Abstract
The MET receptor tyrosine kinase has been implicated in several solid tumor settings, with MET gene amplification present in a subset of patients with epithelial tumors, including non-small cell lung cancer (NSCLC). MET gene amplification also appears to underlie acquired resistance to EGFR kinase inhibitor therapy in ~20% of NSCLC patients that initially respond to such treatment. In vitro studies have demonstrated that cancer cells harboring MET amplification demonstrate striking sensitivity to small molecule selective MET kinase inhibitors, prompting efforts to evaluate such inhibitors in clinical trials. Previous experience with kinase-targeted therapeutics such as the selective tyrosine kinase inhibitors (TKIs) imatinib and erlotinib, in chronic myeloid leukemia and NSCLC, respectively, has revealed that most treatment-responsive patients eventually relapse with drug-resistant disease. Here, we have modeled acquired resistance to the investigational MET kinase inhibitor PF2341066 in MET-amplified NSCLC cell lines to identify specific drug resistance mechanisms that are anticipated in the course of clinical development. Through this analysis, we identified EGFR (epidermal growth factor receptor) pathway engagement as a PF2341066 resistance mechanism in MET-amplified cells following prolonged drug exposure. While combined inhibition of MET and EGFR kinases in MET-dependent NSCLC cells did not detectably enhance their acute PF2341066 sensitivity, this combination dramatically suppressed the eventual emergence of drug-resistant clones following prolonged exposure. Conversely, activating the EGFR pathway with the receptor ligand EGF increased the yield of PF2341066-resistant clones, confirming the significance of this pathway in conferring resistance to MET TKIs. These findings further support an intimate relationship between EGFR and MET signaling pathways in NSCLC, and suggest that a combination treatment strategy with MET and EGFR kinase inhibitors may be beneficial in MET-amplified NSCLC.
Keywords: MET, EGFR, TKI resistance, cross-talk, acquired drug resistance
INTRODUCTION
Many of the human receptor tyrosine kinases (RTKs) mediate extracellular signals that promote proliferation, migration, and survival of cancer cells. Consequently, the RTKs have been long recognized as potentially important targets for the development of targeted cancer therapeutics, and approximately 30 distinct kinase inhibitors have been developed thus far to the level of Phase I clinical evaluation (1). The clinical success of TKIs such as erlotinib (in NSCLC) and imatinib (in chronic myeloid leukemia and gastrointestinal stromal tumors) has prompted intensive efforts to identify and target additional oncogenic kinases as a broad therapeutic strategy for selected patient populations (2–5).
Focal amplification of the MET gene, which encodes the MET RTK, has been identified in a subset of solid tumors—most notably, gastric and NSCLC cancers (6, 7). In NSCLC-derived cell lines, amplified MET is associated with highly expressed and active MET protein, and consequent engagement of established MET effectors, such as the AKT and ERK1,2 kinases (8). Furthermore, inhibition of MET kinase activity in MET-amplified cancer cells, using selective kinase inhibitors, results in decreased cancer cell viability in both cell culture and animal xenografts models, suggesting that MET TKIs may be clinically effective in patients whose tumors harbor MET amplification/activation (8–11). Consequently, at least six MET TKIs are currently undergoing early phase clinical testing as anti-cancer agents in the United States (12).
Although the clinically approved TKIs can yield impressive responses in a subset of treated cancer patients, rapidly acquired drug resistance remains an important limitation to the long-term efficacy of such treatments (13–16). Therefore, it is critical to establish mechanisms by which drug resistance develops and to apply that knowledge to the development of strategies to combat resistance (14, 17–19). One such strategy is to treat tumors with a combination of agents that might prevent the emergence of drug-resistant cells by anticipating specific mechanisms of resistance that might otherwise arise in the context of single agent-based therapies.
In this study, we have established pre-clinical findings suggesting that acquired resistance to MET TKIs in the context of MET-dependent NSCLC cells is associated with either a partial or complete switch to EGFR-dependent signaling for the maintenance of tumor cell survival. Significantly, despite the absence of any detectable sensitivity to EGFR TKIs in these NSCLC cell lines, combined MET/EGFR kinase blockade dramatically suppresses the emergence of drug-resistant clones, pointing to a potential therapeutic strategy to reduce the likelihood of relapse in the treatment of NSCLC patients with MET-amplified tumors, and highlighting an intimate relationship between MET and EGFR signaling in NSCLC.
MATERIALS AND METHODS
Human cancer cell lines and cell viability assays
The EBC-1 and NCI-H1993 cell lines were obtained from the Japanese Health Sciences Foundation (JHSF) and the American Type Culture Collection (ATCC) respectively and cultured according to their recommendations. COR-L 105 cells were obtained from the European Collection of Cell Cultures (ECACC). EBC-1 cells have been authenticated by JHSF using short tandem repeat DNA profiling. ATCC have not published a DNA STR profile for NCI-H1993, so a sample was sent to the Wellcome Trust Sanger Institute (Cambridge, UK) for SNP analysis and comparison with their cancer cell line collection. The only match was with NCI-H1993 in that collection. COR-L 105 cells were similarly genotyped by the Wellcome Trust Sanger Institute and found to match their COR-L 105 stock and no other lines. All cell lines were obtained directly from each repository and passaged for fewer than 6 months after receipt. All drug-resistant clones were removed from drug for a minimum of 10 days before undergoing cell viability assays. Cell viability was measured as previously described (10). In brief, the cells were fixed in 4% formaldehyde and then incubated in the fluorescent DNA-binding dye Syto-60 (Invitrogen, Carlsbad) before analysis on a fluorescent plate reader. The sensitivity of each cell line to various concentrations of compound was calculated as the fraction of viable cells relative to untreated cells following 72 hour exposure. Data were subjected to nonlinear regression analysis using GraphPad Prism Software version 5.2 (GraphPad Software Inc., San Diego, CA) to obtain IC50 values.
Generation of PF2341066-resistant clones
Drug-resistant clones of the PF2341066-sensitive MET amplified NSCLC cell lines, EBC-1 and NCI-H1993, were established by exposing these cells to increasing concentrations of PF2341066 for 3 months. Clones capable of proliferation in a final concentration of 1uM were selected for subsequent experiments, were transferred to separate plates using cloning rings, and treated with 1uM of PF2341066 every 2 weeks thereafter. The drug-resistant cells were designated PR (PF2341066 Resistant).
Protein detection
Immunodetection of proteins following SDS-PAGE was performed using standard protocols. Equal lane loading was assessed using β-tubulin (Sigma) and GAPDH (chemicon) antibodies. The AKT, ERK1/2, phospho-ERK1/2 (T202/Y204), MET, phospho-MET (Y1234/35), STAT3 and phospho-STAT3 (S727) antibodies were from Cell Signaling Technology (Beverly, MA). The phospho-AKT(S473) antibody was from BioSource International (Camarillo, CA). The phospho-EGFR antibody was from Abcam (Cambridge, MA). The PARP and total EGFR antibodies were from BD Biosciences (San Jose, CA). All antibodies were used at a 1:1,000 dilution, except for the β-tubulin and GAPDH antibodies, which were used at 1:10,000 dilution. All drug-resistant clones were removed from drug for a minimum of 10 days before collecting cell lysates for further analysis.
Kinase inhibitors
PHA665752 was synthesized at the Dana Farber Cancer Institute and PF2341066 was synthesized by Pfizer Pharmaceuticals. All compounds were reconstituted in DMSO to a 10mM concentration and stored at −80°C. Additional compounds detailed in the supplementary tables were synthesized by N. Gray or obtained through commercial suppliers.
Giemsa staining of drug resistant colonies
Plates of cultured cells were washed in PBS before adding ice-cold methanol as a fixative. Following fixation the cells were incubated in Giemsa stain (Sigma-Aldrich, USA) for 1 hour before washing in distilled water and air drying. Colonies containing >50 cells were counted under microscopy.
Human growth factor antibody array
Cells were seeded at equivalent density on a 6-well plate and the following day the medium was replaced with 1ml of serum-free medium. 24 hours later the conditioned medium was used to incubate with the antibody array as per the manufacturer’s instructions (RayBiotech Inc, USA). Detection of signal was achieved using HRP-conjugated streptavidin and exposure of the array membrane to x-ray film.
RESULTS
Establishment of MET-amplified NSCLC cells with acquired resistance to a selective MET kinase inhibitor
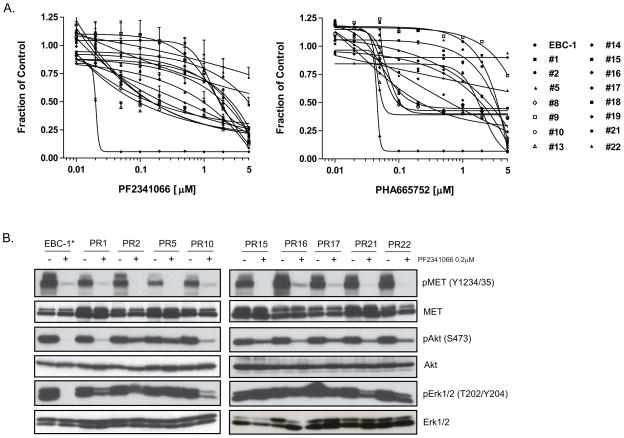
The NSCLC cell line EBC-1 has been previously shown to harbor focal MET gene amplification and is exquisitely sensitive to inhibition of MET kinase activity (Fig. 1A) (10). To explore potential mechanisms of acquired resistance to MET-targeted therapeutics, we employed the selective small molecule MET TKI, PF2341066, which is currently being evaluated in early phase clinical trials (12). PF2341066-resistant clones were generated from EBC-1 cells by exposing the cells to increasing concentrations of PF2341066 for a period of 3 months. The resultant clones (designated EBC-1 PR1 to EBC-1 PR22), following expansion from single cells, demonstrated resistance to PF2341066 treatment, with the majority of clones exhibiting at least 50-fold reduced drug sensitivity, and several clones demonstrating >250-fold reduced drug sensitivity (Fig. 1A, Supp. Table 1). The drug-resistant clones also exhibited cross-resistance to an additional selective MET TKI, PHA665752 (Fig. 1A, Supp. Table 1).
Figure 1.
Generation of PF2341066-resistant NSCLC cells. (A) PF2341066-resistant clones derived by exposing the EBC-1 NSCLC cell line to increasing concentrations of the MET TKI PF2341066 weekly for 3 months exhibit increased resistance to PF2341066 as well as an additional MET TKI, PHA665752. The 15 drug-resistant clones are designated as ‘PR’ (PF2341066 resistant). Cell viability was assayed 72 hrs after treatment with the indicated drug concentrations. Independent triplicate tests were performed and the results reflect the mean and standard deviation of those results. (B) PF2341066-resistant cells maintain AKT and ERK1/2 phosphorylation in the presence of PF2341066. EBC-1 and EBC-1 PR cells were treated for 6 hours with a 200nM concentration of PF2341066. Cell extracts were immunoblotted to detect the indicated proteins. Total proteins are shown to serve as loading controls. The parental cell line EBC-1 is indicated by an asterisk (*).
To explore the biochemical basis for acquired MET TKI resistance in the EBC-1-derived clones, the phosphorylation state of MET and the downstream effectors, AKT and ERK1/2, was examined in the presence or absence of PF2341066. As expected, in the parental EBC-1 cells, acute treatment with PF2341066 causes virtually complete suppression of MET autophosphorylation, as well as phosphorylation of AKT and ERK1/2 (Fig. 1B). Similarly, in each of nine independent EBC-1-derived drug-resistant clones, PF2341066 treatment caused suppression of MET phosphorylation, indicating that the observed drug resistance does not reflect drug efflux or the presence of a mutation that prevents drug binding to MET. However, in contrast to the findings with parental EBC-1 cells, phosphorylation of AKT and ERK1/2 in the drug-resistant clones was maintained in the presence of PF2341066 (Fig. 1B), suggesting an uncoupling of these critical cell survival effectors from MET kinase function in the drug-resistant clones.
Acquired resistance to MET kinase inhibition is associated with a switch to EGFR dependency
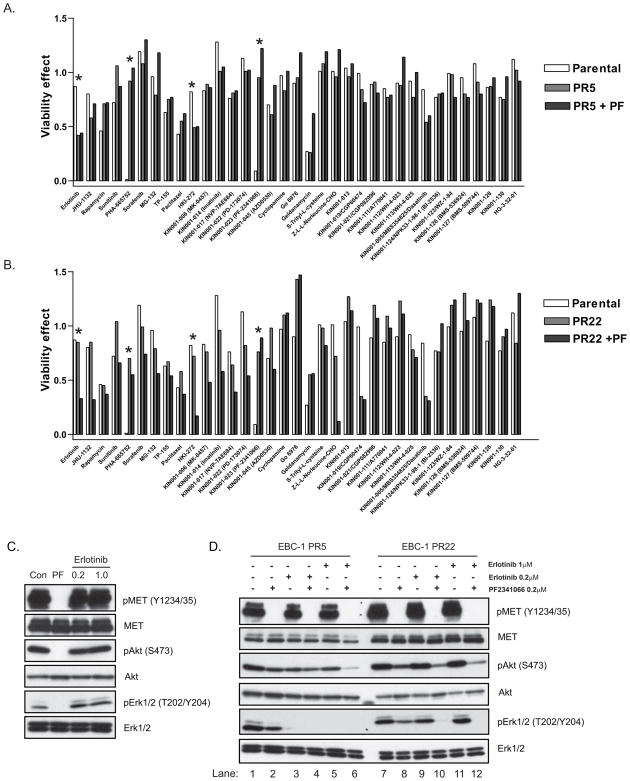
To identify cell survival pathways specifically engaged in the EBC-1-derived PF2341066-resistant cells, clones PR5 and PR22 (>250 fold increased resistance to PF2341066 compared to parental EBC-1 cells) were tested for sensitivity to 35 established and investigational anti-cancer agents (Supp Table 2), in a 72-hour cell viability assay (Fig. 2A,B). Significantly, among all of the tested agents, EBC-1 PR5 cells displayed substantial sensitivity to two different EGFR TKIs, erlotinib and HKI-272 (Fig. 2A), as single agents, whereas EBC-1 PR22 cells displayed sensitivity to both of these compounds only when used in combination with PF2341066 (Fig. 2B). These results suggest that EBC-1 PR5 cells have completely switched their survival dependency from MET to EGFR kinase-mediated signaling, whereas the EBC-1 PR22 cells appear to have become co-dependent on both MET and EGFR signaling to maintain cell survival. Significantly, the EGFR TKI erlotinib had no effect on ERK1/2 or AKT signaling pathways in the EBC-1 parental cell line (Fig. 2C). In contrast, treatment of the EBC-1 PR5 clone with erlotinib resulted in complete abolition of ERK1/2 signaling (Fig. 2D, lanes 3–6). Suppression of downstream signaling was similarly seen in EBC-1 PR22, but only when erlotinib and PF2341066 were tested in combination (Fig. 2D, lanes 10 and 12).
Figure 2.
Engagement of EGFR-dependent survival signaling in PF2341066-resistant NSCLC cells. The parental EBC-1 cell line and MET TKI-resistant clones PR5 (A) and PR22 (B) were treated with the 35 indicated established or investigational anti-cancer compounds in the absence and presence of PF2341066 (0.2μM) (PF), and cell viability was measured after 72 hours. The compounds selected are known inhibitors of key signaling pathways in the proliferation and survival of cancer cell populations. Asterisks indicate results of particular interest. (C) The parental EBC-1 cell line was treated with either PF2341066 (0.2μM) or erlotinib (0.2 or 1μM) for 6 hours. Cell extracts were immunoblotted to detect the indicated proteins. (D) MET TKI-resistant EBC-1 clones PR5 and PR22 were treated for 6 hrs with either PF2341066 (0.2μM), erlotinib (0.2 or 1μM) or both in combination. Cell extracts were immunoblotted to detect the indicated proteins. Total proteins serve as normalization controls.
To determine whether a switch to either partial or complete EGFR dependency occurs in the context of acquired resistance to PF2341066 in the other drug-resistant clones that were isolated, each of the clones was treated with erlotinib alone or in combination with PF2341066 and the effect on cell viability was measured. 13/15 (87%) of the tested EBC-1 PR clones demonstrated the acquisition of sensitivity either to single-agent erlotinib or to the combination of erlotinib and PF2341066 (Supp. Fig. 1), suggesting that in these MET-amplified cells EGFR-mediated signaling is a potent and “preferred” mechanism for maintaining cell survival when the cancer cell population is chronically exposed to a MET TKI.
Combined MET and EGFR blockade prevents the emergence of drug resistant clones
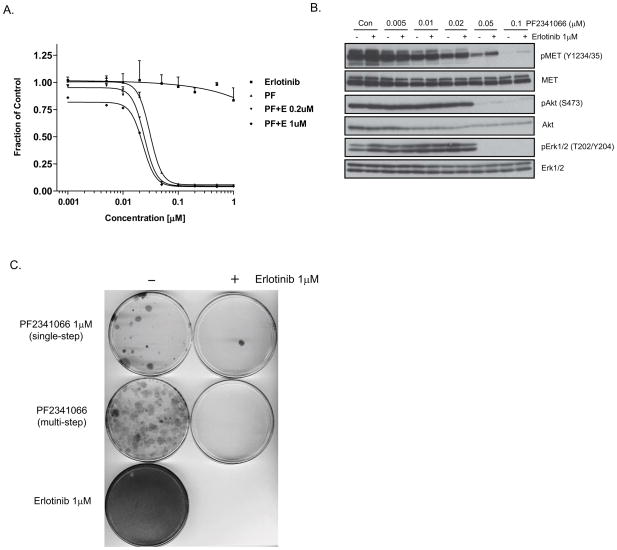
The frequently observed switch to EGFR dependency or the acquisition of co-dependency on MET and EGFR signaling in the context of MET TKI resistance prompted us to examine the potential benefit of combining these inhibitors to prevent the emergence of drug-resistant NSCLC cells. First, we established that the combination of erlotinib and PF2341066 does not detectably increase the IC50 value relative to PF2341066 treatment alone in the setting of EBC-1 cells, as measured by growth inhibition (Fig. 3A). Moreover, combined MET and EGFR kinase blockade did not yield any enhanced effect on suppression of AKT and ERK1/2 activation in these cells (Fig. 3B). We then generated PF2341066-resistant clones as described above by exposing the cells to increasing concentrations of PF2341066 (‘multi-step’) or by exposing cells weekly to a 1μM concentration (‘single-step’), either in the presence or absence of 1μM erlotinib. Erlotinib alone had no effect on cell viability, but the addition of erlotinib to PF2341066 almost completely inhibited the development of PF2341066-resistant clones (Fig. 3C).
Figure 3.
Co-targeting of MET and EGFR kinases to prevent PF2341066 resistance (A) The NSCLC cell line EBC-1 was treated for 72 hours with a range of concentrations of PF2341066 (MET inhibitor; PF), erlotinib (EGFR inhibitor), or a combination of both agents, after which cell viability was determined (B) 1μM of the EGFR TKI erlotinib was added to a range of concentrations of PF2341066 for 6 hour treatment of EBC-1 cells. Cell extracts were immunoblotted to detect the indicated proteins. (C) Inhibition of EGFR signaling dramatically suppresses the emergence of PF2341066-resistant colonies. Giemsa stain of EBC-1 cells plated at equal densities and treated continuously as indicated for 8 weeks.
To further explore the role of EGFR activation in the development of resistance to a MET kinase inhibitor, EBC-1 cells were exposed over 4 weeks to PF2341066 in the presence of the EGFR ligand, epidermal growth factor (EGF). In the acute treatment setting, combined exposure to supplemental EGF and PF2341066 had almost no effect on short-term viability of EBC-1 cells (Supp. Fig. 2A); however, addition of EGF to PF2341066-treated cells led to a persistent low level of ERK1/2 phosphorylation despite the complete suppression of MET autophosphorylation (Supp. Fig. 2B). Furthermore, the combination of supplemental EGF and PF2341066 resulted in a significant increase in the number of PF2341066-resistant clones that were generated following chronic drug exposure (Supp. Fig. 2C). These findings further support a role for EGFR signaling in the emergence of MET TKI resistance in MET-dependent NSCLC cells, and they suggest that ligand-mediated EGFR activation could constitute a potential mechanism.
To further explore a potential role for ligand-dependent activation of EGFR in this setting, a membrane-based antibody array was used to compare the expression of 41 growth factors in conditioned medium prepared from the parental EBC-1 cell line and the PF2341066-resistant clone PR22 (Supp. Fig. 3). This array included 3 well established EGFR ligands – EGF, amphiregulin and TGFα. This analysis revealed significantly increased expression of amphiregulin in the PR22 cell line compared to EBC-1, suggesting that dysregulated amphiregulin expression may contribute to the acquisition of EGFR dependency in the context of resistance to MET TKI treatment.
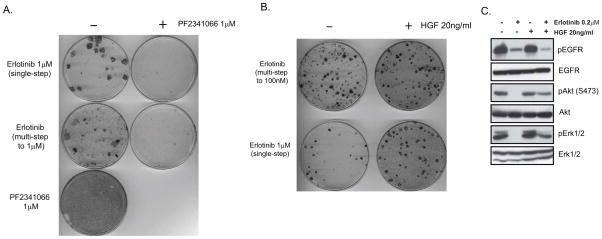
Cooperation between the EGFR and MET signaling pathways has also been shown to underlie the acquisition of resistance to EGFR TKI treatment in the setting of the EGFR TKI-sensitive EGFR mutant NSCLC cell line HCC827, as well as in EGFR mutant NSCLC patients (17). Increased MET signaling, associated with MET gene amplification, was found to yield acquired resistance to an EGFR TKI by driving ERBB3–dependent activation of PI-3 kinase signaling. Therefore, to determine whether we could prevent the emergence of resistance to EGFR TKI treatment in the setting of MET-amplified NSCLC, using a similar combination strategy to that employed with the EBC-1 cell line, we exposed HCC827 cells to either single-step (1μM) or multi-step (5nM to 1μM) erlotinib treatment over 8 weeks, and in the absence and presence of PF2341066 (1μM). HCC827 cells are resistant to PF2341066 treatment, and there was no additive effect seen when it was included in combination with erlotinib (Supp. Fig. 4A). Furthermore, there was no detectable effect on pro-survival signaling pathways in HCC827 cells observed with this combination (Supp. Fig. 4B). However, the addition of PF2341066 to erlotinib almost completely inhibited the establishment of erlotinib-resistant HCC827 clones (Fig. 4A). Conversely, activation of the MET signaling axis with its ligand hepatocyte growth factor (HGF) in erlotinib-treated HCC827 cells (either single-step or multi-step) led to a significant increase in the number of EGFR TKI-resistant clones observed after 8 weeks of treatment (Fig. 4B). Furthermore, the addition of HGF to HCC827 cells previously treated with erlotinib dramatically reversed the inhibition of AKT and ERK1/2 phosphorylation seen following treatment with erlotinib alone (Fig. 4C). It has been recently reported that HGF induces resistance in HCC827 cells to the EGFR TKI gefitinib, and in addition, elevated HGF levels were detected in clinical lung adenocarcinoma cases demonstrating either intrinsic or acquired resistance to that agent (20). Taken together, these findings are consistent with an intimate relationship between EGFR and MET signaling in RTK-addicted NSCLC cells, and suggest that maintaining signaling through AKT or ERK1/2 via cross-talk between these receptors may be a common mechanism by which RTK-addicted cells are able to survive treatment with small molecule inhibitors of these oncogenic kinases.
Figure 4.
Combined EGFR and MET blockade inhibits the development of drug-resistant clones in the EGFR-mutant NSCLC cell line HCC827. (A) Giemsa stain of HCC827 cells plated at equal densities and treated continuously as indicated for 8 weeks. (B) Giemsa stain of HCC-827 cells plated at equal densities and treated as indicated for 8 weeks with erlotinib and HGF weekly. (C) Immunoblot analysis of extracts of HCC827 cells 6 hours following the indicated treatments to detect the phosphorylation status of EGFR, AKT, and ERK1/2. Total proteins serve as normalization controls.
To determine whether the EGFR-mediated resistance mechanisms we observed in the MET-amplified EBC-1 cell line are more broadly relevant in NSCLC cells, PF2341066-resistant clones were generated using another NSCLC cell line harboring MET amplification, NCI-H1993, which is similarly dependent on MET signaling for growth and survival (Fig. 5A) (8). Similar to what was described in the EBC-1 cell line, treatment of the TKI-resistant clones with PF2341066 for 6 hours completely suppressed MET autophosphorylation in NCI-H1993 PR cells; however, relative to effects observed in the parental NCI-H1993 cell line, there was either partial or complete persistence of ERK1/2 phosphorylation in the TKI-resistant cells (Fig. 5B). Furthermore, as seen in the EBC-1 model, whereas the addition of the EGFR TKI erlotinib to PF2341066 did not significantly affect cell viability in the parental cell line, the combination treatment dramatically reduced the number of PF2341066-resistant clones that emerged after 8 weeks (Fig. 5C,D). This finding suggests that EGFR-mediated signaling may be a broadly relevant mechanism of acquired MET TKI resistance in the setting of MET-amplified NSCLC.
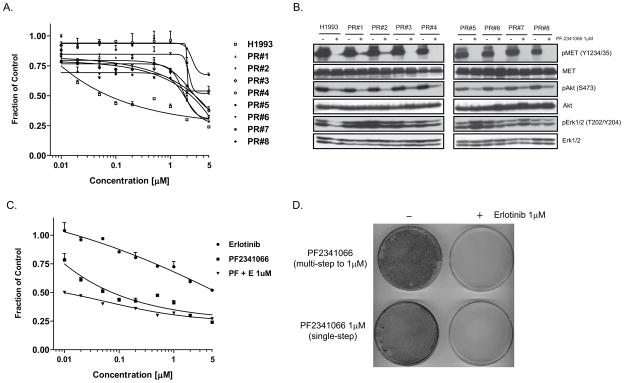
Figure 5.
Combined MET and EGFR blockade suppresses the emergence of drug-resistant clones in the MET-amplified NSCLC cell line NCI-H1993. (A) PF2341066-resistant clones of NCI-H1993 were derived by treating cells with increasing concentrations of the MET TKI PF2341066 weekly for 3 months (to a final concentration of 1μM). Cell viability assays reflect 72 treatment with the indicated concentrations of PF2341066. (B) Immunoblots demonstrating the effect of PF2341066 treatment on signaling pathways in the parental NCI-H1993 cell line versus PF2341066-resistant clones at a 6-hr time-point. (C) NCI-H1993 cells were treated for 72 hours with a range of concentrations of PF2341066, erlotinib (EGFR inhibitor), or a combination of both agents, and the effect on cell viability was measured. (D) NCI-H1993 cells were treated weekly (for 8 weeks) with either a steadily increasing concentration (starting at 10nM, ending at 1μM) or a fixed concentration (1μM) of PF2341066. In addition, erlotinib (1μM) was added weekly to similarly treated plates. All plates were fixed and Giemsa stained at the end of the experiment, and representative plates are shown.
De novo resistance to MET TKI treatment in a NSCLC cell line harboring MET gene amplification
Acquired resistance to EGFR TKI treatment in the setting of EGFR-mutant NSCLC has been associated with two distinct genetic mechanisms--the acquisition of a secondary T790M mutation within the EGFR kinase domain, and focal amplification of the MET gene (17, 21). Significantly, both of these genomic alterations have also been observed in some cases of NSCLC demonstrating de novo resistance to treatment, suggesting that they may contribute to oncogenicity as well as drug resistance (2, 22). De novo drug resistance mechanisms in cancer cell line models have been shown in some cases to be predictive of acquired drug resistance in clinical populations (21).
We previously analyzed 500K genomic SNP array data for a large panel of cancer cell lines, many of which had been tested for sensitivity to PF2341066, and this dataset revealed a NSCLC cell line with elevated copy number for the MET gene, but which was resistant to MET TKI treatment (10). This cell line (NCI-H1573) was shown to harbor MET gene amplification and also demonstrated high levels of MET receptor phosphorylation. However, unlike with the EBC-1 cell line, treatment with the MET kinase inhibitor PF2341066 had no significant effect on cell viability and did not result in complete inhibition of AKT and ERK1/2 signaling, despite completely suppressing MET autophosphorylation (Supp. Fig. 5A,B). These findings were reminiscent of those seen with the PF2341066-resistant EBC-1 clones, and intriguingly, a comparison of gene copy number changes across 360 “cancer genes” revealed that NCI-H1573 cells harbor both MET and EGFR amplification (Supp. Table 3). However, unlike what was observed in a subset of the MET TKI-resistant EBC-1 clones, there was no detectable effect on cell viability when NCI-H1573 cells were treated with erlotinib, either alone or in combination with PF2341066 (Supp. Fig. 5A). In addition, neither erlotinib alone nor in combination with PF2341066 yielded complete suppression of AKT and ERK1/2 signaling (Supp. Fig. 5B).
NCI-H1573 cells were then exposed to an additional 30 compounds known to target critical oncogenic signaling pathways (Supp. Table 4), either as single agents, or in combination with either PF2341066 or PF2341066/erlotinib, to determine whether inhibition of MET and EGFR signaling combined with disruption of a third pathway could inhibit the growth of these cells. However, there was no additional inhibitory effect seen with any of these 30 compounds with the addition of either PF2341066 or the PF2341066/erlotinib combination (Supp. Fig. 5C). These findings implicate additional as yet unidentified mechanisms of resistance to MET TKIs in some NSCLC cells treated with these targeted therapeutics.
Potential benefit of combining EGFR and MET TKIs in NSCLCs with resistance to single agents
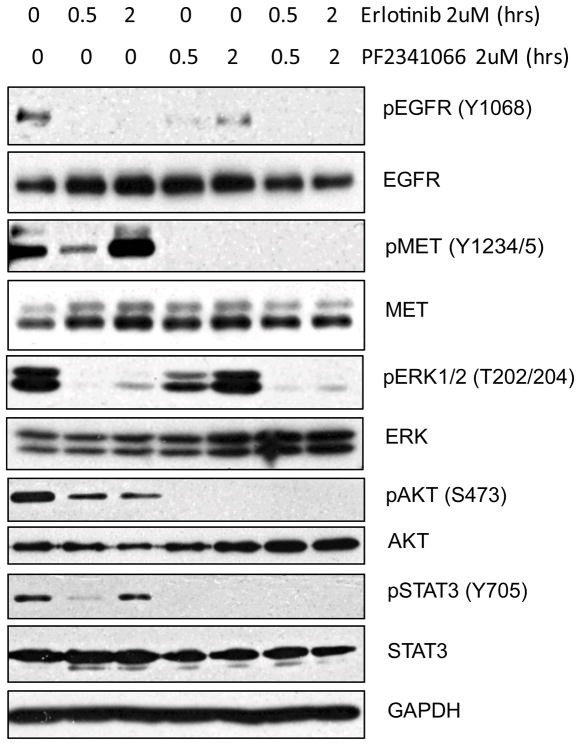
To further explore the potential utility of combining MET and EGFR TKIs in the treatment of NSCLC, we tested a panel of 100 NSCLC-derived cell lines for sensitivity to erlotinib, PF2341066, or a combination of the two inhibitors. While the observed sensitivity to single agent treatments in the various cell lines tested was well correlated with genomic activation of the target kinase, as expected, a few cell lines lacking EGFR or MET genomic activation were largely refractory to single agent treatment, but demonstrated notable sensitivity to the combination treatment (Supp. Table 5). To explore the mechanism underlying sensitivity to the combination treatment, we focused on one of these lines, COR-L 105. By examining the signaling consequence of single or combination TKI treatment of these cells, we determined that the inhibition of either EGFR or MET kinase activity results in only partial inhibition of pro-survival signaling pathways mediated through activated AKT, ERK1/2, and STAT3 (Fig. 6). However the combined inhibition of EGFR and MET signaling led to virtually complete suppression of these pathways. In these cells, phosphorylation of ERK1/2 is clearly coupled to EGFR signaling whereas the phosphorylation of AKT and STAT3 is coupled to MET signaling (Fig. 6). However, unlike in the HCC827 line, resistance to MET TKI is not coupled to ERBB3-dependent activation of PI-3 kinase signaling (data not shown). Interestingly, erlotinib-induced inhibition of EGFR phosphorylation leads to an acute decrease in phospho-MET levels (at 0.5 hrs post treatment) followed by an increase in activated MET at 2 hours of treatment (Fig. 6). Similarly, PF2341066-mediated inhibition of MET phosphorylation initially caused a decrease in phospho-EGFR levels at 0.5 hours followed by an increase at 2 hours post-treatment. These findings highlight the complexity of cross-talk between the MET and EGFR RTKs in NSCLC, potentially involving feedback regulation following TKI treatment.
Figure 6.
Combined inhibition of EGFR and MET activation disrupts survival signaling in the NSCLC cell line Cor-L 105. Immunoblot analysis of Cor-L 105 cell lysates at 0.5 and 2 hours post-treatment with the indicated TKIs to detect phosphorylated forms of EGFR, MET, AKT, ERK1/2, and STAT3. Total proteins served as normalization controls with GAPDH providing an additional loading control.
DISCUSSION
Acquired resistance to selective kinase inhibitors in cancer therapy remains a critical limitation to the efficacy of these agents. Despite the clinical success of several of these drugs, the eventual, and often rapid development of drug resistance demands a more thorough understanding of the underlying mechanisms in order to develop effective strategies to manage or prevent drug resistance. In many cases, cancer cell lines can provide a powerful model system to identify clinically relevant mechanisms underlying acquired drug resistance, with the best example thus far coming from studies of acquired resistance to EGFR TKI therapy in the setting of EGFR-mutant NSCLC (14). Here, we have undertaken this strategy to anticipate potential mechanisms underlying acquired resistance to MET TKIs, which are currently undergoing early phase clinical testing. Amplified MET is observed in approximately 4% of NSCLCs and 15% of gastric cancers (6, 23) and based on pre-clinical findings, it appears to potentially constitute a drug-sensitizing allele in the setting of MET TKI therapy (8–10). Consequently, several small molecule inhibitors of MET kinase have been developed and are currently being evaluated in early phase clinical trials (24).
To anticipate mechanisms of acquired resistance to MET TKI therapy in the setting of MET-amplified NSCLC, we modeled acquired resistance to a selective MET TKI in MET-amplified TKI-sensitive NSCLC-derived cell lines. Through this analysis, we determined that the engagement of EGFR signaling appears to be a common mechanism by which these cells develop resistance to treatment. In the majority of tested drug-resistant clones, we observed either a complete switch from MET to EGFR dependency or the acquisition of co-dependency on these two kinases for sustained cell survival. Analysis of downstream signaling pathways in these cells suggests that the coupling of RTK signals to effectors including AKT and ERK1/2 is a key determinant of sensitivity to these RTK inhibitors—apparently reflecting an “addiction” to these signaling pathways. These observations further reinforce previous findings from others that highlight an intimate relationship between EGFR and MET signaling pathways, particularly in the setting of acquired resistance to EGFR TKI treatment in NSCLC, where MET amplification appears to constitute an important mechanism (17). Considered together with those findings, our studies implicate a balance of partially redundant EGFR- and MET-transduced signals in the survival of a subset of NSCLCs, and highlight the potential importance of combining MET and EGFR TKIs as a front-line therapy in this disease setting to prevent or delay the development of drug resistance. Our findings indicating that this combination treatment, both in the setting of EGFR-mutant or MET-amplified NSCLC-derived cell lines, potently suppresses the emergence of clones demonstrating TKI resistance supports the potential clinical utility of this therapeutic strategy.
The mechanism by which EGFR signaling becomes engaged during the acquisition of MET TKI resistance in the cell line model we have examined appears to involve elevated expression of the EGFR ligand amphiregulin. By analogy, it appears that supplemental HGF, the ligand for MET, can confer resistance to EGFR TKI treatment of EGFR mutant NSCLCs, a finding that was similarly demonstrated in a recent report by Yano et al, and increased HGF levels in tumors were found to be associated with acquired EGFR TKI resistance in NSCLC patients (20). Moreover, another recent report demonstrated that supplemental EGF can promote resistance to MET TKI treatment in the setting of MET-amplified gastric cancers (25), suggesting that the acquisition of resistance to MET TKIs may similarly involve a switch to EGFR signaling dependency in both MET-amplified NSCLCs and gastric cancers. Taken together, these findings highlight the potentially broader role for changes in the levels of RTK ligands in the context of acquired drug resistance.
Recent studies have demonstrated that the activated MET RTK can transactivate EGFR and/or the EGFR-related receptor, ERBB3 via receptor cross-talk in MET-amplified NSCLC cells (8, 17, 26). Together with other studies demonstrating cross-talk between these RTKs both in the context of normal biology and cancer (27), such findings point to an intimate relationship between the MET and EGFR signaling pathways. Our observation that cells with acquired resistance to MET TKIs can exhibit either a switch from MET to EGFR dependency or the acquisition of EGFR/MET co-dependency point to an additional level of complexity in this relationship, and the mechanistic distinction between these signaling states in the various TKI-resistant clones we have generated remains unclear.
With respect to the clinical implications of these findings, our results suggest that prior knowledge of a predominant drug resistance mechanism can potentially be exploited to develop a combination treatment strategy that dramatically reduces the frequency with which drug-resistance develops. Prompted by the discovery of MET gene amplification as a mechanism of acquired resistance to EGFR TKIs in EGFR-mutant NSCLC, oncologists are already examining the potential clinical benefit of combining EGFR and MET TKIs to treat such patients. Our findings suggest that a similar combination strategy could be beneficial for the treatment of NSCLC patients who demonstrate MET amplification at initial diagnosis. Notably, however, we did find one NSCLC line demonstrating MET gene amplification associated with high levels of phosphorylated MET protein, but exhibiting resistance to MET TKI treatment as well as combined MET and EGFR TKI treatment. Therefore, MET-amplified NSCLCs may not be expected to uniformly respond to such treatments. Our observation that a subset of NSCLC-derived cell lines exhibits MET/EGFR co-dependency in the absence of any apparent genomic activation of these RTKs also highlights the potential significance of the EGFR-MET relationship in the context of first-line therapy strategies with EGFR and MET TKIs.
Combination drug treatment strategies for cancer patients have typically been informed by pre-clinical data suggesting either an additive or synergistic effect of combining various agents on cell viability and/or proliferation, with the emphasis being on an enhanced acute effect in the combination setting. Here we propose an alternative rationale, whereby one agent (‘survival modulator’) targets the critical oncogenic kinase maintaining cancer cell survival whereas the other agent (‘resistance modulator’) targets the anticipated resistance mechanism. Thus, in our models of acquired drug resistance, there was no evidence of synergistic effects of MET and EGFR TKIs when examining acute effects on cell viability; however, when drug-resistant clones were quantified after several months of combination treatment, dramatic suppression of drug-resistant colonies was observed. This strategy may prove to be more broadly applicable for the identification of mechanisms underlying resistance to the various kinase-targeted therapeutics currently being developed.
Acknowledgments
We are grateful to members of the Settleman laboratory for helpful discussions throughout the course of these studies. This work was supported by NCI SPORE in Lung Cancer award P20 CA090578 and NIH RO1 CA115830 to J.S.
References
- 1.Zhang J, Yang PL, Gray NS. Targeting cancer with small molecule kinase inhibitors. Nature reviews. 2009;9(1):28–39. doi: 10.1038/nrc2559. [DOI] [PubMed] [Google Scholar]
- 2.Sequist LV, Martins RG, Spigel D, et al. First-line gefitinib in patients with advanced non-small-cell lung cancer harboring somatic EGFR mutations. J Clin Oncol. 2008;26(15):2442–9. doi: 10.1200/JCO.2007.14.8494. [DOI] [PubMed] [Google Scholar]
- 3.Asahina H, Yamazaki K, Kinoshita I, et al. A phase II trial of gefitinib as first-line therapy for advanced non-small cell lung cancer with epidermal growth factor receptor mutations. British journal of cancer. 2006;95(8):998–1004. doi: 10.1038/sj.bjc.6603393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Druker BJ, Guilhot F, O’Brien SG, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. The New England journal of medicine. 2006;355(23):2408–17. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 5.Cohen MH, Farrell A, Justice R, Pazdur R. Approval summary: imatinib mesylate in the treatment of metastatic and/or unresectable malignant gastrointestinal stromal tumors. The oncologist. 2009;14(2):174–80. doi: 10.1634/theoncologist.2008-0255. [DOI] [PubMed] [Google Scholar]
- 6.Nakajima M, Sawada H, Yamada Y, et al. The prognostic significance of amplification and overexpression of c-met and c-erb B-2 in human gastric carcinomas. Cancer. 1999;85(9):1894–902. doi: 10.1002/(sici)1097-0142(19990501)85:9<1894::aid-cncr3>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 7.Beau-Faller M, Ruppert AM, Voegeli AC, et al. MET gene copy number in non-small cell lung cancer: molecular analysis in a targeted tyrosine kinase inhibitor naive cohort. J Thorac Oncol. 2008;3(4):331–9. doi: 10.1097/JTO.0b013e318168d9d4. [DOI] [PubMed] [Google Scholar]
- 8.Lutterbach B, Zeng Q, Davis LJ, et al. Lung cancer cell lines harboring MET gene amplification are dependent on Met for growth and survival. Cancer research. 2007;67(5):2081–8. doi: 10.1158/0008-5472.CAN-06-3495. [DOI] [PubMed] [Google Scholar]
- 9.Smolen GA, Sordella R, Muir B, et al. Amplification of MET may identify a subset of cancers with extreme sensitivity to the selective tyrosine kinase inhibitor PHA-665752. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(7):2316–21. doi: 10.1073/pnas.0508776103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McDermott U, Sharma SV, Dowell L, et al. Identification of genotype-correlated sensitivity to selective kinase inhibitors by using high-throughput tumor cell line profiling. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(50):19936–41. doi: 10.1073/pnas.0707498104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamazaki S, Skaptason J, Romero D, et al. Pharmacokinetic-pharmacodynamic modeling of biomarker response and tumor growth inhibition to an orally available cMet kinase inhibitor in human tumor xenograft mouse models. Drug metabolism and disposition: the biological fate of chemicals. 2008;36(7):1267–74. doi: 10.1124/dmd.107.019711. [DOI] [PubMed] [Google Scholar]
- 12.http://clinicaltrials.gov/.
- 13.Kosaka T, Yatabe Y, Endoh H, et al. Analysis of epidermal growth factor receptor gene mutation in patients with non-small cell lung cancer and acquired resistance to gefitinib. Clin Cancer Res. 2006;12(19):5764–9. doi: 10.1158/1078-0432.CCR-06-0714. [DOI] [PubMed] [Google Scholar]
- 14.Engelman JA, Settleman J. Acquired resistance to tyrosine kinase inhibitors during cancer therapy. Current opinion in genetics & development. 2008;18(1):73–9. doi: 10.1016/j.gde.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Heinrich MC, Corless CL, Blanke CD, et al. Molecular correlates of imatinib resistance in gastrointestinal stromal tumors. J Clin Oncol. 2006;24(29):4764–74. doi: 10.1200/JCO.2006.06.2265. [DOI] [PubMed] [Google Scholar]
- 16.Shah NP, Nicoll JM, Nagar B, et al. Multiple BCR-ABL kinase domain mutations confer polyclonal resistance to the tyrosine kinase inhibitor imatinib (STI571) in chronic phase and blast crisis chronic myeloid leukemia. Cancer cell. 2002;2(2):117–25. doi: 10.1016/s1535-6108(02)00096-x. [DOI] [PubMed] [Google Scholar]
- 17.Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science (New York, NY. 2007;316(5827):1039–43. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 18.O’Hare T, Eide CA, Deininger MW. Bcr-Abl kinase domain mutations, drug resistance, and the road to a cure for chronic myeloid leukemia. Blood. 2007;110(7):2242–9. doi: 10.1182/blood-2007-03-066936. [DOI] [PubMed] [Google Scholar]
- 19.Shah NP, Tran C, Lee FY, Chen P, Norris D, Sawyers CL. Overriding imatinib resistance with a novel ABL kinase inhibitor. Science (New York, NY. 2004;305(5682):399–401. doi: 10.1126/science.1099480. [DOI] [PubMed] [Google Scholar]
- 20.Yano S, Wang W, Li Q, et al. Hepatocyte growth factor induces gefitinib resistance of lung adenocarcinoma with epidermal growth factor receptor-activating mutations. Cancer research. 2008;68(22):9479–87. doi: 10.1158/0008-5472.CAN-08-1643. [DOI] [PubMed] [Google Scholar]
- 21.Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS medicine. 2005;2(3):e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maheswaran S, Sequist LV, Nagrath S, et al. Detection of mutations in EGFR in circulating lung-cancer cells. The New England journal of medicine. 2008;359(4):366–77. doi: 10.1056/NEJMoa0800668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao X, Weir BA, LaFramboise T, et al. Homozygous deletions and chromosome amplifications in human lung carcinomas revealed by single nucleotide polymorphism array analysis. Cancer research. 2005;65(13):5561–70. doi: 10.1158/0008-5472.CAN-04-4603. [DOI] [PubMed] [Google Scholar]
- 24.Comoglio PM, Giordano S, Trusolino L. Drug development of MET inhibitors: targeting oncogene addiction and expedience. Nat Rev Drug Discov. 2008;7(6):504–16. doi: 10.1038/nrd2530. [DOI] [PubMed] [Google Scholar]
- 25.Bachleitner-Hofmann T, Sun MY, Chen CT, et al. HER kinase activation confers resistance to MET tyrosine kinase inhibition in MET oncogene-addicted gastric cancer cells. Molecular cancer therapeutics. 2008;7(11):3499–508. doi: 10.1158/1535-7163.MCT-08-0374. [DOI] [PubMed] [Google Scholar]
- 26.Agarwal S, Zerillo C, Kolmakova J, et al. Association of constitutively activated hepatocyte growth factor receptor (Met) with resistance to a dual EGFR/Her2 inhibitor in non-small-cell lung cancer cells. British journal of cancer. 2009;100(6):941–9. doi: 10.1038/sj.bjc.6604937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fischer OM, Giordano S, Comoglio PM, Ullrich A. Reactive oxygen species mediate Met receptor transactivation by G protein-coupled receptors and the epidermal growth factor receptor in human carcinoma cells. The Journal of biological chemistry. 2004;279(28):28970–8. doi: 10.1074/jbc.M402508200. [DOI] [PubMed] [Google Scholar]