Abstract
Objective
This study examines the hypothesis that chronic inflammation is associated with a higher risk of cardiac death compared to the risk of non-fatal myocardial infarction.
Methods
Cardiac death and non-fatal MI events were identified in the ARIC cohort during follow-up from 1987 through 2001. Markers of inflammation and hemostasis were determined at baseline using standardized procedures. Cox proportional hazard regression and polytomous logistic regression were used to estimate associations.
Results
We observed a positive gradient in incidence of sudden cardiac death, non-sudden cardiac death and non-fatal MI in association with decreasing levels of albumin and increasing levels of white blood cell count and of markers of hemostasis (fibrinogen, von Willebrand factor, factor VIIIc). Associations for von Willebrand factor (vWF) and factor VIIIc were stronger for fatal relative to non-fatal events (3rd versus 1st tertile hazard ratios: vWF: SCD 2.67 (95% CI 1.80, 3.96), NSCD 2.11 (95% CI 1.40, 3.19), non-fatal MI 1.40 (95% CI 1.17, 1.67); factor VIIIc: SCD 2.58 (95% CI 1.77, 3.78), NSCD 2.01 (95% CI 1.38, 2.93), non-fatal MI 1.48 (95% CI 1.24, 1.78). Gradients of association for fibrinogen and white blood cell count, examined over tertiles of distribution and per one SD increase, were similar for the three endpoints. All associations were independent of smoking status.
Conclusion
Von Willebrand factor and factor VIIIc are associated with an increased risk of cardiac death as compared to the risk of non-fatal MI.
Keywords: hemostasis, inflammation, von Willebrand factor, sudden cardiac death, non-fatal myocardial infarction
BACKGROUND
Inflammation and hemostasis play a central role in the development and progression of atherosclerotic lesions [1-3]. Chronic inflammation, associated with conditions such as smoking, diabetes, hypertension, and obesity, promotes expression of pro-inflammatory cytokines, attracting leukocytes and monocytes to the developing plaque [4]. Systemic markers of inflammation are associated positively with risk of cardiovascular events in both cross-sectional and prospective studies [5-11]. Likewise, increased levels of circulating markers of hemostasis are associated with increased risk of secondary cardiovascular events and to a lesser extent, increased risk of primary events [5, 8, 12].
Few prospective studies have explored differences in the association of markers of inflammation and hemostasis with different coronary heart disease outcomes. In this study, we used data from the Atherosclerosis Risk in Communities (ARIC) cohort to examine the association of the following selected markers of inflammation and hemostasis: white blood cell count, serum albumin, plasma fibrinogen, von Willebrand factor and Factor VIIIc, with the risk of non-fatal myocardial infarction, non-sudden cardiac death, and sudden cardiac death.
METHODS
Study population
The ARIC cohort is a biracial cohort, consisting of 15,792 men and women, 45-64 years of age at baseline (1987-1989), selected as probability samples from four US communities located in North Carolina, Mississippi, Minnesota, and Maryland [13]. Follow-up of the ARIC cohort members is conducted annually through telephone interviews. In addition, three triennial follow up visits were conducted after the baseline data collection.
The present study is based on data obtained at baseline (1987-1989) and during follow-up through December 31, 2001. We eliminated from analysis (exclusions not mutually exclusive) persons who did not report a black or white race on their baseline questionnaire (n=48), the small number of persons who were black and residing in the Minnesota or Maryland communities (n=55), persons missing outcome information (n=9), persons with coronary heart disease identified at baseline (n= 766) or those missing baseline CHD information (n=344), as well as persons missing baseline information on the following exposures and selected analysis covariates: fibrinogen (n=276), von Willebrand factor (n=273), factor VIIIc (n=281), white blood cell count (n=246), albumin (n=150), systolic blood pressure (n=14), anti-hypertension medication (n=8), cigarette smoking status (n=11), cigarette years of smoking (n=279), and HDL-cholesterol (n=249). The final analysis cohort consisted of 13,975 ARIC cohort members.
Baseline measurements
Physical examination of the cohort members and assessment of their risk factor was conducted during the baseline visit. Members of the ARIC cohort had blood drawn from an antecubital vein into tubes containing EDTA (lipids), sodium citrate (hemostatic assays), or a separating gel (glucose and albumin). Plasma HDL-cholesterol levels were measured using the method of Warnick et al [14]. Plasma total cholesterol levels were determined enzymatically [15] using a Cobas-Bio analyzer with reagents purchased from Boehringer Mannheim Biochemicals, Indianapolis, IN. White blood cell count was determined in the whole blood sample using a Coulter Counter. Plasma levels of fibrinogen, von Willebrand factor and factor VIIIc were determined in the ARIC Central Hemostasis Laboratory using established procedures [16]. Plasma fibrinogen levels were determined using the thrombin time titration method, with reagents obtained from General Diagnostics Organon Technica Co., Morris Plains, NJ. Von Willebrand factor antigen was determined using ELISA kits from American Bioproducts Co. Factor VIII activity was determined on the basis of a coagulation titration curve established using factor VIII–deficient plasma (George King Biochemical Inc., Overland Park, KS). Reliability coefficients for measurement of the hemostatic factors in a sample of ARIC study participants conducted over several weeks were 0.68 for von Willebrand factor, 0.86 for Factor VIIIc, and 0.72 for fibrinogen [10, 16].
Body mass index was calculated as the ratio of weight in kilograms to height in meters squared. Sitting blood pressure was measured three times using a random zero sphygmomanometer. Blood pressure calculations were made as an average of the second and third measurement. Hypertension was defined as present, based on use of antihypertensive medication within two weeks of baseline data collection, or systolic blood pressure greater than or equal to 140 mm Hg or diastolic blood pressure greater than or equal to 90 mm Hg. Baseline diabetes was defined as either a self-reported physician’s diagnosis of diabetes, use of hypoglycemic medications, non-fasting serum glucose levels greater than or equal to 11.1 mmol/L, or fasting (≥ 8 hr) serum glucose level greater than or equal to 7.0 mmol/L.
Highest attained level of education was defined on the basis of a personal report at baseline of the highest grade completed in school and categorized as low (11th grade or less) or high (12th grade and above).
Outcome ascertainment
Incident sudden cardiac death cases
All deaths attributed to coronary heart disease in the main ARIC adjudication were reviewed to ascertain whether each event was due to sudden cardiac death. These cases underwent a separate physician panel review using a uniform abstraction form to determine whether the death was characterized as a sudden, pulseless condition without a known non-cardiac cause. Adjudication was performed independently by two physicians on the basis of data obtained from death certificates, informant interviews, physician questionnaires, coroner reports, or hospital discharge summaries. This review was performed for both in- and out-of-hospital deaths. Inter-reviewer reliability was evaluated in a subset of cases. The kappa coefficient describing the classification of sudden cardiac death between the two physician reviewers was 0.79. Initial discordant classification was resolved by a third independent review.
Incident non-sudden cardiac death
Incident non-sudden cardiac death cases, identified during follow-up, were defined as those cases of death meeting ARIC study criteria for definite fatal myocardial infarction or definite or possible fatal coronary heart disease [13], which did not meet sudden cardiac death criteria.
Incident non-fatal myocardial infarction
Incident non-fatal myocardial infarction, defined by the ARIC study criteria [13] , was identified during follow-up through annual questionnaires, review of medical discharge records (presence of chest pain, cardiac biomarkers, electrocardiograms), or at follow-up examinations [13, 17].
Statistical analysis
Age-adjusted incidence rates were calculated per tertiles of distribution of von Willebrand factor, Factor VIIIc, fibrinogen, albumin, and white blood cell count. Regression analysis was performed using Cox proportional hazard models of the association of marker tertiles with the hazard of sudden cardiac death, non-sudden cardiac death, or non-fatal MI. The following baseline variables: age, race/ARIC center, body mass index, gender, diabetes, cigarette years of smoking, cigarette smoking status, systolic blood pressure, use of anti-hypertensive medication, total cholesterol and HDL-cholesterol were evaluated for inclusion into regression models on the basis of existing literature and retained according to a 10% change-in-estimate criterion [18]. The race/ARIC center variable was created as a categorical variable encompassing five categories of race and ARIC center. Potential interaction terms were evaluated using partial likelihood ratio tests based on nested models. All continuous variables were evaluated for linearity of association with the main outcome. The Cox proportional hazard assumption was examined for all variables individually and for the final model using the Cox test and on the basis of ln-ln plots and plots of scaled Schoenfeld residuals. Nominal polytomous logistic regression analysis was used to address the question of competing risk of cardiac death in the analysis of the risk of non-fatal MI. All analyses were performed using STATA 10.0 (STATA Corp., College Station, TX).
RESULTS
Cohort participants, whose levels of von Willebrand factor, factor VIIIc, fibrinogen and white blood cell count were in the highest tertiles of distribution, and whose albumin levels were in the lowest tertile of the distribution, shared the following characteristics. They were on average older, had higher BMI values, and had higher baseline glucose levels than those cohort participants whose levels of the markers of inflammation and hemostasis were in the lowest (highest for albumin) tertile of distribution (Table 1). Prevalence of ever smoking and of diabetes and hypertension was higher among those with highest, as compared to the lowest, levels of von Willebrand factor, factor VIIIc, fibrinogen and white blood cell count, and lowest levels of albumin. Those differences were statistically significant at p=0.05.
Table 1.
Baseline characteristics according to tertiles of markers of inflammation and hemostasis, the ARIC cohort study
| Marker Tertile range |
Age (years) |
Gender (% male) |
Race (% Black) |
Smoking (% ever) |
BMI (kg/m2) |
Glucose (mmol/L) |
Diabetes (%) |
Systolic blood pressure (mm Hg) |
HT medication (%) |
|---|---|---|---|---|---|---|---|---|---|
| von Willebrand factor (%) | |||||||||
| <92 | 52.8 (5.5) | 43.9 | 18.9 | 56.6 | 26.8 (4.6) | 5.60(1.30) | 6.2 | 119.1 (17.8) | 23.3 |
| 92-129 | 54.2 (5.7) | 44.2 | 22.6 | 57.0 | 27.4 (5.2) | 5.83 (1.79) | 6.7 | 120.7 (18.4) | 26.6 |
| >129 | 55.2 (5.8) | 42.3 | 35.4 | 57.0 | 28.6 (6.0) | 6.44 (3.05) | 17.6 | 123.3 (19.5) | 35.0 |
| Factor VIIIc (%) | |||||||||
| <112 | 53.0 (5.5) | 48.1 | 17.2 | 60.6 | 26.7 (4.6) | 5.51 (1.04) | 5.0 | 118.4 (17.3) | 21.6 |
| 112-141 | 54.1 (5.8) | 44.3 | 21.5 | 56.3 | 27.5 (5.1) | 5.76 (1.65) | 8.8 | 120.6 (18.6) | 27.0 |
| >141 | 55.0 (5.8) | 37.8 | 38.2 | 53.4 | 28.9 (6.0) | 6.60 (3.19) | 19.9 | 124.2 (19.6) | 36.4 |
| Fibrinogen (μmol/L) | |||||||||
| <7.97 | 53.1 (5.6) | 49.1 | 19.3 | 52.6 | 26.4 (4.5) | 5.70 (1.62) | 7.1 | 118.9 (17.4) | 21.2 |
| 7.97-9.41 | 54.3 (5.8) | 43.8 | 23.6 | 56.7 | 27.5 (4.8) | 5.90 (2.05) | 10.2 | 120.5 (18.4) | 26.7 |
| >9.41 | 54.8 (5.7) | 37.4 | 33.8 | 61.3 | 29.1 (6.2) | 6.27 (2.75) | 16.2 | 123.7 (19.8) | 37.0 |
| WBC count (x10−6) | |||||||||
| ≤5.1 | 53.9 (5.7) | 39.7 | 35.2 | 43.8 | 27.1 (5.1) | 5.73 (1.80) | 7.8 | 120.2 (18.6) | 26.1 |
| 5.1-6.1 | 54.2 (5.7) | 44.2 | 22.1 | 53.3 | 27.7 (5.7) | 5.87 (1.93) | 9.9 | 121.1 (18.2) | 27.0 |
| ≥6.1 | 54.1 (5.8) | 46.2 | 19.5 | 69.6 | 28.1 (5.6) | 6.19 (2.58) | 14.5 | 121.6 (18.9) | 30.7 |
| Albumin (g/L) | |||||||||
| <38 | 54.4 (5.8) | 35.8 | 30.9 | 56.3 | 28.5 (6.1) | 6.08 (2.59) | 12.9 | 120.9 (19.3) | 30.5 |
| 38-40 | 54.0 (5.7) | 46.3 | 21.4 | 57.3 | 27.1 (4.7) | 5.89 (1.98) | 10.3 | 120.5 (18.1) | 26.4 |
| >40 | 53.5 (5.7) | 54.5 | 20.4 | 57.3 | 26.6 (4.3) | 5.79 (1.55) | 8.7 | 121.9 (18.0) | 26.2 |
The age-adjusted rate of incidence of non-fatal MI (NFMI) was consistently higher than that of sudden (SCD) or non-sudden (NSCD) cardiac death overall and across all exposures (overall incidence rates (IR) per 10,000 person-years : IRSCD = 11.7 (95% CI 10.2, 13.4), IRNSCD = 9.8 (95% CI 8.5, 11.4), and IRNFMI= 42.5 (95% CI 39.5, 45.7)). The age-adjusted incidence rates for sudden and non-sudden cardiac death were similar in magnitude across all exposure categories (Table 2). Incidence of sudden cardiac death, non-sudden cardiac death and non-fatal MI increased across increasing tertiles of von Willebrand factor, factor VIIIc, fibrinogen and white blood cell count and across decreasing tertiles of serum albumin, with a greater difference in incidence rates between the second and third tertile, as compared to the first and second tertile for all exposures except albumin.
Table 2.
Age-adjusted incidence rates of sudden cardiac death, non-sudden cardiac death, and non-fatal myocardial infarction in tertiles of markers of inflammation and hemostasis, the ARIC cohort study
| Marker/Range | Sudden cardiac death (n=207) | Non-sudden cardiac death (n=174) | Non-fatal MI (n=737) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Events | p-years | IR per 10,000 (95% CI) |
Events | p-years | IR per 10,000 (95% CI) |
Events | p-years | IR per 10,000 (95% CI) |
|
| Von Willebrand factor (%) | |||||||||
| <92 | 33 | 61,580 | 6.1 (3.9, 8.2) | 32 | 61,580 | 6.1 (4.0, 8.3) | 210 | 60,390 | 36.7 (31.8, 41.7) |
| 92-129 | 55 | 59,014 | 9.4 (6.9, 11.9) | 51 | 59,014 | 8.6 (6.3, 11.0) | 225 | 57,882 | 38.0 (33.2, 42.9) |
| >129 | 119 | 56,667 | 19.8 (16.2, 23.4) | 91 | 56,667 | 14.9 (11.8, 18.0) | 302 | 55,215 | 51.1 (45.5, 56.8) |
| Factor VIIIc (%) | |||||||||
| <112 | 38 | 62,334 | 7.0 (4.7, 9.2) | 44 | 62,334 | 8.2 (5.8, 10.7) | 227 | 61,018 | 38.7 (33.7, 43.7) |
| 112-141 | 52 | 58,986 | 8.9 (6.5, 11.3) | 34 | 58,986 | 5.7 (3.8, 7.6) | 220 | 57,922 | 37.2 (32.4, 42.0) |
| >141 | 117 | 55,941 | 19.9 (16.2, 23.5) | 96 | 55,941 | 16.2 (12.9, 19.4) | 290 | 54,547 | 49.6 (44.0, 55.2) |
| Fibrinogen (μmol/L) | |||||||||
| <7.97 | 38 | 62,181 | 6.7 (4.5, 8.8) | 34 | 62,181 | 6.2 (4.1, 8.4) | 149 | 61,354 | 25.1 (21.0, 29.1) |
| 7.97-9.41 | 45 | 57,909 | 7.7 (5.4, 9.9) | 47 | 57,909 | 8.2 (5.8, 10.5) | 250 | 56,743 | 43.0 (37.9, 48.2) |
| >9.41 | 124 | 57,171 | 21.0 (17.3, 24.8) | 93 | 57,171 | 15.8 (12.6, 19.0) | 338 | 55,390 | 57.9 (51.9, 63.9) |
| WBC (x10−6) | |||||||||
| ≤5.1 | 54 | 61,309 | 9.1 (6.7, 11.5) | 27 | 61,309 | 4.5 (2.8, 6.2) | 169 | 60,472 | 27.8 (23.7, 31.9) |
| 5.1-6.1 | 32 | 43,872 | 7.3 (4.8, 9.9) | 31 | 43,872 | 6.9 (4.5, 9.4) | 158 | 43,075 | 35.8 (30.4, 41.3) |
| ≥6.1 | 121 | 72,081 | 16.9 (13.9, 20.0) | 116 | 72,081 | 16.2 (13.3, 19.2) | 410 | 69,941 | 57.5 (52.1, 62.8) |
| Albumin (g/L) | |||||||||
| <38 | 120 | 78,903 | 14.9 (12.2, 17.5) | 107 | 78,903 | 13.4 (10.9, 16.0) | 351 | 77,051 | 44.2 (39.7, 48.7) |
| 38-40 | 55 | 52,963 | 10.7 (7.8, 13.5) | 42 | 52,963 | 8.0 (5.6, 10.5) | 216 | 51,967 | 41.2 (35.8, 46.5) |
| >40 | 32 | 45,395 | 7.1 (4.6, 9.6) | 25 | 45,395 | 6.0 (3.6, 8.4) | 170 | 44,469 | 38.7 (32.9, 44.4) |
In analyses stratified by gender, men had significantly higher crude rates of incidence of sudden cardiac death and non-fatal MI than women, overall and for all exposure categories. There were no significant gender-differences in rates of non-sudden cardiac death, either overall or across exposure categories. For all exposures examined, the rates of sudden cardiac death and non-sudden cardiac death were higher among blacks, as compared to whites. There were no significant race differences in the rates of non-fatal MI overall and across exposure categories (race and gender stratified data not shown).
We examined the association of the selected markers of hemostasis and inflammation with the risk of non-fatal MI, sudden cardiac death, and non-sudden cardiac death in tertiles of marker distribution using Cox proportional hazard regression models adjusted for the following covariates: age, gender, race ARIC center, cigarette years of smoking, and cigarette smoking status. The hazard ratios for sudden cardiac death, non-sudden cardiac death, and nonfatal MI increased across exposure levels for all exposure categories (Table 3). All hemostatic (von Willebrand factor, Factor VIIIc, fibrinogen) and inflammatory (white blood cell count, albumin) markers examined were associated strongly with the risk of sudden cardiac death and of non-sudden cardiac death. With respect to non-fatal MI, we observed modest associations for von Willebrand factor, factor VIIIc, and white blood cell count, a strong association for fibrinogen, and no association for albumin. Adjustment for the following covariates associated with CHD outcomes: diabetes, total cholesterol, HDL-cholesterol, systolic blood pressure, and use of anti-hypertensive medication, in addition to the demographic variables and smoking, did not alter estimates for the risk of sudden cardiac death, but decreased the estimates for the associations of the highest, as compared to the lowest tertiles, of factor VIIIc and fibrinogen with the risk of non-sudden cardiac death to statistically non-significant levels (HRfactor VIII=1.43 (95% CI 0.97, 2.11, HRfibrinogen= 1.44 (95% CI 0.96, 2.17)). The estimate for the association of factor VIIIc with the risk of non-fatal MI was likewise decreased with full adjustment to a statistically non-significant level (HR= 1.18 (95% CI 0.98, 1.42)). The hazard ratio of the association of the highest, as compared to the lowest, tertile of factor VIIIc with the risk of sudden cardiac death remained statistically significant after adjustment (HR=2.09 (95% CI 1.42, 3.08)).
Table 3.
Tertiles of markers of inflammation and hemostasis and the relative risk of sudden cardiac death, non-sudden cardiac death, and non-fatal MI, the ARIC cohort study
Model 1: adjustment for age
Model 2: adjustment for age, gender, race, ARIC center, baseline smoking status, and cigarette years of smoking
| Marker/Range | Sudden cardiac death (n=207) | Non-sudden cardiac death (n=174) | Non-fatal MI (n=737) | |||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | |
| Von Willebrand factor (%) | ||||||
| <92 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 92-129 | 1.58 (1.03, 2.44) | 1.47 (0.95, 2.06) | 1.47 (0.95, 2.30) | 1.40 (0.90, 2.18) | 1.05 (0.87, 1.27) | 1.04 (0.86, 1.26) |
| >129 | 3.34 (2.26, 4.93) | 2.67 (1.80, 3.96) | 2.55 (1.70, 3.84) | 2.11 (1.40, 3.19) | 1.43 (1.20, 1.71) | 1.40 (1.17, 1.67) |
| Factor VIIIc (%) | ||||||
| <112 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 112-141 | 1.33 (0.88, 2.03) | 1.31(0.86, 2.00) | 0.75 (0.48, 1.17) | 0.74 (0.47, 1.17) | 0.97 (0.81, 1.17) | 1.02 (0.85, 1.23) |
| >141 | 2.97 (2.05, 4.29) | 2.58 (1.77, 3.78) | 2.18 (1.52, 3.13) | 2.01 (1.38, 2.93) | 1.32 (1.11, 1.58) | 1.48 (1.24, 1.78) |
| Fibrinogen (μmol/L) | ||||||
| <7.97 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 7.97-9.41 | 1.17 (0.76, 1.80) | 1.09 (0.70, 1.68) | 1.38 (0.88, 2.14) | 1.24 (0.80, 1.93) | 1.74 (1.42, 2.13) | 1.69 (1.38, 2.07) |
| >9.41 | 3.17 (2.20, 4.57) | 2.56 (1.76, 3.73) | 2.68 (1.80, 3.97) | 2.00 (1.33, 2.99) | 2.38 (1.97, 2.89) | 2.27 (1.86, 2.77) |
| WBC (x10−6) | ||||||
| ≤5.1 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 5.1-6.1 | 0.81 (0.52, 1.25) | 0.96 (0.62, 1.49) | 1.55 (0.92, 2.59) | 1.75 (1.04, 2.94) | 1.30 (1.04, 1.61) | 1.25 (1.00, 1.55) |
| ≥6.1 | 1.89 (1.37, 2.61) | 2.05 (1.45, 2.89) | 3.70 (2.43, 5.62) | 3.55 (2.29, 5.51) | 2.10 (1.76, 2.52) | 1.71 (1.41, 2.07) |
| Albumin (g/L) | ||||||
| <38 | 2.01 (1.36, 2.97) | 1.90 (1.28, 2.83) | 2.76 (1.78, 4.29) | 2.52 (1.62, 3.93) | 1.16 (0.97, 1.70) | 1.24 (1.03, 1.49) |
| 38-40 | 1.41 (0.91, 2.19) | 1.44 (0.93, 2.24) | 1.52 (0.92, 2.49) | 1.52 (0.93, 2.50) | 1.07 (0.87, 1.31) | 1.12 (0.91, 1.37) |
| >40 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
Likelihood ratio tests of nested models with and without gender and race interaction terms, as well as analyses of joint and independent effects, suggested that neither race nor gender modified the association of albumin, white blood cell count, fibrinogen, von Willebrand factor, and factor VIIIc with the risk of sudden cardiac death, or non-sudden cardiac death, or non-fatal MI.
Sensitivity analyses
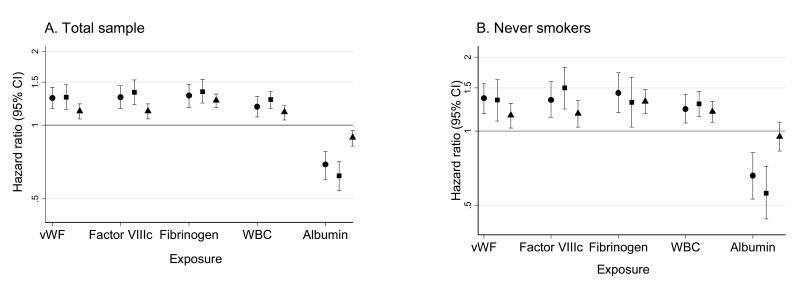
Since cigarette smoking contributes significantly to the level of chronic systemic inflammation, we examined the associations of the selected markers of inflammation and hemostasis with the risk of cardiac death and non-fatal MI among never smokers and among ever-smokers. We did not observe a significant difference in the point estimates among never-smokers as compared to the total sample population, suggesting a negligible effect modification by smoking of the association of the selected markers of inflammation and hemostasis with sudden or non-sudden cardiac death, or non-fatal MI (Figure 1). Likelihood ratio tests of the interaction of smoking status with all exposures were non-significant for all outcomes. Model 2 shown in Table 3, represents association of tertiles of the selected markers with the risk of CHD outcomes adjusted for the demographic covariates and for smoking. Incorporation of baseline diabetes, a condition associated with chronic inflammatory states and advanced atherosclerosis, into this model only slightly attenuated the estimates of the hazard ratios for sudden cardiac death, non-sudden cardiac death and non-fatal MI in association with all selected exposures, except for albumin. Diabetes attenuated the estimate of the association of the lowest tertile of albumin with the risk of non-fatal MI to a statistically non-significant level (hazard ratio 1.20 (95% CI 0.99, 1.44))
Figure 1.
Association of markers of inflammation and hemostasis with the risk of sudden cardiac death, non-sudden cardiac death, and non-fatal MI in the total sample population (A.) and among never smokers (B.). Hazard ratio per one SD difference in exposure. Analysis adjusted for age, gender, race, ARIC center, cigarette years of smoking (total sample only), and cigarette smoking status (total sample only). vwF: von Willebrand factor; WBC: white blood cell count ● sudden cardiac death; ■ non-sudden cardiac death; ▲ non-fatal MI
Inclusion of education, or of median household income, as covariates in the Cox regression models, did not alter the estimates of risk associated with any of the exposures.
Baseline hemostatic and inflammatory factors were also associated with non-cardiac mortality, although the relative strength of the association was lower than for cardiac death. Hazard ratios of non-cardiac death, adjusted for age, gender, race, ARIC center, and smoking, for the highest versus the lowest tertiles of von Willebrand factor and factor VIIIc with the risk of non-cardiac death were 1.55 (95% CI 1.34, 1.78) and 1.63 (95% CI 1.42, 1.88) respectively.
We performed polytomous logistic regression to confirm, or refute, observations obtained with single endpoint models (Table 4). Regression was performed with coronary heart disease events categorized as follows: non-fatal MI not followed by cardiac death, sudden cardiac death not preceded by a non-fatal MI, non-sudden cardiac death not preceded by a non-fatal MI, sudden cardiac death following a non-fatal MI, or non-sudden cardiac death following a non-fatal MI. There were 21 cases of sudden cardiac death and 46 cases of non-sudden cardiac death which were preceded by a non-fatal MI. Those numbers are too small for a meaningful analysis of effects stratified by exposure categories. We therefore, focused analysis on the three independent outcomes: sudden and non-sudden cardiac deaths and non-fatal MI. Results of this multinomial analysis, presented in Table 4, confirmed results from individual analyses suggesting a stronger association of von Willebrand factor and factor VIII with the risk of sudden and non-sudden cardiac death as compared to the risk of non-fatal MI. A formal test of the null hypothesis that the strength of the association is the same for the three outcomes, indicated a significant difference in the association of von Willebrand factor with sudden (p<0.01) and non-sudden (p<0.01) cardiac death as compared to non-fatal MI and a significant difference in the association of factor VIIIc with sudden (p<0.01) and non-sudden (p<0.01) cardiac death as compared to non-fatal MI.
Table 4.
Tertiles of markers of inflammation and hemostasis and the relative risk of sudden cardiac death, non-sudden cardiac death, and non-fatal MI. Odds ratio estimates determined by polytomous logistic regression with no events as the base category. Data presented for independent outcomes only. Analysis adjusted for age, gender, race, ARIC center, baseline smoking status, and cigarette years of smoking.
| Sudden cardiac death (n= 186) |
Non-sudden cardiac death (n= 128) |
Non-fatal MI (n= 670) |
|
|---|---|---|---|
| Von Willebrand factor (%) | |||
| <92 | 1.00 | 1.00 | 1.00 |
| 92-129 | 1.30 (0.82, 2.06) | 1.56 (0.91, 2.67) | 1.01 (0.82, 1.21) |
| >129 | 2.58 (1.71, 3.90) | 2.55 (1.55, 4.21) | 1.37 (1.13, 1.66) |
| Factor VIIIc (%) | |||
| <112 | 1.00 | 1.00 | 1.00 |
| 112-141 | 1.35 (0.86, 2.12) | 0.56 (0.32, 0.97) | 0.98 (0.81, 1.20) |
| >141 | 2.47 (1.64, 3.71) | 1.83 (1.19, 2.80) | 1.31 (1.08, 1.60) |
| Fibrinogen (μmol/L) | |||
| <7.97 | 1.00 | 1.00 | 1.00 |
| 7.97-9.41 | 0.96 (0.61, 1.52) | 0.96 (0.59, 1.57) | 1.57 (1.27, 1.95) |
| >9.41 | 2.25 (1.52, 3.32) | 1.46 (0.93, 2.28) | 1.91 (1.55, 2.36) |
| WBC (x10−6) | |||
| ≤5.1 | 1.00 | 1.00 | 1.00 |
| 5.1-6.1 | 0.90 (0.56, 1.42) | 1.72 (0.97, 3.03) | 1.21 (0.96, 1.53) |
| ≥6.1 | 1.84 (1.28, 2.63) | 2.84 (1.73, 4.65) | 1.50 (1.22, 1.85) |
| Albumin (g/L) | |||
| <38 | 1.70 (1.12, 2.58) | 1.89 (1.14, 3.15) | 1.01 (0.83, 1.23) |
| 38-40 | 1.31 (0.82, 2.09) | 1.32 (0.74, 2.35) | 1.01 (0.82, 1.26) |
| >40 | 1.00 | 1.00 | 1.00 |
DISCUSSION
In this study, we observed a positive association of high white blood cell count, fibrinogen, von Willebrand factor, and of factor VIIIc and a negative association of high albumin with increased incidence of fatal and non-fatal coronary heart disease events during an average 12.4 years of follow up. Our results corroborate observations from published studies [5, 19-24] extending previously reported ARIC study results [5, 22] to a longer follow up time and providing a comparative analysis of the association of those markers with different CHD outcomes. Presented data suggest a stronger association of factor VIII and von Willebrand factor with risk of cardiac death (sudden and non-sudden), as compared to the risk of non-fatal MI.
Coagulation factor VIII, secreted by the liver, and to a lesser extent by the spleen and cells of the lymphatic system, exists in the circulation in the form of a stable complex with von Willebrand factor [25, 26], a multimeric glycoprotein produced primarily by the endothelium in response to injury and inflammatory stimuli [24]. This results in von Willebrand factor and factor VIIIc being highly correlated and showing almost identical associations with coronary heart disease. Binding of von Willebrand factor to the subendothelial matrix and to activated platelets, facilitates platelet accumulation at the site of injury and leads to eventual formation of a thrombus plug [27, 28]. Elevated plasma levels of von Willebrand factor reflect presence of endothelial injury and dysfunction in a manner more specific than that of the other inflammatory and hemostatic markers [29]. In this study, we found that plasma levels of factor VIIIc and von Willebrand factor, although positively associated with all CHD outcomes, were more strongly associated with cardiac death (sudden and non-sudden) as compared to non-fatal MI. Those results would suggest, indirectly, a specific role of endothelial dysfunction in fatal CHD events [30, 31].
Our finding that smoking does not modify the association of von Willebrand factor, factor VIII, fibrinogen, and white blood cell count with coronary heart diseases events, confirms and extends the data of Lee et al. [19] in which the authors observed that the associations of white blood cell count with cardiovascular mortality and with the incidence of coronary heart disease were independent of smoking status.
We observed an inverse association of albumin with cardiac death, but we did not observe an independent association of albumin with incidence of non-fatal MI. These data are in agreement with the report by Kuller et al. [20], suggesting a stronger association of albumin with fatal, as compared to non-fatal CHD events.
Other studies of the association of inflammatory and hemostatic markers with fatal and non-fatal events have not been conclusive. Investigators of the Northwick Park Heart Study, in long-term follow up of persons without prior myocardial infarction [32, 33], observed an association of factor VII activity with fatal ischemic heart disease events, but not with non-fatal events. Those results have not been reproduced by other studies [6, 34, 35].
There were several limitations to this study. The study lacked extensive information (e.g. C-reactive protein) concerning baseline inflammatory status. We examined only baseline exposure status; it was beyond the scope of this study to examine the effect of change in levels of inflammatory and hemostatic markers over the period of follow-up.
The strength of this study lies in its prospective design. We have observed an association of markers of inflammation and hemostasis obtained from blood samples collected at baseline with coronary heart disease events occurring during an extensive (12.4 year) follow up period. The fact that these associations were present for different markers argues against them resulting from chance and is consistent with the presence of a sustained chronic inflammatory burden, which then impacts cardiovascular events. The definition of sudden cardiac death used in this study was constructed to best capture those cases of cardiac death that were truly sudden and unexpected and combined all available cardiac death information.
In conclusion, our observations add to the existing hypotheses concerning different roles that inflammatory and hemostatic processes may play in the development of fatal and non-fatal coronary heart disease outcomes [34, 36]. Furthermore, our results suggest that elevated plasma levels of factor VIIIc and von Willebrand factor may aid in identifying those at increased risk of sudden cardiac death. Cardiac death, particularly sudden cardiac death [37], is often the first manifestation of underlying pathology, yet traditional risk factors do not specifically identify those at increased risk [38-40]. Observations presented here may help clarify the role of inflammation and impairment of hemostasis in the pathology of sudden cardiac death and aid in identifying persons at increased risk and perhaps serve as guidelines for thrombolytic therapy.
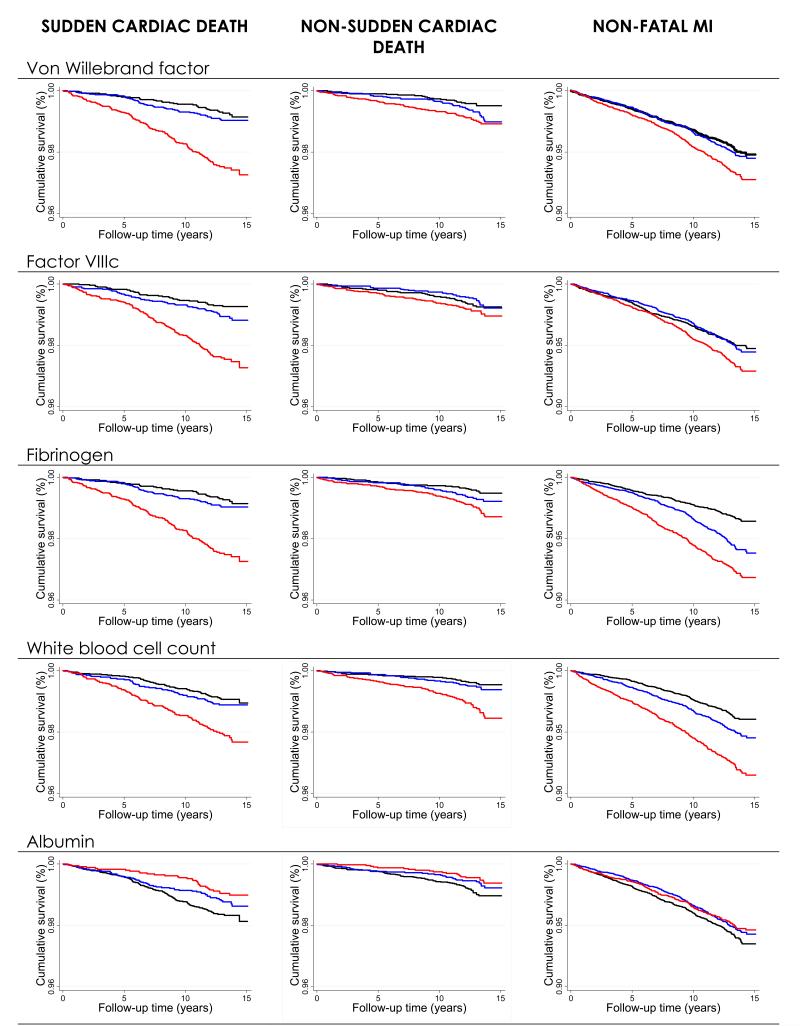
Figure 2.
Kaplan-Meier curves of cumulative survival according to tertiles of von Willebrand factor, Factor VIIIc, fibrinogen, white blood cell count, and albumin. Data adjusted for age and presented for the following outcomes: non-data MI, non-sudden cardiac death, and sudden cardiac death. The ARIC study cohort 1987-2001. 1st tertile ;
1st tertile ;  2nd tertile;
2nd tertile;  3rd tertile
3rd tertile
Acknowledgement
The authors thank the staff and participants of the Atherosclerosis Risk in Communities Study study for their important contributions.
Sources of funding The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts N01-HC-55015, N01-HC-55016, N01-HC-55018, N01-HC-55019, N01-HC-55020, N01-HC-55021, and N01-HC-55022. This work was also supported by a National Research Service Award training grant T32 H2-0007055 (AMK-N).
Footnotes
Disclosures none
References
- 1.Croce K, Libby P. Intertwining of thrombosis and inflammation in atherosclerosis. Curr Opin Hematol. 2007;14(1):55–61. doi: 10.1097/00062752-200701000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Libby P, Ridker PM, Maseri A. Inflammation and Atherosclerosis. Circulation. 2002;105(9):1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 3.Bassuk SS, Rifai N, Ridker PM. High-sensitivity C-reactive protein: Clinical importance. Current Problems in Cardiology. 2004;29(8):439–493. [PubMed] [Google Scholar]
- 4.Packard RR, Libby P. Inflammation in atherosclerosis: from vascular biology to biomarker discovery and risk prediction. Clin Chem. 2008;54(1):24–38. doi: 10.1373/clinchem.2007.097360. [DOI] [PubMed] [Google Scholar]
- 5.Folsom AR, et al. Prospective study of hemostatic factors and incidence of coronary heart disease: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 1997;96(4):1102–8. doi: 10.1161/01.cir.96.4.1102. [DOI] [PubMed] [Google Scholar]
- 6.Salomaa V, et al. Hemostatic factors as predictors of coronary events and total mortality: The FINRISK ’92 Hemostasis Study. Arterioscler Thromb Vasc Biol. 2002;22(2):353–8. doi: 10.1161/hq0202.104078. [DOI] [PubMed] [Google Scholar]
- 7.Rajecki M, et al. Hemostatic factors as predictors of stroke and cardiovascular diseases: the FINRISK ’92 Hemostasis Study. Blood Coagul Fibrinolysis. 2005;16(2):119–24. doi: 10.1097/01.mbc.0000161565.74387.5b. [DOI] [PubMed] [Google Scholar]
- 8.Tzoulaki I, et al. Relative Value of Inflammatory, Hemostatic, and Rheological Factors for Incident Myocardial Infarction and Stroke: The Edinburgh Artery Study. Circulation. 2007;115(16):2119–2127. doi: 10.1161/CIRCULATIONAHA.106.635029. [DOI] [PubMed] [Google Scholar]
- 9.Woodward M, et al. A comparison of the associations between seven hemostatic or inflammatory variables and coronary heart disease. Journal of Thrombosis and Haemostasis. 2007;5(9):1795–1800. doi: 10.1111/j.1538-7836.2007.02677.x. [DOI] [PubMed] [Google Scholar]
- 10.Folsom AR, et al. Association of hemostatic variables with prevalent cardiovascular disease and asymptomatic carotid artery atherosclerosis. The Atherosclerosis Risk in Communities (ARIC) Study Investigators. Arterioscler Thromb. 1993;13(12):1829–36. doi: 10.1161/01.atv.13.12.1829. [DOI] [PubMed] [Google Scholar]
- 11.Folsom AR. Hemostatic risk factors for atherothrombotic disease: an epidemiologic view. Thromb Haemost. 2001;86(1):366–73. [PubMed] [Google Scholar]
- 12.Folsom AR, et al. Prospective Study of Fibrinolytic Factors and Incident Coronary Heart Disease : The Atherosclerosis Risk in Communities (ARIC) Study. Arterioscler Thromb Vasc Biol. 2001;21(4):611–617. doi: 10.1161/01.atv.21.4.611. [DOI] [PubMed] [Google Scholar]
- 13.The ARIC Investigators The Atherosclerosis Risk in Community (ARIC) Study. Design and Objectives. Am. J. Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 14.Warnick GR, et al. HDL cholesterol quantitation by phosphotungstate-Mg2+ and by dextran sulfate-Mn2+-polyethylene glycol precipitation, both with enzymic cholesterol assay compared with the lipid research method. Am J Clin Pathol. 1982;78(5):718–23. doi: 10.1093/ajcp/78.5.718. [DOI] [PubMed] [Google Scholar]
- 15.Siedel J, et al. Reagent for the enzymatic determination of serum total cholesterol with improved lipolytic efficiency. Clin Chem. 1983;29(6):1075–80. [PubMed] [Google Scholar]
- 16.Papp AC, et al. ARIC hemostasis study--I. Development of a blood collection and processing system suitable for multicenter hemostatic studies. Thromb Haemost. 1989;61(1):15–9. [PubMed] [Google Scholar]
- 17.White AD, et al. Sex and race differences in short-term prognosis after acute coronary heart disease events: the Atherosclerosis Risk In Communities (ARIC) study. Am Heart J. 1999;138(3 Pt 1):540–8. doi: 10.1016/s0002-8703(99)70158-4. [DOI] [PubMed] [Google Scholar]
- 18.Mickey RM, Greenland S. The Impact of Confounder Selection Criteria on Effect Estimation. Am. J. Epidemiol. 1989;129(1):125–137. doi: 10.1093/oxfordjournals.aje.a115101. [DOI] [PubMed] [Google Scholar]
- 19.Lee CD, et al. White blood cell count and incidence of coronary heart disease and ischemic stroke and mortality from cardiovascular disease in African-American and White men and women: atherosclerosis risk in communities study. Am J Epidemiol. 2001;154(8):758–64. doi: 10.1093/aje/154.8.758. [DOI] [PubMed] [Google Scholar]
- 20.Kuller LH, et al. The Relation between Serum Albumin Levels and Risk of Coronary Heart Disease in the Multiple Risk Factor Intervention Trial. Am. J. Epidemiol. 1991;134(11):1266–1277. doi: 10.1093/oxfordjournals.aje.a116030. [DOI] [PubMed] [Google Scholar]
- 21.Corti MC, Salive ME, Guralnik JM. Serum albumin and physical function as predictors of coronary heart disease mortality and incidence in older persons. J Clin Epidemiol. 1996;49(5):519–26. doi: 10.1016/0895-4356(95)00562-5. [DOI] [PubMed] [Google Scholar]
- 22.Nelson JJ, et al. Serum Albumin Level as a Predictor of Incident Coronary Heart Disease: The Atherosclerosis Risk in Communities (ARIC) Study. Am. J. Epidemiol. 2000;151(5):468–477. doi: 10.1093/oxfordjournals.aje.a010232. [DOI] [PubMed] [Google Scholar]
- 23.Kannel WB. Overview of hemostatic factors involved in atherosclerotic cardiovascular disease. Lipids. 2005;40(12):1215–20. doi: 10.1007/s11745-005-1488-8. [DOI] [PubMed] [Google Scholar]
- 24.Vischer UM. von Willebrand factor, endothelial dysfunction, and cardiovascular disease. J Thromb Haemost. 2006;4(6):1186–93. doi: 10.1111/j.1538-7836.2006.01949.x. [DOI] [PubMed] [Google Scholar]
- 25.Conlan MG, et al. Associations of factor VIII and von Willebrand factor with age, race, sex, and risk factors for atherosclerosis. The Atherosclerosis Risk in Communities (ARIC) Study. Thromb Haemost. 1993;70(3):380–5. [PubMed] [Google Scholar]
- 26.Sadler JE. Biochemistry and genetics of von Willebrand factor. Annu Rev Biochem. 1998;67:395–424. doi: 10.1146/annurev.biochem.67.1.395. [DOI] [PubMed] [Google Scholar]
- 27.Ruggeri ZM. The role of von Willebrand factor in thrombus formation. Thrombosis Research. 2007;120(Supplement 1):S5–S9. doi: 10.1016/j.thromres.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Constans J, Conri C. Circulating markers of endothelial function in cardiovascular disease. Clinica Chimica Acta. 2006;368(1-2):33–47. doi: 10.1016/j.cca.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 29.Lip GYH, Blann A. von Willebrand factor: a marker of endothelial dysfunction in vascular disorders? Cardiovasc Res. 1997;34(2):255–265. doi: 10.1016/s0008-6363(97)00039-4. [DOI] [PubMed] [Google Scholar]
- 30.Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003;23(2):168–75. doi: 10.1161/01.atv.0000051384.43104.fc. [DOI] [PubMed] [Google Scholar]
- 31.Lerman A, Zeiher AM. Endothelial Function: Cardiac Events. Circulation. 2005;111(3):363–368. doi: 10.1161/01.CIR.0000153339.27064.14. [DOI] [PubMed] [Google Scholar]
- 32.Ruddock V, Meade TW. Factor-VII activity and ischaemic heart disease: fatal and non-fatal events. QJM. 1994;87(7):403–6. [PubMed] [Google Scholar]
- 33.De Stavola BL, Meade TW. Long-term effects of hemostatic variables on fatal coronary heart disease: 30-year results from the first prospective Northwick Park Heart Study (NPHS-I) J Thromb Haemost. 2007;5(3):461–71. doi: 10.1111/j.1538-7836.2007.02330.x. [DOI] [PubMed] [Google Scholar]
- 34.Heinrich J, et al. Fibrinogen and factor VII in the prediction of coronary risk. Results from the PROCAM study in healthy men. Arterioscler Thromb. 1994;14(1):54–9. doi: 10.1161/01.atv.14.1.54. [DOI] [PubMed] [Google Scholar]
- 35.Salomaa V, et al. Haemostatic factors and prevalent coronary heart disease; the FINRISK haemostasis study. Eur Heart J. 1994;15(10):1293–1299. doi: 10.1093/oxfordjournals.eurheartj.a060387. [DOI] [PubMed] [Google Scholar]
- 36.Fibrinogen Studies, C. Plasma Fibrinogen Level and the Risk of Major Cardiovascular Diseases and Nonvascular Mortality: An Individual Participant Meta-analysis. JAMA. 2005;294(14):1799–1809. doi: 10.1001/jama.294.14.1799. [DOI] [PubMed] [Google Scholar]
- 37.Zipes DP, Wellens HJ. Sudden cardiac death. Circulation. 1998;98(21):2334–51. doi: 10.1161/01.cir.98.21.2334. [DOI] [PubMed] [Google Scholar]
- 38.Wannamethee G, et al. Risk factors for sudden cardiac death in middle-aged British men. Circulation. 1995;91:1749–1756. doi: 10.1161/01.cir.91.6.1749. [DOI] [PubMed] [Google Scholar]
- 39.Thorgeirsson G, et al. Risk factors for out-of-hospital cardiac arrest: the Reykjavik study. European Heart Journal. 2005;26:1499–1505. doi: 10.1093/eurheartj/ehi179. [DOI] [PubMed] [Google Scholar]
- 40.Jouven X, et al. Predicting sudden death in the population, the Paris Prospective Study I. Circulation. 1999;99:1978–1983. doi: 10.1161/01.cir.99.15.1978. [DOI] [PubMed] [Google Scholar]