Abstract
Object
Carbamylated erythropoietin (CEPO) is a modified erythropoietin molecule that does not affect hematocrit. In this study, we compared the efficacy of a single dose with triple dose of CEPO treatment of traumatic brain injury (TBI) in rats.
Methods
TBI was induced by controlled cortical impact over the left parietal cortex. CEPO (50 μg/kg) was administered intraperitoneally in rats with TBI at 6 hours (CEPO x 1 group) or 6, 24 and 48 hours (CEPO x 3 group) post injury. Neurological function was assessed using a modified neurological severity score, footfault and Morris water maze tests. Animals were sacrificed 35 days after injury and brain sections stained for immunohistochemistry to assess lesion volume, cell loss, cell proliferation, angiogenesis and neurogenesis after CEPO treatment.
Results
Compared to the vehicle treatment, single treatment of CEPO (6 hours) significantly reduced lesion volume and hippocampal cell loss, enhanced angiogenesis and neurogenesis in the injured cortex and hippocampus, and significantly improved sensorimotor functional recovery and spatial learning in rats after TBI. Importantly, triple dosing of CEPO (6, 24 and 48 hours) further reduced lesion volume and improved functional recovery and neurogenesis compared to the CEPO x 1 group.
Conclusions
Our results indicate that CEPO has considerable therapeutic potential in TBI and related pathologies and furthermore that repeated dosing in the sub-acute phase might have important pharmacological relevance.
Keywords: angiogenesis, carbamylated erythropoietin, functional recovery, neurogenesis, traumatic brain injury
Traumatic brain injury (TBI) is a leading cause of mortality and morbidity throughout the world.32 In addition to primary mechanical damage, secondary injury resulting from biochemical and physiological events evolves over a period of hours to days after the primary insult and ultimately leads to neural cell death. This period of secondary injury provides a window of opportunity for therapeutic intervention with the potential to improve long-term patient outcome. The most prevalent and debilitating features in survivors of TBI are cognitive deficits and motor dysfunctions. Despite advances in basic research as well as improved neurological intensive care in recent years, there is still no effective treatment identified to promote functional recovery in TBI patients.25
Erythropoietin (EPO) and the EPO receptor (EPOR), essential for erythropoiesis, are also expressed in neuronal, astrocytic, and endothelial cells.4 EPO shows neuroprotection in animal models including stroke5,27,34 and TBI.19,22 EPO treatment shows beneficial outcome in a small number of stroke patients.11 However, the higher death rate in a recent stroke clinical trial with EPO raised a major concern over the safety of EPO.12 Although the reason for the higher death rate remains unknown and the large percentage of patients receiving tissue plasminogen activator and patients being treated outside the safety window may contribute to the adverse effect, EPO-increased hematocrit (HCT) may also play a role in the higher mortality. Carbamylated erythropoietin (CEPO) is EPO that is chemically modified by carbamylation. CEPO does not bind to the EPOR and is therefore without hematopoietic side effects.16 Despite the lack of binding to EPOR, CEPO retains full cytoprotective properties, demonstrating that CEPO mediates its beneficial effects via a mechanism different from that of the classical EPOR.3,14,23,39 Similarly to EPO, CEPO shows neuroprotection in rodents after stroke, spinal cord injury, and experimental autoimmune encephalitis (EAE).15,16,28,33,36 Our recent data demonstrate that CEPO is equally as effective as EPO in enhancing spatial learning in rats after TBI.22 However, whether CEPO treatment improves sensorimotor function and reduces lesion volume and cell loss after TBI remains unknown. In addition there have been no studies of the effects of CEPO on angiogenesis and neurogenesis after TBI, or any comparing the efficacy of multiple doses of CEPO with a single dose for treatment of TBI. Accordingly, using a controlled cortical impact (CCI) TBI rat model, we investigated the effects of posttraumatic administration of CEPO on cortical and hippocampal injury, cell proliferation, neurogenesis, angiogenesis, long-term sensorimotor function and spatial learning recovery. We compared the efficacy of a single dose of CEPO with three doses of CEPO for the treatment of TBI in rats.
Methods
All experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of Henry Ford Health System.
TBI Model
A CCI model of TBI in the rat was utilized for the present study.18,21,37 Young adult male Wistar rats (310 ± 13 g) were anesthetized with an intraperitoneal injection of chloral hydrate (350 mg/kg body weight). Rectal temperature was kept at 37 °C with a feedback-regulated water-heating pad. The rats were placed in a stereotactic frame. Two 10-mm-diameter craniotomies were performed adjacent to the central suture, midway between lambda and bregma. The second craniotomy allowed for movement of cortical tissue laterally. The dura mater was kept intact over the cortex. A CCI device was used to induce the injury. Injury was delivered by impacting the left (ipsilateral) cortex with a pneumatic piston containing a 6-mm-diameter tip at a rate of 4 m/second and 2.5 mm of compression. Velocity was measured with a linear velocity displacement transducer.
Experimental Groups and Treatment
Young adult male Wistar rats were divided into 4 groups: Group 1, Sham (n = 8); Group 2, TBI + vehicle (n = 8); Group 3, TBI + CEPO x 1 (n = 8); and Group 4, TBI + CEPO x 3 (n = 8). TBI was induced by CCI over the left parietal cortex. CEPO at a dose of 50 μg/kg body weight (H. Lundbeck A/S, Copenhagen, Denmark) was administered intraperitoneally at 6 hours (for the CEPO) x 1 group) or at 6, 24 and 48 hours (for the CEPO) x 3 group) after TBI. Animals in the TBI + vehicle group received vehicle intraperitoneally at 6, 24, and 48 hours. The doseof CEPO was selected based on previous studies.22,36 For labeling proliferating cells, 5-bromo-2′-deoxyuridine (BrdU, 100 mg/kg; Sigma, St. Louis, MO) was injected intraperitoneally into rats daily for 10 days, starting 1 day after injury. Allrats were sacrificed at 35 days after TBI.
Hematocrit
To determine whether CEPO has any effects on HCT, a blood sample (50 μl) was collected via tail vein before injury, on Day 4 and weekly after TBI up to 5 weeks. HCT was measured in micro-HCT capillary tubes (Fisher Scientific, Pittsburgh, PA) using standard procedures (Readacrit Centrifuge, Clay Adams, Parsippany, NJ).38
Morris Water Maze Test
All functional tests were performed by investigators blinded to the treatment status. To detect spatial learning impairments, a recent version of the Morris water maze (MWM) test was used.8 The procedure was modified from previous versions9,24 and has been found to be useful for chronic spatial memory assessment in rodents with brain injury.8,19 All animals were tested during the last five days (i.e., 31–35 days after TBI) before sacrifice. Data collection was automated by the HVS Image 2020 Plus Tracking System (US HVS Image, San Diego, CA.), as described previously.21 The advantage of this version of the water maze is that each trial takes on the key characteristics of a probe trial because the platform is not in a fixed location within the target quadrant.29
Footfault Test
To evaluate sensorimotor function, the footfault test was carried out before TBI and 1, 4, 7, 14, 21, 28 and 35 days after TBI. The rats were allowed to walk on a grid. With each weight-bearing step, a paw might fall or slip between the wires and, if this occurred, it was recorded as a footfault.2 A total of 50 steps were recorded for each right forelimb and hindlimb.
Modified Neurological Severity Score Test
Neurological functional measurement was performed using the modified neurological severity score (mNSS) test.6 The test was carried out on all rats preinjury and on Days 1, 4, 7, 14, 21, 28, and 35 after TBI. The mNSS is a composite of the motor (muscle status, abnormal movement), sensory (visual, tactile and proprioceptive) and reflex tests and has been employed in previous studies.20 In this TBI model, injury in the left hemispheric cortex of rats causes sensory and motor functional deficiency with elevated scores on motor, sensory, and Beam Balance Tests in the early phase after injury. Beam Balance Test results showing slow recovery in asymmetry deficiency have been reported in unilateral brain injuries including TBI 20 and ischemia.6 This test is suitable for evaluating long-term neurological function after unilateral brain injury.
Tissue Preparation and Measurement of Lesion Volume
On Day 35 after TBI, rats were anesthetized with chloral hydrate administered intraperitoneally, and perfused transcardially with saline solution, followed by 4% paraformaldehyde in 0.1 M phosphate-buffered saline (PBS), pH 7.4. Brains were removed and post-fixed in 4 % paraformaldehyde for 2 days at room temperature. The braintissue was cut into 7 equally spaced (2 mm) coronal blocks, and processed for paraffin sectioning. A series of adjacent 6-μm-thick sections were cut fromeach block in the coronal plane and stained with hematoxylin andeosin (H&E). For lesion volume measurement the 7 brain sections were traced by a microcomputerimaging device (MCID) (ImagingResearch, St. Catharine’s, Ontario, Canada), as previously described.7 The indirect lesion area was calculated (i.e., the intact area of the ipsilateral hemisphere subtractedfrom the area of the contralateral hemisphere),31 and the lesion volume presented as a volume percentage of thelesion compared with the contralateral hemisphere.
Immunohistochemistry
To examine the effect of CEPO on cell proliferation and angiogenesis, coronal sections were histochemically stained with mouse anti-BrdU19 and rabbit anti-human von Willebrandfactor (vWF),38 respectively. For BrdU detection, 6-μm paraffin-embedded coronal sections were deparaffinized and rehydrated. Antigen retrieval was performed by boiling sections in 10-mM citrate buffer (pH 6.0) for 10 minutes.6 After washing with PBS, sections were incubated with 0.3 % H2O2 in PBS for 10 minutes, blocked with 1 % BSA containing 0.3 % Triton-X 100 for 1 hour at room temperature, and incubated with mouse anti-BrdU (1:200; Dako, Carpinteria, CA) at 4 °C overnight. After washing, sections were incubated with biotinylated anti-mouse antibody (1:200; Vector Laboratories, Inc., Burlingame, CA) at room temperature for 30 minutes. After washing, sections were incubated with an avidin-biotin-peroxidase system (ABC kit, Vector Laboratories, Inc., Burlingame, CA), visualized with diaminobenzidine (Sigma, St. Louis, MO) and counterstained with hematoxylin.
To identify vascular structure, brain sections were deparaffinized and then incubated with 0.4% Pepsin solution at 37 °C for 1 hour. After washing, the sections were blocked with 1% BSA at room temperature for 1 hour, and then incubated with rabbit anti-human vWF (1:200; DakoCytomation, Carpinteria, CA) at 4°C overnight. After washing, sections were incubated with biotinylated anti-rabbit antibody (1:200; Vector Laboratories, Inc., Burlingame, CA) at room temperature for 30 minutes. The subsequent procedures were the same as for BrdU staining.
BrdU+ cells and vWF-stained vascular structures in the DG, CA3, and the cortex of ipsilateral hemispheres were examined at x 20 magnification and counted.
Immunofluorescent Staining
Newly generated neurons were identified by double labeling for BrdU and NeuN. After dehydration, tissue sections were boiled in 10-mM citric acid buffer (pH 6) for 10 min. After washing with PBS, sections were incubated in 2.4 N HCl at 37°C for 20 min. Sections were then incubated with 1% BSA containing 0.3% Triton-X-100 in PBS, followed by incubation with mouse anti-NeuN antibody (1:200; Chemicon, Temecula, CA) at 4°C overnight. FITC-conjugated anti-mouse antibody (1:400; Jackson ImmunoResearch, West Grove, PA) was added to sections at room temperature for 2h. Sections were then incubated with rat anti-BrdU antibody (1:200; Dako, Glostrup, Denmark) at 4°C overnight, followed by incubation with Cy3-conjugated anti-rat antibody (1:400; Jackson ImmunoResearch) at room temperature for 2 h. Each of the steps was followed by three 5-min rinses in PBS. Tissue sections were mounted with Vectashield mounting medium (Vector Laboratories). Images were collected with fluorescent microscopy and merged. NeuN/BrdU-colabeled cells in the DG and the cortex were counted at x 40 magnification.
Cell Counting and Quantitation
For quantitative measurements of BrdU+, NeuN+, and NeuN+/BrdU+, we used four slides from each brain, with each slide containing five fields of view in the lesion boundary zone from the epicenter of the injury cavity (bregma −3.3 mm), three fields of view in the ipsilateral CA3 and nine fields of view in the ipsilateral DG in the same section. The fields were digitized under the light microscope (Nikon, Eclipse 80i, Melville, NY) at a magnification of either 200 or 400 using CoolSNAP color camera (Photometrics, Tucson, AZ) interfaced with MetaMorph image analysis system (Molecular Devices, Downingtown, PA). The immunopositive cells were calculated and divided by the measured areas, and presented as numbers per mm2. Cell counts were performed by observers blinded to the individual treatment status of the animals. All counting was performed on a computer monitor to improve visualization and in one focal plane to avoid oversampling.41 To evaluate whether CEPO administered intraperitoneally reduces neuronal damage after TBI, we counted the number of neuronal cells in the DG and CA3 in H&E sections. Counts were averaged and normalized by measuring the linear distance (in mm) of the DG and CA3 for each section. Neurogenesis was evaluated in the DG by calculating the density of BrdU-labeled cells and BrdU/NeuN-colabeled cells. We mainly focused on the ipsilateral DG and its subregions, including the subgranular zone, granular cell layer, and the molecular layer.38
Statistical analyses
All data are presented as means ± SDs. Data on mNSS were first evaluated for normality. The rank data were used for the analysis since data were not normal. Analysis of variance (ANCOVA), PROC MIXED with CONTRAST statement in SAS, was employed to test the group differences on mNSS. The analysis began testing the overall group effect, followed by pair-wise group comparisons if the overall group effect was detected at the 0.05 level; otherwise the pair-wise group comparisons would be considered as exploratory analyses. Data on HCT and sensorimotor function were analyzed by ANOVA for repeated measurements. For lesion volume, cell counting, and vWF-stained vascular density, a one-way ANOVA followed by post hoc SNK tests were used to compare the differences between the CEPO x 1-treated, CEPO x 3- treated, vehicle-treated and sham groups. Statistical significance was set at p < 0.05.
Results
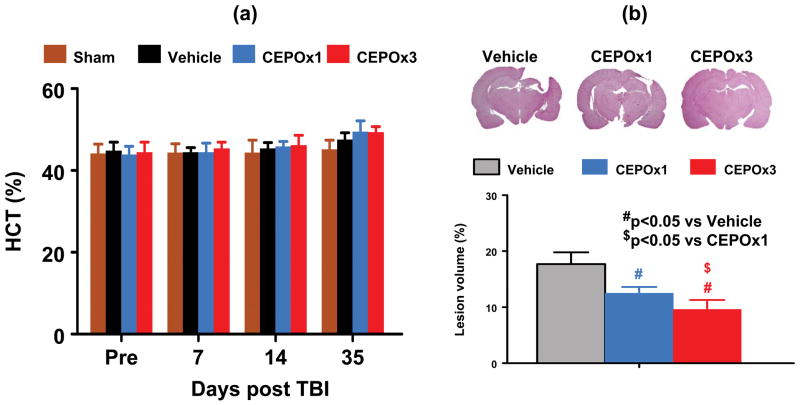
CEPO Does Not Affect HCT
The baseline of HCT was similar for all animals before injury (Fig. 1A, p > 0.05). As compared to the vehicle treatment, the CEPO treatment did not affect HCT in both CEPO x 1 and CEPO x 3 groups during the 35-day study period (p > 0.05).
Fig. 1.
Effect of CEPO on hematocrit and lesion volume. a: CEPO treatment does not affect hematocrit in all groups. b: CEPO treatment significantly reduces lesion volume after TBI. Lesion volume was examined after H&E staining of brain sections on Day 35 after TBI. Data represent mean ± SD. #p < 0.05 vs. the vehicle group. $p < 0.05 vs CEPO x 1 group. N (rats/group) = 8.
CEPO Significantly Reduces Lesion Volume
Lesion volume was measured 35 days post TBI. CEPO treatment initiated at 6 hours post injury in both CEPO x 1 (p <0.001) and CEPO x 3 (p <0.001) groups significantly reduced lesion volume after TBI compared to vehicle controls (Figure 1B). However, animals had a significantly smaller lesion volume in the CEPO x 3 group than in the CEPO x 1 group (p = 0.002).
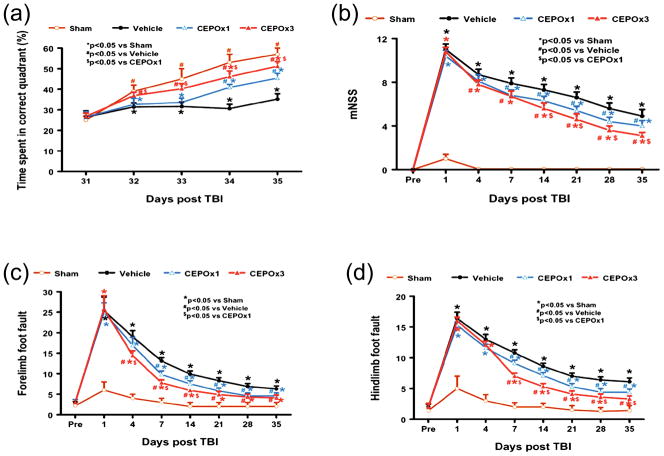
CEPO Significantly Enhances Spatial Learning
The water maze protocol in the present study was used to detect spatial learning deficits. As shown in Figure 2a, the time spent in the correct quadrant (Northeast) by sham rats gradually increased from Days 31 to 35 after surgery. The vehicle-treated rats with TBI were impaired relative to sham-operated rats on Days 32–35 after injury (p < 0.05). The time spent in the correct quadrant by the vehicle-treated rats with TBI was much less than CEPO x 1-treated rats at Day 34 (p = 0.008) and Day 35 (p < 0.001) and CEPO x 3-treated rats at Day 32 (p = 0.033), Day 33 (p = 0.017), Day 34 (p = 0.002) and Day 35(p < 0.001) after injury. However, as compared to the CEPO x 1 group, the CEPO x 3 group showed a significant improvement in spatial learning after injury (i.e., larger percentage of time spent in the correct quadrant) at Day 32 (p = 0.007), Day 33 (p = 0.039), Day 34 (p = 0.036) and Day 35 (p = 0.014 ).
Fig. 2.
Effect of CEPO on functional recovery after TBI. a: Effect of CEPO on spatial learning function 31–35 days after TBI. TBI significantly impairs spatial learning on Days 32–35 compared to sham controls (p < 0.05). Delayed treatment with CEPO improves spatial learning performance measured by a recent version of the water maze test on Days 32–35 (CEPO x 3) or Days 33–35 (CEPO x 1) compared with the vehicle group (p < 0.05). CEPO x 3 treatment shows better spatial learning compared to CEPO x 1 treatment on Days 32–35 after TBI (p < 0.05). b: The line graph shows the functional improvement detected on the modified neurological severity scores (mNSS). CEPO treatment significantly lowers mNSS scores on Days 4–35 (CEPO x 3) or Days 7–35 (CEPO x 1) compared to the vehicle group (p < 0.05). CEPO x 3 treatment shows significantly lowered mNSS scores compared to CEPO x 1 treatment on Days 14–35 after TBI (p < 0.05). c: Effect of CEPO on sensorimotor function (forelimb footfault) after TBI. “Pre” represents pre-injury level. TBI significantly impairs sensorimotor function on Days 1–35 compared to sham controls (p < 0.05). Delayed CEPO treatment significantly reduces forelimb foot faults on Days 4–35 (CEPO x 3) or Days 7–35 (CEPO x 1) compared with the vehicle treated group (p < 0.05). However, CEPO x 3 treatment has a much lower incidence of forelimb footfaults than CEPO x 1 treatment on Days 4–14 after TBI (p < 0.05). d: Effect of CEPO on sensorimotor function (hindlimb footfault) after TBI. “Pre” represents pre-injury level. TBI significantly impairs sensorimotor function on Days 1–35 compared to sham controls (p < 0.05). Delayed CEPO treatment significantly reduces hindlimb foot faults on Days 7–35 (CEPO x 3 and CEPO x 1) compared with the vehicle-treated group (p < 0.05). However, CEPO x 3 treatment has much lower incidence of hindlimb footfaults than CEPO x 1 treatment on Days 7–35 after TBI (p < 0.05). Data represent mean ± SD. *p < 0.05 vs Sham group. #p < 0.05 vs Vehicle group. $p < 0.05 vs CEPO x 1 group. N (rats/group) = 8.
CEPO Significantly Reduces Modified Neurological Severity Score
The higher the modified neurological severity score (mNSS), the worse the neurological function. The rats had no functional deficits in the mNSS test (Score 0) before TBI. Sham operation had minor effects on the mNSS on Day 1 which returned to zero on postoperative Day 4 (Figure 2b). The mNSS score was close to 11 among the vehicle, CEPO x 1 and CEPO x 3 groups on Day 1 post TBI and there was no significant difference, indicating neurological function was severely impaired in all TBI rats. Spontaneous functional recovery was seen in the vehicle-treated animals. However, significantly reduced scores were measured after TBI in the CEPO groups (all p values p < 0.05 on Days 7–35 for CEPO x 1 and on Days 4–35 for CEPO x 3) compared to vehicle treatment. A triple dose of CEPO therapy significantly reduced the mNSS score at Day 14 (p = 0.004), Day 21 (p = 0.004), Day 28 (p = 0.001), and Day 35 (p = 0.001) compared to the single therapy.
CEPO Significantly Reduces the Incidence of Footfaults
The incidence of forelimb footfaults during baseline (preoperatively) was about 4–5 % (Fig. 2c). TBI significantly increased the incidence of right forelimb footfaults contralateral to the TBI from 1–35 days postinjury (all p < 0.05) as compared with the pre-injury baseline. Treatment with CEPO significantly reduced the number of contralateral forelimb footfaults after TBI compared to treatment with vehicle ( all p < 0.05, on days 7–35 for CEPO x 1 and on days 4–35 for CEPO x 3). However, as compared to the CEPO x 1 group, CEPO x 3 group showed a significant reduction in forelimb footfaults at Day 4 (p = 0.008), Day 7 (p <0.001), and Day 14 (p <0.001).
Similar results were found for the contralateral hindlimb (Fig. 2d). As compared to preinjury baseline, TBI significantly increased the incidence of contralateral hindlimb footfaults from Days 1–35 post-injury (all p < 0.05). Treatment with CEPO significantly reduced the number of contralateral hindlimb footfaults from Days 7–35 after TBI compared to treatment with vehicle (all p < 0.05). However, as compared to the CEPO x 1 group, the CEPO x 3 group showed a significant reduction in hindlimb footfaults at Day 7 (p <0.001), Day 14 (p <0.001), Day 21 (p = 0.012), Day 28 (p = 0.049), and Day 35 (p < 0.001).
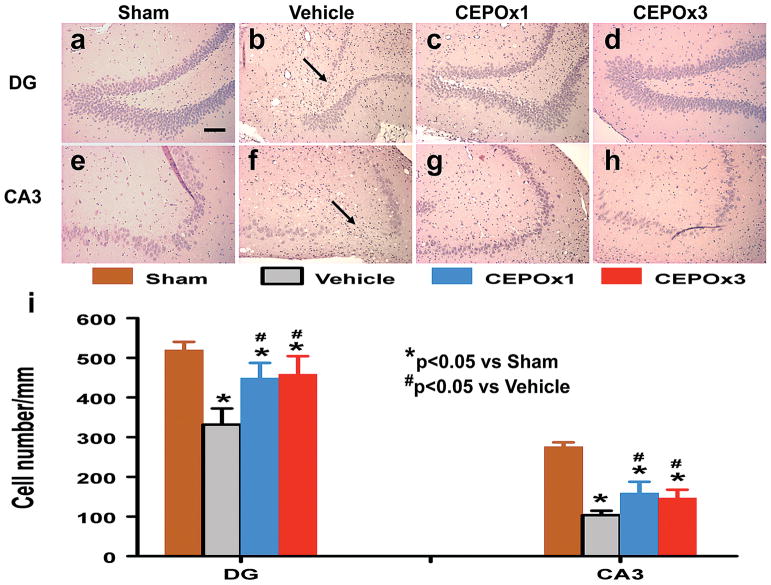
CEPO Significantly Reduces Cell Loss in the CA3 and DG
When examined 35 days post TBI (Figure 3), the neuron counts in the ipsilateral CA3 (p < 0.01) and DG (p < 0.01) had significantly decreased after TBI (Fig. 3b and 3f) compared to the sham groups (Fig. 3a and 3e). As compared to vehicle controls, CEPO treatment significantly increased the neuron counts in the CA3 (Fig. 3c and 3d, p = 0.003 for CEPO x 1 and 0.047 for CEPO x 3) and DG (Fig. 3g and 3h, p = 0.004 for CEPO x 1 and 0.001 for CEPO x 3). However, there was no difference between three doses of CEPO and a single dose of CEPO (p > 0.05).
Fig. 3.
Effect of CEPO on cell number in the ipsilateral DG and CA3 region 35 days after TBI. H&E staining: a–h. Delayed treatment with CEPO (c, d, g, and h) significantly reduces cell loss as compared with the vehicle-treated group (b and f) (p < 0.05). The cell number in the DG and CA3 region is shown in (i). Scale bar = 50 μm (a, applicable to a–h). Data represent mean ± SD. *p < 0.05 vs Sham group. #p < 0.05 vs Vehicle group. N (rats/group) = 8.
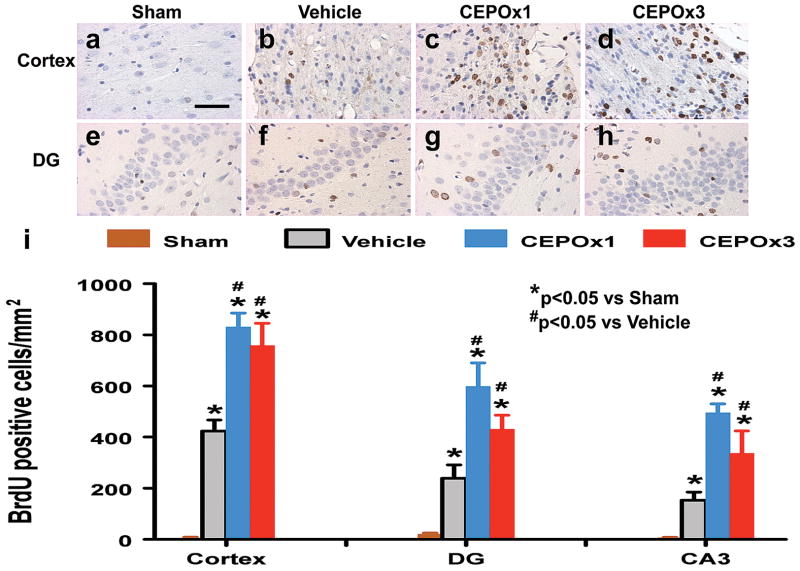
CEPO Significantly Promotes Cell Proliferation
BrdU, an analog of thymidine, is widely used to detect proliferating cells in living tissues.19 BrdU can be incorporated into the newly synthesized DNA of replicating cells during the S phase of the cell cycle, substituting for thymidine during DNA replication. The number of BrdU-positive cells found in the ipsilateral cortex, CA3 and DG areas was significantly increased 35 days after TBI, compared with sham controls (Fig. 4, all p < 0.05). However, CEPO treatment further increased the number of BrdU-positive cells in these regions after TBI compared to vehicle controls (Fig. 4, all p < 0.05). There was no significant difference in the number of BrdU-positive cells in these regions between the CEPO x 1 and CEPO x 3 groups (p > 0.05).
Fig. 4.
Effect of CEPO on cell proliferation in the injured cortex and ipsilateral DG 35 days after TBI. TBI alone (b and f) significantly increases the number of BrdU+ cells (brown-stained) in the ipsilateral cortex and DG compared to sham controls (a and e) (p < 0.05). CEPO treatment significantly increases the number of BrdU+ cells in these regions compared to the vehicle-treated groups (p < 0.05). The number of BrdU+ cells is shown in (i). Scale bar = 50μm (a, applicable to a–h). Data represent mean ± SD. *p < 0.05 vs Sham group. #p < 0.05 vs Vehicle group. N (rats/group) = 8.
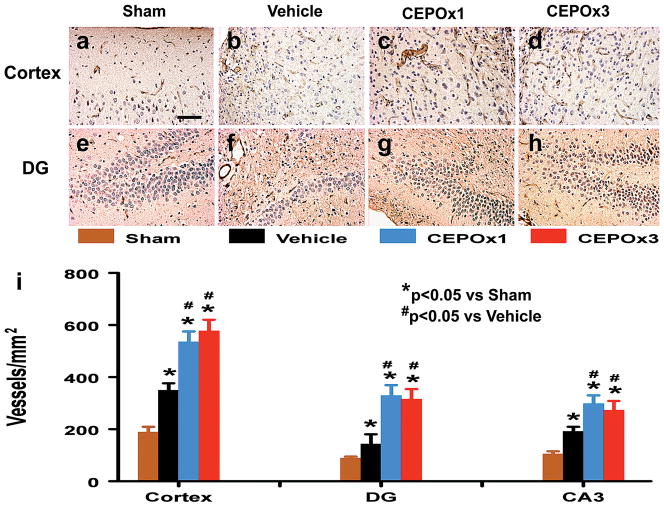
CEPO Significantly Enhances Angiogenesis
vWF-staining has been used to identify vascular structure in the brain after TBI.37 TBI alone significantly increased the density of vessels in the cortex, CA3 and DG of the ipsilateral hemisphere compared to contralateral controls (Fig. 5, all p < 0.05). CEPO treatment significantly increased the vascular density in these regions compared to vehicle treatment (Fig. 5, all p < 0.05). There was no significant difference in the vascular density between the CEPO x 1 and CEPO x 3 groups (p > 0.05).
Fig. 5.
Effect of CEPO on vWF-staining vascular structure in the injured cortex and ipsilateral DG 35 days after TBI. TBI alone (b and f) significantly increases the vascular density (brown-stained) in these regions compared to sham controls (p < 0.05). CEPO treatment (c, d, g, and h) further enhances angiogenesis after TBI compared to the vehicle-treated groups (p < 0.05). The density of vWF-stained vasculature is shown in (i). Scale bar = 50 μm (a, applicable to a–h). Data represent mean ± SD. *p < 0.05 vs Sham group. #p < 0.05 vs Vehicle group. N (rats/group) = 8.
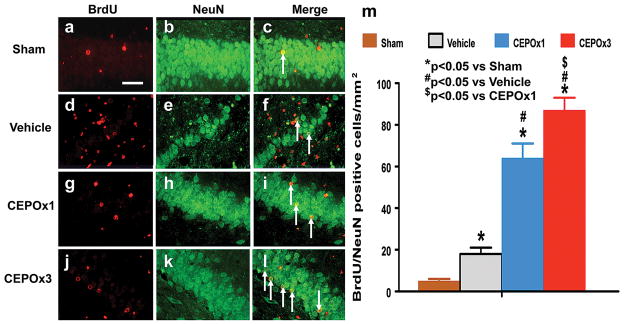
CEPO Significantly Increases Neurogenesis
Newly generated neurons in the DG were identified by double labeling for BrdU (proliferating marker) and NeuN (mature neuronal marker). The number of NeuN/BrdU-colabeled cells (newborn neurons) was significantly higher in the ipsilateral DG after TBI compared to sham controls (Fig. 6c vs 6f, p < 0.001). CEPO treatment significantly increased the number of newborn neurons in the DG (Fig. 6i and 6l, p < 0.001) compared to vehicle controls. The number of newborn neurons was significantly higher in the CEPO x 3 group than in the CEPO x 1 group (Fig. 6i vs 6l, p = 0.018).
Fig. 6.
Double fluorescent staining for BrdU (red) and NeuN (green) to identify newborn neurons (yellow after merge) in the ipsilateral DG 35 days after TBI (f) and CEPO treatment (i and l). The total number of NeuN/BrdU-colabeled cells is shown in (m). Scale bars = 50μm. Data represent mean ± SD. #p < 0.05 vs. the vehicle group. $p < 0.05 vs CEPO x 1 group. N (rats/group) = 8.
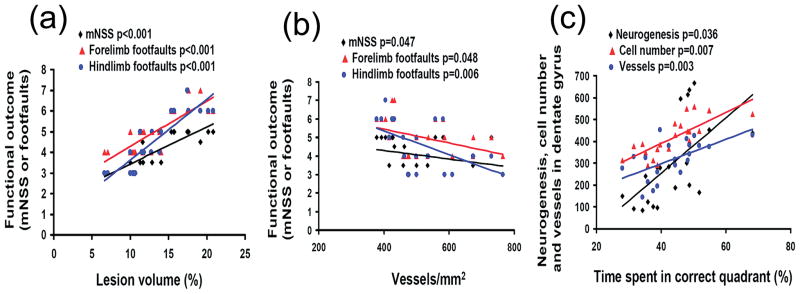
Correlation of Lesion Volume, Angiogenesis and Neurogenesis with Functional Recovery
Figure 7a shows that the lesion volume was significantly correlated to the mNSS score and the incidence of forelimb and hindlimb footfaults on Day 35 after TBI (p < 0.001). The larger the lesion volume, the higher the mNSS score and the incidence of footfaults, suggesting that the cortical tissue loss plays an important role in the functional deficits after TBI. In addition, the cortical angiogenesis was highly correlated to the mNSS score (p = 0.047) and the incidence of forelimb (p = 0.048) and hindlimb (p = 0.006) footfaults examined on Day 35 after TBI (Fig. 7b). Spatial learning assessed by the MWM test was significantly correlated to neurogenesis (p = 0.036), cell number (p = 0.007) and vessels (p = 0.003) in the DG examined on Day 35 after TBI (Fig. 7c).
Fig. 7.
Line graphs showing the correlation of lesion volume, angiogenesis and neurogenesis with functional recovery. The lesion volume was significantly and positively correlated to functional deficits assessed by the mNSS score and the incidence of forelimb and hindlimb footfaults examined on day 35 after TBI (a). The cortical angiogenesis was highly and inversely correlated to functional deficits evaluated by the mNSS score and the incidence of forelimb and hindlimb footfaults examined on day 35 after TBI (b). Spatial learning was significantly and positively correlated to neurogenesis, cell number and vessels in the DG examined 35 days after TBI (c).
Discussion
In this study, we have demonstrated that: 1) CEPO treatment (6 hours post injury) promotes both spatial learning and sensorimotor functional recovery after TBI; 2) improvements in spatial learning and sensorimotor function may derive from the effect of CEPO on reducing hippocampal cell loss, lesion volume, and promoting angiogenesis and neurogenesis as reflected by the correlation analyses; and 3) although a single dose of CEPO shows substantial benefits after TBI, the three-dose paradigm provides a better outcome in terms of reducing lesion volume and enhancing neurogenesis, and improving spatial learning and sensorimotor functional recovery.
The well-documented neuroprotective effects of EPO are commonly associated with unwanted erythrocyte-stimulating effects, with subsequent risk of thromboembolic complications.1 The present data show that CEPO treatment did not elevate HCT. This is consistent with earlier reports16 because CEPO does not bind to the classical EPOR and therefore does not simulate erythropoiesis. Although CEPO does not affect HCT, it is as effective as EPO in neuroprotection.16,22,36 There are two major differences between those earlier studies and the current work. First, in our previous study, CEPO treatment (6 hours) improved spatial learning function in rats after TBI.22 The sensorimotor functional recovery was not evaluated in that study. To the best of our knowledge, the present data demonstrate for the first time that posttraumatic treatment (6 hours) with CEPO significantly reduces the lesion volume and hippocampal cell loss, promotes angiogenesis and neurogenesis and improves sensorimotor functional recovery after TBI. These findings indicate that CEPO treatment provides both neuroprotection and neurorestoration, which may contribute to functional recovery after TBI. Our present study extends our recent study that CEPO treatment (6 hours) effectively reduces infarction and significantly improves functional recovery in rats subjected to embolic middle cerebral artery occlusion.36 Second, and perhaps most importantly, in the present study, we used a multiple-dose paradigm in which three equal doses of drug were administered, with the initial dose given at 6 hours and additional doses given at 24 and 48 hours. This dosing paradigm was used to match as closely as possible the dosing paradigm used in a small but positive clinical study using EPO in stroke.11 We compared the efficacy of a single-dose (6 hours) and multiple-dose paradigm (6, 24 and 48 hours) in terms of lesion volume, cell loss, cell proliferation, angiogenesis, neurogenesis, and spatial learning and sensorimotor functional recovery. The functional recovery in the multiple-dose group was significantly improved compared to the single-dose group. Our data support the use of repeated doses of CEPO for treatment of TBI.
Our recent study indicates that CEPO administered intravenously crosses the blood-brain barrier in rats after stroke.36 The present study shows that the neuroprotective effect of CEPO treatment is localized mainly to the cerebral cortex and hippocampus. In this rat model of CCI injury, TBI caused lesion cavity in the injured cortex and cell loss in the DG and CA3 region of the hippocampus. As compared to the vehicle treatment, CEPO treatment significantly reduces lesion cavity by 31% (single dose) and 45% (triple dose) examined at Day 35 after TBI. Notably, the present study shows that CEPO administered at both dose paradigms has similar efficacy in reducing cell loss in the hippocampus, promoting cell proliferation and angiogenesis. In addition, CEPO treatment also promotes neurogenesis in the DG after TBI. Multiple doses show better effects than a single dose. Under normal conditions, neurogenesis in the subgranular zone of the DG occurs within an angiogenic microenvironment.42 In vivo, neurogenesis and angiogenesis are highly coupled to promote brain remodeling and subsequent improvement of neurological function after brain injury.42 Our present data support the coupling of angiogenesis and neurogenesis, which is reflected by the increased formation of new neurons (NeuN/BrdU-positive cells) in the injured hippocampus where angiogenesis (increased vWF staining) is also enhanced after TBI. The present study demonstrates that CEPO treatment further increases angiogenesis and neurogenesis. Recent studies indicate that the majority of BrdU-positive cells in the DG become granule neurons after TBI, and these neurons exhibit extensive anatomical integration into the CA3 region when cognitive recovery is observed.30 Our present data are in agreement with previous studies that neurogenesis may participate in brain repair and functional recovery.26,30,37 Our recent work demonstrates that CEPO significantly increases neural progenitor cell proliferation and promotes neural progenitor cell differentiation into neurons. These processes are associated with up-regulation of Sonic hedgehog (Shh) because blockage of the Shh signaling pathway with a pharmacological inhibitor, cyclopamine, abolishes the CEPO-induced neurogenesis.35
In addition to its effect on angiogenesis and neurogenesis, CEPO neuroprotection may be mediated through its antiapoptotic effects.3,10,14,17,23,36,39,40 The apoptotic response to trauma may be involved in both acute and delayed patterns of cell death and be regionally distinct after TBI. Our previous study has demonstrated that apoptotic cells in the ipsilateral cortex and hippocampus were observed as early as 2 hours after the impact, peaked at 2 days, and gradually tapered off, although scattered apoptotic cells were still detected at 2 weeks after the impact.13 In our present study, TBI caused a significant cortical lesion and cell loss in the ipsilateral hippocampus. Although a single dose of CEPO initiated at 6 hours post injury significantly reduces lesion volume, additional doses at 24 and 48 hours further reduce lesion volume, which confirmed and extended our recent study that delayed treatment (24 hours) with CEPO provides beneficial effects after TBI.22 Although functional recovery was significantly improved in the multiple-dose group compared to the single-dose group, there was no significant difference in the cell proliferation, angiogenesis and hippocampal cell counting between these two CEPO treatment groups. These findings suggest that an early treatment (6 hours) is important and sufficient to provide significant beneficial effects on histological outcome but later repeated doses are necessary to further improve functional recovery by reducing lesion volume and increasing neurogenesis (or by affecting processes we did not examine in this study, e.g. axonal sprouting). Taken together, these findings indicate that the therapeutic window may not be limited to the first few hours after TBI and can be extended to several hours or even days following TBI.
The cellular mechanisms responsible for the neuroprotective effects of CEPO have not been fully elucidated. CEPO (EPO with all lysines transformed to homocitrulline by carbamylation) does not bind to the homodimeric EPO receptor, suggesting that the tissue protective effects of EPO and CEPO may be mediated by a receptor that includes other components.16 CEPO-induced tissue protection appears to require the common β chain of IL-3/IL-5/GM-CSF receptor (also known as CD131), which can functionally associate with EPOR.16, 23 Recent data indicate that protective effects of EPO and CEPO are mediated by a heteromeric complex that includes the common β-receptor (βcR) subunit as well as an EPO receptor component.3,14,23,39 Our recent data show that the beneficial effect of CEPO may be related to an increase in the expression of brain-derived neurotrophic factor in rat brains after TBI.22 As CEPO signals only through binding to the βcR subunit in the EPOR-βcR heteromeric complex, the mechanism of CEPO-induced protection could differ from that of EPO. For example, unlike EPO, CEPO does not effectively activate the transcription factors STAT-5 or Jak2, a downstream kinase activated directly with ligand binding to EPOR.16 CEPO-induced cardioprotection may be mediated through a PI3K/Akt-depdent mechanism in acute myocardial ischemia/reperfusion injury in the mouse.39 However, further mechanistic studies on CEPO-induced neuroprotective and neurorestorative effects after TBI are warranted.
In conclusion, in the present study, posttraumatic administration (6 hours) of CEPO significantly improves both histological and functional outcomes in rats with TBI, indicating that CEPO has considerable therapeutic potential in TBI patients and that repeated dosing may be needed.
Acknowledgments
Sources of financial support: NINDS grants RO1 NS62002 (Ye Xiong) and PO1 NS42345 (Asim Mahmood, Michael Chopp).
Footnotes
Disclosure/conflict of interest: None. Dr. Thomas N. Sager is a full-time employee of H. Lundbeck A/S.
Author contributions to the study and manuscript preparation include the following. Conception and design: Xiong, Mahmood, Z.G. Zhang, Chopp. Acquisition of data: Xiong, Y. Zhang, Meng, Qu. Analysis and interpretation of data: Xiong, Mahmood, Z.G. Zhang, Chopp. Drafting of the manuscript: Xiong, Chopp. Critical review of the manuscript: Xiong, Sager, Chopp. Review of final version of the manuscript and approved for submission: all authors.
Disclosure
This work was supported by NIH Grant Nos. RO1 NS62002 (to Dr. Xiong) and PO1 NS42345 (to Drs. Mahmood and Chopp). Dr. Thomas N. Sager is a full-time employee of H. Lundbeck A/S. Special thanks to Ms. Susan MacPhee-Gray for editorial assistance.
References
- 1.Adembri C, Massagrande A, Tani A, Miranda M, Margheri M, De Gaudio R, et al. Carbamylated erythropoietin is neuroprotective in an experimental model of traumatic brain injury. Crit Care Med. 2008;36:975–978. doi: 10.1097/CCM.0B013E3181644343. [DOI] [PubMed] [Google Scholar]
- 2.Baskin YK, Dietrich WD, Green EJ. Two effective behavioral tasks for evaluating sensorimotor dysfunction following traumatic brain injury in mice. J Neurosci Methods. 2003;129:87–93. doi: 10.1016/s0165-0270(03)00212-7. [DOI] [PubMed] [Google Scholar]
- 3.Brines M, Grasso G, Fiordaliso F, Sfacteria A, Ghezzi P, Fratelli M, et al. Erythropoietin mediates tissue protection through an erythropoietin and common beta-subunit heteroreceptor. Proc Natl Acad Sci U S A. 2004;101:14907–14912. doi: 10.1073/pnas.0406491101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brines ML, Ghezzi P, Keenan S, Agnello D, de Lanerolle NC, Cerami C, et al. Erythropoietin crosses the blood-brain barrier to protect against experimental brain injury. Proc Natl Acad Sci U S A. 2000;97:10526–10531. doi: 10.1073/pnas.97.19.10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cerami A, Brines ML, Ghezzi P, Cerami CJ. Effects of epoetin alfa on the central nervous system. Semin Oncol. 2001;28:66–70. doi: 10.1016/s0093-7754(01)90216-7. [DOI] [PubMed] [Google Scholar]
- 6.Chen J, Sanberg PR, Li Y, Wang L, Lu M, Willing AE, et al. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke. 2001;32:2682–2688. doi: 10.1161/hs1101.098367. [DOI] [PubMed] [Google Scholar]
- 7.Chen J, Zhang C, Jiang H, Li Y, Zhang L, Robin A, et al. Atorvastatin induction of VEGF and BDNF promotes brain plasticity after stroke in mice. J Cereb Blood Flow Metab. 2005;25:281–290. doi: 10.1038/sj.jcbfm.9600034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi SH, Woodlee MT, Hong JJ, Schallert T. A simple modification of the water maze test to enhance daily detection of spatial memory in rats and mice. J Neurosci Methods. 2006;156:182–193. doi: 10.1016/j.jneumeth.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Day LB, Weisand M, Sutherland RJ, Schallert T. The hippocampus is not necessary for a place response but may be necessary for pliancy. Behav Neurosci. 1999;113:914–924. doi: 10.1037//0735-7044.113.5.914. [DOI] [PubMed] [Google Scholar]
- 10.Doggrell SA. A neuroprotective derivative of erythropoietin that is not erythropoietic. Expert Opin Investig Drugs. 2004;13:1517–1519. doi: 10.1517/13543784.13.11.1517. [DOI] [PubMed] [Google Scholar]
- 11.Ehrenreich H, Hasselblatt M, Dembowski C, Cepek L, Lewczuk P, Stiefel M, et al. Erythropoietin therapy for acute stroke is both safe and beneficial. Mol Med. 2002;8:495–505. [PMC free article] [PubMed] [Google Scholar]
- 12.Ehrenreich H, Weissenborn K, Prange H, Schneider D, Weimar C, Wartenberg K, et al. Recombinant human erythropoietin in the treatment of acute ischemic stroke. Stroke. 2009;40:e647–656. doi: 10.1161/STROKEAHA.109.564872. [DOI] [PubMed] [Google Scholar]
- 13.Kaya SS, Mahmood A, Li Y, Yavuz E, Goksel M, Chopp M. Apoptosis and expression of p53 response proteins and cyclin D1 after cortical impact in rat brain. Brain Res. 1999;818:23–33. doi: 10.1016/s0006-8993(98)01204-9. [DOI] [PubMed] [Google Scholar]
- 14.King VR, Averill SA, Hewazy D, Priestley JV, Torup L, Michael-Titus AT. Erythropoietin and carbamylated erythropoietin are neuroprotective following spinal cord hemisection in the rat. Eur J Neurosci. 2007;26:90–100. doi: 10.1111/j.1460-9568.2007.05635.x. [DOI] [PubMed] [Google Scholar]
- 15.Lapchak PA. Carbamylated erythropoietin to treat neuronal injury: new development strategies. Expert Opin Invest Drugs. 2008;17:1175–1186. doi: 10.1517/13543784.17.8.1175. [DOI] [PubMed] [Google Scholar]
- 16.Leist M, Ghezzi P, Grasso G, Bianchi R, Villa P, Fratelli M, et al. Derivatives of erythropoietin that are tissue protective but not erythropoietic. Science. 2004;305:239–242. doi: 10.1126/science.1098313. [DOI] [PubMed] [Google Scholar]
- 17.Liao ZB, Zhi XG, Shi QH, He ZH. Recombinant human erythropoietin administration protects cortical neurons from traumatic brain injury in rats. Eur J Neurol. 2008;15:140–149. doi: 10.1111/j.1468-1331.2007.02013.x. [DOI] [PubMed] [Google Scholar]
- 18.Lighthall JW. Controlled cortical impact: a new experimental brain injury model. J Neurotrauma. 1988;5:1–15. doi: 10.1089/neu.1988.5.1. [DOI] [PubMed] [Google Scholar]
- 19.Lu D, Mahmood A, Qu C, Goussev A, Schallert T, Chopp M. Erythropoietin enhances neurogenesis and restores spatial memory in rats after traumatic brain injury. J Neurotrauma. 2005;22:1011–1017. doi: 10.1089/neu.2005.22.1011. [DOI] [PubMed] [Google Scholar]
- 20.Lu D, Mahmood A, Qu C, Hong X, Kaplan D, Chopp M. Collagen scaffolds populated with human marrow stromal cells reduce lesion volume and improve functional outcome after traumatic brain injury. Neurosurgery. 2007;61:596–602. doi: 10.1227/01.NEU.0000290908.38438.B2. discussion 602–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahmood A, Lu D, Qu C, Goussev A, Chopp M. Treatment of traumatic brain injury with a combination therapy of marrow stromal cells and atorvastatin in rats. Neurosurgery. 2007;60:546–553. doi: 10.1227/01.NEU.0000255346.25959.99. discussion 553–544. [DOI] [PubMed] [Google Scholar]
- 22.Mahmood A, Lu D, Qu C, Goussev A, Zhang ZG, Lu C, et al. Treatment of traumatic brain injury in rats with erythropoietin and carbamylated erythropoietin. J Neurosurg. 2007;107:392–397. doi: 10.3171/JNS-07/08/0392. [DOI] [PubMed] [Google Scholar]
- 23.Mennini T, De Paola M, Bigini P, Mastrotto C, Fumagalli E, Barbera S, et al. Nonhematopoietic erythropoietin derivatives prevent motoneuron degeneration in vitro and in vivo. Mol Med. 2006;12:153–160. doi: 10.2119/2006-00045.Mennini. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morris RG, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 25.Narayan RK, Michel ME, Ansell B, Baethmann A, Biegon A, Bracken MB, et al. Clinical trials in head injury. J Neurotrauma. 2002;19:503–557. doi: 10.1089/089771502753754037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richardson RM, Sun D, Bullock MR. Neurogenesis after traumatic brain injury. Neurosurg Clin N Am. 2007;18:169–181. xi. doi: 10.1016/j.nec.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 27.Sakanaka M, Wen TC, Matsuda S, Masuda S, Morishita E, Nagao M, et al. In vivo evidence that erythropoietin protects neurons from ischemic damage. Proc Natl Acad Sci U S A. 1998;95:4635–4640. doi: 10.1073/pnas.95.8.4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Savino C, Pedotti R, Baggi F, Ubiali F, Gallo B, Nava S, et al. Delayed administration of erythropoietin and its non-erythropoietic derivatives ameliorates chronic murine autoimmune encephalomyelitis. J Neuroimmunol. 2006;172:27–37. doi: 10.1016/j.jneuroim.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 29.Schallert T. Behavioral tests for preclinical intervention assessment. NeuroRx. 2006;3:497–504. doi: 10.1016/j.nurx.2006.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun D, McGinn MJ, Zhou Z, Harvey HB, Bullock MR, Colello RJ. Anatomical integration of newly generated dentate granule neurons following traumatic brain injury in adult rats and its association to cognitive recovery. Exp Neurol. 2007;204:264–272. doi: 10.1016/j.expneurol.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 31.Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab. 1990;10:290–293. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- 32.Thurman DJ, Alverson C, Dunn KA, Guerrero J, Sniezek JE. Traumatic brain injury in the United States: A public health perspective. J Head Trauma Rehabil. 1999;14:602–615. doi: 10.1097/00001199-199912000-00009. [DOI] [PubMed] [Google Scholar]
- 33.Villa P, van Beek J, Larsen AK, Gerwien J, Christensen S, Cerami A, et al. Reduced functional deficits, neuroinflammation, and secondary tissue damage after treatment of stroke by nonerythropoietic erythropoietin derivatives. J Cereb Blood Flow Metab. 2007;27:552–563. doi: 10.1038/sj.jcbfm.9600370. [DOI] [PubMed] [Google Scholar]
- 34.Wang L, Zhang Z, Wang Y, Zhang R, Chopp M. Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke. 2004;35:1732–1737. doi: 10.1161/01.STR.0000132196.49028.a4. [DOI] [PubMed] [Google Scholar]
- 35.Wang L, Zhang ZG, Gregg SR, Zhang RL, Jiao Z, LeTourneau Y, et al. The Sonic hedgehog pathway mediates carbamylated erythropoietin-enhanced proliferation and differentiation of adult neural progenitor cells. J Biol Chem. 2007;282:32462–32470. doi: 10.1074/jbc.M706880200. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, Zhang ZG, Rhodes K, Renzi M, Zhang RL, Kapke A, et al. Post-ischemic treatment with erythropoietin or carbamylated erythropoietin reduces infarction and improves neurological outcome in a rat model of focal cerebral ischemia. Br J Pharmacol. 2007;151:1377–1384. doi: 10.1038/sj.bjp.0707285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiong Y, Lu D, Qu C, Goussev A, Schallert T, Mahmood A, et al. Effects of erythropoietin on reducing brain damage and improving functional outcome after traumatic brain injury in mice. J Neurosurg. 2008;109:510–521. doi: 10.3171/JNS/2008/109/9/0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiong Y, Mahmood A, Lu D, Qu C, Kazmi H, Goussev A, et al. Histological and functional outcomes after traumatic brain injury in mice null for the erythropoietin receptor in the central nervous system. Brain Res. 2008;1230:247–257. doi: 10.1016/j.brainres.2008.06.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu X, Cao Z, Cao B, Li J, Guo L, Que L, et al. Carbamylated erythropoietin protects the myocardium from acute ischemia/reperfusion injury through a PI3K/Akt-dependent mechanism. Surgery. 2009;146:506–514. doi: 10.1016/j.surg.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 40.Yatsiv I, Grigoriadis N, Simeonidou C, Stahel PF, Schmidt OI, Alexandrovitch AG, et al. Erythropoietin is neuroprotective, improves functional recovery, and reduces neuronal apoptosis and inflammation in a rodent model of experimental closed head injury. FASEB J. 2005;19:1701–1703. doi: 10.1096/fj.05-3907fje. [DOI] [PubMed] [Google Scholar]
- 41.Zhang R, Wang Y, Zhang L, Zhang Z, Tsang W, Lu M, et al. Sildenafil (Viagra) induces neurogenesis and promotes functional recovery after stroke in rats. Stroke. 2002;33:2675–2680. doi: 10.1161/01.str.0000034399.95249.59. [DOI] [PubMed] [Google Scholar]
- 42.Zhang ZG, Chopp M. Neurorestorative therapies for stroke: underlying mechanisms and translation to the clinic. Lancet Neurol. 2009;8:491–500. doi: 10.1016/S1474-4422(09)70061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]