Abstract
Streptococcus sobrinus, one agent of dental caries, secretes a protein that induces lymphocyte polyclonal activation of the host as a mechanism of immune evasion. We have isolated from culture supernatants of this bacterium a protein with murine B-cell-stimulatory properties and subsequently cloned the relevant gene. It contains an open reading frame of 825 bp encoding a polypeptide with 275 amino acid residues and a molecular mass of 30 kDa. The protein displays high sequence homology with NAD+ synthetases from several organisms, including a conserved fingerprint sequence (SGGXD) characteristic of ATP pyrophosphatases. The polypeptide was expressed in Escherichia coli as a hexahistidine-tagged protein and purified in an enzymatically active form. The recombinant NAD+ synthetase stimulates murine B cells after in vitro treatment of spleen cell cultures, as demonstrated by its ability to induce up-regulation of the expression of CD69, an early marker of lymphocyte activation. Stimulation with the recombinant NAD+ synthetase was also observed with other B-cell markers, such as CD19+, B220+, and CD21+. Cell proliferation follows the activation induced by the recombinant NAD+ synthetase.
Streptococcus sobrinus is considered one of the principal agents of dental caries (17, 20, 26, 41), and the treatment of this infectious disease is among the world's most costly health problems, due to its wide distribution (26, 34). The polyclonal-lymphocyte activation of the host, observed in several microbial infections, has been described as a general survival strategy of the infecting microorganism (9, 21, 30, 48). Several microbial molecules have been described as B- and T-cell mitogens (8, 16, 19, 25, 27, 31, 35, 39, 40, 49, 50). It has been reported that S. sobrinus produces a protein that activates murine lymphocytes polyclonally, suppresses specific antibody response to S. sobrinus antigens, and potentiates the growth of the microorganism in the host (18).
The vital and ubiquitous enzyme NAD+ synthetase belongs to the amidotransferase family. This enzyme catalyzes the synthesis of NAD+ from either NH3 or glutamine and nicotinic acid adenine dinucleotide (29, 53), and NAD+ is involved in an enormous variety of biochemical processes, such as the synthesis of various essential molecules and antibiotics, oxidation-reduction reactions, and DNA repair and recombination (37). The NAD+ synthetase of Bacillus subtilis is a member of a σB-dependent general stress regulon (5). NAD+ synthetases are essential for viability in Escherichia coli (3) and Salmonella enterica serovar Typhimurium (23).
In this work, we identify and characterize for the first time the NAD+ synthetase of S. sobrinus as a murine B-cell-stimulatory protein. Our results provide information on the involvement of NAD+ synthetase in the modulation of the host immune system induced by S. sobrinus. As previously suggested and reported for several microorganisms (6, 10, 30, 42, 47), the isolation and the characterization of molecules involved in microbial pathogenicity may be useful in the identification of targets for vaccination.
MATERIALS AND METHODS
Strains and plasmids.
S. sobrinus strain 6715, able to induce caries in rats (15, 43), was a kind gift of B. Klausen from the Department of Oral Diagnosis and Microbiology, Royal Dental College, University of Copenhagen, Denmark. The strain was stored at −70°C in Todd-Hewitt broth medium (Difco Laboratories, Detroit, Mich.) with 10% glycerol. E. coli strain DH-5α (38) and the plasmid pGEM-T Easy vector (Promega Corp., Madison, Wis.) were used for cloning PCR fragments, while E. coli strain M-15 (38) was used as the host for the plasmid pQE-31 (Qiagen, Oslo, Norway) in the protein expression experiments. For immunobiological experiments, 6- to 8-week-old male C57BL/6 mice were bred at the Gulbenkian Institute of Science, Oeiras, Portugal. We followed the guidelines of the European Community for animal studies.
Purification of the protein secreted by S. sobrinus.
S. sobrinus was cultured in Todd-Hewitt broth medium for 2 days at 37°C with an initial inoculum of 108 cells/ml. The cultures were centrifuged at 12,000 × g for 30 min. The supernatants were filtered through 0.22-μm-pore-size filters (Schleicher & Schuell, Dassel, Germany) and concentrated by ultrafiltration (membrane cutoff, 10 kDa) in a Vivaflow 200 system (Cole-Parmer's Masterflex, Vernon Hills, Ill.). This crude extracellular product was subjected to ion-exchange chromatography in DEAE-cellulose (DE54; Amersham Biosciences, Uppsala, Sweden), using a continuous molarity gradient of 0.05 to 1.0 M NaCl in 50 mM Tris-HCl, pH 7.0. The fractions containing B-cell-stimulatory activity were concentrated by vacuum dialysis and resolved by preparative isoelectric focusing in sucrose with a pH gradient of 2.5 to 10, using a mixture of ampholytes of pH 2.5 to 5.0 and 3.0 to 10.0 (Amersham Biosciences). The relevant fractions were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) in 10% gels stained with 0.05% Coomassie blue R250. A protein band of ∼38 kDa was submitted to trypsin digestion and peptide mapping by high-performance liquid chromatography, and the internal amino acid sequences were determined by mass spectrometry at the Protein Chemistry Core Facility (Howard Hughes Medical Institute-Columbia, New York, N.Y.).
Molecular cloning.
Standard protocols were followed for restriction enzyme digestion, agarose gel electrophoresis, and general cloning techniques (38, 51). Genomic DNA from S. sobrinus was obtained by a modification of the method of Sun et al. (45). Briefly, overnight cultures of S. sobrinus in Luria-Bertani medium supplemented with 0.2% glucose were harvested and washed twice with TE buffer (10 mM Tris-HCl, 1 mM EDTA, pH 7.5). About 1 g (wet weight) of bacteria was suspended in 5 ml of TE, heated for 20 min at 60°C, and incubated for 1 h at 37°C in the presence of 40 mg of lysozyme (Sigma, St. Louis, Mo.)/ml and 200 U of mutanolysin (Sigma)/ml. After the addition of 100 μg of proteinase K (Sigma)/ml and 100 μg of RNase (Sigma)/ml, the cell suspension was further incubated for 1 h at 37°C. The cells were lysed by adding SDS to a final concentration of 2.5% (wt/vol). Total DNA was purified by extraction with phenol, phenol-chloroform (1:1), and chloroform and then precipitated with ethanol. The DNA was further purified with a purification kit (Genomic-tip 20/G; Qiagen). The NAD+ synthetase gene was amplified by PCR with the following primers, each at a concentration of 1 μM: NAD1, 5′-ATGA(G/C)(A/T)TT(A/G)CAA(G/C)AA(G/C)A(G/A/T)AT-3′, and NAD9, 5′-TTACTTCCA(G/A/C)AAATCATC(A/G)AA(G/A/C)A-3′.
The PCR temperature profile was carried out by the touchdown method, consisting of an initial denaturation step at 95°C for 5 min, followed by 10 cycles of a denaturing step of 95°C for 30 s, a primer-annealing step at 60°C for 30 s, and an extension step at 72°C for 1 min. In the next 10 cycles, the annealing temperature was decreased by 6°C. Then 10 more cycles were performed using an annealing temperature of 48°C and an extension step of 72°C for 2 min, followed by a final step of incubation at 72°C for 5 min. The 825-bp PCR product was cloned in the pGEM-T Easy vector. The complete double-stranded nucleotide sequence of the PCR product was determined using a primer-walking strategy with a model 310 automated DNA sequencer (ABI- Perkin-Elmer, Foster City, Calif.). In order to subclone the PCR product in the expression vector pQE-31, it was excised from a recombinant pGEM-T plasmid (containing the insert in the correct orientation) with the restriction enzymes SphI and PstI. The relevant band was purified from 1% agarose gels with a Promega gel purification kit and ligated to SphI/PstI-digested pQE-31 with T4 ligase (Amersham Biosciences). The construct was transformed into the competent E. coli strain M-15.
Expression and characterization of recombinant NAD+ synthetase.
Cultures of exponentially growing E. coli M-15 cells (A600 = 0.6 to 0.8) containing the pQE-31 expression construct were induced in Luria-Bertani medium for expression of the fusion protein for 3 h at 37°C by the addition of 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside). The cells were harvested by centrifugation at 5,000 × g for 20 min and resuspended in phosphate buffer (1 mM Na2HPO4 · 0.2H2O, 1 mM NaH2PO4 · H2O, 50 mM NaCl, pH 7.4) containing 10 mM imidazole. The sample was incubated on ice for 30 min in the presence of 100 μg of lysozyme/ml and 1% Triton X-100. After sonication at maximum intensity for three 10-s bursts, the insoluble material was removed by centrifugation at 10,000 × g for 15 min. The supernatant was filtered through a 0.45-μm-pore-size filter (Millipore, Billerica, Mass.) and applied to a His-trap column (Amersham Biosciences). The recombinant NAD+ synthetase was eluted with imidazole under native conditions. The protein concentration was determined by the method of Lowry et al. (28). The purity of the recombinant protein was evaluated by SDS-PAGE in 17.5% gels (38). The NAD+ synthetase activity of the recombinant protein was assayed in 0.5 ml of 60 mM HEPES buffer, pH 8.5, containing 2 mM nicotinic acid adenine dinucleotide, 2 mM ATP, 10 mM NH4Cl, 10 mM MgCl2, and 20 mM KCl. The reaction was started by the addition of 0.5 μg of enzyme. After 5 min of incubation at 37°C, the reaction was stopped by the addition of 0.5 ml of 0.1 M sodium pyrophosphate buffer, pH 8.9, containing 0.5% (wt/vol) semicarbazide hydrochloride (33). The NAD+ formed was measured spectrophotometrically at 340 nm by the alcohol dehydrogenase method (12). The S. sobrinus recombinant enolase was expressed in a similar way (details will be published elsewhere).
Expression of CD69 assay.
A suspension of untreated mouse spleen cells was prepared by homogenizing the organ in cold balanced saline solution supplemented with 3% fetal calf serum (Gibco Biocult, Glasgow, United Kingdom). Total splenic cells were resuspended in 0.2 ml of RPMI containing fetal calf serum and plated in triplicate in 96-well tissue culture plates (2 × 106 cells/well). The cultures were stimulated with 6.25, 12.5, 25, 50, or 100 μg of S. sobrinus recombinant NAD+ synthetase or with 50 μg of S. sobrinus recombinant enolase and incubated for 6 h at 37°C in 7% CO2- 93% humidified air.
Peripheral blood mononuclear cells were obtained from defibrinated blood from normal human donors after centrifugation on a Ficoll (Sigma)-sodium metrizoate gradient by the method of Boyum (13). The mononuclear cells were resuspended in 0.2 ml of RPMI containing fetal calf serum and plated in triplicate in 96-well tissue culture plates (2 × 106 cells/well). The cultures were stimulated with 50 μg of S. sobrinus recombinant NAD+ synthetase and incubated for 6 h at 37°C in 7% CO2- 93% humidified air. After incubation, the plates were centrifuged at 1,000 × g for 10 min, and the cells were incubated for 30 min on ice in the dark with the following monoclonal antibodies diluted in phosphate-buffered saline containing 10 mM azide and 1% bovine serum albumin: goat anti-mouse fluorescein isothiocyanate (FITC)-conjugated immunoglobulin M (IgM) (Southern Biotechnology Associates, Birmingham, Ala.), phycoerythrin-conjugated hamster anti-mouse CD3 (Pharmingen, San Diego, Calif.), FITC-conjugated rat anti-mouse CD19 (Pharmingen), FITC-conjugated rat anti-mouse B220 (Pharmingen), FITC-conjugated rat anti-mouse CD21 (Pharmingen), and biotin conjugated hamster anti-mouse early activation marker (CD69; Pharmingen) for murine spleen cultures and FITC-conjugated mouse anti-human CD19 (Pharmigen), FITC-conjugated mouse anti-human CD3 (Pharmingen), and phycoerythrin-conjugated mouse anti-human CD69 (Pharmingen) for human cultures. The plates were washed with phosphate-buffered saline containing 10 mM azide and 1% bovine serum albumin and centrifuged at 1,000 × g for 10 min, and the cells were incubated with phycoerythrin-conjugated avidin for 20 min. Dead cells were excluded by propidium iodide incorporation. The samples were assayed for immunofluorescence cytometric analysis in a FACScan with Lysis II software (Becton Dickinson, San Jose, Calif.).
Immunosuppression assays.
Mice were immunized by an intraperitoneal injection of 5 × 107 sheep red blood cells. The splenic production of specific IgM antibodies against these cells (primary immune response) was evaluated 5 days later by hemolytic plaque-forming cell assays (11). Treatments with 100 μg of recombinant protein or with phosphate-buffered saline (control mice) were also carried out by intraperitoneal injections 4 days before priming with sheep red blood cells.
MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide; Sigma) proliferation assay.
Mouse spleen cells were obtained by homogenizing the organ in RPMI complete medium, that is, RPMI 1640 medium supplemented with 100 IU of penicillin/ml, 50 μg of streptomycin/ml, 0.05 mM 2-mercaptoethanol, and 10% fetal calf serum (Gibco Biocult). For the isolation of mononuclear cells, 5-ml aliquots of the spleen cell suspension were layered on 2.5 ml of Ficoll (Sigma) and centrifuged at 1,000 × g for 20 min at room temperature. The cells were then gently removed from the medium-Ficoll interface, transferred to a sterile container, and washed in 10 ml of RPMI complete medium. The isolated mononuclear lymphocytes were resuspended in 5 ml of RPMI complete medium, and cell counts were performed.
The MTT assay was performed as previously described (32) with some modifications. Briefly, mononuclear cells (5 × 105/well) were plated in triplicate in 96-well tissue culture plates and stimulated with 25 or 50 μg of S. sobrinus recombinant NAD+ synthetase or with 100 μg of S. sobrinus recombinant enolase and incubated for 24, 48, and 72 h in 7% CO2- 93% humidified air. Then, MTT stock solution (0.5 mg of MTT per ml of phenol red-free cell culture medium) equivalent to 1/10 of the original culture volume was added to each culture being assayed. After incubation for 4 h, the supernatants were decanted. An amount of 0.08 M HCl solution in anhydrous isopropanol equal to the original culture volume was added to each well to dissolve the resulting MTT formazan crystals. The absorbance of the purple solution at 570 nm was measured spectrophotometrically in a Multiskan plate reader (Multiskan EX; Labsystems, Helsinki, Finland). An increase in the number of cells corresponds to an increase in the amount of formazan and results in an increase of absorbance. The percent proliferation was calculated as [(test cell A570 − control cell A570)/control cell A570] × 100.
Nucleotide sequence accession number.
The sequence of the NAD+ synthetase gene from S. sobrinus has been deposited in the EMBL Nucleotide Sequence Database under accession number AJ536592.
RESULTS
Identification and characterization of NAD+ synthetase.
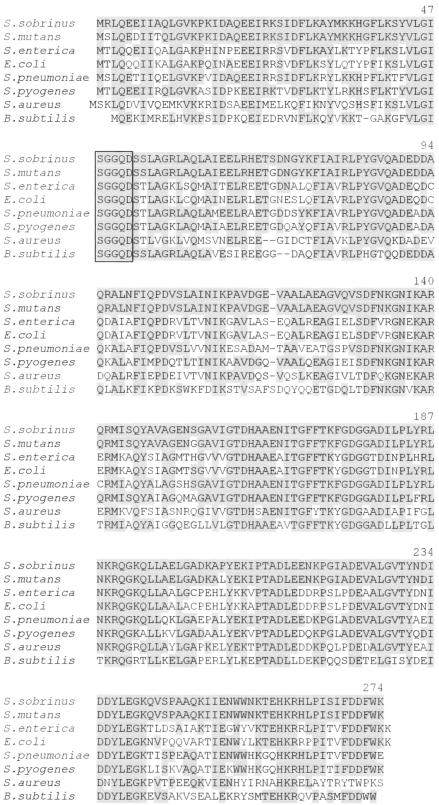
Partial purification of a protein from S. sobrinus that was able to induce both an immunosuppressive response and lymphocyte polyclonal activation in mouse cells was previously described (18). Using a similar purification strategy, we have now isolated the protein that induces the lymphocyte polyclonal activation. It was not possible to achieve direct protein sequencing, suggesting that the N terminus of the protein is blocked. Therefore, the polypeptide was cleaved with trypsin prior to being sequenced, and two adjacent internal amino acid sequences were obtained: N-MISQYAVAGENS-C and N-GAVIGTDHAAENIT-C. A BLAST (4) search of sequences deposited in computer databases of the National Center for Biotechnology Information revealed high similarity of these sequences to those of bacterial NAD+ synthetases (Fig. 1). Several of the sequences were aligned, and we designed a series of degenerate primers, based on either amino acid sequences conserved in the different organisms or the known internal amino acid sequences of the NAD+ synthetase from S. sobrinus, to be used in PCR experiments.
FIG. 1.
Amino acid sequence alignment of the NAD+ synthetases from S. sobrinus, S. mutans, Salmonella enterica, E. coli, Streptococcus pneumoniae, Streptococcus pyogenes, Staphylococcus aureus, and Bacillus subtilis. The consensus fingerprint sequence for the N-type ATP pyrophosphatases is boxed. Amino acid residues identical to those of S. sobrinus are shaded.
In brief, a PCR product of 825 bp was obtained with primers NAD1 and NAD9, which hybridize at genomic DNA regions corresponding to the N terminus and C terminus of the NAD+ synthetase polypeptide, respectively. The PCR fragment was cloned, and both DNA strands were fully sequenced. The deduced primary structure of the 30,271-Da protein, which is highly homologous to NAD+ synthetases from other organisms and includes a typical sequence fingerprint characteristic of ATP-pyrophosphatases (S-G-G-X-D), is shown in Fig. 1. The difference between the polypeptide sequences of the NAD+ synthetases from S. sobrinus and Streptococcus mutans strain UA159 (accession no. AE014133) is limited to six amino acid residues. Four out of the six substitutions, R2S, E6D, A9T, S72G, S154G, and P205L, represent conservative amino acid replacements.
NAD+ synthetase displays immunobiological activity.
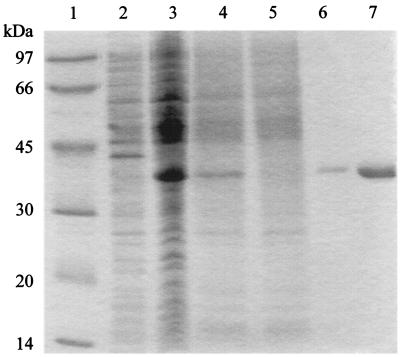
We have produced the NAD+ synthetase of S. sobrinus in a heterologous system. For this, the gene was cloned in the expression vector pQE-31 and transformed into an E. coli strain. The bacteria were induced for expression, and the recombinant protein, which contains 26 extra amino acid residues at the N terminus, including six consecutive histidines, was purified by affinity chromatography. The purified protein was resolved by SDS-PAGE as a single band displaying an apparent molecular mass of 38 kDa (Fig. 2). This value is higher than expected and likely reflects an overestimation due to the acidic nature of the protein (29). Despite the presence of extra N-terminal amino acids, the recombinant protein showed specific enzymatic activity. We observed an activity of 492 nmol of NAD+ synthesized per min per mg of protein. This activity is about twice the activity of the recombinant NAD+ synthetase isolated from Mycobacterium tuberculosis (14). The enzymatic activity was optimal at a temperature of 37°C and could be inactivated by heating the enzyme at 95°C for 10 min.
FIG. 2.
Purification of the S. sobrinus recombinant NAD+ synthetase. Molecular mass standards (lane 1), uninduced cell lysate (lane 2), IPTG-induced cell lysate (lane 3), material loaded into the Ni affinity column (lane 4), column flowthrough (lane 5), and different fractions of the eluted NAD+ synthetase (lanes 6 and 7) were resolved by SDS-PAGE and stained with Coomassie blue.
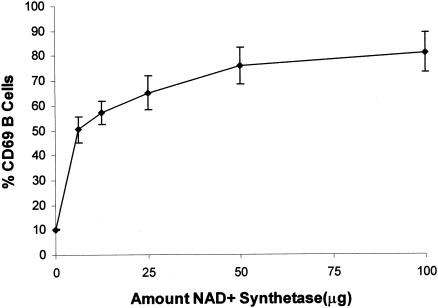
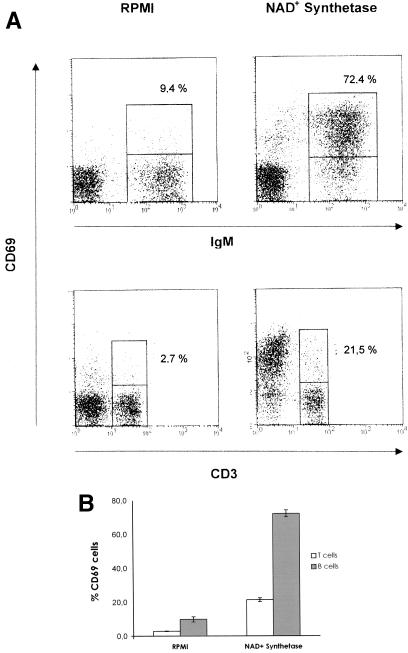
In order to demonstrate the stimulatory activity of the S. sobrinus recombinant NAD+ synthetase on lymphocyte cells, we tested the ability of the protein to induce an in vitro increase in the surface expression of the early activation marker CD69 on B and T cells (46, 52). Titration experiments indicated that the optimal quantity was 50 μg (Fig. 3). Cultures of murine spleen cells were treated in vitro with 50 μg of the S. sobrinus recombinant protein, and the expression of CD69 was evaluated 6 h after NAD+ synthetase stimulation. As shown in Fig. 4, increased expression of CD69 on the surfaces of B (IgM+) and T cells could be observed after stimulation with the recombinant protein. This increase in CD69 expression is more pronounced on B cells than on T cells (Fig. 4B). To exclude the possibility that the His tag of the recombinant protein produced any interference in the results, we evaluated the induction of CD69 expression on mouse lymphocytes after stimulation of spleen cells with the S. sobrinus recombinant enolase as a control protein also carrying a His tag. No significant increase in the expression of the CD69 on B or T cells was observed after 6 h of stimulation with the recombinant enolase (data not shown).
FIG. 3.
NAD+ synthetase dose-response curve measured by the expression of CD69+ analyzed by flow cytometry. Shown are the percentages of the B220+-B-cell population expressing CD69 after incubation of total splenic cells with RPMI (0 μg) or after stimulation with 6.25, 12.5, 25, 50, or 100 μg of recombinant NAD+ synthetase. The standard deviations of triplicate samples, repeated twice, are shown as vertical bars.
FIG. 4.
Analysis of immunobiological activity of the recombinant NAD+ synthetase on mouse lymphocytes by flow cytometry. (A) Typical expression of CD69 on the surfaces of splenic B (IgM+) or T (CD3+) lymphocytes after incubation of total spleen cells with RPMI (control) or after treatment with 50 μg of recombinant NAD+ synthetase. (B) Percentages of T (CD3+) and B (IgM+) lymphocytes expressing CD69 6 h after in vitro stimulation with recombinant NAD+ synthetase. The results represent means ± standard deviations of triplicate samples repeated twice.
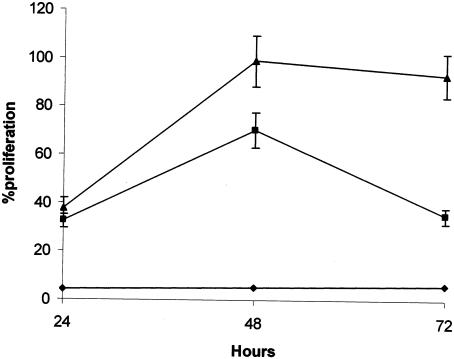
In order to verify whether the increased expression of CD69 on the IgM+-cell population due to stimulation with the recombinant NAD+ synthetase also occurred with other B-cell markers, the expression of CD69 was evaluated in CD19+, B220+, and CD21+ cell populations. As depicted in Table 1, there are no significant differences between the different B cell markers, that is, the recombinant NAD+ synthetase induces activation of all B cells. To investigate whether this activation leads to cell proliferation, murine spleen cells were stimulated with 25 or 50 μg of recombinant NAD+ synthetase for 24, 48, and 72 h. As shown in Fig. 5, the recombinant NAD+ synthetase induces cellular proliferation with a peak response at 48 h for the two concentrations of the protein. In contrast, the recombinant enolase is unable to stimulate proliferation of the cells.
TABLE 1.
Expression of CD69 on mouse B lymphocytes analyzed by flow cytometry
| B-cell marker | % CD69+ cellsa
|
|
|---|---|---|
| RPMI | NAD+ synthetase | |
| CD19 | 11.2 ± 0.3 | 72.1 ± 2.6 |
| B220 | 10.4 ± 0.8 | 65.0 ± 4.5 |
| CD21 | 12.4 ± 1.4 | 70.1 ± 3.7 |
| IgM | 10.4 ± 0.8 | 68.4 ± 3.7 |
Percentage of the CD19+-, B220+-, CD21+-, and IgM+-B-cell populations expressing CD69 after incubation of total spleen cells with RPMI (control) or after stimulation with 50 μg of recombinant NAD+ synthetase. The results represent the means and standard deviations of triplicate samples repeated twice.
FIG. 5.
Effects of recombinant NAD+ synthetase on cell proliferation in vitro. Murine mononuclear spleen cells were stimulated with 25 (▪) or 50 (▴) μg of recombinant NAD+ synthetase or with 100 μg of recombinant enolase (⧫). The standard deviations of triplicate samples, repeated twice, are shown as vertical bars.
In order to investigate the effects of recombinant NAD+ synthetase on human lymphocytes, the expression of the CD69 marker was evaluated in these cells, and it was found that an increase in CD69 expression on lymphocytes, mainly on the B-cell population, could be observed after activation of the cells with the recombinant NAD+ synthetase, corroborating the results obtained with murine cells (data not shown).
We also investigated the immunosuppressive activity of NAD+ synthetase, but no suppressive effect of the protein was observed. We found no differences between the primary immune responses to sheep red blood cells in a control group of mice and mice treated with the recombinant protein (data not shown).
DISCUSSION
A protein with immunostimulatory and immunosuppressive properties secreted by S. sobrinus was previously described and tentatively identified as enolase (18). In this paper, we have cloned and characterized an S. sobrinus protein that is able to induce a B-cell-stimulatory effect in mice. The protein was identified as a NAD+ synthetase, since it displays a high degree of similarity to homologous proteins from other microorganisms. In particular, it is almost identical to the corresponding enzyme of S. mutans, another agent of dental caries (2, 24). It will be interesting to investigate whether some features of these proteins that are absent in other organisms are responsible for their immunological effects.
We could produce in E. coli cells a recombinant NAD+ synthetase that exhibited enzymatic activity. This activity was even higher than that found in the recombinant NAD+ synthetase isolated from M. tuberculosis (14). In addition, the recombinant NAD+ synthetase from S. sobrinus is able to induce B-cell stimulation of mouse lymphocytes. We have shown that the recombinant enzyme increases CD69 expression on the surfaces of lymphocytes, mainly of the B-cell population. Furthermore, the NAD+ synthetase induced cellular proliferation in vitro. Thus, the S. sobrinus NAD+ synthetase likely accounts for the immunostimulatory effects previously described for the proteinaceous factor secreted by the bacterium (18). However, it did not induce immunosuppressive effects in mice. These effects are probably due to other S. sobrinus-secreted proteins, such as enolase. Indeed, we found that S. sobrinus enolase suppresses mouse immune responses to nominal antigens and is able to stimulate the production of interleukin-10 (unpublished data). In conclusion, our results indicate that the suppression and the mitogenic effects previously described for a protein secreted by S. sobrinus (18) must be induced by at least two different proteins, enolase and NAD+ synthetase.
NAD+ synthetase is a member of the amidotransferase family of enzymes, characterized by the presence of two separate domains (glutamine amide transfer and synthetase domains) that can belong to the same polypeptide chain or exist as independent subunits. The glutamine amide transfer domain permits the use of glutamine as a nitrogen source, and the synthetase domain confers specificity and catalyzes the transfer of ammonia to the substrate (54). Their vast similarity with the sequence of B. subtilis, and their relatively small sizes (33), suggest that the NAD+ synthetases of S. sobrinus and S. mutans are strictly ammonia-dependent enzymes. However, we cannot exclude the existence of an as-yet-uncharacterized second subunit bearing the glutamine amide transfer domain. NAD+ synthetase is a vital and ubiquitous enzyme, and NAD+ is an essential component of metabolic pathways in all living cells (3, 5, 23, 37). The B. subtilis synthetase is essential for spore outgrowth and vegetative growth and was also described as a σB-dependent general stress protein (5). The connection between the biological role of NAD+ synthetase and its role in immunostimulation remains to be elucidated. However, it should be noted that other enzymes involved in NAD+ metabolism have been found in connection with parasitic infections (1, 44). The production of NAD glycohydrolase was associated with invasive streptococcal infections (1, 44).
It has been shown that different pathogenic microorganisms produce mitogenic and immunosuppressive proteins which also constitute important virulence factors (7, 8, 18, 19, 39). Such proteins have been generally designated virulence-associated immunomodulatory proteins. They induce polyclonal activation of lymphocytes, as well as the suppression of the host immune response to microbial specific antigens (8, 19, 39). It was also found that specific immunization against virulence-associated immunomodulatory proteins (but not against other antigenic components of the microbe) induces immunoprotection (39, 47). In a mouse model of Trypanosoma cruzi infection, a B-cell-mitogenic protein was isolated from the culture supernatants of the parasite infective forms. This protein was cloned and characterized as a cofactor-independent proline racemase and can ensure parasite evasion at the very beginning of the infection (36). Immunoprotection against the producing microbe was also observed following immunoneutralization of proteins secreted by M. tuberculosis (22). All of these vaccination approaches target molecules that are essential for the survival of the producing microorganism. Thus, immunoneutralization of the effects induced by NAD+ synthetase may prove to be a reasonable strategy for the development of a vaccine against dental caries.
Acknowledgments
We thank Laura Pinto for excellent technical assistance.
This research was supported by Fundação para a Ciência e a Tecnologia from Portugal and the POCTI program of QCA III through research grants to A.V., grants MGI/33790/2000 and MGI/33570/2000 to P.F., and fellowships to I.V.-M. and M. Duarte.
Footnotes
This article is dedicated to the memory of Mário Arala Chaves.
REFERENCES
- 1.Ajdic, D., W. M. McShan, D. J. Savic, D. Gerlach, and J. J. Ferretti. 2000. The NAD-glycohydrolase (nga) gene of Streptococcus pyogenes. FEMS Microbiol. Lett. 191:235-241. [DOI] [PubMed] [Google Scholar]
- 2.Ajdic, D., W. M. McShan, R. E. McLaughlin, G. Savic, J. Chang, M. B. Carson, C. Primeaux, R. Tian, S. Kenton, H. Jia, S. Lin, Y. Qian, S. Li, H. Zhu, F. Najar, H. Lai, J. White, B. A. Roe, and J. J. Ferretti. 2002. Genome sequence of Streptococcus mutans UA159, a cariogenic dental pathogen. Proc. Natl. Acad. Sci. USA 99:14434-14439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allibert, P., J. C. Willison, and P. M. Vignais. 1987. Complementation of nitrogen regulatory (ntr-like) mutations in Rhodobacter capsulatus by an Escherichia coli gene: cloning and sequencing of the gene and characterization of the gene product. J. Bacteriol. 169:260-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altschul, S. F., M. S. Boguski, W. Gish, and J. C. Wooton. 1994. Issues in searching molecular sequences databases. Nat. Genet. 6:119-129. [DOI] [PubMed] [Google Scholar]
- 5.Antelmann, H., R. Schmid, and M. Hecker. 1997. The NAD synthetase NadE (outB) of Bacillus subtilis is a σB-dependent general stress protein. FEMS Microbiol. Lett. 153:405-409. [DOI] [PubMed] [Google Scholar]
- 6.Arala-Chaves, M. P. 1992. Is prophylactic immunostimulation of the host against pathogenic microbial antigens an adequate strategy of immunoprotection? Scand. J. Immunol. 35:495-500. [DOI] [PubMed] [Google Scholar]
- 7.Arala-Chaves, M. P., A. S. Ribeiro, M. M. G. Santarem, and A. Coutinho. 1986. Strong mitogenic effect for murine B lymphocytes of an immunosuppressor substance released by Streptococcus intermedius. Infect. Immun. 54:543-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arala-Chaves, M. P., A. S. Ribeiro, M. Vilanova, M. T. Porto, M. M. G. Santarem, and M. Lima. 1988. Correlation between B cell mitogenicity and immunosuppressor effect of a protein released by porcine monocytes infected with African swine fever virus. Am. J. Vet. Res. 49:1955-1961. [PubMed] [Google Scholar]
- 9.Arala-Chaves, M. P., T. B. Higerd, M. T. Porto, J. Munoz, J. M. Goust, and H. H. Fudenberg. 1979. Evidence for the synthesis and release of strongly immunosuppressive noncytotoxic substances by Streptococcus intermedius. J. Clin. Investig. 64:871-873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balaban, N., T. Goldkorn, R. T. Nhan, L. B. Dang, S. Scott, R. M. Ridgley, A. Rasooly, S. C. Wright, J. W. Larrick, R. Rasooly, and J. R. Carlson. 1998. Autoinducer of virulence as a target for vaccine and therapy against Staphylococcusaureus. Science 280:438-440. [DOI] [PubMed] [Google Scholar]
- 11.Benner, R., F. Meina, G. M. van der Meulen, and W. B. V. Muiswinkel. 1974. Antibody formation in mouse bone marrow. I. Evidence for the development of plaque forming cells in situ. Immunology 26:247-255. [PMC free article] [PubMed] [Google Scholar]
- 12.Bergmeyer, U. 1974. Methods of enzymatic analysis, vol. 4, p. 2048-2050. Academic Press, New York, N.Y.
- 13.Boyum, A. 1968. Separation of leukocytes from blood and bone marrow. Scand. J. Clin. Lab. Investig. 21:7. [PubMed] [Google Scholar]
- 14.Cantoni, R., M. Branzoni, M. Labò, M. Rizzi, and G. Riccardi. 1998. The MTCY428.08 gene of Mycobacterium tuberculosis codes for NAD+ synthetase. J. Bacteriol. 180:3218-3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cole, M. F., S. D. Hsu, M. J. Sheridan, and H. M. Stiles. 1992. Natural transmission of Streptococcus sobrinus in rats: saliva and serum antibody responses to colonization. Infect. Immun. 60:778-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coutelier, J. P., and J. van Snick. 1985. Isotopically restricted activation of B lymphocytes by lactic dehydrogenase virus. Eur. J. Immunol. 15:250-255. [DOI] [PubMed] [Google Scholar]
- 17.De Soet, J. J., C. van Leveren, A. J. Lammens, M. J. A. Pavicic, C. H. E. Homburg, J. M. Cate, and J. Graaff. 1991. Differences in cariogenicity between fresh isolates of Streptococcus sobrinus and Streptococcus mutans. Caries Res. 25:116-122. [DOI] [PubMed] [Google Scholar]
- 18.Ferreira, P., A. Brás, D. Tavares, M. Vilanova, A. Ribeiro, A. Videira, and M. Arala-Chaves. 1997. Purification, and biochemical and biological characterization of an immunosuppressive and lymphocyte mitogenic protein secreted by Streptococcus sobrinus. Int. Immunol. 11:1735-1743. [DOI] [PubMed] [Google Scholar]
- 19.Ferreira, P., R. Soares, A. Ribeiro, and M. P. Arala-Chaves. 1988. Correlation between specific immunosuppresion and polyclonal B cell activation induced by a protein secreted by Streptococcus mutans. Scand. J. Immunol. 27:549-554. [DOI] [PubMed] [Google Scholar]
- 20.Hirose, H., K. Hirose, E. Isogai, H. Miura, and I. Ueda. 1993. Close association between Streptococcus sobrinus in the saliva of young children and smooth-surface caries increment. Caries Res. 27:292-297. [DOI] [PubMed] [Google Scholar]
- 21.Ho, M. K., S. I. Morse, and A. S. Kong. 1981. Studies on a new lymphocyte mitogen from Bordetella pertussis. J. Exp. Med. 153:75-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horwitz, M. A., B. W. Lee, B. J. Dillon, and G. Harth. 1995. Protective immunity against tuberculosis induced by vaccination with major extracellular proteins of Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 92:1530-1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hughes, K. T., B. M. Olivera, and J. R. Roth. 1988. Structural gene for NAD synthetase in Salmonella typhimurium. J. Bacteriol. 170:2113-2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li, Y., P. W. Caufield, I. R. Emanuelsson, and E. Thornqvist. 2001. Differentiation of Streptococcus mutans and Streptococcus sobrinus via genotypic and phenotypic profiles from three different populations. Oral Microbiol. Immunol. 16:16-23. [DOI] [PubMed] [Google Scholar]
- 25.Lima, M., A. Bandeira, D. Portnoi, A. Ribeiro, and M. P. Arala-Chaves. 1992. Protective effect of a T cell dependent immunosuppressive B cell mitogenic protein (F3′ EP-Si, or p90) produced by Streptococcus intermedius. Infect. Immun. 60:3571-3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loesche, W. J. 1986. Role of Streptococcus mutans in human dental decay. Microbiol. Rev. 50:353-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lopes, L. M., M. A. C. Pereira, S. E. Gerken, and N. Vaz. 1990. Polyclonal B activation of B lymphocytes during experimental infection with Schistosoma mansoni. Parasitology 100:83-91. [DOI] [PubMed] [Google Scholar]
- 28.Lowry, O. H., N. Y. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 29.Magni, G., A. Amici, M. Emanuelli, N. Raffaeli, and S. Ruggieri. 1999. Enzymology of NAD+ synthesis. Adv. Enzymol. Relat. Areas Mol. Biol. 73:135-182. [DOI] [PubMed] [Google Scholar]
- 30.Minoprio, M. P., H. Eisen, L. Forni, M. R. Imperio-Lima, M. Joskowicz, and A. Coutinho. 1986. Polyclonal lymphocyte response to murine Trypanosoma cruzi infection. I. Quantitation of both T and B cell response. Scand. J. Immunol. 24:661-668. [DOI] [PubMed] [Google Scholar]
- 31.Miyoshi-Akiyama, T., J. Zhao, H. Kato, K. Kikuchi, K. Totsuka, Y. Kataoka, M. Katsumi, and T. Uchiyama. 2003. Streptococcus dysgalactiae-derived mitogen (SDM), a novel bacterial superantigen: characterization of its biological activity and predicted tertiary structure. Mol. Microbiol. 47:1589-1599. [DOI] [PubMed] [Google Scholar]
- 32.Mosmann, T. 1983. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 65:55-63. [DOI] [PubMed] [Google Scholar]
- 33.Nessi, C., A. Albertini, M. Speranza, and A. Galizzi. 1995. The outB gene of Bacillus subtilis codes for NAD synthetase. J. Biol. Chem. 270:6181-6185. [DOI] [PubMed] [Google Scholar]
- 34.Newbrun, E. 1983. Cariology, 2nd ed. Williams & Wilkins, Baltimore, Md.
- 35.Pahwa, S., R. Pahwa, C. Saxinger, R. C. Gallo, and R. A. Good. 1985. Influence of the human T-lymphotropic virus/lymphoadenopathy-associated virus on function of human lymphocytes: evidence for immunosuppressive effects and polyclonal B cell activation by banded viral preparations. Proc. Natl. Acad. Sci. USA 82:8198-8202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reina-San-Martin, B., W. Degrave, C. Rougeot, A. Cosson, N. Chamond, A. Cordeiro-Da-Silva, M. Arala-Chaves, A. Coutinho, and P. Minoprio. 2000. A B-cell mitogen from a pathogenic trypanosome is a eukaryotic proline racemase. Nat. Med. 6:890-897. [DOI] [PubMed] [Google Scholar]
- 37.Rizzi, M., C. Nessi, A. Mattevi, A. Coda, M. Bolognesi, and A. Galizzi. 1996. Crystal structure of NH3-dependent NAD+ synthetase from Bacillus subtilis. EMBO J. 15:5125-5134. [PMC free article] [PubMed] [Google Scholar]
- 38.Sambrook, J., and D. W. Russel. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 39.Santarem, M. M. G., M. T. Porto, P. Ferreira, R. Soares, and M. P. Arala-Chaves. 1987. Semi-purification of an immunosuppressor substance secreted by Streptococcus mutans which plays a role in the protection of the bacterium in the host. Scand. J. Immunol. 26:755-761. [DOI] [PubMed] [Google Scholar]
- 40.Schmidt, S., S. Balzer, C. Nullen, M. Schmidt, C. Pohl, A. von Rucker, and T. Klockgether. 2002. Immunomodulatory effects of glatiramer acetate on superantigen- and mitogen-induced T-cell stimulation in vitro. Mult. Scler. 8:307-309. [DOI] [PubMed] [Google Scholar]
- 41.Smith, G. E. 1988. Tooth decay in the developing world: could a vaccine help prevent caries? Perspect. Biol. Med. 31:440-453. [DOI] [PubMed] [Google Scholar]
- 42.Soares, R., P. Ferreira, M. M., Santarem, M. T. Silva, and M. P. Arala-Chaves. 1990. Low T and B cell reactivity is an apparently paradoxical request for murine immunoprotection against Streptococcus mutans. Scand. J. Immunol. 31:361-366. [DOI] [PubMed] [Google Scholar]
- 43.Stack, W. E., M. A. Taubman, T. Tsukuda, D. J. Smith, J. L. Ebersole, and R. Kent. 1990. Dental caries in congenitally athymic rats. Oral Microbiol. Immunol. 5:309-314. [DOI] [PubMed] [Google Scholar]
- 44.Stevens, D. L., D. B. Salmi, E. R. McIndoo, and A. E. Bryant. 2000. Molecular epidemiology of nga and NAD glycohydrolase/ADP-ribosyltransferase activity among Streptococcus pyogenes causing streptococcal toxic shock syndrome. J. Infect. Dis. 182:1117-1128. [DOI] [PubMed] [Google Scholar]
- 45.Sun, J., S. Wanda, A. Camilli, and R. Curtiss III. 1994. Cloning and DNA sequencing of the dextranase inhibitor gene (dei) from Streptococcus sobrinus. J. Bacteriol. 176:7213-7222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Swat, W., M. Dessing, H. von Boehmer, and P. Kisielow. 1993. CD69 expression during selection and maturation of CD4+8+ thymocytes. Eur. J. Immunol. 23:739-744. [DOI] [PubMed] [Google Scholar]
- 47.Tavares, D., A. Salvador, P. Ferreira, and M. P. Arala-Chaves. 1993. Immunological activities of a Candida albicans protein which play an important role in the survival of the microorganism in the host. Infect. Immun. 61:1881-1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tavares, D., P. Ferreira, M. Vilanova, A. Videira, and M. Arala-Chaves. 1995. Immunoprotection against systemic candidiasis in mice. Int. Immunol. 7:785-796. [DOI] [PubMed] [Google Scholar]
- 49.Uchiyama, T., and D. M. Jacobs. 1978. Modulation of immune response to bacterial LPS: multifocal effects of LPS induced suppression of the primary antibody response to a T dependent antigen. J. Immunol. 121:2340-2346. [PubMed] [Google Scholar]
- 50.Unnikrishnan, M., D. M. Altmann, T. Proft, F. Wahid, J. Cohen, J. D. Fraser, and S. Sriskandan. 2002. The bacterial superantigen streptococcal mitogenic exotoxin Z is the major immunoactive agent of Streptococcus pyogenes. J. Immunol. 169:2561-2569. [DOI] [PubMed] [Google Scholar]
- 51.Videira, A., M. Tropschug, E. Wachter, H. Schneider, and S. Werner. 1990. Molecular cloning of subunits of complex I from Neurospora crassa. Primary structure and in vitro expression of a 22-kDa polypeptide. J. Biol. Chem. 265:13060-13065. [PubMed] [Google Scholar]
- 52.Yokoyama, W. M., F. Koning, P. J. Kehn, G. M. Pereira, G. Stinge, J. E. Coligan, and E. M. Shevach. 1988. Characterization of a cell surface-expressed disulphide-linked dimer involved in murine T cell activation. J. Immunol. 141:369-376. [PubMed] [Google Scholar]
- 53.Zalkin, H. 1993. NAD synthetase. Methods Enzymol. 113:297-302. [DOI] [PubMed] [Google Scholar]
- 54.Zalkin, H. 1993. The amidotransferases. Adv. Enzymol. Relat. Areas Mol. Biol. 66:203-309. [DOI] [PubMed] [Google Scholar]