Abstract
Fibroblast growth factor 21 (FGF21) was originally identified as a member of the FGF family in homology studies and is a member of the endocrine FGF subfamily that lacks heparin binding domains and is released into the circulation. A potential role as a metabolic regulator emerged when FGF21 was shown to increase glucose uptake in adipocytes. Subsequently, marked elevations in FGF21 expression were observed in mice that ate a ketogenic diet and when fasting, which suggests that FGF21 expression plays a role in the adaptation to metabolic states that require increased fatty acid oxidation. Consistent with this evidence, FGF21 knockout mice were not able to respond appropriately to consumption of a ketogenic diet. FGF21 expression is downstream of peroxisome proliferator-activated receptor (PPAR) α in the liver and PPARγ in adipose tissue. FGF21 concentrations are higher in both rodent and human obesity, and recent data suggest that obesity may be an FGF21-resistant state. Recent data increasingly suggest that FGF21 is an important metabolic regulator that may have potential clinical implications.
INTRODUCTION
The ketogenic diet, which is a high-fat and low-carbohydrate diet, has emerged as an important nonpharmacologic therapeutic option for children with intractable epilepsy (1). Closer analysis of carbohydrate-restricted diets in humans has shown that they are effective in causing weight loss and in reducing insulin resistance (2–5), lowering serum triglyceride concentrations, and raising HDL-cholesterol concentrations (2, 3, 6, 7).
Consumption of a ketogenic diet induces a metabolic state similar to fasting. During periods of fasting, the liver uses fatty acids, and through a series of oxidative reactions produces ketone bodies, which serve as fuel for the organism and are the predominant source of energy for the brain (8, 9). The pattern of improved metabolic variables observed in humans is also seen in normal and diet-induced obese mice fed a ketogenic diet and is also associated with a concomitant suppression of transcription factors and enzymes involved in lipid synthesis in the liver. Furthermore, a ketogenic diet leads to a reversal of high-fat, diet-induced obesity along with increased energy expenditure, improved glucose homeostasis, and increased expression of genes in the fatty acid oxidation pathway (10). Beneficial effects of ketogenic diet are also seen in mice that lack leptin (ob/ob mice) because the prolonged ketogenic diet feeding of these mice leads to the improvement of glycemic and insulinemic profiles in the absence of weight loss (11). Thus, the manipulation of the dietary macronutrient content can have significant effects on metabolic status, and consumption of fat is not necessarily associated with the development of obesity and impaired glucose homeostasis.
Potential mechanisms that explain the effects of a ketogenic diet might be mediated by the brain. Hypothalamic neuropeptides, such as neuropeptide Y (NPY), agouti-related peptide (AgRP), and proopiomelanocortin (POMC), play a role in the regulation of energy-expenditure changes under different dietary conditions. Increases in NPY and AgRP are associated with decreased energy expenditures, whereas increased POMC is associated with increased energy expenditures (10, 12–14). In animals that ate a ketogenic diet, the expression profile revealed an increase in NPY and AgRP and a decrease in POMC, which was consistent with the low peripheral leptin concentrations but inconsistent with the observed increase in energy expenditure (10). This observation led to the exploration of peripheral pathways that might play a principal role in the metabolic changes associated with a ketogenic diet (10) and to the identification of fibroblast growth factor 21 (FGF21) as a key mediator of lipid oxidation in animals that eat a ketogenic diet as well as potentially during fasting (9).
FGF21 ACTION
The encoding complementary DNA of FGF21 was initially identified in mouse embryos and subsequently in humans; its product is a secreted protein of 210 amino acids in mice and 209 amino acids in humans with a 75% correspondence between the 2 species (15). The expression of FGF21 in mice is predominantly in the liver and adipose tissue, whereas a relatively lower expression has also been shown in other tissues, including the thymus, muscle, and pancreas (15–17). The potential role of FGF21 as a metabolic regulator was first considered when it was discovered that it could act in 3T3-L1 cells to increase glucose uptake via expression of glucose transporter-1 (18). FGF21 is a member of the broad FGF family, which includes a total of 22 factors in humans that are involved in the regulation of cell survival, mitogenesis, cell growth, tissue repair, and tumor growth (19). FGF21 belongs to the endocrine subfamily of FGFs that includes FGF19 in humans (FGF15 in mice) and FGF23. These members lack a heparin binding domain, are released from the cell, and require a co-receptor to mediate binding to the various FGF receptor (FGFR) isotypes (20–22). The various subfamilies of high-affinity FGFRs have intrinsic tyrosine kinase activity. FGF21 has a specific affinity for FGFR1 but can also act through different isoforms such as FGFR2 and FGFR4 (18, 23, 24). Unlike the classic FGFs, FGF21 has no known mitogenic properties (18).
The target tissue action specificity of FGF21 derives from the interaction of FGF21 with its receptors; this requires the presence of βKlotho, which serves as a direct binding subunit of the FGF21 receptor and forms a complex with it (23, 24). βKlotho is a type 1 transmembrane protein member of the Klotho family, and it is known to be expressed in the pancreas, adipose tissue, and liver (25). βKlotho and FGFR1 form an inactive complex on the surface of the cells, which is activated upon the binding of FGF21. This activation leads to the phosphorylation of a signaling molecule, FGFR substrate 2 (FRS2), as well as the downstream phosphorylation of extracellular signal-regulated kinase 1 and 2. In adipose tissue, this interaction leads to the increased expression of glucose transporter-1, which leads to enhanced glucose uptake (18, 26). Additional studies of FGF21 action have shown a decrease of serum free fatty acids; we recently showed that this was downstream to the decreased expression of lipolytic genes in white adipose tissue in the short term (Figure 1). These effects were more accentuated in lean mice than in obese mice (27).
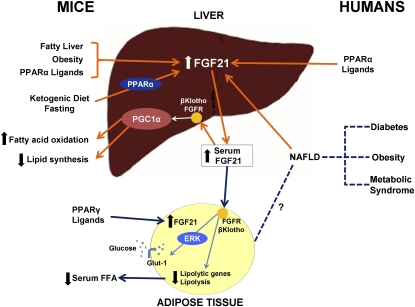
FIGURE 1.
Summary of fibroblast growth factor 21 (FGF21) physiology in mice and humans. In mice, consumption of a ketogenic diet leads to a peroxisome proliferator-activated receptor (PPAR) α–dependent increase of FGF21 in the liver and an increase in serum FGF21 concentrations. FGF21 expression in the liver is also induced by fatty liver disease, obesity, and PPARα ligands in mice. PPARα ligands, such as fenofibrate, also increase FGF21 messenger RNA expression in human hepatocytes. FGF21 interacts with the FGF receptor (FGFR) in the presence of βKlotho in the mouse liver and adipose tissue. This interaction leads to a PPARγ coactivator protein-1α (PGC1α)–dependent up-regulation of fatty acid oxidation and down-regulation of lipid synthesis in the liver. In mouse adipose tissue, the presence of PPARγ ligands leads to the production of FGF21, and the short-term effect of FGF21 results in a decreased expression of lipolytic genes and leads to lower concentrations of circulating free fatty acids (FFA). FGF21-induced phosphorylation of extracellular signal-regulated kinase-1 (ERK) leads to the activation of glucose transporter-1 (Glut-1) and glucose uptake in mouse 3T3-L1 adipocytes and primary human adipocytes. In humans, serum concentrations of FGF21 are higher in diabetes, obesity, metabolic syndrome, and nonalcoholic fatty liver disease (NAFLD). This effect may be mediated by increased FGF21 liver expression.
FGF21 actions in the liver enhance fatty acid oxidation and tricarboxylic acid cycle flux during prolonged fasting. These actions may be mediated by inducing the expression of peroxisome proliferator-activated receptor (PPAR) γ coactivator protein-1α (PGC-1α), which is a transcriptional regulator of energy homeostasis (Figure 1). One group reported that mice that lacked FGF21 were unable to increase PGC-1α expression in response to prolonged fasting and also showed an impaired ability in gluconeogenesis and ketogenesis (28). These data supported a preeminent role for FGF21 in the regulation of carbohydrate and fatty acid metabolism during the progression from fasting to starvation.
FGF21 MEDIATES THE EFFECTS OF KETOGENIC DIET IN MICE
The induction of hepatic gene expression and the increase of circulating serum concentrations of FGF21 have been observed with ketogenic diet feeding and fasting. Refeeding after a fast was followed by rapid FGF21 suppression (9). In mice that consumed a ketogenic diet, the knockdown of FGF21 by using silencing RNA constructs delivered through an adenovirus vector led to a marked metabolic impairment that included hepatosteatosis secondary to the accumulation of triglycerides. These mice also showed hyperlipidemia, including increases in serum triglycerides, cholesterol, and nonesterified fatty acids (9). In addition, FGF21 knockout mice, when placed on a ketogenic diet, showed an atypical response to the diet and a mild weight gain instead of a loss of weight, exaggerated hepatic steatosis, and impaired glucose tolerance with reduced ketone formation (29). Hepatic FGF21 is regulated by PPARα, which is critical to the physiologic regulation of lipid metabolism (9, 30). Mice deficient in PPARα also showed marked impairments when placed on a ketogenic diet. Thus, it appears that hepatic FGF21 production and action are necessary for the appropriate metabolism of lipids during states when fatty acids are the major fuel source.
REGULATION OF FGF21 IN MICE
In mice fed a ketogenic diet, the up-regulation of FGF21 gene expression was accompanied by an increased expression of PPARα-regulated transcripts, which suggests that the induction of FGF21 during ketotic states is PPARα dependent (9) (Figure 1). No induction of FGF21 gene expression was observed in PPARα-null mice during fasting, and a very attenuated induction was seen when these mice were fed a ketogenic diet, which suggests that, during ketosis, an alternative metabolic pathway may be involved that is PPARα independent (9, 30–33). Concentrations of FGF21 were noted to be low in animals with hypothyroidism; recently, we showed that T3 robustly increased FGF21 expression, and this action was dependent on PPARα (34). In addition, FGF21 induced glucose uptake in adipocytes in a PPARγ-dependent manner and led to an increase in glucose transporter-1 messenger RNA (mRNA) expression, whereas PPARγ agonists induced FGF21 expression in adipocytes (35–37) (Figure 1). Recent studies have shown that FGF21 may also be regulated by PGC-1α through the modulation of the heme and Rev-Erbα axis and also by retinoic acid receptor-related orphan receptor α (38, 39).
FGF21 concentrations are elevated in obese mice. This was initially observed in leptin deficient ob/ob mice (40). We recently showed that FGF21 mRNA expression was increased in the liver and white adipose tissue of mice with diet-induced obesity and showed evidence for FGF21 resistance in obesity (27). Furthermore, the systemic administration of FGF21 in diet-induced obese and ob/ob mice was shown to lower the mean body weight predominantly via a reduction in adiposity and to reduce fasting blood glucose and insulin concentrations (41, 42).
FGF21 IN HUMANS
Currently, the role of FGF21 in humans is unclear. A number of studies indicated that FGF21 serum concentrations correlated with the body mass index (43–45). With regard to changes in nutritional status, one small study reported that 7 d of fasting increased FGF21 serum concentrations (46), whereas another study showed a decline in FGF21 serum concentrations with 3 d of fasting (47). In addition, FGF21 serum concentrations were decreased in subjects with anorexia nervosa, which were more consistent with the fall seen with 3 d of fasting (48, 49). Blood samples of children after the ingestion of a ketogenic diet showed no increase in FGF21 concentrations (46). Furthermore, FGF21 serum concentrations were significantly increased in patients under fenofibrate treatment of primary hypertriglyceridemia than in a control group (46), which was consistent with the fact that PPARα ligands, such as fenofibrate, were shown to increase FGF21 mRNA concentrations in human hepatocytes (40).
Studies in humans with type 2 diabetes have shown that FGF21 expression was increased in baseline conditions and during fasting compared with the expression in control subjects (45, 50–52). It was also reported that obese patients had increased FGF21 blood concentrations, whereas FGF21 mRNA expression in visceral fat increased 2-fold in obese patients compared with in control subjects, and a very low–calorie diet induced a significant increase of FGF21 mRNA expression in the subcutaneous fat of obese patients (45). However, one group did not find detectable FGF21 in fat (47). FGF21 serum concentrations in humans have also been positively correlated with adiposity, fasting insulin, and triglycerides but negatively with HDL cholesterol after adjustment for age and body mass index (53). An independent association was also shown between serum FGF21 concentrations and metabolic syndrome (53). A recent study showed that FGF21 serum concentrations were increased in patients with nonalcoholic fatty liver disease (NAFLD) or steatohepatitis and reported, for the first time to our knowledge, that the mRNA expression of FGF21 was up-regulated in the liver of patients with NAFLD (47) (Figure 1). Another 2 groups reported an association between serum FGF21 concentrations and the degree of fatty liver accumulation (54, 55). These findings suggest that the regulation of FGF21 serum concentrations is different between humans and mice, and further investigations need to be performed. For example, measured circulating concentrations may be affected by as yet unidentified binding proteins, or the duration of fasting may lead to variable results.
CONCLUSIONS
Ketogenic diets increasingly gained attention as alternative and effective means for weight loss and which were also associated with marked improvements in insulin sensitivity and glucose tolerance. In mice, FGF21 expression and action were required to mediate an appropriate metabolic response to consumption of a ketogenic diet. FGF21 has also been shown to be an important regulator of energy expenditure and metabolism; in obese rodents, treatment with FGF21 led to improvement in many metabolic variables and was associated with weight loss. Studies in mice have provided useful information on the pathobiological pathways involved in FGF21 actions and regulation. However, data on FGF21 in humans are limited, and future clinical studies are necessary to provide insight into the role of FGF21 in humans, its potential use as a biomarker for NAFLD, and its potential as a therapeutic agent in states of insulin-resistance obesity and metabolic syndrome.
Acknowledgments
The authors’ responsibilities were as follows—EMD: drafted the manuscript and conducted the literature search; and EMF: outlined and critically revised the manuscript. Neither of the authors had a conflict of interest.
REFERENCES
- 1.Wheless JW. History of the ketogenic diet. Epilepsia 2008;49(suppl 8):3–5 [DOI] [PubMed] [Google Scholar]
- 2.Foster GD, Wyatt HR, Hill JO, et al. A randomized trial of a low-carbohydrate diet for obesity. N Engl J Med 2003;348:2082–90 [DOI] [PubMed] [Google Scholar]
- 3.Gardner CD, Kiazand A, Alhassan S, et al. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: the A TO Z Weight Loss Study: a randomized trial. JAMA 2007;297:969–77 [DOI] [PubMed] [Google Scholar]
- 4.Samaha FF, Iqbal N, Seshadri P, et al. A low-carbohydrate as compared with a low-fat diet in severe obesity. N Engl J Med 2003;348:2074–81 [DOI] [PubMed] [Google Scholar]
- 5.Dansinger ML, Gleason JA, Griffith JL, Selker HP, Schaefer EJ. Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: a randomized trial. JAMA 2005;293:43–53 [DOI] [PubMed] [Google Scholar]
- 6.Jenkins DJ, Wong JM, Kendall CW, et al. The effect of a plant-based low-carbohydrate (“Eco-Atkins”) diet on body weight and blood lipid concentrations in hyperlipidemic subjects. Arch Intern Med 2009;169:1046–54 [DOI] [PubMed] [Google Scholar]
- 7.Halton TL, Liu S, Manson JE, Hu FB. Low-carbohydrate-diet score and risk of type 2 diabetes in women. Am J Clin Nutr 2008;87:339–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liebhaber G, Pascher B, Gempel K, Baumeister FA. [Asymptomatic carnitine depletion on ketogenic diet in patients with pharmacoresistant epilepsies] Klin Padiatr 2006;218:260–3 (in German) [DOI] [PubMed] [Google Scholar]
- 9.Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab 2007;5:426–37 [DOI] [PubMed] [Google Scholar]
- 10.Kennedy AR, Pissios P, Otu H, et al. A high-fat, ketogenic diet induces a unique metabolic state in mice. Am J Physiol Endocrinol Metab 2007;292:E1724–39 [DOI] [PubMed] [Google Scholar]
- 11.Badman MK, Kennedy AR, Adams AC, Pissios P, Maratos-Flier E. A very low carbohydrate ketogenic diet improves glucose tolerance in ob/ob mice independent of weight loss. Am J Physiol Endocrinol Metab (Epub ahead of print 8 September 2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hwa JJ, Witten MB, Williams P, et al. Activation of the NPY Y5 receptor regulates both feeding and energy expenditure. Am J Physiol 1999;277:R1428–34 [DOI] [PubMed] [Google Scholar]
- 13.Hoggard N, Rayner DV, Johnston SL, Speakman JR. Peripherally administered [Nle4,D-Phe7]-alpha-melanocyte stimulating hormone increases resting metabolic rate, while peripheral agouti-related protein has no effect, in wild type C57BL/6 and ob/ob mice. J Mol Endocrinol 2004;33:693–703 [DOI] [PubMed] [Google Scholar]
- 14.Small CJ, Liu YL, Stanley SA, et al. Chronic CNS administration of Agouti-related protein (Agrp) reduces energy expenditure. Int J Obes Relat Metab Disord 2003;27:530–3 [DOI] [PubMed] [Google Scholar]
- 15.Nishimura T, Nakatake Y, Konishi M, Itoh N. Identification of a novel FGF, FGF-21, preferentially expressed in the liver. Biochim Biophys Acta 2000;1492:203–6 [DOI] [PubMed] [Google Scholar]
- 16.Johnson CL, Weston JY, Chadi SA, et al. Fibroblast growth factor 21 reduces the severity of cerulein-induced pancreatitis in mice. Gastroenterology 2009;137:1795–804 [DOI] [PubMed] [Google Scholar]
- 17.Izumiya Y, Bina HA, Ouchi N, Akasaki Y, Kharitonenkov A, Walsh K. FGF21 is an Akt-regulated myokine. FEBS Lett 2008;582:3805–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kharitonenkov A, Shiyanova TL, Koester A, et al. FGF-21 as a novel metabolic regulator. J Clin Invest 2005;115:1627–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ornitz DM, Itoh N. Fibroblast growth factors. Genome Biol. 2001;2:reviews30051–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goetz R, Beenken A, Ibrahimi OA, et al. Molecular insights into the klotho-dependent, endocrine mode of action of fibroblast growth factor 19 subfamily members. Mol Cell Biol 2007;27:3417–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harmer NJ, Pellegrini L, Chirgadze D, Fernandez-Recio J, Blundell TL. The crystal structure of fibroblast growth factor (FGF) 19 reveals novel features of the FGF family and offers a structural basis for its unusual receptor affinity. Biochemistry 2004;43:629–40 [DOI] [PubMed] [Google Scholar]
- 22.Jones S. Mini-review: endocrine actions of fibroblast growth factor 19. Mol Pharm 2008;5:42–8 [DOI] [PubMed] [Google Scholar]
- 23.Kharitonenkov A, Dunbar JD, Bina HA, et al. FGF-21/FGF-21 receptor interaction and activation is determined by betaKlotho. J Cell Physiol 2008;215:1–7 [DOI] [PubMed] [Google Scholar]
- 24.Ogawa Y, Kurosu H, Yamamoto M, et al. BetaKlotho is required for metabolic activity of fibroblast growth factor 21. Proc Natl Acad Sci USA 2007;104:7432–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ito S, Kinoshita S, Shiraishi N, et al. Molecular cloning and expression analyses of mouse betaklotho, which encodes a novel Klotho family protein. Mech Dev 2000;98:115–9 [DOI] [PubMed] [Google Scholar]
- 26.Micanovic R, Raches DW, Dunbar JD, et al. Different roles of N- and C- termini in the functional activity of FGF21. J Cell Physiol 2009;219:227–34 [DOI] [PubMed] [Google Scholar]
- 27.Fisher FM, Chui PC, Antonellis PJ, et al. Obesity is an FGF21 resistant state. Diabetes 2010;59:2781–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Potthoff MJ, Inagaki T, Satapati S, et al. FGF21 induces PGC-1alpha and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proc Natl Acad Sci USA 2009;106:10853–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Badman MK, Koester A, Flier JS, Kharitonenkov A, Maratos-Flier E. Fibroblast growth factor 21-deficient mice demonstrate impaired adaptation to ketosis. Endocrinology 2009;150:4931–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inagaki T, Dutchak P, Zhao G, et al. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab 2007;5:415–25 [DOI] [PubMed] [Google Scholar]
- 31.Hashimoto T, Cook WS, Qi C, Yeldandi AV, Reddy JK, Rao MS. Defect in peroxisome proliferator-activated receptor alpha-inducible fatty acid oxidation determines the severity of hepatic steatosis in response to fasting. J Biol Chem 2000;275:28918–28 [DOI] [PubMed] [Google Scholar]
- 32.Kersten S, Seydoux J, Peters JM, Gonzalez FJ, Desvergne B, Wahli W. Peroxisome proliferator-activated receptor alpha mediates the adaptive response to fasting. J Clin Invest 1999;103:1489–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leone TC, Weinheimer CJ, Kelly DP. A critical role for the peroxisome proliferator-activated receptor alpha (PPARalpha) in the cellular fasting response: the PPARalpha-null mouse as a model of fatty acid oxidation disorders. Proc Natl Acad Sci USA 1999;96:7473–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adams AC, Astapova I, Fisher FM, et al. Thyroid hormone regulates hepatic expression of fibroblast growth factor 21 in a PPARalpha-dependent manner. J Biol Chem 2010;285:14078–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muise ES, Azzolina B, Kuo DW, et al. Adipose fibroblast growth factor 21 is up-regulated by peroxisome proliferator-activated receptor gamma and altered metabolic states. Mol Pharmacol 2008;74:403–12 [DOI] [PubMed] [Google Scholar]
- 36.Moyers JS, Shiyanova TL, Mehrbod F, et al. Molecular determinants of FGF-21 activity-synergy and cross-talk with PPARgamma signaling. J Cell Physiol 2007;210:1–6 [DOI] [PubMed] [Google Scholar]
- 37.Wang H, Qiang L, Farmer SR. Identification of a domain within peroxisome proliferator-activated receptor gamma regulating expression of a group of genes containing fibroblast growth factor 21 that are selectively repressed by SIRT1 in adipocytes. Mol Cell Biol 2008;28:188–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Estall JL, Ruas JL, Choi CS, et al. PGC-1alpha negatively regulates hepatic FGF21 expression by modulating the heme/Rev-Erb(alpha) axis. Proc Natl Acad Sci USA 2009;106:22510–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Y, Solt LA, Burris TP. Regulation of FGF21 expression and secretion by retinoic acid receptor-related orphan receptor alpha. J Biol Chem 2010;285:15668–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lundasen T, Hunt MC, Nilsson LM, et al. PPARalpha is a key regulator of hepatic FGF21. Biochem Biophys Res Commun 2007;360:437–40 [DOI] [PubMed] [Google Scholar]
- 41.Coskun T, Bina HA, Schneider MA, et al. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology 2008;149:6018–27 [DOI] [PubMed] [Google Scholar]
- 42.Xu J, Stanislaus S, Chinookoswong N, et al. Acute glucose-lowering and insulin-sensitizing action of FGF21 in insulin resistant mouse models—association with liver and adipose tissue effects. Am J Physiol Endocrinol Metab (Epub ahead of print 25 August 2009) [DOI] [PubMed] [Google Scholar]
- 43.Christodoulides C, Dyson P, Sprecher D, Tsintzas K, Karpe F. Circulating fibroblast growth factor 21 is induced by peroxisome proliferator-activated receptor agonists but not ketosis in man. J Clin Endocrinol Metab 2009;94:3594–601 [DOI] [PubMed] [Google Scholar]
- 44.Chavez AO, Molina-Carrion M, Abdul-Ghani MA, Folli F, Defronzo RA, Tripathy D. Circulating fibroblast growth factor-21 is elevated in impaired glucose tolerance and type 2 diabetes and correlates with muscle and hepatic insulin resistance. Diabetes Care 2009;32:1542–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mraz M, Bartlova M, Lacinova Z, et al. Serum concentrations and tissue expression of a novel endocrine regulator fibroblast growth factor-21 in patients with type 2 diabetes and obesity. Clin Endocrinol (Oxf) 2009;71:369–75 [DOI] [PubMed] [Google Scholar]
- 46.Galman C, Lundasen T, Kharitonenkov A, et al. The circulating metabolic regulator FGF21 is induced by prolonged fasting and PPARalpha activation in man. Cell Metab 2008;8:169–74 [DOI] [PubMed] [Google Scholar]
- 47.Dushay J, Chui PC, Gopalakrishnan GS, et al. Increased fibroblast growth factor 21 in obesity and nonalcoholic fatty liver disease. Gastroenterology 2010;139:456–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dostalova I, Kavalkova P, Haluzikova D, et al. Plasma concentrations of fibroblast growth factors 19 and 21 in patients with anorexia nervosa. J Clin Endocrinol Metab 2008;93:3627–32 [DOI] [PubMed] [Google Scholar]
- 49.Kliewer SA, Mangelsdorf DJ. Fibroblast growth factor 21: from pharmacology to physiology. Am J Clin Nutr 2010;91:254S–7S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matuszek B, Lenart-Lipinska M, Duma D, Solski J, Nowakowski A. Evaluation of concentrations of FGF-21 - a new adipocytokine in type 2 diabetes. Endokrynol Pol 2010;61:50–4 [PubMed] [Google Scholar]
- 51.Li L, Yang G, Ning H, Yang M, Liu H, Chen W. Plasma FGF-21 levels in type 2 diabetic patients with ketosis. Diabetes Res Clin Pract 2008;82:209–13 [DOI] [PubMed] [Google Scholar]
- 52.Chen WW, Li L, Yang GY, et al. Circulating FGF-21 levels in normal subjects and in newly diagnose patients with type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes 2008;116:65–8 [DOI] [PubMed] [Google Scholar]
- 53.Zhang X, Yeung DC, Karpisek M, et al. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes 2008;57:1246–53 [DOI] [PubMed] [Google Scholar]
- 54.Yilmaz Y, Eren F, Yonal O, et al. Increased serum FGF21 levels in patients with nonalcoholic fatty liver disease. Eur J Clin Invest 2010;40:887–92 [DOI] [PubMed] [Google Scholar]
- 55.Li H, Fang Q, Gao F, et al. Fibroblast growth factor 21 levels are increased in nonalcoholic fatty liver disease patients and are correlated with hepatic triglyceride. J Hepatol 2010;53:934–40 [DOI] [PubMed] [Google Scholar]