Abstract
Background
Whether unvaccinated children and adolescents differ from those vaccinated in terms of health is subject to some discussion.
Method
We evaluated data on diseases that are preventable by vaccination, infectious and atopic diseases, and vaccinations received that had been collected between 2003 and 2006 in a representative sample of 17 641 subjects aged 0 to 17 years in the framework of the German Health Interview and Examination Survey for Children and Adolescents (Kinder- und Jugendgesundheitssurvey, KiGGS).
Results
Evaluable data on vaccinations were available for 13 453 subjects aged 1–17 years from non-immigrant families. 0.7% of them (95% confidence interval: 0.5%–0.9%) were not vaccinated. The lifetime prevalence of diseases preventable by vaccination was markedly higher in unvaccinated than in vaccinated subjects. Unvaccinated children aged 1–5 years had a median number of 3.3 (2.1–4.6) infectious diseases in the past year, compared to 4.2 (4.1–4.4) in vaccinated children. Among 11- to 17-year-olds, the corresponding figures were 1.9 (1.0–2.8) (unvaccinated) versus 2.2 (2.1–2.3) (vaccinated). The lifetime prevalence of at least one atopic disease among 1- to 5-year-olds was 12.6% (5.0%–28.3%) in unvaccinated children and 15.0% (13.6%–16.4%) in vaccinated children. In older children, atopy was more common, but its prevalence was not found to depend on vaccination status: among 6- to 10-year-olds, the prevalence figures were 30.1% (12.9%–55.8%) for unvaccinated children versus 24.4% (22.8%–26.0%) for vaccinated children, and the corresponding figures for 11- to 17-year-olds were 20.3% (10.1%–36.6%) versus 29.9% (28.4%–31.5%).
Conclusion
The prevalence of allergic diseases and non-specific infections in children and adolescents was not found to depend on vaccination status.
Protective vaccinations are among the most important and effective preventive measures in modern medicine (1). They make a substantial contribution to reducing the probability of contracting many infectious diseases as well as their severity. The immediate goal of a vaccination is to protect the vaccinated person from a disease. Achieving high rates of vaccinated persons additionally results in the so called herd immunity.
In Germany, recommendations for vaccinations are made by the German Standing Vaccination Committee (Ständige Impfkommission, STIKO) at the Robert Koch Institute (2). For infants, children, and adolescents, the current vaccination schedule includes standard vaccinations to protect against diphtheria, pertussis, tetanus, Haemophilus influenzae type b, hepatitis B, poliomyelitis, pneumococci, meningococci, measles, mumps, rubella, and varicella. For girls aged 12–17, vaccination against human papillomavirus is also recommended (3). The benefits, efficacy, and safety of protective vaccinations are widely scientifically proven. Furthermore, modern vaccines are well tolerated (4, 5). In spite of all this, some parents and doctors have reservations against vaccinations. The fear is that vaccinations overburden, stress, or weaken a child’s immune system and may therefore cause harm. As a result they think that vaccinated children are more prone to falling ill than non-vaccinated children. In addition, vaccinations are deemed to be responsible for the occurrence or increased incidence of other diseases, including chronic diseases (6, 7).
The current exploratory study uses data from the German Health Interview and Examination Survey for Children and Adolescents (KiGGS) to investigate and compare the occurrence of vaccine preventable diseases, infections, and atopic disorders in vaccinated and unvaccinated children.
Methods
The German Health Interview and Examination Survey for Children and Adolescents (KiGGS) was conducted from May 2003 to May 2006 by the Robert Koch Institute. The objective of the interview and examination survey was to collect representative data on the health status of children and adolescents aged up to 17 years. In total, 17 641 children and adolescents (8656 girls, 8985 boys) and their parents participated in the study (response rate 66.6%), who were randomly selected in 167 German locations (8, 9). The study was approved by the Charité-Universitätsmedizin Berlin ethics committee.
Data on sociodemographic characteristics, vaccine preventable diseases, and infections were collected from a parent questionnaire. From the data submitted by parents on their own school education, professional qualifications, job positions, and net household incomes, the social strata index by Winkler (10) was determined (“high,” “medium,” or “low”). Lifetime prevalence calculations for pertussis, measles, mumps, and rubella were based on the question: “Has your child ever had … ?” The parent questionnaire also collected data on the following infections: cold/flu-like infection, tonsillitis, herpesvirus infection, bronchitis (not when asthma was present), gastrointestinal infection, cystitis and/or urethritis, purulent conjunctivitis (bacterial conjunctivitis). The parents of children up to age 11 years were also asked whether their child had ever had croup. From these data, the median number of infections for the preceding year was calculated for every study subject.
A standardized, computer-assisted personal interview (CAPI) of the accompanying parent by a doctor yielded data on medical diagnoses of atopic disorders (allergic rhinoconjunctivitis, atopic eczema, bronchial asthma) and several other diseases. The questions were: “Has a doctor at any time diagnosed your child with disease X?” Subjects for whom at least one atopic disorder ever was reported were assigned the characteristic “atopic disorder.” The questions about diseases were followed by data collection on the basis of medical records in the vaccination card, about data concerning the administered vaccinations and the timing of the vaccination. The data were evaluated with regard to the STIKO recommendations. Children and adolescents were defined as unvaccinated if at the time of the KiGGS survey no documentation existed for any vaccination against diphtheria, pertussis, tetanus, Haemophilus influenzae type b, hepatitis B, poliomyelitis, measles, mumps, or rubella. By contrast, children who had by then received at least one vaccination according to their vaccination card were categorized as vaccinated. Immigrant families were excluded from the analyses in order to avoid misclassification because of the often missing or incomplete vaccination documents.
The software packages SPSS (version 14, prevalences) and SAS (version 9.2, medians) were used for our statistical analyses. The stratified and clustered study design of KiGGS was taken into account by applying the analytical methods for complex samples (SPSS), which provide correct 95% confidence intervals, or by applying the survey procedures in SAS (11). The data were evaluated in consideration of the correlation of the subjects within a community and by including weighting and correcting for deviations of the KiGGS participants from the population structure of 0–17-year-olds (as of 31 December 2004) with regard to age, sex, region (east/west/Berlin), and nationality (12). Differences between groups in terms of prevalences or medians that reached significance were tested at the 5% significance level by means of Fisher’s exact test.
Results
Of the 17 641 individuals in the KiGGS, 14 148 children and adolescents were aged 1–17 years and lived in non-immigrant families. A vaccination card was available for 13 499 of these individuals (95.4%). Of these, 41 were not evaluated because their vaccination card was illegible or seemingly incomplete (follow-up document). Furthermore, five unvaccinated subjects were not evaluated who, according to their parents, had not been vaccinated at the planned date because of frequent illnesses. Evaluable vaccination data were therefore available for 13 453 children and adolescents aged 1–17 years (95.1%).
For 94 of these children and adolescents, 48 girls and 46 boys, no vaccination had been documented at the time of the KiGGS survey. This equals a proportion of 0.7% (95% confidence interval [CI] 0.5 to 0.9). By comparison, 99.3% (13 359 children and adolescents, 6572 girls and 6787 boys) had received at least one vaccination according to the available documentation (95% CI 99.1 to 99.5). The proportion of unvaccinated children and adolescents was highest in the age group of 1–5-year-olds, at 1.1% (95% CI 0.7 to 1.7, n = 44). In 6–10-year-olds, the proportion was 0.5% (95% CI 0.3 to 0.8, n = 20), and in 11–17-year-olds, 0.6% (95% CI 0.4 to 1.0, n = 30). A slightly higher proportion of unvaccinated children and adolescents was observed in families with a high socioeconomic status compared with families with a lower socioeconomic status. Unvaccinated children and adolescents also tended to live in the old German states to a slightly higher extent than in the new ones. The proportion of unvaccinated subjects was identical for boys and girls (Table 1).
Table 1. Description of the study population (n = 13 453) by vaccination status and ?sociodemografic characteristics as percentages (95% confidence interval).
| Unvaccinated (no vaccination) | n | Vaccinated (at least one vaccination) | n | |
| Total | 0.7 (0.5 to 0.9) | 94 | 99.3 (99.1 to 99.5) | 13 359 |
| Age (years) | ||||
| 1–5 | 1.1 (0.7 to 1.7) | 44 | 98.9 (93.3 to 99.3) | 3886 |
| 6–10 | 0.5 (0.3 to 0.8) | 20 | 99.5 (99.2 to 99.7) | 4149 |
| 11–17 | 0.6 (0.4 to 1.0) | 30 | 99.4 (99.0 to 99.6) | 5324 |
| p = 0.0013 | ||||
| Sex | ||||
| Girls | 0.7 (0.5 to 1.0) | 48 | 99.3 (99.0 to 99.5) | 6572 |
| Boys | 0.7 (0.5 to 1.0) | 46 | 99.3 (99.0 to 99.5) | 6787 |
| p = 0.7566 | ||||
| Socioeconomic status | ||||
| Low | 0.6 (0.4 to 1.0) | 22 | 99.4 (99.0 to 99.6) | 3054 |
| Medium | 0.7 (0.5 to 1.0) | 41 | 99.3 (99.0 to 99.5) | 6454 |
| High | 0.8 (0.5 to 1.3) | 29 | 99.2 (98.7 to 99.5) | 3769 |
| p = 0.7045 | ||||
| Place of ‧residence | ||||
| New German states | 0.6 (0.4 to 1.0) | 30 | 99.4 (99.0 to 99.6) | 5052 |
| Old German states | 0.7 (0.5 to 1.0) | 64 | 99.3 (99.0 to 99.5) | 8307 |
| p = 0.2856 | ||||
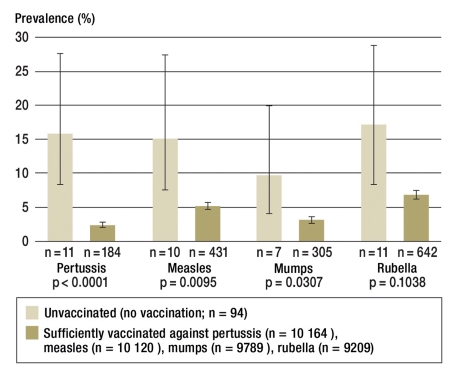
Figure 1 shows the percentages of 1–17-year-old children and adolescents who according to their parents had had pertussis, measles, mumps, and/or rubella. The comparison is between unvaccinated children and adolescents and those who, according to STIKO recommendations for children’s respective age groups, had been sufficiently vaccinated against the respective infectious disease at the time of the survey.
Figure 1.
Lifetime prevalence of vaccine preventable infectious diseases by disease specific vaccine status (as percentages with 95% confidence intervals)
The proportion of children and adolescents who had had pertussis, measles, mumps, and/or rubella was much higher in unvaccinated children than in those who had been vaccinated against the respective disease to a sufficient extent. For pertussis, the lifetime prevalence in unvaccinated subjects was 15.8% (95% CI 8.5 to 27.6, n = 11), in those with sufficient vaccination cover only 2.3% (95% CI 2.0 to 2.8), n = 184). For measles, the lifetime prevalence was 15.0 (95% CI 7.7 to 27.4, n = 10) in unvaccinated subjects and 5.2% (95% CI 4.7 to 5.8, n = 431) in sufficiently vaccinated ones. For mumps, the lifetime prevalence in unvaccinated subjects was 9.6% (95% CI 4.2 to 20.2, n = 7) and in vaccinated ones, 3.1% (95% CI 2.6 to 3.7, n = 305). For rubella, the lifetime prevalence was 17.0% (95% CI 9.4 to 29.0, n = 11) for unvaccinated subjects and 6.8% (95% CI 6.0 to 7.6, n = 642) for vaccinated ones. Differentiated stratified analyses confirmed the described higher proportions of subjects with the respective disease among unvaccinated subjects for boys and girls and for different age groups (data not shown).
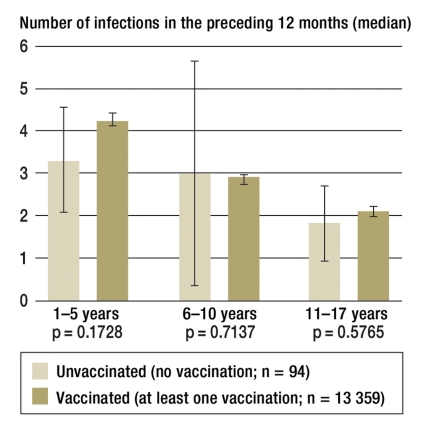
Relative to the year preceding the KiGGS survey, children aged 1–5 years had the highest numbers of infections. The median number in unvaccinated subjects was 3.3 (95% CI 2.1 to 4.6) and in vaccinated ones, 4.2 (95% CI 54.1 to 4.4). For 6–10-year-olds, the numbers were 3.0 (95% CI 0.4 to 5.7) in unvaccinated subjects and 2.9 (95% CI 2.7 to 3.0) in vaccinated subjects. In 11–17-year-olds, the median number of infections was 1.9 (95% CI 1.0 to 2.8) and 2.2 (95% CI 2.1 to 2.3) (figure 2). The slight differences between the vaccination status groups did not reach significance. Even when only cold/flu-like infections were considered, the parents of unvaccinated children and adolescents reported a similar number of 1.3 (95% CI 0.9 to 1.7) for infections as parents of vaccinated children and adolescents, with 1.4 (95% CI 1.4 to 1.5). Differentiated analyses stratified by sex did not show any differences between girls and boys (data not shown).
Figure 2.
Median number of infections in the year preceding the KiGGS study by vaccination status and age (median and 95% confidence interval)
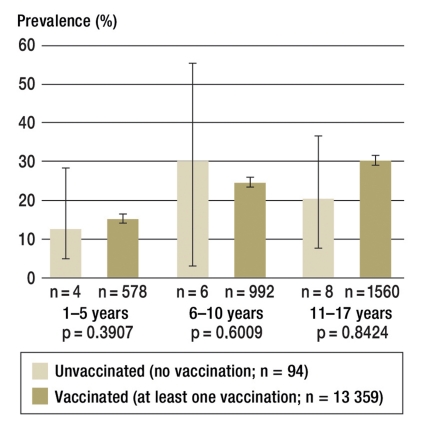
Table 2 shows the lifetime prevalence of medically diagnosed atopic disorders in unvaccinated and vaccinated children and adolescents in different age groups. Independent of their vaccination status, the prevalences increased considerably with age. Important differences between unvaccinated and vaccinated children and adolescents were not seen, however. The lifetime prevalence of at least one atopic disorder in the 1–5-year-olds was 12.6% (95% CI 5.0 to 28.3, n = 4) in unvaccinated subjects and 15.0% (95% CI 13.6 to 16.4, n = 578) in vaccinated subjects. According to CAPI, at least one atopic disorder was diagnosed by a doctor in 6–10-year-olds in 30.1% (95% CI 12.9 to 55.8, n = 6) of unvaccinated subjects and 24.4% (95% CI 22.8 to 26.0, n = 992) of vaccinated subjects; in 11–17-year-olds, in 20.3% (95% CI 10.1 to 36.6, n = 8) in unvaccinated subjects and 29.9% (95% CI 28.4 to 31.5, n = 1560) in vaccinated subjects (Figure 3). Differentiated analyses stratified by sex did not show any differences between boys and girls (data not shown).
Table 2. Lifetime prevalence of atopic disorders by vaccination status and age as percentages (with 95% confidence interval).
| Unvaccinated (no vaccination) | n | Vaccinated (at least one vaccination) | n | ||
| Allergic rhinoconjunctivitis | |||||
| 1 to 5 years | 5.0 (0.9 to 23.8) | 1 | 2.7 (2.2 to 3.4) | 100 | p=1.0000 |
| 6 to 10 years | 8.7 (2.3 to 27.7) | 3 | 10.0 (8.9 to 11.2) | 386 | p=0.4296 |
| 11 to 17 years | 8.2 (2.8 to 22.0) | 3 | 17.4 (16.1 to 18.7) | 891 | p=0.4630 |
| atopic excema | |||||
| 1 to 5 years | 12.6 (5.0 to 28.3) | 4 | 12.2 (10.9 to 13.7) | 482 | p=0.8143 |
| 6 to 10 years | 26.4 (10.2 to 53.3) | 4 | 15.6 (14.3 to 17.1) | 653 | p=0.5503 |
| 11 to 17 years | 6.4 (1.5 to 23.5) | 2 | 15.3 (14.0 to 16.6) | 800 | p=0.3045 |
| Bronchial asthma | |||||
| 1 to 5 years | 0 | 0 | 1.8 (1.4 to 2.3) | 60 | p=1.0000 |
| 6 to 10 years | 0 | 0 | 4.6 (3.9 to 5.4) | 182 | p=1.0000 |
| 11 to 17 years | 8.4 (2.8 to 22.3) | 2 | 7.0 (6.2 to 7.8) | 375 | p=0.1655 |
Figure 3.
Lifetime prevalence of at least one atopic disorder by vaccination status and age (as percentages with 95% confidence intervals)
Discussion
The KiGGS study is a German nationwide cross sectional study that is representative for the resident population. The study investigated the health status of children and adolescents up to 17 years of age. The participation rate was very high for a study of this type (12).
In total, 13 453 individuals were included in the analyses. The excluded individuals had not completed the first year of life at the time of the data collection, or they lived in immigrant families, or their vaccination data were not evaluable. We excluded such subjects in order to minimize misclassifications with regard to participants’ vaccination status. Furthermore, data from unvaccinated children were excluded if the recommended vaccinations had not taken place because of illness, as reported by the parents. This approach was chosen to distinguish children who were not vaccinated because of poor health from those who are categorized as unvaccinated in the context of this study.
In spite of any attempt to rule out misclassifications we cannot entirely exclude the possibility that some children without a vaccination card were vaccinated without adequate documentation. A systematic question regarding this issue will therefore be included in future KiGGS studies.
Study data regarding diseases may also depend on people’s ability to remember and on the assessment of those surveyed as well as on a medical diagnosis. We therefore cannot completely rule out that the true prevalence may be over- or underestimated. Questionnaire surveys or interviews with doctors are, however, standard procedures internationally and have been used in many studies. Furthermore, the information documents morbidity for which advice or treatment had been provided in the health care system.
On the basis of the KiGGS data, 0.7% (95% CI 0.5 to 0.9) of all children and adolescents aged 1–17 years who don’t come from immigrant families had never received a vaccine in Germany. This proportion was no different for girls or boys and was highest in children aged up to 5 years. With regard to the importance of vaccinations the low proportion of children and adolescents who had never been vaccinated is appreciated. The US National Immunization Survey (NIS) for 2001 showed that 0.3% (95% CI 0.2 to 0.4) of children aged 19–35 months were unvaccinated (13). In spite of limitations in terms of the comparability of the healthcare system in both countries, KiGGS data indicate that the proportion of unvaccinated children in Germany is higher (1.1% [95% CI 0.7 to 1.7] of 1–5-year-olds) than in the US.
Because of the low proportion of unvaccinated persons in the population, the numbers even in the large KiGGS study are small, so that statistical evaluation—especially subgroup analyses—is hindered by small case numbers.
As expected, vaccine preventable diseases affected those who had been vaccinated against a particular disease to a lower degree than unvaccinated persons. The KiGGS data do not show at what age the disease first occurred. This means that vaccinated subjects may have contracted the disease before vaccine induced immunity had developed. Looking only at the time interval after vaccine induced immunity had developed the probability of contracting a disease can be expected to be notably lower in those vaccinated against that disease. Outbreak investigations for measles and pertussis, for example, have reported high effectiveness of vaccinations in more than 97% of children who were sufficiently vaccinated (14– 16). In future KiGGS studies, the questions regarding vaccine preventable diseases will be extended to include the age at which the disease first manifested.
Some parents—and doctors—fear that vaccinated children are protected against specific infections, but that their immune systems reacts less to non-specific diseases and that vaccinated children contract infections such as colds, bronchitis, or gastrointestinal infections more often than unvaccinated children. However, the KiGGS data did not show any notable differences in the numbers of infections. By contrast, in unvaccinated as well as vaccinated subjects, a notable drop in the number of infections was observed with increasing age.
Another fear associated with protective vaccinations is that they might possibly promote the development of allergies. The KiGGS data did not show statistically significant differences in the prevalence of atopic disorders in unvaccinated subjects compared with vaccinated subjects. The prevalences in both groups increased, as expected, with age. No doctors’ diagnoses of bronchial asthma were reported for unvaccinated children aged 1–10 years. Because of the low incidence of asthma and given the fact that diagnosing asthma in this age group is difficult, no cases of asthma were to be expected within the small group of unvaccinated children. Furthermore, differences in the awareness of symptoms or parents’ advice seeking behaviors may explain different prevalences in doctors’ diagnoses. Studies that enumerated cases at the symptom level have continuously observed higher prevalences of asthma than KiGGS (17, 18).
In recent years, a number of scientific articles were published investigating potential associations between vaccinations and allergies. In a review article by Bernsen et al. from 2006, which summarized study results about the association of diphtheria/tetanus/pertussis vaccination, measles/mumps/rubella vaccination, and Haemophilus influenzae type b vaccination with atopic disorders, the authors conclude that according to the available evidence, recommended protective vaccinations do not increase the risk of atopic disorders in children (19). In an international study from 2008, which included 2184 children aged 1–2 years, Grüber et al. investigated the effect of vaccinations in the first year of life on the severity of atopic eczema and allergic sensitization in the second year of life. They did not find an increased risk for allergies (20). The current guideline for allergy prevention (as of March 2009) recommends vaccinations according to STIKO recommendations for children and adolescents with and without allergy risk (21).
In addition to atopic disorders, we further compared diseases—such as obstructive bronchitis, pneumonia and otitis media, heart disease, anemia, epilepsy, and attention deficit hyperactivity disorder (ADHD)—in unvaccinated and vaccinated subjects. No relevant differences in the lifetime prevalences were found, neither for different age groups nor between girls and boys. Schneeweiß et al. conducted a comprehensive literature review of vaccine safety, the central part of which was the evaluation of vaccine critical arguments on the basis of the current state of scientific knowledge. None of the hypotheses were found to be valid (5).
Key Messages.
On the basis of representative KiGGS data, 0.7% of children and adolescents aged 1–17 years from non-immigrant families in Germany have never been vaccinated.
The evaluation showed that vaccinated children and unvaccinated children differed substantially only in terms of the lifetime prevalence of vaccine preventable diseases; as is to be expected the risk of such diseases is notably lower in vaccinated subjects.
In the largest study in children and adolescents so far none of the often anticipated health differences—such as allergies and the number of infections—were observed in vaccinated and unvaccinated subjects aged 1–17 years.
Acknowledgments
Translated from the original German by Dr Birte Twisselmann.
Footnotes
Conflict of interest statement
Drs Schmitz and Reiter declare that no conflict of interests exists according to the guidelines of the International Committee of Medical Journal Editors.
Dr Schlaud was the lead investigator of an epidemiological study of deaths in children aged 2–24 months (TOKEN Study) in 2004–2009, which was jointly funded by the Federal Ministry of Health, the Paul Ehrlich-Institute, Sanofi Pasteur, and Glaxo Smith Kline.
Dr Poethko-Müller was responsible for coordinating the study mentioned before.
References
- 1.Reiter S. Über die Bedeutung von Schutzimpfungen. Public Health Forum. 2009;17:2.e1–2e3. [Google Scholar]
- 2. http://www.rki.de/cln_160/nn_199596/DE/Content/Infekt/Impfen/STIKO/stiko__node.html?__nnn=true:
- 3.Robert Koch-Institut. Epidemiologisches Bulletin. Nr. 30. Berlin: 2009. [Google Scholar]
- 4.Heininger U. Risiken von Infektionskrankheiten und der Nutzen von Impfungen. Bundesgesundheitsblatt - Gesundheitsforschung - Gesundheitsschutz. 2004;47:1129–1135. doi: 10.1007/s00103-004-0951-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schneeweiß B, Pfleiderer M, Keller-Stanislawski B. Impfsicherheit heute. Dtsch Arztebl. 2008;105:590–595. doi: 10.3238/arztebl.2008.0590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meyer C, Reiter S. Impfgegner und Impfskeptiker: Geschichte, Hintergründe, Thesen, Umgang. Bundesgesundheitsblatt - Gesundheitsforschung - Gesundheitsschutz. 2004;47:1182–1188. doi: 10.1007/s00103-004-0953-x. [DOI] [PubMed] [Google Scholar]
- 7.Heininger U. An internet-based survey on parental attitudes towards immunization. Vaccine. 2006;24:6351–6355. doi: 10.1016/j.vaccine.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 8.Hölling H, Kamtsiuris P, Lange M, et al. Der Kinder- und Jugendgesundheitssurvey (KiGGS): Studienmanagement und Durchführung der Feldarbeit. Bundesgesundheitsblatt - Gesundheitsforschung - Gesundheitsschutz. 2007;50:557–566. doi: 10.1007/s00103-007-0216-8. [DOI] [PubMed] [Google Scholar]
- 9.Kurth BM. Der Kinder- und Jugendgesundheitssurvey (KiGGS): Ein Überblick über Planung, Durchführung und Ergebnisse unter Berücksichtigung von Aspekten eines Qualitatsmanagements. Bundesgesundheitsblatt - Gesundheitsforschung - Gesundheitsschutz. 2007;50:533–546. doi: 10.1007/s00103-007-0214-x. [DOI] [PubMed] [Google Scholar]
- 10.Lange M, Kamtsiuris P, Lange C, et al. Messung soziodemographischer Merkmale im Kinder- und Jugendgesundheitssurvey (KiGGS) und ihre Bedeutung am Beispiel der Einschätzung des allgemeinen Gesundheitszustands. Bundesgesundheitsblatt - Gesundheitsforschung - Gesundheitsschutz. 2007;50:578–589. doi: 10.1007/s00103-007-0219-5. [DOI] [PubMed] [Google Scholar]
- 11. www.spss.com/de/media/collateral/statistics/complex-samples.pdf:
- 12.Kamtsiuris P, Lange M, Schaffrath Rosario A. Der Kinder- und Jugendgesundheitssurvey (KiGGS): Stichprobendesign, Response und Nonresponse-Analyse. Bundesgesundheitsblatt - Gesundheitsforschung - Gesundheitsschutz. 2007;50:547–556. doi: 10.1007/s00103-007-0215-9. [DOI] [PubMed] [Google Scholar]
- 13.Smith PJ, Chu SY, Barker LE. Children who have received no vaccines: who are they and where do they live? Pediatrics. 2004;114:187–195. doi: 10.1542/peds.114.1.187. [DOI] [PubMed] [Google Scholar]
- 14.Wichmann O, Hellenbrand W, Sagebiel D, et al. Large measles outbreak at a German public school, 2006. Pediatr Infect Dis J. 2007;26:782–786. doi: 10.1097/INF.0b013e318060aca1. [DOI] [PubMed] [Google Scholar]
- 15.Bisgard KM, Rhodes P, Connelly BL, et al. Pertussis vaccine effectiveness among children 6 to 59 months of age in the United States, 1998-2001. Pediatrics. 2005;116:e285–e294. doi: 10.1542/peds.2004-2759. [DOI] [PubMed] [Google Scholar]
- 16.Robert Koch-Institut (ed) Infektionsepidemiologisches Jahrbuch für 2008. Berlin: 2009. [Google Scholar]
- 17.Maziak W, Behrens T, Brasky TM, et al. Are asthma and allergies in children and adolescents increasing? Results from ISAAC phase I and phase III surveys in Münster, Germany. Allergy. 2003;58:572–579. doi: 10.1034/j.1398-9995.2003.00161.x. [DOI] [PubMed] [Google Scholar]
- 18.Punekar YS, Sheikh A. Establishing the incidence and prevalence of clinician-diagnosed allergic conditions in children and adolescents using routinely collected data from general practices. Clin Exp Allergy. 2009;39:1209–1216. doi: 10.1111/j.1365-2222.2009.03248.x. [DOI] [PubMed] [Google Scholar]
- 19.Bernsen RMD, van der Wouden JC, Nagelkerke NJD, de Jongste JC. Early life circumstances and atopic disorders in childhood. Clinical and Experimental Allergy. 2006;36:858–865. doi: 10.1111/j.1365-2222.2006.02518.x. [DOI] [PubMed] [Google Scholar]
- 20.Grüber C, Warner J, Hill D, Bachau V, the EPAAC Study Group Early atopic disease and early childhood immunization - is there a link? Allergy. 2008;63:1464–1472. doi: 10.1111/j.1398-9995.2008.01696.x. [DOI] [PubMed] [Google Scholar]
- 21.Muche-Borowski C, Kopp M, Reese I, et al. Klinische Leitlinie: Allergieprävention. Dtsch Arztebl Int. 2009;106:625–631. doi: 10.3238/arztebl.2009.0625. [DOI] [PMC free article] [PubMed] [Google Scholar]