ 1%) in 721.221 cells compared to classical MHC class I heavy chain (39). Together, these findings support the existence of intermediate PLCs containing tapasin, ERp57, β2m and calreticulin (Figures 7C and 7D). It is presently unclear whether the observed binding of calreticulin to TAP in the 721.221 cells is dominated by N233-dependent interactions, C95-dependent interactions, or both. Thus, while intermediate heavy chain-depleted complexes are depicted as being tapasin(N233)- and tapasin(C95)-linked (Figures 7C and 7D), further investigations will be needed to verify both possibilities.
Despite recruitment of β2m into intermediate PLCs in a heavy chain-independent manner, the PLC does not appear to be the site for initial heavy chain-β2m assembly, as PLCs deficient in β2m recruitment (via the tapasin(C95A) mutation) were competent for early assembly of heterodimers (Figure 6A and 6B). Rather, the intermediate PLCs that are enriched in β2m (relative to heavy chains) may serve as a platform for peptide exchange that is coupled to β2m exchange from unstable heavy chain-β2m heterodimers that are recruited into the PLC. Indeed, the presence of excess exogenous β2m has been shown to facilitate peptide exchange from MHC class I molecules in vitro (40). Interactions of sub-optimally assembled heavy chain-β2m heterodimers with intermediate β2m-enriched PLC could result in peptide and β2m exchange from the pre-assembled heterodimers, simultaneously recruiting heavy chains into the PLC. Previous in vitro studies have demonstrated interactions between heavy chains and tapasin with sub-stoichiometric levels of β2m (unless β2m is added in excess (41)). Additionally, β2m-deficient cells are able to recruit heavy chains with low efficiency (Figure 3 and (42)). These findings, taken together with the findings that β2m can be recruited to the PLC independently of heavy chains (Figure 5), lead to the postulate that ERp57- and/or calreticulin-dependent recruitment of β2m into the PLC can stabilize weaker binding between heavy chains and tapasin, and serve as a point of contact for stable heavy chain recruitment to the PLC. Thus, distinct interactions may be important for stabilizing heavy chain and β2m recruitment to the PLC.
The findings described here also show that the conserved heavy chain glycan is not central to calreticulin recruitment into the PLC. The finding that the heavy chain glycan impacts the efficiency with which heavy chain is recruited into the PLC (Figure 5) raises the possibility that the heavy chain glycan is engaged by the glycan binding site of calreticulin within the tapasin(C95)-dependent mode of calreticulin recruitment (Figure 7B). However, if β2m is an important point of contact between heavy chain and the PLC as suggested above, an alternative possibility is that the heavy chain glycan mutant has disrupted interactions with β2m within the PLC, also suggested by the finding of reduced efficiency of early heterodimer assembly in cells in the context of HA-HLA-A2(N86Q) compared to HA-HLA-A2 (Figure 6E). Further studies are needed to resolve these two possibilities. Thus, while structural studies of the tapasin-ERp57 heterodimers (36) provide a valuable starting point for the construction of model for a mature PLC, whether calreticulin in fact interacts with the MHC class I glycan within the PLC as suggested (36) remains to be established, as well as the nature of interactions between tapasin-ERp57, β2m and calreticulin.
Tapasin facilitated an early step in the assembly of heterodimers, and this step did not require tapasin-associated ERp57 or calreticulin (Figure 6A and 6B). Tapasin stabilizes TAP and increases peptide transport by TAP (43, 44), and by this mechanism, tapasin could impact an early step in heterodimer assembly. However, we have previously shown that the tapasin(C95A) mutant induces MHC class I assembly via a mechanism independent of its effect on TAP stabilization and peptide transport (16). It is possible that tapasin facilitates heterodimer formation simply by localizing heavy chains in the vicinity of the peptide source (TAP). Thus, there are at least two distinct levels of tapasin-assisted assembly, an early step that impacts heterodimer formation, and a later step involving calreticulin and ERp57, that could impact the generation of optimally-assembled heterodimers. The conserved MHC class I glycan also facilitates an early step in the assembly of heterodimers (Figure 6D–6F). The MHC class I glycan likely facilitates heterodimer formation by directly stabilizing heavy chain-β2m complexes, and/or by facilitating calreticulin recruitment, which could enhance the formation or stability of heterodimers.
In summary, the studies described here provide evidence for distinct and important roles for different glycans within the assembly complex. Furthermore, evidence is presented for the presence of intermediate complexes that are heavy chain-depleted. These studies provide new insights into the sequence and nature of assembly events within the MHC class I assembly pathway.
Acknowledgments
We thank Dr. Sanjeeva Joseph Wijeyesakere for docking studies of putative calreticulin-tapasin-ERp57 complex structures that assisted with the development of the model proposed in Figure 7, Dr. Naveen Bangia for the M553 cell line, Dr. Robert DeMars for 721.221 cells, Dr. Kathleen Collins for the HA-HLA-A2 retroviral construct and BOSC cells. We thank Dr. Clay Brown (University of Michigan High Throughput Protein (HTP) laboratory) and Dr. Nasir Salam for assistance with the retroviral HLA-B*3503 construct. We thank Dr. Matt Androlewicz, Dr. Peter Cresswell, Dr. Soldano Ferrone, Dr. Ted Hansen, Dr. Robert Tampe, and Dr. Jonathan Yewdell for the antibody reagents that were critical for this work. We thank the University of Michigan DNA sequencing core for sequencing analyses, and the University of Michigan hybridoma core for antibody production.
This work was supported by NIH grants (AI044115 and AI066131) to MR and by financial support from the University of Michigan Rheumatic Diseases Core Center and the University of Michigan Diabetes Research and Training Center.
References
- 1.Wearsch PA, Cresswell P. The quality control of MHC class I peptide loading. Curr Opin Cell Biol. 2008;20:624–631. doi: 10.1016/j.ceb.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schrag JD, Bergeron JJ, Li Y, Borisova S, Hahn M, Thomas DY, Cygler M. The Structure of calnexin, an ER chaperone involved in quality control of protein folding. Mol Cell. 2001;8:633–644. doi: 10.1016/s1097-2765(01)00318-5. [DOI] [PubMed] [Google Scholar]
- 3.Frickel EM, Riek R, Jelesarov I, Helenius A, Wuthrich K, Ellgaard L. TROSY-NMR reveals interaction between ERp57 and the tip of the calreticulin P-domain. Proc Natl Acad Sci U S A. 2002;99:1954–1959. doi: 10.1073/pnas.042699099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Helenius A, Aebi M. Roles of N-linked glycans in the endoplasmic reticulum. Annu Rev Biochem. 2004;73:1019–1049. doi: 10.1146/annurev.biochem.73.011303.073752. [DOI] [PubMed] [Google Scholar]
- 5.Sadasivan B, Lehner PJ, Ortmann B, Spies T, Cresswell P. Roles for calreticulin and a novel glycoprotein, tapasin, in the interaction of MHC class I molecules with TAP. Immunity. 1996;5:103–114. doi: 10.1016/s1074-7613(00)80487-2. [DOI] [PubMed] [Google Scholar]
- 6.Harris MR, Yu YY, Kindle CS, Hansen TH, Solheim JC. Calreticulin and calnexin interact with different protein and glycan determinants during the assembly of MHC class I. J Immunol. 1998;160:5404–5409. [PubMed] [Google Scholar]
- 7.Harris MR, Lybarger L, Myers NB, Hilbert C, Solheim JC, Hansen TH, Yu YYL. Interactions of HLA-B27 with the peptide loading complex as revealed by heavy chain mutations. International Immunology. 2001;13:1275–1282. doi: 10.1093/intimm/13.10.1275. [DOI] [PubMed] [Google Scholar]
- 8.Sadasivan BK, Cariappa A, Waneck GL, Cresswell P. Assembly, peptide loading, and transport of MHC class I molecules in a calnexin-negative cell line. Cold Spring Harb Symp Quant Biol. 1995;60:267–275. doi: 10.1101/sqb.1995.060.01.031. [DOI] [PubMed] [Google Scholar]
- 9.Scott JE, Dawson JR. MHC class I expression and transport in a calnexin-deficient cell line. J Immunol. 1995;155:143–148. [PubMed] [Google Scholar]
- 10.Gao B, Adhikari R, Howarth M, Nakamura K, Gold MC, Hill AB, Knee R, Michalak M, Elliott T. Assembly and antigen-presenting function of MHC class I molecules in cells lacking the ER chaperone calreticulin. Immunity. 2002;16:99–109. doi: 10.1016/s1074-7613(01)00260-6. [DOI] [PubMed] [Google Scholar]
- 11.Ireland BS, Brockmeier U, Howe CM, Elliott T, Williams DB. Lectin-deficient calreticulin retains full functionality as a chaperone for class I histocompatibility molecules. Mol Biol Cell. 2008;19:2413–2423. doi: 10.1091/mbc.E07-10-1055. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Del Cid N, Jeffery E, Rizvi SM, Stamper E, Peters LR, Brown WC, Provoda C, Raghavan M. Modes of calreticulin recruitment to the major histocompatibility complex class I assembly pathway. J Biol Chem. 2010;285:4520–4535. doi: 10.1074/jbc.M109.085407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dick TP, Bangia N, Peaper DR, Cresswell P. Disulfide bond isomerization and the assembly of MHC class I-peptide complexes. Immunity. 2002;16:87–98. doi: 10.1016/s1074-7613(02)00263-7. [DOI] [PubMed] [Google Scholar]
- 14.Peaper DR, Cresswell P. The redox activity of ERp57 is not essential for its functions in MHC class I peptide loading. Proc Natl Acad Sci U S A. 2008;105:10477–10482. doi: 10.1073/pnas.0805044105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vigneron N, Peaper DR, Leonhardt RM, Cresswell P. Functional significance of tapasin membrane association and disulfide linkage to ERp57 in MHC class I presentation. Eur J Immunol. 2009;39:2371–2376. doi: 10.1002/eji.200939536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rizvi SM, Raghavan M. Mechanisms of function of tapasin, a critical major histocompatibility complex class I assembly factor. Traffic. 2010;11:332–347. doi: 10.1111/j.1600-0854.2009.01025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belicha-Villanueva A, McEvoy S, Cycon K, Ferrone S, Gollnick SO, Bangia N. Differential contribution of TAP and tapasin to HLA class I antigen expression. Immunology. 2008;124:112–120. doi: 10.1111/j.1365-2567.2007.02746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shimizu Y, Geraghty DE, Koller BH, Orr HT, DeMars R. Transfer and expression of three cloned human non-HLA-A,B,C class I major histocompatibility complex genes in mutant lymphoblastoid cells. Proc Natl Acad Sci U S A. 1988;85:227–231. doi: 10.1073/pnas.85.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pear WS, Nolan GP, Scott ML, Baltimore D. Production of high-titer helper-free retroviruses by transient transfection. Proc Natl Acad Sci U S A. 1993;90:8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roeth JF, Williams M, Kasper MR, Filzen TM, Collins KL. HIV-1 Nef disrupts MHC-I trafficking by recruiting AP-1 to the MHC-I cytoplasmic tail. J Cell Biol. 2004;167:903–913. doi: 10.1083/jcb.200407031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Androlewicz MJ, Ortmann B, van Endert PM, Spies T, Cresswell P. Characteristics of peptide and major histocompatibility complex class I/beta 2-microglobulin binding to the transporters associated with antigen processing (TAP1 and TAP2) Proc Natl Acad Sci U S A. 1994;91:12716–12720. doi: 10.1073/pnas.91.26.12716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brodsky FM, Bodmer WF, Parham P. Characterization of a monoclonal anti-beta 2-microglobulin antibody and its use in the genetic and biochemical analysis of major histocompatibility antigens. Eur J Immunol. 1979;9:536–545. doi: 10.1002/eji.1830090709. [DOI] [PubMed] [Google Scholar]
- 23.Ogino T, Wang X, Kato S, Miyokawa N, Harabuchi Y, Ferrone S. Endoplasmic reticulum chaperone-specific monoclonal antibodies for flow cytometry and immunohistochemical staining. Tissue Antigens. 2003;62:385–393. doi: 10.1034/j.1399-0039.2003.00114.x. [DOI] [PubMed] [Google Scholar]
- 24.Stam NJ, Spits H, Ploegh HL. Monoclonal antibodies raised against denatured HLA-B locus heavy chains permit biochemical characterization of certain HLA-C locus products. J Immunol. 1986;137:2299–2306. [PubMed] [Google Scholar]
- 25.Simone LC, Wang X, Tuli A, Solheim JC. Effect of a tapasin mutant on the assembly of the mouse MHC class I molecule H2-K(d) Immunol Cell Biol. 2010;88:57–62. doi: 10.1038/icb.2009.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diedrich G, Bangia N, Pan M, Cresswell P. A role for calnexin in the assembly of the MHC class I loading complex in the endoplasmic reticulum. J Immunol. 2001;166:1703–1709. doi: 10.4049/jimmunol.166.3.1703. [DOI] [PubMed] [Google Scholar]
- 27.Ortmann B, Androlewicz MJ, Cresswell P. MHC class I/beta 2-microglobulin complexes associate with TAP transporters before peptide binding. Nature. 1994;368:864–867. doi: 10.1038/368864a0. [DOI] [PubMed] [Google Scholar]
- 28.Suh WK, Cohen-Doyle MF, Fruh K, Wang K, Peterson PA, Williams DB. Interaction of MHC class I molecules with the transporter associated with antigen processing. Science. 1994;264:1322–1326. doi: 10.1126/science.8191286. [DOI] [PubMed] [Google Scholar]
- 29.Peaper DR, Wearsch PA, Cresswell P. Tapasin and ERp57 form a stable disulfide-linked dimer within the MHC class I peptide-loading complex. EMBO J. 2005;24:3613–3623. doi: 10.1038/sj.emboj.7600814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindquist JA, Hammerling GJ, Trowsdale J. ER60/ERp57 forms disulfide-bonded intermediates with MHC class I heavy chain. FASEB J. 2001;15:1448–1450. doi: 10.1096/fj.00-0720fje. [DOI] [PubMed] [Google Scholar]
- 31.Kienast A, Preuss M, Winkler M, Dick TP. Redox regulation of peptide receptivity of major histocompatibility complex class I molecules by ERp57 and tapasin. Nat Immunol. 2007;8:864–872. doi: 10.1038/ni1483. [DOI] [PubMed] [Google Scholar]
- 32.Martayan A, Sibilio L, Setini A, Lo Monaco E, Tremante E, Fruci D, Colonna M, Giacomini P. N-linked glycosylation selectively regulates the generic folding of HLA-Cw1. J Biol Chem. 2008;283:16469–16476. doi: 10.1074/jbc.M709175200. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Q, Tector M, Salter RD. Calnexin recognizes carbohydrate and protein determinants of class I major histocompatibility complex molecules. J Biol Chem. 1995;270:3944–3948. doi: 10.1074/jbc.270.8.3944. [DOI] [PubMed] [Google Scholar]
- 34.Radcliffe CM, Diedrich G, Harvey DJ, Dwek RA, Cresswell P, Rudd PM. Identification of specific glycoforms of major histocompatibility complex class I heavy chains suggests that class I peptide loading is an adaptation of the quality control pathway involving calreticulin and ERp57. J Biol Chem. 2002;277:46415–46423. doi: 10.1074/jbc.M202466200. [DOI] [PubMed] [Google Scholar]
- 35.Wearsch PA, Jakob CA, Vallin A, Dwek RA, Rudd PM, Cresswell P. Major histocompatibility complex class I molecules expressed with monoglucosylated N-linked glycans bind calreticulin independently of their assembly status. J Biol Chem. 2004;279:25112–25121. doi: 10.1074/jbc.M401721200. [DOI] [PubMed] [Google Scholar]
- 36.Dong G, Wearsch PA, Peaper DR, Cresswell P, Reinisch KM. Insights into MHC class I peptide loading from the structure of the tapasin-ERp57 thiol oxidoreductase heterodimer. Immunity. 2009;30:21–32. doi: 10.1016/j.immuni.2008.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carreno BM, Solheim JC, Harris M, Stroynowski I, Connolly JM, Hansen TH. TAP associates with a unique class I conformation, whereas calnexin associates with multiple class I forms in mouse and man. J Immunol. 1995;155:4726–4733. [PubMed] [Google Scholar]
- 38.Solheim JC, Harris MR, Kindle CS, Hansen TH. Prominence of beta 2-microglobulin, class I heavy chain conformation, and tapasin in the interactions of class I heavy chain with calreticulin and the transporter associated with antigen processing. J Immunol. 1997;158:2236–2241. [PubMed] [Google Scholar]
- 39.Shimizu Y, DeMars R. Production of human cells expressing individual transferred HLA-A,-B,-C genes using an HLA-A,-B,-C null human cell line. J Immunol. 1989;142:3320–3328. [PubMed] [Google Scholar]
- 40.Pedersen LO, Hansen AS, Olsen AC, Gerwien J, Nissen MH, Buus S. The interaction between beta 2-microglobulin (beta 2m) and purified class-I major histocompatibility (MHC) antigen. Scand J Immunol. 1994;39:64–72. doi: 10.1111/j.1365-3083.1994.tb03341.x. [DOI] [PubMed] [Google Scholar]
- 41.Rizvi SM, Raghavan M. Direct peptide-regulatable interactions between MHC class I molecules and tapasin. Proc Natl Acad Sci U S A. 2006;103:18220–18225. doi: 10.1073/pnas.0605131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bangia N, Lehner PJ, Hughes EA, Surman M, Cresswell P. The N-terminal region of tapasin is required to stabilize the MHC class I loading complex. Eur J Immunol. 1999;29:1858–1870. doi: 10.1002/(SICI)1521-4141(199906)29:06<1858::AID-IMMU1858>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 43.Lehner PJ, Surman MJ, Cresswell P. Soluble tapasin restores MHC class I expression and function in the tapasin-negative cell line .220. Immunity. 1998;8:221–231. doi: 10.1016/s1074-7613(00)80474-4. [DOI] [PubMed] [Google Scholar]
- 44.Garbi N, Tiwari N, Momburg F, Hammerling GJ. A major role for tapasin as a stabilizer of the TAP peptide transporter and consequences for MHC class I expression. Eur J Immunol. 2003;33:264–273. doi: 10.1002/immu.200390029. [DOI] [PubMed] [Google Scholar]