Abstract
Multipotent mesenchymal stromal cells (MSCs) hold tremendous promise for tissue engineering and regenerative medicine, yet with so many sources of MSCs, what are the primary criteria for selecting leading candidates? Ideally, the cells will be multipotent, inexpensive, lack donor site morbidity, donor materials should be readily available in large numbers, immunocompatible, politically benign and expandable in vitro for several passages. Bone marrow MSCs do not meet all of these criteria and neither do embryonic stem cells. However, a promising new cell source is emerging in tissue engineering that appears to meet these criteria: MSCs derived from Wharton’s jelly of umbilical cord MSCs. Exposed to appropriate conditions, umbilical cord MSCs can differentiate in vitro along several cell lineages such as the chondrocyte, osteoblast, adipocyte, myocyte, neuronal, pancreatic or hepatocyte lineages. In animal models, umbilical cord MSCs have demonstrated in vivo differentiation ability and promising immunocompatibility with host organs/tissues, even in xenotransplantation. In this article, we address their cellular characteristics, multipotent differentiation ability and potential for tissue engineering with an emphasis on musculoskeletal tissue engineering.
Keywords: differentiation, musculoskeletal, regenerative medicine, umbilical cord stroma
An ideal cell source for tissue engineering should satisfy requirements including ease of access, sufficient cell number and immunocompatibility. Mature autologous cells have commonly been used in previous studies, owing to the advantages of already being differentiated and immunocompatible. In contrast to mature autologous cells, embryonic stem cells (ESCs) are a major area of contemporary research interest, and offer significant potential to treat or even cure a plethora of diseases [1,2]. They can differentiate into all cell types of the body including germ cells [3–5]. By contrast, bone marrow mesenchymal stromal cells (BMSCs) have a more limited differentiation potential. The self-renewal ability of ESCs also allows for a theoretically unlimited number of undifferentiated cells to be produced for tissue engineering applications. However, ESCs suffer from a series of constraints including ethical concerns, limited availability, potential for teratoma formation upon transplantation and immune rejection [6]. Induced pluripotent stem cells are an alternative to ESCs, and have been generated from many cell types including cells from umbilical cords [7]. Although the use of patient-specific induced pluripotent stem cells will overcome the immune rejection obstacle [8–11], a great deal of work remains before these cells can be developed into a safe therapy in humans.
Adult BMSCs are the most extensively investigated mesenchymal stromal cells (MSCs) for tissue engineering applications and are considered the ‘gold standard’ cell source in musculoskeletal tissue engineering. BMSCs have the ability to differentiate in vitro and in vivo into connective tissues such as cartilage, bone and adipose tissues. The main advantage of using BMSCs is to provide an autologous cell source for tissue engineers to avoid potential immune rejection. Autologous and allogeneic BMSCs also exhibit immunosuppressive capabilities [12–14]. BMSCs are relatively easy to access, and have limited self-renewal ability. The first tissue engineering studies to treat bone defects in clinical studies used human BMSCs (hBMSCs) [15,16]. In a 7-year clinical trial [15,16], this integration of implants into host bone was maintained and no further fractures were observed, thus demonstrating the feasibility of a tissue engineering approach in human bone regeneration with long-term durability. However, there are some limitations to the applications of BMSCs, including the low number of hBMSCs in marrow, their proliferation ability and the fact that their differentiation potential decreases significantly with age [17]. Moreover, the invasive harvesting procedure may lead to complications and morbidity [18].
There are a number of other sources of MSCs that are gaining popularity in the literature. However, for the sake of brevity, we refer readers to outstanding reviews on adipose-derived stem cells (ADSCs) [19–21] and dental pulp stem cells [22–24].
Human umbilical cord mesenchymal stromal cells
Great importance has been given to the identification of other cell sources that can be utilized for tissue engineering and regenerative medicine [25–27]. To date, other than BMSCs, MSCs have been identified in a variety of tissues, such as adipose tissue, blood, synovial fluid, dermis, muscle, dental pulp and umbilical cords, and can be differentiated along several mesenchymal lineages [21,24,28–33]. However, there are significant differences in their proliferation and differentiation abilities, and in harvesting procedures among these MSCs [34]. With the discovery of new MSC sources, a standard definition of these cells is needed. The International Society of Cellular Therapy proposes that cells must meet three criteria to be classified as MSCs [35]. The criteria are:
Be plastic adherent
Express CD105, CD73 and CD90 cell surface markers and not express hematopoietic stem cell markers
Be capable of osteoblast, adipocyte and chondrocyte differentiation in vitro
In recent years, the Wharton’s jelly of human umbilical cords has been discovered as a source of MSCs [36]. Human umbilical cord mesenchymal stromal cells (hUCMSCs) are a multipotent stromal cell population that are plastic adherent, share MSC surface markers such as CD73, CD90 and CD105, and are nonhematopoietic cells [37]. Nomenclature of these cells varies among investigators, and names used for cells derived from the Wharton’s jelly include umbilical cord matrix stem cells [33,38], human umbilical cord stroma cells [36], Wharton’s Jelly cells [37], umbilical cord stem cells [39], umbilical cord stromal cells [40], UCMSCs [41], umbilical cord tissue-derived cells [42] and various other combinations. Cell naming can also be specific to the site of isolation within the Wharton’s Jelly, for example human umbilical cord perivascular cells (hUCPVCs) [43,44]. As these cells are further characterized, a standard naming system for these cells may develop. Although the nomenclature of these cells varies among investigators, cells isolated from Wharton’s jelly are referred to as UCMSCs later.
Before the 21st century, umbilical cord research focused on the structure of umbilical cords and the characterization of extracellular matrix and stromal cells, going back as early as the 1940s [45,46]. In 1994, only two publications dealt with the subject of umbilical cord and MSCs (including blood derived), and that number increased to 349 publications in 2009 [47]. Following the recognition of their neural differentiation ability, demonstrated early in the current decade [48], it has been reported that hUCMSCs may differentiate along several cell lineages in all three germ layers including chondrogenic, osteogenic, adipogenic, myogenic, pancreatic, neurogenic and hepatogenic [36,38,48–50]. Additionally, hUCMSCs do not appear to form teratomas when transplanted [51]. In vivo transplantation of these cells has been demonstrated to prevent progressive deterioration with brain injury and to rescue the eye from retinal disease in a rodent model [52,53]. Recent tissue engineering studies with hUCMSCs have focused on cardiovascular tissue engineering [49,54–58] and musculoskeletal tissue engineering [59–61]. In this article, we have emphasized the mesenchymal differentiation of hUCMSCs and their potential for musculoskeletal tissue engineering. Cell biology and transplantation reviews of hUCMSCs can be found in detail elsewhere in the literature [37,39,40].
Cell characterization
Cell isolation & growth
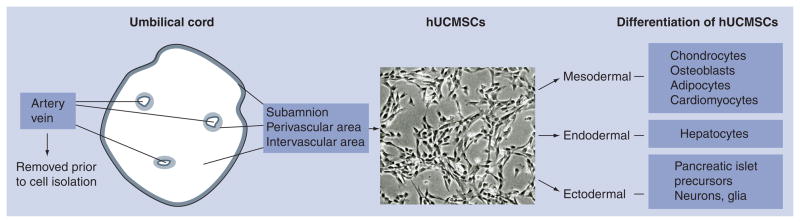
The human umbilical cord includes one vein, two arteries and a surrounding connective tissue known as Wharton’s jelly laying deep to the amnion (Figure 1). For more physiological information about the umbilical cord, see cited references [62,63]. The connective tissue may be divided into three zones: subamniotic, intervascular and perivascular stroma [36]. Various methods have been used to liberate cells from the umbilical cord tissue. Enzyme digestion of trypsin and/or collagenase has been recently utilized to extract hUCMSCs with fast isolation and high yield [33,36,64,65]. Moretti et al. provided a diagram mapping the many isolation methods, including steps such as removal of blood vessels, enzymatic digestion or explant culture [47]. Protocols can be modified to target the Wharton’s jelly cells or specific zones within the stroma. For example, Fong et al. modified their protocol to obtain a population of Wharton’s jelly cells using a specific enzymatic digestion process [51,66]. Currently there is no standard operating procedure for isolation of hUCMSCs from the umbilical cord. As a result, isolation methods may differ in their ability to release the MSCs from their niche, differ in the number of cells found at initial isolation or enrich one or more cells types found in the mixed MSC population. Thus, cell populations that are isolated may vary from group to group.
Figure 1. Structure of umbilical cord and differentiation schematic diagram of human mesenchymal stromal cells.
hUCMSC: Human umbilical cord mesenchymal stromal cell.
Human umbilical cord mesenchymal stromal cells resemble fibroblasts in their morphology (Figure 1) and have been defined as myofibroblasts based on their positive expression of vimentin, desmin and/or α-smooth muscle actin in native tissue or with in vitro cultured cells [48,67,68]. As an extraembryonic cell source, hUCMSCs have a rapid proliferation rate with a shorter doubling time than adult stem cells [37]. hUCMSCs also maintain their expansion and multipotent differentiation properties longer in vitro, compared with hMSCs [69]. Following cell harvesting, hUCMSCs have been passaged for seven times to achieve a 300-fold increase in cell number while the differentiation potential was maintained [36]. However, the quality of the cells can be affected by the length of culture time and cell seeding density [70]. The rapid proliferation rate, accompanied by the wide availability of umbilical cords, is highly desirable for tissue engineering as it is possible to obtain a large number of cells in a short time.
Cell phenotype
Human umbilical cord mesenchymal stromal cells represent an adherent cell population with a nonhematopoietic and nonendothelial (CD34 negative) phenotype and share a similar set of surface markers with adult MSCs, including CD10 [33], CD13 [33,38,71,72], CD29 [33,65,71,72], CD44 [33,36,38,65,71,72], CD49e [33], CD51 [65], CD73/SH3 [36,38,65,71], CD90 (Thy-1) [33,38,72], CD105/SH2 [33,36,38,65,71], CD106 [71], CD117 [38,48], CD166 [38,71,72] and HLA-1/HLA-ABC [33,71,72], and are negative for hematopoietic markers (e.g., CD14, CD31, CD34, CD38, CD45 and HLA-DR) [33,36,38,48,49,54,65,71–76]. Besides MSC surface markers, transcription factors such as Oct-4, Nanog and Sox-2 have been reported to exist in porcine umbilical cord cells, albeit at low levels of transcription [75]. Recent studies revealed that hUCMSCs also expressed these three markers [77,78] and other pluripotent markers including Rex-1, SSEA-3, SSEA-4, Tra-1–60 and Tra-1–81 [77]. More importantly, these pluripotent stem cell markers were retained for at least nine passages during subculture [77]. The presence of these pluripotent markers was also observed in transcriptome profiling of hUCMSCs using DNA microarrays [79]. It is important to note that the pluripotent markers (POUF1, NANOG, SOX2 and LIN28), in this study and in other studies, were expressed in low levels, generally orders of magnitude less than ESCs. Moreover, there is no demonstration that these transcription factors function in MSCs. The presence of low level gene expression of pluripotent transcription factors is insufficient to demonstrate pluripotency [80]. However, if a group would be able to demonstrate with blastocyst injection of a clonal cell line that the distribution of MSCs within all three cell types, including the germline, is observed, that would provide a much greater argument for pluripotency. Until such a demonstration is made, the most appropriate conclusion at this stage would be that UCMSCs most likely are not pluripotent cells.
For a complete table of all cell markers, see the review by Moretti et al. [47]. Regarding the ‘stemness’ of UCMSCs, UCMSCs have not been shown to be stem cells by the strict definition (permanently engraft and self-renew and produce differentiated progeny). However, recent work from Méndez-Ferrer et al., in the mouse, indicates that progress is being made to better identify the stem cell populaton within MSCs [81]. It remains to be seen whether the identifiers for MSCs found in mouse bone marrow will be found in other regions or other species where MSC-like cells have been derived.
Cell populations from different zones
Human UCMSCs represent a heterogeneous cell population. Thus, it might be important to isolate cells from each zone and characterize them to better understand their respective properties. hUCPVCs have been isolated through a delicate enzyme digestion of stroma surrounding the vessels [44]. These cells have shown a surface phenotype and multipotency similar to BMSCs and hUCMSCs [64,82], including CD44, CD73, CD90, CD105 and HLA-ABC. However, CD146, a surface antigen widely expressed in endothelial cells, was expressed at a higher rate of 52% with hUCPVCs compared with hBMSCs (15%) [82]. Only one group has isolated cells from the subamniotic zone due to the difficulty of isolating these cells from the surrounding tissue [83]. In situ characterization indicated that the cell number in the subamniotic area was less than 10% of the population from the whole cord and the cells had a stronger staining of α-smooth muscle actin and pancytokeratin [36]. After isolation, subamniotic cells expressed stem cell (SSEA-4, Oct-4 and Nanog) and MSC markers (CD73 and CD105), and were capable of adipogenic, osteogenic and chondrogenic differentiation [83]. However, more data are required to characterize this subpopulation.
Differentiation & tissue engineering
Human UCMSCs may have the ability to differentiate along several cell lineages of mesodermal or ectodermal origin (Figure 1) [36,38,48,49,84,85]. Furthermore, recent studies reported that hUCMSCs were able to differentiate both in vivo and in vitro into hepatocyte-like cells and pancreatic islet precursors of endodermal origin [38,50]. Exposed to a hepatogenic culture medium, hUCMSCs expressed hepatic markers including albumin, α-fetoprotein, cytokeratin-19, connexin-32 and dipeptidyl peptidase IV [38]. This study provided the foundation for the application of hUCMSCs for liver regeneration. Work from Chao et al. suggested that UCMSCs may be differentiated into pancreatic islet precursors, produce human insulin and respond to a glucose challenge for up to 12 weeks in a xenotransplantation Type I diabetes mellitus model [50]. In the following sections, we focus specifically on mesenchymal differentiation of hUCMSCs for the application of musculoskeletal tissue engineering.
Chondrogenic differentiation & cartilage tissue engineering
Cartilage, which plays a critical role in load bearing and distribution, has limited self-regeneration capabilities after injury. Compared with the current clinical approaches such as microfracture, artificial prosthesis and cell-based implantation, tissue engineering may be an ideal method to fully restore the cartilage function [86]. Cell dedifferentiation of mature chondrocytes during monolayer expansion makes it difficult to obtain enough cells for tissue engineering [87]. Thus, the application of MSCs in tissue engineering is promising mainly due to their self-renewal and differentiation ability. Chondrogenic differentiation of MSCs can be triggered by the TGF-β isoforms (TGF-β1, β2 and β3) and be enhanced by the addition of dexamethasone [88–90]. IGF-I, an important anabolic agent, remains controversial with its role in chondrogenic differentiation [90–92]. However, a recent study reported that IGF-I and TGF-β had equal chondroinductive ability with MSCs after the removal of insulin from insulin–transferrin–selenious acid Premix (1X serum substitute Premix), a substitute for fetal bovine serum in serum-free chondrogenic medium [92]. In addition, we showed that switching from a chondrogenic medium with TGF-β to an anabolic medium with IGF-I not only significantly increased collagen production relative to remaining in the chondrogenic medium, but also significantly increased the gene expression and the synthesis of collagen II [93].
Like other MSCs, hUCMSCs are able to differentiate along a chondrogenic lineage under the stimulation of TGF-βs in 3D cell pellets and polyglycolic acid (PGA) scaffolds [36,60,65,82,94]. In a more recent study, the chondrogenic ability of hUCMSCs was initiated at the mRNA level by the upregulation of SOX-9, a key regulatory gene for chondrogenesis [41]. At the extracellular protein level, evidence of chondrogenic differentiation was supported by Alcian blue staining for glycosaminoglycans (GAGs) [65,82], and immunohistochemical staining for type II collagen [36,65]. However, the chondrogenic differentiation studies of hUCMSCs have had inconsistent results [36,65]. In a study by Wang et al., untreated cells in the control group (no TGF-β1) also demonstrated positive staining for GAGs and collagen type II [65]. By contrast, no type II collagen was observed with the control group in a study by Karahuseyinoglu et al. [36], although only a trace of type II collagen was detected with hBMSCs, in contrast to the formation of abundant type II collagen in previous hBMSC studies [95,96]. Type I collagen was also identified in this study in both hBMSCs and hUCMSCs. Nevertheless, larger pellets were observed in the hUCMSC group with a better filamentous extracellular matrix than hBMSCs [36,82]. Moreover, the GAG quantification revealed that the perivascular cells, a subpopulation of hUCMSCs, produced a quantity of GAGs comparable to hBMSCs after 21 days, although the perivascular groups had fewer GAGs than hBMSC groups during the first week. Therefore, the better differentiation ability compared with hBMSCs and the coexistence of type I and II collagen in both cell pellets and biomaterial-based culture indicate that hUCMSCs may be considered as a MSC source for fibrocartilage tissue engineering applications such as for the temporomandibular (TMJ) disc, intervertebral disc and knee meniscus.
The first full-length study of hUCMSCs for musculoskeletal tissue engineering was a comparison of hUCMSCs to TMJ condylar cartilage cells in vitro for TMJ regeneration [60]. Both types of cells were cultured in PGA scaffolds over a 4-week period. The hUCMSC constructs formed a fibrocartilage-like tissue similar to the native TMJ cartilage and outperformed TMJ condylar cartilage cells with higher cellularity and biosynthesis of collagen and GAGs. Based on the limited availability of TMJ cells relative to umbilical cord cells and the superior biosynthesis of hUCMSCs, hUCMSCs were suggested as a promising alternative to mature condylar cartilage cells for TMJ tissue engineering. In a 3D comparison of hUCMSCs and hBMSCs for cartilage tissue engineering, hUCMSCs also demonstrated superior extracellular matrix synthesis with more GAGs and collagen than hBMSCs, while hBMSCs appeared to progress further along the chondrogenic lineages with higher type II collagen at both the gene expression and protein levels [97]. This study further supported the suitability of hUCMSCs for fibrocartilage tissue engineering for tissues such as TMJ cartilages and the intervertebral disc. After the establishment of hUCMSCs for fibrocartilage tissue engineering, the effects of cell seeding densities on cell proliferation, differentiation and matrix synthesis were explored [59]. hUCMSCs were seeded in PGA scaffolds at three densities: 5 (low), 25 (middle) or 50 (high) million cells/ml of scaffold. After a 4-week culture period, the high and medium density groups possessed significantly higher cell numbers and extracellular matrix content per construct and per cell than the lower density group. More importantly, constructs in the high and medium density groups maintained their mechanical integrity, which was confirmed by unconfined compression testing. Therefore, a seeding density greater than 25 million cells/ml was recommended for related future fibrocartilage tissue engineering studies. In summary, the formation of fibrocartilage-like tissue with the coexistence of type I and II collagen and aggrecan indicates that hUCMSCs differentiate along a fibrocartilage lineage and may be an excellent cell source candidate for fibrocartilage regeneration. A further modified chondrogenic environment, perhaps including the investigation of factors such as bioactive chemical signals (e.g., growth factors and aggrecan), oxygen tension, coculture, mechanical stimulation and/or in vivo regulation, will be needed to enhance chondrogenesis to apply hUCMSCs in hyaline cartilage tissue engineering.
Osteogenic differentiation & bone tissue engineering
Unlike cartilage, bone is a vascularized tissue with an innate self-healing and remodeling ability [98]. Although bone defects can be treated by autologous bone grafts, the current gold standard, this method is limited by donor site morbidity, malformation and infection [99]. Moreover, there is no satisfactory treatment for some severe bone injuries [100]. As a tissue engineering approach, BMSCs can be incorporated into biomaterials for direct implantation, with or without prior in vitro culture in osteogenic media. A typical osteogenic medium includes osteogenic signals of dexamethasone and/or BMP-2, in addition to β-glycerophosphate and ascorbic acid to aid in matrix synthesis. Exposure of BMSCs to this medium before implantation allows the delivery of more mature osteogenic cells to defects, thus accelerating the in vivo bone regeneration [100].
Osteogenic induction of hUCMSCs in 2D monolayer has been accomplished by treatment with dexamethasone and β-glycerophosphate [36,64,65,71,72,82]. During the course of osteogenic differentiation, Runx2, a master gene of osteogenesis, and its transcriptional coactivator for osteocalcin expression, TAZ, were activated [41], osteocalcin and osteopontin genes were up-regulated [41,65,71], positive alkaline phosphatase (ALP) activity was observed [64,65,82], the bone-specific immunostaining of osteonectin, osteocalcin and bone sialoprotein-2 was visualized [36], and mineralization was verified by von Kossa staining and alizarin red staining [36,64,65,71,72,82]. Like hBMSCs, mineralization was observed with hUCMSCs after 2 weeks [36,65,71,72] and similar side-by-side gene expression was observed during this period [71]. However, it appeared that hUCMSCs had less mineralization with larger bone nodules than hBMSCs as evidenced by alizarin red and von Kossa staining [36,71]. Similarly, after exposure to dihydroxyvitamin D3 for 6 weeks, hUCMSCs exhibited weak osteogenesis, with few ALP-positive cells or little mineralization [70]. Exposed to osteogenic medium, perivascular cells differentiated faster along the osteogenic lineage with the formation of bone nodules after only 4–5 days [64]. In a direct comparison of hUCMSCs and hUCPVCs, the cell types were phenotypically similar, except for CD146 expression (37.9% of perivascular cells, 16.2% in hUCMSCs) [101]. After exposure to osteogenic medium, positive von Kossa staining was slightly stronger in perivascular cells than in hUCMSCs. It will be interesting to further evaluate a subpopulation excluding hUCPVCs from hUCMSCs, which may clarify how hUCPVCs contributed to the osteogenic activity of hUCMSCs. In BMP-2-induced differentiation, hUCMSCs had similar expression of osteogenic phenotypes and signal transduction pathways compared with hBMSCs [102]. The differentiation of hUCMSCs could be triggered by osteoinductive materials. Cells cultured on calcium phosphate cement were shown to proliferate and undergo osteogenic differentiation [103]. When hUCMSCs were cultured for 7 days in monolayer with a medium containing demineralized bone matrix [104], their proliferation was inhibited, their morphology appeared shortened and flattened, and ALP activity increased significantly. These phenomena indicated that hUCMSCs may differentiate along the osteogenic lineage [104], while long-term culture will be needed to verify the presence of late stage osteogenic markers.
In monolayer culture, hUCMSCs demonstrated osteogenic differentiation ability after exposure to chemical signals and osteoinductive biomaterials. In 3D biomaterials, hUC-MSCs demonstrated their osteogenic differentiation, such as the upregulation of Runx2 and osteonectin and evidence of mineralization [61,105,106], while hBMSCs had higher osteogenic activity with 4.1-times more mineral content than hUCMSCs when cells were cultured in polycaprolactone–tricalcium–phosphate scaffolds [105]. hUCMSCs are capable of in vitro osteogenic differentiation when seeded on polycaprolactone–collagen–hydroxyapatite nanofibrous scaffolds [107], and when encapsulated in alginate microbeads [108], injectable calcium phosphate–alginate hydrogel paste [109] and fast-resorbable calcium phosphate bone cement [103]. In a pair of bone tissue engineering studies, hUCMSC seeding densities were compared on PGA scaffolds, and the effect of switching from osteogenic medium (dexamethasone and vitamin D3) to anabolic medium (IGF-I) with poly(L-lactic acid) scaffolds was investigated. It was discovered that with these nonwoven meshes a seeding density of 25 million cells/ml or higher was preferred and that changing to anabolic medium under the prescribed conditions was not beneficial [106,110]. In vivo osteogenic differentiation has been examined in a mouse model. Subcutaneous implantation of hUCMSCs demonstrated ectopic bone formation by von Kossa staining in 2 months and micro-CT and inferior osteogenesis of hUCMSCs compared with hBMSCs [105]. In another study, a porous biomimetic bone scaffold material, nano-hydroxyapatite–collagen–poly(L-lactic acid) composite, was seeded with hUCMSCs and then implanted into nude mice subcutaneously. Osteoblasts were detected by transmission electron microscopy after 12 weeks [61]. However, there was no additional technique used to investigate if these cells were host mouse cells or differentiated hUCMSCs.
Our group has evaluated hUCMSCs in osteochondral tissue engineering, where chondrogenesis and osteogenesis were regionally promoted within a given construct. One approach seeded hUCMSCs into poly(L-lactic acid) scaffolds with either chondrogenic or osteogenic medium for 3 weeks [111]. These constructs were then sutured together, with or without a thin layer of undifferentiated hUCMSCs in between. Although differentiation was limited, the primary finding was that the inclusion of hUCMSCs in this ‘sandwich’ approach noticeably improved the integration of the two constructs, evidenced by histology. In another approach, microsphere-based scaffolds were constructed to release TGF-β from one side of a cylindrical scaffold and BMP-2 from the other side, with a gradual and continuous transition in release from one side to the other [112]. While differentiation was again limited, and there was evidence of calcium deposition throughout the scaffold, it was encouraging to see regionalized Safranin-O staining on the TGF-β side of the construct, demonstrating that regionalized chondrogenesis may be possible in an osteochondral approach. Although these two studies have provided two alternative approaches for the inclusion of hUCMSCs in osteochondral tissue engineering, the high priority at this stage is to evaluate the in vivo performance of hUCMSCs in osteochondral tissue engineering. If hUCMSCs are able to respond to inductive signals from surrounding native tissues in osteochondral defects, they may hold significant promise in regenerating an integrated osteochondral tissue.
In summary, hUCMSCs may share an osteogenic differentiation pathway similar to hBMSCs, namely the activation of the Runx2 gene and TAZ and the upregulation of osteocalcin and osteopontin, leading to mineralization. However, the osteogenic capability of hUCMSCs seems inferior to hBMSCs based on strategies employed to date. It will be valuable to make a side-by-side comparison among hUCMSCs, hUCPVCs and hBMSCs. The success of in vivo bone tissue engineering is promising using subcutaneous mouse models and further segment grafts in a larger animal model will be necessary to evaluate the functionality of engraftment when exposed to physiological mechanical and chemical cues.
Side-by-side comparisons of BMSCs & UCMSCs for bone & cartilage tissue engineering
In 2009, hBMSCs and hUCMSCs were passaged, seeded onto PGA scaffolds and cultured in chondrogenic medium, all under virtually identical conditions [97]. The major findings were that the hUCMSCs were far superior in terms of the quantity of collagen produced (per construct and on a per cell basis), although they were inferior in terms of collagen II gene expression and synthesis. The following year, hBMSCs and hUCMSCs were again compared under virtually identical conditions, seeded in the microsphere-based gradient scaffolds described in the previous section [112]. In this osteochondral application, the hUCMSCs were superior in terms of collagen synthesis and ALP activity, but were inferior in terms of Sox9 and Runx2 gene expression.
Collectively, these studies told us that under the prescribed conditions in vitro, hUCMSCs were inferior in terms of their differentiation capacity, but had a much greater capacity for tissue formation. However, it must be noted that these findings apply to a specific set of in vitro conditions, and that if hUCMSCs are indeed able to respond favorably to inductive signals in vivo, hUCMSCs may hold the potential for superior osteochondral regeneration in terms of the rate and quality of tissue regeneration.
Myogenic differentiation & muscle tissue engineering
Skeletal muscle provides voluntary motor control. Muscular disease and atrophy can permanently damage the muscle, and one category of muscular disease is progressive muscular dystrophy. With no effective treatment for progressive muscular dystrophy, stem cell therapy and tissue engineering could potentially be treatment options. To date, there has been limited investigation of UCMSCs differentiation into skeletal myocytes. Current techniques for myogenic differentiation of UCMSCs are exposure to myogenic medium [113], gene transfection [114], coculture [114], and injection into injured or dystrophic in vivo models [113,115]. After exposure to myogenic medium containing 5-azacytidine, a genomic DNA demethylating agent, Myf5 and MyoD were expressed after 7–11 days [113]. UCMSCs were also injected into an injured muscle, and skeletal muscle differentiation was observed by immunofluorescence of HLA-1 and sarcomeric tropomyosin antigens [113]. UCMSCs have been transfected with MyoD transcription factor. After 5 days, the cells exhibited functional markers of fusion machinery, muscle cell-specific structural proteins and muscle cell-specific enzymes [114]. Lastly, a dystrophic murine model was used to evaluate systemic exposure to hUCMSCs, and the cells’ ability to engraft into the damaged muscle [115]. While hUCMSCs were able to reach the muscle, they possessed a lesser degree of differentiation than human ADSCs, as analyzed by dysferlin and human-dystrophin expression. The authors hypothesized that the niche difference between hUCMSCs and human ADSCs may have been the reason for the difference in in vivo differentiation. Further research is needed to determine the best differentiation technique for these cells, and if in vivo differentiation is possible.
Adipose differentiation & tissue engineering
Traditional autologous fat tissue transplantation suffers from the loss of graft volume with time [116]. When tissue engineering is applied to adipose tissue regeneration, the usage of mature adipocytes is limited by the inferior in vitro proliferation and expansion ability of these cells, and stem cells have been proposed as substitute cells [117]. Like hBMSCs, adipose differentiation of hUCMSCs has been induced in monolayer culture by dexamethasone, insulin, indomethacin and isobutylmethylxan-thine, and has been verified by lipoid deposits that were detected by Oil Red O staining [36,65,71–72,82]. Adipogenic differentiation has also been observed in hUCPVCs [64,82]; however, cyclic stretch-induced TGF-β1/Smad signaling inhibited expression of adipocyte markers [118]. Following adipogenic differentiation, adipose-related genes, such as peroxisome proliferator-activated receptor-γ2 [65], lipoprotein lipase [71] and plasminogen activator inhibitor-1 [36], were detected in hUCMSCs by reverse transcription-PCR. Compared with hBMSCs, hUCMSCs took a longer period to achieve adipose differentiation (40 vs 21 days, respectively) [36]. A similar observation was made between fetal MSCs and adult MSCs. In addition, hBMSCs formed more homogeneous lipid droplets and had a more round morphology than the hUCMSCs [36]. However, in a 2-week study by Lu et al., no significant difference was found in the percentage of adipogenic positive cells between hUCMSCs and hBMSCs (69.4 vs 57.3%, respectively) [71]. After long-term exposure to adipogenic medium, 90% of hUCMSCs differentiated toward the adipogenic lineage and formed mature adipocytes [119]. Therefore, the adipose differentiation ability of hUCMSCs makes them a promising cell source for regenerative medicine, despite the fact that hUCMSCs have not yet been used for adipose tissue engineering.
Undifferentiated hUCMSCs & cardiovascular tissue engineering
Detailed reviews regarding the application of hUCMSCs in cardiovascular tissue engineering have appeared [120,121], so a brief overview is provided. hUCMSCs were utilized for cardiovascular tissue engineering prior to the identification of multipotent differentiation [49], and they have been shown to be a suitable cell source for this application [54–58]. In monolayer culture, undifferentiated hUCMSCs expressed positive staining of α-smooth muscle actin and vimentin with the deposition of type I and III collagen after 2 weeks in a culture medium containing only Dulbecco’s modified Eagle’s medium and 10% fetal bovine serum. After exposure to the culture medium, the hUCMSCs were phenotypically similar to the native cells in heart valve leaflets [49]. This similarity provided the rationale for using these cells for cardiovascular regeneration [49]. The culture of hUCMSCs on 3D PGA/poly-4-hydroxybutyrate [49,54,56–58] or poly-4-hydroxybutyrate [55] scaffolds produced a layered tissue with type I and III collagen, GAGs, elastin and a mechanical stiffness similar to native cardiovascular tissue. These tissue engineered constructs were endothelialized, under cyclic strain and growth factor perfusion, with human umbilical cord blood endothelial progenitor cells (UC-EPCs) to provide a functional endothelial layer [57]. Hence, the approach using hUCMSCs for cardiovascular tissue engineering is attractive, since UC-EPCs can also be obtained at birth along with hUCMSCs. This alone will not solve the problem of tissue rejection. However, if the umbilical cord is collected from children who have a congenital heart defect, then UC-EPCs and UCMSCs may be used to construct autologous bilaminar constructs.
Discussion
In this review, hUCMSCs were introduced as an alternative to ESCs and BMSCs, with clear advantages over each (Table 1). hUCMSCs are noncontroversial, in contrast to ESCs. In terms of availability and harvesting efficiency, hUCMSCs offer a major advantage over ESCs, as there is an endless supply of umbilical cords with 4.1 million births in the USA alone in 2005 [122]. The ample supply of the cords and the cryopreservation of UCMSCs will allow cells to be banked for future use as an autologous or allogeneic (with tissue typing) cell source. Practically speaking, hUCMSCs are much easier and less expensive to harvest and culture than ESCs. The umbilical cord stroma can be digested by trypsin, type I and II collagenase, and hyaluronidase in 4 h [33,36,64,65]. Among these enzymes, type II collagenase has been proved to be the strongest and most efficient with all hUCMSCs released [36], while the cocktail of type I collagen and hyaluronidase [33] may only release a part of the cells from the stroma, which might isolate a different cell type. It must also be noted that the hUCPVCs reported by Sarugaser et al. represented a subpopulation with great intrinsic osteogenic ability [64], although they share most of the surface markers with hUCMSCs, and should be distinguished from the whole population of hUCMSCs when comparing hUCMSCs to other types of stem cells.
Table 1.
Criteria for cell source selection in tissue engineering.
| Factor | Mature cells | ADSCs | BMSCs | ESCs† | UCMSCs | Ref. |
|---|---|---|---|---|---|---|
| Highly available deciduous tissue source | X | [122] | ||||
| Politically noncontroversial | X | X | X | X | [48] | |
| Easy to obtain and to use | X | X | X | X | [134] | |
| No donor site morbidity | X | X | [134] | |||
| Multiple passaging | X | X | [33,36] | |||
| Multipotent | X | X | X | X | [36,65,135] | |
| Low immunogenicity | X | X | X | X | [33,42,43, 53,123] |
Includes embryonic germ cells.
ADSC: Adipose-derived stromal cell; BMSC: Bone marrow mesenchymal stromal cell; ESC: Embryonic stem cell; Mature: Fully differentiated cells from their host tissue; UCMSC: Umbilical cord mesenchymal stromal cell.
As for their differentiation ability, hUCMSCs differentiate along mesenchymal lineages and perhaps across germ layer boundaries, which translates to a broad potential for curing a range of diseases such as diabetes and Parkinson’s disease. Currently, most of the differentiation efforts were conducted in vitro in monolayer culture, which is different from the 3D architecture and microenvironment of scaffolds and native tissues. The differences between 2D and 3D environments are certain to affect cellular migration, proliferation and differentiation. For example, Zhang et al. reported that hUCMSCs had slightly better proliferative and osteogenic potential than adult hBMSCs in 2D monolayer culture; however, when these cells were cultured in 3D scaffolds both in in vitro culture and in vivo implantation, the adult hBMSCs outperformed the hUCMSCs [105]. Another feature of the contemporary literature is that all differentiation studies evaluated extracellular protein markers biochemically, and only examined several genes for a specific lineage at the mRNA level. An examination of gene expression over the time course of differentiation may reveal both the expression of genes associated with the initial lineage commitment at early time points and, later, the expression of genes associated with the fully differentiated cell [5]. These cell products, which indicate a fully differentiated cell, are critically important to produce the biomechanical and physiological properties required in many tissue engineering applications. For example, a couple of studies used osteopontin as a differentiation marker to confirm osteogenic differentiation, although osteopontin is not a bone-specific marker, also being expressed in other tissues such as kidney and placenta [65,71]. Based on the current limitations with gene expression data, it appears that hUCMSCs share a gene activation at the initial commitments similar to other MSCs. For instance, the SOX9, Runx2 and PPARγ genes play the key role in chondrogenic, osteogenic and adipogenic differentiation, respectively, and all of these genes were upregulated after exposure to differentiation signals at the earlier stage of differentiation and their expression continued to increase with time. At the late stage, lower type II collagen at both the mRNA and protein levels with chondrogenesis in hUCMSCs relative to hBMSCs, along with the inferior mineralization with osteogenesis, and no presence of mature adipocytes with adipogenesis, indicate that hUCMSCs may follow a different differentiation pathway from other MSCs or require a longer time course to reach mature phenotypes. These puzzles can be further clarified by side-by-side comparison with other MSCs using cDNA microarray and reverse transcription-PCR analysis.
In addition to the differentiation ability, the self-renewal ability is the other key characteristic of stromal cells. The self-renewal capability of stromal cells not only plays a key role in tissue homeostasis in vivo, but also has particular implications for tissue engineering, requiring a large amount of undifferentiated cells for large defects. hUCMSCs, as fetus-derived MSCs, have a better in vitro expansion capability without the loss of differentiation ability than adult stem cells, perhaps due to their longer telomeres [105]. The original phenotypic markers of hUCMSCs were positive throughout a nine-passage period, including MSC surface markers (e.g., CD73, CD90 and CD105) and transcription factors (e.g., Nanog, Oct-4 and Sox2), and the differentiation ability showed no significant differences among these passages [77]. Therefore, great self-replenishing activity of hUCMSCs and their extensive availability make it possible to obtain a tremendous number of cells in a short time (orders of magnitude larger than BMSCs) to meet the urgent need of tissue engineering applications.
With regard to their immunocompatibility, hUCMSCs suppress the proliferation of activated splenocytes and T cells appear to be immune suppressive like other MSCs and more immunocompatible than ESCs [43,123–127]. In vitro immune characterization of hUCMSCs revealed that the immunosuppression properties can be elucidated by evidence from the literature [123]. First, hUCMSCs suppress the proliferation of immune cells such as Con-A-stimulated rat splenocytes, human peripheral blood mononuclear cells and purified T cells. Second, hUCMSCs synthesize HLA-G6, an immunosuppressive isoform of HLAs; while hUCMSCs do not express HLA-G5, a critical isoform to the immunosuppressive function of adult hBMSCs [128]. Third, immune response-related costimulatory molecules including CD40, CD80 and CD86 are not detected on hUCMSCs. Finally, hUCMSCs produce IL-6, which is involved with MSC immune regulation [129]. The in vitro results support the earlier finding that hUCMSCs can be tolerated in animal models [33,42,43,64,123,130]. In an allogeneic study by Cho et al., the immunogenicity of UCMSCs was only observed after repeated injection of MHC-mismatched unactivated porcine UCMSCs into the same inflamed region [42]. In xenotransplantation studies, UCMSCs survived without immune response and displayed a rescue phenomenon in retinal and Parkinson’s disease [53,130]. Moreover, hUCMSCs can also be used as an autologous MSC source, which can be cryogenically frozen, and thus it is possible to avoid immunorejection. Even if evidence of immunogenicity is discovered after differentiation [131], allogeneic hUCMSCs would be a viable option with the availability of cord cell banks, as routine tissue-typing (HLA/MHC matching) with a tremendous donor selection would be straightforward and would thus reduce any potential immunogenicity along with immuno-suppressants, as is done for transplant patients (but without waiting indefinitely for a donor). With all of these characteristics, we believe that hUCMSCs will be a suitable, attractive and potentially preferred cell source for tissue engineering. Although hUCMSCs possess a number of advantages over other cell sources, there are limitations that warrant further investigation. The greatest limitation to date is the relative paucity of characterization data relative to the current gold standard, BMSCs. For example, methods to differentiate BMSCs are established and well-documented, whereas we have only begun to scratch the surface with regard to identifying the signaling strategies tailored to hUCMSC differentiation. Although there is evidence that hUCMSCs proliferate faster, can be passaged further and synthesize matrix at a higher rate, the key limitation is that the current literature is currently not able to provide compelling and agreed-upon evidence that hUCMSCs can match the differentiation capacity of BMSCs in a 3D in vitro or in vivo environment. The burden will be on the growing number of investigators joining the field of hUCMSC biology and tissue engineering to identify the set of conditions specific to hUCMSCs that will signal differentiation along desired lineages in a 3D environment.
En route to the application of hUCMSCs in tissue engineering, a number of other concerns remain to be addressed, including cell–biomaterial surface interaction, cell culture environment and safety. Tissue engineering strategies require hUCMSCs to adhere efficiently to biomaterials. Indeed, high seeding efficiency with hUCMSCs, coupled with fast proliferation, can reduce the quantity of cords and the cost of culture reagents significantly. Tissue engineering strategies also require an optimal culture environment that can maintain cell growth, differentiation potential, phenotype and genomic stability [132]. Currently, the most common medium for hUCMSCs involves the use of bovine serum, which may cause disease transmission and immune reaction. As tissue engineering using hUCMSCs is translated into clinical practice, a serum-free medium may be preferred to avoid these problems. In fact, a low-serum medium has been successfully used to culture hUCMSCs. It includes only 2% bovine serum, 1X serum substitute, and growth factors containing EGF and PDGF [33]. In this medium, cells grew robustly and most of the initial surface markers were maintained after four passages. In the future, human autologous serum may be an option to replace this 2% animal serum, although clearly a serum-free medium would be ideal. Moreover, to engineer large-scale tissues, typical scale-up problems need to be addressed as the diffusion of nutrients and wastes is hindered in large constructs [133].
Future perspective
Umbilical cord mesenchymal stromal cells represent an exciting MSC source for tissue engineering applications with clear advantages over other cell sources including, but not limited to, the ease of access, extensive availability, noninvasive harvesting and in vitro expansion ability. Although their degree of ‘stemness’ is still unclear due to the lack of data on long-term engraftment and self-renewal in the literature [37], the in vivo differentiation of these cells including osteogenic, endothelial, neurogenic, hepatic and pancreatic lineages, accompanied by their great in vitro expansion ability, certainly supports the contention that these cells may indeed act as true therapeutic cells. Investigations of hUCMSCs in musculoskeletal tissue engineering have shown encouraging results based on the in vitro and in vivo evaluations while more in vivo tissue engineering studies with large animal models are warranted en route to clinical trials. Following these initial current successes with these cells, we believe that hUCMSCs have the potential to be successfully used in other tissue engineering areas such as pancreas and neural tissue regeneration. Future work should include the optimization of cell culture environment, scaffold design and evaluation of in vivo regenerative medicine performance, all of which can be integrated in stem cell-based tissue engineering strategies toward clinical application.
Footnotes
Financial & competing interests disclosure
The authors gratefully acknowledge support from the Arthritis Foundation, both from the national organization and from the Kansas Chapter. They further acknowledge support from the NSF CAREER Award (MS Detamore). ML Weiss is supported by NIH grant NS-34160, and a KSU Targeted Excellence grant. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
- 1.Vats A, Bielby RC, Tolley NS, Nerem R, Polak JM. Stem cells. Lancet. 2005;366(9485):592–602. doi: 10.1016/S0140-6736(05)66879-1. [DOI] [PubMed] [Google Scholar]
- 2.Brignier AC, Gewirtz AM. Embryonic and adult stem cell therapy. J Allergy Clin Immunol. 2010;125(2 Suppl 2):S336–S344. doi: 10.1016/j.jaci.2009.09.032. [DOI] [PubMed] [Google Scholar]
- 3.Kaufman DS, Hanson ET, Lewis RL, et al. Hematopoietic colony-forming cells derived from human embryonic stem cells. Proc Natl Acad Sci USA. 2001;98(19):10716–10721. doi: 10.1073/pnas.191362598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Assady S, Maor G, Amit M, Itskovitz-Eldor J, Skorecki KL, Tzukerman M. Insulin production by human embryonic stem cells. Diabetes. 2001;50(8):1691–1697. doi: 10.2337/diabetes.50.8.1691. [DOI] [PubMed] [Google Scholar]
- 5.Green H, Easley K, Iuchi S. Marker succession during the development of keratinocytes from cultured human embryonic stem cells. Proc Natl Acad Sci USA. 2003;100(26):15625–15630. doi: 10.1073/pnas.0307226100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blum B, Benvenisty N. The tumorigenicity of human embryonic stem cells. Adv Cancer Res. 2008;100:133–158. doi: 10.1016/S0065-230X(08)00005-5. [DOI] [PubMed] [Google Scholar]
- 7.Cai J, Li W, Su H, et al. Generation of human induced pluripotent stem cells from umbilical cord matrix and amniotic membrane mesenchymal cells. J Biol Chem. 2010;285(15):11227–11234. doi: 10.1074/jbc.M109.086389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448(7151):313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 9.Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 10.Maherali N, Sridharan R, Xie W, et al. Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell Stem Cell. 2007;1(1):55–70. doi: 10.1016/j.stem.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 11.Wernig M, Meissner A, Foreman R, et al. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448(7151):318–324. doi: 10.1038/nature05944. [DOI] [PubMed] [Google Scholar]
- 12.Di Nicola M, Carlo-Stella C, Magni M, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99(10):3838–3843. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 13.Bartholomew A, Sturgeon C, Siatskas M, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30(1):42–48. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- 14.Kode JA, Mukherjee S, Joglekar MV, Hardikar AA. Mesenchymal stem cells: immunobiology and role in immunomodulation and tissue regeneration. Cytotherapy. 2009;11(4):377–391. doi: 10.1080/14653240903080367. [DOI] [PubMed] [Google Scholar]
- 15.Quarto R, Mastrogiacomo M, Cancedda R, et al. Repair of large bone defects with the use of autologous bone marrow stromal cells. N Engl J Med. 2001;344(5):385–386. doi: 10.1056/NEJM200102013440516. [DOI] [PubMed] [Google Scholar]
- 16.Marcacci M, Kon E, Moukhachev V, et al. Stem cells associated with macroporous bioceramics for long bone repair: 6- to 7-year outcome of a pilot clinical study. Tissue Eng. 2007;13(5):947–955. doi: 10.1089/ten.2006.0271. [DOI] [PubMed] [Google Scholar]
- 17.Mueller SM, Glowacki J. Age-related decline in the osteogenic potential of human bone marrow cells cultured in three-dimensional collagen sponges. J Cell Biochem. 2001;82(4):583–590. doi: 10.1002/jcb.1174. [DOI] [PubMed] [Google Scholar]
- 18.Lee SY, Miwa M, Sakai Y, et al. In vitro multipotentiality and characterization of human unfractured traumatic hemarthrosis-derived progenitor cells: a potential cell source for tissue repair. J Cell Physiol. 2007;210(3):561–566. doi: 10.1002/jcp.20890. [DOI] [PubMed] [Google Scholar]
- 19.Schaffler A, Buchler C. Concise review: adipose tissue-derived stromal cells – basic and clinical implications for novel cell-based therapies. Stem Cells. 2007;25(4):818–827. doi: 10.1634/stemcells.2006-0589. [DOI] [PubMed] [Google Scholar]
- 20.Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100(9):1249–1260. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13(12):4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakashima M, Reddi AH. The application of bone morphogenetic proteins to dental tissue engineering. Nat Biotechnol. 2003;21(9):1025–1032. doi: 10.1038/nbt864. [DOI] [PubMed] [Google Scholar]
- 23.Sloan AJ, Waddington RJ. Dental pulp stem cells: what, where, how? Int J Paediatr Dent. 2009;19(1):61–70. doi: 10.1111/j.1365-263X.2008.00964.x. [DOI] [PubMed] [Google Scholar]
- 24.Gronthos S, Brahim J, Li W, et al. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81(8):531–535. doi: 10.1177/154405910208100806. [DOI] [PubMed] [Google Scholar]
- 25.Tuan RS, Boland G, Tuli R. Adult mesenchymal stem cells and cell-based tissue engineering. Arthritis Res Ther. 2003;5(1):32–45. doi: 10.1186/ar614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choumerianou DM, Dimitriou H, Kalmanti M. Stem cells: promises versus limitations. Tissue Eng Part B Rev. 2008;14(1):53–60. doi: 10.1089/teb.2007.0216. [DOI] [PubMed] [Google Scholar]
- 27.Secco M, Zucconi E, Vieira NM, et al. Mesenchymal stem cells from umbilical cord: do not discard the cord! Neuromuscul Disord. 2008;18(1):17–18. doi: 10.1016/j.nmd.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 28.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276(5309):71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 29.Childs R, Chernoff A, Contentin N, et al. Regression of metastatic renal-cell carcinoma after nonmyeloablative allogeneic peripheral-blood stem-cell transplantation. N Engl J Med. 2000;343(11):750–758. doi: 10.1056/NEJM200009143431101. [DOI] [PubMed] [Google Scholar]
- 30.Jones EA, English A, Henshaw K, et al. Enumeration and phenotypic characterization of synovial fluid multipotential mesenchymal progenitor cells in inflammatory and degenerative arthritis. Arthritis Rheum. 2004;50(3):817–827. doi: 10.1002/art.20203. [DOI] [PubMed] [Google Scholar]
- 31.Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W. β-catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell. 2001;105(4):533–545. doi: 10.1016/s0092-8674(01)00336-1. [DOI] [PubMed] [Google Scholar]
- 32.Seale P, Asakura A, Rudnicki MA. The potential of muscle stem cells. Dev Cell. 2001;1(3):333–342. doi: 10.1016/s1534-5807(01)00049-1. [DOI] [PubMed] [Google Scholar]
- 33.Weiss ML, Medicetty S, Bledsoe AR, et al. Human umbilical cord matrix stem cells: preliminary characterization and effect of transplantation in a rodent model of Parkinson’s disease. Stem Cells. 2006;24(3):781–792. doi: 10.1634/stemcells.2005-0330. [DOI] [PubMed] [Google Scholar]
- 34.Guilak F, Awad HA, Fermor B, Leddy HA, Gimble JM. Adipose-derived adult stem cells for cartilage tissue engineering. Biorheology. 2004;41(3–4):389–399. [PubMed] [Google Scholar]
- 35.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 36.Karahuseyinoglu S, Cinar O, Kilic E, et al. Biology of stem cells in human umbilical cord stroma: in situ and in vitro surveys. Stem Cells. 2007;25(2):319–331. doi: 10.1634/stemcells.2006-0286. [DOI] [PubMed] [Google Scholar]
- 37.Troyer DL, Weiss ML. Wharton’s jelly-derived cells are a primitive stromal cell population. Stem Cells. 2008;26(3):591–599. doi: 10.1634/stemcells.2007-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Campard D, Lysy PA, Najimi M, Sokal EM. Native umbilical cord matrix stem cells express hepatic markers and differentiate into hepatocyte-like cells. Gastroenterology. 2008;134(3):833–848. doi: 10.1053/j.gastro.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 39.Weiss ML, Troyer DL. Stem cells in the umbilical cord. Stem Cell Rev. 2006;2(2):155–162. doi: 10.1007/s12015-006-0022-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Can A, Karahuseyinoglu S. Concise review: human umbilical cord stroma with regard to the source of fetus-derived stem cells. Stem Cells. 2007;25(11):2886–2895. doi: 10.1634/stemcells.2007-0417. [DOI] [PubMed] [Google Scholar]
- 41.Ciavarella S, Dammacco F, De Matteo M, Loverro G, Silvestris F. Umbilical cord mesenchymal stem cells: role of regulatory genes in their differentiation to osteoblasts. Stem Cells Dev. 2009;18(8):1211–1220. doi: 10.1089/scd.2008.0340. [DOI] [PubMed] [Google Scholar]
- 42.Cho PS, Messina DJ, Hirsh EL, et al. Immunogenicity of umbilical cord tissue derived cells. Blood. 2008;111(1):430–438. doi: 10.1182/blood-2007-03-078774. [DOI] [PubMed] [Google Scholar]
- 43.Ennis J, Götherström C, Le Blanc K, Davies JE. In vitro immunologic properties of human umbilical cord perivascular cells. Cytotherapy. 2008;10(2):174–181. doi: 10.1080/14653240801891667. [DOI] [PubMed] [Google Scholar]
- 44.Sarugaser R, Ennis J, Stanford WL, Davies JE. Isolation, propagation, and characterization of human umbilical cord perivascular cells (HUCPVCs) Methods Mol Biol. 2009;482:269–279. doi: 10.1007/978-1-59745-060-7_17. [DOI] [PubMed] [Google Scholar]
- 45.Hadidian Z, Pirie NW. The preparation and some properties of hyaluronic acid from human umbilical cord. Biochem J. 1948;42(2):260–265. doi: 10.1042/bj0420260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bowles HE, McKee RD. Ruptures of the umbilical cord with a case of intrapartum rupture or all three vessels. Calif Med. 1949;70(5):422. [PMC free article] [PubMed] [Google Scholar]
- 47.Moretti P, Hatlapatka T, Marten D, et al. Mesenchymal stromal cells derived from human umbilical cord tissues: primitive cells with potential for clinical and tissue engineering applications. Adv Biochem Eng Biotechnol. 2009;123:29–54. doi: 10.1007/10_2009_15. [DOI] [PubMed] [Google Scholar]
- 48.Mitchell KE, Weiss ML, Mitchell BM, et al. Matrix cells from Wharton’s jelly form neurons and glia. Stem Cells. 2003;21(1):50–60. doi: 10.1634/stemcells.21-1-50. [DOI] [PubMed] [Google Scholar]
- 49.Kadner A, Hoerstrup SP, Tracy J, et al. Human umbilical cord cells: a new cell source for cardiovascular tissue engineering. Ann Thorac Surg. 2002;74(4):S1422–S1428. doi: 10.1016/s0003-4975(02)03910-3. [DOI] [PubMed] [Google Scholar]
- 50.Chao KC, Chao KF, Fu YS, Liu SH. Islet-like clusters derived from mesenchymal stem cells in Wharton’s Jelly of the human umbilical cord for transplantation to control Type 1 diabetes. PLoS ONE. 2008;3(1):e1451. doi: 10.1371/journal.pone.0001451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fong CY, Richards M, Manasi N, Biswas A, Bongso A. Comparative growth behaviour and characterization of stem cells from human Wharton’s jelly. Reprod Biomed Online. 2007;15(6):708–718. doi: 10.1016/s1472-6483(10)60539-1. [DOI] [PubMed] [Google Scholar]
- 52.Jomura S, Uy M, Mitchell K, Dallasen R, Bode CJ, Xu Y. Potential treatment of cerebral global ischemia with Oct-4+ umbilical cord matrix cells. Stem Cells. 2007;25(1):98–106. doi: 10.1634/stemcells.2006-0055. [DOI] [PubMed] [Google Scholar]
- 53.Lund RD, Wang S, Lu B, et al. Cells isolated from umbilical cord tissue rescue photoreceptors and visual functions in a rodent model of retinal disease. Stem Cells. 2007;25(3):602–611. doi: 10.1634/stemcells.2006-0308. [DOI] [PubMed] [Google Scholar]
- 54.Kadner A, Zund G, Maurus C, et al. Human umbilical cord cells for cardiovascular tissue engineering: a comparative study. Eur J Cardiothorac Surg. 2004;25(4):635–641. doi: 10.1016/j.ejcts.2003.12.038. [DOI] [PubMed] [Google Scholar]
- 55.Sodian R, Lueders C, Kraemer L, et al. Tissue engineering of autologous human heart valves using cryopreserved vascular umbilical cord cells. Ann Thorac Surg. 2006;81(6):2207–2216. doi: 10.1016/j.athoracsur.2005.12.073. [DOI] [PubMed] [Google Scholar]
- 56.Schmidt D, Mol A, Neuenschwander S, et al. Living patches engineered from human umbilical cord derived fibroblasts and endothelial progenitor cells. Eur J Cardiothorac Surg. 2005;27(5):795–800. doi: 10.1016/j.ejcts.2005.01.064. [DOI] [PubMed] [Google Scholar]
- 57.Schmidt D, Mol A, Odermatt B, et al. Engineering of biologically active living heart valve leaflets using human umbilical cord-derived progenitor cells. Tissue Eng. 2006;12(11):3223–3232. doi: 10.1089/ten.2006.12.3223. [DOI] [PubMed] [Google Scholar]
- 58.Schmidt D, Asmis LM, Odermatt B, et al. Engineered living blood vessels: functional endothelia generated from human umbilical cord-derived progenitors. Ann Thorac Surg. 2006;82(4):1465–1471. doi: 10.1016/j.athoracsur.2006.05.066. discussion 1471. [DOI] [PubMed] [Google Scholar]
- 59.Wang L, Seshareddy K, Weiss ML, Detamore MS. Effect of initial seeding density on human umbilical cord mesenchymal stromal cells for fibrocartilage tissue engineering. Tissue Eng Part A. 2009;15(5):1009–1017. doi: 10.1089/ten.tea.2008.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bailey MM, Wang L, Bode CJ, et al. A comparison of human umbilical cord matrix stem cells and temporomandibular joint condylar chondrocytes for tissue engineering temporomandibular joint condylar cartilage. Tissue Eng. 2007;13(8):2003–2010. doi: 10.1089/ten.2006.0150. [DOI] [PubMed] [Google Scholar]
- 61.Diao Y, Ma Q, Cui F, Zhong Y. Human umbilical cord mesenchymal stem cells: osteogenesis in vivo as seed cells for bone tissue engineering. J Biomed Mater Res A. 2008;91(1):123–131. doi: 10.1002/jbm.a.32186. [DOI] [PubMed] [Google Scholar]
- 62.Heifetz SA. The umbilical cord: obstetrically important lesions. Clin Obstet Gynecol. 1996;39(3):571–587. doi: 10.1097/00003081-199609000-00007. [DOI] [PubMed] [Google Scholar]
- 63.Hill LM, Kislak S, Runco C. An ultrasonic view of the umbilical cord. Obstet Gynecol Surv. 1987;42(2):82–88. [PubMed] [Google Scholar]
- 64.Sarugaser R, Lickorish D, Baksh D, Hosseini MM, Davies JE. Human umbilical cord perivascular (HUCPV) cells: a source of mesenchymal progenitors. Stem Cells. 2005;23(2):220–229. doi: 10.1634/stemcells.2004-0166. [DOI] [PubMed] [Google Scholar]
- 65.Wang HS, Hung SC, Peng ST, et al. Mesenchymal stem cells in the Wharton’s jelly of the human umbilical cord. Stem Cells. 2004;22(7):1330–1337. doi: 10.1634/stemcells.2004-0013. [DOI] [PubMed] [Google Scholar]
- 66.Fong CY, Subramanian A, Biswas A, et al. Derivation efficiency, cell proliferation, freeze-thaw survival, stem-cell properties and differentiation of human Wharton’s jelly stem cells. Reprod Biomed Online. 2010;21(3):391–401. doi: 10.1016/j.rbmo.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 67.Nanaev AK, Kohnen G, Milovanov AP, Domogatsky SP, Kaufmann P. Stromal differentiation and architecture of the human umbilical cord. Placenta. 1997;18(1):53–64. doi: 10.1016/s0143-4004(97)90071-0. [DOI] [PubMed] [Google Scholar]
- 68.Kobayashi K, Kubota T, Aso T. Study on myofibroblast differentiation in the stromal cells of Wharton’s jelly: expression and localization of α-smooth muscle actin. Early Hum Dev. 1998;51(3):223–233. doi: 10.1016/s0378-3782(97)00123-0. [DOI] [PubMed] [Google Scholar]
- 69.Bongso A, Fong CY, Gauthaman K. Taking stem cells to the clinic: major challenges. J Cell Biochem. 2008;105(6):1352–1360. doi: 10.1002/jcb.21957. [DOI] [PubMed] [Google Scholar]
- 70.Majore I, Moretti P, Stahl F, Hass R, Kasper C. Growth and differentiation properties of mesenchymal stromal cell populations derived from whole human umbilical cord. Stem Cell Rev. 2010 doi: 10.1007/s12015-010-9165-y. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 71.Lu LL, Liu YJ, Yang SG, et al. Isolation and characterization of human umbilical cord mesenchymal stem cells with hematopoiesis-supportive function and other potentials. Haematologica. 2006;91(8):1017–1026. [PubMed] [Google Scholar]
- 72.Wu KH, Zhou B, Lu SH, et al. In vitro and in vivo differentiation of human umbilical cord derived stem cells into endothelial cells. J Cell Biochem. 2007;100(3):608–616. doi: 10.1002/jcb.21078. [DOI] [PubMed] [Google Scholar]
- 73.Hoerstrup SP, Kadner A, Breymann C, et al. Living, autologous pulmonary artery conduits tissue engineered from human umbilical cord cells. Ann Thorac Surg. 2002;74(1):46–52. doi: 10.1016/s0003-4975(02)03649-4. discussion 52. [DOI] [PubMed] [Google Scholar]
- 74.Eblenkamp M, Aigner J, Hintermair J, et al. Umbilical cord stromal cells (UCSC). Cells featuring osteogenic differentiation potential. Orthopade. 2004;33(12):1338–1345. doi: 10.1007/s00132-004-0730-4. [DOI] [PubMed] [Google Scholar]
- 75.Carlin R, Davis D, Weiss M, et al. Expression of early transcription factors Oct4, Sox2 and Nanog by porcine umbilical cord (PUC) matrix cells. Reprod Biol Endocrinol. 2006;4(1):8. doi: 10.1186/1477-7827-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu KH, Zhou B, Yu CT, et al. Therapeutic potential of human umbilical cord derived stem cells in a rat myocardial infarction model. Ann Thorac Surg. 2007;83(4):1491–1498. doi: 10.1016/j.athoracsur.2006.10.066. [DOI] [PubMed] [Google Scholar]
- 77.Jo CH, Kim OS, Park EY, et al. Fetal mesenchymal stem cells derived from human umbilical cord sustain primitive characteristics during extensive expansion. Cell Tissue Res. 2008;334(3):423–433. doi: 10.1007/s00441-008-0696-3. [DOI] [PubMed] [Google Scholar]
- 78.Zhang P, Luo X, Wang H. Clinical transplantation of a tissue-engineered airway. Lancet. 2009;373(9665):718. doi: 10.1016/S0140-6736(09)60430-X. author reply 718–719. [DOI] [PubMed] [Google Scholar]
- 79.Fong CY, Chak LL, Biswas A, et al. Human Wharton’s jelly stem cells have unique transcriptome profiles compared to human embryonic stem cells and other mesenchymal stem cells. Stem Cell Rev. 2010 doi: 10.1007/s12015-010-9166-x. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 80.Liedtke S, Stephan M, Kogler G. Oct4 expression revisited: potential pitfalls for data misinterpretation in stem cell research. Biol Chem. 2008;389(7):845–850. doi: 10.1515/BC.2008.098. [DOI] [PubMed] [Google Scholar]
- 81.Méndez-Ferrer S, Michurina TV, Ferraro F, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466(7308):829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Baksh D, Yao R, Tuan RS. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells. 2007;25(6):1384–1392. doi: 10.1634/stemcells.2006-0709. [DOI] [PubMed] [Google Scholar]
- 83.Kita K, Gauglitz GG, Phan TT, Herndon DN, Jeschke MG. Isolation and characterization of mesenchymal stem cells from the sub-amniotic human umbilical cord lining membrane. Stem Cells Dev. 2010;19(4):491–502. doi: 10.1089/scd.2009.0192. [DOI] [PubMed] [Google Scholar]
- 84.Anzalone R, Lo Iacono M, Corrao S, et al. New emerging potentials for human Wharton’s jelly mesenchymal stem cells: immunological features and hepatocyte-like differentiative capacity. Stem Cells Dev. 2010;19(4):423–438. doi: 10.1089/scd.2009.0299. [DOI] [PubMed] [Google Scholar]
- 85.Fan CG, Zhang QJ, Zhou JR. Therapeutic potentials of mesenchymal stem cells derived from human umbilical cord. Stem Cell Rev. 2010 doi: 10.1007/s12015-010-9168-8. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 86.Gao J, Yao JQ, Caplan AI. Stem cells for tissue engineering of articular cartilage. Proc Inst Mech Eng. 2007;221(5):441–450. doi: 10.1243/09544119JEIM257. [DOI] [PubMed] [Google Scholar]
- 87.Darling EM, Athanasiou KA. Rapid phenotypic changes in passaged articular chondrocyte subpopulations. J Orthop Res. 2005;23(2):425–432. doi: 10.1016/j.orthres.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 88.Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res. 1998;238(1):265–272. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- 89.Mackay AM, Beck SC, Murphy JM, Barry FP, Chichester CO, Pittenger MF. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng. 1998;4(4):415–428. doi: 10.1089/ten.1998.4.415. [DOI] [PubMed] [Google Scholar]
- 90.Im GI, Jung NH, Tae SK. Chondrogenic differentiation of mesenchymal stem cells isolated from patients in late adulthood: the optimal conditions of growth factors. Tissue Eng. 2006;12(3):527–536. doi: 10.1089/ten.2006.12.527. [DOI] [PubMed] [Google Scholar]
- 91.Spagnoli A, Longobardi L, O’Rear L. Cartilage disorders: potential therapeutic use of mesenchymal stem cells. Endocr Dev. 2005;9:17–30. doi: 10.1159/000085719. [DOI] [PubMed] [Google Scholar]
- 92.Longobardi L, O’Rear L, Aakula S, et al. Effect of IGF-I in the chondrogenesis of bone marrow mesenchymal stem cells in the presence or absence of TGF-β signaling. J Bone Miner Res. 2006;21(4):626–636. doi: 10.1359/jbmr.051213. [DOI] [PubMed] [Google Scholar]
- 93.Wang L, Detamore MS. Insulin-like growth factor-I improves chondrogenesis of predifferentiated human umbilical cord mesenchymal stromal cells. J Orthop Res. 2009;27(8):1109–1115. doi: 10.1002/jor.20848. [DOI] [PubMed] [Google Scholar]
- 94.Naughton BA. Cells isolated from Wharton’s jelly of the human umbilical cord develop a cartilage phenotype when treated with TGF-β in vitro. FASEB J. 1997;11:A19. (Abstr. No. 108) [Google Scholar]
- 95.Barry F, Boynton RE, Liu B, Murphy JM. Chondrogenic differentiation of mesenchymal stem cells from bone marrow: differentiation-dependent gene expression of matrix components. Exp Cell Res. 2001;268(2):189–200. doi: 10.1006/excr.2001.5278. [DOI] [PubMed] [Google Scholar]
- 96.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 97.Wang L, Tran I, Seshareddy K, Weiss ML, Detamore MS. A comparison of human bone marrow-derived mesenchymal stem cells and human umbilical cord-derived mesenchymal stromal cells for cartilage tissue engineering. Tissue Eng Part A. 2009;15(8):2259–2266. doi: 10.1089/ten.tea.2008.0393. [DOI] [PubMed] [Google Scholar]
- 98.Motoki DS, Mulliken JB. The healing of bone and cartilage. Clin Plast Surg. 1990;17(3):527–544. [PubMed] [Google Scholar]
- 99.Habal MB. Bone repair by regeneration. Clin Plast Surg. 1996;23(1):93–101. [PubMed] [Google Scholar]
- 100.Salgado AJ, Coutinho OP, Reis RL. Bone tissue engineering: state of the art and future trends. Macromol Biosci. 2004;4(8):743–765. doi: 10.1002/mabi.200400026. [DOI] [PubMed] [Google Scholar]
- 101.Martin-Rendon E, Sweeney D, Lu F, Girdlestone J, Navarrete C, Watt SM. 5-azacytidine-treated human mesenchymal stem/progenitor cells derived from umbilical cord, cord blood and bone marrow do not generate cardiomyocytes in vitro at high frequencies. Vox Sang. 2008;95(2):137–148. doi: 10.1111/j.1423-0410.2008.01076.x. [DOI] [PubMed] [Google Scholar]
- 102.Hou T, Xu J, Wu X, et al. Umbilical cord Wharton’s Jelly. a new potential cell source of mesenchymal stromal cells for bone tissue engineering. Tissue Eng Part A. 2009;15(9):2325–2334. doi: 10.1089/ten.tea.2008.0402. [DOI] [PubMed] [Google Scholar]
- 103.Xu HH, Zhao L, Detamore MS, Takagi S, Chow LC. Umbilical cord stem cell seeding on fast-resorbable calcium phosphate bone cement. Tissue Eng Part A. 2010;16(9):2743–2753. doi: 10.1089/ten.tea.2009.0757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Honsawek S, Dhitiseith D, Phupong V. Effects of demineralized bone matrix on proliferation and osteogenic differentiation of mesenchymal stem cells from human umbilical cord. J Med Assoc Thai. 2006;89(Suppl 3):S189–S195. [PubMed] [Google Scholar]
- 105.Zhang ZY, Teoh SH, Chong MS, et al. Superior osteogenic capacity for bone tissue engineering of fetal compared to perinatal and adult mesenchymal stem cells. Stem Cells. 2009;27(1):126–131. doi: 10.1634/stemcells.2008-0456. [DOI] [PubMed] [Google Scholar]
- 106.Wang L, Singh M, Bonewald LF, Detamore MS. Signalling strategies for osteogenic differentiation of human umbilical cord mesenchymal stromal cells for 3D bone tissue engineering. J Tissue Eng Regen Med. 2009;3(5):398–404. doi: 10.1002/term.176. [DOI] [PubMed] [Google Scholar]
- 107.Gauthaman K, Venugopal JR, Yee FC, et al. Osteogenic differentiation of human Wharton’s jelly stem cells on nanofibrous substrates in vitro. Tissue Eng Part A. 2010 doi: 10.1089/ten.TEA.2010.0224. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 108.Penolazzi L, Tavanti E, Vecchiatini R, et al. Encapsulation of mesenchymal stem cells from Wharton’s jelly in alginate microbeads. Tissue Eng Part C Methods. 2010;16(1):141–155. doi: 10.1089/ten.TEC.2008.0582. [DOI] [PubMed] [Google Scholar]
- 109.Zhao L, Weir MD, Xu HH. An injectable calcium phosphate–alginate hydrogel–umbilical cord mesenchymal stem cell paste for bone tissue engineering. Biomaterials. 2010;31(25):6502–6510. doi: 10.1016/j.biomaterials.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang L, Dormer NH, Bonewald LF, Detamore MS. Osteogenic differentiation of human umbilical cord mesenchymal stromal cells in polyglycolic acid scaffolds. Tissue Eng Part A. 2010;16(6):1937–1948. doi: 10.1089/ten.TEA.2009.0706. [DOI] [PubMed] [Google Scholar]
- 111.Wang L, Detamore MS. Human umbilical cord mesenchymal stromal cells in a sandwich approach for osteochondral tissue engineering. J Tissue Eng Regen Med. 2010 doi: 10.1002/term.370. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dormer NH, Singh M, Wang L, Berkland CJ, Detamore MS. Osteochondral interface tissue engineering using macroscopic gradients of bioactive signals. Ann Biomed Eng. 2010;38(6):2167–2182. doi: 10.1007/s10439-010-0028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Conconi MT, Burra P, Di Liddo R, et al. CD105+ cells from Wharton’s jelly show in vitro and in vivo myogenic differentiative potential. Int J Mol Med. 2006;18(6):1089–1096. [PubMed] [Google Scholar]
- 114.Kocaefe C, Balci D, Hayta BB, Can A. Reprogramming of human umbilical cord stromal mesenchymal stem cells for myogenic differentiation and muscle repair. Stem Cell Rev. 2010;6(4):512–522. doi: 10.1007/s12015-010-9177-7. [DOI] [PubMed] [Google Scholar]
- 115.Vieira NM, Zucconi E, Bueno CR, Jr, et al. Human multipotent mesenchymal stromal cells from distinct sources show different in vivo potential to differentiate into muscle cells when injected in dystrophic mice. Stem Cell Rev. 2010;6(4):560–566. doi: 10.1007/s12015-010-9187-5. [DOI] [PubMed] [Google Scholar]
- 116.Patrick CW., Jr Tissue engineering strategies for adipose tissue repair. Anat Rec. 2001;263(4):361–366. doi: 10.1002/ar.1113. [DOI] [PubMed] [Google Scholar]
- 117.Gomillion CT, Burg KJ. Stem cells and adipose tissue engineering. Biomaterials. 2006;27(36):6052–6063. doi: 10.1016/j.biomaterials.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 118.Turner NJ, Jones HS, Davies JE, Canfield AE. Cyclic stretch-induced TGFβ1/Smad signaling inhibits adipogenesis in umbilical cord progenitor cells. Biochem Biophys Res Commun. 2008;377(4):1147–1151. doi: 10.1016/j.bbrc.2008.10.131. [DOI] [PubMed] [Google Scholar]
- 119.Karahuseyinoglu S, Kocaefe C, Balci D, Erdemli E, Can A. Functional structure of adipocytes differentiated from human umbilical cord stroma-derived stem cells. Stem Cells. 2008;26(3):682–691. doi: 10.1634/stemcells.2007-0738. [DOI] [PubMed] [Google Scholar]
- 120.Breymann C, Schmidt D, Hoerstrup SP. Umbilical cord cells as a source of cardiovascular tissue engineering. Stem Cell Rev. 2006;2(2):87–92. doi: 10.1007/s12015-006-0014-y. [DOI] [PubMed] [Google Scholar]
- 121.Schmidt D, Hoerstrup SP. Tissue engineered heart valves based on human cells. Swiss Med Wkly. 2007;137(Suppl 155):80S–85S. [PubMed] [Google Scholar]
- 122.Martin JA, Hamilton BE, Sutton PD, et al. Births: final data for 2005. Natl Vital Stat Rep. 2007;56(6):1–103. [PubMed] [Google Scholar]
- 123.Weiss ML, Anderson C, Medicetty S, et al. Immune properties of human umbilical cord Wharton’s jelly-derived cells. Stem Cells. 2008;26(11):2865–2874. doi: 10.1634/stemcells.2007-1028. [DOI] [PubMed] [Google Scholar]
- 124.Drukker M. Immunogenicity of human embryonic stem cells: can we achieve tolerance? Springer Semin Immunopathol. 2004;26(1–2):201–213. doi: 10.1007/s00281-004-0163-5. [DOI] [PubMed] [Google Scholar]
- 125.Drukker M, Benvenisty N. The immunogenicity of human embryonic stem-derived cells. Trends Biotechnol. 2004;22(3):136–141. doi: 10.1016/j.tibtech.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 126.Najar M, Raicevic G, Boufker HI, et al. Adipose-tissue-derived and Wharton’s jelly-derived mesenchymal stromal cells suppress lymphocyte responses by secreting leukemia inhibitory factor. Tissue Eng Part A. 2010;16(11):3537–3546. doi: 10.1089/ten.TEA.2010.0159. [DOI] [PubMed] [Google Scholar]
- 127.Najar M, Raicevic G, Boufker HI, et al. Mesenchymal stromal cells use PGE2 to modulate activation and proliferation of lymphocyte subsets: combined comparison of adipose tissue, Wharton’s Jelly and bone marrow sources. Cell Immunol. 2010;264(2):171–179. doi: 10.1016/j.cellimm.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 128.Selmani Z, Naji A, Zidi I, et al. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells. 2008;26(1):212–222. doi: 10.1634/stemcells.2007-0554. [DOI] [PubMed] [Google Scholar]
- 129.Patel SA, Sherman L, Munoz J, Rameshwar P. Immunological properties of mesenchymal stem cells and clinical implications. Arch Immunol Ther Exp (Warsz) 2008;56(1):1–8. doi: 10.1007/s00005-008-0001-x. [DOI] [PubMed] [Google Scholar]
- 130.Weiss ML, Mitchell KE, Hix JE, et al. Transplantation of porcine umbilical cord matrix cells into the rat brain. Exp Neurol. 2003;182(2):288–299. doi: 10.1016/s0014-4886(03)00128-6. [DOI] [PubMed] [Google Scholar]
- 131.Chen X, McClurg A, Zhou GQ, McCaigue M, Armstrong MA, Li G. Chondrogenic differentiation alters the immunosuppressive property of bone marrow-derived mesenchymal stem cells and the effect is partially due to the upregulated expression of B7 molecules. Stem Cells. 2007;25(2):364–370. doi: 10.1634/stemcells.2006-0268. [DOI] [PubMed] [Google Scholar]
- 132.Mannello F, Tonti GA. Concise review: no breakthroughs for human mesenchymal and embryonic stem cell culture: conditioned medium, feeder layer, or feeder-free; medium with fetal calf serum, human serum, or enriched plasma; serum-free, serum replacement nonconditioned medium, or ad hoc formula? All that glitters is not gold! Stem Cells. 2007;25(7):1603–1609. doi: 10.1634/stemcells.2007-0127. [DOI] [PubMed] [Google Scholar]
- 133.Galban CJ, Locke BR. Analysis of cell growth kinetics and substrate diffusion in a polymer scaffold. Biotechnol Bioeng. 1999;65(2):121–132. doi: 10.1002/(sici)1097-0290(19991020)65:2<121::aid-bit1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 134.Seshareddy K, Troyer D, Weiss ML. Method to isolate mesenchymal-like cells from Wharton’s Jelly of umbilical cord. Methods Cell Biol. 2008;86:101–119. doi: 10.1016/S0091-679X(08)00006-X. [DOI] [PubMed] [Google Scholar]
- 135.Kim JW, Kim SY, Park SY, et al. Mesenchymal progenitor cells in the human umbilical cord. Ann Hematol. 2004;83(12):733–738. doi: 10.1007/s00277-004-0918-z. [DOI] [PubMed] [Google Scholar]