Abstract
FoxD4L1/D5 is a forkhead transcription factor that functions as both a transcriptional activator and repressor. FoxD4L1/D5 acts upstream of several other neural transcription factors to maintain neural fate, regulate neural plate patterning and delay the expression of neural differentiation factors. To identify a more complete list of downstream genes that participate in these earliest steps of neural ectodermal development, we carried out a microarray analysis comparing gene expression in control animal cap ectodermal explants (ACs), which will form epidermis, to that in FoxD4L1/D5-expressing ACs. Forty-four genes were tested for validation by RT-PCR of ACs and/or in situ hybridization assays in embryos; 86% of those genes up-regulated and 100% of those genes down-regulated in the microarray were altered accordingly in one of these independent assays. Eleven of these 44 genes are of unknown function, and we provide herein their developmental expression patterns to begin to reveal their roles in ectodermal development.
Keywords: BMP signaling, FGF signaling, cement gland, olfactory placode, epidermis, Elf-1, FoxD4L1.1, FoxD5
Introduction
During embryonic development, the formation of neural versus non-neural ectoderm is controlled by the differential activation of three main signaling pathways (reviewed in Stern, 2005; Itoh and Sokol, 2007; Rogers et al., 2009). BMP signaling is required to activate an epidermal fate in the embryonic ectoderm, and reduction of BMP signaling by secretion of BMP antagonists from the Organizer/Node converts the embryonic ectoderm to a neural fate (Hawley et al., 1995). Wnt signaling during gastrulation also represses neural fate (Christian et al., 1991; Heeg-Truesdell and Labonne, 2006) and promotes epidermal fate, in part by regulating the duration of the BMP/SMAD1 signal (Fuentealba et al., 2007). Like BMP, Wnt signaling is prevented in the presumptive neural ectoderm by the secretion of Wnt antagonists from the Organizer. FGF signaling promotes a neural fate and contributes to the down-regulation of both Bmp gene expression and BMP signaling (Delaune et al., 2005; Kuroda et al., 2005; Marchal et al., 2009).
In response to these signaling pathways, the presumptive neural ectoderm of Xenopus initially expresses a large number of transcription factors that have overlapping expression domains (reviewed in Yan et al., 2009; Rogers et al., 2009). One of these is FoxD5, whose gene nomenclature was recently changed to foxd4l1.1 (http://www.xenbase.org/gene/showgene.do?method=display&geneId=479246&); therefore we will refer to it as FoxD4L1/D5 here on out. FoxD4L1/D5 is expressed broadly throughout the newly induced neural ectoderm adjacent to the blastopore lip, gradually becomes confined to the mid-regions of the neural plate, and at neural tube stages is expressed only at the midbrain-hindbrain region and in the tail bud (Solter et al., 1999; Fetka et al., 2000 Sullivan et al., 2001). We recently showed that FoxD4L1/D5 plays an essential role in maintaining an immature neural fate by regulating a number of other neural transcription factors (Yan et al., 2009). Knock-down of endogenous FoxD4L1/D5 in the neural ectoderm by targeted injection of anti-sense morpholino oligonucleotides (MOs) reduces the size of the neural plate and causes a loss of 11 other neural transcription factors. Thus, FoxD4L1/D5 is necessary for either the induction or the maintenance of the expression of these genes in the neural ectoderm. In contrast, increased expression of FoxD4L1/D5 by mRNA injection into a neural progenitor blastomere: 1) increases the expression of Gem and Zic2; 2) initially represses Sox2, Sox3 and Sox11 and then either increases their endogenous expression level (Sox11) or expands their expression domains (Sox2, Sox3); and 3) represses the expression of Zic1, Zic3, SoxD and Xiro1–3. Ventral expression of FoxD4L1/D5 also promotes ectopic expression of some neural genes, decreases epidermal genes (TFAP2, Epi-cytokeratin), and locally reduces BMP signaling as evidenced by the loss of nuclear phosphoSMAD1/5/8 staining (Yan et al. 2009). Thus, FoxD4L1/D5 is a key regulator of neural fate via both transcription and cell-cell signaling. However, we do not yet know the molecular details of how this is accomplished.
To identify a broader cast of molecules that may be involved in the gene network by which FoxD4L1/D5 regulates neural ectodermal fate, microarray expression analyses were performed on animal cap ectodermal explants (ACs) that expressed FoxD4L1/D5. A subset of the candidate downstream genes identified by microarray was retested by RT-PCR analysis of FoxD4L1/D5 mRNA-injected ACs and/or by in situ hybridization assays of FoxD4L1/D5 mRNA-injected embryos. Together these validated the microarray results for 41/44 tested genes. Several of the genes downstream of FoxD4L1/D5 include molecules in the BMP and FGF signaling pathways, indicating that one function of FoxD4L1/D5 is to maintain a signaling environment in the embryonic ectoderm that favors a neural fate. Several of the up-regulated genes are normally expressed in the neural ectoderm, and several of the down-regulated genes are normally expressed in the ectoderm bordering the neural plate or in the epidermis. These results confirm a role for FoxD4L1/D5 in maintaining a neural versus non-neural ectodermal fate. This study additionally provides the developmental expression patterns of genes of unknown function that are regulated by FoxD4L1/D5.
Results and Discussion
FoxD4L1/D5 is a member of the forkhead/winged helix family of transcription factors that functions as both an activator and a repressor (Sullivan et al., 2001; Yan et a l., 2009). Our previous work demonstrated that FoxD4L1/D5 acts upstream of 11 other transcription factors (Gem, Sox2, Sox3, Sox11, SoxD, Zic1–3, Xiro1–3) in the nascent neural ectoderm to maintain an immature neural fate, regulate neural plate patterning and delay the onset of expression of bHLH neural differentiation transcription factors (Yan et al., 2009). To identify a more complete list of downstream genes that may coordinately regulate the earliest steps of neural ectodermal development, we compared the gene expression profile of control, untreated ACs, which would eventually form epidermis, to that of FoxD4L1/D5-expressing ACs. Assays were performed in ACs to avoid the influence of endogenous signaling centers in whole embryos. We did not treat the ACs with neuralizing factors to elicit a neural ectodermal response because previous work showed that FoxD4L1/D5-expressing ACs express a subset of neural genes in the absence of early mesoderm genes (Sullivan et al., 2001). However, it should be noted that none of the 11 genes listed above that are regulated by FoxD4L1/D5 in whole embryo assays (Yan et al., 2009) were altered >2-fold in this microarray assay, suggesting that these genes require the endogenous neuralizing environment for FoxD4L1/D5 to optimally affect their transcription. Finally, it should be noted that although FoxD5 is maternally expressed, its transcripts are not detected by RT-PCR in uninjected ACs harvested at the same stages as the microarray analyses (Sullivan et al., 2001).
Increased levels of FoxD4L1/D5 alter the expression of numerous genes
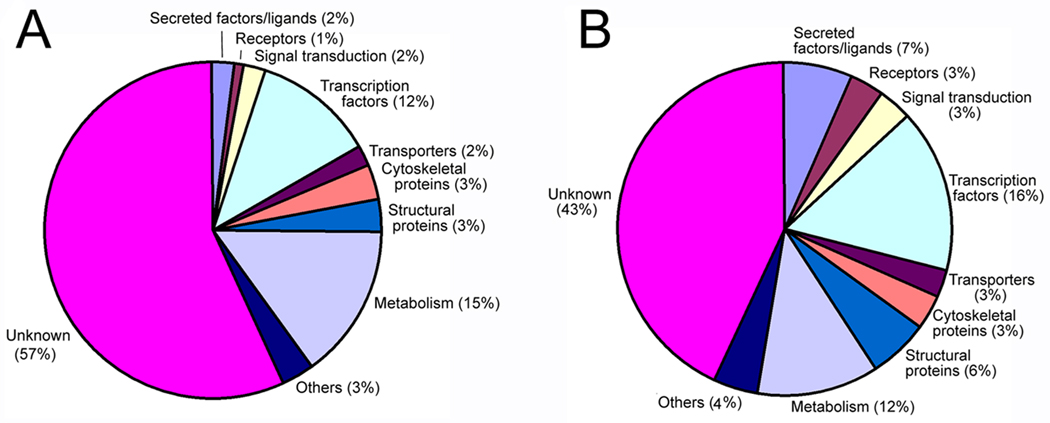
Increasing the level of FoxD4L1/D5 in ACs significantly changed by >2-fold the expression of 216 genes represented on the array. Of these, 95 were up-regulated (Supplemental Table 1) and 121 were down-regulated (Supplemental Table 2). Of the up-regulated genes, more than half are of unknown function. The majority of the remainder includes proteins involved in metabolic processes and transcription factors, with smaller percentages involved in signaling pathways and more general cellular functions (Fig. 1A). Of the down-regulated genes, a little less than half are of unknown function, 16% are transcription factors, 12% are involved in metabolic processes, 13% are involved in signaling pathways, and the remainder represent more general cellular functions (Fig. 1B).
Figure 1.
Gene Ontology analysis of the genes whose expression levels in non-neuralized ACs were significantly altered >2 fold by FoxD4L1/D5 in a microarray analysis. A. Percentages of genes up-regulated by FoxD4L1/D5 in each of several GO functional classes. B. Percentages of genes down-regulated by FoxD4L1/D5 in each of several GO functional classes. The “Others” category includes metal ion binding proteins, inhibitors, kinases and undefined nuclear factors.
Validation of microarray results by RT-PCR and ISH assays
To predict the level of false-positive identification of downstream genes by the microarray approach, we assayed the expression of a subset of putative downstream genes in ACs by RT-PCR and in whole embryos by ISH. Because FoxD4L1/D5 is known to regulate other transcription factors and BMP signaling (Yan et al., 2009), we selected genes for validation if they are known to be involved in transcription or signaling. We also chose genes of unknown function that displayed the greatest fold-changes (Tables 1 and 2).
Table 1.
Changes in expression levels of selected FoxD5 up-regulated genes
| Array Number | Gene Name | Accession Number |
Array Fold↑ |
RT-PCR Forward primer |
RT-PCR Reverse primer |
PCR Fold increase* |
ISH, percentage of embryos |
|
|---|---|---|---|---|---|---|---|---|
| Dorsal clone |
Ventral clone |
|||||||
| Xl.403.1.S1_at | FoxB1/Fkh-5 | AF064810 | 4.842 | tcctctccatccatgagtcc | attgtaaggggaacgtgctg | 5.75 | 80.4%↑ (46) | NC (21) |
| Xl.20011.1.S1_at | FGF-8b | AF461177 | 4.591 | gaccaactaagccgacggctcatc | tttatgaggttctgtggtgtggtgtc | 31.67 | 80.0%↑ (30) | NC (25) |
| Xl.637.1.A1_at | Egr1 | AF250345 | 4.501 | gagatgttagccttgtatctgc | gtactgttgatagtcttgaggtcc | 7.33 | nd | nd |
| Xl.3540.1.S1_at | Fullback | AF131890 | 3.943 | gcccacttcatcctcctgtagtag | tgctcccaactggaaactgtagg | 2.45 | 58.1%↑ (31) | NC (25) |
| Xl.1775.1.S1_at | Brat/VegT | U89707 | 3.868 | acccatatagtgcgatgtca | ctcccaatgcagggttttta | 8.62 | 88.9%↑ (18) | 94.4%↑ (18) |
| Xl.24509.1.A1_at | MGC132176 | BC108806 | 3.480 | gaggcaacaaaggaagtcca | ggagttccagggggaaataa | 3.93 | 75.0%↑ (20) | NC (24) |
| Xl.16539.1.A1_at | LOC496224 | BC088686 | 3.436 | gtggtacccagaggtggaga | cgattggtgtccttgttcct | 0.95 | 65.4%↑ (26) | NC (27) |
| Xl.25938.1.A1_at | EST | BC097640 | 3.301 | gtttagctggcttggctttg | aacaggattgggctgaacac | 1.82 | 59.3%↑ (27) | NC (27) |
| Xl.18191.1.A1_at | XEvl | BI312773 | 3.290 | tagtggtccaccacctcctc | ccacaaggacttgagcttcc | 1.34 | nd | nd |
| Xl.22268.1.S1_at | EST | BG161338 | 3.050 | aggctgtggcagaagctaaa | agccttcttttgagcagcag | 1.07 | NC (35) | NC (24) |
| Xl.15365.1.A1_at | EST | BJ051730 | 2.930 | tgctgacacctgaacagtcc | cacggtgtagctgcgaataa | 0.28 | 84.8%↑ (66) | 41.2%↑ (17) |
| Xl.15990.1.A1_at | EST | BJ043686 | 2.872 | ttggggatttgctattccag | gagcaatgcctaaccccata | 0.94 | 52.4%↑ (21) | NC (27) |
| Xl.9731.1.A1_at | EST | CB983184 | 2.842 | atactgggctgacctgtgct | gcaccggagttcactgtttt | 0.49 | 64.0%↑ (25) | NC (25) |
| Xl.14214.1.A1_at | MGC68716 | BC060483 | 2.820 | gggggtcggcaatacttaat | tcagtctggcagccctagtt | 4.02 | NC (30) | NC (24) |
| Xl.2755.1.S1_at | XSpr-2 | AY062263 | 2.226 | caaactgttgcctctcatgag | cacttacacctccggcagcgc | 2.79 | 89.1%↑ (46) | 63.8%↑ (58) |
| Xl.12111.1.S1_at | Prickle-b | BC073208 | 2.212 | gtggatcacgcacaaatgac | cttttgcccatccgtacact | 7.06 | nd | nd |
| Xl.1265.1.S1_at | Oct-1 | X17190 | 2.152 | ctggagggacccctaacatt | agtgcgttctggatggaatc | 1.36 | 82.1%↑ (39) | NC (47) |
| Xl.457.1.S1_at | Derriere | AF065135 | 2.122 | tggcagagttgtggctatca | ctatggctgctatggttcctt | 1.82 | 50.9%↑ (53) | 67.6%↑ (34) |
| Xl.410.1.S1_at | XLef-1 | AF287147 | 2.109 | attatccccagcagcaacag | gcaccactgggactttgttt | 1.22 | NC (30) | NC (29) |
| Xl.1182.1.S1_at | FGFR2 | X65943 | 2.086 | cccccttgtgacttggacta | ccctgaagaactggcttctg | 1.54 | 52.2%↑ (46) | NC (45) |
| Xl.5454.1.S1_at | Xmc | AF394111 | 2.016 | ctggtgttacagaccaagggg | acctgtgcttttgccactc | 2.54 | 82.9%↑ (35) | NC (41) |
denotes average of three independent experiments
increased or expanded expression
nd, not detected
NC, no change.
Sample size is shown in parentheses.
Table 2.
Changes in expression levels of selected FoxD5 down-regulated genes
| Array Number | Gene Name | Accession Number |
Array Fold ↓ |
RT-PCR Forward primer |
RT-PCR Reverse primer |
PCR Fold decrease* |
ISH, percentage of embryos | |
|---|---|---|---|---|---|---|---|---|
| Dorsal clone |
Ventral clone |
|||||||
| Xl.450.1.S1_at | Xash1 | M98272 | 4.831 | aacttcaatggcttcggcta | agcgtctccactttgctcat | 5.56 | 81.8%↓ (33) | NC (33) |
| Xl.12881.2.A1_at | MGC81002 | BC073481 | 4.810 | ccagtggctggagatagagc | gctctgaggtctccacgttc | 1.45 | 58.3%↓ (24) | NC (21) |
| Xl.14513.1.A1_at | Dlx3 | BC123268 | 4.505 | atgagtggcccctatgagaagaag | ggttctctgtaatggacaaacgg | 0.99 | 64.3%↓ (28) | 50.0%↓ (16) |
| Xl.620.1.S1_s_at | Frzb3 | AF136184 | 4.385 | gattgaacatgtgctgctga | atggtgtctccacctccttg | 2.78 | nd | nd |
| Xl.7307.1.S1_at | LOC443682 | BC041544 | 4.149 | aatgtttgctgggggtcata | aggtgcccttatcagcaatg | 5.56 | 61.5%↓ (26) | 52.8%↓ (36) |
| Xl.23326.1.S1_at | MGC53193 | BC045031 | 3.788 | gaaagtgacattgccggact | ctgagccctcacatcagtca | 3.85 | 78.3%↓ (23) | 52.8%↓ (39) |
| Xl.792.1.S1_at | GATA2 | M76564 | 3.745 | ggaaggaattcagaccagaaatagga | actccagcatggtgacggctatgg | 3.57 | 77.4%↓ (31) | 48.3%↓ (29) |
| Xl.3326.2.S1_a_at | BMP7 | BC057702 | 3.175 | atctccgcagtgttcgatct | atgatccagtcttgccaacc | 1.69 | 71.8%↓ (39) | 35.7%↓ (28) |
| Xl.838.1.S1_at | Dlx3-a | BC116461 | 3.115 | atttggctctgcctgagaga | aaggctttgctgctgttgtt | 2.00 | nd | nd |
| Xl.1076.1.S1_at | MGC52564 | U76752 | 3.003 | aaagaagcaggcaggaacaa | tttctgtgccattcgatcag | 4.76 | 83.3%↓ (36) | NC (18) |
| Xl.793.1.A1_at | GATA3 | M76565 | 2.985 | - | - | - | 66.7%↓ (33) | 52.0%↓ (25) |
| Xl.15156.1.S1_at | MGC52940 | BC045214 | 2.976 | tgctgaagacggagtcaatg | ttcaaagagccgaggtgagt | 1.54 | 80.6%↓ (36) | 37.5%↓ (16) |
| Xl.4619.1.S1_s_at | BMP4 | BC060340 | 2.976 | gcatgtaaggataagtcgatc | gatctcagactcaacggcac | 0.93 | 68.2%↓ (22) | NC (59) |
| Xl.1016.1.S1_at | FGFR4c | AF288453 | 2.849 | gcctttgatatcaccaag | agaggatggcactggatc | 2.27 | 69.7%↓ (33) | 58.7%↓ (63) |
| Xl.2143.1.S1_at | TFAP-2 | M59455 | 2.681 | - | - | - | 68.8%↓ (16) | 93.8%↓ (65)** |
| Xl.5908.1.S1_x_at | Xpo | X58487 | 2.611 | ctccggttggtaagcacatt | ggcaacatatcagggcagtt | 1.11 | 76.5%↓ (17) | 39.0%↓ (59) |
| Xl.2206.1.S1_at | Neptune | AF353715 | 2.597 | ttgtcatcggaccacctaca | atatcctctgtgggcactgg | 1.85 | 78.3%↓ (23) | 63.0%↓ (73) |
| Xl.139.1.S1_at | FoxI1/XFD-2 | X74316 | 2.451 | ccagaactgaaatcttagcaa | taacaaagataaagccagaggt | 3.85 | NC (21) | 60.5%↓ (43) |
| Xl.3606.1.S1_at | Szl | AF059570 | 2.309 | gattgaacagtggctgctga | atgtctccacctccttgtgg | 5.00 | 86.0%↑ (57) | 63.5%↑ (63) |
| Xl.262.1.S1_at | Dlx6 | D10259 | 2.242 | gctacatgcctggctactcc | ctcctgtgcagccactgtaa | 1.61 | 66.7%↓ (15) | 58.9%↓ (56) |
| Xl.563.1.S2_at | Xblimp1/Prdm1 | AF182280 | 2.119 | gcgagcatgaaagacaaaca | agcgggtatgggagagtctt | 1.23 | 77.8%↓ (18) | 87.2%↓ (47) |
| Xl.21868.1.S1_at | Elf-1 | BC044973 | 2.110 | ggtgctacaaccccaaagaa | agcagaggtgaagtgctggt | 2.27 | 58.6%↓ (41) | NC (20) |
| Xl.1047.1.S1_at | Lim5/Lhx5 | L42546 | 2.088 | tgagctctgggccttacagt | ttttctgaagggtggtgtcc | 1.35 | 78.0%↓ (41) | 47.2%↓ (36) |
denotes average of three independent experiments
decreased expression
increased expression; nd, not detected
not done
NC, no change
reported in Yan et al, 2009.
Sample size is shown in parentheses.
For the selected FoxD4L1/D5 up-regulated genes (Table 1), 13/21 were significantly increased >1.5 fold in the RT-PCR analysis of FoxD4L1/D5-expressing ACs, consistent with the microarray analysis. For 6/21 genes there was no detectable change in expression level (0.9−1.49 fold). Two genes (EST-BJ051730, EST-CB983184) that scored as up-regulated by the microarray analysis were down-regulated >2-fold in the RT-PCR assay. To analyze whether FoxD4L1/D5 alters the expression of these genes in whole embryos, we injected FoxD4L1/D5 mRNA into a single dorsal 16-cell blastomere to cause the FoxD4L1/D5-expressing clone to overlap with the endogenous expression domain of each target gene. The expression of Prickle-b, Egr1 and XEvl proved below detection by ISH and therefore could not be analyzed by this method. Of the 18 remaining genes, the expression of 15 were either increased or expanded in >50% of the embryos in which FoxD4L1/D5 was expressed in a dorsal clone (Table 1; Fig. 2A). These included 10 that also showed an increase by RT-PCR, 3 that showed no change by RT-PCR and 2 (EST-BJ051730, EST-CB983184) that showed a decrease by RT-PCR. Further analysis is required to explain why EST-BJ051730 and EST-CB983184 are up-regulated by microarray and ISH analyses but down-regulated by RT-PCR analysis. We also tested whether FoxD4L1/D5 could ectopically induce these genes by injecting mRNA into a single ventral 16-cell blastomere; 4/18 genes were induced in the ventral epidermis by FoxD4L1/D5 (Table 1; Fig. 2A). In total, 18/21 genes (85.7%) were confirmed to be increased in expression by FoxD5 by at least one independent assay. Only the FoxD4L1/D5 up-regulation of XEvl (Wanner et al., 2005), EST-BG161338 and XLef1 (Molenaar et al., 1998) by microarray analyses were not confirmed by either RT-PCR or ISH analyses.
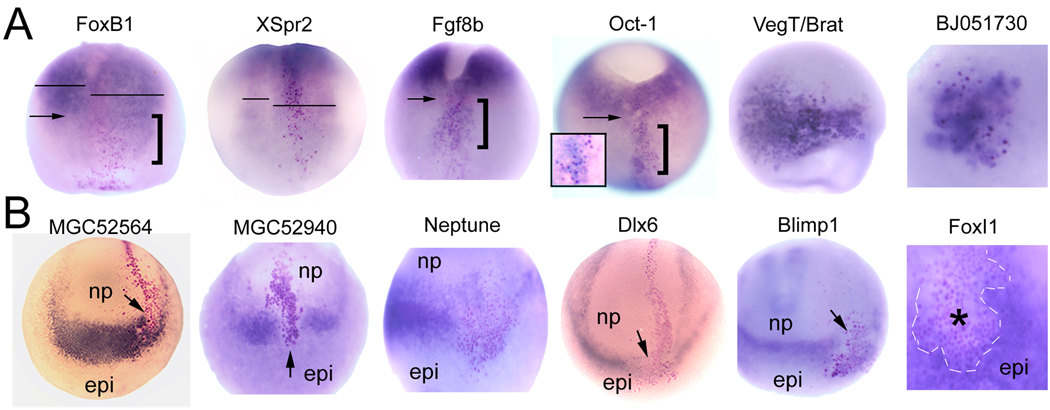
Figure 2. Whole mount in situ hybridization assays of embryos injected with FoxD4L1/D5 mRNA.
A. Examples of FoxD4L1/D5 causing an increase in downstream gene expression. In neural plate stage embryos, the expression domains of FoxB1 and XSpr2 are broader on the FoxD4L1/D5-injected side (right; indicated by βGal-expressing (red) cells). Lines indicate width of neural plate on injected (right) and control (left) sides of embryos. [Dorsal views with anterior to the bottom.] The FoxB1 expression domain also extends more anterior (bracket) compared to anterior limit on control side (arrow). At gastrula stages, the endogenous domains of Fgf8b and Oct-1 surround the yolk plug (*); the anterior extent of the domain is indicated by an arrow on the control side. The cells expressing FoxD4L1/D5 (red nuclei) in the neural plate (bracket) also express increased levels of Fgf8b and Oct-1. [Dorsal views with anterior to the bottom.] Inset in Oct-1 embryo: higher magnification showing FoxD4L1/D5-cells with red nuclei and blue cytoplasm. At gastrula stages, VegT/Brat and BJ051739 are ectopically induced in the animal cap ectoderm. [Animal pole views.]
B. Examples of FoxD4L1/D5 causing a decrease in downstream gene expression. In neural plate stage embryos, MGC52564, MGC52940, Neptune, Dlx6 and Blimp1 are expressed in the border zone that separates the anterior neural plate (np) from the epidermis (epi). For each gene, this region shows decreased expression at the site of FoxD4L1/D5-expressing cells (red nuclei, arrows; for Neptune, the stripe of expression is interrupted between arrows). [MGC52564, MGC52940, Dlx6, Blimp1 are anterior views with dorsal to the top; control side is on the left. Neptune is a side view with anterior to top and dorsal to right.] In a gastrula embryo, the normal high level of FoxI1 expression in the animal cap ectoderm (epi) is greatly reduced in the FoxD4L1/D5-expressing clone (*), which is outlined for clarity.
For the 23 selected down-regulated genes, 21 were tested by RT-PCR (Table 2). TFAP2 was not tested because we previously demonstrated down-regulation by FoxD4L1/D5 (Yan et al., 2009); Gata3 could not be tested because 3 different primer sets produced inappropriately sized products. Of the remaining 21 genes, 15 were significantly decreased >1.5 fold, consistent with the microarray analysis. For 6/21 genes there was no detectable change in expression level. The expression of Frb3 and Dlx3-a proved below detection by ISH and therefore could not be analyzed by this method. Of the 21 remaining genes, 20 showed decreased expression in >50% of FoxD4L1/D5 mRNA-injected embryos (Table 2; Fig. 2B). Expression of Szl was strongly induced both dorsally and ventrally, as previously reported (Yan et al., 2009); surprisingly, this response is opposite to the microarray and RT-PCR data suggesting that there are additional factors in the whole embryo that allow for Szl induction by FoxD4L1/D5. It is interesting that although Szl and Frb3 are considered paralogues (Hufton et al., 2006), we did not detect up-regulation of Frb3 by ISH. In total, all 23 genes were confirmed to be decreased in expression by FoxD4L1/D5 by at least one independent method.
Developmental roles of validated FoxD4L1/D5 downstream genes
Previous studies of FoxD4L1/D5 indicate that it plays multiple roles in early neural ectodermal development, including: 1) increasing the expression of neural ectoderm genes; 2) reducing the expression of neural differentiation genes; 3) reducing the expression of epidermis specific genes; and 4) reducing BMP signaling (Solter et al., 1999; Fetka et al., 2000; Sullivan et al., 2001; Yan et al., 2009). Analyses of the Gene Ontology function and/or published literature of those genes validated to be downstream of FoxD4L1/D5 demonstrate concordance with these previous findings, reveal a newly described involvement in mesoderm and endoderm specification and a newly described involvement in FGF signaling (Tables 3, 4).
Table 3.
Description of function and expression patterns of validated up-regulated genes
| Gene Name | Protein Description | Expression patterns | References |
|---|---|---|---|
| FoxB1/Fkh5 | Transcription factor | Posterior NE, DMR | Gamse and Sive, 2001 |
| FGF-8b | Peptide growth factor | Mesoderm, anterior NP, MHR | Fletcher et al., 2006 |
| Egr1 | DNA binding | DBL, mesoderm | Panitz et al., 1998 |
| Fullback | p75-like receptor | Posterior NE, mesoderm, DMR | Bromley et al., 2004 |
| Brat/VegT | Transcription factor | DBL, endomesoderm |
Horb and Thompsen, 1997; Zhang and King, 1996 |
| MGC132176 | Similar to heme- oxygenase | AC, NP, BZ, NT, MHR, NC, CG, lens, Oto, BA, Neph |
This study |
| LOC496224 | Similar to uridine-cytidine kinase |
AC, NP, BZ, NT, retina, lens, Oto, BA, somites |
This study |
| BC097640 | Similar to Solute carrier family 43, member 2 |
AC, NP, BZ, CG, PL, NT, retina, pineal, lens, Oto, BA, heart, somites, Neph, BL |
This study |
| BJ051730 | Unknown | AC, NP, BZ, NT, MHR, NC, lens, Oto, BA, somites, Neph |
This study |
| BJ043686 | Unknown | AC, NP, BZ, NT, lens, Oto, BA, somites, Neph |
This study |
| CB983184 | Similar to Rapamycin- associated protein-1 |
AC, NP, BZ, NT, retina, Olf, lens, Oto, BA, somites, heart |
This study |
| MGC68716 | Similar to Claudin-5 | AC, NP, BZ, NT, pineal, Oto, BA, heart, somites, notochord |
This study |
| XSpr2 | Zinc finger protein | DBL, Mesoderm, brain | Ossipova et al., 2002 |
| Prickle-b | LIM domain-PCP pathway | Posterior NE, dorsal mesoderm | Wallingford et al., 2002 |
| Oct-1 | Transcription factor | Ectoderm, anterior NT | Veenstra et al., 1995 |
| Derriere | Peptide growth factor | Endoderm, mesoderm | Sun et al., 1999 |
| FGFR2 | FGF receptor | MHR | Friesel and Brown, 1992 |
| Xmc | Marginal coil protein | DBL, mesoderm | Frazzetto et al., 2002 |
Legend: AC, animal cap ectoderm; BA, branchial arches; BL, ventral blood islands; BZ, ectoderm bordering the neural plate; CG, cement gland; DBL, dorsal blastopore lip; DMR, diencephalon-midbrain region; HB, hindbrain; MHR, midbrain-hindbrain region; NC, neural crest; NE, neural ectoderm; Neph, nephric mesoderm; NP, neural plate; NT, neural tube; Olf, olfactory, Oto, otocyst; PL, placodes.
Table 4.
Description of function and expression patterns of validated down-regulated genes
| Gene Name | Protein Description | Expression | References |
|---|---|---|---|
| Xash1 | Transcription factor | Brain, retina | Ferreiro et al., 1993 |
| MGC81002 | Unknown | AC, NP, BZ, NT, Epi, retina, Oto, BA |
This study |
| Frzb3 | Secreted protein | Ventral mesoderm, ectoderm | Hufton et al., 2006 |
| LOC443682 | Predicted sulfotransferase | AC, Epi, ANR, BZ, Neph | This study |
| MGC53193 | Predicted cytokeratin | AC, Epi, ANR, BZ, NT, lens, Oto, BA, Ph |
This study |
| GATA2 | Transcription factor | Mesoderm |
Bertwistle et al., 1996; Zon et al., 1991 |
| BMP7 | Peptide growth factor | Ventral mesoderm, ectoderm | Hawley et al., 1995 |
| Dlx3-a | Transcription factor | Ectoderm, CG | Papalopulu and Kintner, 1993 |
| MGC52564 | XAG-1; Secreted protein | Ectoderm, CG | Sive et al., 1989 |
| GATA3 | Transcription factor | Mesoderm, brain |
Bertwistle et al., 1996; Zon et al., 1991 |
| MGC52940 | Predicted Villin1 | AC, Epi, BZ, CG, Olf, Ph | This study |
| BMP4 | Peptide growth factor | Ventral mesoderm, ectoderm | Fainsod et al., 1994 |
| FGFR4c | FGF receptor | Ectoderm, NT, brain, eyes | Golub et al., 2000 |
| TFAP-2 | Transcription factor | AC, Epi, NC |
Luo et al., 2003; Winning et al., 1991 |
| Xpo | Transcription factor | Posterior mesoderm, posterior ectoderm |
Sato and Sargent, 1991 |
| Neptune | Transcription factor | Ectoderm, BZ | Takeda et al., 2005 |
| FoxI1/ XFD-2 | Transcription factor | AC, Epi, BZ | Matsuo-Takasaki et al., 2005 |
| Szl | Secreted protein | Ventral marginal zone | Salic et al., 1997 |
| Dlx6 | Transcription factor | AC, Epi, BZ | Luo et al., 2001 |
| Xblimp-1/ Prdm-1 |
Transcription factor | Non-neural ectoderm, endomesoderm, BZ |
de Souza et al., 1999 Rossi et al., 2008 |
| Elf-1 | Transcription factor | AC, Epi, NP, BZ, lens, BA | This study |
| Lim5/Lhx5 | Transcription factor | Ectoderm, NP, brain | Toyama et al., 1995 |
AC, animal cap ectoderm; ANR, anterior neural ridge; BA, branchial arches; BZ, border zone of neural plate; CG, cement gland; Epi, epidermis; NC, neural crest; NP, neural plate; NT, neural tube; Olf, olfactory placode/pit; Oto, otocyst, Ph, anterior pharynx.
FoxD4L1/D5 up-regulates genes expressed in the neural ectoderm
FoxB1/Fkh5, a forkhead transcription factor, is expressed in the posterior neural ectoderm during gastrulation and in the diencephalon-midbrain region in the neural tube (Gamse and Sive, 2001). Fullback, a p75-related neurotrophin receptor, is expressed in the posterior neural ectoderm during gastrula and neural plate stages, and later is expressed in the diencephalon-midbrain region (Bromley et al., 2004). Prickle-b, which is involved in the non-canonical Wnt-PCP pathway during convergent extension movements (Takeuchi et al., 2003), is expressed in the posterior neural ectoderm during gastrulation (Wallingford et al., 2002). Oct-1, a POU homeodomain transcription factor, is expressed in the neural ectoderm during gastrulation, and then becomes enriched in the anterior neural tube (Veenstra et al., 1995). Thus, FoxD4L1/D5 up-regulates other neural genes with which it shares common expression domains (Table 3); this would be expected of down-stream targets that are positively regulated by FoxD4L1/D5.
FoxD4L1/D5 down-regulates transcription factors that promote neural differentiation
Xash1, a bHLH transcription factor, is expressed in differentiating neural progenitors (Ferreiro et al., 1993). It was significantly down-regulated by FoxD4L1/D5 (Table 4), in accord with a previous study showing that FoxD4L1/D5 down-regulates other bHLH neural differentiation genes (Ngnr1, NeuroD) (Sullivan et al 2001).
FoxD4L1/D5 down-regulates epidermal-specific genes
Many genes validated to be down-regulated by FoxD4L1/D5 (Bmp4, Bmp7, Dlx3, Dlx3-a, Dlx6, Elf-1, FoxI1, Lim5, Neptune, TFAP-2, Xblimp-1) are known to be involved in non-neural ectodermal development (Table 4). This is consistent with a previous report that FoxD4L1/D5 represses epidermal fate (Yan et al., 2009). However, Elf1 and Lim5 are also later expressed in the neural plate; it will be interesting to determine whether they are later involved in neural differentiation, as predicted by their down-regulation by FoxD4L1/D5. This would be in accord with a previous study that showed that some neural genes (Zic, Xiro) that promote bHLH expression of neural differentiation genes are down-regulated by FoxD4L1/D5 (Yan et al., 2009).
FoxD4L1/D5 down-regulates genes involved in BMP signaling
Several genes related to BMP signaling were down-regulated by FoxD4L1/D5 (Table 4). These include two ligands (Bmp4, Bmp7) and several genes whose expression is known to depend on BMP signaling (Dlx3, Dlx6, Szl, TFAP-2). Recent studies show that GATA2 also acts downstream of BMP in ectodermal cells (Dalgin et al., 2007). These results are in accord with a previous report that FoxD4L1/D5 reduced nuclear phospho-SMAD 1/5/8 expression in ventral epidermal cells (Yan et al., 2009). The microarray data support the suggestion that FoxD4L1/D5 interferes with the BMP signaling pathway, but the molecular mechanism is not yet known.
FoxD4L1/D5 down-regulates genes involved in mesoderm and endoderm development
Similar to our findings with non-neural ectodermal genes, we found that genes involved in mesoderm and endoderm development (Bmp4, Bmp7, Frzb3, GATA2, GATA3, Xpo) were down-regulated by FoxD4L1/D5 (Table 4). BMP4 and BMP7 promote ventral mesodermal fates (Nishimatsu and Thomsen, 1998). Frzb3 is a putative Wnt inhibitor (AF136184, Genbank). GATA2 and GATA3 are primarily expressed in blood lineages derived from ventral mesoderm, although there may be some neural expression of GATA3 (Zon et al., 1991). Xpo is expressed in posterior mesoderm and ectoderm during gastrulation and neurulation in Xenopus (Sato and Sargent, 1991). These results are consistent with the conclusion that FoxD4L1/D5 down-regulates genes that specify tissues other than neural ectoderm.
However, there were a few striking examples of mesoderm- and endoderm-associated genes that were strongly up-regulated by FoxD4L1/D5 (Table 3). Brat/VegT is a T-box transcription factor that is essential in establishing the endoderm in Xenopus (Horb and Thomsen, 1997; Zhang et al., 1998), and Derriere is a Nodal factor involved in mesoderm induction (Sun et al., 1999) that is directly regulated by Brat/VegT (White et al., 2002). Egr1, a zinc finger protein, is involved in early mesoderm induction in Xenopus (Panitz et al., 1998), and Xmc is a novel coil-coiled protein initially expressed in the dorsal marginal zone at the onset of gastrulation and later strongly expressed in the mesoderm flanking the neural plate (Frazzetto et al., 2002). This inconsistency requires experimental evaluation, but there are two possible explanations based on previous studies. First, many of these genes are expressed early in the dorsal blastopore lip region, as is FoxD4L1/D5; perhaps FoxD4L1/D5 regulates genes involved in setting up the dorsal axis, as previously suggested (Sullivan et al., 2001). Second, while FoxD4L1/D5 does not induce Xbra expression in ACs harvested at neural plate stages, it does induce muscle-specific actin in ACs harvested at tail bud stages and in ectopically induced dorsal axes in whole embryos (Sullivan et al., 2001). Because FoxD4L1/D5 is normally expressed in the mesoderm of the tail bud (Solter et al., 1999; Sullivan et al., 2001), these observations suggest that FoxD4L1/D5 may play a later role in mesoderm formation in the tail bud that is reflected in the up-regulation of the mesoderm-associated genes reported herein. Perhaps FoxD4L1/D5 secondarily influences mesoderm gene expression by altering FGF signaling, as discussed in the next section.
FoxD4L1/D5 alters genes involved in FGF signaling
FGF signaling has been implicated in promoting neural induction, regulating patterning of the midbrain and maintaining neural stem cell proliferation (Umemori, 2009), as well as epidermal and mesodermal formation. Two members of the FGF pathway that are expressed in the neural ectoderm were highly up-regulated by FoxD4L1/D5 (Table 3). FGF8-b, a splice form of FGF8, mediates early mesoderm and posterior neural tissue formation (Fletcher et al., 2006), and FgfR2 is expressed in the midbrain-hindbrain region (Blak et al., 2005). Both share expression domains in the neural ectoderm with FoxD4L1/D5 (Solter et al., 1999; Fetka et al., 2000). One member of the FGF pathway, FgfR4-c, which causes ectodermal cells to develop into epidermis (Umbhauer et al., 2000) was down-regulated by FoxD4L1/D5 (Table 4). These results are consistent with the proposal that FoxD4L1/D5 promotes neural ectodermal fates and suppresses epidermal fates. In addition, three genes (Fullback, Xmc, XSpr2) regulated by the FGF signaling pathway (Frazzetto et al., 2002; Ossipova et al., 2002; Sasai et al., 2004; Chung et al., 2005) were up-regulated by FoxD4L1/D5. Fullback and Xmc roles in neural and mesoderm formation were discussed above. Although XSpr2 is required for gastrulation movements of the mesoderm (Nutt et al 2001; Zhao et al., 2003), it also is later expressed in the brain (Ossipova et al., 2002; Fig. 2A); in which of these tissues its expression is influenced by FoxD4L1/D5 requires further study. One FGF regulated gene, Elf-1, which encodes an ETS domain transcription factor, was down-regulated by FoxD4L1/D5. This is surprising, because in mouse Elf-1 is activated by FGF8 signaling in the midbrain (Lee et al., 1997). The expression of Elf-1 has not been reported in Xenopus. By RT-PCR, we observed low levels of Elf-1 expression at the 1-cell, blastula and early gastrula stages, high levels at neural plate through tail bud stages and decreased levels at larval stages (Fig. 3B). By ISH, we detected weak expression in the animal cap ectoderm, the neural plate and border zone, anterior neural tube, lens and branchial arch mesoderm (Fig. 5B). Only at gastrula and neural plate stages does Elf-1 expression overlap with that of FoxD4L1/D5. In total, these results suggest novel roles for FoxD4L1/D5 in promoting FGF signaling in the neural ectoderm and perhaps the mesoderm, and in repressing FGF signaling in the epidermis.
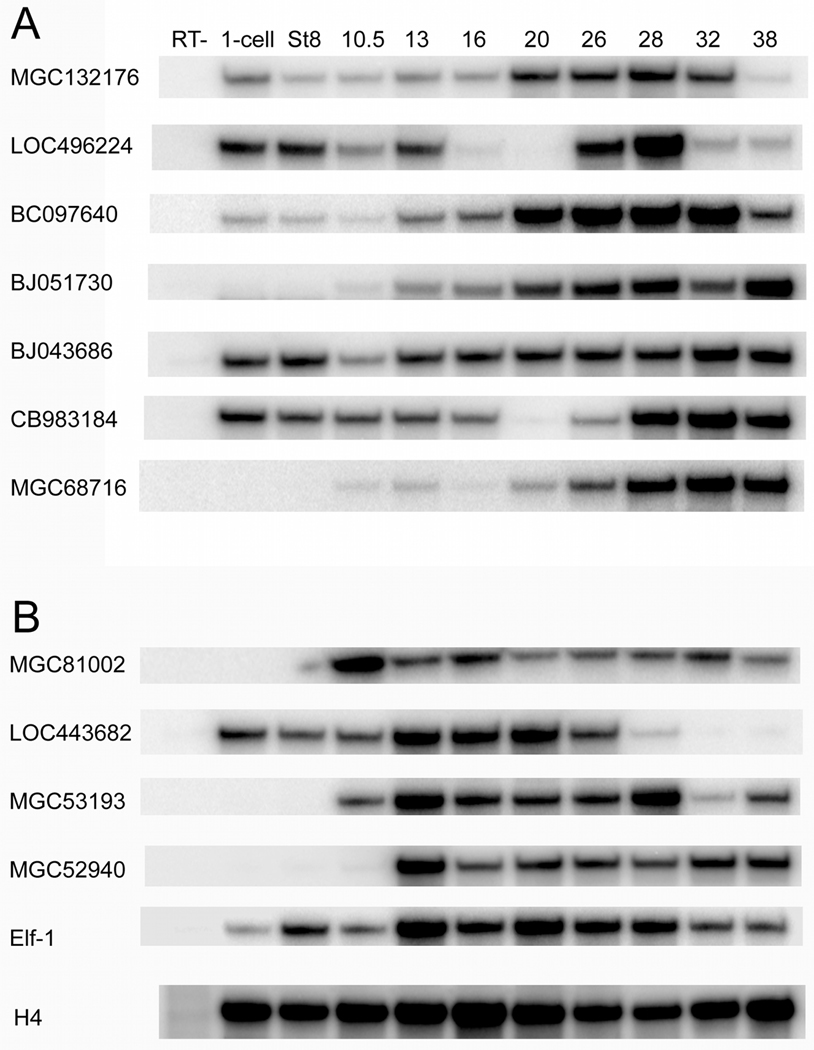
Figure 3.
RT-PCR assays illustrate the temporal expression patterns of uncharacterized up-regulated (A) and down-regulated (B) genes in normal, unmanipulated embryos. RT-, minus RT step; numbers across the top indicate the developmental stages (st). H4 is an internal control.
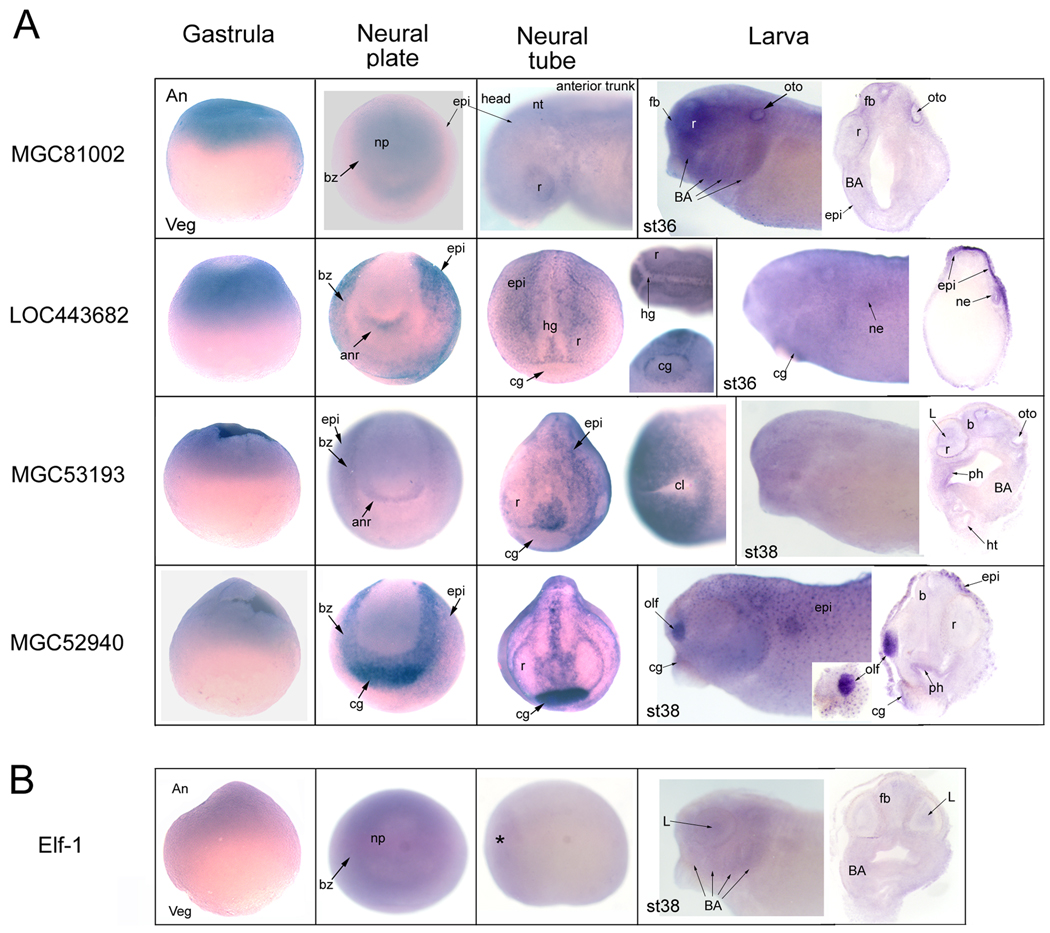
Figure 5.
Whole mount in situ hybridization assays illustrating the expression patterns of 5 genes that are down-regulated by FoxD4L1/D5. Abbreviations are the same as in Figure 4.
A. Down-regulated, validated genes of unknown function. Gastrula stages are side views oriented with animal pole to the top and vegetal pole to the bottom. Transcripts for all 4 genes are detected in the animal cap ectoderm. At neural plate stages, embryos are oriented in an anterior frontal view, with dorsal to the top. MGC81002 is weakly detected in the neural plate, the ectoderm bordering it and epidermis (epi). At neural tube stages, it is detected diffusely in the neural tube, retina and epidermis. [Side view]. At larval stages, MGC81002 is additionally detected in the otocyst and branchial arch mesoderm. [Side view and transverse sections]. At neural plate stages, LOC443682 is strongly expressed in the epidermis, the ectoderm bordering the neural plate and the anterior neural ridge (anr). At neural tube stages, LOC443682 is detected throughout the epidermis, with enhanced expression bordering the retina, hatching gland (hg) and cement gland. [Left embryo is a dorso-anterior view with anterior to the bottom; top right embryo is a dorsal view of the head with anterior to the left, and bottom right embryo is a ventral view of the head with anterior to the top.] At larval stages, LOC443682 is detected throughout the epidermis, most strongly on the dorsal side, and in the nephric mesoderm. [Side view and transverse sections]. At neural plate stages, MGC53193 is detected in the epidermis, border of the neural plate and anterior neural ridge. At neural tube stages, MGC53193 is strongly expressed in the head epidermis and tissue surrounding the cloaca (cl). [Left embryo is a frontal view with dorsal to the top and right embryo is a ventral view with posterior to the left.] At larval stages, MGC53193 is weakly detected in the epidermis of whole mount embryos [side view], but transverse sections reveal additional weak expression in brain (b), lens, otocyst, and anterior pharynx (ph). At neural plate stages, MGC52940 is strongly expressed in the ectoderm bordering the neural plate and cement gland, and weakly in the epidermis. At neural tube stages, MGC52940 expression is pronounced in a dorsal midline stripe in the epidermis, surrounding the retinas, in a patch over the forebrain (between the retinas) and in the cement gland. [Anterior-frontal view with dorsal to the top.] At larval stages, MGC52940 is highly expressed in the olfactory placode and in cells dotted throughout the epidermis; it is weakly expressed in the cement gland and anterior pharynx. [Side view and transverse sections].
B. Expression pattern of Xenopus Elf-1. Elf-1 is weakly expressed in the animal cap ectoderm at gastrulation, throughout the neural plate and border zone, and then is barely detectable in the anterior neural tube (*). At larval stages it is weakly detected in the brain, lens and branchial arch mesoderm [side views and transverse sections].
Expression patterns of FoxD4L1/D5-regulated genes of unknown function
A large number of the genes represented on the Affymetrix Xenopus laevis GeneChip (v1.0) have not been annotated or fully sequenced, and therefore are of unknown function (Fig. 1). We performed a BLAST search of the 12 genes of unknown function that we validated to identify their predicted gene functions. Of the seven up-regulated, validated genes, 2 remain unknown, 2 have high similarity to metabolic enzymes (heme-oxygenase, uridine-cytidine kinase), one is similar to a solute carrier for membrane transport (Bodoy et al., 2005), one is similar to a Rapamycin-associated protein, which is a serine-threonine kinase involved in cell cycle progression (Sabatini et al., 1994), and one is similar to Claudin-5, an integral membrane protein associated with tight junctions (Sirotkin et al., 1997) (Table 3). Of the 5 down-regulated, validated genes, one remains unknown, one is a cytokeratin, which is an epidermis-enriched cytoskeletal protein, one is the same as XAG-1, which is a secreted protein specific to the cement gland (Sive et al., 1989), one is predicted to be a metabolic enzyme (sulfotransferase) and one is similar to Villin1, which is an actin regulatory protein (Hesterberg and Weber, 1983) (Table 4). Using whole embryo RT-PCR, we determined the temporal pattern of gene expression (Fig. 3) and using whole embryo ISH, we determined the expression patterns of 11 of these validated genes (Figs. 4 and 5); we do not report the expression of MGC52564 because XAG-1 expression is already characterized (Sive et al., 1989). In Supplemental Figure 1 we present the expression pattern of the non-validated gene BG161338 because it is a novel gene.
Figure 4.
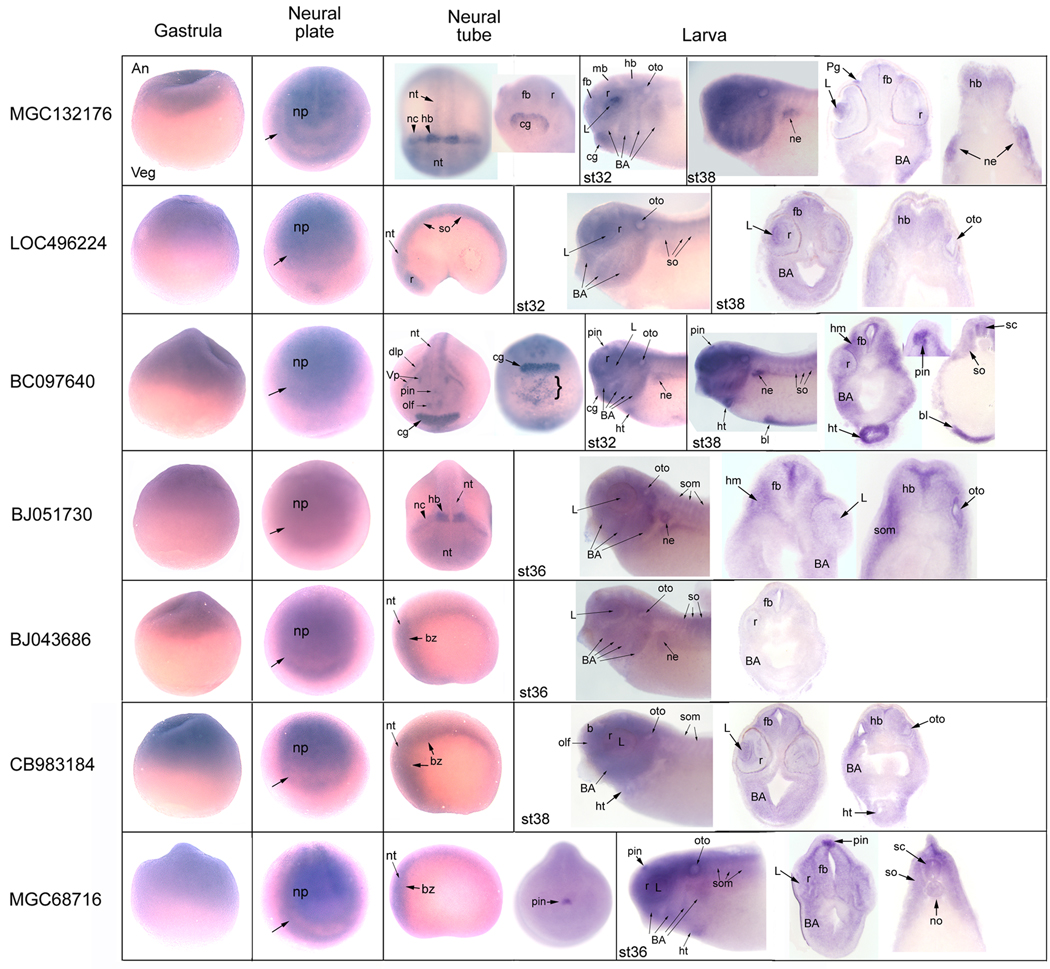
Whole mount in situ hybridization assays illustrating the expression patterns of 7 uncharacterized genes that are up-regulated by FoxD4L1/D5. Gastrula stages are side views oriented with animal cap (An) to the top and vegetal pole (Veg) to the bottom. Transcripts for all 7 genes are detected in the animal cap ectoderm. At neural plate stages, embryos are oriented in an anterior frontal view, with dorsal to the top. Transcripts for all 7 genes are detected in the neural plate (np) and in the ectoderm bordering the neural plate (arrow) that will give rise to the cranial placodes and neural crest. At neural tube stages, MGC132176 is expressed throughout the neural tube (nt), with intense staining in a band in the midbrain-hindbrain (hb) region, in adjacent neural crest (nc) and the dorsal rim of the cement gland (cg). [Left embryo is a dorsal view with anterior at the bottom and right embryo is a ventral view of the head.] At larval stages (st32−38), MGC132176 is detected throughout the brain and retina (r), in the lens (L), otocyst (oto), cement gland, branchial arch (BA) and nephric mesoderm (ne). [This and all larval stages are side views with anterior to the left.] Transverse sections of st38 embryo confirm the labeled tissues identified in whole mount embryos, and identify the profundal ganglion (Pg). At neural tube stages, LOC496224 is expressed throughout the neural tube, retina and somites (so). [Side view with anterior to the left.] At larval stages, LOC496224 is additionally expressed in the lens, otocyst and branchial arches. BC097640 is expressed throughout the neural tube, with strong expression in the pineal primordium (pin), cement gland, several cranial placodes (dlp, dorso-lateral placode; olf, olfactory placode; Vp, trigeminal placode), and scattered cells on the ventral midline (bracket) caudal to the cement gland. [Left embryo is an anterior frontal view with dorsal to the top; right embryo is a ventral view with anterior to the top.] At larval stages, BC097640 is additionally expressed in the lens, otocyst, branchial arches, head mesoderm (hm), heart (ht), ventral blood islands (bl), somites and nephric mesoderm. At neural tube stages, BJ051730 is weakly expressed throughout the neural tube, with strong expression in the midbrain-hindbrain region and adjacent neural crest. [Dorsal anterior-view from the top.] At larval stages, BJ051730 is additionally expressed in the lens, otocyst, branchial arches, somites and nephric mesoderm. At neural tube stages, BJ043686 is expressed in the neural tube and the ectoderm bordering it (bz). [Side view with anterior to the left.] At larval stages, BJ043686 is additionally expressed at low levels in the lens, otocyst, somites, branchial arch, head and nephric mesoderm. At neural tube stages, CB983184 is expressed in the neural tube and the ectoderm bordering it. At larval stages, CB983184 is additionally expressed in the olfactory pit, lens, retina, otocyst, somites, branchial arch mesoderm and heart. At neural tube stages, MGC68716 is expressed in the neural tube, the ectoderm bordering it and pineal primordium. At larval stages, MGC68716 is additionally expressed in the otocyst, somites, branchial arch mesoderm and heart. Additional abbreviations: fb, forebrain; mb, midbrain.
Uncharacterized genes that are up-regulated by FoxD4L1/D5 are all expressed in the neural ectoderm
Transcripts for MGC132176, LOC496224, BC097640, BJ043686 and CB983184 were detected by RT-PCR at the 1-cell and blastula stages (Fig. 3A), consistent with the reported maternal expression of FoxD4L1/D5 (Solter et al., 1999; Fetka et al., 2000 Sullivan et al., 2001). At gastrula stages (10.0–12.5), all 7 up-regulated, validated genes are expressed uniformly in the animal cap ectoderm (Figs. 3A; 4). Each is also detected at the neural plate stages (13–16) (Fig. 3A); by ISH analyses each is expressed throughout the neural plate and the neural plate border zone of ectoderm that will give rise to cranial placodes, hatching gland and neural crest (Fig. 4). Thus in general, the expression domains of these genes overlap with that of FoxD4L1/D5, which is maternally expressed in the animal pole at cleavage and blastula stages and zygotically expressed in the neural ectoderm. However, FoxD4L1/D5 expression is not detected in the neural plate border zone or the lateral neural plate, indicating that other factors also regulate these genes.
FoxD4L1/D5 expression wanes at neural tube (20–28) and larval (st32–38) stages except at the midbrain-hindbrain boundary and in the tail bud (Solter et al., 1999; Fetka et al., 2000 Sullivan et al., 2001). In contrast, all 7 up-regulated, validated genes are strongly expressed at most of these stages (Fig. 3A), and spatially their expression domains are more extensive than those of FoxD4L1/D5. MGC132176 is expressed throughout the neural tube, with strong expression in the midbrain-hindbrain region, in the associated neural crest, the dorsal edge of the cement gland, several placode derivatives, branchial arch and nephric mesoderm (Fig. 4). LOC496224 is expressed throughout the neural tube, some placode derivatives, somites, and branchial arch mesoderm (Fig. 4). BC097640 is expressed in the neural tube with enhanced staining in the pineal, cement gland, several cranial placodes, heart, somites, branchial arch and nephric mesoderm and ventral blood islands (Fig. 4). BJ051730 is expressed diffusely throughout the neural tube with enhanced expression in the midbrain-hindbrain region, in the adjacent neural crest, several placode derivatives, somites, branchial arch and nephric mesoderm (Fig. 4). At early stages, BJ043686 is weakly expressed throughout the neural tube and the border zone containing the cranial placodes and neural crest (Fig. 4). At late tail bud through larval stages, BJ043686 expression is detected in some placode derivatives, somites, branchial arch and nephric mesoderm (Fig. 4). At early stages, CB983184 is expressed in the anterior neural tube and border zone containing the cranial placodes and neural crest (Fig. 4). At late tail bud through larval stages, CB983184 is expressed diffusely in the brain, retina, some placode derivatives, somites, branchial arch and cardiac mesoderm (Fig. 4). At early stages, MGC68716 is weakly expressed in the anterior neural tube, with intense staining in the pineal primordium (Fig. 4). At late tail bud through larval stages, MGC68716 is additionally expressed in some placode derivatives, somites, notochord, branchial arch and cardiac mesoderm (Fig. 4). In summary, while the maternal, blastula, gastrula and neural plate expression patterns of these 7 genes overlap with that of FoxD4L1/D5, starting at neural plate stages their expression domains are far more extensive than FoxD4L1/D5, which is only maintained in the midbrain-hindbrain region. This indicates that these genes may be initially regulated by FoxD4L1/D5, but their later expression is likely regulated by other factors.
Unknown genes that are down-regulated by FoxD4L1/D5 are all expressed in non-neural ectoderm
Only LOC443682 expression was detected by RT-PCR at the 1-cell and blastula stages (Fig. 3B). All 4 down-regulated, validated genes are expressed at gastrula stages uniformly through the animal cap ectoderm (Figs. 3B, 5A; Table 4), overlapping with FoxD4L1/D5 expression. RT-PCR analysis shows strong expression continuing through neural plate and neural tube stages (Fig. 3B), but at larval stages the expression of LOC443682 wanes.
While temporally, FoxD4L1/D5 expression overlaps initially with these four genes, spatially it only overlaps significantly at gastrula stages. At neural plate/tube stages, MGC81002 is weakly expressed in the dorsal epidermis, neural plate/tube and border zone (Fig. 5A). By late tail bud and larval stages, MGC81002 is additionally expressed in the otocyst and branchial arch mesoderm (Fig. 5A). At neural plate/tube stages, LOC443682 is strongly expressed in the epidermis, border zone and anterior neural ridge (Fig. 5A). At later stages, LOC443682 is additionally expressed in the nephric mesoderm (Fig. 5A). At neural plate/tube stages, MGC53193 is expressed in the epidermis, border zone and anterior neural ridge (Fig. 5A). At tail bud stages, MGC53193 expression is notable in tissue surrounding the cloaca; at larval stages it is weakly expressed in brain, some placode derivatives, branchial arch mesoderm and the anterior pharynx (Fig. 5A). At neural plate stages, MGC52940 is strongly expressed in the border zone surrounding the neural plate and cement gland, and is weakly expressed throughout the epidermis (Fig. 5A). At later stages, MGC52940 is strongly expressed in the olfactory placode and scattered cells throughout the epidermis and is weakly expressed in the anterior pharynx (Fig. 5A).
Summary
The expression patterns of these uncharacterized genes are consistent with previous studies showing that FoxD4L1/D5 promotes neural plate genes and represses non-neural ectodermal genes (Yan et al., 2009). The expression domains of all of the up-regulated genes overlap with FoxD4L1/D5 expression at maternal, blastula, gastrula and neural plate stages, but they are more extensive than that of FoxD4L1/D5 at neural tube to larval stages. This indicates that only their early phases of expression might be regulated by FoxD4L1/D5. The down-regulated genes are highly expressed in the epidermis and non-neural ectoderm that surrounds the neural plate border and gives rise to placodes and neural crest, suggesting that FoxD4L1/D5 may restrict their expression from encroaching into the neural ectodermal domain. It is now important to functionally characterize these novel genes to further elucidate the gene regulatory network that controls early ectodermal fate decisions. In particular, we need to test which of these genes are directly regulated by FoxD4L1/D5, and which ones are regulated by transcriptional activation or repression. These new FoxD4L1/D5 targets should be valuable in understanding the molecular mechanisms of embryonic neural development.
Experimental Procedures
Embryos and microinjections
Fertilized Xenopus laevis eggs were obtained by either in vitro fertilization (for ACs) or gonadotropin-induced natural mating of adult frogs (for targeted blastomere microinjections) as described elsewhere (Moody, 2000). FoxD4L1/D5 and nuclear β-galactosidase (nβgal) mRNAs were synthesized by in vitro transcription (Ambion, mMessage mMachine kit). For ACs, FoxD4L1/D5 mRNA (150pg) was injected into the animal poles of both cells at the 2-cell stage. ACs were dissected at stages 8.5–9, cultured in 1X MAB and collected at stages 11.5–12.5. For whole embryo in situ hybridization (ISH) assays, one blastomere of the 16-cell embryo having a defined neural or epidermal lineage (Moody, 1987) was injected with a mixture of FoxD4L1/D5 (150pg) and nβgal (100pg) mRNAs. Embryos were cultured in 50% Steinberg’s solution and collected at stages 12–14.
Microarray analyses
Four independent samples of FoxD4L1/D5 mRNA-injected ACs were collected. Each sample was derived from one different male and two different females and contained ∼100 ACs. For each FoxD4L1/D5-expressing sample, a control, uninjected sample from sibling embryos also was collected. All samples were processed in parallel for cDNA labeling and chip hybridization to reduce inter-sample variations. Total RNAs were extracted from ACs with the RNeasy mini kit (Qiagen). Integrity of RNAs was assessed using the Agilent 2100 bioanalyzer (Agilent Technologies) and only samples with an integrity number > 8.0 were used. Total RNAs were labeled and fragmented with the Ovation Biotin RNA Amplification and Labeling System (NuGEN Technologies, Inc.). Briefly, 50ng total RNA was used for first- and second-strand cDNA syntheses. The synthesized cDNAs were amplified, purified with a PCR purification kit (Qiagen), then labeled and fragmented following the Ovation kit instructions. The labeled cDNAs were purified with the Dye Ex kit (Qiagen).
Chip hybridization and statistical analyses were performed by the NINDS-NIMH Microarray Consortium at The Translational Genomics Research Institute (T-GEN, Tempe, AZ). The Affymetrix GeneChip® Xenopus laevis Genome Array (v1.0) bears 15503 probe sets representing about 14400 transcripts. These chips have been successfully used for analyzing gene expression in the Organizer, endoderm, neural crest and regenerating hind limb (Grow et al., 2006; Hufton et al., 2006; Sinner et al., 2006; Pearl et al., 2008; Zhao et al., 2008). The chips were washed and scanned as recommended by the Ovation Biotin RNA Amplification and Labeling System User Guide (version 1.0). GCOS software was used to determine signal intensities and detection calls for each gene. The replicate correlation value for the four control samples was 0.94 and for the FoxD4L1/D5-injected samples it was 0.95, indicating consistency between replicates despite random genetic variance between parental frogs. Using GeneSpring software, all experimental arrays were normalized to their matched control array. Genes were then filtered for at least 2 present calls out of 8 calls. The remaining list was tested for significant changes by ANOVA (p< 0.05). The raw and processed data are available (GEO accession number GSE11143).
RT-PCR
Animal poles of 2-cell embryos were injected with FoxD4L1/D5 mRNA and ACs collected as described for the microarray analyses. Total RNA was extracted the with RNeasy mini kit (Qiagen). Semi-quantitative RT-PCR within linear ranges was performed as previously described (Yan et al., 2009). Primers are listed in Tables 1 and 2; some primer sequences were obtained from published papers, whereas the others were designed with the Primer3 software (Rozen and Skaletsky, 2000). Reverse transcription was performed with 1.0µg of RNA using the SuperScript first-strand synthesis system (Invitrogen). Standard PCR amplifications were performed with PCR Supermix (Invitrogen) including 1.0µCi α-[32P]dATP (Amersham) in the reaction mixture. All PCR assays were repeated at least 3 times. Bands were visualized with a Storm 860 phosphorimager (Molecular Dynamics), the band intensities were measured with the ImageQuant analysis program and the relative expression levels of the genes were calculated as compared to an internal control gene (H4). To determine the developmental expression of unknown FoxD4L1/D5 downstream genes, wild type embryos were snap-frozen at a series of developmental stages. Total RNAs were extracted and reverse-transcribed as above. Standard PCR amplifications (primers in Tables 1, 2) were performed to show the expression levels of each gene, with H4 as a loading control. These analyses were repeated three times with independent samples.
Whole mount in situ hybridization
Plasmids encoding a subset of the putative downstream genes identified in the microarray assay were either obtained from the laboratory in which they were originally reported (Tables 3, 4), or purchased from Open Biosystems (Thermo Scientific). Anti-sense digoxigenin-labeled RNA probes were synthesized by in vitro transcription (Ambion, Megascript kit). FoxD4L1/D5 mRNA-injected embryos were fixed, stained for expression of the nβGal lineage tracer and processed for whole mount ISH according to standard protocols (Sive et al., 2000). Embryos were analyzed for whether the expression domain of the putative downstream gene was expanded or decreased in size or staining intensity within the clone of FoxD4L1/D5-expressing cells. The uninjected side of the same embryo was used as an internal control. To analyze the gene expression patterns of putative downstream genes with unknown function, uninjected wild type embryos were fixed at gastrulation (stages 10.0–12.5), neural plate (stages 13–16), early neural tube (stages 20–24), tail bud (stages 25–28) and larval (stages 32–38) stages and processed for ISH as above.
Supplementary Material
Supplemental Figure 1: Expression pattern of BG161338, which was up-regulated in the microarray assay, but not validated by RT-PCR or ISH assays.
(A) RT-PCR analysis of unmanipulated embryos shows that BG161338 is highly expressed at all developmental stages investigated.
(B) ISH analysis shows that at gastrula stages, BG161338 is expressed in the animal cap ectoderm (an) and the marginal zone (bracket) that will give rise to mesoderm; side view with animal to the top. Veg, vegetal pole. At neural plate stages, it is strongly expressed in the neural plate (np) and the ectoderm bordering the neural plate (arrow); anterior view with dorsal to the top. At neural tube stages it is strongly expressed throughout the neural tube 9nt), in the migrating neural crest (nc) and in the lens (L); anterior view with dorsal to the top. At larval stages it is additionally expressed in the otocyst (oto), branchial arch mesoderm (BA), somites (so), nephric mesoderm (ne) and ventral blood islands (bl); side view with anterior to the left.
Acknowledgments
We thank our many colleagues the Xenopus community who supplied ISH clones for several of the genes investigated herein. This work was partially funded by the NIH, the NSF and the George Washington University School of Medicine and Health Sciences.
Granting Agencies: NIH Grant number: NS23158
NSF Grant number: IOS-0817902
References
- Bertwistle D, Walmsley ME, Read EM, Pizzey JA, Patient RK. GATA factors and the origins of adult and embryonic blood in Xenopus: responses to retinoic acid. Mech. Dev. 1996;57:199–214. doi: 10.1016/0925-4773(96)00547-3. [DOI] [PubMed] [Google Scholar]
- Blak AA, Naserke T, Weisenhorn DM, Prakash N, Partanen J, Wurst W. Expression of Fgf receptors 1, 2, and 3 in the developing mid- and hindbrain of the mouse. Dev. Dyn. 2005;233:1023–1030. doi: 10.1002/dvdy.20386. [DOI] [PubMed] [Google Scholar]
- Bodoy S, Martin L, Zorzano A, Palacin M, Estevez R, Bertran J. Identification of LAT4, a novel amino acid transporter with system L activity. J. Biol. Chem. 2005;280:12002–12011. doi: 10.1074/jbc.M408638200. [DOI] [PubMed] [Google Scholar]
- Bromley E, Knapp D, Wardle FC, Sun BI, Collins-Racie L, LaVallie E, Smith JC, Sive HL. Identification and characterisation of the posteriorly-expressed Xenopus neurotrophin receptor homolog genes fullback and fullback-like. Gene Expr. Patterns. 2004;5:135–140. doi: 10.1016/j.modgep.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Christian JL, McMahon JA, McMahon AP, Moon RT. Xwnt-8, a Xenopus Wnt-1/int-1-related gene responsive to mesoderm-inducing growth factors, may play a role in ventral mesodermal patterning during embryogenesis. Development. 1991;111:1045–1055. doi: 10.1242/dev.111.4.1045. [DOI] [PubMed] [Google Scholar]
- Chung HA, Hyodo-Miura J, Nagamune T, Ueno N. FGF signal regulates gastrulation cell movements and morphology through its target NRH. Dev. Biol. 2005;282:95–110. doi: 10.1016/j.ydbio.2005.02.030. [DOI] [PubMed] [Google Scholar]
- Dalgin G, Goldman DC, Donley N, Ahmed R, Eide CA, Christian JL. GATA-2 functions downstream of BMPs and CaM KIV in ectodermal cells during primitive hematopoiesis. Dev. Biol. 2007;310:454–469. doi: 10.1016/j.ydbio.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza FS, Gawantka V, Gómez AP, Delius H, Ang SL, Niehrs C. The zinc finger gene Xblimp1 controls anterior endomesodermal cell fate in Spemann's organizer. EMBO J. 1999;18:6062–6072. doi: 10.1093/emboj/18.21.6062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaune E, Lemaire P, Kodjabachian L. Neural induction in Xenopus requires early FGF signalling in addition to BMP inhibition. Development. 2005;132:299–310. doi: 10.1242/dev.01582. [DOI] [PubMed] [Google Scholar]
- Fainsod A, Steinbeisser H, De Robertis EM. On the function of BMP-4 in patterning the marginal zone of the Xenopus embryo. EMBO J. 1994;13:5015–5025. doi: 10.1002/j.1460-2075.1994.tb06830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreiro B, Skoglund P, Bailey A, Dorsky R, Harris WA. XASH1, a Xenopus homolog of achaete-scute: a proneural gene in anterior regions of the vertebrate CNS. Mech. Dev. 1993;40:25–36. doi: 10.1016/0925-4773(93)90085-c. [DOI] [PubMed] [Google Scholar]
- Fetka I, Doederlein G, Bouwmeester T. Neuroectodermal specification and regionalization of the Spemann organizer in Xenopus. Mech. Dev. 2000;93:49–58. doi: 10.1016/s0925-4773(00)00265-3. [DOI] [PubMed] [Google Scholar]
- Fletcher RB, Baker JC, Harland RM. FGF8 spliceforms mediate early mesoderm and posterior neural tissue formation in Xenopus. Development. 2006;133:1703–1714. doi: 10.1242/dev.02342. [DOI] [PubMed] [Google Scholar]
- Frazzetto G, Klingbeil P, Bouwmeester T. Xenopus marginal coil (Xmc), a novel FGF inducible cytosolic coiled-coil protein regulating gastrulation movements. Mech. Dev. 2002;113:3–14. doi: 10.1016/s0925-4773(01)00664-5. [DOI] [PubMed] [Google Scholar]
- Friesel R, Brown SA. Spatially restricted expression of fibroblast growth factor receptor-2 during Xenopus development. Development. 1992;116:1051–1058. doi: 10.1242/dev.116.4.1051. [DOI] [PubMed] [Google Scholar]
- Fuentealba LC, Eivers E, Ikeda A, Hurtado C, Kuroda H, Pera EM, De Robertis EM. Integrating patterning signals: Wnt/GSK3 regulates the duration of the BMP/Smad1 signal. Cell. 2007;131:980–993. doi: 10.1016/j.cell.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamse JT, Sive H. Early anteroposterior division of the presumptive neurectoderm in Xenopus. Mech. Dev. 2001;104:21–36. doi: 10.1016/s0925-4773(01)00358-6. [DOI] [PubMed] [Google Scholar]
- Golub R, Adelman Z, Clementi J, Weiss R, Bonasera J, Servetnick M. Evolutionarily conserved and divergent expression of members of the FGF receptor family among vertebrate embryos, as revealed by FGFR expression patterns in Xenopus. Dev. Genes Evol. 2000;210:345–357. doi: 10.1007/s004270000076. [DOI] [PubMed] [Google Scholar]
- Grow M, Neff AW, Mescher AL, King MW. Global analysis of gene expression in Xenopus hind limbs during stage-dependent complete and incomplete regeneration. Dev. Dyn. 2006;235:2667–2685. doi: 10.1002/dvdy.20897. [DOI] [PubMed] [Google Scholar]
- Hawley SH, Wünnenberg-Stapleton K, Hashimoto C, Laurent MN, Watabe T, Blumberg BW, Cho KW. Disruption of BMP signals in embryonic Xenopus ectoderm leads to direct neural induction. Genes Dev. 1995;9:2923–2935. doi: 10.1101/gad.9.23.2923. [DOI] [PubMed] [Google Scholar]
- Heeg-Truesdell E, LaBonne C. Neural induction in Xenopus requires inhibition of Wnt-beta-catenin signaling. Dev. Biol. 2006;298:71–86. doi: 10.1016/j.ydbio.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Hesterberg LK, Weber K. Demonstration of three distinct calcium-binding sites in villin, a modulator of actin assembly. J. Biol. Chem. 1983;258:365–369. [PubMed] [Google Scholar]
- Horb ME, Thomsen GH. A vegetally localized T-box transcription factor in Xenopus eggs specifies mesoderm and endoderm and is essential for embryonic mesoderm formation. Development. 1997;124:1689–1698. doi: 10.1242/dev.124.9.1689. [DOI] [PubMed] [Google Scholar]
- Hufton AL, Vinayagam A, Suhai S, Baker JC. Genomic analysis of Xenopus organizer function. BMC Dev. Biol. 2006;6:27. doi: 10.1186/1471-213X-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K, Sokol SY. Early development of epidermis and neural tissue. In: Moody SA, editor. Principles of Developmental Genetics. San Diego: Academic Press; 2007. pp. 241–258. [Google Scholar]
- Kuroda H, Fuentealba L, Ikeda A, Reversade B, De Robertis EM. Default neural induction: neuralization of dissociated Xenopus cells is mediated by Ras/MAPK activation. Genes Dev. 2005;19:1022–1027. doi: 10.1101/gad.1306605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SM, Danielian PS, Fritzsch B, McMahon AP. Evidence that FGF8 signalling from the midbrain-hindbrain junction regulates growth and polarity in the developing midbrain. Development. 1997;124:959–969. doi: 10.1242/dev.124.5.959. [DOI] [PubMed] [Google Scholar]
- Luo T, Lee YH, Saint-Jeannet JP, Sargent TD. Induction of neural crest in Xenopus by transcription factor AP2alpha. Proc. Natl. Acad. Sci. USA. 2003;100:532–537. doi: 10.1073/pnas.0237226100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo T, Matsuo-Takasaki M, Lim JH, Sargent TD. Differential regulation of Dlx gene expression by a BMP morphogenetic gradient. Int. J. Dev. Biol. 2001;45:681–684. [PubMed] [Google Scholar]
- Marchal L, Luxardi G, Thomé V, Kodjabachian L. BMP inhibition initiates neural induction via FGF signaling and Zic genes. Proc. Natl. Acad. Sci. USA. 2009;106:17437–17442. doi: 10.1073/pnas.0906352106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo-Takasaki M, Matsumura M, Sasai Y. An essential role of Xenopus Foxi1a for ventral specification of the cephalic ectoderm during gastrulation. Development. 2005;132:3885–3894. doi: 10.1242/dev.01959. [DOI] [PubMed] [Google Scholar]
- Molenaar M, Roose J, Peterson J, Venanzi S, Clevers H, Destrée O. Differential expression of the HMG box transcription factors XTcf-3 and XLef-1 during early Xenopus development. Mech. Dev. 1998;75:151–154. doi: 10.1016/s0925-4773(98)00085-9. [DOI] [PubMed] [Google Scholar]
- Moody SA. Fates of the blastomeres of the 16-cell stage Xenopus embryo. Dev. Biol. 1987;119:560–578. doi: 10.1016/0012-1606(87)90059-5. [DOI] [PubMed] [Google Scholar]
- Moody SA. Cell lineage analysis in Xenopus embryos. Methods Mol. Biol. 2000;135:331–347. doi: 10.1385/1-59259-685-1:331. [DOI] [PubMed] [Google Scholar]
- Nishimatsu S, Thomsen GH. Ventral mesoderm induction and patterning by bone morphogenetic protein heterodimers in Xenopus embryos. Mech. Dev. 1998;74:75–88. doi: 10.1016/s0925-4773(98)00070-7. [DOI] [PubMed] [Google Scholar]
- Nutt SL, Dingwell KS, Holt CE, Amaya E. Xenopus Sprouty2 inhibits FGF-mediated gastrulation movements but does not affect mesoderm induction and patterning. Genes Dev. 2001;15:1152–1166. doi: 10.1101/gad.191301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossipova O, Stick R, Pieler T. XSPR-1 and XSPR-2, novel Sp1 related zinc finger containing genes, are dynamically expressed during Xenopus embryogenesis. Mech Dev. 2002;115:117–122. doi: 10.1016/s0925-4773(02)00086-2. [DOI] [PubMed] [Google Scholar]
- Panitz F, Krain B, Hollemann T, Nordheim A, Pieler T. The Spemann organizer-expressed zinc finger gene Xegr-1 responds to the MAP kinase/Ets-SRF signal transduction pathway. EMBO J. 1998;17:4414–4425. doi: 10.1093/emboj/17.15.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papalopulu N, Kintner C. Xenopus Distal-less related homeobox genes are expressed in the developing forebrain and are induced by planar signals. Development. 1993;117:961–975. doi: 10.1242/dev.117.3.961. [DOI] [PubMed] [Google Scholar]
- Pearl EJ, Barker D, Day RC, Beck CW. Identification of genes associated with regenerative success of Xenopus laevis hindlimbs. BMC Dev. Biol. 2008;8:66. doi: 10.1186/1471-213X-8-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers CD, Moody SA, Casey ES. Neural induction and factors that stabilize a neural fate. Birth Defects Res. C Embryo Today. 2009;87:249–262. doi: 10.1002/bdrc.20157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi CC, Hernandez-Lagunas L, Zhang C, Choi IF, Kwok L, Klymkowsky M, Artinger KB. Rohon-Beard sensory neurons are induced by BMP4 expressing non-neural ectoderm in Xenopus laevis. Dev. Biol. 2008;314:351–361. doi: 10.1016/j.ydbio.2007.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- Sabatini DM, Erdjument-Bromage H, Lui M, Tempst P, Snyder SH. RAFT1: a mammalian protein that binds to FKBP12 in a rapamycin-dependent fashion and is homologous to yeast TORs. Cell. 1994;78:35–43. doi: 10.1016/0092-8674(94)90570-3. [DOI] [PubMed] [Google Scholar]
- Salic AN, Kroll KL, Evans LM, Kirschner MW. Sizzled: a secreted Xwnt8 antagonist expressed in the ventral marginal zone of Xenopus embryos. Development. 1997;124:4739–4748. doi: 10.1242/dev.124.23.4739. [DOI] [PubMed] [Google Scholar]
- Sasai N, Nakazawa Y, Haraguchi T, Sasai Y. The neurotrophin-receptor-related protein NRH1 is essential for convergent extension movements. Nature Cell Biol. 2004;6:741–748. doi: 10.1038/ncb1158. [DOI] [PubMed] [Google Scholar]
- Sato SM, Sargent TD. Localized and inducible expression of Xenopus-posterior (Xpo), a novel gene active in early frog embryos, encoding a protein with a 'CCHC' finger domain. Development. 1991;112:747–753. doi: 10.1242/dev.112.3.747. [DOI] [PubMed] [Google Scholar]
- Sinner D, Kirilenko P, Rankin S, Wei E, Howard L, Kofron M, Heasman J, Woodland HR, Zorn AM. Global analysis of the transcriptional network controlling Xenopus endoderm formation. Development. 2006;133:1955–1966. doi: 10.1242/dev.02358. [DOI] [PubMed] [Google Scholar]
- Sirotkin H, Morrow B, Saint-Jore B, Puech A, Das Gupta R, Patanjali SR, Skoultchi A, Weissman SM, Kucherlapati R. Identification, characterization, and precise mapping of a human gene encoding a novel membrane-spanning protein from the 22q11 region deleted in velo-cardio-facial syndrome. Genomics. 1997;42:245–251. doi: 10.1006/geno.1997.4734. [DOI] [PubMed] [Google Scholar]
- Sive HL, Grainger RM, Harland RM. Early Development of Xenopus laevis, a Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2000. [Google Scholar]
- Sive HL, Hattori K, Weintraub H. Progressive determination during formation of the anteroposterior axis in Xenopus laevis. Cell. 1989;58:171–180. doi: 10.1016/0092-8674(89)90413-3. [DOI] [PubMed] [Google Scholar]
- Sölter M, Köster M, Hollemann T, Brey A, Pieler T, Knöchel W. Characterization of a subfamily of related winged helix genes, XFD-12/12'/12" (XFLIP), during Xenopus embryogenesis. Mech. Dev. 1999;89:161–165. doi: 10.1016/s0925-4773(99)00195-1. [DOI] [PubMed] [Google Scholar]
- Stern CD. Neural induction: old problem, new findings, yet more questions. Development. 2005;132:2007–2021. doi: 10.1242/dev.01794. [DOI] [PubMed] [Google Scholar]
- Sullivan SA, Akers L, Moody SA. foxD5a, a Xenopus winged helix gene, maintains an immature neural ectoderm via transcriptional repression that is dependent on the C-terminal domain. Dev. Biol. 2001;232:439–457. doi: 10.1006/dbio.2001.0191. [DOI] [PubMed] [Google Scholar]
- Sun BI, Bush SM, Collins-Racie LA, LaVallie ER, DiBlasio-Smith EA, Wolfman NM, McCoy JM, Sive HL. derrière: a TGF-beta family member required for posterior development in Xenopus. Development. 1999;126:1467–1482. doi: 10.1242/dev.126.7.1467. [DOI] [PubMed] [Google Scholar]
- Takeda M, Kurauchi T, Yamazaki T, Izutsu Y, Maéno M. Neptune is involved in posterior axis and tail formation in Xenopus embryogenesis. Dev. Dyn. 2005;234:63–73. doi: 10.1002/dvdy.20518. [DOI] [PubMed] [Google Scholar]
- Takeuchi M, Nakabayashi J, Sakaguchi T, Yamamoto TS, Takahashi H, Takeda H, Ueno N. The prickle-related gene in vertebrates is essential for gastrulation cell movements. Curr. Biol. 2003;13:674–679. doi: 10.1016/s0960-9822(03)00245-8. [DOI] [PubMed] [Google Scholar]
- Toyama R, Curtiss PE, Otani H, Kimura M, Dawid IB, Taira M. The LIM class homeobox gene lim5: implied role in CNS patterning in Xenopus and zebrafish. Dev. Biol. 1995;170:583–593. doi: 10.1006/dbio.1995.1238. [DOI] [PubMed] [Google Scholar]
- Umbhauer M, Penzo-Méndez A, Clavilier L, Boucaut J, Riou J. Signaling specificities of fibroblast growth factor receptors in early Xenopus embryo. J. Cell Sci. 2000;113:2865–2875. doi: 10.1242/jcs.113.16.2865. [DOI] [PubMed] [Google Scholar]
- Umemori H. Weaving the neuronal net with target-derived fibroblast growth factors. Dev. Growth Differ. 2009;51:263–270. doi: 10.1111/j.1440-169X.2008.01079.x. [DOI] [PubMed] [Google Scholar]
- Veenstra GJ, Beumer TL, Peterson-Maduro J, Stegeman BI, Karg HA, van der Vliet PC, Destrée OH. Dynamic and differential Oct-1 expression during early Xenopus embryogenesis: persistence of Oct-1 protein following down-regulation of the RNA. Mech. Dev. 1995;50:103–117. doi: 10.1016/0925-4773(94)00328-k. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Goto T, Keller R, Harland RM. Cloning and expression of Xenopus Prickle, an orthologue of a Drosophila planar cell polarity gene. Mech. Dev. 2002;116:183–186. doi: 10.1016/s0925-4773(02)00133-8. [DOI] [PubMed] [Google Scholar]
- Wanner SJ, Danos MC, Lohr JL, Miller JR. Molecular cloning and expression of Ena/Vasp-like (Evl) during Xenopus development. Gene Expr. Patterns. 2005;5:423–428. doi: 10.1016/j.modgep.2004.09.004. [DOI] [PubMed] [Google Scholar]
- White RJ, Sun BI, Sive HL, Smith JC. Direct and indirect regulation of derrière, a Xenopus mesoderm-inducing factor, by VegT. Development. 2002;129:4867–4876. doi: 10.1242/dev.129.20.4867. [DOI] [PubMed] [Google Scholar]
- Winning RS, Shea LJ, Marcus SJ, Sargent TD. Developmental regulation of transcription factor AP-2 during Xenopus laevis embryogenesis. Nucleic Acids Res. 1991;19:3709–3714. doi: 10.1093/nar/19.13.3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan B, Neilson KM, Moody SA. FoxD5 plays a critical upstream role in regulating neural ectodermal fate and the onset of neural differentiation. Dev. Biol. 2009;329:80–95. doi: 10.1016/j.ydbio.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Houston DW, King ML, Payne C, Wylie C, Heasman J. The role of maternal VegT in establishing the primary germ layers in Xenopus embryos. Cell. 1998;94:515–524. doi: 10.1016/s0092-8674(00)81592-5. [DOI] [PubMed] [Google Scholar]
- Zhang J, King ML. Xenopus VegT RNA is localized to the vegetal cortex during oogenesis and encodes a novel T-box transcription factor involved in mesodermal patterning. Development. 1996;122:4119–4129. doi: 10.1242/dev.122.12.4119. [DOI] [PubMed] [Google Scholar]
- Zhao H, Tanegashima K, Ro H, Dawid IB. Lrig3 regulates neural crest formation in Xenopus by modulating Fgf and Wnt signaling pathways. Development. 2008;135:1283–1293. doi: 10.1242/dev.015073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Cao Y, Zhao C, Postlethwait J, Meng A. An SP1-like transcription factor Spr2 acts downstream of Fgf signaling to mediate mesoderm induction. EMBO J. 2003;22:6078–6088. doi: 10.1093/emboj/cdg593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zon LI, Mather C, Burgess S, Bolce ME, Harland RM, Orkin SH. Expression of GATA-binding proteins during embryonic development in Xenopus laevis. Proc. Natl. Acad. Sci. USA. 1991;88:10642–10646. doi: 10.1073/pnas.88.23.10642. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Expression pattern of BG161338, which was up-regulated in the microarray assay, but not validated by RT-PCR or ISH assays.
(A) RT-PCR analysis of unmanipulated embryos shows that BG161338 is highly expressed at all developmental stages investigated.
(B) ISH analysis shows that at gastrula stages, BG161338 is expressed in the animal cap ectoderm (an) and the marginal zone (bracket) that will give rise to mesoderm; side view with animal to the top. Veg, vegetal pole. At neural plate stages, it is strongly expressed in the neural plate (np) and the ectoderm bordering the neural plate (arrow); anterior view with dorsal to the top. At neural tube stages it is strongly expressed throughout the neural tube 9nt), in the migrating neural crest (nc) and in the lens (L); anterior view with dorsal to the top. At larval stages it is additionally expressed in the otocyst (oto), branchial arch mesoderm (BA), somites (so), nephric mesoderm (ne) and ventral blood islands (bl); side view with anterior to the left.