Abstract
Background: Exposure to polyunsaturated fatty acids (PUFAs) in early life may influence adiposity development.
Objective: We examined the extent to which prenatal n−3 (omega-3) and n−6 (omega-6) PUFA concentrations were associated with childhood adiposity.
Design: In mother-child pairs in the Project Viva cohort, we assessed midpregnancy fatty acid intakes (n = 1120), maternal plasma PUFA concentrations (n = 227), and umbilical cord plasma PUFA concentrations (n = 302). We performed multivariable regression analyses to examine independent associations of n−3 PUFAs, including docosahexaenoic and eicosapentaenoic acids (DHA + EPA), n−6 PUFAs, and the ratio of n−6:n−3 PUFAs, with child adiposity at age 3 y measured by the sum of subscapular and triceps skinfold thicknesses (SS + TR) and risk of obesity (body mass index ≥95th percentile for age and sex).
Results: Mean (±SD) DHA + EPA intake was 0.15 ± 0.14 g DHA + EPA/d, maternal plasma concentration was 1.9 ± 0.6%, and umbilical plasma concentration was 4.6 ± 1.2%. In children, SS + TR was 16.7 ± 4.3 mm, and 9.4% of children were obese. In the adjusted analysis, there was an association between each SD increase in DHA + EPA and lower child SS + TR [−0.31 mm (95% CI: −0.58, −0.04 mm) for maternal diet and −0.91 mm (95% CI: −1.63, −0.20 mm) for cord plasma] and lower odds of obesity [odds ratio (95% CI): 0.68 (0.50, 0.92) for maternal diet and 0.09 (0.02, 0.52) for cord plasma]. Maternal plasma DHA + EPA concentration was not significantly associated with child adiposity. A higher ratio of cord plasma n−6:n−3 PUFAs was associated with higher SS + TR and odds of obesity.
Conclusion: An enhanced maternal-fetal n−3 PUFA status was associated with lower childhood adiposity.
INTRODUCTION
Two types of polyunsaturated fatty acids (PUFAs), the n−3 (omega-3) and n−6 (omega-6) PUFAs, are essential in the human diet. During pregnancy, an adequate maternal intake of these nutrients, especially the long-chain n−3 PUFAs docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) that are primarily in fish, promotes the optimal development of the fetal brain and immune system (1, 2). Unfortunately, many women in the United States consume substantially less of these nutrients than recommended (3). Although women can convert the parent n−3 PUFA α-linolenic acid (ALA) into EPA and DHA, this conversion is limited and probably inadequate to support optimal tissue concentrations of DHA in the mother and fetus (4). In contrast, dietary sources of the parent n−6 PUFA linoleic acid (LA) and the long-chain arachidonic acid (AA) are abundant in developed countries, and the median intake of LA is similar to the recommended Adequate Intake (5).
Emerging evidence suggests that the composition of PUFAs in the maternal diet may influence offspring somatic growth and the deposition of adipose tissue. Body composition evolves dramatically during early development, with fat contributing ≈1–2% of body weight in a 1000-g preterm infant to upwards of 20% of body weight in a 3500-g term infant (6). Evidence from animal and cell culture studies confirmed that early PUFA exposure may influence adipose tissue development. Exposure to n−6 AA promotes maturation of adipocytes, whereas n−3 DHA blocks this process (7, 8). Offspring of mother rats fed a diet rich in n−3 PUFAs during pregnancy and lactation had lower body weight, adipose tissue mass, and leptin concentrations than did offspring of mothers given a diet very high in n−6 PUFAs and low in n−3 PUFAs (9, 10). In another rodent study, body weight in offspring of mothers supplemented with n−6 PUFAs was higher than in offspring of mothers supplemented with n−6 and n−3 PUFAs (11). Because the fat cell number remains stable throughout adulthood, early influences on adipocyte differentiation may have a lifelong influence on adiposity (12).
Limited human data also suggested that offspring growth was influenced by prenatal PUFA status. We previously reported that, in a cohort of pregnant women in the United States, a higher prenatal intake of long chain n−3 PUFAs was associated with lower birth-weight-for-gestational-age (13). Observational studies in some, but not all, other populations have had similar findings (14–17). However, these studies had no direct measure of adiposity other than weight and did not report on anthropometric measures after birth. These observational findings were supported by evidence from a small intervention trial in which children whose mothers were randomly assigned to receive prenatal supplementation with long-chain n−3 PUFAs had a lower attained body weight at 21 mo of age (18).
In the current study, we used data from a longitudinal prebirth cohort study to examine associations of prenatal n−3 and n−6 PUFA status reflected by maternal diet, maternal blood concentrations, and umbilical cord blood concentrations with child adiposity at age 3 y. We hypothesized that a higher prenatal n−3 PUFA exposure would be inversely associated with child adiposity, whereas an increased ratio of n−6:n−3 fatty acids would be directly related to child adiposity.
SUBJECTS AND METHODS
Study design and participants
Mothers and children were participants in Project Viva, which was a prospective observational cohort study in eastern Massachusetts into which we recruited pregnant women from 1999 to 2002 (13). All women provided written informed consent to participate in the study, and institutional review boards of participating institutions approved the study. All procedures were in accordance with the Declaration of Helsinki.
Of 2128 live births, 1579 children were eligible for the 3-y visit because their mothers provided information on prenatal diet and subsequently enrolled the children for follow-up beyond age 6 mo. We collected information from 1401 (89%) children at age 3 y. We excluded 9 women from the current analysis who reported prenatal cod liver–oil or other fish-oil supplement use. We further restricted the analysis to the 1250 mother-child pairs with a measure of child adiposity at age 3 y and some information on prenatal fatty acid status, including 1120 mother-child pairs with information on midpregnancy diet, 1178 mother-child pairs with information on late pregnancy diet, 227 mother-child pairs with measured maternal midpregnancy plasma fatty acid concentrations, and 302 mother-child pairs with measured umbilical cord plasma fatty acid concentrations. The 1250 mothers included in this analysis, compared with excluded mothers, were more likely to be white (73% compared with 57% of mothers, respectively), were more educated (71% compared with 56% of mothers, respectively, had at least a college degree), and had lower mean prepregnancy body mass index (BMI; in kg/m2) (24.6 compared with 25.3, respectively). Prenatal fatty acid intake was similar in included and excluded women.
Dietary assessment and blood fatty acid assays
At a mean of 29 wk gestation, participants completed a semiquantitative food-frequency questionnaire (FFQ), which we modified for use in pregnancy from a well-validated instrument used in several large cohorts of nonpregnant adults (19, 20) and validated against erythrocyte concentrations of fatty acids in pregnant women (21, 22). The FFQ quantified the average frequency of consumption of >140 specified foods and beverages during the preceding 3 mo.
Participants provided additional information on diet during the month before delivery in a brief dietary questionnaire completed in the hospital after delivery. The 9 questions queried frequency of consumption of major dietary contributors to n−3 and trans fatty acid intake (4 groupings of fish, poultry, beef, pork, or lamb, margarine, baked products, and deep-fried foods). This brief postdelivery questionnaire did not allow us to estimate the intake of total n−3 PUFAs, n−3 ALA, or n−6 fatty acids.
We calculated nutrient intakes by multiplying a weight assigned to the frequency of consumption of each food item from the FFQ by the nutrient composition for the portion size specified. For each time point, we used the sum of contributions to intake across all foods to generate the total intake of a variety of nutrients for each participant (23). We obtained nutrient estimates from the Harvard nutrient-composition database, which was based on US Department of Agriculture publications and is continually supplemented by other published sources and personal communications from laboratories and manufacturers (24–26). We energy adjusted by using the nutrient residuals method (27).
We collected maternal blood during the routine nonfasting midpregnancy clinical blood draw. We collected blood from the umbilical vein from infants after delivery who were delivered on a weekday at one of the 2 study hospital sites. We refrigerated blood samples immediately, and, within 24 h, separated the plasma from erythrocytes and stored aliquots in liquid nitrogen. We retained erythrocytes and plasma from the maternal blood and plasma only from the cord blood. We quantified fatty acid concentrations by using gas-liquid chromatography (22).
Child outcomes
At the 3-y visit, trained research assistants measured child height and weight with a calibrated stadiometer (Shorr Productions, Olney, MD) and scale (Seca model 881; Seca Corp, Hanover, MD) from which we calculated BMI. We determined age- and sex-specific BMI percentiles and z scores by using US reference data (28). We defined child obesity as BMI ≥95th percentile for age and sex, and used BMI <85th percentile as the comparison. The research assistants measured the subscapular skinfold thickness (SS) and triceps skinfold thickness (TR) with Holtain calipers (Holtain Ltd, Crosswell, United Kingdom), and calculated the sum (SS + TR) and ratio (SS:TR) of the 2 thicknesses. Research assistants followed standardized techniques for all measurements (29) and participated in biannual in-service training (IJ Shorr; Shorr Productions, Olney, MD). The inter- and intrarater measurement error was within published reference ranges (30).
We also collected venous blood from children, which we separated into erythrocytes and plasma and stored in liquid nitrogen. We measured concentrations of leptin in plasma for 702 of the 1250 children by using a radioimmunoassay (31).
Covariates
With the use of interviews and mailed questionnaires we obtained information on maternal demographic characteristics, medical history, and lifestyle habits, paternal height and weight, and child sex, behaviors, and diet. From the prenatal clinical record, we obtained results of the maternal glucose tolerance testing, which we used to classify women as having normal or impaired glucose tolerance or gestational diabetes mellitus (32), and serial pregnancy weights, which we used to categorize gestational weight gain according to 2009 Institute of Medicine guidelines (33). We obtained child gestation length and birth weight from the hospital medical record and determined the birth-weight-for-gestational-age (fetal growth) z score according to a 1999–2000 US national birth reference (34).
Analysis
We determined the child obesity prevalence and mean SS + TR by maternal characteristics by using chi-square and unadjusted linear regression analyses. We examined Spearman's correlations among fatty acids in maternal diet, maternal plasma, and umbilical cord plasma. We performed multivariable linear analyses to estimate the independent relation of prenatal fatty acid status with SS + TR and BMI z score. We checked models for normality and equal variance of residuals. We performed multivariable logistic regression analyses to examine associations with child obesity (BMI ≥95th compared with <85th percentiles).
Exposures of primary interest were n−3 PUFAs [ALA, DHA and EPA (DHA + EPA), and total n−3 PUFAs]; n−6 PUFAs (LA, AA, and total n−6 PUFAs), n−6:n−3 ratios (total n−6:n−3 PUFAs, LA:ALA, and AA:DHA + EPA). We fit separate models for each exposure and outcome, in which we modeled the effect of a 1-SD increase of each fatty acid measure in diet or blood. Before performing multivariable analyses, we examined scatter plots and exposures categorized into quartiles to ensure that the relations between exposures and outcomes were linear. In models with umbilical cord plasma fatty acid concentrations as the primary exposure, we excluded infants born before 34 completed weeks of gestation because the fatty acid transfer across the placenta occurs primarily in the late third trimester. We also used as exposures the proportion of women who consumed ≥200 mg DHA/d (compared with <200 mg DHA/d) as recommended in consensus guidelines (35), fish intake in servings per week, and fish intake above compared with within current US federal guidelines (>2 compared with ≤ 2 servings of fish/wk) (36). In secondary analyses, we examined associations of prenatal fatty acid status with the child 3-y blood leptin concentration. We reported β coefficients and 95% CIs for all continuous outcomes and odds ratios (ORs) with 95% CIs for obesity.
To control for confounding, we considered maternal and child covariates that were of a priori interest as independent predictors of child adiposity or that may be related to maternal diet during pregnancy. In all models, we included covariates as categorical variables as presented in Table 1, except for the length of gestation and child age at the 3-y assessment, which we included as continuous measures. Associations of prenatal fatty acid status with child-adiposity outcomes were similar in unadjusted models and in models adjusted for maternal and child characteristics. In the current article, we presented results from the multivariable models (see supplemental Table X under “Supplemental data” in the online issue for results from the unadjusted models). Additional adjustment for maternal annual household income, gestational diabetes and impaired glucose tolerance, alcohol consumption during pregnancy, and prudent compared with western diet patterns during pregnancy (37), paternal BMI, and child sleep duration, television viewing, and consumption of sugar-sweetened beverages or fish at age 3 y did not change the coefficients for prenatal fatty acid status substantially, and thus, we did not include these factors in our final models. We performed all analyses with SAS version 9.2 software (SAS Institute, Cary, NC).
TABLE 1.
Measures of child adiposity at age 3 y by maternal characteristics in 1250 mother-child pairs in Project Viva1
| Maternal characteristics | n | Children with obesity (BMI ≥ 95th percentile) at age 3 y | Child age 3 y SS + TR2 |
| n (%) | mm | ||
| Overall | 1250 | 116 (9) | 16.7 ± 4.3 |
| Age at enrollment | |||
| 14–24 y | 95 | 9 (9) | 16.4 ± 4.4 |
| 25–34 y | 771 | 68 (9) | 16.8 ± 4.3 |
| ≥35 y | 384 | 39 (10) | 16.7 ± 4.3 |
| P | — | 0.78 | 0.75 |
| Race-ethnicity | |||
| Non-Hispanic white | 909 | 78 (9) | 16.9 ± 4.1 |
| Black | 151 | 19 (13) | 15.9 ± 4.8 |
| Hispanic | 74 | 13 (18) | 17.3 ± 5.9 |
| Other race | 113 | 6 (5) | 16.2 ± 4.2 |
| P | — | 0.02 | 0.02 |
| Previous live births | |||
| 0 | 593 | 42 (7) | 16.7 ± 4.0 |
| ≥1 | 657 | 74 (11) | 16.8 ± 4.6 |
| P | — | 0.01 | 0.66 |
| Marital status | |||
| Married or cohabiting | 1163 | 106 (9) | 16.7 ± 4.2 |
| Single, divorced, or widowed | 83 | 10 (12) | 17.1 ± 5.6 |
| P | — | 0.40 | 0.40 |
| Education | |||
| Less than college | 364 | 43 (12) | 16.8 ± 4.9 |
| College graduate | 883 | 73 (8) | 16.7 ± 4.0 |
| P | — | 0.05 | 0.75 |
| Prepregnancy BMI | |||
| <25 kg/m2 | 807 | 45 (6) | 16.5 ± 4.0 |
| 25 to <30 kg/m2 | 271 | 33 (12) | 16.8 ± 4.4 |
| ≥30 kg/m2 | 168 | 37 (22) | 17.6 ± 5.5 |
| P | — | <0.0001 | 0.008 |
| Height | |||
| <1.6 m | 226 | 22 (10) | 16.8 ± 4.1 |
| 1.6 to <1.65 m | 341 | 29 (9) | 16.7 ± 4.5 |
| 1.65 to <1.7 m | 309 | 32 (10) | 16.7 ± 4.1 |
| ≥1.7 m | 374 | 33 (9) | 16.7 ± 4.4 |
| P | — | 0.87 | 0.98 |
| Gestational weight gain per 2009 Institute of Medicine guidelines | |||
| Inadequate | 143 | 13 (9) | 16.4 ± 5.0 |
| Adequate | 366 | 24 (7) | 16.7 ± 4.5 |
| Excessive | 725 | 77 (11) | 16.8 ± 4.1 |
| P | — | 0.09 | 0.60 |
| Tobacco use | |||
| Never smoked | 832 | 67 (8) | 16.4 ± 3.9 |
| Quit before pregnancy | 253 | 25 (10) | 17.2 ± 4.5 |
| Smoked during pregnancy | 132 | 21 (16) | 17.9 ± 5.6 |
| P | — | 0.01 | 0.0001 |
| Duration of any breastfeeding | |||
| ≤2 mo | 298 | 37 (13) | 17.5 ± 4.8 |
| >2 to <6 mo | 327 | 31 (10) | 16.7 ± 4.2 |
| ≥6 to <11 mo | 235 | 26 (11) | 16.9 ± 4.3 |
| ≥11 mo | 327 | 17 (5) | 16.0 ± 3.7 |
| P | — | 0.01 | 0.0004 |
SS + TR, sum of subscapular and triceps skinfold thicknesses. P values were derived from a chi-square test for obesity and by linear regression for skinfold thickness.
Values are means ± SDs.
RESULTS
The mean (±SD) maternal age at study enrollment was 32.4 ± 5.1 y, and more than one-third of women were overweight or obese before pregnancy. In children at 3 y of age, SS + TR was 16.7 ± 4.3 mm, BMI z score was 0.46 ± 1.03, and 9.4% of children were obese (BMI ≥95th percentile for age and sex). Participant characteristics were generally associated with child adiposity in the expected directions (Table 1).
In Table 2, we report the means ± SDs for maternal dietary fatty acid intake and plasma fatty acid concentrations. In Table 3, we present correlations of maternal dietary fish and fatty acid intake with corresponding concentrations in maternal and umbilical cord plasma. As expected, fish consumption was a primary contributor to DHA + EPA intake, and maternal fish intake was correlated with maternal and cord plasma DHA + EPA concentrations (Table 3). The maternal dietary intake of DHA + EPA was correlated with the intake of AA (r = 0.36), and the intake of total n−3 PUFAs was correlated with the intake of total n−6 PUFAs (r = 0.76) (Table 3).
TABLE 2.
Adjusted associations of maternal and child fatty acid status with child adiposity at age 3 y in 1250 mother-child pairs in Project Viva1
| Fatty acid measure | Values | Obesity (BMI ≥95th vs <85th percentiles)2 | BMI z score3 | SS + TR (mm)3 |
| mm | ||||
| Maternal dietary intake during midpregnancy (n = 1120) | ||||
| Total n−3 fatty acids (g/d) | 1.16 ± 0.424 | 0.77 (0.60, 0.99) | −0.03 (−0.09, 0.03) | −0.34 (−0.59,-0.08) |
| DHA + EPA (g/d) | 0.15 ± 0.14 | 0.68 (0.50, 0.92) | −0.04 (−0.10, 0.03) | −0.31 (−0.58,-0.04) |
| ALA (g/d) | 0.99 ± 0.40 | 0.85 (0.67, 1.08) | −0.01 (−0.08, 0.05) | −0.26 (−0.51, 0.00) |
| Total n−6 fatty acids (g/d) | 12.27 ± 3.18 | 0.88 (0.70, 1.10) | −0.01 (−0.07, 0.05) | −0.20 (−0.45, 0.05) |
| AA (g/d) | 0.09 ± 0.03 | 0.82 (0.64, 1.06) | −0.02 (−0.08, 0.05) | −0.23 (−0.50, 0.04) |
| LA (g/d) | 12.16 ± 3.17 | 0.88 (0.70, 1.10) | −0.01 (−0.07, 0.05) | −0.20 (−0.45, 0.05) |
| n−6:n−3 ratio | 11.13 ± 2.53 | 1.19 (0.94, 1.50) | 0.04 (−0.02, 0.10) | 0.32 (0.07, 0.58) |
| AA:DHA + EPA ratio | 10.32 ± 52.91 | 1.19 (1.00, 1.42) | 0.03 (−0.03, 0.10) | 0.01 (−0.25, 0.26) |
| LA:ALA ratio | 13.07 ± 3.04 | 1.02 (0.79, 1.31) | 0.02 (−0.05, 0.08) | 0.20 (−0.05, 0.46) |
| DHA intake, ≥200 vs <200 mg/d (%) | 12.1 | 0.82 (0.37, 1.84) | −0.02 (−0.21, 0.17) | −0.37 (−1.15, 0.42) |
| Fish intake (effect per serving/wk) | 1.58 ± 1.38 | 0.77 (0.62, 0.95) | −0.03 (−0.09, 0.03) | −0.16 (−0.34, 0.03) |
| Fish intake, >2 vs ≤2 servings/wk (%) | 22.3 | 0.53 (0.27, 1.03) | −0.07 (−0.22, 0.08) | −0.21 (−0.81, 0.40) |
| Relative concentration of midpregnancy maternal plasma lipids (n = 227) | ||||
| Total n−3 fatty acids (% of total fatty acids) | 2.8 ± 0.7 | 0.52 (0.16, 1.75) | −0.01 (−0.16, 0.15) | −0.50 (−1.21, 0.22) |
| DHA + EPA (% of total fatty acids) | 1.9 ± 0.6 | 0.68 (0.22, 2.16) | 0.01 (−0.14, 0.17) | −0.39 (−1.11, 0.33) |
| ALA (% of total fatty acids) | 0.61 ± 0.19 | 0.51 (0.18, 1.44) | −0.04 (−0.19, 0.10) | −0.44 (−1.12, 0.24) |
| Total n−6 fatty acids (% of total fatty acids) | 37.3 ± 3.8 | 1.89 (0.76, 4.73) | 0.16 (0.02, 0.30) | 0.35 (−0.29, 0.99) |
| AA (% of total fatty acids) | 6.2 ± 1.2 | 1.49 (0.59, 3.74) | 0.12 (−0.03, 0.27) | 0.64 (−0.06, 1.33) |
| LA (% of total fatty acids) | 28.5 ± 3.7 | 1.80 (0.72, 4.48) | 0.14 (0.00, 0.28) | 0.16 (−0.49, 0.81) |
| n−6:n−3 ratio | 14.1 ± 3.3 | 2.15 (0.90, 5.15) | 0.07 (−0.08, 0.22) | 0.63 (−0.04, 1.30) |
| AA:DHA + EPA ratio | 3.4 ± 0.9 | 2.16 (0.77, 6.02) | 0.07 (−0.08, 0.22) | 0.84 (0.15, 1.54) |
| LA:ALA ratio | 51.2 ± 18.8 | 2.27 (0.72, 7.16) | 0.10 (−0.05, 0.24) | 0.27 (−0.39, 0.92) |
| Maternal dietary intake during the month before delivery (n = 1178) | ||||
| DHA + EPA (g/d) | 0.11 ± 0.11 | 0.84 (0.62, 1.15) | −0.04 (−0.10, 0.02) | −0.20 (−0.48, 0.07) |
| DHA intake, ≥200 vs <200 mg/d (%) | 2.7 | 0.95 (0.20, 4.52) | −0.11 (−0.49, 0.28) | −0.76 (−2.41, 0.90) |
| Fish intake, >2 vs ≤2 servings/wk (%) | 21.0 | 0.60 (0.32, 1.11) | −0.07 (−0.22, 0.08) | −0.25 (−0.88, 0.38) |
| Relative concentration of umbilical cord plasma lipids (n = 3025) | ||||
| Total n−3 fatty acids (% of total fatty acids) | 5.1 ± 1.4 | 0.07 (0.01, 0.48) | −0.14 (−0.28, 0.01) | −0.92 (−1.65,-0.20) |
| DHA + EPA (% of total fatty acids) | 4.6 ± 1.2 | 0.09 (0.02, 0.52) | −0.14 (−0.29, 0.01) | −0.91 (−1.63,-0.20) |
| ALA (% of total fatty acids) | 0.17 ± 0.07 | 0.46 (0.19, 1.13) | −0.05 (−0.18, 0.09) | −0.46 (−1.12, 0.21) |
| Total n−6 fatty acids (% of total fatty acids) | 31.4 ± 2.7 | 0.68 (0.39, 1.18) | −0.09 (−0.22, 0.04) | −0.23 (−0.86, 0.40) |
| AA (% of total fatty acids) | 15.5 ± 2.1 | 0.64 (0.35, 1.17) | −0.12 (−0.25, 0.02) | −0.23 (−0.90, 0.44) |
| LA (% of total fatty acids) | 11.1 ± 1.6 | 0.78 (0.41, 1.50) | −0.02 (−0.15, 0.12) | −0.06 (−0.72, 0.60) |
| n−6:n−3 ratio | 6.5 ± 1.6 | 3.81 (1.40,10.36) | 0.09 (−0.05, 0.23) | 0.76 (0.09, 1.42) |
| AA:DHA + EPA ratio | 3.6 ± 0.9 | 2.63 (1.09, 6.31) | 0.05 (−0.10, 0.19) | 0.69 (0.02, 1.36) |
| LA:ALA ratio | 74.2 ± 32.8 | 1.80 (1.03, 3.15) | 0.00 (−0.13, 0.13) | 0.21 (−0.43, 0.86) |
| Child diet, age 3 y (n = 1149) | ||||
| Fish intake (effect per serving/wk) | 0.86 ± 1.19 | 1.00 (0.81, 1.22) | 0.03 (−0.03, 0.10) | 0.07 (−0.14, 0.29) |
| Fish intake, >2 vs ≤2 servings/wk (%) | 9.2 | 1.01 (0.44, 2.31) | 0.09 (−0.12, 0.30) | 0.14 (−0.73, 1.02) |
SS + TR, sum of subscapular and triceps skinfold thicknesses; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; ALA, α-linolenic acid; AA, arachidonic acid; LA, linoleic acid. The effect estimate represents the effect per SD increase of polyunsaturated fatty acid unless otherwise noted. Values were adjusted for maternal race-ethnicity, smoking, parity, adequacy of gestational weight gain, prepregnancy BMI, height, education, age, marital status, and breastfeeding duration and child length of gestation, sex, and age at 3-y assessment by using multivariable linear or logistic regression analysis.
Values are odds ratios; 95% CIs in parentheses.
Values are β coefficients; 95% CIs in parentheses.
Mean ± SD (all such values).
n = 246 for total n−3, EPA, DHA + EPA, n−6:n−3 ratio, and AA:DHA + EPA ratio from cord blood plasma.
TABLE 3.
Spearman's correlation coefficients for maternal dietary fatty acid intake and fatty acid concentrations in maternal and cord blood in 1250 mother-child pairs in Project Viva1
| Midpregnancy maternal dietary intake |
|||||||
| Total n−3 PUFAs | DHA + EPA | Total n−6 PUFAs | AA | Total n−6:n−3 ratio | AA:DHA + EPA ratio | Fish intake | |
| Fatty acid intake in maternal midpregnancy diet (n = 1250) | |||||||
| Total n−3 PUFAs | 1.0 | 0.32*** | 0.76*** | 0.39*** | −0.69*** | −0.21*** | 0.29*** |
| DHA + EPA | 1.0 | −0.01 | 0.36*** | −0.51*** | −0.94*** | 0.84*** | |
| Total n−6 PUFAs | 1.0 | 0.21*** | −0.10** | 0.08* | 0.00 | ||
| AA | 1.0 | −0.34*** | −0.06dagger | 0.29*** | |||
| Total n−6:n−3 ratio | 1.0 | 0.43*** | −0.45*** | ||||
| AA:DHA + EPA ratio | 1.0 | −0.80*** | |||||
| Fatty acid concentrations in maternal midpregnancy plasma (n = 227) | |||||||
| Total n−3 PUFAs | 0.17 * | 0.50*** | 0.03 | 0.07 | −0.25*** | −0.50*** | 0.47*** |
| DHA + EPA | 0.15* | 0.50*** | 0.00 | 0.09 | −0.24*** | −0.51*** | 0.48*** |
| Total n−6 PUFAs | 0.21** | 0.16* | 0.25*** | 0.12dagger | −0.03 | −0.14* | 0.09 |
| AA | 0.02 | 0.06 | 0.00 | 0.12dagger | −0.01 | 0.00 | 0.01 |
| Total n−6:n−3 ratio | −0.10 | −0.47*** | 0.06 | −0.03 | 0.25*** | 0.47*** | −0.46*** |
| AA:DHA + EPA ratio | −0.14* | −0.50*** | 0.01 | 0.03 | 0.25*** | 0.56*** | −0.51*** |
| Fatty acid concentrations in umbilical cord plasma (n = 3022) | |||||||
| Total n−3 PUFAs | 0.13dagger | 0.37*** | −0.07 | 0.09 | −0.27*** | −0.34*** | 0.32*** |
| DHA + EPA | 0.13dagger | 0.36*** | −0.08 | 0.08 | −0.27*** | −0.33*** | 0.32*** |
| Total n−6 PUFAs | 0.06 | −0.03 | 0.15* | 0.10dagger | 0.03 | 0.06 | −0.06 |
| AA | 0.00 | −0.14* | 0.03) | 0.05 | 0.01 | 0.17* | −0.11dagger |
| Total n−6:n−3 ratio | −0.12dagger | −0.40*** | 0.12dagger | −0.06 | 0.29*** | 0.38*** | −0.36*** |
| AA:DHA + EPA ratio | −0.14dagger | −0.45*** | 0.09 | −0.07 | 0.28*** | 0.44*** | −0.39*** |
| 3-y fish intake | 0.09** | 0.34*** | −0.05 | 0.14*** | −0.20*** | −0.32*** | 0.33*** |
PUFA, polyunsaturated fatty acid; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; AA, arachidonic acid. ***P <0.001, **0.001 ≤ P < 0.01, *0.01 ≤ P < 0.05, †0.05 ≤ P < 0.10.
n = 246 for total n−3, EPA, DHA + EPA, n−6:n−3 ratio, and AA:DHA + EPA ratio from cord blood plasma.
In Table 2, we report associations of each fatty acid measure with child outcomes at age 3 y. We observed inverse relations of several indicators of prenatal n−3 PUFA status with child-adiposity measures (Table 2). For example, higher DHA + EPA concentrations in the midpregnancy diet was associated with lower SS + TR concentrations [β = −0.31 mm (95% CI: −0.58, −0.04 mm)] and with a reduced odds of childhood obesity [odds ratio: 0.68 (95% CI: 0.50, 0.92)]. Higher DHA + EPA concentrations in umbilical cord plasma was similarly associated with lower adiposity [β = −0.91 mm (95% CI: −1.63, −0.20 mm) for skinfold thicknesses; odds ratio 0.09 (95% CI: 0.02, 0.52 for obesity)]. Late pregnancy diet and maternal second trimester plasma also suggested that a higher prenatal n−3 PUFA status was associated with lower childhood adiposity but with wider CIs that did not exclude a null effect (Table 2). Associations of the prenatal n−3 PUFA status with the BMI z score as a continuous outcome were less strong and generally null (Table 2). Further adjustment for the fetal growth z score did not markedly change any of the effect estimates (eg, the estimated association of DHA + EPA intake with SS + TR went from −0.31 to −0.29 mm).
The mean (±SD) child plasma leptin concentration was 1.92 ± 1.90 ng/mL, and as expected, leptin concentrations were directly correlated with contemporaneous SS + TR (Spearman's r = 0.21, P < 0.0001). Maternal midpregnancy n−3 PUFA intake was inversely associated with child blood leptin concentrations [β = −0.14 ng/mL (95% CI: −0.27, −0.01 ng/mL) for total n−3 PUFAs, β =−0.10 ng/mL (95% CI: −0.25, 0.04 ng/mL) for DHA + EPA, and β = −0.11 ng/mL (95% CI −0.22, −0.01 ng/mL) per weekly fish serving]. In the small number of participants with both measures, n−3 PUFA concentrations in maternal (n = 122) and umbilical cord blood (n = 153) were not related to child leptin concentrations (data not shown).
Higher maternal intake and cord plasma concentrations of total n−6 PUFAs and AA were generally not significantly associated with adiposity outcomes (Table 2). However, in maternal plasma, a higher AA concentration was associated with a somewhat higher SS + TR [0.64 mm ( 95% CI: −0.06, 1.33 mm)], BMI z score [0.12 (95% CI: −0.03, 0.27)], and leptin concentrations [0.15 ng/mL (95% CI: 0.00, 0.29 ng/mL)], and a higher LA concentration was associated with a higher BMI z score [0.14 (95% CI: 0.00, 0.28)], although all CIs included a null association.
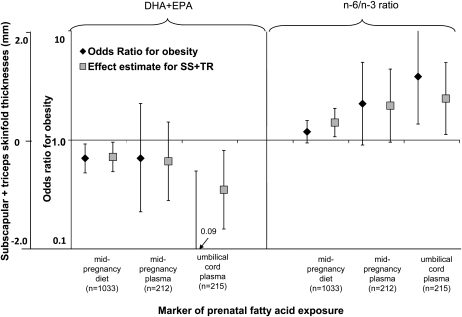
Ratios of total n−6:n−3 fatty acids and of AA:DHA + EPA in umbilical cord plasma lipids were directly associated with the sum of skinfold thicknesses and obesity (Table 2). We also observed significant direct associations of the maternal dietary n−6:n−3 ratio with child skinfold thickness, of maternal dietary AA:DHA + EPA with odds of child obesity, and of maternal plasma AA:DHA + EPA with child skinfold thickness. In Figure 1, we present the covariate-adjusted associations of measures of prenatal DHA + EPA and n−6:n−3 status with SS + TR and odds for obesity in children.
FIGURE 1.
Associations of pre- and perinatal docosahexaenoic acid and eicosapentaenoic acid (DHA+EPA) status and of n−6:n−3 polyunsaturated fatty acid ratio with child age 3 y subscapular and triceps skinfold thickness (SS+TR) and odds of obesity. Effect estimates represent β coefficients (for linear regression) or odds ratios (for logistic regression) and 95% CIs per SD increase of energy-adjusted DHA+EPA or n−6:n−3 ratio adjusted for maternal race-ethnicity, smoking, parity, adequacy of gestational weight gain, prepregnancy BMI, height, education, age, and marital status and child length of gestation, sex, breastfeeding duration, and age at 3-y assessment.
Prenatal dietary intake and blood concentrations of trans fatty acids were not significantly associated with child adiposity (data not shown). Child fish intake at 3 y was not significantly associated with contemporaneous adiposity (Table 2) or with blood leptin concentrations [−0.02 ng/mL (95% CI: −0.16, 0.13)]. When further adjusted for child fish intake, estimates for maternal fatty acid status were not substantially different (results not shown).
DISCUSSION
In this cohort of children followed since pregnancy, higher n−3 PUFA concentrations in the maternal diet and in umbilical cord plasma phospholipids were associated with lower adiposity in children at age 3 y as measured by the sum of skinfold thicknesses, odds of obesity, and the concentration of leptin, which is a biomarker directly correlated with adiposity. Fish intake is a primary source of n−3 PUFAs, and a greater maternal prenatal fish intake was also associated with lower child adiposity. Associations were similar but weaker with the BMI z score. These associations persisted after adjustment for a number of maternal and child factors associated with adiposity.
These results extended and expanded findings from studies in animals and human infants and suggested that a higher intake of n−3 PUFAs, especially the long-chain fatty acids DHA and EPA, may influence weight gain in early life (13, 38, 39). Only a few investigators have examined pre- or perinatal PUFA status and child weight or adiposity after birth in humans. Helland et al (40) did not find a difference in lengths, weights, or head circumferences from birth through age 12 mo in children of mothers who were randomly assigned to receive cod liver oil (2632 mg n−3 PUFAs/d and 235 mg n−6 PUFAs/d) compared with corn oil (350 mg n−3 PUFAs/d and 4747 mg n−6 PUFAs/d) during pregnancy and lactation. However, growth data were only available for 288 infants of the 590 mothers who were randomly assigned (44%). In a small randomized trial, children of women given a supplement that contained 200 mg DHA during pregnancy and lactation had BMI z scores at age 21 mo that were 0.76 lower than the BMI z scores of children of mothers given a vitamin supplement without DHA (18). In the current study, we had several measures of prenatal fatty acid status (ie, maternal diet, maternal blood, and umbilical cord blood) and followed a large population of children to age 3 y with several measures of adiposity including skinfold thicknesses and blood leptin concentrations.
We also showed that a higher n−6:n−3 PUFA ratio in the maternal diet, in maternal blood, or in cord blood was associated with higher child adiposity at age 3 y. In addition to their direct adipogenic effects, as shown in animal and cell culture studies (7–10), n−6 PUFAs may compete with n−3 PUFAs for enzymes necessary for elongation and desaturation and, thus, limit the conversion of the parent n−3 PUFA ALA to DHA and EPA. A high intake of n−6 relative to n−3 PUFAs may have more substantial effects on adipose tissue development and body weight than absolute n−6 PUFA concentrations (9, 10, 41). However, we saw no strong or consistent evidence that the prenatal n−6 PUFA status itself predicted childhood adiposity. Thus, the association of a higher n−6:n−3 ratio with greater adiposity probably reflected the effects of a lower n−3 PUFA status rather than of a higher n−6 PUFA status.
Associations of the prenatal fatty acid status with the child BMI z score as a continuous measure were generally null, although often in the same direction as associations with skinfold thicknesses. Especially in young children, skinfold thicknesses may provide a more direct measure of adiposity than BMI, which includes both fat and lean mass (32, 42). Associations were stronger when we used obesity as an outcome (ie, BMI >95th percentile), which also supported the idea that fatty acids may influence excess adiposity. One infant feeding trial similarly showed that PUFAs may influence body composition without an effect on overall size (43). In that study, preterm infants given formula supplemented with DHA and AA had no differences in weights, lengths, or head circumferences but had lower body fat and greater lean body mass than did infants given unsupplemented formula (43).
Early childhood leptin concentrations are directly correlated with contemporaneous adiposity (31). Similar to our results, the supplementation of maternal rats during gestation and lactation with n−3 PUFAs was associated with lower serum leptin concentrations in offspring than supplementation with n−6 PUFAs (10). The inverse relation we observed between n−3 PUFAs and leptin provided additional support for the observation of the association of higher n−3 PUFA concentrations with lower childhood adiposity.
We previously reported that maternal midpregnancy erythrocyte fatty acid concentrations were modestly associated with dietary intake and cord plasma concentrations (22). However, correlations of dietary fatty acid intake were stronger with corresponding concentrations in plasma than in erythrocytes (eg, for DHA + EPA: Spearman's r = 0.50 with plasma, and r = 0.35 with erythrocytes; for total n−6 PUFAs: r = 0.25 for plasma, and r = 0.17 for erythrocytes) and therefore, in the current analysis, we used plasma as a biomarker for maternal fatty acid status.
Strengths of this study include a relatively large study population and measurements of a number of maternal and child characteristics potentially associated with fatty acid intake and early childhood adiposity. We used a comprehensive validated FFQ and analyzed maternal and infant biomarkers of fatty acid status. We assessed adiposity in children with research-standard anthropometric measurements and measured blood leptin concentrations. We had information on many potential confounders and other contributors to child adiposity.
Nevertheless, dietary intake was self-reported and may have under- or overestimated true intake. However, FFQs are generally good at assessing relative intakes across a group of individuals. Fatty acid intakes in this population were similar to other US and European populations, with a high n−6:n−3 ratio above 10:1 (44). As in all observational studies, unmeasured confounders may exist. However, the adjustment for measured confounders, including sociodemographic characteristics, overall maternal diet quality, and child obesogenic behaviors, did not appreciably change estimates. Results may not be generalizable to populations with different education, socioeconomic status, or racial-ethnic distributions who may have different dietary patterns and obesity risks. However, we previously showed that maternal dietary intake and blood fatty acid status did not substantially vary by prepregnancy BMI or race-ethnicity (22).
In conclusion, these results suggested that higher prenatal fish intake and exposure to elongated n−3 PUFAs were associated with lower adiposity in early childhood. In this cohort, as in other populations in North America and Western Europe, the prenatal intake of elongated n−3 fatty acids was well below recommended concentrations. A higher n−3 PUFA intake may be associated with lower rates of obesity as well as less atopy and improved neurocognitive development. Results of ongoing trials will provide additional evidence regarding whether interventions to optimize maternal n−3 PUFA status could contribute to the prevention of adiposity-related outcomes in childhood (45).
Supplementary Material
Acknowledgments
We thank the participants and staff of Project Viva.
The authors' responsibilities were as follows—EO and MWG: designed the study, secured funding, managed data collection, and supervised the study; EO, SMAD, and SLR-S: analyzed the data; DRG: secured funding and managed data collection; ZEJ: secured funding; SMAD: drafted the manuscript; and all authors: provided critical revisions for important intellectual content. Mead Johnson Nutrition had no role in the design, implementation, analysis, presentation, or interpretation of results. None of the authors had a conflict of interest.
REFERENCES
- 1.McCann JC, Ames BN. Is docosahexaenoic acid, an n−3 long-chain polyunsaturated fatty acid, required for development of normal brain function? An overview of evidence from cognitive and behavioral tests in humans and animals. Am J Clin Nutr 2005;82:281–95 [DOI] [PubMed] [Google Scholar]
- 2.Chapkin RS, Kim W, Lupton JR, McMurray DN. Dietary docosahexaenoic and eicosapentaenoic acid: emerging mediators of inflammation. Prostaglandins Leukot Essent Fatty Acids 2009;81:187–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nesheim M, Yaktine A, Seafood choices: balancing benefits and risks. Washington, DC: The National Academies Press, 2007 [Google Scholar]
- 4.Brenna JT, Salem N, Jr, Sinclair AJ, Cunnane SC. alpha-Linolenic acid supplementation and conversion to n−3 long-chain polyunsaturated fatty acids in humans. Prostaglandins Leukot Essent Fatty Acids 2009;80:85–91 [DOI] [PubMed] [Google Scholar]
- 5.Harris WS, Mozaffarian D, Rimm E, et al. Omega-6 fatty acids and risk for cardiovascular disease: a science advisory from the American Heart Association Nutrition Subcommittee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Cardiovascular Nursing; and Council on Epidemiology and Prevention. Circulation 2009;119:902–7 [DOI] [PubMed] [Google Scholar]
- 6.Ziegler EE, O'Donnell AM, Nelson SE, Fomon SJ. Body composition of the reference fetus. Growth 1976;40:329–41 [PubMed] [Google Scholar]
- 7.Gaillard D, Negrel R, Lagarde M, Ailhaud G. Requirement and role of arachidonic acid in the differentiation of pre-adipose cells. Biochem J 1989;257:389–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim HK, Della-Fera M, Lin J, Baile CA. Docosahexaenoic acid inhibits adipocyte differentiation and induces apoptosis in 3T3-L1 preadipocytes. J Nutr 2006;136:2965–9 [DOI] [PubMed] [Google Scholar]
- 9.Korotkova M, Gabrielsson BG, Holmang A, Larsson BM, Hanson LA, Strandvik B. Gender-related long-term effects in adult rats by perinatal dietary ratio of n−6/n−3 fatty acids. Am J Physiol Regul Integr Comp Physiol 2005;288:R575–9 [DOI] [PubMed] [Google Scholar]
- 10.Korotkova M, Gabrielsson B, Lonn M, Hanson LA, Strandvik B. Leptin levels in rat offspring are modified by the ratio of linoleic to alpha-linolenic acid in the maternal diet. J Lipid Res 2002;43:1743–9 [DOI] [PubMed] [Google Scholar]
- 11.Massiera F, Saint-Marc P, Seydoux J, et al. Arachidonic acid and prostacyclin signaling promote adipose tissue development: a human health concern? J Lipid Res 2003;44:271–9 [DOI] [PubMed] [Google Scholar]
- 12.Spalding KL, Arner E, Westermark PO, et al. Dynamics of fat cell turnover in humans. Nature 2008;453:783–7 [DOI] [PubMed] [Google Scholar]
- 13.Oken E, Kleinman KP, Olsen SF, Rich-Edwards JW, Gillman MW. Associations of seafood and elongated n−3 fatty acid intake with fetal growth and length of gestation: results from a US pregnancy cohort. Am J Epidemiol 2004;160:774–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olsen SF, Hansen HS, Secher NJ, Jensen B, Sandstrom B. Gestation length and birth weight in relation to intake of marine n−3 fatty acids. Br J Nutr 1995;73:397–404 [DOI] [PubMed] [Google Scholar]
- 15.Grandjean P, Bjerve KS, Weihe P, Steuerwald U. Birthweight in a fishing community: significance of essential fatty acids and marine food contaminants. Int J Epidemiol 2001;30:1272–8 [DOI] [PubMed] [Google Scholar]
- 16.Rump P, Mensink RP, Kester AD, Hornstra G. Essential fatty acid composition of plasma phospholipids and birth weight: a study in term neonates. Am J Clin Nutr 2001;73:797–806 [DOI] [PubMed] [Google Scholar]
- 17.Halldorsson TI, Meltzer HM, Thorsdottir I, Knudsen V, Olsen SF. Is high consumption of fatty fish during pregnancy a risk factor for fetal growth retardation? A Study of 44,824 Danish Pregnant Women. Am J Epidemiol 2007;166:687–96 [DOI] [PubMed] [Google Scholar]
- 18.Lucia Bergmann R, Bergmann KE, Haschke-Becher E, et al. Does maternal docosahexaenoic acid supplementation during pregnancy and lactation lower BMI in late infancy? J Perinat Med 2007;35:295–300 [DOI] [PubMed] [Google Scholar]
- 19.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol 1992;135:1114–26 [DOI] [PubMed] [Google Scholar]
- 20.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol 1985;122:51–65 [DOI] [PubMed] [Google Scholar]
- 21.Fawzi WW, Rifas-Shiman SL, Rich-Edwards JW, Willett WC, Gillman MW. Calibration of a semi-quantitative food frequency questionnaire in early pregnancy. Ann Epidemiol 2004;14:754–62 [DOI] [PubMed] [Google Scholar]
- 22.Donahue SM, Rifas-Shiman SL, Olsen SF, Gold DR, Gillman MW, Oken E. Associations of maternal prenatal dietary intake of n−3 and n−6 fatty acids with maternal and umbilical cord blood levels. Prostaglandins Leukot Essent Fatty Acids 2009;80:289–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rifas-Shiman SL, Willett WC, Lobb R, Kotch J, Dart C, Gillman MW. PrimeScreen, a brief dietary screening tool: reproducibility and comparability with both a longer food frequency questionnaire and biomarkers. Public Health Nutr 2001;4:249–54 [DOI] [PubMed] [Google Scholar]
- 24.Hu FB, Bronner L, Willett WC, et al. Fish and omega-3 fatty acid intake and risk of coronary heart disease in women. JAMA 2002;287:1815–21 [DOI] [PubMed] [Google Scholar]
- 25.Iso H, Rexrode KM, Stampfer MJ, et al. Intake of fish and omega-3 fatty acids and risk of stroke in women. JAMA 2001;285:304–12 [DOI] [PubMed] [Google Scholar]
- 26.Agricultural Research Service USDoA USDA nutrient database for standard reference, release 13. Nutrient data laboratory homepage. 1999. Available from: http://www.nal.usda.gov/fnic/foodcomp (cited 1 July 2000)
- 27.Willett W. Nutritional epidemiology, 2nd ed New York, NY: Oxford University Press, 1998 [Google Scholar]
- 28.National Center for Health Statistics CDC growth charts, United States. 2000. Available at: http://www.cdc.gov/growthcharts/ (cited 16 February 2009)
- 29.Shorr IJ. How to weigh and measure children. New York, NY: UN, 1986 [Google Scholar]
- 30.Mueller WH, Martorell R. Reliability and accuracy of measurement : Lohman TG, Roche AF, Martorell R, Anthropometric standardization reference manual. Champaign, IL: Human Kinetics Books, 1988:83–6 [Google Scholar]
- 31.Mantzoros CS, Rifas-Shiman SL, Williams CJ, Fargnoli JL, Kelesidis T, Gillman MW. Cord blood leptin and adiponectin as predictors of adiposity in children at 3 years of age: a prospective cohort study. Pediatrics 2009;123:682–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wright CS, Rifas-Shiman SL, Rich-Edwards JW, Taveras EM, Gillman MW, Oken E. Intrauterine exposure to gestational diabetes, child adiposity, and blood pressure. Am J Hypertens 2009;22:215–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Institute of Medicine and National Research Council of the National Academies Weight gain during pregnancy: reexamining the guidelines. Washington, DC: National Academies Press, 2009 [PubMed] [Google Scholar]
- 34.Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr 2003;3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koletzko B, Cetin I, Brenna JT. Dietary fat intakes for pregnant and lactating women. Br J Nutr 2007;98:873–7 [DOI] [PubMed] [Google Scholar]
- 36.US Department of Health and Human Services and US Environmental Protection Agency What you need to know about mercury in fish and shellfish. 2004. Updated 2/4/2005. Available from: http://www.cfsan.fda.gov/∼dms/admehg3.html (cited 12 November 2008)
- 37.Rifas-Shiman SL, Rich-Edwards JW, Kleinman KP, Oken E, Gillman MW. Dietary quality during pregnancy varies by maternal characteristics in Project Viva: a US cohort. J Am Diet Assoc 2009;109:1004–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carlson SE, Cooke RJ, Werkman SH, Tolley EA. First year growth of preterm infants fed standard compared to marine oil n−3 supplemented formula. Lipids 1992;27:901–7 [DOI] [PubMed] [Google Scholar]
- 39.Lapillonne A, Carlson SE. Polyunsaturated fatty acids and infant growth. Lipids 2001;36:901–11 [DOI] [PubMed] [Google Scholar]
- 40.Helland IB, Saugstad OD, Smith L, et al. Similar effects on infants of n−3 and n−6 fatty acids supplementation to pregnant and lactating women. Pediatrics 2001;108:E82. [DOI] [PubMed] [Google Scholar]
- 41.Ailhaud G, Guesnet P, Cunnane SC. An emerging risk factor for obesity: does disequilibrium of polyunsaturated fatty acid metabolism contribute to excessive adipose tissue development? Br J Nutr 2008;100:461–70 [DOI] [PubMed] [Google Scholar]
- 42.Eisenmann JC, Heelan KA, Welk GJ. Assessing body composition among 3- to 8-year-old children: anthropometry, BIA, and DXA. Obes Res 2004;12:1633–40 [DOI] [PubMed] [Google Scholar]
- 43.Groh-Wargo S, Jacobs J, Auestad N, O'Connor DL, Moore JJ, Lerner E. Body composition in preterm infants who are fed long-chain polyunsaturated fatty acids: a prospective, randomized, controlled trial. Pediatr Res 2005;57:712–8 [DOI] [PubMed] [Google Scholar]
- 44.King C, Fewtrell M. Fishing for brain power? Arch Dis Child Fetal Neonatal Ed 2008;93:F4–6 [DOI] [PubMed] [Google Scholar]
- 45.Hauner H, Vollhardt C, Schneider KT, Zimmermann A, Schuster T, Amann-Gassner U. The impact of nutritional fatty acids during pregnancy and lactation on early human adipose tissue development. Rationale and design of the INFAT study. Ann Nutr Metab 2009;54:97–103 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.