Abstract
The increased incidence and severity of Clostridium difficile infection (CDI) in older adults (age, ⩾65 years) corresponds with the emergence of the BI/NAP1 strain, making elucidation of the host immune response extremely important. We therefore infected germ-free C57BL/6 mice aged 7–14 months with a BI/NAP1 strain and monitored the mice for response. Infected mice were moribund 48–72 h after infection and developed gross and histological cecitis and colitis and elevated concentrations of keratinocyte chemoattractant, interleukin 1β, monocyte chemotactic protein 1, and granulocyte colony-stimulating factor and decreased levels of interferon γ, interleukin 12 p40, interleukin 12 p70, and interleukin 10 compared with controls. We conclude that aged, germ-free C57BL/6 mice are susceptible to fulminant CDI from a BI/NAP1 strain and represent a novel model to further elucidate the host immune response to acute CDI.
The incidence, severity, and mortality of Clostridium difficile infection (CDI) have increased, especially in individuals ⩾65 years of age, possibly in association with the emergence of the epidemic restriction endonuclease analysis and pulse field gel electrophoresis BI/NAP1 strain [1]. This strain has been present since the 1980s but was not previously responsible for outbreaks 2]. It hyperproduces toxins A and B, secretes a binary toxin, hypersporulates, and has developed high-level fluoroquinolone resistance, which suggests that these traits have led to increased virulence and spread [2].
A murine model of infection is extremely important to help elucidate the host immune response to CDI and to better understand the pathogenesis induced by BI/NAP1 as well as additional C. difficile strains. The traditional animal model of CDI, the Syrian hamster, is limited by an inability to study the host cytokine response due to a lack of host-specific reagents. Previous germ-free CDI experiments with murine strains other than C57BL/6, particularly in the 1980s and early 1990s, resulted in minimal intestinal pathogenesis to severe cecal and colon ulceration with 100% mortality [3, 4]. The majority of these studies, however, utilized a highly toxigenic laboratory strain, VPI 10463, which has high levels of toxin A and B production, has low sporulation rates, and has never been found to cause human colitis. No gnotobiotic murine studies have been performed with the BI/NAP1 strain, and it has not yet been determined whether germ-free C57BL/6 mice are susceptible to CDI. Additionally, the murine mucosal cytokine response to CDI has not been documented.
Therefore, we undertook this study to determine whether germ-free C57BL/6 mice—a commonly investigated strain that can be bred for transgenic and knockout mouse studies—inoculated with a clinically relevant BI/NAP1 strain could be a beneficial model to study the pathogenesis of and host mucosal cytokine responses to acute CDI.
Methods. UVA13, a human-infecting C. difficile isolate that was previously cultured from a fecal specimen from a patient with CDI at the University of Virginia Hospital and typed as BI/NAP1 by the Hines Reference Laboratory at the Hines Veterans Affairs Hospital (Hines, IL) with the use of restriction endonuclease analysis and a protocol that has been described elsewhere [5], was inoculated into chopped meat broth and incubated anaerobically at 38°C for 24 h. Sixteen germ-free C57BL/6 mice (age, 7–14 months) from the National Gnotobiotic Rodent Resource Center at the University of North Carolina were orally gavaged with 330 μL of incubated broth containing a total of 1 X 108 organisms. Similarly, VPI 11186, a nontoxigenic strain of C. difficile, was incubated in broth for 24 h, and 2 germ-free mice were orally gavaged with 1 X 108 organisms. Six germ-free mice received a similar dosage of uninoculated broth and served as uninfected controls. Sterility was confirmed in germ-free mice by aerobic and anaerobic culture and Gram staining of stool samples, as outlined elsewhere [6].
Mice were monitored every 8 h after inoculation for clinical signs and symptoms of disease, including diarrhea and impaired physical condition and behavior. Weights were obtained as a measure of disease at the time of euthanasia. Mice that were determined to be moribund according to Animal Care and Use Committee policy and approved protocol were weighed and euthanized. (See Appendix 1, which appears only in the online version of the Journal .)
Cecal and colon segments were collected from euthanized mice and prepared for histological analysis by use of hematoxylin-eosin stain and antibodies directed against myeloperoxidase. Histological specimens were scored by 2 blinded investigators (S.W.P. and R.F.) on a scale of 0–3 (minimal score, 0; maximal score, 3) on the basis of each of the following criteria: inflammatory cell infiltration, mucosal hypertrophy, vascular congestion, epithelial disruption, and submucosal edema. (See Appendix 2, which appears only in the online version of the Journal .)
Cecal and colon segments were homogenized and assayed for the cytokines granulocyte colony-stimulating factor (GCSF), interleukin 1β (IL-1β), keratinocyte chemoattractant (KC), tumor necrosis factor α (TNF-α), interferon γ (IFN-γ), monocyte chemotactic protein 1 (MCP-1), interleukin 12 p40 (IL-12p40), interleukin 12 p70 (IL-12p70), interleukin 6 (IL6), interleukin 17 (IL-17), interleukin 13 (IL-13), and interleukin 10 (IL-10) by use of bead-based Luminex immunoanalysis (Bio-Rad).
Mean differences in cecal and colon histopathological scores and cytokine levels were analyzed with SPSS software (version 17; SPSS) and evaluated using an independent Student t test. Results for which P⩽ .05 were determined to be significant.
Results. All mice given UVA13 died or were moribund (necessitating euthanasia) within 72 h, whereas mice given VPI 11186 or broth were asymptomatic. Germ-free mice administered UVA13 developed diarrhea and/or wet tail, accompanied by weight loss. Eleven (69%) of 16 UVA13-infected mice were euthanized and processed for histological and cytokine analysis (5 UVA13-infected mice died and could not be processed). Broth control mice (N= 6) and mice infected with the nontoxigenic strain (N= 2) were euthanized after 72 h, processed, and assessed for comparison of histological scores and cytokine levels.
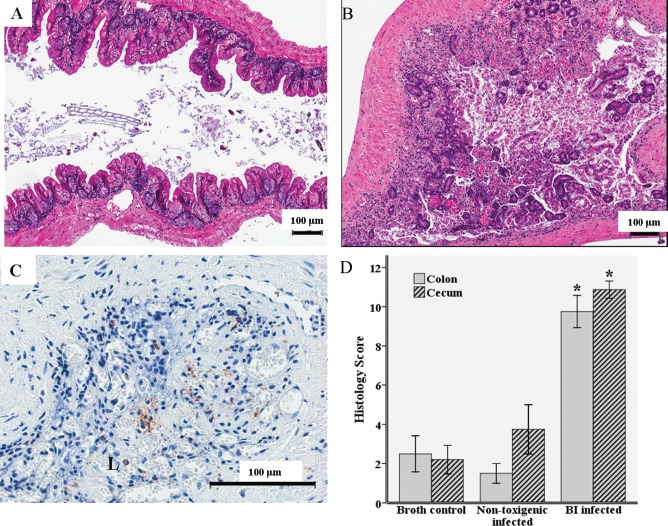
In a gross comparison, UVA13-infected mice had shorter ceca than those of controls, with evidence of purulent, hemorrhagic, or loose cecal contents with less pronounced alterations in colon segments. Histopathological scores were significantly higher in UVA13-infected mice than in control mice, with evidence of both cecal and colon ulceration, loss of mucosal architecture, epithelial exfoliation, inflammatory cell in filtration, edema, and hemorrhage in the lamina propria (Figure 1). Although neutrophilic infiltration was demonstrated with myeloperoxidase staining, it was relatively minor, and there was evidence of myeloperoxidase both within neutrophils and extravasated within the lumen (Figure 1).
Figure 1.
Histopathological analysis of cecal and colon segments 72 h after oral inoculation with 1 X 108colony-forming units of UVA13 (BI/NAP1 [BI] strain of Clostridium difficile), VPI 11186 (nontoxigenic C. difficile strain), or broth. A, Hematoxylin-eosin stain of a cecal segment from a germ-free mouse infected with the nontoxigenic strain. B, Hematoxylin-eosin stain of a cecal segment from a germ-free mouse infected with the BI strain. C, Myeloperoxidase stain of a cecal segment from a germ-free mouse infected with the BI strain (brown indicates myeloperoxidase activity). D, Histopathological differences in the cecum and colon between BI-infected mice (N = 11), mice infected with the nontoxigenic strain (N = 2), and broth controls (N = 6) as determined by inflammatory cell infiltration, mucosal hypertrophy, vascular congestion, epithelial disruption, and submucosal edema, with a maximum score of 15. *P ⩽ .001 for BI-infected mice compared with broth controls. L, lumen.
We next examined cecal and colon segments to determine mucosal cytokine concentrations produced after infection. UVA13 infection led to elevated cecal and colonic concentrations of KC, MCP-1, IL-1β, and G-CSF compared with those in controls; concentrations were greater in the cecum than in the colon (Figure 2). Additionally, BI/NAP1 infection led to lower levels of cecal and colonic IL-12p40, IL-12p70, IFN-γ, and IL-10 (Figure 2). There were no differences in the concentrations of the pro-inflammatory cytokines TNF-α, IL-6, IL-13, and IL-17.
Figure 2.
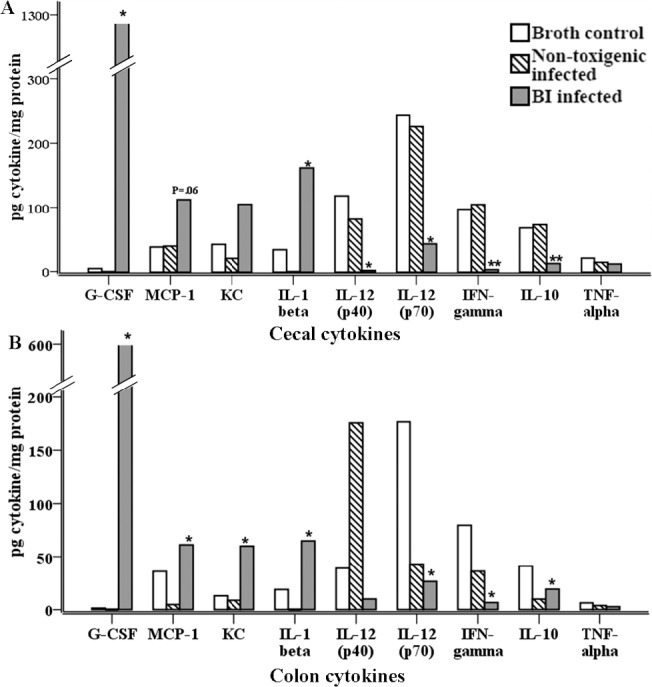
Cytokine concentrations in the cecum and colon 72 h after oral inoculation with 1 X 108 colony-forming units of UVA13 (BI/NAP1 [BI] strain of Clostridium difficile), VPI 11186 (nontoxigenic strain), or broth. Cecal (A) and colon (B) segments were homogenized in a proteinase inhibitor cocktail and assayed for the cytokines granulocyte colony-stimulating factor (G-CSF), monocyte chemotactic protein 1 (MCP-1), keratinocyte chemoattractant (KC), interleukin 1β (IL-1β), interleukin 12 p40 (IL-12p40), interleukin 12 p70 (IL-12p70), interferon γ (IFN-γ), interleukin 10 (IL-10), tumor necrosis factor α (TNF-α), interleukin 6 (IL-6), interleukin 17 (IL-17), and interleukin 13 (IL-13) by use of Luminex immunoanalysis (Bio-Rad). There were no significant differences in IL-6, IL-17, or IL-13 concentrations between the groups (data not shown). * P⩾ .05 for BI-infected mice (N = 11) compared with broth controls (N = 6); ** P ⩽ .001 for BI-infected mice (N = 11) compared with broth controls (N = 6).
Discussion. The results of this study indicate that acute infection with a clinically relevant BI/NAP1 C. difficile strain leads to weight loss, symptomatic disease, cecal and colonic ulceration, a relatively minor neutrophil infiltration, a measurable cytokine response, and eventual death in monoassociated C57BL/6 mice. The clinical and histopathological findings are in agreement with those of Vernet and colleagues [4], who previously observed that germ-free C3H/He mice infected with C. difficile that secreted elevated levels of toxin A in vitro developed cecal and colon ulceration and 100% mortality. The findings of other studies demonstrate that the BI/NAP1 strain hyperproduces toxins A and B in vitro, and those of additional studies, as well as of work in our laboratory, indicate that VPI 10463, which was used in the majority of previous studies involving CDI in germ-free mice, produces even greater levels of toxin A in vitro compared with UVA13 [2].
Previous studies of the host cytokine response to toxins A and B have been limited to data from cell culture and small intestinal loops directly exposed to purified toxins A or B, and those studies have shown a predominant inflammatory response to toxin exposure. Binding of the toxins to their respective receptor leads to induction of mitogen-activated protein kinase, translocation of nuclear factor κB into the nucleus, inhibition of the Rho family of proteins, and activation of submucosal neurons, all of which have the effect of activation of pro-inflammatory cytokines, recruitment of neutrophils, epithelial cell apoptosis, and disruption of tight junctions. Not only does toxin exposure lead to the secretion of some pro-inflammatory cytokines, it may also lead to decreased levels of protective cytokines such as transforming growth factor β (TGF-β) [7]. We demonstrated increased production of many, but not all, proinflammatory cytokines produced by innate immune cells and epithelial cells, as well as decreased mucosal production of IL-10, another protective cytokine. We did not measure TGF-β concentrations.
The elevations of IL-1β, KC (human interleukin 8 [IL-8] equivalent), and MCP-1 in our study of BI/NAP1 CDI are in agreement with the findings of cell culture and murine intestinal loop studies that show elevations in the same cytokines in response to toxin exposure. Our results are also in agreement with cytokine data in studies of CDI in humans, which show elevated fecal IL-8 levels in patients with clinically severe CDI [8]. Similarly, decreased levels of IL-10, which are normally thought to play a role in maintaining the host barrier defense and suppressing inflammation, in our BI/NAP1-infected mice are in agreement with our preliminary data from rabbit intestinal loops showing decreased IL-10 levels after toxin A exposure (C.A.W., oral communication, April 2010). These similarities of cytotoxic profiles further validate the clinical relevance of our model.
Interestingly, there was no difference in the pro-inflammatory cytokine TNF-α, which has previously been shown to be increased in toxin A enteritis [9]. In addition, the significantly lower levels of IFN-γ in our severely infected mice are in contrast to those found in studies of small intestinal loops exposed to purified toxin A by Ishida and colleagues [9], who hypothesized that IFN-γ is the crucial mediator of toxin-induced enteritis after noting attenuated disease in IFN-γ knockout mice. The interrelationship among cytokines likely explains the low levels of TNF-α, as IFN-γ is partially responsible for TNF-α expression in murine intestinal loops exposed to toxin A [9]. Additionally, IL-12, which was significantly lower in our BI/ NAP1-infected mice, has been shown to induce IFN-γ expression in macrophages, T cells, and intestinal loops and therefore may be partially responsible for the low levels of IFN-γ found [9–11].
The differences in cytokine expression between our model of CDI and studies involving pure toxin exposure of cell culture and intestinal loops may in part be due to a lack of direct host-pathogen interaction in the latter models. Toxin A-negative, toxin B-positive C. difficile strains lead to severe human CDI despite the limited pathogenesis observed when purified toxin B is inoculated into murine, hamster, and rabbit small intestinal loops [5]. On the other hand, purified toxin A within murine, hamster, and rabbit small intestinal loops leads to severe disease. Further lending credence to the importance of the pathogen-host interaction is a recent study indicating that toxin B is responsible for pathogenesis as opposed to toxin A. In this study, Lyras and colleagues [12] found that hamsters exposed to a toxin A-positive, toxin B-negative C. difficile showed no evidence of disease, whereas disease was observed in hamsters exposed to a toxin A-negative, toxin B-positive variant. Additionally, C. difficile surface layer proteins lead to pro-inflammatory IL-1β and IL-6 secretion, which highlights the importance of host-pathogen recognition and signaling and the interaction of the toxin and the C. difficile -derived components [13].
While we were performing our germ-free studies, Chen and colleagues [14] discovered a murine model of CDI using conventional C57BL/6 mice, which leads to ulcerative cecitis, colitis, and a moribund state after exposure to 6 antibiotics and VPI 10463, whereas a BI/NAP1 strain given in a similar manner leads to symptomatic disease and cecal and colon pathogenesis, but no mortality. Histological analysis of the cecum and colon of BI/NAP1-infected mice in the study by Chen and colleagues [14] demonstrated subcutaneous edema and neutrophilic infiltration but little epithelial disruption, whereas mice given VPI 10463 showed destruction of the host architecture in addition to edema and neutrophil margination, indicating that epithelial disruption is necessary to induce mortality, which is similar to the results found for gnotobiotic mice [4, 14]. Why the BI/ NAP1 strain was able to induce epithelial destruction and death in our monoassociated mice, as opposed to in the conventional mice in the study by Chen and colleagues [14], is unknown, but possibilities include the greater number of organisms used to colonize our mice (1 X 108 vs 1 X 105 colony-forming units), age-related differences in the host immune response, less developed mucosal responses in germ-free mice, or an influence by the retained host microbiota after antibiotics [15]. While microbiota analysis was not performed on fecal pellets before or after exposure to antibiotics, one can hypothesize that exposure to 6 different antibiotics led to decreased normal bacterial concentrations and altered composition, although antibiotics do not induce a sterile state.
We have thus shown that BI/NAP1 CDI in an aged germ-free C57BL/6 mouse leads to symptomatic disease, a moribund state, and profound histopathology in the cecum and colon. Additionally, as a result of CDI, certain cecal and colonic cytokines are produced, which had not previously been documented. Differences in cytokine production between in vivo infection and small intestinal loop studies or in vitro toxin studies highlight the importance of the host-pathogen relationship in CDI. Use of this model in concurrence with other newly established conventional C57BL/6 mouse models will lead to a greater understanding of the host-pathogen interaction and the role of the host microbiota in CDI.
Acknowledgment
We thank Maureen Bower for her assistance with gnotobiotic animal work and Lane Smith for his assistance with images.
Footnotes
Potential conflicts of interest: none reported.
Presented in part: 47th Annual Meeting of the Infectious Diseases Society of America, Philadelphia, PA, 29 October-1 November 2009 (oral presentation).
Financial support: National Institute of Allergy and Infectious Diseases, National Institutes of Health (grants 5T32 AI 007046–33, UO1 AI075526, P40 RR018603, and P30 DK34987); Crohn's&Colitis Foundation of America.
References
- 1.Zilberberg MD, Shorr AF, Kollef MH. Increase in adult Clostridium difficile -related hospitalizations and case-fatality rate, United States, 2000–2005. Emerg Infect Dis. 2008;14(6):929–931. doi: 10.3201/eid1406.071447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McDonald LC, Killgore GE, Thompson A, et al. An epidemic, toxin gene-variant strain of Clostridium difficile. N Engl J Med. 2005;353(23):2433–2441. doi: 10.1056/NEJMoa051590. [DOI] [PubMed] [Google Scholar]
- 3.Corthier G, Muller MC, Wilkins TD, Lyerly D, L'Haridon R. Protection against experimental pseudomembranous colitis in gnotobiotic mice by use of monoclonal antibodies against Clostridium difficile toxin A. Infect Immun. 1991;59(3):1192–1195. doi: 10.1128/iai.59.3.1192-1195.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vernet A, Corthier G, Dubos-Ramare F, Parodi AL. Relationship between levels of Clostridium difficile toxin A and toxin B and cecal lesions in gnotobiotic mice. Infect Immun. 1989;57(7):2123–2127. doi: 10.1128/iai.57.7.2123-2127.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sambol SP, Merrigan MM, Lyerly D, Gerding DN, Johnson S. Toxin gene analysis of a variant strain of Clostridium difficile that causes human clinical disease. Infect Immun. 2000;68(10):5480–5487. doi: 10.1128/iai.68.10.5480-5487.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim SC, Tonkonogy SL, Albright CA, et al. Variable phenotypes of enterocolitis in interleukin 10-deficient mice monoassociated with two different commensal bacteria. Gastroenterology. 2005;128(4):891–906. doi: 10.1053/j.gastro.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 7.Johal SS, Solomon K, Dodson S, Borriello SP, Mahida YR. Differential effects of varying concentrations of Clostridium difficile toxin A on epithelial barrier function and expression of cytokines. J Infect Dis. 2004;189(11):2110–2119. doi: 10.1086/386287. [DOI] [PubMed] [Google Scholar]
- 8.Steiner TS, Flores CA, Pizarro TT, Guerrant RL. Fecal lactoferrin, interleukin-1b, and interleukin-8 are elevated in patients with severe Clostridium difficile colitis. Clin Diagn Lab Immunol. 1997;4(6):719–722. doi: 10.1128/cdli.4.6.719-722.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ishida Y, Maegawa T, Kondo T, et al. Essential involvement of IFN-g in Clostridium difficile toxin A-induced enteritis. J Immunol. 2004;172(5):3018–3025. doi: 10.4049/jimmunol.172.5.3018. [DOI] [PubMed] [Google Scholar]
- 10.uddu P, Fantuzzi L, Borghi P, et al. IL-12 induces IFN-g expression and secretion in mouse peritoneal macrophages. J Immunol. 1997;159(7):3490–3497. [PubMed] [Google Scholar]
- 11.Way SS, Havenar-Daughton C, Kolumam GA, Orgun NN, Murali-rishna K. IL-12 and type-I IFN synergize for IFN-g production by CD4 T cells, whereas neither are required for IFN-g production by CD8 T cells after Listeria monocytogenes infection. J Immunol. 2007;178(7):4498–4505. doi: 10.4049/jimmunol.178.7.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lyras D, O'Connor JR, Howarth PM, et al. Toxin B is essential for virulence of Clostridium difficile. Nature. 2009;458(7242):1176–1179. doi: 10.1038/nature07822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ausiello CM, Cerquetti M, Fedele G, et al. Surface layer proteins from Clostridium difficile induce inflammatory and regulatory cytokines in human monocytes and dendritic cells. Microbes Infect. 2006;8(11):2640–2646. doi: 10.1016/j.micinf.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 14.Chen X, atchar K, Goldsmith JD, et al. A mouse model of Clostridium difficile -associated disease. Gastroenterology. 2008;135(6):1984–1992. doi: 10.1053/j.gastro.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 15.Umesaki Y, Setoyama H. Structure of the intestinal flora responsible for development of the gut immune system in a rodent model. Microbes Infect. 2000;2(11):1343–1351. doi: 10.1016/s1286-4579(00)01288-0. [DOI] [PubMed] [Google Scholar]