Abstract
Background: Excessive weight gain during pregnancy is a major risk factor for postpartum weight retention and future weight gain and obesity in women, but few adequately powered randomized controlled trials have examined the efficacy of a behavioral weight-control intervention during pregnancy.
Objective: This study examined whether a behavioral intervention during pregnancy could decrease the proportion of women who exceeded the 1990 Institute of Medicine (IOM) recommendations for gestational weight gains and increase the proportion of women who returned to pregravid weights by 6 mo postpartum.
Design: This study was a randomized, assessor-blind, controlled trial. Participants were pregnant (13.5 wk gestation), normal-weight (NW; n = 201) and overweight or obese (OW/OB; n = 200) women whose average age was 28.8 y. Participants were randomly assigned within the 1990 IOM weight category (NW compared with OW/OB) to standard care (n = 200) or to a behavioral intervention to prevent excessive gestational weight gain (n = 201). The intervention included one face-to-face visit; weekly mailed materials that promoted an appropriate weight gain, healthy eating, and exercise; individual graphs of weight gain; and telephone-based feedback. The retention at the 6-mo postpartum assessment was 82%.
Results: Intent-to-treat analyses showed that the intervention, compared with standard care, decreased the percentage of NW women who exceeded IOM recommendations (40.2% compared with 52.1%; P = 0.003) and increased the percentages of NW and OW/OB women who returned to their pregravid weights or below by 6 mo postpartum (30.7% compared with 18.7%; P = 0.005).
Conclusion: A low-intensity behavioral intervention during pregnancy reduced excessive gestational weight gains in NW women and prevented postpartum weight retention in NW and OW/OB women. This trial was registered at clinicaltrials.gov as NCT01117961.
INTRODUCTION
Excessive weight gain during pregnancy is a major determinant of high postpartum weight retention and long-term obesity in women (1–11) and is linked with several other adverse maternal and fetal outcomes, including gestational hypertension, diabetes, preeclampsia, and cesarean delivery in the mother and macrosomia and long-term obesity in the offspring (12–20). In 1990, the National Academy of Science Institute of Medicine (IOM) formulated body mass index (BMI; in kg/m2)–specific recommended ranges for healthy weight gains during pregnancy, and in 2009 these ranges were made more restrictive for obese pregnant women (21–23). However, nearly one-half of normal-weight (NW) and two-thirds of overweight or obese (OW/OB) women have been shown to exceed even the more liberal 1990 guidelines for gestational weight gain (24).
Several researchers and government bodies have called for empirical studies that evaluate interventions that occur during pregnancy with the specific aim of promoting healthy weight gain during pregnancy and preventing postpartum weight retention and the diseases that follow (22, 25). Although behavioral treatment has been effective in promoting weight loss with other populations (26, 27), much less is known about how best to optimize weight gain during pregnancy and prevent postpartum weight retention. The primary goal of this study was to evaluate the effects of a behavioral lifestyle intervention delivered during pregnancy to decrease the proportion of women who exceeded recommendations for gestational weight gains and increase the proportion who returned to pregravid weights by 6 mo postpartum.
SUBJECTS AND METHODS
Identifying and enrolling study participants
Study participants were recruited by trained research assistants or nurses during the first prenatal visit of patients at 1 of 6 obstetric offices that represented socioeconomic and ethnic diversity in Providence, Rhode Island, from 2006 to 2008. Referral slips were presented to research staff who phone-screened patients for eligibility. All other aspects of the study were conducted outside of the clinic at a research center. Clinic staff and physicians were blinded to subject randomization to prevent contamination.
Women who were eligible and willing to participate in the study provided written informed consent and completed a baseline assessment at the research center after which they were randomly assigned into the intervention or standard care. Randomization was computer-generated (by the study statistician) in randomly varying block sizes and stratified by clinic and BMI category; allocation was concealed in opaque envelopes prepared by the study statistician. The unblinded study research coordinator enrolled and randomly assigned participants into groups. BMI categories were based on the 1990 IOM cutoffs (21) (ie, NW BMI: 19.8–26.0; OW/OB BMI: 26.1–40.0). Participant BMIs were calculated on the basis of self-reported weights and heights at the last menstrual period. Participants were assessed at study entry, 30 wk gestation, and 6 mo postpartum and were paid $25 for attending assessments. The procedures followed in this study were in accordance with the ethical standards of the institutional committees on human experimentation and were approved by the Institutional Review Boards at the Miriam Hospital (Providence, RI), the Women and Infants Hospital (Providence, RI), and California Polytechnic State University (San Luis Obispo, CA).
Study eligibility
Eligibility criteria included gestational age between 10 and 16 wk, BMI between 19.8 and 40, nonsmoking, adults (aged >18 y), fluency in English, access to a telephone, and a singleton pregnancy. Participants with self-reported major health or psychiatric diseases, weight loss during pregnancy, or a history of ≥3 miscarriages were excluded. Physician consent was required from individuals who endorsed items on the Physical Activity Readiness Questionnaire (28).
Standard care
Women in standard care attended their regularly scheduled visits with their prenatal care providers, which typically occurred monthly until 28 wk gestation, biweekly for 28–36 wk gestation, weekly until delivery, and at 6 wk postpartum. Women received standard nutrition counseling provided by physicians, nurses, nutritionists, and counselors from the Women, Infants, and Children's state program. Women were weighed by nurses at each clinical visit; weight graphs were not provided. In addition, women attended a brief (15 min) face-to-face visit at study entry with the study interventionist and received study newsletters at 2-mo intervals during pregnancy and postpartum that provided general information about pregnancy-related issues (eg, prenatal vitamins and maternity clothes); these contacts were designed to improve retention in the study.
Behavioral intervention during pregnancy
Participants in the intervention received all aspects of standard care plus a behavioral lifestyle intervention designed to prevent excessive weight gains during pregnancy; no intervention was provided postpartum. The Fit for Delivery intervention was developed out of a preliminary study done by Polley et al (29) and based on the 1990 IOM guidelines for nutrition and weight during pregnancy and was designed with an eventual dissemination in mind. Well-established behavioral principles rooted in social learning theory (30) were used to promote changes in eating and physical activity. The intervention included one face-to-face visit with an interventionist at the onset of treatment who discussed appropriate weight gains during pregnancy, physical activity (30 min of walking most days of the week), and calorie goals (20 kcal/kg); emphasis was placed on decreasing high fat foods, increasing physical activity, and daily self-monitoring of eating, exercise, and weight (31). Body-weight scales, food records, and pedometers were provided to promote adherence to daily self-monitoring. Automated postcards that prompted healthy eating and exercise habits were mailed weekly. In addition, after each clinic visit, women were sent personalized graphs of their weight gains with feedback. All women in the intervention received 3 brief (ie, 10–15 min) supportive phone calls from the dietitian during the intervention. Women who were over or under weight-gain guidelines during any 1-mo interval received additional brief, supportive phone calls (2 calls/mo) that provided structured meal plans, and specific goals until weight gains returned to appropriate amounts.
Measures
Demographic and weight-history information was obtained at study entry. The primary endpoints were the proportion of women with an excessive gestational weight gain on the basis of the 1990 IOM guidelines and the proportion of women at (±0.9 kg) or below their pregravid weights at 6 mo postpartum. The 1990 IOM guidelines were used because these data were collected before the 2009 revisions. Pregravid weight was based on a self-report at the time of study enrollment. Although the validity of self-reported prepregnancy weight has been shown to be good, especially if collected early in pregnancy (18, 32, 33), a measured weight from the year before pregnancy was available from the clinical records of 203 of the 401 participants (109 NW and 94 OW/OB women) to assess the validity of recalled prepregnancy weights. The correlation between the participant self-reported and physician measured weights was 0.95 (P = 0.0001) with a mean discrepancy of 0.5 ± 3.0 kg and no significant (P = 0.64) differences between NW and OW/OB subjects. These data provided evidence that participants’ self-reported weight information was a valid indication of their prepregnancy weight, even across weight strata. Heights were measured by trained research staff with a stadiometer at study entry. Total gestational weight gain was computed on the basis of the pregravid weight and weight at the last clinic visit (on calibrated scales) before delivery. On the basis of the 1990 IOM guidelines (21), we classified gestational weight gain as excessive in NW women whose weight gains were >35 lb (15.9 kg) and in overweight women whose gains were >25 lb (11.4 kg). Because the 1990 IOM recommendation for obese women provided only a lower limit of gain, similar to other studies, we combined overweight and obese women in our analysis and set the upper weight gain goal of 25 lb (11.4 kg) (34–36). Postpartum weight, changes in demographics, and breastfeeding status (any breastfeeding compared with formula only) were obtained by a blinded research assistant at the 6-mo postpartum visit. Obstetric records were abstracted after delivery to obtain maternal and fetal complications.
Statistical analyses
A power analysis was performed on the basis of a pilot study (29). It was shown that 200 subjects in each group at baseline, with the assumption of a 10% attrition rate and intent-to-treat analyses, would provide ≥90% power to detect a ≥25% difference between the intervention and standard care in proportions that exceeded weight-gain guidelines. In analyses of postpartum weight retention, with the conservative assumption of 30% attrition, an 81% power was provided to detect a 13.6% increase in the proportion of participants who achieved their preconception weights.
The a priori analysis plan followed an intent-to-treat principle by using all randomly assigned participants and conservatively assumed that those participants lost to follow-up were treatment failures. However, analyses that used data of completers yielded near-identical results. t and chi-square tests were used to compare completers with noncompleters and intervention compared with control groups. A multiple logistic regression analysis was used to examine the effects of treatment group and BMI category and their interaction on the proportion of women who exceeded the IOM recommended amount. All models were adjusted for clinic and other determinant confounders of estimates of excessive gestational gain, including total weeks of gestation at delivery, race, age, and parity. Income (r = 0.54, P = 0.0001) and education (r = 0.41, P = 0.001) were highly correlated with race and were, therefore, not included in the models. Analyses that substituted these variables for race yielded the same findings. Women with miscarriages (n = 6) were excluded from the primary analyses; patients with gestational diabetes (n = 32) were also excluded because of the extra nutritional counseling and contact provided and effects of insulin on weight. However, similar results were obtained with their inclusion. Multiple logistic regression analysis was also used to examine the effect of treatment group on the proportion of women who achieved their preconception weights at 6 mo postpartum, with adjustment for the same determinant confounders and weight category. Subsequent models examined the interaction between weight and treatment and the inclusion of 6-mo postpartum breastfeeding status. Women with subsequent pregnancy (n = 5) were excluded from these postpartum analyses.
Repeated-measures analysis of variance was used for secondary aims that assessed the effects of treatment group and BMI category on total weight gain and postpartum weight loss, with adjustment for the same covariates. Logistic regression analyses with similar covariates were also used to compare the intervention with standard care on differential delivery complications and maternal and fetal outcomes. Both R (version 2.11.1; Palo Alto, CA) and SPSS (PASW version 18.0.1; IBM, Somers, NY) statistical packages were used.
RESULTS
Participant enrollment and baseline characteristics
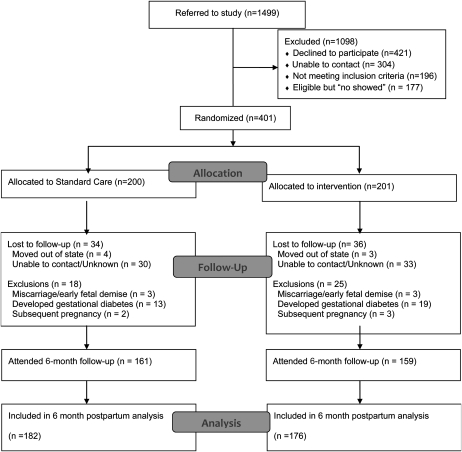
The participant flow into the Fit for Delivery Study is summarized in Figure 1. Of the 1499 potential participants approached, 421 individuals declined to participate and 304 individuals could not be contacted by the research team because of phone numbers that were out of service or because of incorrect contact information; 177 individuals were eligible but did not show up for the enrollment visit, and 196 individuals were ineligible. In full, 401 participants were randomly assigned into the intervention (n = 201) and control groups (n = 200), including 200 OW/OB and 201 NW subjects. The 2 study groups did not significantly differ on key baseline measures (Table 1). With the exclusion of subjects with miscarriages (n = 6), 94.9% (n = 375) of participants attended the 30-wk assessment visit (188 intervention and 187 control subjects), and 82% (n = 320) of participants attended the 6-mo postpartum assessment (159 intervention and 161 control subjects). Completers (n = 320) of the 6-mo postpartum assessment were significantly older (29.1 ± 5.1 compared with 27.1± 5.8 y; P = 0.005) than noncompleters (n = 75), but no other significant differences were shown.
FIGURE 1.
Flow diagram.
TABLE 1.
Baseline characteristics
| Variable | Standard care (n = 200) | Intervention (n = 201) | p1 |
| Age (y) | 28.80 ± 5.22 | 28.6 ± 5.2 | 0.72 |
| Race (%) | 0.19 | ||
| Non-Hispanic white | 67.5 | 68.7 | — |
| Latina and Hispanic | 19.6 | 19.6 | — |
| Non-Hispanic African American | 9.6 | 7.1 | — |
| Other | 3.3 | 4.6 | — |
| Marital status (%) | 0.57 | ||
| Married | 66.5 | 71.7 | — |
| Divorced | 3.0 | 3.0 | — |
| Widowed or never married | 30.5 | 25.3 | — |
| Education (%) | 0.72 | ||
| High school | 16.2 | 12.1 | — |
| Some college | 27.9 | 25.2 | — |
| College degree | 31.5 | 35.4 | — |
| Graduate degree | 24.4 | 27.3 | — |
| Employment (%) | 0.79 | ||
| Unemployed | 18.5 | 15.9 | — |
| Professional | 49.0 | 46.8 | — |
| Clerical | 25.5 | 27.9 | — |
| Trade and crafts | 7.0 | 9.4 | — |
| Childbearing history (%) | 0.51 | ||
| Primiparous | 76.6 | 76.3 | — |
| Multiparous | 23.4 | 23.7 | — |
| Community-based clinic (%) | 25.4 | 27.0 | 0.81 |
| Weeks’ gestation at study entry | 13.5 ± 1.8 | 13.6 ± 1.8 | 0.57 |
| BMI (kg/m2) | 26.48 ± 5.9 | 26.32 ± 5.6 | 0.79 |
P values represent the results of t tests for continuous measures and chi-square tests for categorical variables.
Mean ± SD (all such values).
Excessive weight gain during pregnancy and postpartum weight retention
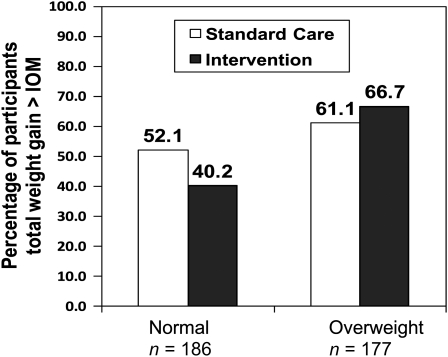
Weight-change variables are summarized in Table 2. As shown in Figure 2, the intent-to-treat analyses showed a significant interaction between treatment group and BMI category for excessive total gestational weight gain [odds ratio (OR): 0.38; 95% CI: 0.15, 0.97; P = 0.04]. In NW women, those in the intervention group were significantly less likely than women who received standard care to gain above the IOM recommendations (OR: 0.38; 95% CI: 0.20, 0.87; P = 0.003) but there was no significant effect in OW/OB women (OR: 1.4; 95% CI: 0.70, 2.7; P = 0.33). In the overall model, exceeding weight-gain goals was also significantly related to a higher gestational age at delivery (OR: 1.2; 95% CI: 1.1, 1.4; P = 0.0005) but not to race, parity, age, or clinic site (weeks of gestation at delivery data are shown in Table 3).
TABLE 2.
Weight changes during pregnancy by treatment group and BMI category1
| NW |
OW/OB |
|||
| Standard care (n = 94) | Intervention (n = 92) | Standard care (n = 90) | Intervention (n = 87) | |
| Total weight gain, pregravid to delivery (kg)2 | 16.2 ± 4.63 | 15.3 ± 4.4 | 15.1 ± 7.5 | 14.7 ± 6.9 |
| Exceeded IOM recommendations (%)4 | 52.1 | 40.2 | 61.1 | 66.7 |
| Gain (kg)2 | 19.7 ± 3.1 | 19.5 ± 3.3 | 19.6 ± 5.5 | 18.2 ± 4.8 |
| Within IOM recommendations (%)4 | 35.1 | 45.7 | 24.4 | 20.7 |
| Gain (kg)2 | 13.6 ± 1.3 | 13.5 ± 1.5 | 10.1 ± 1.9 | 10.2 ± 2.5 |
| Below IOM recommendations (%)4 | 12.8 | 14.1 | 13.3 | 12.6 |
| Gain (kg)2 | 9.1 ± 1.5 | 9.4 ± 1.3 | 4.0 ± 3.3 | 3.4 ± 3.9 |
| Clinic visits at which subjects exceeded IOM recommendations (%) | 35.1 | 26.0 | 63.0 | 58.0 |
| Exceeded IOM recommendations at some point during pregnancy (%)5 | 55.3 | 51.1 | 68.9 | 72.4 |
| Subjects who exceeded IOM recommendations during pregnancy but were within recommendations at delivery (%) | 3.2 | 10.9 | 7.8 | 5.7 |
| 6 mo postpartum6 | ||||
| Weight loss since delivery (kg)7 | 12.6 ± 4.7 | 12.7 ± 4.0 | 10.4 ± 5.9 | 11.3 ± 5.5 |
| Net weight retention (kg)7 | 3.3 ± 3.5 | 2.1 ± 4.7 | 4.3 ± 6.2 | 3.7 ± 5.9 |
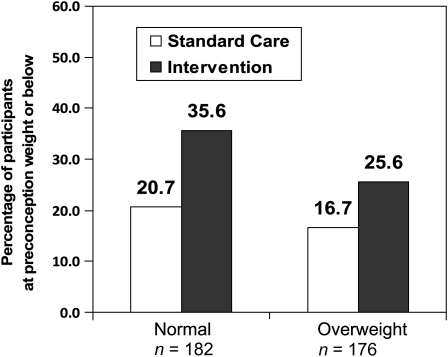
| Subjects at or below prepregnancy weight (%)8 | 20.7 | 35.6 | 16.7 | 25.6 |
IOM, Institute of Medicine; NW, normal weight; OW/OB, overweight or obese. All models were adjusted for clinic, total weeks of gestation at delivery, race, age, and parity.
On the basis of analyses of completers: n = 91 NW standard care, n = 89 NW intervention, n = 83 OW/OB standard care, and n = 80 NW intervention. Repeated-measures analysis showed no significant effects of treatment group on continuous measures of weight changes during pregnancy.
Mean ± SD (all such values).
Intent-to-treat analyses showed a significant interaction between treatment group and BMI category for excessive total gestational weight gain compared with all other weight-gain categories [odds ratio (OR): 0.38; 95% CI: 0.15, 0.97; P = 0.04].
Multiple logistic regression intent-to-treat analysis indicated a significant main effect for BMI category (OR: 0.20; 95% CI: 0.10, 0.43; P = 0.0001).
Six-month postpartum analysis excluded participants who became pregnant (n = 5).
On the basis of analyses of completers: n = 81 NW standard care, n = 72 NW intervention, n = 68 OW/OB standard care, and n = 70 OW/OB intervention. Repeated-measures analysis showed no significant effects of treatment group on continuous measures of weight changes postpartum.
Intent-to-treat analyses showed that the intervention increased percentages of NW and OW/OB women who achieved their preconception weights or below at 6 mo postpartum (OR: 2.1; 95% CI: 1.3, 3.5; P = 0.005); there was no significant weight × treatment group interaction (P = 0.71).
FIGURE 2.
Percentages of women whose total weight gain exceeded 1990 Institute of Medicine (IOM) recommendations. On the basis of multiple logistic regression analysis, the overall BMI category by treatment interaction odds ratio (OR) was 0.38 (95% CI: 0.15, 0.97; P = 0.04); the OR for the treatment effect in normal-weight women was 0.38 (95% CI: 0.20, 0.87; P = 0.003); no significant treatment effect in overweight women was observed (P = 0.33).
TABLE 3.
Effect of treatment group and BMI category on pregnancy outcomes1
| Normal weight |
Overweight |
|||
| Control group (n = 92) | Intervention group (n = 90) | Control group (n = 86) | Intervention group (n = 81) | |
| Infant birth weight (g) | 3271 ± 4672 | 3367 ± 459 | 3442 ± 629 | 3430 ± 650 |
| Low birth weight, <2500 g (n) | 5 | 4 | 4 | 5 |
| Macrosomia, >4000 g (n) | 3 | 6 | 14 | 14 |
| Weeks’ gestation at delivery | 38.4 ± 2.0 | 39.0 ± 1.7 | 38.7 ± 2.1 | 38.4 ± 2.7 |
| Preterm delivery, <36 wk (n) | 13 | 6 | 7 | 10 |
| Cesarean delivery (n) | 25 | 24 | 42 | 33 |
| Preeclampsia (n) | 9 | 3 | 11 | 17 |
| Maternal hypertension (n) | 11 | 3 | 11 | 17 |
| Gestational diabetes (n) | 6 | 8 | 7 | 11 |
Multiple logistic regression analyses indicated a significant treatment-by-weight interaction for maternal gestational hypertension [odds ratio (OR): 0.15; 95% CI: 0.02, 0.75; P = 0.02] and significant main effects for weight category, with lower odds of macrosomia (OR: 0.26; 95% CI: 0.06, 0.99; P = 0.05) and cesarean delivery (OR: 0.38; 95% CI: 0.26, 0.74; P = 0.004) for normal-weight women than for overweight or obese women.
Mean ± SD (all such values).
As shown in Figure 3, intent-to-treat analyses also showed that the intervention increased percentages of NW and OW/OB women who achieved their preconception weights or below at 6 mo postpartum (OR: 2.1; 95% CI: 1.3, 3.5; P = 0.005). Overall, 30.7% (54 of 176 subjects) of the intervention group but only 18.7% (34 of 182 subjects) of the standard care group were at their preconception weights or below by 6 mo postpartum. There was no significant weight × treatment group interaction (P = 0.71). Similar effects for treatment (P = 0.004) were observed in the analysis of completers that was adjusted for breastfeeding at 6 mo postpartum; breastfeeding was also significantly related to a higher odds of women achieving their 6-mo preconception weights or below (OR: 2.4; 95% CI: 1.4, 4.2; P = 0.002); 40.5% (53 of 131 subjects) of breastfeeders compared with 22.3% (35 of 157 subjects) of exclusive formula feeders achieved their preconception weights or below at 6 mo postpartum. Breastfeeding was not significantly related to the weight category or treatment group. Repeated-measures analyses showed no significant effects of treatment group on continuous measures of weight changes during pregnancy and the postpartum period.
FIGURE 3.
Percentages of women who returned to their preconception weights (±0.9 kg) or below at 6 mo postpartum. On the basis of multiple logistic regression analysis, the odds ratio for the main effect for treatment was 2.1 (95% CI: 1.3, 3.5; P = 0.005); there was no significant interaction with weight category (P = 0.71).
Pregnancy complications and fetal outcomes
Pregnancy complications are displayed in Table 3. A significant treatment-by-weight interaction was observed for maternal gestational hypertension (OR: 0.15; 95% CI: 0.02, 0.75; P = 0.02). Post hoc analyses indicated that the intervention was associated with a lower odds of gestational hypertension in NW individuals (OR: 0.21; 95% CI: 0.05, 0.96; P = 0.003) but had no significant effect in OW/OB women (OR: 1.8; 95% CI: 0.71, 4.6; P = 0.20). Main effects for the weight category were also observed, and NW women had a lower odds of macrosomia (OR: 0.26; 95% CI: 0.06, 0.99; P = 0.05) and cesarean delivery (OR: 0.38; 95% CI: 0.26, 0.74; P = 0.004) relative to OW/OB women. No other significant main or treatment effects or interactions were observed.
DISCUSSION
To our knowledge, this was the first adequately powered randomized trial to test the effects of a behavioral lifestyle intervention during pregnancy to reduce excessive gestational weight gains and postpartum weight retention in NW and OW/OB women. This study showed that it was possible to promote adherence to IOM recommendations in NW women by a low-intensity behavioral intervention that aimed to decrease high-fat foods and increase physical activity and daily self-monitoring of eating, exercise, and weight. The intervention had no significant effect on OW/OB women during pregnancy; however, by 6 mo postpartum, the intervention had increased the percentages of NW and OW/OB women who returned to their preconception weights.
Our positive findings for NW women during pregnancy were consistent with preliminary work in a smaller randomized trial by Polley et al (29) that used similar intervention methods and findings from an adequately powered, nonrandomized study by Olson et al (37) that showed effects in low-income NW women. By contrast, other randomized (38, 39) and nonrandomized (40) research involving NW women showed no significant effects for interventions that targeted gestational weight gain; however, these studies lacked dietary and physical activity strategies (39), lacked behavioral strategies (29, 38), used infrequent contact (40), or had a small sample size (38). Overall, findings from the current study and other evidence (29, 37) suggested that a low-intensity, partially mail-based behavioral intervention that targets dietary intake, physical activity, and weight monitoring can promote healthy pregnancy weight gain and prevent high postpartum weight retention in NW women. Because most childbearing women are NW (41), future research is now needed to disseminate and test the effects of this largely automated program into clinical practice.
The intervention in the current study did not significantly reduce excessive gestational weight gain in OW/OB women. These negative findings were consistent with several other studies of obese women (29, 38, 39, 42, 43), including a recent randomized trial (42). More intensive interventions that involved frequent contact (eg, weekly nutritional counseling or physician-provided feedback) and an emphasis on caloric restriction (18–25 kcal/kg) have met with more success (37, 44–50). However, much of the research in this area has been nonrandomized (37, 46–50) or implemented in countries with health care systems that can support time-intensive interventions (44, 47, 49). The recent revisions to the IOM guidelines have made recommended gestational weight gains even more restrictive for obese women than previous guidelines (11–20 lb total gain instead of 15–25 lb). Because excessive gestational weight gain can lead to a lifetime of unhealthy weight for the mother and her offspring (11, 19), future research is needed to determine how best to prevent excessive gestational weight gain in obese pregnant women and in the context of US health care.
Although the intervention increased the percentage of patients who met the pregnancy weight-gain guidelines in NW women only, it was effective in reducing the 6-mo postpartum weight retention in NW and OW/OB women. Overweight women may have learned strategies during their pregnancy that they implemented more effectively after the pregnancy was over. Two other preliminary studies have examined the effects of a pregnancy intervention on postpartum weight retention, and findings were mixed (29, 37). Because the intervention in the current study ceased after delivery, findings from the current study underscore the potential for interventions during pregnancy to exert an ongoing influence in NW and OW/OB women, even after treatment termination.
The intervention had no adverse effects on incidences of pregnancy and birth complications. By contrast, the intervention was associated with a reduced odds of gestational hypertension in NW women. Other research has documented a lack of adverse effects of pregnancy weight-control interventions on maternal and fetal outcomes (29, 38–40, 45). However, this area requires further investigation in larger studies.
Strengths of this study included its randomized, blinded design, the inclusion of NW and OW/OB women, follow-up from early in pregnancy through 6 mo postpartum, and the use of intervention strategies that may have practical relevance during prenatal care in the clinical setting. A high proportion (82%) of women who were randomly assigned completed the study through 6 mo postpartum. A limitation of the study was self-reported prepregnancy weight, which is a problem that exists throughout pregnancy research. In pregnant and nonpregnant samples, heavier women are more likely to underreport their weight and underestimate their BMI (51). Although underreporting may have led us to misclassify some OW/OB women as NW, this would have occurred equally in intervention and control groups. Moreover, we were able to validate the recalled prepregnancy weights against objectively measured weights before pregnancy for approximately one-half of our sample. Our sample was also self-selected, and findings may not generalize to the population at large.
In conclusion, the results of this study showed that a low-intensity behavioral intervention reduced excessive gestational weight gain in NW women and increased the percentages of NW and OW/OB women who returned to their preconception weights by 6 mo postpartum. Future research is needed to examine the effectiveness of this approach in the context of prenatal care. Ways to optimize gestational weight gain in OW/OB women remains another important avenue of inquiry. Pregnancy weight-gain interventions that target diet, physical activity, weight monitoring, and behavioral strategies have the potential to prevent long-term weight gain, obesity, and related comorbities in women.
Acknowledgments
We thank Theresa Scholl and Naomi Stotland for serving as the data safety officers on this project and for their helpful suggestions on the manuscript.
The authors’ responsibilities were as follows—SP, RW, MGP, and BA: designed research; FD and SP: conducted research; AS: analyzed data; SP, RRW, MGP, and BA: wrote the manuscript; SP: had primary responsibility for final content; and all authors: read and approved the final manuscript. None of the authors had a conflict of interest.
REFERENCES
- 1.Rooney BL, Schauberger CW, Mathiason MA. Impact of perinatal weight change on long-term obesity and obesity-related illnesses. Obstet Gynecol 2005;106:1349–56 [DOI] [PubMed] [Google Scholar]
- 2.Ashley JM, St Jeor ST, Perumean-Chaney S, Schrage J, Bovee V. Meal replacements in weight intervention. Obes Res 2001;9(suppl 4):312S–20S [DOI] [PubMed] [Google Scholar]
- 3.Rooney BL, Schauberger CW. Excess pregnancy weight gain and long-term obesity: one decade later. Obstet Gynecol 2002;100:245–52 [DOI] [PubMed] [Google Scholar]
- 4.Long-term weight development in women: a 15-year follow-up of the effects of pregnancy. Obes Res 2004;12:1166–78 [DOI] [PubMed] [Google Scholar]
- 5.Gore SA, Brown DM, West DS. The role of postpartum weight retention in obesity among women: a review of evidence. Ann Behav Med 2003;26:149–59 [DOI] [PubMed] [Google Scholar]
- 6.Gunderson EP, Abrams B, Selvin S. The relative importance of gestational gain and maternal characteristics associated with the risk of becoming overweight after pregnancy. Int J Obes Relat Metab Disord 2000;24:1660–8 [DOI] [PubMed] [Google Scholar]
- 7.Weight development over time in parous women--the SPAWN study--15 years follow-up. Int J Obes Relat Metab Disord. 2003;27:1516–22 [DOI] [PubMed] [Google Scholar]
- 8.Parker JD, Abrams B. Differences in postpartum weight retention between black and white mothers. Obstet Gynecol 1993;81:768–74 [PubMed] [Google Scholar]
- 9.Stotland NE, Cheng YW, Hopkins LM, Caughey AB. Gestational weight gain and adverse neonatal outcome among term infants. Obstet Gynecol 2006;108:635–43 [DOI] [PubMed] [Google Scholar]
- 10.Amorim AR, Rossner S, Neovius M, Lourenco PM, Linne Y. Does excess pregnancy weight gain constitute a major risk for increasing long-term BMI? Obesity (Silver Spring) 2007;15:1278–86 [DOI] [PubMed] [Google Scholar]
- 11.Mamun AA, Kinarivala M, O'Callaghan MJ, Williams GM, Najman JM, Callaway LK. Associations of excess weight gain during pregnancy with long-term maternal overweight and obesity: evidence from 21 y postpartum follow-up. Am J Clin Nutr 2010;91:1336–41 [DOI] [PubMed] [Google Scholar]
- 12.The effects of cigarette smoking and gestational weight change on birth outcomes in obese and normal-weight women. Am J Public Health 1997;87:591–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abrams B, Altman SL, Pickett KE. Pregnancy weight gain: still controversial. Am J Clin Nutr 2000;71(suppl):1233s–41s [DOI] [PubMed] [Google Scholar]
- 14.Shepard MJ, Saftlas AF, Leo-Summers L, Bracken MB. Maternal anthropometric factors and risk of primary cesarean delivery. Am J Public Health 1998;88:1534–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cogswell ME, Serdula MK, Hungerford DW, Yip R. Gestational weight gain among average-weight and overweight women-what is excessive? Am J Obstet Gynecol 1995;172:705–12 [DOI] [PubMed] [Google Scholar]
- 16.Witter FR, Caufield LE, Stolzfus RJ. Influence of maternal anthropometric status and birth weight on the risk of cesarean delivery. Obstet Gynecol 1995;85:947–51 [DOI] [PubMed] [Google Scholar]
- 17.Oken E, Rifas-Shiman SL, Field AE, Frazier AL, Gillman MW. Maternal gestational weight gain and offspring weight in adolescence. Obstet Gynecol 2008;112:999–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oken E, Taveras EM, Kleinman KP, Rich-Edwards JW, Gillman MW. Gestational weight gain and child adiposity at age 3 years. Am J Obstet Gynecol. 2007;196:322.e1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mamun AA, O'Callaghan M, Callaway L, Williams G, Najman J, Lawlor DA. Associations of gestational weight gain with offspring body mass index and blood pressure at 21 years of age: evidence from a birth cohort study. Circulation 2009;119:1720–7 [DOI] [PubMed] [Google Scholar]
- 20.Margerison Zilko CE, Rehkopf D, Abrams B. Association of maternal gestational weight gain with short- and long-term maternal and child health outcomes. Am J Obstet Gynecol. 2010;202:574.e1–8 [DOI] [PubMed] [Google Scholar]
- 21.Institute of Medicine Subcommittee on Nutritional Status and Weight Gain during Pregnancy. Nutrition during pregnancy. Washington, DC: National Academy of Sciences, 1990 [Google Scholar]
- 22.Rasmussen KM, Yaktine AL. Weight gain during pregnancy: reexamining the guidelines. Washington, DC: National Academies Press, 2009 [PubMed] [Google Scholar]
- 23.National Institutes of Health Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults–the evidence report. Obes Res 1998;6(suppl 2):51S–209S [PubMed] [Google Scholar]
- 24.Chu SY, Callaghan WM, Bish CL, D'Angelo D. Gestational weight gain by body mass index among US women delivering live births, 2004-2005: fueling future obesity. Am J Obstet Gynecol. 2009;200:271.e1–7 [DOI] [PubMed] [Google Scholar]
- 25.American College of Obstetricians and Gynecologists ACOG Committee Opinion number 315, September 2005. Obesity in pregnancy. Obstet Gynecol 2005;106:671–5 [DOI] [PubMed] [Google Scholar]
- 26.Wadden TA, Crerand CE, Brock J. Behavioral treatment of obesity. Psychiatr Clin North Am 2005;28:151–70, ix [DOI] [PubMed] [Google Scholar]
- 27.Wing RR. Behavioral interventions for obesity: recognizing our progress and future challenges. Obes Res 2003;11(suppl):3S–6S [DOI] [PubMed] [Google Scholar]
- 28.Thomas S, Reading J, Shephard RJ. Revision of the Physical Activity Readiness Questionnaire (PAR-Q). Can J Sport Sci 1992;17:338–45 [PubMed] [Google Scholar]
- 29.Polley BA, Wing RR, Sims CJ. Randomized controlled trial to prevent excessive weight gain in pregnant women. Int J Obes Relat Metab Disord 2002;26:1494–502 [DOI] [PubMed] [Google Scholar]
- 30.Bandura A. Social learning theory. Englewood Cliffs, NJ: Prentice-Hall, 1977 [Google Scholar]
- 31.National Academy of Sciences, Institute of Medicine, Food and Nutrition Board Nutrition during pregnancy and lactation. Washington, DC: National Academy Press, 1992 [Google Scholar]
- 32.Stevens-Simon C, Roghmann KJ, McAnarney ER. Relationship of self-reported prepregnant weight and weight gain during pregnancy to maternal body habitus and age. J Am Diet Assoc 1992;92:85–7 [PubMed] [Google Scholar]
- 33.Yu SM, Nagey DA. Validity of self-reported pregravid weight. Ann Epidemiol 1992;2:715–21 [DOI] [PubMed] [Google Scholar]
- 34.Brawarsky P, Stotland NE, Jackson RA, et al. Pre-pregnancy and pregnancy-related factors and the risk of excessive or inadequate gestational weight gain. Int J Gynaecol Obstet 2005;91:125–31 [DOI] [PubMed] [Google Scholar]
- 35.Stotland NE, Haas JS, Brawarsky P, Jackson RA, Fuentes-Afflick E, Escobar GJ. Body mass index, provider advice, and target gestational weight gain. Obstet Gynecol 2005;105:633–8 [DOI] [PubMed] [Google Scholar]
- 36.Cogswell ME, Scanlon KS, Fein SB, Schieve LA. Medically advised, mother's personal target, and actual weight gain during pregnancy. Obstet Gynecol 1999;94:616–22 [DOI] [PubMed] [Google Scholar]
- 37.Olson CM, Strawderman MS, Reed RG. Efficacy of an intervention to prevent excessive gestational weight gain. Am J Obstet Gynecol 2004;191:530–6 [DOI] [PubMed] [Google Scholar]
- 38.Asbee SM, Jenkins TR, Butler JR, White J, Elliot M, Rutledge A. Preventing excessive weight gain during pregnancy through dietary and lifestyle counseling: a randomized controlled trial. Obstet Gynecol 2009;113:305–12 [DOI] [PubMed] [Google Scholar]
- 39.Jeffries K, Shub A, Walker SP, Hiscock R, Permezel M. Reducing excessive weight gain in pregnancy: a randomised controlled trial. Med J Aust 2009;191:429–33 [DOI] [PubMed] [Google Scholar]
- 40.Kinnunen TI, Pasanen M, Aittasalo M, et al. Preventing excessive weight gain during pregnancy - a controlled trial in primary health care. Eur J Clin Nutr 2007;61:884–91 [DOI] [PubMed] [Google Scholar]
- 41.Vahratian A. Prevalence of overweight and obesity among women of childbearing age: results from the 2002 National Survey of Family Growth. Matern Child Health J 2009;13:268–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guelinckx I, Devlieger R, Mullie P, Vansant G. Effect of lifestyle intervention on dietary habits, physical activity, and gestational weigth gain in obese pregnant women: a randomized controlled trial. Am J Clin Nutr 2010;91:373–80 [DOI] [PubMed] [Google Scholar]
- 43.Kinnunen TI, Pasanen M, Aittasalo M, Fogelholm M, Weiderpass E, Luoto R. Reducing postpartum weight retention–a pilot trial in primary health care. Nutr J 2007;6:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wolff S, Legarth J, Vangsgaard K, Toubro S, Astrup A. A randomized trial of the effects of dietary counseling on gestational weight gain and glucose metabolism in obese pregnant women. Int J Obes (Lond) 2008;32:495–501 [DOI] [PubMed] [Google Scholar]
- 45.Thornton YS. Preventing excessive weight gain during pregnancy through dietary and lifestyle counseling: a randomized controlled trial. Obstet Gynecol 2009;114:173; author reply 173–4 [DOI] [PubMed] [Google Scholar]
- 46.Shirazian T, Monteith S, Friedman F, Rebarber A. Lifestyle modification program decreases pregnancy weight gain in obese women. Am J Perinatol 2010;27:411–4 [DOI] [PubMed] [Google Scholar]
- 47.Claesson IM, Sydsjo G, Brynhildsen J, et al. Weight gain restriction for obese pregnant women: a case-control intervention study. BJOG 2008;115:44–50 [DOI] [PubMed] [Google Scholar]
- 48.Artal R, Catanzaro RB, Gavard JA, Mostello DJ, Friganza JC. A lifestyle intervention of weight-gain restriction: diet and exercise in obese women with gestational diabetes mellitus. Appl Physiol Nutr Metab 2007;32:596–601 [DOI] [PubMed] [Google Scholar]
- 49.Mottola MF, Giroux I, Gratton R, et al. Nutrition and exercise prevents excess weight gain in overweight pregnant women. Med Sci Sports Exerc (Epub ahead of print 13 November 2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Algert S, Shragg P, Hollingswroth DR. Moderate caloric restriction in obese women with gestational diabetes. Obstet Gynecol 1985;65:487–91 [PubMed] [Google Scholar]
- 51.Gorber SC, Tremblay M, Moher D, Gorber B. A comparison of direct vs. self-report measures for assessing height, weight and body mass index: a systematic review. Obes Rev 2007;8:307–26 [DOI] [PubMed] [Google Scholar]