Abstract
The Obg family of GTPases is widely conserved and predicted to play an as-yet-unknown role in translation. Recent reports provide circumstantial evidence that both eukaryotic and prokaryotic Obg proteins are associated with the large ribosomal subunit. Here we provide direct evidence that the Caulobacter crescentus CgtAC protein is associated with the free large (50S) ribosomal subunit but not with 70S monosomes or with translating ribosomes. In contrast to the Bacillus subtilis and Escherichia coli proteins, CgtAC does not fractionate in a large complex by gel filtration, indicating a moderately weak association with the 50S subunit. Moreover, binding of CgtAC to the 50S particle is sensitive to salt concentration and buffer composition but not guanine nucleotide occupancy of CgtAC. Assays of epitope-tagged wild-type and mutant variants of CgtAC indicate that the C terminus of CgtAC is critical for 50S association. Interestingly, the addition of a C-terminal epitope tag also affected the ability of various cgtAC alleles to function in vivo. Depletion of CgtAC led to perturbations in the polysome profile, raising the possibility that CgtAC is involved in ribosome assembly or stability.
GTP-binding proteins play roles in diverse cellular processes ranging from signal transduction to vesicle fusion. While much attention has been focused on the role of the eukaryotic Ras-like GTPases, less attention has been paid to a large number of GTPase subfamilies that are conserved among all living organisms sequenced to date (4, 5, 27, 33). On the basis of the evolutionary relationships and emerging experimental data, it has been proposed that these relatively widely conserved GTP-binding proteins act as translation factors (27).
The Obg subfamily is a distinct group of monomeric GTP-binding proteins that share a conserved GTP-binding domain. Bacteria encode one Obg protein and Archaea typically encode two related Obg proteins, whereas eukaryotes usually encode four Obg proteins, including three distinct protein types. The bacterial and the eukaryotic mitochondrial Obg proteins are likely to be homologous, as sequences flanking the GTP-binding domain are also conserved. The biochemical features of the Obg proteins are distinct from those of the well-characterized Ras-like proteins. For example, all bacterial Obg proteins examined thus far bind guanine nucleotides with modest (in the micromolar range) affinity (28, 47, 52). More strikingly, the Caulobacter crescentus and the Escherichia coli Obg proteins, CgtAC and CgtAE, respectively (CgtAE is also called ObgE or YhbZ), have rapid GDP and GTP exchange rate constants but relatively slow GTP hydrolysis rates (28). To date, we and others have favored models for Obg function that involve Obg acting as a sensor of intracellular GTP/GDP pools (28, 39). We further proposed that in vivo, rapid exchange of guanine nucleotides might be inhibited and that GTP hydrolysis may play a role in regulating the activation of the Obg proteins (30). The inhibition of guanine nucleotide exchange could be accomplished by the association of Obg in a complex.
Accumulating evidence suggests that the Obg proteins are ribosome associated. The Bacillus subtilis Obg protein fractionates in a large cytoplasmic complex by gel filtration, coelutes with ribosomal proteins, and interacts specifically with the 50S ribosome subunit protein L13 (42). Similarly, the E. coli CgtAE protein also fractionates with a large RNA-containing complex by gel filtration and interacts with L13 (K. Pu and J. R. Maddock, unpublished data). CgtAE has been shown genetically to interact with RrmJ (FtsJ) (47), a methyltransferase that modifies the 23S rRNA (3). Overexpression of CgtAE suppresses both the growth defect and the polysome profile defect of the ΔrrmJ mutant, suggesting that CgtAE plays an active role in ribosome assembly or stability (47).
In this report we demonstrate that the C. crescentus CgtAC protein is associated with the 50S ribosomal subunit but not with the 70S monosomes or with polyribosomes, indicating that CgtAC is not associated with translating ribosomes. The observed association is dependent on both the salt concentration and the magnesium counter ion used in the assay. Moreover, the C-terminal acid domain of CgtAC, which is important for function, is also critical for 50S association, and the addition of a C-terminal epitope tag affects both function and ribosome association. Finally, the long-term effect of CgtAC depletion is a reduction in 70S monosomes and polyribosomes.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The bacterial strains used in this study are listed in Table 1. E. coli was grown at 37°C in Luria-Bertani broth or on Luria-Bertani agar containing antibiotics as required (see below). C. crescentus strains were derived from CB15N and were grown in PYE medium at 30°C. All DNA manipulation was performed in E. coli strain DH5α. Plasmids were introduced into C. crescentus by conjugal transfer via the E. coli strain S17 (12). Antibiotics were used at the following concentrations: for C. crescentus, oxytetracycline (Tet) (concentration, 1 μg ml−1), naladixic acid (20 μg ml−1), ampicillin (Amp) (10 μg ml−1), or kanamycin (Kan) (5 μg ml−1); and for E. coli, tetracycline (12 μg ml−1), Kan (30 μg ml−1), or Amp (100 μg ml−1). The ability of cgtAC alleles to function in C. crescentus strain JM1108 was assayed on PYE agar plates containing 0.2% xylose (PYE+Xyl) or 0.2% glucose (PYE+Glu), Tet, and Kan. No antibiotics were added in the final liquid culture for polyribosome preparation.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristics | Reference or source |
|---|---|---|
| Strains | ||
| E. coli | ||
| DH5a | Δ(lac)U169 φ80 Δ(lacZ)M15 hsdR17 endA1 gyrA96 recA1 relA1 supE44 thi-1 | 17 |
| S17-1 | F−recA; RP4-2 Tcr::Mu Tmr::Tn7 | 44 |
| C. crescentus | ||
| CB15N | Synchronizable derivative of wild type (also known as NA1000) | 13 |
| JM1108 | Pxyl::cgtA integrated at the cgtA locus | 30 |
| Plasmids | ||
| pMR20 | Mini-RK2 cloning vector; Tetr | 45 |
| pCR2.1-TOPO | PCR cloning vector; Kanr Ampr | Invitrogen |
| pT7blue-3 | Blunt cloning vector; Kanr Ampr | Novagen |
| pET28a(+) | Expression vector for N-terminal His-tagged fusions; Kanr | Novagen |
| pFAGa-3HA-TRP | Source of 3HA tag | 31 |
| pJM625 | Full-length cgtA in pET28a | 28 |
| pJM746 | Full-length cgtAT192A in pET28a | 30 |
| pJM747 | Full-length cgtAT193A in pET28a | 30 |
| pJM1402 | 1.06-kb NcoI-BamHI PCR fragment containing cgtA1-347 in pT7blue-3 | This study |
| pJM1759 | 2.25-kb PstI-HindIII cgtA in pMR20; NcoI site at inititating codon | 30 |
| pJM2374 | 2.3-kb NcoI-HindIII cgtA1-347 in PCR2.1-TOPO | This study |
| pJM2383 | cgtA1-347-3HA in pMR20 | This study |
| pJM2384 | cgtAT192A1-347-3HA in pMR20 | This study |
| pJM2385 | cgtAT193A1-347-3HA in pMR20 | This study |
| pJM2395 | 1.09-kb NcoI-PacI PCR fragment containing cgtA in PCR2.1-TOPO | This study |
| pJM2403 | cgtA-3HA in pMR20 | This study |
| pJM2404 | cgtAT192A-3HA in pMR20 | This study |
| pJM2405 | cgtAT193A-3HA in pMR20 | This study |
| cgtA1-347 in pMR20 | This study |
Construction of epitope-tagged CgtAC-3HA.
Relevant plasmids used in this study are listed in Table 1. C-terminal deletion cgtAC1-347 was generated by PCR amplification using primers cgtA-NcoI (5′-GGACCCCATGGAATTCTTGGACCA) and cgtA-347delta (5′-TCTAGAGGATCCCCTCGTCGACGTGATCCT), whereas cgtAC1-334 was generated using primers cgtA-NcoI and cgtA-334delta (5′-TCTAGAGGATCCCGAGATCACCGCGACGGAT). Through a series of subclones, a C-terminal BamHI-HindIII influenza virus hemagglutinin tag (3HA tag) from pFA6a-3HA-TRP1 (31) was added to create the cgtA1-347-3HA fusion construct (pJM2383). 3HA-tagged mutant alleles (T192A and T193A) were generated by substitution of the NcoI-XhoI fragments from the appropriate mutant allele clones into pJM2383 (30). The cgtA1-347 and the cgtA1-347-3HA fusions were placed under the control of the cgtA promoter by replacement of the NcoI-HindIII fragment from pJM1759 (a modified C. crescentus pMR20 plasmid that contains the cgtA promoter and cgtA with an engineered Nco1 site at the initiating codon) with cgtA1-347 or cgtA1-347-3HA. The full-length cgtA-3HA construct was generated by PCR amplification of cgtAC through the use of CgtA-NcoI and C-PacIXbaI-CgtA (5′ GCTCTAGATCTTAATTAACGGCGTCCAGCC), tagging with 3HA, and expression from the cgtA promoter in a manner analogous to that used for the cgtA1-347 and cgtA1-347-3HA fusion constructs. The final constructs of all 3HA-tagged cgtA alleles were verified by DNA sequencing of the cgtA gene, junction regions of the PcgtA promoter, and the 3HA tag.
Affinity purification of CgtAC antibody.
Polyclonal rabbit anti-CgtAC antibody was affinity purified from crude antiserum with Affi-Gel 15 (Bio-Rad) agarose beads coupled with purified CgtAC protein (28). All steps were performed at 4°C unless otherwise specified. Approximately 25 mg of purified CgtAC protein was incubated with 1 ml of Affi-Gel 15 beads in a final volume of 1.5 ml of HEPES buffer (100 mM, pH 8) for 30 min. The coupling efficiency was at least 95%, as determined by a Bradford assay (Bio-Rad) (catalog no. 500-000). The remaining active sites on the Affi-Gel were subsequently blocked with 10 mM ethanolamine-HCl (1 M stock, pH 8) for 1 h. The CgtAC-coupled Affi-Gel (CgtAC-Affi-Gel) was equilibrated with phosphate-buffered saline (100 mM NaCl, 80 mM Na2HPO4, 20 mM NaH2PO4, pH 7.5) containing 0.05% Tween 20 (PBST) and 0.2% sodium azide.
For immunoaffinity purification of anti-CgtAC antibody, approximately 0.3 ml of CgtAC-Affi-Gel beads was combined with 0.5 ml of crude antiserum and brought to a final volume of 1 ml with PBST and 10% bovine serum albumin (BSA). The mixture was incubated overnight with gentle shaking. The beads were incubated on ice for 5 min, pelleted in a 1.75-ml microcentrifuge tube (with a small cut 2 to 4 mm from the bottom) at 2,000 × g for 3 min, and then washed five times with 1 ml of PBST. Anti-CgtAC was eluted from beads three times with 0.1 ml of glycine-HCl (50 mM [pH 2.5] in 0.1% Tween 20), once with 0.1 ml of BSA (10% in PBST), and once with 0.1 ml of Tris-HCl (1.5 M, pH 7.5) by centrifugation at 2,000 × g for 3 min. Eluted samples were combined and stored at −80°C in 10-μl aliquots. The specificity and titer of the purified anti-CgtAC were tested by immunoblot analysis.
Immunoblot analysis.
Proteins were separated by sodium dodecyl sulfate-12% polyacrylamide gel electrophoresis (SDS-12% PAGE) and electroblotted to polyvinylidene difluoride membranes (NEN Life Science Products) with a Hoeffer semidry transfer apparatus as recommended by the manufacturer. The membranes were blocked with 10% skim milk in PBST, probed with either a 1:2,000 dilution of affinity-purified polyclonal rabbit anti-CgtA or a 1:1,000 dilution of monoclonal mouse anti-HA (ascites fluid, clone 16B12; BAbCO), and washed with PBST; bound antibody was detected with a 1:20,000 dilution of horseradish peroxidase-conjugated goat anti-rabbit (Pierce) or rabbit anti-mouse (Sigma) antibody, respectively, and visualized by fluorography using ECL (Amersham Pharmacia Biotech) as recommended by the manufacturer.
Preparation of C. crescentus cell lysates.
C. crescentus cell lysates were prepared according to Ohta et al. (38) and Flessel et al. (15) with the following modifications. Briefly, 500 ml of PYE cultures of C. crescentus cells was grown at 30°C to an optical density at 600 nm (OD600) of 0.6 to 0.8. Chloramphenicol was added to a final concentration of 100 μg/ml 3 min prior to harvesting. Cells were immediately chilled by pouring over an equal volume of crushed ice and harvested by centrifugation (10,000 × g, 15 min). The cell pellet was washed with 20 ml of ice-cold TE (100 mM Tris-HCl [pH 7.5], 1 mM EDTA) and resuspended in 5 ml of SETS buffer (100 mM NaCl, 1 mM EDTA, 100 mM Tris-HCl [pH 7.5], 17% sucrose) containing 100 mg of lysozyme (Sigma)/ml and 100 μg of phenylmethylsulfonyl fluoride (10 mg/ml of stock in ethanol)/ml. All subsequent operations were performed at 4°C. After 30 min of lysozyme treatment, MgCl2 (1 M stock) was added to restore the magnesium concentration to 20 mM unless otherwise indicated. Spheroplasts were monitored under a phase-contrast microscope and centrifuged for 20 min at 10,000 × g. The pellet was resuspended in 2 ml of TM buffer (10 mM Tris-HCl [pH 7.5], 10 mM MgCl2) containing 100 μg of chloramphenicol/ml, 100 μg of phenylmethylsulfonyl fluoride/ml, and Complete EDTA-free protease inhibitor cocktail (Roche Molecular Biochemicals) (1 tablet/50 ml). The spheroplasts were disrupted by the addition of sodium deoxycholate (10% stock) and Brij 58 (10% stock) to achieve a final concentration of 1% each. The crude lysate was frozen in liquid nitrogen and slowly thawed in an ice-water bath. After centrifugation at 20,000 × g for 20 min, the clarified lysate was stored at −80°C in 0.5-ml aliquots. UV absorbance of the cell lysate was determined in a 1-ml quartz cuvette.
Polyribosome fractionation.
Cell lysates (200 μl) at an OD260 of approximately 20 were subjected to ultracentrifugation in 10 ml of 15 to 45% sucrose (ARCOS) RNase- and DNase-free gradients for 3 h at 41,000 rpm (210,000 × g) and 0°C in a Beckman SW41Ti rotor. Higher-resolution separation was performed using 20 to 40% sucrose gradients at a lower speed (84,000 × g) (Fig. 1B). Sucrose solutions contained 100 mM NH4Cl in TM buffer, and the gradients were prepared (10). The resulting polyribosomes were fractionated by a Brandel gradient fractionator (model BR-186) (2-mm-path-length flow cell) connected to a syringe pump (model SYR-101). The syringe was filled with 50% sucrose in distilled water, and the pump flow rate was set to 0.75 ml/min. The UV absorbance (280 nm) of the sample was monitored and recorded by an ISCO UA-5 detector. A total of 10 μl from each fraction was analyzed by SDS-PAGE and immunoblotting. In experiments examining the effects of excess guanine nucleotides on CgtA-ribosome association, the cell lysates were preincubated with 10 mM GDP or GTP and sedimented through a sucrose gradient in standard buffer with additional 250 μM GDP or GTP, respectively. A parallel blank control (loaded with 200 μl of 10 mM GDP on top of the sucrose gradient containing 250 μM GDP) was used, and the resulting UV absorbance profile confirmed that the distribution of guanine nucleotide in the sucrose gradient after ultracentrifugation was unaltered (data not shown).
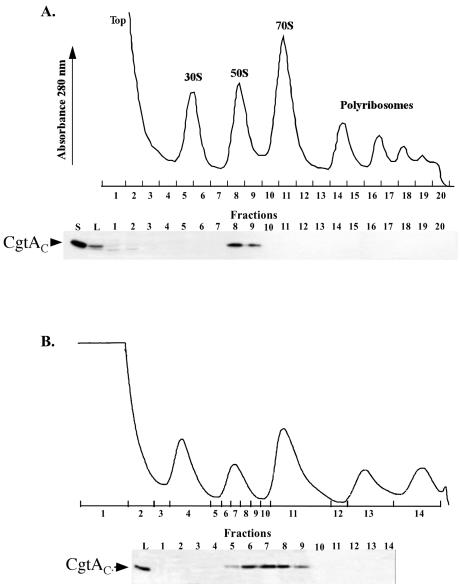
FIG. 1.
CgtAC cofractionates with the 50S ribosomal subunit by sucrose density centrifugation. CB15N cells were grown to logarithmic phase in PYE medium and harvested, and cell lysates were prepared and sedimented through a 15 to 45% sucrose gradient at 210,000 × g for 3 h (A) or a 20 to 40% sucrose gradient at 84,000 × g for 12 h (B). UV profiles were monitored at 280 nm. The positions of the 30S, 50S, and 70S monosome and polyribosome peaks relative to those of the fractions collected are indicated. Immunoblots of relevant fractions (10 μl of each 1-ml fraction) separated by SDS-12% PAGE and the levels of CgtAC detected with anti-CgtAC antibodies are shown below each polyribosome trace. S, 10 ng of purified CgtAC; L, 1 μl of cell lysate (OD260 of 0.1).
Gel filtration chromatography.
C. crescentus cell lysates (0.5 ml) were loaded onto a 100-ml (1.5 by 70 cm) Toyopearl HW-55S column (TosoHaas) eluted (0.4 ml/min) with TMA buffer (10 mM TrisHCl [pH 7.5], 10 mM MgCl2, 100 mM NH4Cl) at 4°C. Fractions (1 ml) were collected from elution volumes of between 20 and 100 ml. When indicated, the cell lysates were preincubated with 10 mM GDP or GTP for 30 min on ice prior to loading and subsequently eluted with TMA buffer containing 1 mM GTP or 1 mM GDP, respectively. Samples (20 μl) from alternate fractions were subjected to SDS-PAGE followed by immunoblot analysis. The column was calibrated with a 0.5-ml mixture of thyroglobulin (Sigma) (669 kDa, 0.25 mg), β-amylase (Sigma) (200 kDa, 0.25 mg), BSA (Sigma) (66 kDa, 0.25 mg), and bovine carbonic anhydrase (Sigma) (29 kDa, 0.25 mg) under the same elution conditions.
RESULTS
CgtAC cosediments with free 50S ribosomal subunits.
In B. subtilis, Obg fractionates as a large cytoplasmic complex containing ribosomal proteins and interacts with the 50S ribosomal subunit protein L13, according to the results of an affinity blot assay (42). The E. coli CgtAE protein also interacts with L13 and purifies in a large RNA-containing complex (Pu and Maddock, unpublished) but does not associate with the large ribosomal subunit after sucrose density centrifugation (25). To examine the relationship between the ribosome and C. crescentus CgtAC protein, we separated cell lysates by ultracentrifugation through a sucrose gradient (10, 15, 38), monitored the polyribosome profile by UV absorbance, and detected the migration of CgtAC by immunoblot analysis (Fig. 1). As expected, the majority of the cellular proteins and small molecules were in the top of the gradient, followed by the peaks for the 30S and 50S subunits and the 70S ribosomes and polyribosomes. CgtAC was located in fractions corresponding to the peak of free 50S ribosomal subunits (Fig. 1A). To examine whether CgtAC cosedimented with ribosomal intermediate particles adjacent to the 50S peak (32, 40), the profile of CgtAC was examined after sucrose density centrifugation under conditions that result in a greater separation of the ribosomal subunits. Under these conditions, CgtAC also coeluted with the mature 50S subunit, although low levels of CgtAC were found in fractions preceding the 50S peak, perhaps due to the dissociation of CgtAC from the 50S subunits during centrifugation or due to an interaction with the pre-50S subunits (Fig. 1B).
Salt dependence of the association of CgtAC with 50S ribosomal subunits.
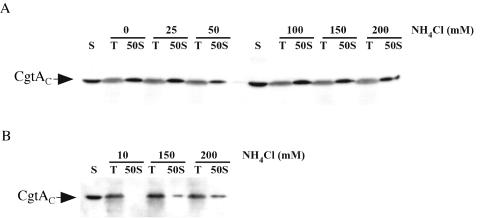
Ammonium chloride (NH4Cl) has been widely employed as a salt in buffers used for polyribosome profiles, although specific assay conditions differ widely among different reports (36). To test the effects of NH4Cl concentration on CgtAC-50S subunit association, lysates of C. crescentus were sedimented through sucrose gradients containing 10 mM Tris-HCl (pH 7.5) supplemented with 10 mM MgCl2 and concentrations of NH4Cl ranging from 0 to 200 mM. No difference in gradient profile was observed regardless of the concentration of NH4Cl present (Fig. 2A). Moreover, when separated in the absence of NH4Cl, CgtAC was still found predominantly in 50S fraction peaks with a trace amount of CgtAC at the top of the gradient (Fig. 2A) and in fractions prior the 50S peak (data not shown). Thus, CgtAC bound to free 50S ribosomal subunits independently of the NH4Cl concentrations used.
FIG. 2.
Salt dependence of the association of CgtAC with free 50S ribosomal subunits. CB15N cell lysates (200 μl, OD260 of 20) were sedimented through 15 to 45% sucrose gradients containing 0 to 200 mM NH4Cl (as indicated) in 10-mM Tris-HCl (pH 7.5) buffer supplemented with either 10 mM MgCl2 (A) or 10 mM MgSO4 (B). Polyribosome profiles similar to that shown in Fig. 1 were obtained, and the fractions corresponding to the top (T) of the gradients and the 50S peaks (50S) were separated by SDS-12% PAGE. Each lane was loaded with 10 μl of a 1-ml fraction, and the levels of CgtAC were detected by immunoblotting using anti-CgtAC antibodies. S, 2 ng of purified CgtAC.
Information regarding the source of the Mg2+ ion present in sucrose gradient buffers also differs among different reports. To address whether the source of the Mg2+ ion was important in the binding of CgtAC to the 50S subunit, we examined the consequences of the use of various levels of NH4Cl in 10 mM Tris-HCl (pH 7.5) supplemented with 10 mM MgSO4. Under these conditions, we observed an NH4Cl concentration-dependent 50S association of CgtAC with the 50S peak. In buffers containing low levels of NH4Cl, CgtAC did not bind to the 50S subunit and was exclusively found at the top of the gradient (Fig. 2B). Some CgtAC-50S cofractionation was observed in buffers containing higher NH4Cl concentrations; even in the presence of 200 mM NH4Cl, however, a significant amount of CgtAC was in the top of the gradient (Fig. 2B). Thus, the combination of the Mg2+ counter ion used and NH4Cl concentration is critical for the observed association of CgtAC with the 50S ribosomal subunit.
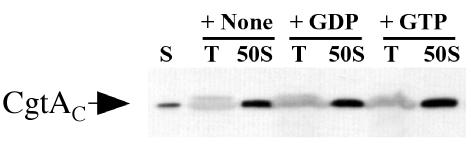
Inclusion of excess GTP or GDP does not affect the cosedimentation of CgtAC with the 50S subunit.
As a guanine nucleotide protein, CgtAC undergoes conformational changes among the GTP-bound, GDP-bound, and apo states (30). It is therefore possible that the guanine nucleotide occupancy of CgtAC could affect its association with free 50S subunits. To examine whether this is the case, C. crescentus cell lysates preincubated with either 10 mM GDP or GTP were sedimented through 15 to 45% sucrose gradients in standard buffers containing 250 μM GDP or GTP, respectively. Because in vitro CgtAC binds guanine nucleotides with moderate affinity (∼1 μM) and displays a rapid guanine nucleotide exchange rate (28), we predicted that under these conditions the majority of the CgtAC protein would be nucleotide bound. As observed in the absence of guanine nucleotides (Fig. 2), the majority of CgtAC cofractionated with the 50S ribosomal subunit irrespective of the presence of either GDP or GTP (Fig. 3). Therefore, it appears that CgtAC binds to the free 50S ribosomal subunit independently of its nucleotide occupancy state.
FIG. 3.
The addition of excess guanine nucleotides does not affect the association of CgtAC and free 50S ribosomal subunits. CB15N cell lysates (200 μl, OD260 of 20) were preincubated on ice without (+None) or with 10 mM GDP (+GDP) or 10 mM GTP (+GTP) for 30 min prior to sedimentation through a 15 to 45% sucrose gradient supplemented with no nucleotide, 250 μM GDP, or 250 μM GTP, respectively. Polyribosome profiles similar to that shown in Fig. 1 were obtained, and the fractions corresponding to the top (T) of the gradients and the 50S peaks (50S) were separated by SDS-12% PAGE. Each lane was loaded with 10 μl of a 1-ml fraction, and the levels of CgtAC were detected by immunoblotting using anti-CgtAC antibodies. S, 2 ng of purified CgtAC.
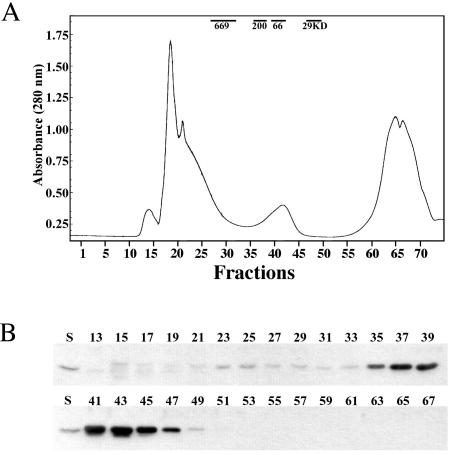
CgtAC is not associated with the 50S particle by gel filtration.
The bacterial 50S ribosomal subunit consists of an ∼1,000-kDa 23S rRNA molecule, an ∼40-kDa 5S rRNA molecule, and over 31 ribosomal proteins with an average molecular mass of 15 kDa each (34). Thus, the overall size of the 50S subunit complex exceeds 1.4 MDa. To examine whether the CgtAC protein binds tightly to the 50S particle, we examined the elution of CgtAC from C. crescentus cell lysates separated by gel filtration on a 100-ml Toyopearl HW-55S gel filtration column (fractionation range of 1 to 103 kDa for globular proteins) (Fig. 4). The majority of CgtAC eluted in fractions corresponding to 30 to 200 kDa, peaking at approximately 50 kDa (Fig. 4), indicating that a significant amount of CgtAC fractionates as monomers or small complexes. To assay whether the guanine nucleotide binding state of CgtAC affected its chromatographic migration, cell lysates were preincubated with 10 mM GDP or GTP and eluted in buffer containing 1 mM GDP or 1 mM GTP, respectively. Under these conditions, no change in CgtAC elution was detected (data not shown). Thus, we conclude that, regardless of its nucleotide occupancy, CgtAC does not bind tightly to the 50S ribosomal subunit.
FIG. 4.
Gel filtration chromatography of a C. crescentus CB15N cell lysate reveals that the majority of CgtAC elutes as a monomer or in a small complex. CB15N cell lysates (0.5 ml) were fractionated through a 100-ml (1.5 by 70 cm) Toyopearl HW-55S column (TosoHaas) at a flow rate of 0.4 ml/min in TMA buffer (10 mM Tris-HCl [pH 7.5], 10 mM MgCl2, 100 mM NH4Cl). (A) The elution of samples was monitored by UV absorbance at 280 nm, and 1-ml fractions were collected using elution volumes between 20 and 100 ml. The elution positions of control proteins are indicated. (B) The indicated fractions (10 μl each) were separated by SDS-12% PAGE. The levels of CgtAC were detected by immunoblotting using anti-CgtAC antibodies. S, 2 ng of CgtA.
C-terminally epitope-tagged CgtAC variants are impaired for in vivo function.
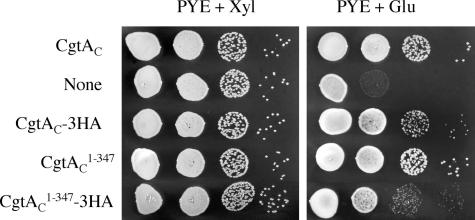
CgtAC is 354-amino-acid tripartite protein possessing an N-terminal glycine-rich domain called the Obg-fold (amino acids 1 to 159) (2), a GTP-binding domain (amino acids 160 to 314), and a C-terminal acidic domain (EEEIDDDEDHVDE; amino acids 335 to 347). We have previously shown that the N-terminal Obg-fold is critical for CgtAC function (29). To address whether the charged C terminus of CgtAC is also important for function, we made C-terminal truncation constructs and examined their ability to support growth as the sole expressed source of CgtAC. To do this, we took advantage of a C. crescentus strain (JM1108) in which the chromosomal cgtAC is under the control of the Pxyl promoter such that expression of chromosomal cgtAC is repressed by a change of carbon source from xylose (Xyl) to glucose (Glu). JM1108 cells containing a plasmid-borne cgtAC allele grow in PYE+Glu (30) (Fig. 5). Episomal expression of cgtAC lacking the terminal 7 amino acids (cgtAC1-347) also supports growth (Fig. 5), whereas CgtAC lacking the acidic domain (cgtAC1-334) does not (data not shown). Thus, modest C-terminal deletions are not deleterious to CgtAC function, whereas removal of the acidic domain results in a nonfunctional protein.
FIG. 5.
Modification of the CgtAC C terminus affects function. Serial dilutions of cultured JM1108 cells containing pMR20 plasmids expressing cgtAC, no protein (None), cgtAC-3HA, cgtAC1-347, or cgtAC1-347-3HA were spotted onto PYE+Xyl and PYE+Glu plates. The plates were incubated at 30°C for 2 days.
To examine whether CgtAC1-347 associates with the 50S ribosomal subunit, we epitope-tagged full-length CgtAC and cgtAC1-347 so that they could be specifically identified in cell lysates expressing untagged chromosomal cgtAC. The full-length protein, CgtAC-3HA, supports growth of JM1108 on PYE+Glu, albeit at a slightly reduced rate compared to untagged CgtAC (Fig. 5). Likewise, the cgtAC1-347-3HA allele supports growth but at a lower rate than that with its untagged counterpart (Fig. 5). Moreover, whereas the growth rates of cells expressing CgtAC or CgtAC1-347 were indistinguishable, cells expressing the cgtAC1-347-3HA allele grew more slowly than those with the cgtAC-3HA allele. Thus, it appears that the C-terminal addition of the 3HA tag results in a partial loss of CgtAC function and that the combination of deletion of the seven C-terminal amino acids and addition of the 3HA tag further perturbs CgtAC, underlining the importance of the C-terminal acidic domain to CgtAC function. To test whether the observed differences in in vivo function were caused by a variation in protein expression, JM1108 cells harboring 3HA-tagged cgtAC alleles were grown in PYE+Xyl and analyzed by immunoblotting using monoclonal anti-HA and/or affinity-purified anti-CgtAC antibodies. All 3HA-tagged proteins (including the T192A and T193A variants described below) containing full-size CgtAC or C-terminally truncated CgtAC1-347 were expressed at similar levels, confirming that the growth differences among strains growing in glucose were not due to changes in steady-state protein levels (data not shown).
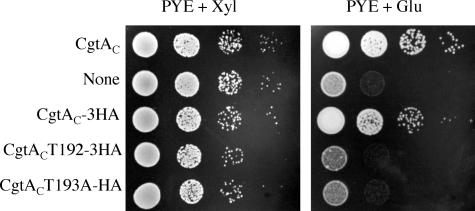
There are two conserved threonine residues within the GTP-binding domain of CgtAC. The first, T192, is critical for CgtAC function, whereas the adjacent threonine, T193, is not essential but exhibits a minor defect in binding GDP and GTP (30). As expected, plasmid-encoded CgtACT192A-3HA does not support growth of JM1108 on PYE+Glu (Fig. 6). Interestingly, whereas plasmid-encoded CgtACT193A supports growth of JM1108 in PYE+Glu (30), CgtACT193A-3HA does not (Fig. 6). We propose that the slight defect in guanine nucleotide binding caused by the T193A mutation and the reduction in function caused by the addition of the C-terminal 3HA tag are negatively synergetic.
FIG. 6.
Growth of tagged CgtAC GTP-binding domain mutants. Serial dilutions of cultured JM1108 cells containing pMR20 plasmids expressing cgtAC, no protein (None), cgtAC-3HA, cgtACT192A-3HA, or cgtACT193A-3HA were spotted onto PYE+Xyl and PYE+Glu plates. The plates were incubated at 30°C for 2 days.
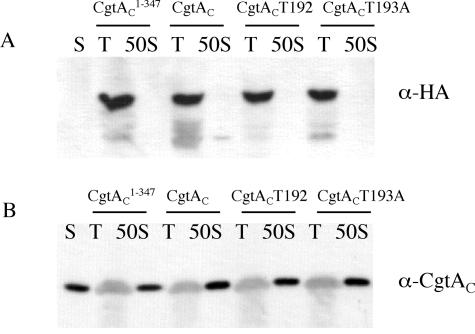
To examine whether the tagged CgtAC variants were associated with the 50S ribosome subunit in the context of wild-type CgtAC, polyribosome profiles of JM1108 expressing the tagged CgtAC were examined by immunoblot analysis. In JM1108 cells expressing plasmid-encoded CgtAC-3HA, the chromosomally encoded CgtAC was found in the 50S fractions (Fig. 7B) whereas the CgtAC-3HA was in the top of the gradient (Fig. 7A). Not surprisingly, the plasmid-encoded 3HA-tagged mutant CgtAC proteins CgtAC1-347-3HA, CgtACT192-3HA, and CgtACT193A-3HA were also found in the top of the gradient in their respective cell lysates (Fig. 7). Thus, the 3HA-tagged proteins either were unable to bind the 50S subunits, were displaced during the sucrose density centrifugation, or could not compete with wild-type CgtA in binding with 50S ribosomal subunits.
FIG. 7.
The C-terminally 3HA-tagged CgtAC proteins failed to cosediment with free 50S ribosomal subunits in the cell lysates. C. crescentus JM1108 cells expressing cgtAC1-347-3HA (second and third lanes), cgtAC-3HA (fourth and fifth lanes), cgtACT192A-3HA (sixth and seventh lanes), and cgtACT193A-3HA (eighth and ninth lanes) episomally from pMR20 were grown to logarithmic phase in PYE+Xyl medium and harvested. Cell lysates were independently sedimented through a 15 to 45% sucrose gradient prepared in 10 mM Tris-HCl (pH 7.5)-10 mM MgCl2-100 mM NH4Cl. A total of 10 μl of each 1-ml fraction from the top of the gradient (T) and the free 50S ribosomal subunit peak (50S) was separated by SDS-12% PAGE. The results of immunoblotting with duplicate gels prepared using monoclonal anti-HA antibody (A) and anti-CgtAC (B) antibodies are shown. S, 2 ng of purified CgtAC.
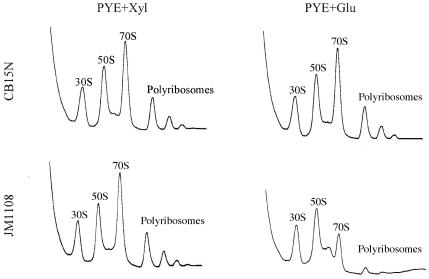
Depletion of CgtAC in C. crescentus resulted in a modest reduction of 70S ribosomes and polyribosomes.
To further investigate the relationship between ribosomes and the function of CgtAC, the effects of depleting CgtAC on polyribosome profiles were examined. Wild-type cells (CB15N) and cells containing cgtA under the control of the Pxyl promoter (JM1108) were grown to mid-exponential phase in PYE+Xyl, washed with PYE, resuspended at low density in either PYE+Xyl or PYE+Glu medium, and grown at 30°C for 6 h, a time when the vast majority of CgtAC protein is depleted, cell viability decreases, and cell growth is slowed (30). Lysates were subjected to sedimentation through a sucrose gradient. In CB15N cells, a shift from PYE+Xyl to PYE+Glu had no effect on the resulting polyribosome profile (Fig. 8). JM1108 cells grown in PYE+Xyl had profiles similar to that of CB15N (Fig. 8). In contrast, JM1108 cells grown in PYE+Glu contained reduced levels of 70S monosomes and polyribosomes whereas the levels of free 30S and 50S ribosomal subunits were not affected (Fig. 8). These data demonstrate that a long-term consequence of CgtAC depletion (either direct or indirect) is a reduction in translating ribosomes. A minor 60S peak is observed in all of our preparations but is particularly visible in JM1108 grown in glucose, due to the reduction of the 70S peak. A similarly sized peak has been reported previously (50), and is predicted to be due to alternate 30S-50S couples.
FIG. 8.
Long-term depletion of CgtAC reduces the levels of 70S ribosomes and polyribosomes. Wild-type C. crescentus cells (CB15N) and cells containing cgtA under the control of the Pxyl promoter (JM1108) were grown to mid-exponential phase in PYE+Xyl, washed with PYE, resuspended at low density in either PYE+Xyl or PYE+Glu medium, and grown at 30°C for 6 h. Cell lysates were sedimented through 15 to 45% sucrose gradients, and the resulting UV absorbance profiles were recorded.
DISCUSSION
Ribosome assembly occurs through the coordinated assembly of specific proteins on the nascent rRNA coupled with temporal rRNA processing and modification. In eukaryotes, there are approximately 200 proteins required for the biogenesis and export of the ribosomal subunits (see reference 49 for a review). Putative remodeling proteins such as helicases, AAA ATPases, and GTPases are included in these ribosomal assembly factors. In E. coli, active small and large ribosomal subunits can be spontaneously assembled in vitro without the need for ribosome assembly factors (8, 20). This in vitro assembly, however, requires elevated temperatures, high Mg2+ levels, and extended incubation times, indicating that in vivo, additional factors are necessary for optimal ribosomal assembly.
GTP-binding proteins associated with ribosomal precursor particles are likely candidates for such assembly factors. Interestingly, the eukaryotic Obg protein, Nog1p, copurifies with a wide range of pre-60S intermediates (1, 14, 18, 37) and is critical for 60S assembly (22, 24). Therefore, it is possible that the Obg proteins in archaea and bacteria also play a role in large ribosome biogenesis. In this study, we demonstrated that CgtAC is associated with the free 50S ribosomal subunits but not with the mature 70S monosomes or with polyribosomes. Since CgtAC is not a protein associated with translating ribosomes but is exclusively associated with the free mature or pre-50S ribosomal subunits, it is a strong candidate for a bacterial ribosomal assembly factor.
The conditional dependence on NH4Cl concentration for CgtAC binding to the 50S subunit is of interest. In the presence of 10 mM MgCl2, CgtAC cosediments with the 50S subunit at similar levels over a wide range of NH4Cl concentrations. In buffers containing 10 mM MgSO4, however, the majority of CgtAC remained in top of the gradient and the amount of CgtA detected in the fractions of the 50S subunit increased in correspondence to the addition of excess NH4Cl. Recently, it was reported that the E. coli CgtAE protein was not detected in ribosomal fractions when the cell lysates were prepared and sedimented in buffers containing 100 mM (NH4)2Ac and 15 mM MgAc (25). It is possible that divalent anions such as SO42− or Ac2− might inhibit CgtAC binding to 50S subunits.
The total intracellular ionic concentration in bacterial cells is estimated to be in the range of 150 to 550 mM, depending on the osmolarity of the medium (7, 21, 46); therefore, the buffers containing 100 mM NH4Cl used in this study should represent an ionic strength close to that of the intracellular ionic environment. In sucrose gradient sedimentation, the CgtAC-50S ribosomal subunit complex withstands NH4Cl concentrations ranging from 0 to 200 mM, suggesting that CgtAC binds free 50S ribosomal subunits in vivo. In gel filtration experiments, however, CgtAC from cell lysates did not elute with the 50S ribosomal particle in buffer containing 100 mM NH4Cl. This paradox could be explained by the equilibrium between the CgtAC-50S subunit complexes and free CgtAC monomers. In single-phase solutions such as cell lysates and sucrose gradients, CgtAC would have access to the 50S subunits and the binding equilibrium would favor the CgtAC-50S complexes. In a multiphase environments such as gel filtration, however, dissociated 50S subunits and CgtAC protein would be separated into two mobile phases. The large 50S complex would elute in the void volumes, whereas the free CgtAC would migrate slowly through the porous resin. Interestingly, the B. subtilis Obg and E. coli CgtAE proteins have been shown to fractionate in a large cytoplasmic complex by gel filtration (42) (Pu and Maddock, unpublished). Perhaps the use of low-salt buffers in these studies increased the stability of the protein-50S complex. Alternatively, there may be species-specific differences in the affinity of the Obg proteins with the 50S ribosomal subunits.
The addition of excess GDP or GTP to the cell lysates had no detectable effects on the association of CgtAC with 50S ribosomal particles. CgtAC has a moderate affinity for both GTP and GDP and rapidly exchanges its bound nucleotide in vitro (28). Therefore, in vivo, unless exchange is inhibited in the CgtAC-50S complex the occupancy state of CgtAC should reflect that of the nucleotide pools. The results in this study suggest that the state of guanine nucleotide occupancy of CgtAC does not affect its binding or dissociating free 50S ribosomal subunits. It will be of interest to examine the association of CgtAC with 50S ribosomal subunits in cells at the stationary phase, when the intracellular levels of GDP should increase relative to that of GTP.
The details of CgtAC association with the 50S ribosomal subunit are unknown. Since the B. subtilis and E. coli Obg proteins associate with L13 (42) (Pu and Maddock, unpublished), it is likely that interaction with L13 mediates at least part of this interaction. In addition, CgtAE interacts with SpoT in a yeast two-hybrid screen (Pu and Maddock, unpublished); therefore, contacts with SpoT may also be critical for ribosome association. In this study, we uncovered a requirement for the C-terminal seven amino acids for both optimal CgtAC function and for association with the 50S ribosomal particles. Interestingly, strains expressing full-length or slightly truncated cgtAC alleles epitope tagged with 3HA grew more slowly than the cells expressing their untagged counterparts, indicating that the addition of the tag was also detrimental for protein function. Moreover, none of the CgtAC-3HA proteins associated with the 50S ribosomal particle when coexpressed with wild-type CgtAC. Thus, either deletion of C-terminal sequences or the addition of a C-terminal tag affects CgtAC function and/or ribosome association.
CgtAC may play a role in ribosome assembly. The majority of CgtAC associates with a 50S particle but not with the 70S monoribosomes or with polyribosomes. The 50S peak is composed of newly synthesized 50S ribosomal subunits, recycled 50S ribosomal subunits (i.e., subunits dissociated from runoff mature ribosomes after transcription), and late pre-50S ribosomal precursors (23, 32, 35). The long-term effect of CgtAC depletion was a reduction in the level of mature 70S ribosomes and polyribosomes, whereas the levels of both free 30S and 50S subunits were relatively unchanged. It is possible that CgtAC acts at a late step in 50S subunit maturation. Such a role would be consistent with the ability of the E. coli CgtAE protein to act as a high-copy-number suppressor of both the growth and polysome defect of an rRNA methyltransferase mutant, ΔrrmJ (47).
The bacterial Obg proteins clearly play a cellular role beyond that of a translation factor. Obg proteins display essential functions in a wide variety of distinct cellular processes, such as cell growth and differentiation (39, 48, 51), DNA replication (26), chromosome segregation, and cell division (9, 11, 25, 43). In addition, the B. subtilis Obg protein is necessary for activation of the general stress response transcription factor, σB (41). Under conditions of environmental stress (i.e., heat, ethanol, salt, or acid treatment) or during energy depletion (i.e., decrease of cellular ATP concentration), σB is activated through the coordinate activities of the Rsb proteins and Obg. Obg in turn, interacts directly with RsbT, RsbW, and RsbX (42). A widespread role for Obg function in stress response, however, cannot be mediated through σB, as most bacteria do not have a σB-regulated stress response pathway. In E. coli, for example, this activity is primarily regulated by σS, a sigma factor that regulates many cellular responses that accompany entry into stationary phase, nutrient starvation (stringent condition), or shift to high osmolarity or low pH (for a review, see reference 19). The activation of σS is controlled, in part, by the alarmone (p)ppGpp (16). (p)ppGpp is synthesized by the ribosome-associated synthase, RelA. When cells enter into the stationary phase or are starved for one or more amino acids, RelA is activated and SpoT [a (p)ppGpp synthetase-hydrolase] is inactivated, resulting in increasing levels of (p)ppGpp (see reference 6 for a review). Intriguingly, the E. coli CgtAE protein interacts directly with SpoT (Pu and Maddock, unpublished), raising the possibility that CgtAE is also involved in stress response. One intriguing possibility is that the Obg proteins are involved in coordinating ribosome assembly and stress response. Studies are in progress to directly test this possibility.
Acknowledgments
We are particularly grateful to the members of the Maddock lab and to Sue Sullivan for critical reading of the manuscript.
This work was supported by grant GM-55133 from the National Institutes of Health.
Footnotes
Present address: NABI Biopharmaceuticals, Rockville, MD 20852.
REFERENCES
- 1.Bassler, J., P. Grandi, O. Gadal, T. Lessmann, E. Petfalski, D. Tollervey, J. Lechner, and E. Hurt. 2001. Identification of a 60S preribosomal particle that is closely linked to nuclear export. Mol. Cell 8:517-529. [DOI] [PubMed] [Google Scholar]
- 2.Buglino, J., V. Shen, P. Hakimian, and C. D. Lima. 2002. Structural and biochemical analysis of the Obg GTP-binding protein. Structure 10:1581-1592. [DOI] [PubMed] [Google Scholar]
- 3.Caldas, T., E. Binet, P. Bouloc, A. Costa, J. Desgres, and G. Richarme. 2000. The FtsJ/RrmJ heat shock protein of Escherichia coli is a 23 S ribosomal RNA methyltransferase. J. Biol. Chem. 275:16414-16419. [DOI] [PubMed] [Google Scholar]
- 4.Caldon, C. E., and P. E. March. 2003. Function of the universally conserved bacterial GTPases. Curr. Opin. Microbiol. 6:135-139. [DOI] [PubMed] [Google Scholar]
- 5.Caldon, C. E., P. Yoong, and P. E. March. 2001. Evolution of a molecular switch: universal bacterial GTPases regulate ribosome function. Mol. Microbiol. 41:289-297. [DOI] [PubMed] [Google Scholar]
- 6.Chatterji, D., and A. K. Ojha. 2001. Revisiting the stringent response, ppGpp and starvation signaling. Curr. Opin. Microbiol. 4:160-165. [DOI] [PubMed] [Google Scholar]
- 7.Csonka, L. N. 1989. Physiological and genetic responses of bacteria to osmotic stress. Microbiol. Rev. 53:121-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Culver, G. M., and H. F. Noller. 2000. In vitro reconstitution of 30S ribosomal subunits using complete set of recombinant proteins. Methods Enzymol. 318:446-460. [DOI] [PubMed] [Google Scholar]
- 9.Czyz, A., R. Zielke, G. Konopa, and G. Wegrzyn. 2001. A Vibrio harveyi insertional mutant in the cgtA (obg, yhbZ) gene, whose homologues are present in diverse organisms ranging from bacteria to humans and are essential genes in many bacterial species. Microbiology 147:183-191. [DOI] [PubMed] [Google Scholar]
- 10.Davies, E., and S. Abe. 1995. Methods for isolation and analysis of polyribosomes. Methods Cell Biol. 50:209-222. [DOI] [PubMed] [Google Scholar]
- 11.Dutkiewicz, R., M. Slominska, G. Wegrzyn, and A. Czyz. 2002. Overexpression of the cgtA (yhbZ, obgE) gene, coding for an essential GTP-binding protein, impairs the regulation of chromosomal functions in Escherichia coli. Curr. Microbiol. 45:440-445. [DOI] [PubMed] [Google Scholar]
- 12.Ely, B. 1979. Transfer of drug resistance factors to the dimorphic bacterium Caulobacter crescentus. Genetics 91:371-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evinger, M., and N. Agabian. 1977. Envelope-associated nucleoid from Caulobacter crescentus stalked and swarmer cells. J. Bacteriol. 132:294-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fatica, A., A. D. Cronshaw, M. Dlakic, and D. Tollervey. 2002. Ssf1p prevents premature processing of an early pre-60S ribosomal particle. Mol. Cell 9:341-351. [DOI] [PubMed] [Google Scholar]
- 15.Flessel, C. P., P. Ralph, and A. Rich. 1967. Polyribosomes of growing bacteria. Science 158:658-660. [DOI] [PubMed] [Google Scholar]
- 16.Gentry, D. R., V. J. Hernandez, L. H. Nguyen, D. B. Jensen, and M. Cashel. 1993. Synthesis of the stationary-phase sigma factor σs is positively regulated by ppGpp. J. Bacteriol. 175:7982-7989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557-580. [DOI] [PubMed] [Google Scholar]
- 18.Harnpicharnchai, P., J. Jakovljevic, E. Horsey, T. Miles, J. Roman, M. Rout, D. Meagher, B. Imai, Y. Guo, C. J. Brame, J. Shabanowitz, D. F. Hunt, and J. L. J. Woolford. 2001. Composition and functional characterization of yeast 66S ribosome assembly intermediates. Mol. Cell 8:505-515. [DOI] [PubMed] [Google Scholar]
- 19.Hengge-Aronis, R. 1999. Interplay of global regulators and cell physiology in the general stress response of Escherichia coli. Curr. Opin. Microbiol. 2:148-152. [DOI] [PubMed] [Google Scholar]
- 20.Herold, M., and K. H. Nierhaus. 1987. Incorporation of six additional proteins to complete the assembly map of the 50S subunit from Escherichia coli ribosomes. J. Biol. Chem. 262:8826-8833. [PubMed] [Google Scholar]
- 21.Ingraham, J. 1987. Effect of temperature, pH, water activity, and pressure on growth, p. 1543-1554. In F. C. Neidhardt, J. L. Ingraham, K. B. Low, B. Magasanik, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella typhimurium: cellular and molecular biology. American Society for Microbiology, Washington, D.C.
- 22.Jensen, B. C., Q. Wang, C. T. Kifer, and M. Parsons. 2003. The NOG1 GTP-binding protein is required for biogenesis of the 60 S ribosomal subunit. J. Biol. Chem. 278:32204-32211. [DOI] [PubMed] [Google Scholar]
- 23.Kaempfer, R. 1968. Ribosomal subunit exchange during protein synthesis. Proc. Natl. Acad. Sci. USA 61:106-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kallstrom, G., J. Hedges, and A. Johnson. 2003. The putative GTPases Nog1p and Lsg1p are required for 60S ribosomal subunit biogenesis and are localized to the nucleus and cytoplasm, respectively. Mol. Cell. Biol. 23:4344-4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kobayashi, G., S. Moriya, and C. Wada. 2001. Deficiency of essential GTP-binding protein ObgE in Escherichia coli inhibits chromosome partition. Mol. Microbiol. 41:1037-1051. [DOI] [PubMed] [Google Scholar]
- 26.Kok, J., K. A. Trach, and J. A. Hoch. 1994. Effects on Bacillus subtilis of a conditional lethal mutation in the essential GTP-binding protein Obg. J. Bacteriol. 176:7155-7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leipe, D. D., Y. I. Wolf, E. V. Koonin, and L. Aravind. 2002. Classification and evolution of P-loop GTPases and related ATPases. J. Mol. Biol. 317:41-72. [DOI] [PubMed] [Google Scholar]
- 28.Lin, B., K. L. Covalle, and J. R. Maddock. 1999. The Caulobacter crescentus CgtA protein displays unusual guanine nucleotide binding and exchange properties. J. Bacteriol. 181:5825-5832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin, B., and J. R. Maddock. 2001. The N-terminal domain of the Caulobacter crescentus CgtA protein does not function as a guanine nucleotide exchange factor. FEBS Lett. 489:108-111. [DOI] [PubMed] [Google Scholar]
- 30.Lin, B., J. M. Skidmore, A. Bhatt, S. M. Pfeffer, L. Pawloski, and J. R. Maddock. 2001. Alanine scan mutagenesis of the switch I domain of the Caulobacter crescentus CgtA protein reveals critcal amino acids required for in vivo function. Mol. Microbiol. 39:924-934. [DOI] [PubMed] [Google Scholar]
- 31.Longtine, M. S., A. McKenzie III, D. J. Demarini, N. G. Shah, A. Wach, A. Brachat, P. Philippsen, and J. R. Pringle. 1998. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14:953-961. [DOI] [PubMed] [Google Scholar]
- 32.Mangiarotti, G., D. Apirion, D. Schlessinger, and L. Silengo. 1968. Biosynthetic precursors of 30S and 50S ribosomal particles in Escherichia coli. Biochemistry 7:456-472. [DOI] [PubMed] [Google Scholar]
- 33.Mittenhuber, G. 2001. Comparative genomics and evolution of genes encoding bacterial (p)ppGpp synthetases/hydrolases (the Rel, RelA and SpoT proteins). J. Mol. Microbiol. Biotechnol. 3:585-600. [PubMed] [Google Scholar]
- 34.Moore, P. B. 1998. The three-dimensional structure of the ribosome and its components. Annu. Rev. Biophys. Biomol. Struct. 27:35-58. [DOI] [PubMed] [Google Scholar]
- 35.Nakada, D., and A. Kaji. 1967. Function and properties of the “native” 30S and 50S ribosomal subunits of Escherichia coli. Proc. Natl. Acad. Sci. USA 57:128-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nierhaus, K. H. 1990. Reconstitution of ribosomes, p. 161-189. In G. Spedding (ed.), Ribosomes and protein synthesis. A practical approach. IRL Press at Oxford University Press, Oxford, United Kingdom.
- 37.Nissan, T. A., J. Bassler, E. Petfalski, D. Tollervey, and E. Hurt. 2002. 60S pre-ribosome formation viewed from assembly in the nucleolus until export to the cytoplasm. EMBO J. 21:5539-5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ohta, N., M. Sanders, and A. Newton. 1978. Characterization of unstable poly(A)-RNA in Caulobacter crescentus. Biochim. Biophys. Acta 517:65-75. [DOI] [PubMed] [Google Scholar]
- 39.Okamoto, S., and K. Ochi. 1998. An essential GTP-binding protein functions as a regulator of differentiation in Streptomyces coelicolor. Mol. Microbiol. 30:107-119. [DOI] [PubMed] [Google Scholar]
- 40.Rohl, R., and K. H. Nierhaus. 1982. Assembly map of the large subunit (50S) of Escherichia coli ribosomes. Proc. Natl. Acad. Sci. USA 79:729-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scott, J. M., and W. G. Haldenwang. 1999. Obg, and essential GTP binding protein of Bacillus subtilis, is necessary for stress activation of transcription factor σB. J. Bacteriol. 181:4653-4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scott, J. M., J. Ju, T. Mitchell, and W. G. Haldenwang. 2000. The Bacillus subtilis GTP binding protein Obg and regulators of the σB stress response transcription factor cofractionate with ribosomes. J. Bacteriol. 182:2771-2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sikora-Borgula, A., M. Slominska, P. Trzonkowski, R. Zielke, A. Mysliwski, G. Wegrzyn, and A. Czyz. 2002. A role for the common GTP-binding protein in coupling of chromosome replication to cell growth and division. Biochem. Biophys. Res. Commun. 292:333-338. [DOI] [PubMed] [Google Scholar]
- 44.Simon, R., U. Priefer, and A. Puhler. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Biotechnology 1:784-790. [Google Scholar]
- 45.Stephens, C., C. Mohr, C. Boyd, J. Maddock, J. Gober, and L. Shapiro. 1997. Identification of the fliI and fliJ components of the Caulobacter flagellar type III protein secretion system. J. Bacteriol. 179:5355-5366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stock, J., B. Rauch, and S. Roseman. 1977. Periplasmic space in Salmonella typhimurium and Escherichia coli. J. Biol. Chem. 252:7850-7861. [PubMed] [Google Scholar]
- 47.Tan, J., U. Jakob, and J. C. Bardwell. 2002. Overexpression of two different GTPases rescues a null mutation in a heat-induced rRNA methyltransferase. J. Bacteriol. 184:2692-2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trach, K., and J. A. Hoch. 1989. The Bacillus subtilis spoOB stage 0 sporulation operon encodes an essential GTP-binding protein. J. Bacteriol. 171:1362-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tschochner, H., and E. Hurt. 2003. Pre-ribosomes on the road from the nucleolus to the cytoplasm. Trends Cell Biol. 13:255-263. [DOI] [PubMed] [Google Scholar]
- 50.van Diggelen, O. P., and L. Bosch. 1973. The association of ribosomal subunits of Escherichia coli. 1. Two types of association products differing in their apparent sedimentation coefficient. Eur. J. Biochem. 39:499-510. [DOI] [PubMed] [Google Scholar]
- 51.Vidwans, S. J., K. Ireton, and A. D. Grossman. 1995. Possible role for the essential GTP-binding protein Obg in regulating the initiation of sporulation in Bacillus subtilis. J. Bacteriol. 177:3308-3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Welsh, K. M., K. A. Trach, C. Folger, and J. A. Hoch. 1994. Biochemical characterization of the essential GTP-binding protein Obg of Bacillus subtilis. J. Bacteriol. 176:7161-7168. [DOI] [PMC free article] [PubMed] [Google Scholar]