Abstract
Exploratory behaviors during learning determine what is studied and when, helping to optimize subsequent memory performance. We manipulated how much control subjects had over the position of a moving window through which they studied objects and their locations, in order to elucidate the cognitive and neural determinants of exploratory behaviors. Our behavioral, neuropsychological, and neuroimaging data indicate volitional control benefits memory performance, and is linked to a brain network centered on the hippocampus. Increases in correlated activity between the hippocampus and other areas were associated with specific aspects of memory, suggesting that volitional control optimizes interactions among specialized neural systems via the hippocampus. Memory is therefore an active process intrinsically linked to behavior. Furthermore, brain structures typically seen as passive participants in memory encoding (e.g., the hippocampus) are actually part of an active network that controls behavior dynamically as it unfolds.
Keywords: active learning, spatial memory, recognition memory, visual learning, brain-network dynamics, hippocampus, exploratory behavior, amnesia
Influential theories of human development, perception, and action all emphasize the crucial role of an individual's control over what he does, how and when1-4. Successful “active learning” educational practices5 emphasize the importance of the individual's control over learning. We see the positive effects of such control in everyday life, when we experience the difficulty of extracting information from a website when someone else is controlling the scrollbar, when it is difficult to learn a route as a car passenger rather than the driver, etc. Yet, human learning and memory are predominantly studied using experimental paradigms in which the participant is more a passive recipient of information than an active learner.
Several empirical findings hint at the importance of active learning for memory, and suggest relevant cognitive and neural mechanisms. For instance, introspective evaluations of how well particular paper-and-pencil test items have been committed to memory are useful in allocating additional attention to the material that will benefit most from it6. However, introspection is not necessarily crucial, as active control can potentially interact with learning and memory automatically, without the need for overt decision-making. For instance, changes in the focus of attention occur dynamically during ongoing visual exploration of the environment, with memory interacting with attentional systems to determine the information that is attended versus ignored7. Because memory encoding and visual attention are of limited bandwidth8, it seems that attentional systems must interact with memory processing to optimize learning as part of online exploratory control9. This conjecture draws support from many findings showing that brain regions involved in encoding include not only historically memory-linked medial temporal lobe structures such as the hippocampus10, 11, but also frontal cortical regions associated with online manipulation of material and strategic planning/prediction12-15, and parietal cortical structures associated with the control of visual attention16, 17. However, the interactions among these systems in the online control of behaviors contributing to learning are little understood.
Here we used a dynamic visual learning task to study the effects of moment-to-moment active control over visual exploration on learning efficacy and to uncover the supporting brain processes. We devised a paradigm for manipulating the extent of control that subjects had over their visual input, inspired by the pioneering studies of Held and Hein18. In these previous studies, pairs of juvenile cats moved around a circular environment with movement controlled by only one member of the pair. The other cat passively rode in a gondola that was physically “yoked” to the first, providing similar visual stimulation to both animals, but differing completely in the ability to control the input. Animals who had control over their movement showed benefits in the development of learned visuomotor behaviors18.
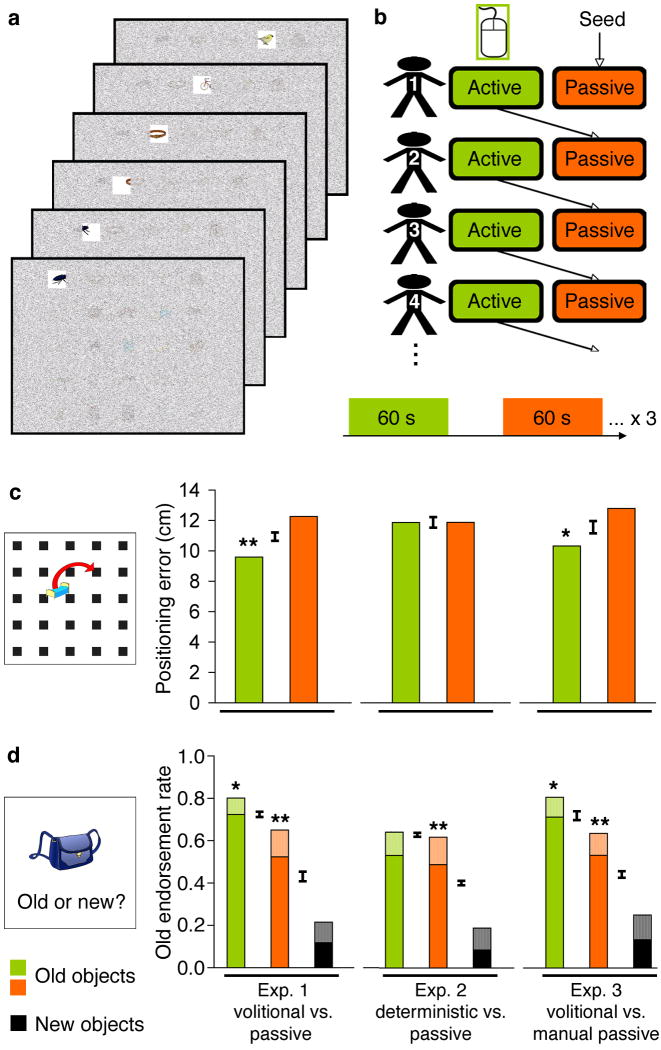
In the studies reported here, adult humans studied arrays of common objects arranged on a grid, viewing one object at a time through a small moving window (Fig. 1A). Each subject participated in two viewing conditions, one with self-initiated active control of window position using a computer mouse or joystick and the other a passive condition. The self-controlled, active movements of one subject were recorded and played back as the passive condition for the next subject (Fig. 1B), such that the visual information displayed during the active condition for subject n was the same as that displayed during the passive condition for subject n+1. Visual stimulation for active versus passive learning was therefore matched via the combination of “yoking” the window movements in the two viewing conditions across pairs of subjects and the precise control of viewing location provided by the window. In this way, subjects viewed exactly the same visual information in the same order for the same durations in both conditions; any observed differences in performance outcome could thus be attributed to the effects of active control (relative to passive viewing).
Figure 1. Volitional control enhances spatial and object-specific memory.
Objects were viewed through a moving window, shown here (a) moving rightward to uncover the topmost row of objects in a 5×5 grid. Window position was under continuous active control for half of the object arrays and was delivered passively for the other half (b). Passive positions were determined by the active control of the previous experimental subject, so that object viewing was matched across conditions. Results are shown for the spatial recall memory test (c) and for the object recognition memory test (d) for Exps. 1-3. All experiments involved self-controlled (active) and passive viewing, with the task requirements for each condition varied across experiments as indicated in the text. High-confidence responses for the object recognition test are indicated with solid bars and low-confidence responses with dithered bars. The false-alarm rate to new items is also provided for high and low confidence levels. Error bars indicate the standard error of the difference between conditions represented by adjacent bars, and therefore correspond to the within-subjects statistical tests employed. *P<0.05 versus the right-adjacent condition. **P<0.01 versus the right-adjacent condition.
We conjectured that this manipulation would allow us to identify the neural processes that support the online control of visual exploration and learning. Based on the considerations discussed above, we predicted that online control would require interaction of brain structures traditionally associated with memory with those traditionally associated with directing attention and strategic planning/prediction, and that damage to memory-linked structures would thus disrupt benefits associated with control. We first sought to characterize the relevant phenomena in a series of behavioral experiments, and then identified relevant neurophysiological substrates via both neuropsychological assessment of individuals with brain lesions and functional neuroimaging of brain activity in healthy individuals.
Results
Behavioral performance
The active condition for subjects in Exp. 1 (N=20) was a volitional control condition, in which they were able to move the window during self-controlled viewing without constraints placed on their viewing patterns, just with instructions to memorize both the objects and the object locations. Tested after studying 150 objects, half under volitional control and half in a passive condition, subjects showed superior performance for information acquired in the volitional condition both on tests for spatial memory and for item (object) memory. In the test for spatial recall of object location, subjects were better able to place volitionally studied objects in their original locations: mean distance error was significantly less for volitionally studied vs passively studied objects (Fig. 1C), with a mean decrease in error of 22% (P<0.01). Furthermore, significantly more volitionally studied objects were placed in precisely the correct location relative to passively studied objects (28% versus 19%, respectively, P=0.01). In the test for object recognition memory, involving “old” vs “new” decisions, recognition was significantly better than chance, as estimated by the false-alarm rate to new objects, for both volitionally studied and passively studied objects (Fig. 1D). But, item recognition was significantly better for the volitional control vs the passive condition (mean difference = 23%; P=0.02; Fig. 1D). These effects provide striking evidence for the power of volitional control in enhancing subsequent memory performance, even when the very same visual information was viewed for the same amount of time and in the same order in the volitional condition as in the passive condition.
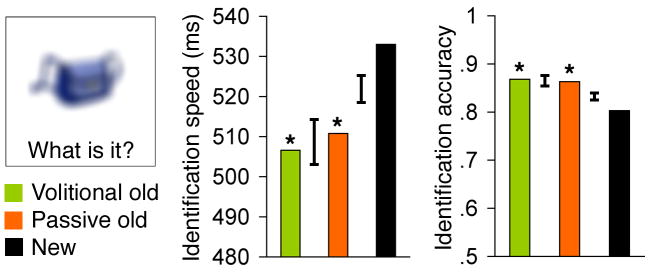
In order to rule out the possibility that the beneficial effects of volitional control of viewing that we observed here were not on memory per se but rather merely a byproduct of effects on perception19, we investigated whether volitionally controlled viewing in our experiment actually led to superior perceptual processing for the studied objects. Speed and accuracy of perceptual identification during a perceptual priming test were superior for objects that had been studied than for new objects (Fig. 2). Critically, however, this effect of prior exposure was no different for objects studied volitionally vs. passively. Thus, volitional control of viewing did not enhance perceptual processing.
Figure 2. No perceptual benefit due to volitional control.
Mean identification speed and accuracy are provided for the perceptual identification priming test, in which degraded viewing conditions rendered identification difficult. Error bars indicate the within-subjects standard error of the difference between conditions represented by adjacent bars. Differences between old objects studied volitionally and studied passively were unreliable (P>0.65). *P<0.05 for old relative to new objects.
We also investigated whether effects of volition could be attributed to factors related just to manual control, required for manipulating window position in the volitional but not the passive condition. In Exp. 2 (N=20) the volitional condition was replaced with an active condition that required manual control, but with constraints on the order and duration of viewing. Manual control of viewing was thus deterministic here rather than volitional, because, although subjects actively moved the window, they did not control what to look at or when. Deterministic viewing did not benefit memory relative to passive viewing on either the spatial recall or object recognition test (Fig. 1CD), suggesting that it was volitional control (rather than manual control) that was responsible for the volitional memory benefit in Exp 1.
Another control condition for influences from manual factors was provided in Exp. 3 (in which neuroimaging data were also collected, as reported below). The active condition was volitional, as in Exp. 1, but the passive condition was modified such that manual responses were required but did not actually control the movements of the viewing window. In this condition, each subject moved a joystick in an effort to mimic the window movements, which, as in the preceding experiments, were “yoked” to the volitionally controlled movements made by the previous subject. Again, beneficial effects of volitional control over this comparison condition were seen on memory performance, of similar magnitude to those observed in Exp. 1, for both spatial recall and object recognition tests (Fig. 1CD), thus indicating that volitional benefits vs. passive study are observed even when both conditions involve motor activity.
To summarize the behavioral findings of Exp. 1-3, volitional study benefited memory relative to passive study, and these effects could not be attributed either to motor control per se or to facilitated perception. Instead, they were associated with unconstrained visual exploration controlled by the individual (i.e., volitional control). Note that the overall level of performance for studied objects did not differ across all the various passive conditions (all P's > 0.3). Moreover, the benefit gained from volitional control over passive viewing did not differ for Exp. 1 versus Exp. 3 for either spatial or object memory tests (P's > 0.75). The consistency of the benefits of volitional control on subsequent memory performance despite the differences among experimental protocols is very striking.
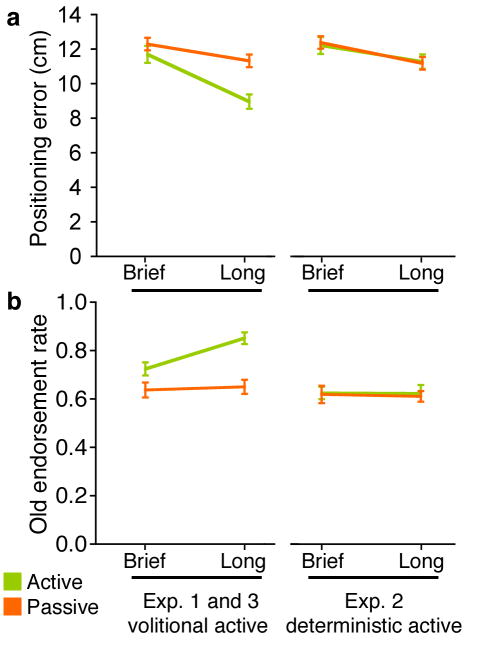
One further analysis of the accuracy of memory for each object as a function of the total duration for which it was viewed during study provides evidence of the causal role of volitional control in memory benefits. Separate analyses were run for objects tested for spatial recall versus item recognition. A median-split on study viewing duration for the remaining objects was calculated, forming “brief” and “long” study categories, separately for active and passive conditions. Data from Exp. 1 and 3 were pooled because of similar accuracy levels in each experiment (see above).
Fig. 3A shows that longer viewing duration led to disproportionately lower error for the volitional relative to the passive objects in the spatial recall test (brief/long-by-volitional/passive interaction [F(1,35)=9.8, P=0.004]). This disproportionate benefit was not observed for deterministic versus passive objects in Exp. 2 [F(1,19)=0.02; P=0.89], nor was there an overall benefit for deterministic versus passive [F(1,19)=0.12; P=0.73]. Likewise, in the object recognition test (Fig. 3B) there was disproportionately higher hit rates with longer viewing for volitional versus passive study [brief/long-by-volitional/passive interaction F(1,25)=8.0, P=0.009], but not for deterministic versus passive study [F(1,19)=0.01; P=0.93]. Unlike effects on spatial recall, effects of longer viewing in isolation [F(1,25)=40.0, P<0.001] on object recognition appeared to be driven entirely by the interaction effect concerning volitional objects; that is, brief versus long did not differ for passive objects (P=0.72). Taken altogether, the findings that volitional viewing led to disproportionate benefits to both spatial and item memory performance, and that an object selected for more study under volitional control was subject to superadditive memory benefits relative to items selected for longer study under passive or deterministic control conditions, validate the causal role of volitional control in producing memory benefits.
Figure 3. Volitional control caused disproportionate memory enhancement with increasing study durations.
Spatial positioning error (a) and old hit rates (b) are provided as a function of how long an object was studied (brief versus long based on median split). Average duration ranges for each condition are provided in Supplementary Table 1. Error bars indicate standard error.
Volitional control in hippocampal amnesia patients
We used two complementary methods to identify the neural bases for the enhancing effects of volitional control on subsequent memory performance: (1) evaluating the necessity of an intact hippocampal system for producing these effects, by testing amnesic patients with hippocampal lesions (Exp. 4), and (2) assessing neural activity associated with these effects in healthy individuals, using functional magnetic resonance imaging (fMRI) (Exp 3).
Recordings from hippocampal neurons in rodents engaged in exploratory behavior reliably reveal place-specific activity20. Interestingly, such activity can be modulated based on the potential for self-controlled exploration21, 22, with active exploration producing independent and higher-fidelity spatial representations relative to passive exploration22. These rodent data suggest a role for the hippocampus, not just in the formation of new memory10, 23, but possibly also in the beneficial effects of volitional control on memory.
In Exp. 4, effects of volitional control were assessed in three amnesic patients with severe hippocampal damage, such that residual hippocampal volume in each patient was more than two Z-scores lower than the mean volume for a comparison population24, 25 (Table S2). Neuropsychological examination confirmed severe memory impairment in each patient (see Methods), and none of the patients showed any systematic impairment in standard neuropsychological tests of executive function, suggesting frontal cortex integrity25.
Hippocampal damage has been shown unequivocally to disrupt expressions of long-term memory for episodes, and we therefore safely predicted that memory would be disrupted overall in amnesic patients. The primary question of interest was therefore how hippocampal damage would affect the memory benefits associated with volitional control. It is conceivable that volitional benefits derive primarily from strategic planning and executive control processes mediated by prefrontal cortex, and that amnesic patients would thus show intact volitional benefits due to the integrity of their prefrontal cortex and executive processes. However, if volitional benefits require interactions between the hippocampus and other structures, then the amnesic patients would be expected to show impairments in the benefits gained from volitional control. The paradigm was modified for amnesic patients in order to assure subsequent memory performance above chance levels, including the use of several smaller object grid sizes (2×2, 3×3, and 4×4), and brief retention intervals (see Methods). Three comparison subjects with no known brain damage, but otherwise matched to amnesic patients, also participated (see Methods).
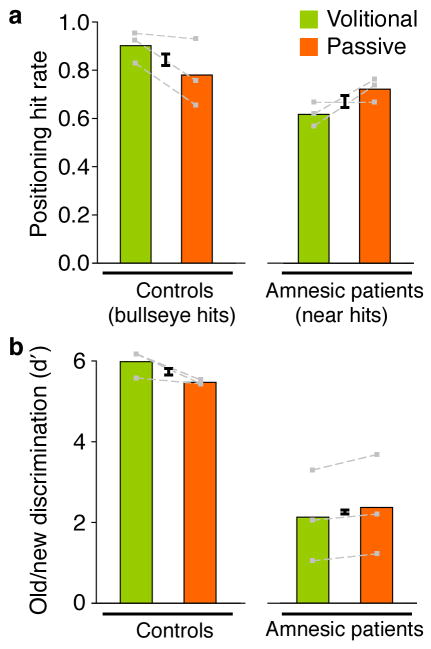
Performance averaged across all object grid sizes is shown in Fig. 4 for amnesic patients and comparisons. Comparison subjects performed at near-ceiling levels, but nonetheless each comparison showed higher performance for volitional relative to passive for both spatial recall and object recognition tests. Amnesic patients showed impaired performance on both spatial and item memory tests, as expected. Critically, amnesic patients failed to derive any benefits from volitional study. In fact, they tended to show poorer memory performance for volitionally studied than for passively studied objects, on both the spatial recall test (2 of 3 patients) and the object recognition test (3 of 3 patients). The qualitative difference between comparison subjects and amnesic patients in the effects of volitional vs. passive study on memory performance was confirmed by reliable interactions between group and study condition for both the spatial recall test [F(1,4)=9.8; P=0.03] and the object recognition test [F(1,4)=14.2; P=0.02]. Likewise, comparison subjects showed significantly more of an advantage on their subsequent memory for volitionally studied than passively studied objects than did amnesic patients on the spatial recall test [t(4)=3.1, P=0.04] and the object recognition test [t(4)=3.7, P=0.02]. Accordingly, these findings suggest that the hippocampus may be necessary for the beneficial effects of volitional study.
Figure 4. No volitional benefits to memory performance in hippocampal amnesia.
Performance on the spatial recall test (a) is quantified for comparison subjects as the proportion of objects placed in precisely the correct location (“bullseye hits”). Performance in amnesic subjects is quantified as the proportion of objects placed within a perimeter of one-object-length from the correct location (“near hits”), given that bullseye hits were at floor levels in patients (0.11 volitional and 0.12 passive). Performance on the object recognition test (b) is quantified as discrimination sensitivity (d′), a normalized measure of hits minus false alarms. Error bars indicate the standard error of the difference between conditions represented by adjacent bars, and therefore correspond to within-subjects volitional/passive difference error. Performance values for each subject are indicated by gray dots connected by gray lines. Performance for each grid size and subject is shown in Table S2.
Functional brain imaging
fMRI was used to assess neural activity throughout the brain in young, healthy subjects during volitional and passive study in Exp. 3 (N=16; behavioral data described above). Because the passive condition included manual responses (that did not actually control the movements of the viewing window, see above), neural activity related to hand movements was approximately matched across viewing conditions. Note that performance did not suffer due to the manual responses required in the passive condition, as passive performance was matched with that in Exps. 1 and 2, which did not involve passive manual responses (see above and Fig. 1).
Given the findings from Exp. 4 demonstrating the necessary role for of the hippocampus in producing the beneficial effects of volitional control on subsequent memory performance, as well as support from the rodent literature implicating the hippocampus21, 22, our primary fMRI analysis strategy sought to characterize how the hippocampus interacts with the rest of the brain in volitional vs. passive learning conditions. What are the brain networks involving the hippocampus that are specifically associated with volitional control and its effects on subsequent memory performance?
The hippocampus was defined anatomically in each subject bilaterally (including hippocampus proper, CA1, CA3, dentate gyrus, and subiculum). Importantly, the activity in the hippocampus defined in this manner was significantly greater during volitional study than during passive study, as determined via a standard univariate contrast [1.7% difference in signal, t(15)=4.0; P=0.001]. We then assessed functional connectivity of the hippocampus with other brain regions26 using a standard approach whereby correlations between activation of the hippocampus and activations in all other brain regions were assessed as a function of viewing condition. A network of structures, shown in Fig. 5, showed more highly correlated activity with the hippocampus in the volitionally controlled viewing condition than in the passive viewing condition. We also performed a standard univariate contrast (non-connectivity) between these conditions, as described in Suppl. Fig. 1.
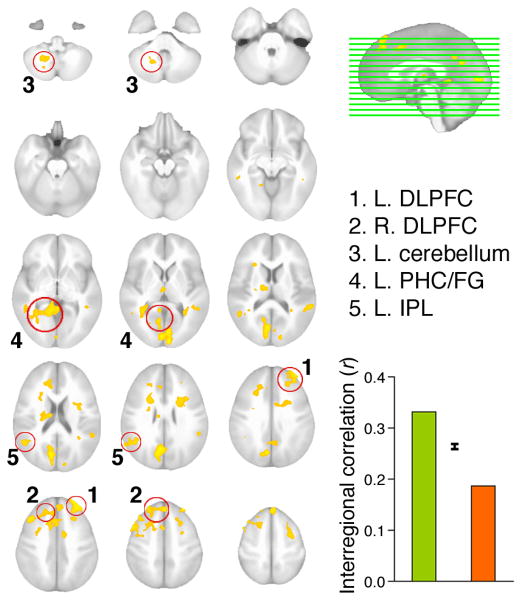
Figure 5. A coordinated brain network for volitional control.
Regions that exhibited significantly greater correlated activity with the hippocampus for the volitional condition than for the passive condition are shown overlaid on anatomical MRI images in the transverse plane. Positions of transverse slices are indicated by horizontal lines on the sagittal midline image. Five structures are highlighted for which activity predicted different aspects of memory performance (Supplementary Figure 2). The correlation with the hippocampus averaged across all identified regions is shown for the volitional (green) and passive (orange) conditions, with error bars indicating the standard error of the within-subjects volitional/passive difference. All regions are described in Supplementary Table 3.
Volitional control was associated with enhanced coordination of the activity of the hippocampus with bilateral dorsolateral and medial prefrontal cortex, left ventrolateral parietal cortex, and left cerebellum. These structures have been implicated in executive and attentional control during memory tasks12, 14, 17, 27, 28, and prefrontal and parietal cortex regions that overlap those identified here have been associated with static, introspective judgments about the success of learning29. Furthermore, the prefrontal, parietal, and cerebellar regions identified here have been identified elsewhere as part of an executive “default” network that exhibits intrinsically correlated activity in the absence of sensory stimulation30, 31. Collectively, these structures comprise a volitional control network, and the coordination of this network is seen to be associated with optimized learning as evidenced by later memory (Fig. 1CD).
We then used brain activity to determine the nature of the volitional network's contributions to learning. The neuroanatomically discrete elements of the volitional network defined based on their coordinated activity could conceivably support a unitary volitional control process, with each element affecting memory in roughly the same way. Alternatively, different elements of the network may support distinct processes whose effects on memory result from the coordination of these different processes. We reasoned that if the latter were true, then the volitional network could be fractionated based on selective associations with different aspects of learning and memory. We therefore used activity within each discrete element of the volitional network (Fig. 5) to predict performance on the spatial vs object memory tests.
Enhancements in correlation between activity of the hippocampus, bilateral dorsolateral prefrontal cortex (DLPFC), and cerebellum that were due to volitional control were associated with benefits to spatial accuracy during the subsequent spatial recall test (R2adj=0.66, P=0.001; Supp. Fig. 2). This relationship is consistent with evidence that spatial memory requires interactions between prefrontal cortex and hippocampus32 via their dense, reciprocal anatomical connections33, and that fronto-cerebellar circuits participate in strategic planning/control that might benefit the organization of spatial memories28, 30, 31.
A different set of regions was identified in association with benefits for object recognition memory, including increases in correlation due to volitional control between activity of the hippocampus, ventral lateral parietal cortex (LPC), and posterior parahippocampal cortex (PHC), including anteromedial fusiform gyrus (FG) (R2adj=0.53, P=0.003; Supp. Fig. 2). Interestingly, other evidence implicates ventral LPC in the direction of attention to salient stimulus features16, 17. Furthermore, PHC supports spatial navigation and processing of elements in scenes34, and FG activity is sensitive to categories of visual objects35. These results suggest an interaction between spatial and object-specific information processing in the direction of attention to stimulus features critical for item-memory encoding. Thus, structures within the volitional network could be selectively associated with discrete aspects of subsequent memory performance.
Discussion
We studied learning and memory in the context of online control of visual exploration, which, to our knowledge, has not been previously attempted in human neuroscience experiments. The results provide new insights into a fundamental determinant of memory that we operationally define as volitional control. Volitional control was expressed when subjects were allowed to select the specific information for study on a moment-by-moment basis, which provided substantial benefits to later memory performance relative to passively taking in the very same information. Volitional control is not just “active” control; actively controlling the viewing window along a pre-determined route (“deterministic” control) did nothing to aid memory relative to passive viewing of the same information (Exp. 2). The key ingredient in volitional control's benefit to memory performance was that the directing of the where and when of exploratory behaviors was controlled completely by the individual.
Note that no claims are being made here about the necessity of any particular phenomenological states during volitional control for obtaining the observed memory benefits (i.e., “feelings” of volition, “meta-memory” monitoring6, etc.). Other goal-directed behaviors that appear “purposive” have similarly been studied in nonhuman animals without phenomenological presumptions36. Indeed, “meta-memory” phenomena in humans have analogs in nonhuman animal behavior37, raising the possibility that “meta-memory” feelings in humans are epiphenomenal to the act of exerting control and benefiting from it. In the current work, subjects showed no systematic awareness of the benefits of volitional control on memory upon debriefing (χ2=0.05, P=0.82; see Methods). In contrast, all subjects reported higher difficulty for the spatial recall test than the object recognition test. This result is counter to expectation if the volitional benefits were due to ongoing overt meta-memory judgments, as subjects typically report feeling that these judgments are helpful6, and is instead consistent with recent evidence for implicit/unconscious determinants of complex, goal-oriented behaviors that appear to involve overt choices.38
On the contrary, the data support the notion that volitional control is an omnipresent determinant of exploratory behaviors that occur whenever an organism is unconstrained in interactions with the environment. Importantly, interaction with the environment occurred during the passive learning conditions in all experiments as well as during “deterministic” active control (Exp. 2), but these interactions were not volitionally controlled, and as a result they did not benefit subsequent memory performance.
A network of brain regions was identified that showed greater correlated activity with the hippocampus during volitional than passive study. Furthermore, functional subdivisions within this network were associated with benefits of volitional control on subsequent spatial versus object-specific memory (Supp. Fig. 2), conforming with the known functional roles of the individual brain regions comprising the network. This suggests that volitional control and its effects on memory can be attributed to the coordination of distributed and functionally distinct neural processes, dependent upon interaction of the involved brain structures with the hippocampus. Amnesic patients with hippocampal damage failed to show any benefits from volitional control (despite above-chance overall memory performance); if anything, volitional control was detrimental to subsequent memory performance in hippocampal amnesia.
Collectively, our neuroimaging and neuropsychological data indicate that it is the interplay between multiple cortical areas and the hippocampus that produces optimized learning with volitional control. We propose that volitional control is advantageous for learning because distinct neural systems related to planning/predicting, attention, and object processing can be updated in an iterative fashion via communication with the hippocampus, such that exploratory behaviors become more finely tuned to the most critical environmental information during the course of exploratory behavior.
The role of the hippocampus in this scheme is of particular interest. Rather than just being engaged in relational memory binding of incoming information23, 39, automatically and obligatorily, the current findings suggest that the hippocampus actually has a more active role in acquiring information, presumably involved in directing what information should be obtained next from the environment based on the information already obtained. Such a role is consistent with, and perhaps underlies, the recently hypothesized role of the hippocampus in advantageous decision-making and the planning of future actions40-42. The hypothesized role of the hippocampus in the online control of information acquisition is consistent with the extensive bidirectional connections that exist between the hippocampus and all higher order association cortices in the mammalian brain43, thus suggesting that the hippocampus may be ideally suited to integrate memory signals with strategic control/planning processes, attentional control processes, and representations of goal states44, all mediated by these association cortices. Indeed, this conjecture is supported by our functional imaging data, which show greater correlated activity between the hippocampus and these association cortices for volitional control relative to passive learning.
Additional studies will be needed to specify the moment-to-moment dynamics of the interaction of the hippocampus with the rest of the volitional control network. Such work will need to have the same emphasis on active learning in more naturalistic experiments as was employed here, rather than more typical experiments in which subjects are passive recipients of information. This strategy will also serve to bring research on the neural mechanisms of learning and memory in humans into closer correspondence with work on model organisms in which active exploration of the environment is a much more natural part of the inquiry.
Methods
In Exp. 1-3, subjects (all between the ages of 18 and 28, 59% female, recruited from the University of Illinois community) attempted to memorize six 25-object arrays, each arranged as a 5×5 grid presented on a computer monitor. Objects were common, nameable, and obtained from the set described by45. The entire display was occluded by a semi-transparent mask of Gaussian noise that permitted subjects to determine the overall arrangement of objects perifoveally, but only one object could be viewed clearly at a time through a small window. The viewing window was sized such that it could uncover one object at a time, and objects were spaced at a distance of approximately one window from edge to edge.
The position of the viewing window was under the continuous control of a computer mouse for the active-control conditions in Exps. 1-2. Control was provided by an MRI-compatible joystick in Exp. 3. Window positions for the passive conditions were determined by the movements made by the active-control condition for the previous experimental subject, except for the first subject. For the first subject in each experiment, passive condition window movements were provided by an additional subject who was not administered the passive condition or memory tests (a “seed”). Each subject completed a practice session to become accustomed to active and passive control before the experimental session.
No constraints were placed on subjects' active movements in Exp. 1 (volitional control). For Exp. 2, subjects were required to move the window in a predetermined pattern at specified intervals (deterministic control). Movements were made in a “snaking” pattern from upper left to bottom right. An auditory cue was used to indicate when the subject should move to the next object. The duration for each object was randomized from 500 ms to 3500 ms such that the distribution of viewing times approximated the distribution obtained in the volitional condition in Exp. 1. For Exp. 3, the active condition was volitional as in Exp. 1, and the passive condition was altered such that manual input was required (manual-passive condition). As in Exps. 1-2, the window movements in the passive condition were provided by the active condition of the previous experimental subject. Subjects were required to move the joystick to mimic the window movements, as though they were in control of the window. Movements were continuously recorded and visual inspection of movement patterns overlaid on window movement patterns indicated that subjects were highly accurate in mimicking window movement trajectories.
In Exps. 1-3, three 25-object arrays were viewed actively and three were viewed passively in alternating order. Each array was viewed for a total of 60 s, and a 20 s break was provided between each viewing period. Objects were counterbalanced across viewing conditions, such that the objects viewed actively for one subject were the objects viewed passively for the next subject. Thus, across all subjects, the same objects were viewed for the same durations in the active vs. passive conditions.
The viewing duration for each object was computed based on the window positions for each subject, and all objects that were viewed for less than 200 ms total during the entire study period (that is, the sum of all individual “fixations” on an object) were excluded from analyses of performance in the memory tests, such that memory performance was not effected by the inclusion of partially viewed and non-viewed objects (leading to removal of the same objects for the active and passive conditions). This same criterion was used for Exps. 1-4, and less than 1% of objects overall were excluded based on partial viewing.
Subjects were administered the spatial memory test followed by the item recognition test after all object arrays were studied. Spatial recall memory was tested for 50 randomly selected objects, half studied actively and half passively. A grid of 25 black squares was shown in the same spatial positions as where objects were studied. One object appeared at a randomly selected screen location on each test trial, and subjects used the manual input device to drag the object to the location at which it was studied. There were no time constraints, but subjects were encouraged to guess if the correct answer was not known after approximately 10 s. Spatial-positioning error was calculated as the distance between where the subject placed the object and where it was located at study.
Object recognition memory was tested for the remaining 100 objects, half of which had been studied actively and half passively. An additional set of 100 similar-format objects that did not appear during learning was used as foils. Objects appeared at the center of the screen for 1,500 ms each, with a 2,500 ms ISI. Subjects pressed one of four buttons in response to each object to discriminate old from new objects with a 4-point confidence scale (confident and unconfident for both old and new).
In Exp. 1, half of the subjects were administered a test of perceptual processing rather than a test of recognition memory. As for the recognition test, 100 old objects appeared with 100 new objects. All objects were blurred with a 15 mm FWHM Gaussian kernel and shown for 150 ms each, with a 3,850 ms ISI. Subjects were required to press a button to each object to indicate the latency at which they were able to verbally identify the object. Speed and accuracy were emphasized. Misses occurred when subjects failed to press the button, indicating the inability to identify the object. Debriefing after Exp. 1 was used to determine if subjects were aware of any influence of volitional control on their memory performance (subjects indicating “no influence”, “positive influence”, or “negative influence”). Of the 20 subjects, 17 showed better spatial memory for volitional objects, yet upon debriefing only 9 of these subjects reported feeling that volitional control aided memory. Moreover, two of the three subjects who failed to show a volitional benefit nonetheless reported feeling a benefit.
fMRI methods
Neural activity was measured during learning using fMRI in Exp. 3. Each 60 s viewing period of an object array was divided in half, with a 20 s break between each half. An additional 20 s break period was inserted before and after the periods during which arrays were viewed. Visual stimuli were displayed via MRI-compatible LCD goggles. All six object-arrays were viewed during a single functional run. Functional MR images were collected using a Siemens Allegra 3T head-only MRI system. During the learning portion of the experiment, 248 volumes were collected (TR = 2500 ms; TE = 25 ms; FOV = 22 cm; voxel size = 3.4 × 3.4 × 3.0 mm) acquired perpendicular to the long axis of the hippocampus. MR images were not collected and the learning paradigm did not begin until after the scanner reached steady-state. A structural MR image was collected after the functional run while subjects were administered the spatial test and the object recognition test (MP-RAGE T1-weighted scans, voxel size = 1.5 ×1.1 × 1.1 mm, 192 axial slices). All subjects were right-handed, and controlled the input device using the right hand (thus producing contra-lateralized, left motor-related activity, Supp. Fig. 1).
Analyses of fMRI data were accomplished via the AFNI software package46. Preprocessing steps included volume registration through time (motion correction), correction of slice-timing discrepancies, co-registration of functional and structural images, transformation to stereotactic space (MNI-305), removal of linear signal drift, and spatial smoothing of functional data with a 5 mm FWHM Gaussian kernel.
The hippocampus (hippocampus proper, CA1, CA3, dentate gyrus and subiculum) was defined bilaterally for each subject individually in his native anatomical space based on anatomical criteria47. This method was chosen for characterizing hippocampal activity because it has been shown to increase sensitivity to signal in medial temporal lobe (MTL) structures over that obtained by defining the hippocampus after stereotactic transformation, due to considerable anatomical variability in MTL structures across subjects48, 49.
The analysis of functional connectivity was performed by first averaging the timeseries of hippocampal fMRI signal spatially over the entire extent of bilateral hippocampus (although hippocampal subregions were also considered, see below). The activity timeseries in this “seed” region was linearly detrended and regressed against the signal at each non-hippocampal brain voxel. Also entered into the regression was a vector coding for blocks of active and passive viewing (boxcar function) convolved with a canonical hemodynamic response function. Nuisance variables were also entered, including the T1* and T0 components of the MR signal, as well as estimates of brain movement (X, Y, Z, roll, pitch, and yaw). Voxels within the hippocampus were masked from the regression analysis using each subject's anatomical data. Clusters were identified that showed a significant difference in correlation with the hippocampus for the volitional versus the passive condition in a second-order analysis across subjects. The voxel-wise statistical threshold was set to P<0.001 and a cluster extent threshold of 31 contiguous supra-threshold voxels was determined via Monte Carlo simulation50. The combined statistical threshold (voxel-wise and spatial-extent) was P<0.01. The analysis was performed such that both positive and negative differences in correlation between volitional and passive conditions could be identified, but only positive differences were found. The clusters that were identified are described in Supp. Table 3.
We also defined the anterior and posterior extent of the hippocampus in each hemisphere based on previously published criteria48, 49, and performed separate functional connectivity analyses using each of the four hippocampal subregions (anterior/posterior by left/right) as the “seed” region. The four resultant functional connectivity maps were not significantly different, as determined by finding no significant clusters for all main effects and interactions in a voxel-wise repeated-measures ANOVA that treated each subregion separately with laterality (left/right) and location (anterior/posterior) included as factors (with a combined voxel-wise and spatial-extent threshold set to P<0.01, as described above). We thus report the functional connectivity map from the analysis that averaged over the entire hippocampus defined bilaterally.
Stepwise linear regression was performed to examine relationships between fMRI activity in the clusters listed in Supp. Table 3 and performance in the spatial test and the object recognition test. The difference in correlation with the hippocampus between volitional and passive conditions for each cluster was used to predict the volitional vs. passive difference in spatial positioning error in one model and the volitional vs. passive difference in hit rate in the recognition test in another model. Difference scores were z-transformed before analysis. The selectivity of the structures identified in association with one memory type for each model was confirmed by examining the relationship between these structures and the other memory type (Supp. Fig. 2).
Neuropsychological methods
In Exp. 4, patients with hippocampal amnesia were tested along with comparison subjects that were matched to amnesic patients in age, gender, handedness, educational attainment, and scores on the Wechsler Adult Intelligence Scale-III. Damage was due in two patients to anoxic episodes (patients 2363 and 1846) and in one patient (1951) as a result of herpes simplex encephalitis, as reported in24, 25 and summarized in Table S2. Damage in patients 2363 and 1846 is primarily limited to the hippocampus, whereas medial temporal lobe cortex is more extensively damaged in patient 195125. Severe memory impairment was expressed in each patient, with performance on the Wechsler Memory Scale-III at least 25 points lower than performance on the Wechsler Adult Intelligence Scale-III, and the average delay score on the memory scale more than two SD below the population mean. Amnesic patients were recruited from the University of Iowa department of Neurology participant pool. Comparison subjects were recruited from the University of Illinois community. The experiment comprised a series of study-test blocks such that memory was tested at a brief delay from study. Each study display comprised a 5×5 grid of black squares, and displays varied in how many objects were located on the grid. Object set sizes included 4, 9, and 16, orderly arranged on the 5×5 grid at locations specified to the subject before each study trial (and held constant across trials for each set size). There were five blocks, with one object set size per block. Set sizes of 4 and 9 were used on two blocks and 16 on one block. The first size tested was 4, then 9, and finally then 16. Each block comprised four study-test sessions, half with volitional study and half with passive study, and half with spatial recall tests and half with object recognition tests. New objects were used for each block. Recognition testing was yes/no without confidence ratings. Window position for volitional study was under continuous control via a computer mouse, as in Exp. 1. Subjects were familiarized with volitional and passive viewing conditions during two practice sessions, including one additional block using only two objects on the grid, and all subjects showed mastery of controlling the viewing window before the experimental blocks. Window movements for the passive conditions for each patient were yoked to the corresponding volitional conditions of the previous patient, as in the prior experiments. Window movements in the passive conditions for each comparison subject were taken from the passive window movements for the corresponding matched patient. Passive window movements were thus yoked to volitional window movements in amnesic patients, and passive window movements were matched between amnesic patients and comparisons.
Supplementary Material
Acknowledgments
Research was supported by an NIH Pathway to Independence award (K99-NS069788) and a Beckman Institute Postdoctoral Fellowship Award to J.L.V., by funds from the Kiwanis Foundation to D.T.T., and by NIH grants MH062500 to N.J.C., and NS19632 to D.T.T.
Footnotes
Author contributions: J.L.V., N.J.C., B.D.G., and K.D.F. conceived the experiments. J.L.V. designed and performed the experiments and analyzed data. D.T.T. provided access and support for testing patients with amnesia. All authors co-wrote the paper, discussed results, and commented on the manuscript.
References
- 1.Gibson JJ. The ecological approach to visual perception. Houghton Mifflin; Boston: 1979. [Google Scholar]
- 2.Held R. Plasticity in sensory-motor systems. Sci Am. 1965;213:84–94. doi: 10.1038/scientificamerican1165-84. [DOI] [PubMed] [Google Scholar]
- 3.Neisser U. Cognition and reality: Principles and implications of cognitive psychology. W.H. Freeman & Company; San Francisco: 1976. [Google Scholar]
- 4.Piaget J. The origins of intelligence in children. Routledge and Kegan Paul; London: 1953. [Google Scholar]
- 5.National-Research-Council. How People Learn. National Academies Press; Washington, DC: 1999. [Google Scholar]
- 6.Metcalfe J. Metacognitive judgments and control of study. Cur Dir Psychol Sci. 2009;18:159–163. doi: 10.1111/j.1467-8721.2009.01628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mackworth NH, Morandi AJ. The gaze selects informative details within pictures. Percep Psychophysiol. 1967;2:547–552. [Google Scholar]
- 8.Jonides J, et al. The mind and brain of short-term memory. Annu Rev Psychol. 2008;59:193–224. doi: 10.1146/annurev.psych.59.103006.093615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chun MM, Turk-Browne NB. Interactions between attention and memory. Curr Opin Neurobiol. 2007;17:177–184. doi: 10.1016/j.conb.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 10.Cohen NJ, Squire LR. Preserved learning and retention of pattern-analyzing skill in amnesia: dissociation of knowing how and knowing that. Science. 1980;210:207–210. doi: 10.1126/science.7414331. [DOI] [PubMed] [Google Scholar]
- 11.Squire LR, Zola-Morgan S. The medial temporal lobe memory system. Science. 1991;253:1380–1386. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- 12.Koechlin E, Hyafil A. Anterior prefrontal function and the limits of human decision-making. Science. 2007;318:594–598. doi: 10.1126/science.1142995. [DOI] [PubMed] [Google Scholar]
- 13.Sakagami M, Watanabe M. Integration of cognitive and motivational information in the primate lateral prefrontal cortex. Ann N Y Acad Sci. 2007;1104:89–107. doi: 10.1196/annals.1390.010. [DOI] [PubMed] [Google Scholar]
- 14.Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283:1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- 15.Tanji J, Hoshi E. Role of the lateral prefrontal cortex in executive behavioral control. Physiol Rev. 2008;88:37–57. doi: 10.1152/physrev.00014.2007. [DOI] [PubMed] [Google Scholar]
- 16.Cabeza R, Ciaramelli E, Olson IR, Moscovitch M. The parietal cortex and episodic memory: an attentional account. Nat Rev Neurosci. 2008;9:613–625. doi: 10.1038/nrn2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uncapher MR, Wagner AD. Posterior parietal cortex and episodic encoding: insights from fMRI subsequent memory effects and dual-attention theory. Neurobiol Learn Mem. 2009;91:139–154. doi: 10.1016/j.nlm.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Held R, Hein A. Movement-produced stimulation in the development of visually guided behavior. J Comp Physiol Psychol. 1963;56:872–876. doi: 10.1037/h0040546. [DOI] [PubMed] [Google Scholar]
- 19.Harman KL, Humphrey GK, Goodale MA. Active manual control of object views facilitates visual recognition. Curr Biol. 1999;9:1315–1318. doi: 10.1016/s0960-9822(00)80053-6. [DOI] [PubMed] [Google Scholar]
- 20.O'Keefe J, Nadel L. The hippocampus as a cognitive map. Clarendon Press; Oxford: 1978. [Google Scholar]
- 21.Foster TC, Castro CA, McNaughton BL. Spatial selectivity of rat hippocampal neurons: dependence on preparedness for movement. Science. 1989;244:1580–1582. doi: 10.1126/science.2740902. [DOI] [PubMed] [Google Scholar]
- 22.Song EY, Kim YB, Kim YH, Jung MW. Role of active movement in place-specific firing of hippocampal neurons. Hippocampus. 2005;15:8–17. doi: 10.1002/hipo.20023. [DOI] [PubMed] [Google Scholar]
- 23.Eichenbaum H, Cohen NJ. From conditioning to conscious recollection: Memory systems of the brain. Oxford University Press; Oxford: 2004. [Google Scholar]
- 24.Allen JS, Tranel D, Bruss J, Damasio H. Correlations between regional brain volumes and memory performance in anoxia. J Clin Exp Neuropsychol. 2006;28:457–476. doi: 10.1080/13803390590949287. [DOI] [PubMed] [Google Scholar]
- 25.Konkel A, Warren DE, Duff MC, Tranel DN, Cohen NJ. Hippocampal amnesia impairs all manner of relational memory. Front Hum Neurosci. 2008;2:15. doi: 10.3389/neuro.09.015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Friston KJ. Functional and effective connectivity in neuroimaging: A synthesis. Hum Brain Mapp. 1994;2:56–78. [Google Scholar]
- 27.Kesner RP. The posterior parietal cortex and long-term memory representation of spatial information. Neurobiol Learn Mem. 2009;91:197–206. doi: 10.1016/j.nlm.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bellebaum C, Daum I. Cerebellar involvement in executive control. Cerebellum. 2007;6:184–192. doi: 10.1080/14734220601169707. [DOI] [PubMed] [Google Scholar]
- 29.Chua EF, Schacter DL, Sperling RA. Neural correlates of metamemory: a comparison of feeling-of-knowing and retrospective confidence judgments. J Cogn Neurosci. 2009;21:1751–1765. doi: 10.1162/jocn.2009.21123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Habas C, et al. Distinct cerebellar contributions to intrinsic connectivity networks. J Neurosci. 2009;29:8586–8594. doi: 10.1523/JNEUROSCI.1868-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krienen FM, Buckner RL. Segregated fronto-cerebellar circuits revealed by intrinsic functional connectivity. Cereb Cortex. 2009;19:2485–2497. doi: 10.1093/cercor/bhp135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kesner RP, Rogers J. An analysis of independence and interactions of brain substrates that subserve multiple attributes, memory systems, and underlying processes. Neurobiol Learn Mem. 2004;82:199–215. doi: 10.1016/j.nlm.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 33.Simons JS, Spiers HJ. Prefrontal and medial temporal lobe interactions in long-term memory. Nat Rev Neurosci. 2003;4:637–648. doi: 10.1038/nrn1178. [DOI] [PubMed] [Google Scholar]
- 34.Epstein RA. Parahippocampal and retrosplenial contributions to human spatial navigation. Trends Cogn Sci. 2008;12:388–396. doi: 10.1016/j.tics.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martin A. The representation of object concepts in the brain. Annu Rev Psychol. 2007;58:25–45. doi: 10.1146/annurev.psych.57.102904.190143. [DOI] [PubMed] [Google Scholar]
- 36.Tolman EC. Purposive behavior in animals and men. Century Co; New York City: 1932. [Google Scholar]
- 37.Metcalfe J, Jacobs WJ. People's study time allocation and its relation to animal foraging. Behav Processes. 83:213–221. doi: 10.1016/j.beproc.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Custers R, Aarts H. The unconscious will: How the pursuit of goals operates outside of conscious awareness. Science. 2010;329:47–50. doi: 10.1126/science.1188595. [DOI] [PubMed] [Google Scholar]
- 39.Shimamura AP, Wickens TD. Superadditive memory strength for item and source recognition: the role of hierarchical relational binding in the medial temporal lobe. Psychol Rev. 2009;116:1–19. doi: 10.1037/a0014500. [DOI] [PubMed] [Google Scholar]
- 40.Buckner RL. The role of the hippocampus in prediction and imagination. Annu Rev Psychol. 61:27–48. C21–28. doi: 10.1146/annurev.psych.60.110707.163508. [DOI] [PubMed] [Google Scholar]
- 41.Gupta R, et al. Declarative memory is critical for sustained advantageous complex decision-making. Neuropsychologia. 2009;47:1686–1693. doi: 10.1016/j.neuropsychologia.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bird CM, Burgess N. The hippocampus and memory: insights from spatial processing. Nat Rev Neurosci. 2008;9:182–194. doi: 10.1038/nrn2335. [DOI] [PubMed] [Google Scholar]
- 43.Van Hoesen GW, Rosene DL, Mesulam MM. Subicular input from temporal cortex in the rhesus monkey. Science. 1979;205:608–610. doi: 10.1126/science.109926. [DOI] [PubMed] [Google Scholar]
- 44.Kennedy PJ, Shapiro ML. Motivational states activate distinct hippocampal representations to guide goal-directed behaviors. Proc Natl Acad Sci U S A. 2009;106:10805–10810. doi: 10.1073/pnas.0903259106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roission B, Pourtois G. Revisiting Snodgrass and Vanderwart's object set: The role of surface detail in basic-level object recognition. Perception. 2004;3:217–236. doi: 10.1068/p5117. [DOI] [PubMed] [Google Scholar]
- 46.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 47.Insausti R, et al. MR volumetric analysis of the human entorhinal, perirhinal, and temporopolar cortices. AJNR Am J Neuroradiol. 1998;19:659–671. [PMC free article] [PubMed] [Google Scholar]
- 48.Reber PJ, Wong EC, Buxton RB. Encoding activity in the medial temporal lobe examined with anatomically constrained fMRI analysis. Hippocampus. 2002;12:363–376. doi: 10.1002/hipo.10018. [DOI] [PubMed] [Google Scholar]
- 49.Voss JL, Hauner KK, Paller KA. Establishing a relationship between activity reduction in human perirhinal cortex and priming. Hippocampus. 2009;19:773–778. doi: 10.1002/hipo.20608. [DOI] [PubMed] [Google Scholar]
- 50.Forman SD, et al. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.