Abstract
Objective
To assess the safety of interleukin-6 receptor inhibition and to collect preliminary data on clinical and immunologic efficacy of tocilizumab in patients with systemic lupus erythematosus (SLE).
Methods
In an open label, dose-escalating, Phase I study, 16 patients with mild to moderate disease activity were treated bi-weekly for 12 weeks with one of three doses (2 mg/kg [n=4], 4 mg/kg [n=6], 8 mg/kg [n=6]) of tocilizumab and followed for 8 additional weeks.
Results
The infusions were well tolerated. Tocilizumab led to dose-related decreases in absolute neutrophil count with a median decrease of 38% in the 4 mg/kg and 56% in the 8 mg/kg dose groups. Neutrophil counts returned to normal after cessation of treatment. One subject was withdrawn because of neutropenia. Infections occurred in 11 patients; none was associated with neutropenia. Disease activity showed a significant improvement with 8/15 evaluable patients having a decrease of 4 or more points in the modified SELENA-SLEDAI score. Arthritis improved in all seven patients with arthritis at baseline and resolved in four. Anti-dsDNA antibody levels decreased by a median 47% in the 4 and 8 mg/kg dose groups compared to a 7.8% decrease in IgG levels. These changes together with a significant decrease in circulating plasma cells suggest a specific effect of tocilizumab on autoantibody producing cells.
Conclusion
Although neutropenia may limit the maximum dose of tocilizumab in SLE, the observed clinical and serological response data are promising and warrant further studies to establish the optimal dosing regimen and efficacy.
Autoantibody production, complement activation, immune complex deposition, and leukocyte infiltration of target organs are key immunopathogenic events in systemic lupus erythematosus (SLE). Multiple cytokines have been implicated in regulating disease activity or organ involvement in SLE. Among these, interleukin (IL)-6, which exerts pleiotropic effects on numerous cell types (1) is thought to play an important role. In murine models of lupus an age-associated increase of serum IL-6 and abnormal expression of the IL-6 receptor have been described (2–4). Exogenous IL-6 increased autoantibody production and accelerated the progression of glomerulonephritis (5, 6), whereas, blocking IL-6 or its receptor prevented increases in anti-dsDNA antibody levels, progression of proteinuria and improved mortality (7–9).
Lupus patients have elevated serum IL-6 levels (10–13) that correlated with disease activity or anti-DNA levels in some, but not all studies. Moreover, neutralization of IL-6 led to a significant decrease in spontaneous immunoglobulin (12) and anti-dsDNA production in vitro (14). Several studies have demonstrated increased urinary excretion of IL-6 in patients with active proliferative lupus nephritis (13, 15, 16). IL-6 excretion decreased following cyclophosphamide treatment, suggesting that IL-6 may have an important role in lupus nephritis. Based on these data, we hypothesized that blocking the effect of IL-6 may be beneficial in SLE.
Tocilizumab, a humanized monoclonal antibody (mAb) against the α-chain of the IL-6 receptor, prevents the binding of IL-6 to membrane bound and soluble IL-6 receptor (17). The safety and efficacy of tocilizumab has been evaluated in clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis and Castleman’s disease (18). Here we report the data of a pilot clinical study using tocilizumab in SLE.
MATERIALS AND METHODS
Study design
This was a phase I open label, dose-escalating pilot study to evaluate the safety and tolerance of a tocilizumab in patients with SLE and to obtain preliminary evidence of its potential efficacy. The study was approved by the Institutional Review Board of NIAMS/NIDDK, National Institutes of Health (NIH). All patients signed informed consent.
Patient selection
Between 2003–2005, we enrolled 16 adult (age >18 years) patients fulfilling the American College of Rheumatology classification criteria for SLE (19, 20) at the NIH Clinical Center. All subjects had moderately active lupus defined by either of these two (a and b) sets of criteria:
-
chronic glomerulonephritis with inadequate response to at least 6 months of adequate immunosuppressive therapy (with pulse methylprednisolone, cyclophosphamide, azathioprine, cyclosporine, mycophenolate mofetil, or high dose daily corticosteroids, methotrexate or intravenous immunoglobulin IVIg), and
less than 30% increase in serum creatinine compared to lowest level during treatment,
proteinuria ≤1.5x baseline before treatment,
≤ 2+ cellular casts in the urinary sediment, and
extra-renal disease activity not exceeding a score of 10 on the non-renal components of the SELENA-SLEDAI (Safety of Estrogens in Lupus Erythematosus National Assessment Systemic Lupus Erythematosus Disease Activity Index) (21).
moderately active extra-renal lupus defined as an extra-renal SELENA-SLEDAI score in the range of 3–10. The SELENA-SLEDAI score must have been stable for at least two weeks prior to screening.
Because two of the main effects of IL-6 are on inflammatory responses and antibody production, we required the presence of at least one serologic marker of autoantibody production or systemic inflammation. Eligible subjects had to have one or more of the following: a) serum anti-dsDNA antibody level of ≥30 IU, b) IgG anticardiolipin antibody levels of ≥20 GPL, c) CRP >0.8 mg/dL, or d) ESR >25 mm/hr for men; ESR >42 mm/hr for women. Subjects had to be on stable doses of prednisone <0.3 mg/kg/day (or its equivalent) for at least 2 weeks before the first treatment. Subjects were required to use an effective form of contraception throughout the study.
The main exclusion criteria included pregnancy, any therapy with human or murine antibodies or any experimental therapy within 3 months, therapy with cyclophosphamide; pulse methylprednisolone or IVIg within 4 weeks; or azathioprine, mycophenolate mofetil, cyclosporine, or methotrexate within 2 weeks of first study treatment. Subjects with any the following laboratory abnormalities were also excluded: serum creatinine >3.0 mg/dL, WBC <3,500/μL, ANC <3,000/μL, absolute lymphocyte count ≤500/μL, Hgb <8.0 g/dL, platelets <50,000/μL, ALT and/or AST >1.5 x upper limit of normal (ULN), alkaline phosphatase >1.5 ULN, or more than 1000 EBV genome equivalents/106 PBMC.
Treatment
Tocilizumab was provided by Chugai Pharmaceutical Co., Ltd. Study subjects were assigned to ascending dose groups to receive intravenous tocilizumab every two weeks for 12 weeks with one of three doses as follows: group 1 (n= 4): 2 mg/kg, group 2 (n= 6): 4 mg/kg, group 3 (n= 6): 8 mg/kg.
Concomitant immunosuppressive therapy
All patients must have been on ≤0.3 mg/kg/day prednisone at study entry. Prednisone dose was kept stable during the first 7 weeks of treatment. After that, gradual tapering to 0.15 mg/kg/day was allowed. Temporary increases in prednisone dosage were allowed based on disease activity. Patients had to be withdrawn if they required ≥0.5 mg/kg/day of prednisone (or equivalent) or if they did not respond to a 2 weeks course of ≤0.5 mg/kg/day prednisone. The following medications were allowed provided that they were administered at stable doses at least two weeks before and during the study: hydroxychloroquine, non-steroidal anti-inflammatory drugs, ACE-inhibitors, and angiotensin receptor antagonists.
Safety and toxicity
Patients were monitored during the infusion and every 2–4 weeks for the study period of 5 months. Safety assessments included unexpected toxicities, adverse events encountered during or after the drug infusion, along with changes in vital sign measurements and clinical laboratory data. Adverse events were graded according to the National Cancer Institute Common Toxicity Criteria guidelines. Upon occurrence of a Grade 3 or 4 toxicity, at least possibly related to study drug, tocilizumab was discontinued unless it resolved spontaneously within 9 days. Patients withdrawn from treatment were followed for 8 weeks after withdrawal.
Efficacy
Clinical and laboratory data were collected for preliminary evaluation of the potential activity of tocilizumab in SLE and on measures of inflammation. Parameters used for efficacy measures were assessed at initial screening, prior to study drug infusions (weeks 0, 2, 4, 6, and 12), and during follow-up visits (Weeks 14, 16, and 20). The following criteria were used to assess efficacy:
Lupus Activity Indices: Systemic Lupus Activity Measure (SLAM) (22) and modified SELENA-SLEDAI. This index was modified to omit one of the standard parameters (“low complement”) because tocilizumab had been shown to lower complement levels in rheumatoid arthritis patients independent of disease activity (Tocilizumab Investigator’s Brochure).
Serologic markers of SLE (anti-dsDNA antibodies, anti-cardiolipin, ANA, ENA and quantitative immunoglobulins (IgG, IgA, IgM) and inflammation: C-reactive protein (CRP), ESR, fibrinogen and ferritin.
Measurement of complement activation products (CAP)
Complement activation products iC3b, C4d and C5b-9 were measured in plasma by ELISA (Quidel, Corp, San Diego, CA, USA) according to manufacturer’s instructions.
Flow cytometry
Flow cytometric analysis was performed using a FACSCalibur flow cytometer (Becton Dickinson) and CellQuest Pro software (Becton Dickinson).
Statistical analysis
Safety analysis included every patient who received one or more infusions of the study drug. Analysis of efficacy included patients who received at least three infusions of tocilizumab.
Continuous variables were summarized with means, standard deviations, medians, and ranges. Categorical variables were summarized by frequency and count of patients in corresponding categories. All statistical tests were performed at the 0.05 significance level and all confidence intervals were 95% two-sided intervals.
No formal sample size calculation was performed, as this study was designed predominantly to explore the tolerability and safety of these regimens. The choice of 16 patients was based on the experience with similar studies in the past. The study was not powered to detect differences between groups or to confirm efficacy. Therefore, there is a risk of false positive results because of the large number of endpoints being considered. As a consequence, efficacy analyses comparing the dose groups are exploratory in nature.
RESULTS
Baseline characteristics
Sixteen SLE patients (13 females, 3 males) from various ethnic backgrounds and moderate disease activity were enrolled in the study. Baseline demographic and clinical characteristics are shown in Table 1. Mean mSELENA-SLEDAI score was 11, 10, and 8 for the 2, 4, and 8 mg/kg dose groups, respectively. All but one patient were taking prednisone. All twelve patients taking hydroxycholoroquine had been on stable doses for at least three months prior to study treatment. Fifteen patients received 7 infusions of tocilizumab (four at 2 mg/kg, six at 4 mg/kg, and five at 8 mg/kg). One patient in the 8 mg/kg dose group was withdrawn after the first infusion because of Grade 3 neutropenia.
Table 1.
Baseline characteristics
| Safety analysis | ||||
|---|---|---|---|---|
| All patients (n=16) | 2 mg/kg (n=4) | 4 mg/kg (n=6) | 8 mg/kg (n=6) | |
| Age (years), median (min - max) | 36.5 (23 – 54) | 34.5 (23 – 37) | 47.5 (26 – 54) | 38.0 (28 – 47) |
| Female, n (%) | 13 (81.3%) | 4 (100%) | 4 (66.7%) | 5 (83.3%) |
| Ethnicity, n (%) | ||||
| Caucasian | 9 (56.3%) | 1 (25%) | 4 (66.7%) | 4 (66.7%) |
| African-American | 5 (31.3%) | 1 (25%) | 2 (33.3%) | 2 (33.3%) |
| Other1 | 2 (12.4%) | 2 (50%) | 0 (0%) | 0 (0%) |
| Duration of SLE (years), median (min - max) | 14.5 (1.3 – 23.6) | 11 (1.3 – 22.8) | 16.7 (6.5 – 23.6) | 15.6 (4.9 – 19.9) |
| Efficacy analysis | ||||
| All patients3 (n=15) | 2 mg/kg (n=4) | 4 mg/kg (n=6) | 8 mg/kg (n=5) 3 | |
| Renal involvement, n (%) | 5 (33.3%) | 2 (50%) | 1 (16.7%) | 2 (40%) |
| mSELENA-SLEDAI2, median (min - max) | 8 (4 – 15) | 11.5 (4 – 15) | 10 (4 – 14) | 8 (6 – 10) |
| SLAM, median (min - max) | 8 (2 – 12) | 8 (4 – 12) | 8 (2 – 9) | 7 (2 – 10) |
| Anti-dsDNA | ||||
| positive, n (%) | 13 (86.7%) | 4 (100%) | 4 (67%) | 5 (100%) |
| level (IU/mL)3, median (min - max) | 192 (0 – 1849) | 124 (37 – 457) | 121.5 (0 – 872) | 201 (51 – 1849) |
| Prednisone (mg/day), median (min - max) | 7.5 (0 – 20) | 7.5 (1.25 – 10) | 6.25 (2.5 – 15 | 10 (0 – 20) |
1 Asian, 1 Hispanic;
hypocomplementemia was excluded from the scoring (see Methods);
1 African-American male was withdrawn after the first dose
Safety
The infusions were well tolerated without any clinically significant infusion reaction. All patients experienced one or more tocilizumab treatment-related adverse events; however, most of these were mild and resolved spontaneously.
Serious adverse events
There were two serious adverse events which occurred in the same patient in the 8 mg/kg tocilizumab dose group. The first was a hospitalization for acute gastroenteritis 10 days after the second infusion. A detailed diagnostic workup failed to identify any specific cause. The patient recovered spontaneously and completed the treatment course. The same patient had pyelonephritis 8 weeks after the last dose which responded well to antibiotics. In addition to these two serious adverse events, two subjects had severe neutropenia, with an absolute neutrophil count between 500 – 1000/uL (CTC Grade 3) in the 8 mg/kg dose group.
Infections
Eleven patients (4 in the 2 mg/kg group, 4 in the 4 mg/kg group and 3 at 8 mg/kg tocilizumab dose group) had a total of 16 infections between the start of treatment and the end of follow up (Table 2). Three patients experienced repeated infections. The majority of these were upper respiratory tract (5) or urinary tract infections (3). Ten of the 16 infectious episodes were treated with systemic antibiotics or antivirals. No infection led to withdrawal from the study, but tocilizumab was delayed by two weeks when one patient in the 2 mg/kg dose group had herpes zoster keratitis. This responded to antiviral treatment and did not recur after resuming tocilizumab. All other patients continued their tocilizumab therapy uninterrupted while being treated for their infection.
Table 2.
Treatment-emergent infections observed during the study.
| Dose | Subject | Infection | Timepoint* (Week) | SAE | Treatment |
|---|---|---|---|---|---|
| 2 mg/kg | 001 | urinary tract infection | 14 | no | oral antibiotics |
| 002 | folliculitis | 2 | no | topical antibiotics | |
| upper respiratory infection | 5 | no | oral antibiotics | ||
| otitis media | 5 | no | oral antibiotics | ||
| urinary tract infection | 20 | no | oral antibiotics | ||
| 003 | sinusitis | 16 | no | oral antibiotics | |
| 004 | upper respiratory infection | 13 | no | oral antibiotics | |
| 4 mg/kg | 005 | upper respiratory infection | 15 | no | oral antibiotics |
| 007 | herpes keratitis | 5 | no | tocilizumab delayed by 2 weeks; antiviral | |
| 009 | upper respiratory infection | 8 | no | symptomatic | |
| 010 | oral candidiasis | 2 | no | topical antifungal | |
| 8 mg/kg | 013 | urinary tract infection | 12 | no | oral antibiotics |
| labial herpes simplex | 13 | no | none | ||
| acute pyelonephritis | 20 | yes | intravenous antibiotics | ||
| 014 | upper respiratory infection | 1 | no | symptomatic | |
| 020 | fungal vaginosis | 3 | no | oral antifungal |
Tocilizumab was given every two weeks until Week 12.
Laboratory Changes
Chemistries
A slight increase in mean AST within the normal range was seen during the study period. Mean total cholesterol, HDL-cholesterol, LDL-cholesterol and triglycerides increased slightly during the early treatment period but there were no clinically or statistically significant changes by the end of treatment (not shown). Mean albumin levels increased from a mean of 3.45 to 3.77 g/dL (p=0.001, Student’s t-test).
Hematologic changes
Mean platelet counts decreased slightly but the changes were not clinically significant. Conversely, hematocrit and hemoglobin values showed gradual and sustained increases with a maximum mean increase in hemoglobin of 1.54 g/dL at Week 16 in the 8 mg/kg dose group (Supplementary Figure 1).
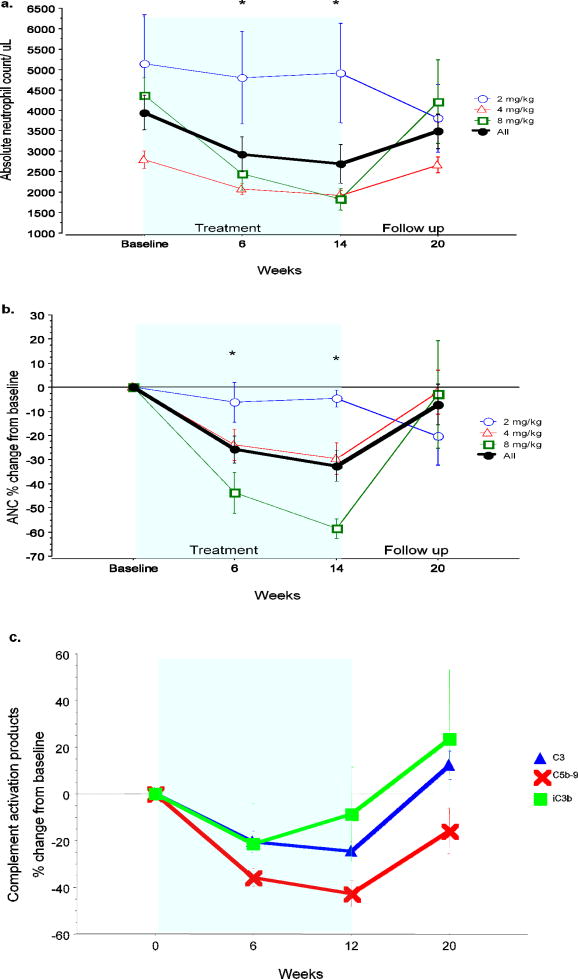
White blood cell counts decreased in all dose groups, largely because of the dose-related reduction in absolute neutrophil counts. Most of the change in neutrophils occurred following the first dose and reached its maximum at the end of treatment and returned towards baseline by Week 20 (Figure 1a and 1b). The median decrease at 14 weeks (2 weeks after the last treatment) was 2% (range 0 – 15%) in the 2 mg/kg, 38% (range 7 – 42%) in the 4 mg/kg and 56% (range 48 – 72%) in the 8 mg/kg tocilizumab dose groups. Two patients in the 8 mg/kg dose group had CTC grade 3 neutropenia (ANC < 1,000/uL): one subject was withdrawn from the study after the first dose, whereas another had it two weeks after the last dose. Neither patient had any infections or other clinical consequences related to the neutropenia and their neutrophil counts recovered spontaneously off treatment.
Figure 1. Changes in absolute neutrophil counts, and complement products.
There was a dose dependent decrease in absolute neutrophil counts. a. Absolute and b. proportional change from baseline. Values returned to baseline after treatment. c. Tocilizumab led to a similar decrease in complement C3 levels and complement activation products iC3b and C5b-9 (terminal activation complex). All returned to baseline after treatment. Values show the mean ± 1 standard error of the mean (SEM). *p< 0.005 for the whole cohort compared to baseline (repeated measures ANOVA)
Efficacy
Fifteen patients were included in the preliminary efficacy analysis; all completed the seven infusions and follow up.
Inflammatory markers
All acute phase reactants decreased promptly and significantly confirming the biologic activity of tocilizumab. Most changes occurred after the first dose and were maintained during the treatment period as shown for ESR and fibrinogen (p<0.001 at 6 and 14 weeks for both) in Supplementary Figure 2.
Serologic response
Complement C3 and C4 showed clear dose-related decreases. Since a decrease in complement levels can be caused by decreased production or increased consumption, we measured complement activation products iC3b, C4d and C5b-9 in ten patients in the 4 and 8 mg/kg dose group. All of these decreased in their absolute levels or relative to C3 (Figure 1c) or C4 with the terminal attack complex decreasing most significantly.
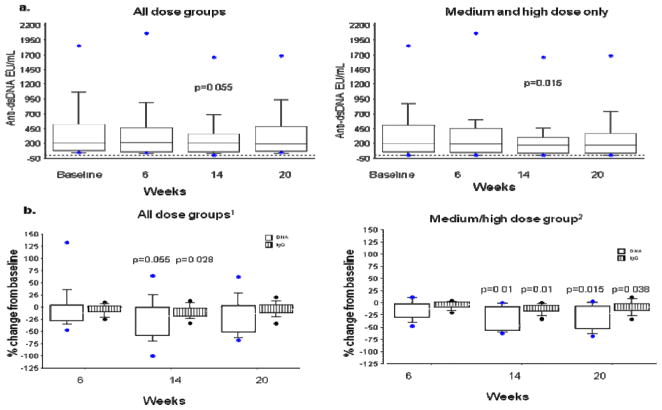
There were no significant changes in IgA and IgM levels, but by the end of the treatment, IgG levels decreased from baseline by a median of 130 mg/dL (minimum −660, maximum + 230 mg/dL; p=0.04, Wilcoxon signed rank test). Next we evaluated the effect of tocilizumab on anti-dsDNA levels. Thirteen subjects had detectable anti-dsDNA antibodies at baseline ranging from 37 to 1849 IU/mL (normal < 25). The median decrease in anti-dsDNA antibody levels at week 14 was −9 IU/mL (minimum −417, maximum + 125; p=0.03, Wilcoxon signed rank test). To adjust for the wide range of baseline values, we also evaluated the proportional change in IgG and anti-dsDNA levels in these thirteen patients and found that the relative change in anti-dsDNA was twice as much (median −17.6%) as that of IgG (median −7.91%). In contrast to the 4 and 8 mg/kg groups, where we did not observe any increase in anti-dsDNAs, 2/4 patients in the 2 mg/kg dose group had an increase in their anti-dsDNA at week 14. Therefore, we performed a subgroup analysis of the nine subjects in the combined medium/high dose groups (4 and 8 mg/kg) who had anti-dsDNA antibody levels at baseline (ranging from 51 to 1849 IU/mL). In these patients anti-dsDNA antibody levels decreased by a median of −113 IU/mL (minimum −417, maximum +1; p=0.01, Wilcoxon signed rank test) at week 14. Analysis of the proportional changes in these subjects showed a small, albeit statistically significant change in IgG levels (median change −7.8% ; min −32.7 %, max 0.0 %; p=0.01) at the end of treatment (week 14). However, this was much smaller than the −46.8% change in anti-dsDNA levels (min −62.7%, max 0.52%; p=0.01) (Figure 2). In fact, 5/9 subjects had more than 45% decrease in the level of anti-dsDNA. Levels of other autoantibodies measured, such as antinuclear antibodies, anti-SSa, anti-SSb, anticardiolipin antibodies, did not change significantly.
Figure 2. Serologic changes.
a. Anti-dsDNA levels decreased during treatment mainly in the medium/high dose group.
b. The proportional change in anti-dsDNA levels was substantially higher than the small non-significant decrease in IgG levels, especially in the medium/high dose group. Only patients with anti-dsDNA at baseline are included in this analysis. 1n=13 for the entire cohort, 2n=9 medium/high dose group.
Box plots show the 10th, 25th, 50th (median), 75th and 90th percentiles. Values above the 90th and below the 10th percentile are plotted as points. (End of treatment values were compared to baseline by the Wilcoxon signed rank test)
Changes in peripheral plasma cell frequency
There were no significant changes in total lymphocytes or in overall T or B lymphocyte counts. The frequency of CD38highCD19low IgD- plasma cells was significantly expanded in SLE patientsat baseline compared with normal controls (mean 5.3 vs 1.2%) (Figure 3). The response to tocilizumab by the end of treatment in a representative patient is shown in Figure 3. For the entire cohort a significant reduction of plasma cells was seen as early as 6 weeks (mean 3.05%) which was maintained throughout the treatment (mean 3.43% at 12 weeks) and follow up (mean 3.46% at 20 weeks).
Figure 3. Changes in circulating plasma cells.
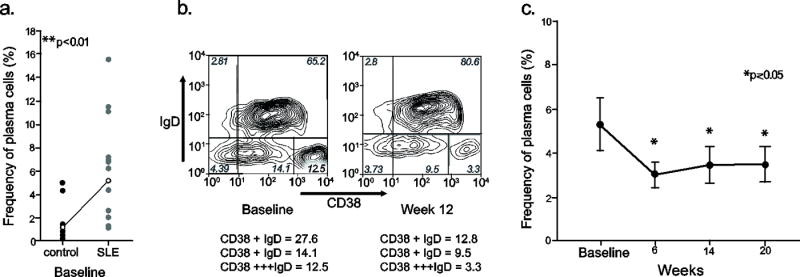
a. Patients had a significantly higher proportion of plasma cells at baseline compared to healthy controls (lines show the means of the groups; Student’s t-test) b. Flow cytometry showing a change in circulating CD38+++IgD- plasma cells before and after therapy in a representative patient. c. The frequency of plasma cells significantly decreased with tocilizumab treatment (means ± standard error of means. p value was calculated from repeated measures of ANOVA with the Bonferroni/Dunn correction for multiple comparisons).
Clinical response
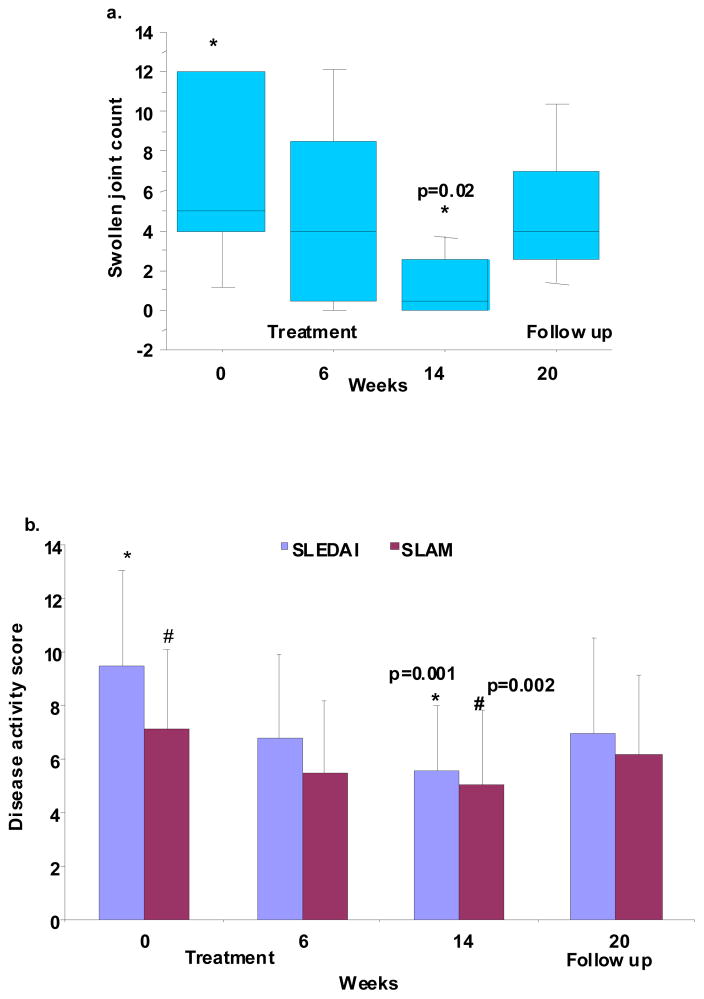
Disease activity showed a modest but significant improvement by the end of treatment (Figure 4). SLAM scores decreased from 7.1 at baseline to 5.0 at week 14 (p=0.002) and mSELENA-SLEDAI scores decreased from 9.5 to 5.5 (p=0.0001). The decrease in SLAM was mainly related to improvement of erythrocyte sedimentation rate, fatigue, and hematocrit score, whereas the decrease in mSELENA-SLEDAI was mainly because of improvement in arthritis and rash. A clinically significant improvement in mSELENA-SLEDAI score was predefined as a decrease of ≥4 points from baseline. This was achieved by 8/15 patients (3 each in the 2 and 4 mg/kg and 2 in the 8 mg/kg group). From these, 5 also achieved the recently proposed (23) criteria of an improvement of ≥7 points. Using a similar approach for SLAM, 5 subjects had ≥4 point improvement (1 in the 2 mg/kg and 2 each in the 4 and 8 mg/kg groups) and none had an improvement of ≥7 points. There was a significant decrease from baseline in both physician and patient global rating of disease activity (mean change from baseline ± standard deviation: −1.7 ±1.2 for physicians and −1.7±1.7 for patients; p<0.002 for both). Interestingly, both physician and patient global assessment decreased significantly in the 2 and 4 but not in the 8 mg/kg dose groups.
Figure 4. Clinical efficacy.
a. Improvement in arthritis. Swollen joint counts improved in all patients with arthritis at baseline (n=7) and completely resolved in 4 patients Box plots show the 10th, 25th, 50th (median), 75th and 90th percentiles. Values above the 90th and below the 10th percentile are plotted as points. (End of treatment values were compared to baseline by the Wilcoxon signed rank test)
b. Improvement in overall disease activity. mSLEDAI and SLAM scores significantly decreased at the end of treatment (means ± SD, repeated measures of ANOVA)
Seven patients had arthritis at baseline: 4 in the 4 mg/kg and 3 in the 8 mg/kg group. Mean swollen joint counts improved from 7.7 to 5.4 at six weeks and 1.1 at the end of treatment, with complete resolution of arthritis in four patients. At the last follow up, 5/7 patients had a reactivation of arthritis (mean swollen joint count 5.4). Six patients had an inflammatory rash at the start of treatment which resolved in three, between weeks 2 to 6. Fatigue, as assessed by SLAM, was present in 7 patients at baseline and resolved in 6 patients. There were three patients with no fatigue at baseline who reported fatigue on at least one occasion during the treatment period; none of these was sustained. Five patients had renal disease at baseline. All had moderate proteinuria, four had pyuria and three had hematuria. None had cellular casts. Other causes of these abnormalities, such as infection, diabetes and uncontrolled hypertension were excluded before the attribution of these abnormalities to SLE. There was no significant change in mean proteinuria during the study. The number of patients with active urinary sediment was too low to assess efficacy, but 2/2 patients with significant hematuria (urinary RBC > 10/hpf) at baseline (determined as the average of screening and pre-treatment values) and 3/4 with significant pyuria (urinary WBC > 10/hpf) had more than 50% improvement at the end of treatment (average of week 12 and 14).
Most patients (12/15) were taking 10 mg or less prednisone daily which was kept stable during the study. Prednisone dose was successfully decreased in the three patients, who took 15 (n=1) or 20 mg (n=2) daily at study entry, by an average of 7.5 mg. There was no flare during the treatment period. One subject in the 4 mg/kg dose group experienced a flare 2 weeks after her last dose and required an increase in her prednisone dose from 7.5 to 15 mg daily. Two other patients in the 2 mg/kg dose group required an increase in prednisone at the last follow up visit; one also received methotrexate for arthritis and rash.
DISCUSSION
Our data provide the first evidence that blocking IL-6 receptor by tocilizumab has an acceptable safety profile and suggest a possible immunologic and clinical benefit in SLE. Patients tolerated the infusions well with no infusion reactions. Infections were the most common adverse events with two thirds of the patients having at least one infection during the five month study period. Although the rate of infections was higher than expected, consistent with other studies using tocilizumab (24–26), most of these were mild and resolved with or without antibiotics on continued tocilizumab therapy. Larger controlled studies are necessary to obtain a better estimate of the risk of infection associated with tocilizumab compared to other drugs in SLE. Liver enzyme abnormalities and increases in serum lipids have been described with tocilizumab previously (25–27). We have not observed any clinically important liver function test abnormalities possibly because patients with significant elevations in liver function tests and those taking potentially hepatotoxic medications were excluded from the study.
The main adverse event in this study was dose-related neutropenia. Absolute neutrophil counts decreased by a median 38% and 56% in the 4 mg/kg and 8 mg/kg dose groups, respectively. Most of the changes occurred after the first dose and remained relatively stable during the treatment. Similar decreases in neutrophil counts were observed in other studies. In the OPTION study, one-third of patients with rheumatoid arthritis treated with 8 mg/kg tocilizumab every 4 weeks in combination with stable MTX had an ANC below the lower limit of normal at least once during the study (24). This is comparable to what we have observed in lupus patients who received 4 mg/kg/tocilizumab every two weeks. It is very important to note that neutropenia did not temporally correlate with infections in either study. The cause of decline in neutrophil count is not clear and requires further investigation. It may be related to decreased production or, more likely, to changes in neutrophil trafficking or both.
IL-6 has a major role in regulating acute phase responses of inflammation and blocking IL-6 is expected to reverse these effects. Accordingly, elevated markers of inflammation decreased promptly following the first dose and remained stable during the treatment phase. The anti-inflammatory effect of tocilizumab was further supported by an improvement in hemoglobin and serum albumin levels. In this study we did not see a significant improvement in proteinuria, and, therefore, the improvement in albumin levels is reflection of decreased systemic inflammation. The most common form of anemia in lupus is anemia of chronic inflammatory disease. Since red blood cells play a major role in immune complex clearance, correcting anemia may increase the clearance of circulating immune complexes and thus may contribute to a decrease in disease activity (28), in addition to improving oxygen carrying capacity and tissue oxygenation.
Tocilizumab treatment was associated with hypocomplementemia in previous studies but it was not known if this was explained by decreased production or increased consumption of complement components. Our data showing that complement activation products decrease to a similar or higher degree than C3 and C4 provide the first evidence that the tocilizumab associated hypocomplementemia represents decreased production rather than increased activation. Since hypocomplementemia can also herald active lupus, complement activation products, rather than complements should be used as markers of lupus activity in future studies with tocilizumab.
Fifteen patients were available for evaluation of efficacy. Although the number of patients was too small to carry out a formal comparison among the three dose groups we did not detect any obvious differences in clinical outcomes among them. The four patients in the 2 mg/kg group had similar trends as patients in the other two groups, with more individual variations and delayed onset of response. Overall disease activity, measured by two disease activity indices and both patient and physician global assessments, improved by the end of the treatment period. Although, the improvement was modest it was statistically significant and consistent across all measures. We a priori defined a decrease of 4 points in mSELENA-SLEDAI score as a clinically important change. This was achieved by more than half (8/15) of the patients. One third of the patients also met the more stringent criteria of a decrease of 7 points, which was recently proposed by a consensus panel (23). The slightly better response in mSELENA-SLEDAI than in the SLAM scores, reflects differences in how the two indices weigh certain manifestations of lupus, such as complete or partial resolution of arthritis and resolution of (even mild) hematuria or pyuria. Most of the changes occurred during the first six weeks and reached maximum levels by 12 week with an increase in disease activity 4–8 weeks after the last treatment. From the individual manifestations arthritis improved the most, with a complete resolution of arthritis in 4/7 patients. Five patients were enrolled in the study with renal involvement. All had chronic glomerulonephritis characterized mainly by proteinuria which did not change during the study. Proteinuria is multifactorial at this stage with significant contribution of non-inflammatory processes, such as hemodynamic abnormalities and fibrosis which may not be altered by IL-6 receptor blockade or may require more time to respond. Although we have seen some improvement in urine sediments, the number of patients with active sediment at baseline and the short duration of the study prevents any conclusion about the potential efficacy of tocilizumab in lupus nephritis.
One of the major biologic actions of IL-6 is its ability to stimulate B-lymphocyte differentiation into immunoglobulin secreting cells. Notably, we observed a statistically significant decrease in circulating plasma cells during treatment as well as a significant decline in anti-dsDNA antibody levels. This change seems to be specific since levels of other autoantibodies did not change and there was only a small, albeit statistically significant, decrease in total IgG levels. The clinical significance of the decrease in anti-dsDNA is unclear but these results are consistent with previous ex vivo observations that IL-6 blockade may alter the B cell abnormalities found in lupus.
There are limitations to our study. First, the 3-month treatment period is too short to estimate long-term toxicities of tocilizumab in SLE. Second, we excluded patients who are on concomitant immunosuppressive treatments and thus this study may not reflect everyday practice. Third, given the open label design and the lack of control group all efficacy data should be considered preliminary. Fourth, because of the small number of patients we could not make formal comparisons among the three dose groups. However, our data suggest that neutropenia is more severe in the 8 mg/kg dose group, therefore, future studies should consider evaluating doses equivalent to 4 mg/kg given every two weeks.
This pilot study provides the first data that tocilizumab can effectively block IL-6 in patients with SLE. The improvements in inflammatory markers as well as clinical and serologic manifestations of lupus activity are encouraging and should be explored further in controlled studies using tocilizumab.
Supplementary Material
a. Platelet counts decreased whereas b. hemoglobin increased during treatment. Values returned to baseline after treatment. Values show the mean ± 1 standard error of the mean (SEM).
Inflammatory markers such as a. erythrocyte sedimentation rate and b. fibrinogen levels decreased significantly after the first treatment and remained low for the duration of therapy. Values show the mean ± 1 standard error of the mean (SEM). For the whole cohort * p<0.001 (repeated measures of ANOVA).
Acknowledgments
We would like to thank Drs. Larissa Lapteva, and Sarah Okada for their assistance with patient evaluations, Ms. Margaret Brown and Tuyet-Hang Pham for their technical assistance and the Nursing Staff of the NIH Clinical Center 5SW Day Hospital for their dedicated care of the patients. The study was supported by the Intramural Research Program of the National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health and was performed under a Collaborative Research And Development Agreement with Chugai Pharmaceutical Co., Ltd.
References
- 1.Kishimoto T. IL-6: from laboratory to bedside. Clin Rev Allergy Immunol. 2005;28(3):177–86. doi: 10.1385/CRIAI:28:3:177. [DOI] [PubMed] [Google Scholar]
- 2.Kobayashi I, Matsuda T, Saito T, Yasukawa K, Kikutani H, Hirano T, et al. Abnormal distribution of IL-6 receptor in aged MRL/lpr mice: elevated expression on B cells and absence on CD4+ cells. Int Immunol. 1992;4(12):1407–1412. doi: 10.1093/intimm/4.12.1407. [DOI] [PubMed] [Google Scholar]
- 3.Suzuki H, Yasukawa K, Saito T, Narazaki M, Hasegawa A, Taga T, et al. Serum soluble interleukin-6 receptor in MRL/lpr mice is elevated with age and mediates the interleukin-6 signal. Eur J Immunol. 1993;23(5):1078–1082. doi: 10.1002/eji.1830230515. [DOI] [PubMed] [Google Scholar]
- 4.Tang B, Matsuda T, Akira S, Nagata N, Ikehara S, Hirano T, et al. Age-associated increase in interleukin 6 in MRL/lpr mice. Int Immunol. 1991;3(3):273–278. doi: 10.1093/intimm/3.3.273. [DOI] [PubMed] [Google Scholar]
- 5.Ryffel B, Car BD, Gunn H, Roman D, Hiestand P, Mihatsch MJ. Interleukin-6 exacerbates glomerulonephritis in (NZB × NZW)F1 mice. Am J Pathol. 1994;144(5):927–937. [PMC free article] [PubMed] [Google Scholar]
- 6.Yang G, Liu H, Jiang M, Jiang X, Li S, Yuan Y, et al. Experimental study on intramuscular injection of eukaryotic expression vector pcDNA3- IL-6 on BXSB mice. Chin Med J. 1998;111(1):38–42. [PubMed] [Google Scholar]
- 7.Finck BK, Chan B, Wofsy D. Interleukin 6 promotes murine lupus in NZB/NZW F1 mice. J Clin Invest. 1994;94(2):585–591. doi: 10.1172/JCI117373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liang B, Gardner DB, Griswold DE, Bugelski PJ, Song XY. Anti-interleukin-6 monoclonal antibody inhibits autoimmune responses in a murine model of systemic lupus erythematosus. Immunology. 2006;119(3):296–305. doi: 10.1111/j.1365-2567.2006.02433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mihara M, Takagi N, Takeda Y, Ohsugi Y. IL-6 receptor blockage inhibits the onset of autoimmune kidney disease in NZB/W F1 mice. Clin Exp Immunol. 1998;112(3):397–402. doi: 10.1046/j.1365-2249.1998.00612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chun HY, Chung JW, Kim HA, Yun JM, Jeon JY, Ye YM, et al. Cytokine IL-6 and IL-10 as biomarkers in systemic lupus erythematosus. J Clin Immunol. 2007;27(5):461–6. doi: 10.1007/s10875-007-9104-0. [DOI] [PubMed] [Google Scholar]
- 11.Grondal G, Gunnarsson I, Ronnelid J, Rogberg S, Klareskog L, Lundberg I. Cytokine production, serum levels and disease activity in systemic lupus erythematosus. Clin Exp Rheumatol. 2000;18(5):565–570. [PubMed] [Google Scholar]
- 12.Linker-Israeli M, Deans RJ, Wallace DJ, Prehn J, Ozeri-Chen T, Klinenberg JR. Elevated levels of endogenous IL-6 in systemic lupus erythematosus. A putative role in pathogenesis. J Immunol. 1991;147(1):117–123. [PubMed] [Google Scholar]
- 13.Peterson E, Robertson AD, Emlen W. Serum and urinary interleukin-6 in systemic lupus erythematosus. Lupus. 1996;5(6):571–575. doi: 10.1177/096120339600500603. [DOI] [PubMed] [Google Scholar]
- 14.Klashman DJ, Martin RA, Martinez-Maza O, Stevens RH. In vitro regulation of B cell differentiation by interleukin-6 and soluble CD23 in systemic lupus erythematosus B cell subpopulations and antigen-induced normal B cells. Arthritis Rheum. 1991;34(3):276–286. doi: 10.1002/art.1780340305. [DOI] [PubMed] [Google Scholar]
- 15.Iwano M, Dohi K, Hirata E, Kurumatani N, Horii Y, Shiiki H, et al. Urinary levels of IL-6 in patients with active lupus nephritis. Clin Nephrol. 1993;40(1):16–21. [PubMed] [Google Scholar]
- 16.Tsai CY, Wu TH, Yu CL, Lu JY, Tsai YY. Increased excretions of beta2-microglobulin, IL-6, and IL-8 and decreased excretion of Tamm-Horsfall glycoprotein in urine of patients with active lupus nephritis. Nephron. 2000;85(3):207–214. doi: 10.1159/000045663. [DOI] [PubMed] [Google Scholar]
- 17.Nishimoto N, Kishimoto T. Humanized antihuman IL-6 receptor antibody, tocilizumab. Handb Exp Pharmacol. 2008;(181):151–60. doi: 10.1007/978-3-540-73259-4_7. [DOI] [PubMed] [Google Scholar]
- 18.Straub RH, Harle P, Yamana S, Matsuda T, Takasugi K, Kishimoto T, et al. Anti-interleukin-6 receptor antibody therapy favors adrenal androgen secretion in patients with rheumatoid arthritis: a randomized, double-blind, placebo-controlled study. Arthritis Rheum. 2006;54(6):1778–85. doi: 10.1002/art.21826. [DOI] [PubMed] [Google Scholar]
- 19.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40(9):1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 20.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25(11):1271–7. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 21.Petri M, Kim MY, Kalunian KC, Grossman J, Hahn BH, Sammaritano LR, et al. Combined oral contraceptives in women with systemic lupus erythematosus. N Engl J Med. 2005;353(24):2550–8. doi: 10.1056/NEJMoa051135. [DOI] [PubMed] [Google Scholar]
- 22.Liang MH, Socher SA, Larson MG, Schur PH. Reliability and validity of six systems for the clinical assessment of disease activity in systemic lupus erythematosus. Arthritis Rheum. 1989;32(9):1107–18. doi: 10.1002/anr.1780320909. [DOI] [PubMed] [Google Scholar]
- 23.The American College of Rheumatology response criteria for systemic lupus erythematosus clinical trials: measures of overall disease activity. Arthritis Rheum. 2004;50(11):3418–26. doi: 10.1002/art.20628. [DOI] [PubMed] [Google Scholar]
- 24.Smolen JS, Beaulieu A, Rubbert-Roth A, Ramos-Remus C, Rovensky J, Alecock E, et al. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet. 2008;371(9617):987–97. doi: 10.1016/S0140-6736(08)60453-5. [DOI] [PubMed] [Google Scholar]
- 25.Nishimoto N, Hashimoto J, Miyasaka N, Yamamoto K, Kawai S, Takeuchi T, et al. Study of active controlled monotherapy used for rheumatoid arthritis, an IL-6 inhibitor (SAMURAI): evidence of clinical and radiographic benefit from an x ray reader-blinded randomised controlled trial of tocilizumab. Ann Rheum Dis. 2007;66(9):1162–7. doi: 10.1136/ard.2006.068064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maini RN, Taylor PC, Szechinski J, Pavelka K, Broll J, Balint G, et al. Double-blind randomized controlled clinical trial of the interleukin-6 receptor antagonist, tocilizumab, in European patients with rheumatoid arthritis who had an incomplete response to methotrexate. Arthritis Rheum. 2006;54(9):2817–29. doi: 10.1002/art.22033. [DOI] [PubMed] [Google Scholar]
- 27.Choy EH, Isenberg DA, Garrood T, Farrow S, Ioannou Y, Bird H, et al. Therapeutic benefit of blocking interleukin-6 activity with an anti-interleukin-6 receptor monoclonal antibody in rheumatoid arthritis: a randomized, double-blind, placebo-controlled, dose-escalation trial. Arthritis Rheum. 2002;46(12):3143–50. doi: 10.1002/art.10623. [DOI] [PubMed] [Google Scholar]
- 28.Kiss E, Kavai M, Csipo I, Szegedi G. Recombinant human erythropoietin modulates erythrocyte complement receptor 1 functional activity in patients with lupus nephritis. Clin Nephrol. 1998;49(6):364–9. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
a. Platelet counts decreased whereas b. hemoglobin increased during treatment. Values returned to baseline after treatment. Values show the mean ± 1 standard error of the mean (SEM).
Inflammatory markers such as a. erythrocyte sedimentation rate and b. fibrinogen levels decreased significantly after the first treatment and remained low for the duration of therapy. Values show the mean ± 1 standard error of the mean (SEM). For the whole cohort * p<0.001 (repeated measures of ANOVA).