Abstract
This study addresses the hypothesis that renal interstitial (RI) cyclic guanosine 3′5′-monophosphate (cGMP), a modulator of pressure-natriuresis (P-N), exerts its effect through a relationship with renal interstitial hydrostatic pressure (RIHP). Increasing renal perfusion pressure (RPP) in Sprague-Dawley rats led to increases in RIHP (5.2 ± 0.6 to 10.9 ± 1.6 mm Hg; P<0.01), UNaV (0.062 ± 0.009 to 0.420 ± 0.068 μmol/min/g; P<0.01), and RI cGMP (3.5 ± 0.8 to 9.5 ± 1.7 fmol/min; P<0.01), and these effects were blocked by partial renal decapsulation. Infusion of cGMP into the RI compartment of decapsulated animals restored natriuresis (0.067 ± 0.010 to 0.310 ± 0.061 μmol/min/g; P<0.01). These changes were independent of changes in glomerular filtration rate (GFR). Artificially increasing RIHP in normotensive animals increased RI cGMP (4.1 ± 0.6 to 6.9 ± 0.7 fmol/min; P<0.01) and UNaV (0.071 ± 0.013 to 0.179 ± 0.039 μmol/min/g; P<0.05). Co-infusion of organic anion transport-inhibitor probenecid, or soluble guanylyl cyclase inhibitor ODQ, abolished these effects. Infusion of cGMP into the RI compartment of normotensive animals increased RIHP (6.7 ± 0.4 to 10.3 ± 0.9 mm Hg; P<0.001). Exogenous RI cGMP delivery did not affect total, cortical, or medullary renal blood flow. These studies suggest that extracellular RI cGMP is required for the natriuresis observed after increases in RPP and RIHP, and that cGMP acts via a tubule mechanism. The results support an intra-renal positive-feedback loop wherein RI cGMP increases RIHP, which in turn increases RI cGMP, contributing to the reinforcement of P-N.
Keywords: sodium, kidney, cyclic GMP, natriuresis, hydrostatic pressure
INTRODUCTION
Pressure-natriuresis (P-N), wherein a rise in renal perfusion pressure (RPP) leads to natriuresis and a diuresis, is a primary mechanism by which the kidney regulates blood pressure.1 Multiple factors are involved in this response, but the mechanisms linking them are unknown.2
Guanosine cyclic 3′5′-monophosphate (cGMP) is an important modulator of P-N. Previous in vivo studies demonstrated that renal interstitial (RI) cGMP levels and urine sodium (Na+) excretion (UNaV) increased in parallel in response to an increase in RPP. These responses were abolished when 1-H-[1,2,4] oxadiazolo-[4,2-α] quinoxalin-1-one (ODQ), an inhibitor of the cGMP-producing enzyme soluble guanylyl cyclase (sGC), was infused into the RI compartment.3 Blocking intracellular cGMP export into the extracellular compartment with the organic anion transporter (OAT)-inhibitor probenecid (PB) inhibited Na+ transport in vitro in human renal proximal tubule (RPT) cells and abolished P-N in vivo in the rat.4, 5 These studies suggested that the production of cGMP and its transport into the RI compartment are critical steps in P-N.
Renal interstitial hydrostatic pressure (RIHP) is another modulator of P-N.6 Studies in rats demonstrate that RIHP increases after an increase in RPP.2, 7 This may occur through the transmission of high pressure from the renal medullary vasculature to the RI compartment.8 The increased RIHP may inhibit passive renal tubular Na+ reabsorption and stimulate the production of natriuretic substances.2, 9
The following studies explore the relationship between RI cGMP and RIHP, two modulators of P-N. They test the hypothesis that RI cGMP stimulates P-N by modulating RIHP, and introduce the concept of a positive feedback loop that reinforces P-N.
METHODS
General Methods
(Please see http://hyper.ahajournals.org online supplement for details.)
Animal Preparation
All studies were approved by the Animal Care and Use Committee at the University of Virginia and performed in accordance with the NIH Guide on the Care and Use of Laboratory Animals. The experiments were conducted on 12-week-old, 225–250 g female Sprague-Dawley rats (Harlan, Teklad; n=181) that were housed in a vivarium under controlled conditions (temperature 21 ± 1°C, humidity 60 ± 10%, light 8:00 AM to 8:00 PM) and received a normal Na+ diet (0.28% NaCl).
Renal Cortical Interstitial Infusion
A micro-infusion catheter (PE-10) was inserted under the renal capsule into the cortex for RI infusion of pharmacologic agent or vehicle (V) at 2.5 μL/min with a syringe pump (Harvard, model 55–222). Vetbond tissue adhesive (3M Animal Care Products) was used to secure the tubing and prevent interstitial pressure loss.
RI Fluid Microdialysis Technique
RI cGMP was collected using microdialysis probes that were constructed and utilized as described previously.10
Renal Blood Flow (RBF), Renal Cortical Blood Flow (RCBF), and Renal Medullary Blood Flow (RMBF) Measurement
Following acute uninephrectomy, a flow probe was secured around the left renal artery and connected to a dual-channel flowmeter (Transonic Systems Inc.) for measuring RBF. RBF is reported as milliliters per minute per gram kidney weight (mL/min/g). Needle probes were placed into the cortex and medulla and connected to a laser Doppler flowmeter (Advance Laser Flowmeter ALF 21D) to measure RCBF and RMBF.
Measurement of GFR, FENa, and FELi
Glomerular filtration rate (GFR) was measured by inulin clearance utilizing a previously described method, and is reported as milliliters per minute per gram kidney weight (mL/min/g).11 Tubular Na+ reabsorption was determined by calculating FeNa and RPT Na+ reabsorption was estimated using FELi.
RIHP Measurement
RIHP measurement was performed utilizing a variant of a technique described by Nakamura et al.12 An opening was made in the renal capsule using a cautery device (Medtronic). A segment of PE-50 tubing was inserted 2 mm into the renal cortical interstitium and secured, while the other end was attached to a BP analyzer. Studies that involved RIHP, UNaV, and RI cGMP measurements were separated into two groups in order to avoid potential renal trauma associated with the insertion of both a RIHP catheter and a RI microdialysis catheter. One group of rats had only a RIHP catheter, while a second group had a RI microdialysis probe to measure RI cGMP levels and a ureteral catheter to measure UNaV. Mean arterial pressure (MAP) was measured in both groups.
Pressure-Natriuresis Model
A variant of the P-N model of Roman and Cowley was employed.13 RPP was increased during the experimental period by tying off the infrarenal aorta and clamping the superior mesenteric artery.
Assays
Cyclic Nucleotides
RI cGMP levels were measured using an enzyme immunoassay (Cayman Chemical).
Urinary and Plasma Na+ and Li+ Concentrations
Urinary and plasma Na+ and Li+ concentrations were measured using a flame photometer (Instrumentation Laboratory-943). Urine flow and UNaV are reported as milliliters per minute per gram kidney weight (mL/min/g) and micromoles per minute per gram kidney weight (μmol/min/g), respectively.
Specific Protocols
(1) Effects of Renal Decapsulation With and Without Intrarenal cGMP Administration on RIHP, UNaV, RI cGMP, RBF, and GFR After Increasing RPP
Rats (n=42) were anesthetized with ketamine and xylazine, underwent an acute uninephrectomy, and were studied according to our P-N protocol. Three groups were evaluated: (1) intact renal capsule: rats received RI V infusion during both the 30-min control and 30-min high RPP periods and served as time controls. RIHP, UNaV, and RI cGMP levels were recorded for each period. (2) Partial renal decapsulation: the renal capsule was partially removed prior to the onset of the control period. Partial decapsulation was performed in order to increase stability of the RIHP catheter (anchored to the renal capsule). Animals received RI V infusion during both the 30-min control and the 30-min high RPP periods. RIHP, UNaV, and RI cGMP levels were recorded for each period. (3) Partial renal decapsulation + RI cGMP infusion: rats received RI infusion of cGMP (18 μg/kg/min) during the 30-min high RPP period following a 30-min control period with infusion of V. UNaV and RI cGMP levels were recorded for each period. RBF and GFR were measured in a separate series of animals (n=24), also evaluated in the three groups previously described.
(2) Effects of RI Albumin Administration in the Presence and Absence of Probenecid or ODQ on RIHP, UNaV, and RI cGMP
Rats (n=51) were anesthetized with ketamine and xylazine and subjected to acute uninephrectomy. Three groups were evaluated: (1) 2% albumin: rats received no RI infusion during the 30-min control period, and received a 100 μL bolus infusion of 2% albumin into the RI compartment over 30 seconds at the onset of the 30-min experimental period (a variant of the method described by Granger et al.).14 (2) 2% albumin + probenecid (PB): rats received no RI infusion during the 30-min control period and received a 2% albumin bolus followed by the RI infusion of PB (10 μg/kg/min) throughout the 30-min experimental period. (3) 2% albumin + ODQ: rats received no RI infusion during the 30-min control period, and received a 2% albumin bolus followed by the RI infusion of ODQ (0.12 mg/kg/min) throughout the 30-min experimental period. RIHP, UNaV, and RI cGMP were recorded for each period.
(3) Effects of Intrarenal cGMP Administration on RIHP, RBF, RCBF, and RMBF
Rats (n=30) were anesthetized with pentobarbital and underwent an acute uninephrectomy. Two groups were evaluated: (1) time control: rats received RI V infusion during both the 1-h control and 1-h experimental periods. (2) cGMP: rats received RI cGMP infusion (18 μg/kg/min) for 1-h during the experimental period after a 1-h control infusion of V. RIHP and RI cGMP levels were recorded for each period. In a separate group of animals (n=7), cGMP (18 μg/kg/min) was infused into the RI compartment for 1-h during the experimental period after a 1-h control infusion of V, and RBF, RCBF, and RMBF were measured during both periods.
(4) Effects of Intrarenal cGMP Administration on UNaV, Urine Flow Rate, MAP, GFR, FENa, and FELi
Rats (n=15) were anesthetized with pentobarbital and studied with both kidneys intact. Inulin and lithium chloride in V were infused throughout the study via the internal jugular catheter. cGMP (18 μg/kg/min) was infused into the RI compartment of the left (experimental) kidney for 1-h after a 1-h control infusion of V. The right (control) kidney received RI V infusion during both the 1-h control and the 1-h experimental periods and served as the time control. UNaV, urine flow, MAP, GFR, FENa, and FELi were recorded for each period.
Statistical Analysis
Results are expressed as mean ± standard error (SE). Paired Student’s t test was used for comparing two periods within the same group of animals. Paired Student’s t test was also used for experiments utilizing two intact kidneys in the same group of animals [Protocol 4]. Two-sample Student’s t test was used when comparing two different groups of animals. When multiple comparisons were made across more than two treatment groups, 1-way analysis of variance (ANOVA) with a repeat measures term was used. The ANOVA test was followed by post hoc testing using Dunnett’s test. P<0.05 was considered statistically significant.
RESULTS
Effects of Renal Decapsulation With and Without Intrarenal cGMP Administration on RIHP, UNaV, RI cGMP, RBF, and GFR After Increasing RPP
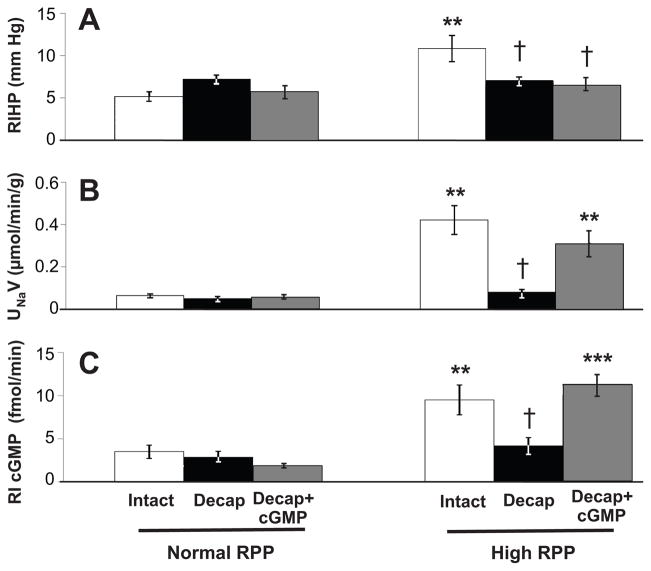
Figure 1A demonstrates that in rats with an intact renal capsule, RIHP increased (5.2 ± 0.6 to 10.9 ± 1.6 mm Hg; P<0.01) after an increase in RPP (96.5 ± 3.6 to 154.3 ± 4.8 mm Hg; P<0.001). In rats that underwent renal decapsulation, including those that received intrarenal cGMP administration, there was not a significant increase in RIHP despite a significant increase in RPP (99.6 ± 2.5 to 165.6 ± 5.2 mm Hg and 98.3 ± 2.3 to 155.9 ± 3.8 mm Hg, respectively; P<0.001 for each) (see Figure S1A, http://hyper.ahajournals.org, for RPP data). As shown in Figure 1B, rats with an intact capsule demonstrated an increase in UNaV (0.062 ± 0.009 to 0.420 ± 0.068 μmol/min/g; P<0.01) following a significant increase in RPP (96.8 ± 2.6 to 156.6 ± 3.8 mm Hg; P<0.001). Decapsulated rats had no change in UNaV despite a significant increase in RPP (98.1 ± 1.9 to 158.1 ± 2.3 mm Hg; P<0.001). However, RI infusion of cGMP in decapsulated animals restored the increase in UNaV (0.067 ± 0.010 to 0.310 ± 0.061 μmol/min/g; P<0.01) following an increase in RPP (96.6 ± 1.0 to 154.4 ± 3.1 mm Hg; P<0.001). There was no significant difference between the increase in natriuresis seen in animals with an intact capsule and in those with a partial decapsulation with RI infusion of cGMP (see Figure S1B, http://hyper.ahajournals.org, for RPP data). Figure 1C demonstrates that RI cGMP levels increased significantly (3.5 ± 0.8 to 9.5 ± 1.7 fmol/min; P<0.01) in rats with an intact capsule following an increase in RPP (96.8 ± 2.6 to 156.6 ± 3.8 mm Hg; P<0.001). There was no change in RI cGMP levels in decapsulated rats despite a significant increase in RPP (98.1 ± 1.9 to 158.1 ± 2.3 mm Hg; P<0.001). Rats that received RI infusion of cGMP had a significant increase in RI cGMP levels (1.9 ± 0.4 to 11.3 ± 1.1 fmol/min; P<0.001) (see Figure S1B, http://hyper.ahajournals.org, for RPP data).
Figure 1.
A, Renal interstitial hydrostatic pressure (RIHP) in uninephrectomized, anesthetized rats during normal or high renal perfusion pressure (RPP) in rats with an intact renal capsule (white bars, n=7), after partial decapsulation (Decap) (black bars, n=6), or after partial decapsulation with RI cortical infusion of cGMP during the experimental period (gray bars, n=7). B, Urine Na+ excretion (UNaV) in response to normal or high RPP in rats under conditions in A (intact capsule, n=8; partial decapsulation, n=7; partial decapsulation with RI cortical infusion of cGMP during the experimental period, n=7). Results are reported per gram (g) kidney weight. C, RI cGMP levels in response to normal or high RPP in rats under conditions in A (intact capsule, n=8; partial decapsulation, n=7; partial decapsulation with RI cortical infusion of cGMP during the experimental period, n=7). Data are shown as mean ± SE. ***P<0.001, **P<0.01 vs own control; †P<0.05 vs intact capsule (ANOVA with Dunnett’s).
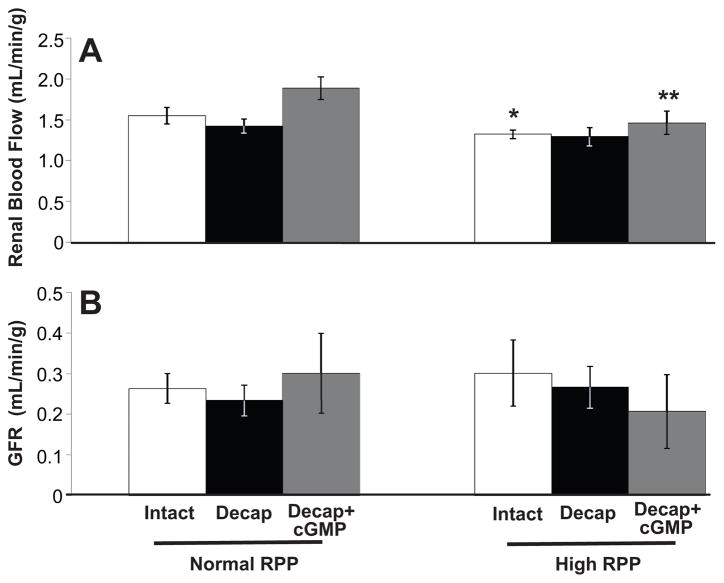
As shown in Figure 2A, RBF decreased (1.5 ± 0.1 to 1.3 ± 0.1 mL/min/g; P<0.05) in rats with an intact capsule following an increase in RPP (93.6 ± 2.8 to 146.3 ± 2.2 mm Hg; P<0.001). There was no change in RBF in decapsulated animals after a significant increase in RPP (91.3 ± 5.9 to 135.3 ± 6.8 mm Hg; P<0.001). RBF decreased (1.9 ± 0.1 to 1.5 ± 0.1 mL/min/g; P<0.01) after a significant increase in RPP (93.1 ± 2.2 to 136.4 ± 3.6 mm Hg; P<0.001) in animals that underwent a partial decapsulation followed by RI infusion of cGMP. There were no significant differences in RBF between the group of animals that had been decapsulated versus the animals with an intact capsule, or between the animals that had been decapsulated and infused with cGMP versus the animals with an intact capsule. Figure 2B demonstrates that GFR did not differ significantly in the three groups of animals (intact capsule, decapsulated, or decapsulated with RI cGMP infusion) (see Figure S2, http://hyper.ahajournals.org, for RPP data).
Figure 2.
A, Renal blood flow (RBF) in uninephrectomized, anesthetized rats during normal or high renal perfusion pressure (RPP) in rats with an intact renal capsule (white bars, n=8), after partial decapsulation (Decap) (black bars, n=8), or after partial decapsulation with RI cortical infusion of cGMP during the experimental period (gray bars, n=8). Results are reported per gram (g) kidney weight. B, Glomerular filtration rate (GFR) in response to normal or high RPP in rats under conditions in A (intact capsule, n=8; partial decapsulation, n=8; partial decapsulation with RI cortical infusion of cGMP during the experimental period, n=8). Results are reported per gram (g) kidney weight. Data are shown as mean ± SE. **P<0.01, *P<0.05 vs own control.
Effects of RI Albumin Administration in the Presence and Absence of Probenecid or ODQ on RIHP, UNaV, and RI cGMP
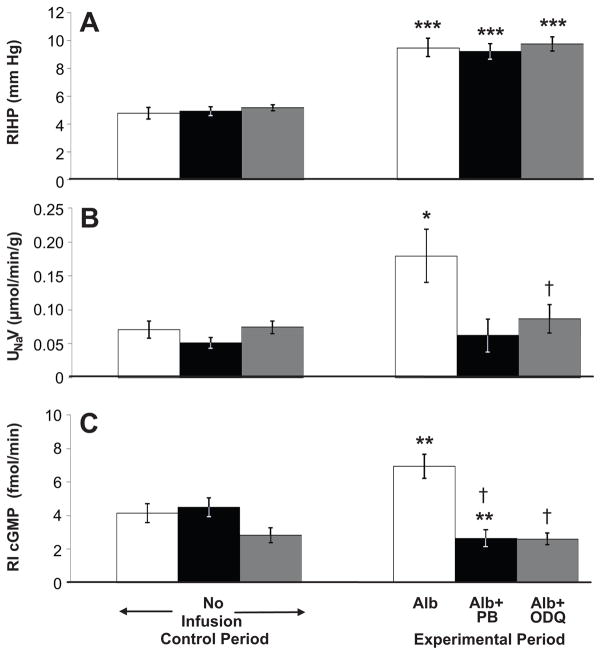
As seen in Figure 3A, RIHP increased in rats that received either a RI bolus of 2% albumin alone, 2% albumin + PB, or 2% albumin + ODQ (4.8 ± 0.4 to 9.5 ± 0.7 mm Hg, P<0.001; 4.9 ± 0.3 to 9.2 ± 0.6 mm Hg, P<0.001; 5.2 ± 0.2 to 9.7 ± 0.5 mm Hg, P<0.001 respectively). MAP was not significantly different between the control and experimental periods for each group of animals. The MAP for animals receiving albumin and ODQ was significantly different from the MAP for animals receiving an albumin bolus alone (see Figure S3A, http://hyper.ahajournals.org, for MAP data). Figure 3B demonstrates that RI infusion of 2% albumin increased UNaV (0.071 ± 0.013 to 0.179 ± 0.039 μmol/min/g; P<0.05), while there was no change in rats that received 2% albumin + PB, or 2% albumin + ODQ. UNaV was significantly different between the animals receiving albumin and ODQ and those receiving an albumin bolus alone, whereas it was not significantly different between the animals receiving albumin and PB and those receiving only an albumin bolus. MAP was not significantly different between the control and experimental periods for each group of animals (see Figure S3B, http://hyper.ahajournals.org, for MAP data). Figure 3C demonstrates that the RI bolus of 2% albumin increased RI cGMP levels (4.1 ± 0.6 to 6.9 ± 0.7 fmol/min; P<0.01) while there was no effect following RI infusion of 2% albumin + ODQ. The RI infusion of 2% albumin + PB significantly decreased RI cGMP levels (4.5 ± 0.6 to 2.6 ± 0.5 fmol/min; P<0.01). MAP was not significantly different between the control and experimental periods for each group of animals (Figure S3B, http://hyper.ahajournals.org).
Figure 3.
A, Renal interstitial hydrostatic pressure (RIHP) in uninephrectomized, anesthetized rats during control and experimental periods in response to RI bolus of 2% albumin (Alb) during the experimental period (white bars, n=6), RI bolus of albumin followed by RI infusion of probenecid (PB) (black bars, n=7), and RI bolus of albumin followed by RI infusion of ODQ (gray bars, n=8). B, Urine Na+ excretion (UNaV) in response to RI infusions as for A (Albumin bolus alone, n=8; Albumin bolus + PB, n=7; Albumin bolus + ODQ, n=13). Results are reported per gram (g) kidney weight. C, RI cGMP levels in response to RI infusions as for A (Albumin bolus alone, n=10; Albumin bolus + PB, n=6; Albumin bolus + ODQ, n=13). Data are shown as mean ± SE. ***P<0.001, **P<0.01, *P<0.05 vs own control; †P<0.05 vs albumin bolus alone (ANOVA with Dunnett’s).
Effects of Intrarenal cGMP Administration on RIHP, RBF, RCBF, and RMBF
Figure 4 demonstrates that RI infusion of cGMP increased RIHP (6.7 ± 0.4 to 10.3 ± 0.9 mm Hg; P<0.001) while RI V infusion had no effect. MAP was not significantly different between the control and experimental periods (Figure S4A, http://hyper.ahajournals.org). RI cGMP infusion significantly increased RI cGMP levels (4.1 ± 0.7 to 8.8 ± 0.5 fmol/min; P<0.001) while RI V infusion had no effect (Figure S4B, http://hyper.ahajournals.org). MAP was not significantly different between the control and experimental periods for these animals (Figure S4C, http://hyper.ahajournals.org). RI infusion of cGMP had no effect on total RBF (Figure S5A, http://hyper.ahajournals.org), RCBF, or RMBF (Figure S5B, http://hyper.ahajournals.org), and MAP was not significantly different between the control and experimental periods for these animals (Figure S5C, http://hyper.ahajournals.org).
Figure 4.
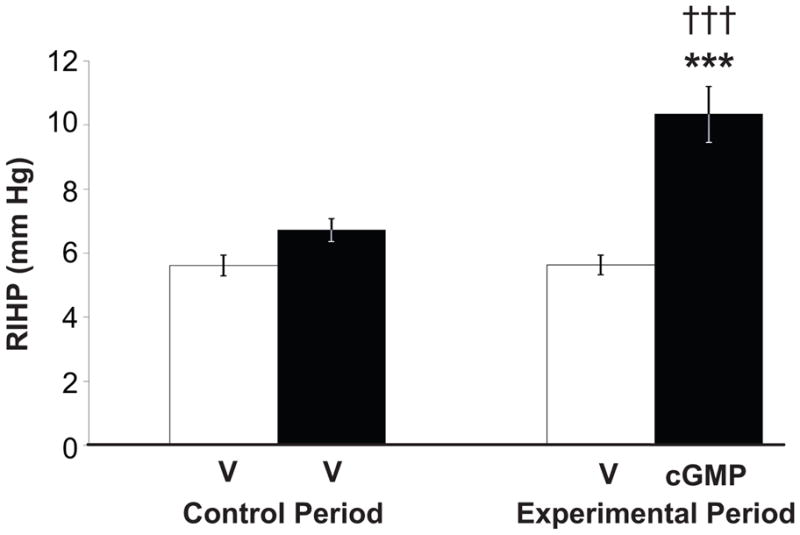
Renal interstitial hydrostatic pressure (RIHP) in uninephrectomized, anesthetized rats during control and experimental periods in response to renal interstitial (RI) cortical infusion of D5W vehicle (V) during both periods (time control, white bars, n=8) or cGMP during the experimental period (black bars, n=10). Data are shown as mean ± SE. ***P<0.001 vs own control; †††P<0.001 vs time control (Two-sample Student’s t test).
Effects of Intrarenal cGMP Administration on UNaV, Urine Flow Rate, MAP, GFR, FENa, and FELi
RI infusion of cGMP increased UNaV (0.052 ± 0.007 to 0.107 ± 0.012 μmol/min/g; P<0.001) while RI V infusion had no effect (Figure S6A, http://hyper.ahajournals.org). RI infusion of cGMP increased urine flow rate (0.0017 ± 0.0002 mL/min to 0.0032 ± 0.0003 mL/min/g; P<0.001), while RI V infusion had no effect (Figure S6B, http://hyper.ahajournals.org). MAP was not influenced by cGMP infusion (Figure S6C, http://hyper.ahajournals.org). RI infusion of either V or cGMP had no significant effect on GFR (Figure S6D, http://hyper.ahajournals.org). RI infusion of cGMP significantly increased FENa (0.14 ± 0.04 to 0.24 ± 0.03%; P<0.05) while RI V infusion had no effect (Figure S6E, http://hyper.ahajournals.org). RI infusion of cGMP significantly increased FELi (22.0 ± 2.0 to 35.7 ± 3.4%; P<0.01) while RI V infusion had no effect (Figure S6F, http://hyper.ahajournals.org).
DISCUSSION
RI cGMP is an important modulating factor in P-N. Studies from our laboratory have suggested that RI cGMP acts through a mechanism that is independent of changes in renal hemodynamics and involves protein kinase G.3, 15 In vitro studies in human RPT cells, and in vivo studies in rats demonstrate that the production of cGMP by sGC and its export into the extracellular compartment though a probenecid-sensitive OAT-inhibitor are critical mechanisms for natriuretic responses to increased RPP.4, 5, 15
RIHP is another modulating factor of P-N. Increases in RPP lead to increases in RIHP, and the subsequent natriuresis is abolished by renal decapsulation.7 Increases in RIHP made by RI volume expansion lead to increases in natriuresis.6, 16 Our studies address the relationship between these two important modulating factors of the P-N relationship.
The major findings of the present studies are: (1) decreasing RIHP by renal decapsulation prevents the rise in RI cGMP levels and UNaV in response to increased RPP, and natriuresis can be restored in decapsulated animals with exogenous RI cGMP replacement; (2) directly increasing RIHP without changing MAP increases RI cGMP and UNaV, both of which can be blocked by inhibiting transport of cGMP from the intracellular to the extracellular compartment with PB, or by decreasing cGMP production with sGC inhibitor ODQ; and (3) exogenous RI cGMP administration increases both RIHP and UNaV. These findings are observed in the absence of increases in GFR, RBF, RCBF, and RMBF, suggesting a tubular rather than hemodynamic mechanism. These findings suggest that extracellular RI cGMP modulates P-N at least in part by augmenting RIHP, and that an increase in RIHP is required for the rise in RI cGMP levels observed after an increase in RPP.
The mechanism by which RI cGMP increases RIHP is unclear. Some investigators have suggested that an increase in RPP leads to increased RIHP through changes in renal medullary blood flow.2, 8, 17 Intrarenal infusions of vasodilatory substances are associated with increases in RIHP that are necessary for natriuresis.18, 19 While cGMP is known to be involved in vasodilation in other vascular beds, our experiments do not support this mechanism for the increase observed in RIHP. Here we show that intrarenal infusion of cGMP does not increase RBF, RCBF, and RMBF. Further studies are currently underway in our laboratory evaluating relationships between RI cGMP, RCBF, and RMBF during P-N.
Of note, in our studies we measure cortical RIHP. Previous studies have shown that while renal decapsulation significantly reduces cortical RIHP, it does not have as great an effect on medullary RIHP.7 In fact, medullary RIHP still increases significantly when renal perfusion pressure is increased, even in the absence of a renal capsule. It will be very important that we differentiate between cortical and medullary interstitial pressures in future studies to help understand the roles that each play in the P-N mechanism, and to define their relationships with RI cGMP.
The mechanism whereby an increase in RIHP leads to the production of cGMP is unknown. Studies have documented the presence of mRNA in rat renal tissues for the three isoforms of nitric oxide synthase (NOS).20 Studies in mouse inner medullary collecting duct cells have shown that shear stress, or the pressure exerted by a fluid flowing past cells in culture, can stimulate NOS to produce NO.21
Immunohistochemistry in human kidney preparations demonstrates the presence of neuronal NOS (NOS1) in the cortical and medullary segments of the nephron. sGC also appears to be present in these same portions of the nephron.22 These data suggest that the enzymes necessary for cGMP production exist in the rodent and human kidney, and that an increase in interstitial pressure and the resultant shear stress might stimulate NOS and lead to cGMP production by sGC. NO is able to diffuse across many cellular membranes, and therefore NO produced in the renal interstitium could diffuse into surrounding renal tubule cells.23
The precise mechanism by which cGMP inhibits Na+ reabsorption is unclear. Our data suggest that cGMP exits the renal tubule cell through one or more PB-sensitive anion channels.4, 5 Multidrug-resistance protein 5 has been shown to export cGMP, and localizes to the basolateral membranes of canine kidney cells.24, 25 cGMP may exit the tubule cell through this channel, and inhibit Na+ reabsorption by binding to a plasma membrane-bound receptor or channel involved in renal Na+ regulation, such as Na,K-ATPase. Both in vitro and in vivo animal studies have suggested that cGMP may act at this important Na+ pump to reduce Na+ reabsorption.26–28 Some investigators have suggested that stimulation of the NO-cGMP pathway may internalize this pump, thereby inactivating it.26
The segment of the nephron involved in RI cGMP-mediated inhibition of Na+ reabsorption requires further delineation. Studies from our laboratory have suggested that the proximal tubule is involved, as RI cGMP administration increases both FENa and FELi.4, 29 In vitro studies in human RPT cells using a Na+-sensitive fluorescent indicator have demonstrated that exogenous administration of cGMP decreases cellular Na+ uptake.5 The RPT would be an effective site of action, as this nephron segment reabsorbs the majority of filtered Na+.30
PERSPECTIVES
These studies suggest that an increase in RIHP is required for the rise in RI cGMP and UNaV seen in the P-N response, and lend support to the hypothesis that extracellular transport of cGMP is required for natriuresis. Furthermore, the direct RI administration of cGMP leads to an increase in RIHP through an unknown mechanism. These findings suggest a positive-feedback loop wherein an increase in RPP leads to increased RIHP and RI cGMP, natriuresis, and a further increase in RIHP. Such a feedback loop might allow small changes in RPP to translate into a larger and sustained natriuresis and diuresis, enhancing normal homeostatic protection against a rise in BP. Further studies are underway to determine the mechanisms by which cGMP induces natriuresis and increases RIHP.
Supplementary Material
Acknowledgments
Dr. Lieb is the recipient of the Warren Trust Fellowship of the Consortium for Southeastern Hypertension Control, and thanks them for their support and encouragement. The authors also wish to thank Drs. Susanna Keller and Shetal Padia for their insightful comments during the production of this manuscript.
SOURCES OF FUNDING
This work was supported by the National Institutes of Health, grants R01-HL-081891 and 5T32-DK-007646 to R.M.C., and 1F32-DK-082110 to D.C.L.
As per the National Institutes of Health:
“[This research] was supported by Award Number F32DK082110 from the National Institute of Diabetes and Digestive and Kidney Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institutes of Health.”
Footnotes
DISCLOSURES
None.
Publisher's Disclaimer: This is an un-copyedited author manuscript that was accepted for publication in Hypertension, copyright The American Heart Association. This may not be duplicated or reproduced, other than for personal use or within the “Fair Use of Copyrighted Materials” (section 107, title 17, U.S. Code) without prior permission of the copyright owner, The American Heart Association. The final copyedited article, which is the version of record, can be found at http://hyper.ahajournals.org. The American Heart Association disclaims any responsibility or liability for errors or omissions in this version of the manuscript or in any version derived from it by the National Institutes of Health or other parties.
References
- 1.Roman RJ. Pressure diuresis mechanism in the control of renal function and arterial pressure. Fed Proc. 1986;45:2878–2884. [PubMed] [Google Scholar]
- 2.Evans RG, Majid DS, Eppel GA. Mechanisms mediating pressure natriuresis: what we know and what we need to find out. Clin Exp Pharmacol Physiol. 2005;32:400–409. doi: 10.1111/j.1440-1681.2005.04202.x. [DOI] [PubMed] [Google Scholar]
- 3.Jin XH, McGrath HE, Gildea JJ, Siragy HM, Felder RA, Carey RM. Renal interstitial guanosine cyclic 3′, 5′-monophosphate mediates pressure-natriuresis via protein kinase G. Hypertension. 2004;43:1133–1139. doi: 10.1161/01.HYP.0000123574.60586.7d. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed F, Kemp BA, Howell NL, Siragy HM, Carey RM. Extracellular renal guanosine cyclic 3′5′-monophosphate modulates nitric oxide and pressure-induced natriuresis. Hypertension. 2007;50:958–963. doi: 10.1161/HYPERTENSIONAHA.107.092973. [DOI] [PubMed] [Google Scholar]
- 5.Sasaki S, Siragy HM, Gildea JJ, Felder RA, Carey RM. Production and role of extracellular guanosine cyclic 3′, 5′ monophosphate in sodium uptake in human proximal tubule cells. Hypertension. 2004;43:286–291. doi: 10.1161/01.HYP.0000112421.18551.1e. [DOI] [PubMed] [Google Scholar]
- 6.Granger JP. Regulation of sodium excretion by renal interstitial hydrostatic pressure. Fed Proc. 1986;45:2892–2896. [PubMed] [Google Scholar]
- 7.Garcia-Estan J, Roman RJ. Role of renal interstitial hydrostatic pressure in the pressure diuresis response. Am J Physiol. 1989;256:F63–70. doi: 10.1152/ajprenal.1989.256.1.F63. [DOI] [PubMed] [Google Scholar]
- 8.Mattson DL. Importance of the renal medullary circulation in the control of sodium excretion and blood pressure. Am J Physiol Regul Integr Comp Physiol. 2003;284:R13–27. doi: 10.1152/ajpregu.00321.2002. [DOI] [PubMed] [Google Scholar]
- 9.Williams JM, Sarkis A, Lopez B, Ryan RP, Flasch AK, Roman RJ. Elevations in renal interstitial hydrostatic pressure and 20-hydroxyeicosatetraenoic acid contribute to pressure natriuresis. Hypertension. 2007;49:687–694. doi: 10.1161/01.HYP.0000255753.89363.47. [DOI] [PubMed] [Google Scholar]
- 10.Siragy HM, Carey RM. The subtype-2 (AT2) angiotensin receptor regulates renal cyclic guanosine 3′, 5′-monophosphate and AT1 receptor-mediated prostaglandin E2 production in conscious rats. J Clin Invest. 1996;97:1978–1982. doi: 10.1172/JCI118630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muchant DG, Thornhill BA, Belmonte DC, Felder RA, Baertschi A, Chevalier RL. Chronic sodium loading augments natriuretic response to acute volume expansion in the preweaned rat. Am J Physiol. 1995;269:R15–22. doi: 10.1152/ajpregu.1995.269.1.R15. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura T, Alberola AM, Granger JP. Role of renal interstitial pressure as a mediator of sodium retention during systemic blockade of nitric oxide. Hypertension. 1993;21:956–960. doi: 10.1161/01.hyp.21.6.956. [DOI] [PubMed] [Google Scholar]
- 13.Roman RJ, Cowley AW., Jr Characterization of a new model for the study of pressure-natriuresis in the rat. Am J Physiol. 1985;248:F190–198. doi: 10.1152/ajprenal.1985.248.2.F190. [DOI] [PubMed] [Google Scholar]
- 14.Granger JP, Haas JA, Pawlowska D, Knox FG. Effect of direct increases in renal interstitial hydrostatic pressure on sodium excretion. Am J Physiol. 1988;254:F527–532. doi: 10.1152/ajprenal.1988.254.4.F527. [DOI] [PubMed] [Google Scholar]
- 15.Jin XH, Siragy HM, Carey RM. Renal interstitial cGMP mediates natriuresis by direct tubule mechanism. Hypertension. 2001;38:309–316. doi: 10.1161/01.hyp.38.3.309. [DOI] [PubMed] [Google Scholar]
- 16.Wilcox CS, Sterzel RB, Dunckel PT, Mohrmann M, Perfetto M. Renal interstitial pressure and sodium excretion during hilar lymphatic ligation. Am J Physiol. 1984;247:F344–351. doi: 10.1152/ajprenal.1984.247.2.F344. [DOI] [PubMed] [Google Scholar]
- 17.Roman RJ, Cowley AW, Jr, Garcia-Estan J, Lombard JH. Pressure-diuresis in volume-expanded rats. Cortical and medullary hemodynamics. Hypertension. 1988;12:168–176. doi: 10.1161/01.hyp.12.2.168. [DOI] [PubMed] [Google Scholar]
- 18.Haas JA, Hammond TG, Granger JP, Blaine EH, Knox FG. Mechanism of natriuresis during intrarenal infusion of prostaglandins. Am J Physiol. 1984;247:F475–479. doi: 10.1152/ajprenal.1984.247.3.F475. [DOI] [PubMed] [Google Scholar]
- 19.Hartupee DA, Burnett JC, Jr, Mertz JI, Knox FG. Acetylcholine-induced vasodilation without natriuresis during control of interstitial pressure. Am J Physiol. 1982;243:F325–329. doi: 10.1152/ajprenal.1982.243.4.F325. [DOI] [PubMed] [Google Scholar]
- 20.Shin SJ, Lai FJ, Wen JD, Lin SR, Hsieh MC, Hsiao PJ, Tsai JH. Increased nitric oxide synthase mRNA expression in the renal medulla of water-deprived rats. Kidney Int. 1999;56:2191–2202. doi: 10.1046/j.1523-1755.1999.00795.x. [DOI] [PubMed] [Google Scholar]
- 21.Cai Z, Xin J, Pollock DM, Pollock JS. Shear stress-mediated NO production in inner medullary collecting duct cells. Am J Physiol Renal Physiol. 2000;279:F270–274. doi: 10.1152/ajprenal.2000.279.2.F270. [DOI] [PubMed] [Google Scholar]
- 22.Jarry A, Renaudin K, Denis MG, Robard M, Buffin-Meyer B, Karam G, Buzelin F, Paris H, Laboisse CL, Vallette G. Expression of NOS1 and soluble guanylyl cyclase by human kidney epithelial cells: morphological evidence for an autocrine/paracrine action of nitric oxide. Kidney Int. 2003;64:170–180. doi: 10.1046/j.1523-1755.2003.00078.x. [DOI] [PubMed] [Google Scholar]
- 23.Stefansson BV, Bjornson AL, Haraldsson B, Nilsson UA. A new method for monitoring nitric oxide production using Teflon membrane microdialysis. Free Radic Biol Med. 2005;39:249–256. doi: 10.1016/j.freeradbiomed.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 24.Wijnholds J, Mol CA, van Deemter L, de Haas M, Scheffer GL, Baas F, Beijnen JH, Scheper RJ, Hatse S, De Clercq E, Balzarini J, Borst P. Multidrug-resistance protein 5 is a multispecific organic anion transporter able to transport nucleotide analogs. Proc Natl Acad Sci U S A. 2000;97:7476–7481. doi: 10.1073/pnas.120159197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu HL, Gavrilyuk V, Wolde HM, Baughman VL, Pelligrino DA. Regulation of rat pial arteriolar smooth muscle relaxation in vivo through multidrug resistance protein 5-mediated cGMP efflux. Am J Physiol Heart Circ Physiol. 2004;286:H2020–2027. doi: 10.1152/ajpheart.01105.2003. [DOI] [PubMed] [Google Scholar]
- 26.Guzman NJ, Fang MZ, Tang SS, Ingelfinger JR, Garg LC. Autocrine inhibition of Na+/K(+)-ATPase by nitric oxide in mouse proximal tubule epithelial cells. J Clin Invest. 1995;95:2083–2088. doi: 10.1172/JCI117895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang M, Knox FG. Nitric oxide reduces the molecular activity of Na+,K+-ATPase in opossum kidney cells. Kidney Int. 1999;56:627–634. doi: 10.1046/j.1523-1755.1999.00583.x. [DOI] [PubMed] [Google Scholar]
- 28.Zhang C, Mayeux PR. NO/cGMP signaling modulates regulation of Na+-K+-ATPase activity by angiotensin II in rat proximal tubules. Am J Physiol Renal Physiol. 2001;280:F474–479. doi: 10.1152/ajprenal.2001.280.3.F474. [DOI] [PubMed] [Google Scholar]
- 29.Park J, Kemp BA, Howell NL, Gildea JJ, Keller SR, Carey RM. Intact microtubules are required for natriuretic responses to nitric oxide and increased renal perfusion pressure. Hypertension. 2008;51:494–499. doi: 10.1161/HYPERTENSIONAHA.107.103036. [DOI] [PubMed] [Google Scholar]
- 30.Greenberg A, Cheung AK National Kidney Foundation. Primer on kidney diseases. 3. San Diego, Calif: Academic Press; 2001. Ralph Erskine Conrad Memorial Fund. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.