Abstract
The inhibition of vascular endothelial growth factor (VEGF) signaling with antibodies or small molecules achieves clinical benefits in diverse solid malignancies. Nonetheless, therapeutic effects are usually not sustained, and most patients eventually succumb to progressive disease, indicating that anti-angiogenic strategies require additional optimization. Vaccination with lethally irradiated, autologous tumor cells engineered to secrete granulocyte-macrophage colony stimulating factor (GM-CSF) and antibody blockade of cytotoxic T lymphocyte associated antigen-4 (CTLA-4) trigger a tumor vasculopathy in some long-term responding subjects. These reactions are characterized by disrupted tumor blood vessels in association with lymphocyte and granulocyte infiltrates and zonal areas of ischemic tumor necrosis. However, the mechanisms underlying this immune mediated destruction of the tumor vasculature remain to be clarified. Here, we show that GM-CSF secreting tumor cell vaccines and CTLA-4 blockade elicit a functionally important humoral reaction against multiple angiogenic cytokines. Antibodies to angiopoietin-1 and -2 block Tie-2 binding, downstream signaling, endothelial cell tube formation, and macrophage chemotaxis. Antibodies to macrophage inhibitory factor (MIF) attenuate macrophage Tie-2 expression and matrix metalloproteinase-9 (MMP-9) production. Together, these results delineate an immunotherapy induced host response that broadly targets the angiogenic network in the tumor microenvironment.
Keywords: Immunotherapy, angiogenesis, GM-CSF, CTLA-4, angiopoietin
Introduction
Substantial evidence indicates that the angiogenic switch plays a decisive role during tumor development (1). Since oxygen and other essential metabolites diffuse from the existing vasculature for only a limited distance, progressive tumor growth and systemic dissemination require the acquisition of additional blood supplies. Whereas several mechanisms may contribute to the angiogenic switch, the generation of new blood vessels from pre-existing vascular structures is the most intensively studied (2). Angiogenesis is now understood to reflect the integration of multiple pro- and anti-angiogenic factors and to involve the concerted activities of not only vascular elements, but also myeloid cell populations (3).
Among the components of the angiogenic network, VEGF-A was the first to be validated as a target for cancer therapy (4). Antibodies and small molecule inhibitors of VEGF function mediate anti-tumor activity alone or in combination with chemotherapy in carcinomas of the colon, kidney, lung, breast, and liver, but the overall magnitude of the benefit is modest, and most patients still succumb to progressive disease (5, 6). Several factors might limit the efficacy of VEGF-A targeted treatments, including the activation of other soluble or cellular angiogenic factors and a switch to non-angiogenic modes of accessing a vascular supply (7). The relative importance of these pathways to therapeutic resistance in patients, however, remains to be determined.
Tumor pathogenesis may involve an impaired wound healing response (8). Since tissue damage normally elicits a coordinated immune and vascular reaction, immunologic mechanisms might be able to modulate tumor angiogenesis. Indeed, Coley’s toxins, one of the first immunotherapies to be developed, evoke hemorrhagic tumor necrosis through a cascade of cytokines and cells that perturb the tumor vasculature (9, 10). More recently, vaccination against VEGF, VEGFR, and tumor-associated macrophage gene products was shown to elicit protective tumor immunity in several murine models (11–13).
In this context, we reported that vaccination with lethally irradiated, autologous tumor cells engineered to secrete GM-CSF and antibody blockade of CTLA-4 engendered a coordinated cellular and humoral response that effectuated clinically significant tumor destruction in some patients with advanced solid malignancies (14–18). Metastases resected following therapy revealed the stimulation of dense intra-tumoral infiltrates composed of CD4+ and CD8+ T cells and antibody-producing B cells in long-term responding patients. Disrupted tumor blood vessels were also observed in association with lymphocyte and granulocyte infiltrates and zonal areas of ischemic tumor necrosis. These findings suggested that multiple immune effector mechanisms might participate in tumor destruction.
Through antibody based screening of tumor-derived cDNA expression libraries, we previously characterized several tumor-associated gene products that were recognized by high titer antibodies and cytotoxic T cells, and were linked with therapy induced tumor necrosis (14). Here, we employed a similar approach to uncover a potent humoral reaction against multiple angiogenic cytokines.
Materials and Methods
Clinical protocols
The Phase I trials of vaccination with lethally irradiated, autologous tumor cells engineered to secrete GM-CSF in advanced melanoma and non-small cell lung carcinoma patients have been described (15, 16, 19). The Phase I trials of the fully human anti-CTLA-4 blocking monoclonal antibody (Ipilimumab®) in previously vaccinated melanoma and ovarian carcinoma patients have also been reported (17, 18). All clinical protocols received approval from the Dana-Farber/Harvard Cancer Center Institutional Review Board, the Food and Drug Administration, and the Recombinant DNA Advisory Committee.
cDNA library construction and screening
A cDNA expression library was generated from B16 cells employing previously described methods (20). In brief, total RNA was isolated using guanidine isothiacyanate, mRNA purified over oligo-dT cellulose columns, and cDNA synthesized with Superscript II Reverse Transcriptase (RT, Invitrogen). The cDNA was cloned into the Lambda Zap vector and the library screened according to the manufacturer’s instructions (ZAP-cDNA Gigapack III Gold cloning and picoBlue Immunoscreening kits, Stratagene). K008 serum diluted 1:500 in TBST was pre-cleared against a negative control phage expression library overnight and then used for screening. Positive clones were developed with mouse HRP-conjugated anti-human antibodies (Zymed) at a 1:2000 dilution and then isolated through three rounds of purification. Phagemids were excised, purified (Qiagen kit), and sequenced by the Dana-Farber Cancer Institute Core Facility. Inserts were compared using BLAST search protocols to the Entrez Nucleotide database maintained by the NCBI.
Anti-cytokine antibodies
For immunoblotting, 250 ng of recombinant VEGF-A and bFGF (R&D Systems) were run on a 10% reducing SDS polyacrylamide gel, transferred to a nylon membrane, probed with patient sera (1:100 dilution in 2% milk) followed by a HRP-conjugated goat anti-human IgG (Zymed) at a 1:2000 dilution, and developed with a Chemiluminescence reagent (Perkin-Elmer). Control anti-VEGF-A and bFGF antibodies (R&D Systems) were used at a 1:2000 dilution.
Anti-cytokine antibodies were measured by coating ELISA plates (Nunc) overnight at 4°C with recombinant human angiopoietin-1, -2, MIF, bFGF, PLGF, VEGF-A, VEGF-B, VEGF-C, and VEGF-D protein (R&D Systems) dissolved in a carbonate buffer, pH 9.6. Next, the wells were blocked overnight at 4°C with 2% milk, washed, and then incubated in duplicate or triplicate with 100 μl of patient or donor sera diluted 1:100 in 2% milk overnight at 4°C. A goat anti-human IgG conjugated to alkaline-phosphatase (Jackson Laboratories) was added at room temperature, the plate developed with pNPP substrate (Sigma-Aldrich), and the absorbance at 450 nm determined.
In some experiments, immunoglobulins were enriched from plasma through an initial clearing on glass beads (Sigma) followed by treatment with recombinant Protein G agarose (Invitrogen), according to the manufacturer’s protocol. For absorption studies, the clarified plasma was incubated with plate-bound angiopoietin-1 or -2 overnight at 4°C and then purified with Protein G agarose. Absorption was repeated until the anti-angiopoietin titer became undetectable in ELISA.
Angiopoietin/Tie-2 binding
ELISA plates were coated with 20 ng per well of angiopoietin-1 or -2 in a carbonate buffer overnight at 4°C. After washing and blocking overnight with milk, sera diluted 1:7 in 2% milk were added for 8 hours at room temperature, and then 7.5 ng of recombinant Tie2-Fc (R&D Systems) in 2% milk was added overnight at room temperature (final sera dilution 1:10). After washing, Tie-2 specific binding was determined with an anti-Tie-2 mAb (R&D Systems), a HRP conjugated anti-mouse IgG, and developing reagents. Absorbance at 450 nm was measured. In some experiments, Tie-2-Fc was first coated on the plate and the specific binding of angiopoietin-1 and -2 was determined using comparable procedures.
Tube forming assays
Human umbilical vein endothelial cells (HUVECs) were obtained from Cambrex and early passage cells (<12) were used for all experiments. Tube forming assays were conducted using 10,000 cells/well maintained in Ham F12 media containing 15% FBS in 96-well cell culture plates and a Fibrin Gel In Vitro Angiogenesis Assay Kit (Chemicon International) according to the manufacturer’s instructions. At least two replicate wells were used for each experimental condition. In some experiments, 250 ng of recombinant angiopoietin-1, -2, or MFG-E8 were added to the culture media, and patient sera were used at a 1:10 dilution. Tube formation was assayed by pattern recognition according to manufacturer’s instructions, using at least 3 random view-fields per well by an observer who was blinded to the treatment conditions. Values were assigned as follows: 0, cells isolated or in a sheet-like monoloayer; 1, cells begin to migrate and align themselves; 2, capillary tubes visible. No sprouting; 3, sprouting of new capillary tubes visible; 4, closed polygons begin to form; 5, complex mesh like structures develop.
Angiopoietin induced migration
Migration assays were performed with transwells containing 16-μm pore-size inserts (Corning, Corning, NY) coated with Basement Membrane Extract Cultrex (BD Biosciences). Monocytes were isolated from peripheral blood mononuclear cells using CD14 microbeads (Miltenyi Biotech) and cultured for five days with M-CSF (20 μg/mL added on days 0, 2, 4). Recombinant angiopoietin-1 or angiopoietin-2 (100 ng/mL) and patient sera diluted 1:100 were placed in serum-free RPMI medium (500 μL) in the bottom compartment of the chamber, and 100 μL of monocytes (104 cells/mL) was added to the top compartment. The chambers were incubated at 37°C in humidified air with 5% CO2 for 24 hours. Migrated cells were labeled with 5 μg/mL calcein-AM (Molecular Probes) in RPMI at 37°C for 1 hour and counted under a fluorescence microscope. The number of cells migrated in the absence of angiopoietin-1/2 (-) was used as a reference value and set to 1. Results are expressed as a migration index relative to the reference value. Spontaneous migration was typically 1–6% of input cell number.
Phospho-ERK-1/2 activation
Subconfluent HUVECs were serum starved in 1% BSA supplemented Ham’s F-12 medium for six hours, treated with angiopoietins and/or patient sera as indicated, washed twice in ice-cold PBS, and lysed in Cell Signaling Technology buffer supplemented with 1 mM phenylmethylsulfonyl fluoride. Immunblotting was performed as above using antibodies to phospho-p44/42 ERK-1/2 (Cell Signaling). The PathScan(R) Phospho-p44/42 MAPK (Thr202/Tyr204) Sandwich ELISA Kit (Cell Signaling Technology) was also used according to the manufacturer’s instruction.
MIF induced responses
Human monocytes obtained as above were incubated for 48 hours (2.5 × 105/well in 12 well plates) with recombinant MIF (200 ng/mL; R&D Systems) with or without MEL15 sera (10%) in serum-free RPMI medium. The cells were stained with PE-conjugated anti-Tie2 mAb (R&D Systems), and analyzed with a FW501 flow cytometer (Beckman-Coulter) and FlowJo software (Tree Star). In some experiments, monocytes were treated for one day with recombinant MIF in the presence of anti-MIF neutralizing antibodies (20 μg/mL: R&D Systems), control IgG, or MEL15 sera diluted 1:100. MMP-9 levels were measured using ELISA systems according to the manufacture’s instruction (R&D Systems)
Statistics
The exact Wilcoxon rank sum test was used to compare the vaccinated and control subjects for antibodies to angiopoietin-1 and -2, and Tie-2/angiopoietin binding. The t-test was used to compare MMP-9 levels in response to MIF.
Results
Vaccination stimulated blocking antibodies to angiogenic cytokines
In earlier work aimed at identifying antigens associated with immune mediated tumor destruction, we screened cDNA expression libraries prepared from densely infiltrated metastases with sera from patients who achieved durable clinical responses to vaccination with lethally irradiated, autologous tumor cells engineered to secrete GM-CSF alone or with subsequent CTLA-4 antibody blockade. These experiments established the immunogenicity of multiple tumor-associated gene products, including ATP6S1, melanoma inhibitor of apoptosis protein, opioid growth factor receptor, MHC class I chain-related protein A, focal adhesion kinase, AKT-2, upstream binding factor (UBF), and HIDA (20–24). Interestingly, ATP6S1 was also found to elicit high titer antibodies in mice immunized with irradiated, GM-CSF secreting B16 melanoma cells, suggesting that some tumor antigens might be recognized in both human and murine systems (20). Consistent with this possibility, a screen of a B16 cDNA expression library with sera from mice vaccinated with GM-CSF secreting B16 cells yielded MIDA and UBF-2, the murine orthologs of the human antigens HIDA and UBF (unpublished findings).
To explore further the idea that vaccine targets might show conserved immunogenicity, we screened a B16 murine melanoma cDNA expression library with sera from long term responding melanoma patient K008. This subject underwent harvest of a pulmonary lesion for vaccine manufacture, and harbored a soft tissue metastasis at the time of initiating therapy with irradiated, autologous, GM-CSF secreting tumor cells. After the fifth immunization, a subcutaneous nodule in K008 was noted to become hemorrhagic, which reflected the induction of a tumor vasculopathy with brisk lymphocyte and granulocyte infiltrates, as revealed through pathologic examination of the resected lesion (16). Without any additional treatment, K008 remained disease free for fourteen years following receipt of the six vaccinations.
Sera obtained from K008 after completion of immunization detected 13 gene products in the B16 library, and these included proteins linked to transcription, translation, DNA repair, protein homeostasis, the cytoskeleton, and the surface membrane (Supplemental Table 1). Functionally, these targets were similar to antigens identified in previous studies using the human cDNA expression libraries. However, the B16 screen also yielded VEGF-A, a potent angiogenic factor with a key role in tumor pathogenesis (25), and we elected to investigate this target further. Consistent with the high sequence conservation of the cytokine between mice and humans, K008 sera also recognized recombinant human VEGF-A protein in immunoblotting (Figure 1A). Antibodies to VEGF-A were not observed in several healthy donors, whereas K008 sera failed to react with basic fibroblast growth factor (bFGF), underscoring the specificity of the anti-VEGF-A response. K008 sera did not detect VEGF-A in an ELISA format, precluding more detailed quantitative analysis. The reasons for this discordance in reactivity remain to be clarified, but might include differences in the recognition of denatured linear versus conformation epitopes.
Figure 1.
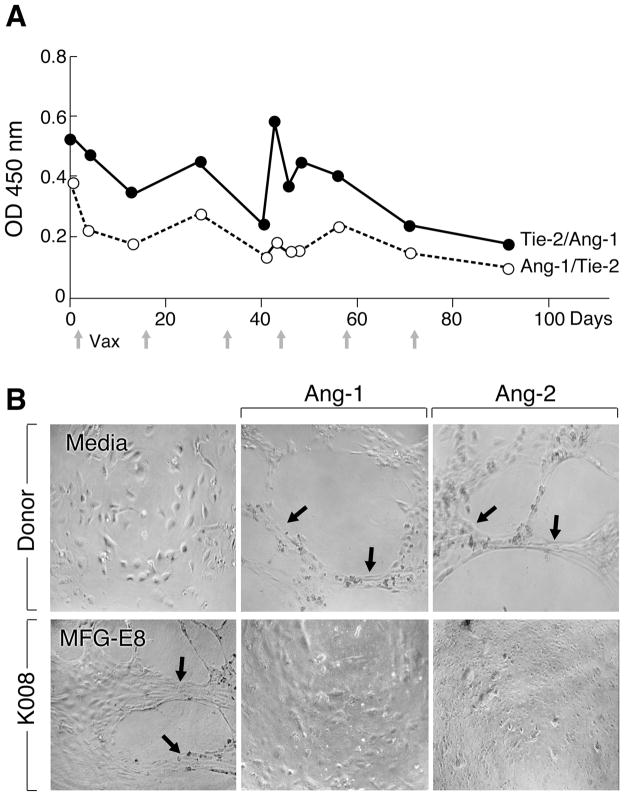
Vaccinated melanoma patient K008 developed antibodies to multiple angiogenic cytokines. (A). Sera from K008, but not healthy donors (n=3) recognize VEGF-A, but not bFGF by immunoblotting (sera at 1:100). Control anti-cytokine antibodies are shown for comparison. (B). Longitudinal analysis of antibodies to VEGF family members in K008. Antigen specific IgG levels were determined with an ELISA (sera at 1:100). Arrows denote vaccinations. Day 0 is pre-vaccination. (C). Vaccination stimulated potent humoral reactions to angiopoietin-1 and -2 in K008. A subcutaneous nodule developed hemorrhagic necrosis after the fifth immunization. Day 0 is pre-vaccination.
As VEGF-A is closely related to several other proteins that contribute to angiogenesis, we investigated whether K008 sera recognized other human VEGF family members (Figure 1B, C). An ELISA revealed that K008 harbored antibodies to VEGF-B at the time of study enrollment. While these varied somewhat during the course of immunization, a clear association with therapy was not evident. Antibodies to VEGF-C and placental growth factor (PLGF) were of a relatively low titer, whereas no reactivity against VEGF-D was observed. Together, these humoral reactions did not appear sufficient to account for the therapy induced tumor vasculopathy.
Since angiogenesis involves the coordinated activities of several soluble proteins, we expanded our survey to include angiopoietin-1 and -2. These growth factors cooperate with VEGF during tumor progression, but their precise functions in endothelial cell and pericyte biology, and the signaling pathways triggered after cognate binding to the Tie-2 receptor tyrosine kinase remain under active study (26, 27). Intriguingly, vaccination increased antibodies to both angiopoietin-1 and -2 in K008, and these peaked concurrently with the development of hemorrhage in the subcutaneous nodule (Figure 1C). The maximal reactions were detectable in sera dilutions of 1:10,000.
To explore whether the anti-angiopoietin antibodies modulated cytokine activity, we established an ELISA to measure ligand-receptor interactions in vitro. Recombinant Tie-2 protein was coated on the plate and the ability of K008 sera to inhibit cognate angiopoietin-1 binding was then determined (Figure 2A). The vaccine stimulated increase in antibody titer was associated with diminished angiopoietin-1/Tie-2 binding. Similar results were obtained when angiopoietin-1 was initially applied to the dish and the binding of Tie-2 was subsequently evaluated. Additional studies showed that K008 sera mediated comparable inhibition of angiopoietin-2/Tie-2 interactions as a function of immunization (not shown).
Figure 2.
Vaccine induced antibodies to angiopoietin-1 and -2 manifest blocking activity. (A). Post-vaccination K008 sera inhibit the binding of Tie-2 and angiopoietin-1 as measured with an ELISA (sera at 1:10). Day 0 is pre-vaccination. Full time-course studies were performed twice with similar results, whereas high and low blocking samples were analyzed two additional times, with equivalent findings. (B). Sera from K008, but not healthy donors inhibit angiopoietin-1 and -2 induced tube formation with HUVECs. K008 sera failed to block MFG-E8 stimulated tube formation. This experiment was performed two times with similar results. Pre-vaccination sera from K008 were not available for this assay. Representative high power fields are shown. Tube forming scores (averages of six replicates) were: donor (media) 0, (angiopoetin-1) 3.5, (angiopoietin-2) 3; K008 (MFG-E8) 2.67, (angiopoietin-1) 0.83, (angiopoietin-2) 0.33.
To examine whether the ability of the anti-angiopoietin antibodies to block Tie-2 binding in vitro attenuated cytokine biologic activity, we established tube formation assays using HUVECs. While minimal tube formation was elicited in the absence of endothelial growth factor supplementation, both angiopoietin-1 and -2 stimulated responses, which were not affected by the addition of sera from healthy donors lacking anti-angiopoietin antibodies (Figure 2B). Sera obtained from K008 after vaccination markedly inhibited angiopoietin-1 and -2 induced tube formation, but did not influence tube formation elicited with milk fat globule EGF-8, a protein highly expressed in melanomas that promotes angiogenesis through binding αvβ3 and αvβ5 integrins on endothelial cells (28). Unfortunately, insufficient sera from K008 prior to vaccination were available for evaluation in the tube formation assays. Nonetheless, because previous work in murine models showed that antagonizing angiopoietin function stimulated tumor destruction (29, 30), our findings raise the possibility that anti-angiopoietin-1 and -2 antibodies in K008 might contribute to the tumor vasculopathy.
Anti-angiopoietin antibodies in vaccinated cancer patients
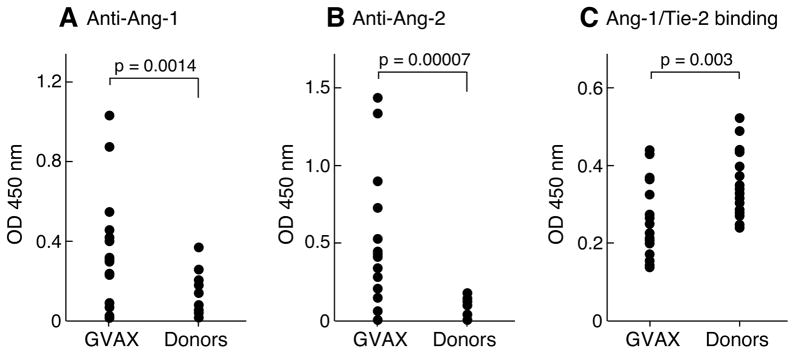
To determine whether other patients mounted humoral reactions to angiopoietins, we tested a panel of stage IV melanoma, non-small cell lung carcinoma, and ovarian carcinoma patients that were enrolled on our Phase I clinical trials of vaccination with lethally irradiated, autologous tumor cells engineered to secrete GM-CSF (Figure 3A-C, Supplemental Table 2). Immunized subjects harbored higher titers of antibodies to angiopoietin-1 and -2 compared to healthy donors (anti-angiopoietin-1 antibodies, vaccinated versus donors, p=0.0014; anti-angiopoietin-2 antibodies, vaccinated versus donors, p=0.00007). These humoral reactions were evident in sera dilutions ranging from 1:400 to 1:12,500 (Supplemental Table 3). The sera from immunized patients also manifested greater blockade of angiopoietin-1/Tie-2 interactions in vitro compared to the donors (vaccinated versus donors, p=0.003). Although angiopoietin-1 and -2 share 55% sequence identity, one subject (MEL15) mounted humoral responses to angiopoietin-2 alone, indicating that unique determinants may be targeted in some patients. Further studies are required, however, to delineate the specific epitopes evoking immune recognition in the vaccinated cohort.
Figure 3.
Vaccinated patients harbor neutralizing antibodies to angiopoietin-1 and -2. (A, B). Anti-angiopoietin-1 and -2 IgG antibodies were measured with an ELISA (sera 1:100) in normal donors (n=16) and vaccinated patients (n=16). (C). Antibodies in vaccinated patients block the binding of angiopoietin-1 and Tie-2.
Longitudinal analysis revealed that immunization enhanced antibody titers in eleven patients, and these ranged from three to thirty fold increases (Supplemental Table 4). Eight of the subjects with enhanced antibody responses achieved prolonged survival as a function of treatment, which ranged from a minimum of 4.5 through 14 years. Based on these favorable clinical courses, we wondered whether the humoral reactions to angiopoietins might be functionally active.
Two patients with sufficient sera available were selected for more detailed characterization. M30 is an advanced melanoma patient with visceral and brain metastases who was treated with vaccination, brain irradiation, and DTIC and achieved a complete response that is ongoing at nine years following vaccination. L19 is a stage IV non-small cell lung carcinoma patient who was surgically rendered without evidence of disease following vaccination and remains disease free ten years after study enrollment.
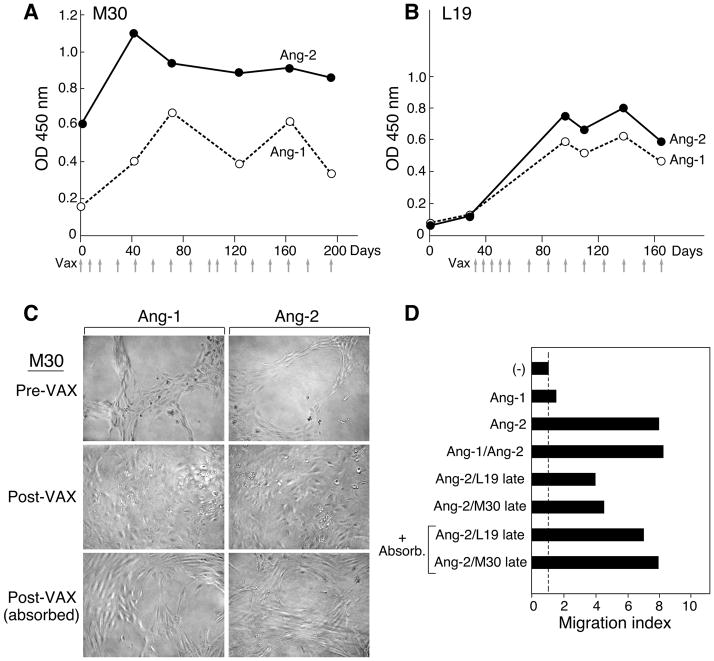
Both M30 and L19 displayed increases in antibodies to angiopoietin-1 and -2 as a consequence of immunization (Figure 4A, B). Late in the treatment course, however, antibody titers declined slightly; this might reflect a reduction in tumor burden, consistent with the clinical course, or less likely a waning of the immune response. Post-vaccination sera from M30 attenuated angiopoietin-1 and -2 driven endothelial cell tube formation (Figure 4C), consistent with the results obtained for K008, whereas pre-treatment sera failed to. Moreover, the blocking activity was attenuated by absorption of the sera with recombinant cytokine, demonstrating that anti-angiopoietin antibodies, and not other factors present in the sera, mediated the inhibition. In contrast, sera obtained from L19 after vaccination elicited tube formation in the absence of supplemental growth factors (not shown), which precluded application of this assay to monitor functional activity.
Figure 4.
Vaccination stimulated functional antibodies to angiopoietin-1 and -2. (A). Longitudinal analysis of metastatic melanoma patient M30 (sera 1:100). Day 0 is pre-vaccination. (B). Longitudinal analysis of metastatic non-small cell lung carcinoma patient L19. Day 0 is pre-vaccination. (C). Post-vaccination sera from M30 specifically blocks angiopoietin-1 and -2 induced tube formation. Serum was absorbed against recombinant cytokine as indicated. This experiment was performed twice with similar results. Tube forming scoring (average of two replicates): Pre-VAX (angiopoietin-1 and -2) 3.5, post-VAX (angiopoietin-1 and -2) 0, post-VAX absorbed (angiopoietin-1 and -2) 1.5. (D). Post-vaccination sera from M30 and L19 specifically block angiopoietin-2 stimulated migration of Tie-2 expressing monocytes. Cell migration in response to cytokine as compared to media is depicted. Serum was absorbed against recombinant cytokine as indicated. Similar results were observed in a second experiment.
However, recent studies have highlighted a key role for Tie-2 expressing macrophages in tumor angiogenesis, and demonstrated that these cells migrate towards angiopoietin-2 in vitro (31–33). Consistent with this work, we found that angiopoietin-2, but not angiopoietin-1 displayed chemotactic activity towards Tie-2 expressing monocytes obtained from normal donor peripheral blood (Figure 4D). Furthermore, post-vaccination sera obtained from both L19 and M30 diminished angiopoietin-2 induced monocyte chemotaxis, and this inhibition was decreased with angiopoietin-2 absorption.
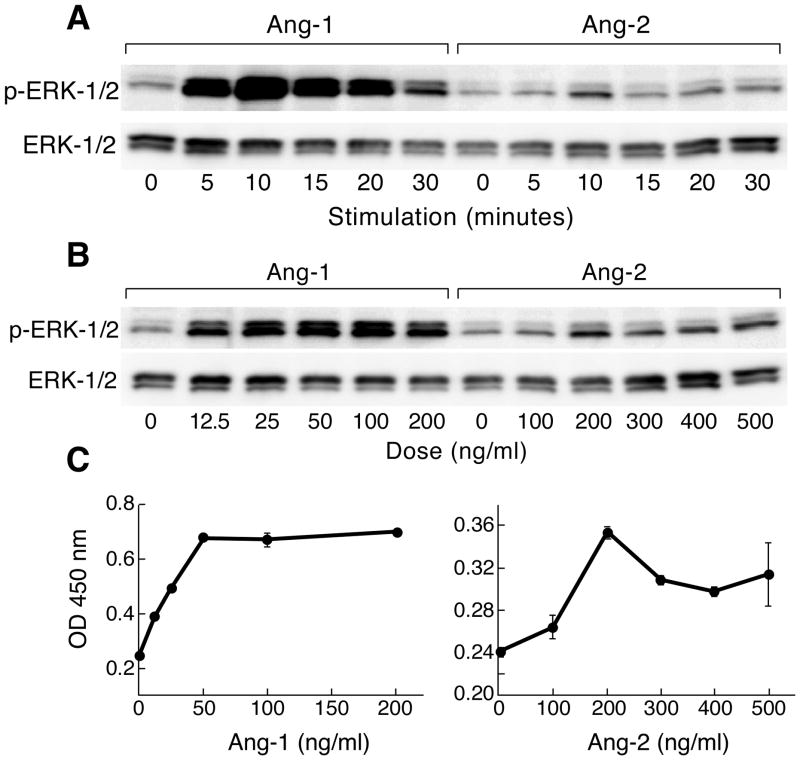
To examine the mechanisms underlying the ability of L19 and M30 sera to decrease angiopoietin-1 and-2 responses, we characterized downstream Tie-2 signaling. HUVECs treated with recombinant angiopoietin-1 and -2 showed rapid ERK phosphorylation, which was maximal at ten minutes (Figure 5A). Peak levels were observed with 50 ng/ml of angiopoietin-1 and 200 ng/ml of angiopoietin-2 (Figure 5B, C). Using these optimized conditions, we found that post-vaccination, but not pre-vaccination sera from both L19 and M30 inhibited angiopoietin-1 and-2 triggered ERK phosphorylation (Figure 6A, B). Moreover, the blocking activity was decreased with cytokine absorption, underscoring the specificity of the response.
Figure 5.
Angiopoetin-1 and -2 trigger ERK phosphorylation. (A). HUVECs were treated with 200 ng/ml of angiopoietin-1 or 500 ng/ml angiopoietin-2 for varying times. Cell lysates were analyzed for phospho-ERK with immunoblotting. (B). HUVECs were treated for ten minutes with varying concentrations of angiopoietin-1 and -2, and then phospho-ERK was assayed with immunoblotting. (C). Phospho-ERK levels from (B) were analyzed with an ELISA.
Figure 6.
Vaccine induced antibodies block angiopioetin-1/2 signaling. HUVECs were treated for ten minutes with 50 ng/ml of angiopoietin-1 or 200 ng/ml of angiopoietin-2 in the presence of PBS or 1 mg/ml of purified immunoglobulins from pre- or post-vaccination sera (with and without absorption with recombinant cytokine). Cell lysates were assayed for phospho-ERK with an ELISA. (A). Stage IV melanoma patient M30. (B). Stage IV lung carcinoma patient L19. Similar results were observed in two experiments.
Therapy induced antibodies to MIF antagonize angiogenesis
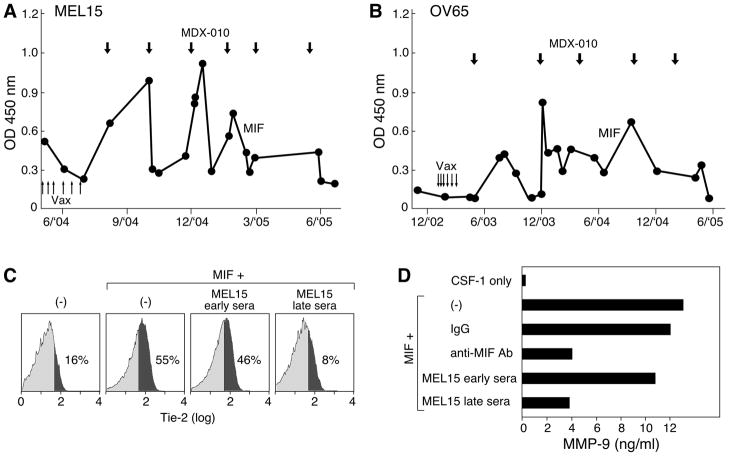
Some patients who manifested prolonged survival after immunization did not generate anti-angiopoietin reactions, suggesting that other pathways may contribute to therapeutic activity. Moreover, three patients who developed increased anti-angiopoietin antibody titers as a function of vaccination did not show clinical benefits, indicating that these humoral responses may not be sufficient for tumor destruction (Supplemental Table 2). In this context, our earlier investigations of MEL15, a stage IV melanoma patient who achieved an ongoing partial response (50+ months) to irradiated, autologous GM-CSF secreting tumor cell vaccines followed by CTLA-4 blockade identified MIF as a target of high titer antibodies (23). This cytokine also contributes to tumor blood vessel formation, as illustrated by the reduction in intestinal polyp associated angiogenesis following the introgression of a null MIF allele into mice harboring a mutant adenomatous polyposis coli gene (34). Longitudinal analysis of MEL15 revealed that immunotherapy engendered an increase in anti-MIF antibody titers (Figure 7A). Furthermore, OV65, an advanced ovarian carcinoma patient who manifested a striking clinical response to the combination treatment, similarly showed augmented anti-MIF humoral reactions (Figure 7B). The anti-MIF antibody titers in both of these subjects fluctuated in association with the periodic infusion of CTLA-4 blockade, which raises the possibility of a dynamic interplay of anti-tumor humoral reactions and immune regulation. Three additional melanoma, myeloid leukemia, and lung cancer patients also generated antibodies to MIF as a function of treatment (not shown).
Figure 7.
Immunotherapy stimulated blocking antibodies to MIF. (A). Longitudinal analysis of anti-MIF antibodies in metastatic melanoma patient MEL15 (sera 1:500). Upward arrows denote vaccinations, downward arrows indicate anti-CTLA-4 mAb (MDX-010) infusions. (B). Longitudinal analysis of stage IV ovarian carcinoma patient OV65. (C). MEL15 sera obtained after immunotherapy inhibited MIF induced Tie-2 expression on monocytes. (D). Late MEL15 sera attenuated MIF stimulated MMP-9 production. Similar results were observed in three experiments; p=0.0314 for MEL15 early versus late sera.
Since MEL15 and OV65 manifested coordinated humoral reactions to MIF and the angiopoietins, we wondered whether these cytokines might functionally interact in an angiogenic network. Indeed, MIF enhanced the expression of Tie-2 on peripheral blood monocytes cultured with colony stimulating factor-1, whereas this activity was blocked with sera obtained from MEL15 after immunotherapy (Figure 7C). MEL-15 late sera also diminished the ability of MIF to stimulate MMP-9 production from monocytes (35), at levels comparable to the addition of an anti-MIF monoclonal antibody (Figure 7D). Together, these results raise the possibility that the therapy induced anti-MIF humoral responses might cooperate with the anti-angiopoietin antibodies to perturb tumor angiogenesis.
Discussion
The detailed analysis of patients achieving sustained clinical benefits from lethally irradiated, autologous GM-CSF secreting tumor cell vaccines and CTLA-4 antibody blockade affords a rich opportunity to delineate mechanisms of therapeutic immunity and identify antigens associated with immune mediated tumor destruction. Previous investigations revealed the development of dense intra-tumoral T and B cell infiltrates that accomplished extensive tumor necrosis, and uncovered several tumor-associated gene products that served as targets for these reactions (14). Nonetheless, a tumor vasculopathy linked with zonal areas of ischemic tumor necrosis was also observed, yet the basis for this immune targeting of tumor blood vessels was not evident.
Since many key principles of irradiated, GM-CSF secreting tumor cell vaccines are conserved between murine and human systems (36), we screened a B16 murine melanoma cDNA expression library with sera from a long-term surviving melanoma patient who manifested hemorrhagic tumor necrosis as a function of vaccination. Among the gene products identified through this approach was VEGF-A, a validated target for anti-angiogenic therapies (4). Further studies established that vaccination stimulated a coordinated humoral reaction to angiopoietin-1 and -2, and MIF, whereas these antibodies blocked the ability of the cytokines to promote angiogenesis in several in vitro assays. Together, these results support the idea that humoral immunity might contribute to disruption of the angiogenic network in vivo and provoke ischemic tumor necrosis. Interestingly, long-term follow-up of the patients has not revealed any toxicities that might be related to the anti-angiogenic responses, suggesting that a favorable therapeutic index may have been achieved.
Our finding that sustained responses to immunotherapy may involve neutralizing antibodies to diverse angiogenic cytokines suggests that concurrently targeting multiple arms of the angiogenic network might enhance clinical efficacy. In this context, therapeutic agents that target the angiopoietins demonstrate considerable promise in model systems and early clinical trials (29, 37, 38), whereas combined angiopoietin and VEGF inhibition displays synergistic effects in some experiment settings (39). Moreover, we showed here that blockade of the angiopoietins and MIF inhibited not only the direct stimulation of endothelial cells, but also the recruitment and activation of pro-angiogeneic myeloid elements.
The pathways underlying the generation of antibodies that antagonize the function of soluble angiogenic factors remain to be clarified. Analysis of sera samples obtained prior to vaccination revealed the presence of nascent antibody responses, but these manifested limited blocking activity. Thus, while tumor development may provoke immune recognition, the endogenous reactions appear to be insufficient to impede disease progression. The autologous tumor cell vaccines employed in our clinical trials derive from single cell suspensions of metastatic lesions, and thus include a mixture of vascular components, stromal cells, and cancer cells. The engineered local production of GM-CSF enhances the capture and presentation of these elements by recruited dendritic cells, which likely contributes to the breach of tolerance to some self-antigens. Consistent with this idea, experiments in murine models illustrate that vaccination can engender protective immunity against angiogenic moieties (11–13). Nevertheless, the selection for antibodies with blocking activity in cancer patients is surprising. Whether humoral immunity plays a more general role in tissue homeostasis, perhaps during wound healing, is an intriguing issue that warrants further investigation.
In addition to the impact on tumor blood vessel development, the generation of neutralizing antibodies to angiogenic cytokines might potentiate tumor immunity. Accumulating evidence indicates that VEGF, angiopoietins, and MIF attenuate anti-tumor lymphocyte cytotoxicity through modulating dendritic cells, macrophages, NK cells, and FoxP3 expressing regulatory T cells (40). An inhibition of immunosuppressive circuits with therapy induced anti-cytokine antibodies might allow for enhanced intra-tumoral infiltration with activated CD4+ and CD8+ effector T cells. The increase in tumor cell killing might in turn establish a more immunogenic environment for the presentation of angiogenic factors, thereby creating a feed-forward amplification loop that coordinates cellular and humoral pathways for further tumor destruction. Such interplay of host reactivity and angiogenesis suggests that immunotherapies might be effectively combined with anti-angiogenic treatments to improve patient outcomes.
Supplementary Material
Acknowledgments
Supported by the HHMI (J.S.), Uehara Memorial Foundation, the Kanae Foundation for the Promotion of Medical Sciences (M.J.), CA78378, CA111506, AI29530, the Leukemia & Lymphoma Society, the Melanoma Research Alliance, and the Research Foundation for the Treatment of Ovarian Cancer (G.D.).
We thank the Dana-Farber Cell Manipulation Core Facility for help with the patient samples.
Footnotes
There are no conflicts of interest.
References
- 1.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;863:353–64. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 2.Carmeliet P, Jain R. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–57. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 3.Shojaei F, Zhong C, Wu X, Yu L, Ferrara N. Role of myeloid cells in tumor angiogenesis and growth. Trends Cell Biol. 2008;188:372–8. doi: 10.1016/j.tcb.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;4387070:967–74. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 5.Ellis LM, Hicklin DJ. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat Rev Cancer. 2008;88:579–91. doi: 10.1038/nrc2403. [DOI] [PubMed] [Google Scholar]
- 6.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;35023:2335–42. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 7.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;88:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;31526:1650–9. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 9.Nauts H, Fowler G, Bogatko F. A review of the influence of bacterial infection and of bacterial products (Coley’s toxins) on malignant tumors in man. Acta Medica Scandinavica. 1953:5–103. [PubMed] [Google Scholar]
- 10.Old LJ. Tumor necrosis factor. Science. 1985;230:630–2. doi: 10.1126/science.2413547. [DOI] [PubMed] [Google Scholar]
- 11.Wei YQ, Huang MJ, Yang L, et al. Immunogene therapy of tumors with vaccine based on Xenopus homologous vascular endothelial growth factor as a model antigen. Proc Natl Acad Sci U S A. 2001;9820:11545–50. doi: 10.1073/pnas.191112198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niethammer AG, Xiang R, Becker JC, et al. A DNA vaccine against VEGF receptor 2 prevents effective angiogenesis and inhibits tumor growth. Nat Med. 2002;812:1369–75. doi: 10.1038/nm1202-794. [DOI] [PubMed] [Google Scholar]
- 13.Luo Y, Zhou H, Krueger J, et al. Targeting tumor-associated macrophages as a novel strategy against breast cancer. J Clin Invest. 2006;1168:2132–41. doi: 10.1172/JCI27648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hodi FS, Dranoff G. Combinatorial cancer immunotherapy. Adv Immunol. 2006;90:337–60. doi: 10.1016/S0065-2776(06)90009-1. [DOI] [PubMed] [Google Scholar]
- 15.Soiffer R, Hodi FS, Haluska F, et al. Vaccination with irradiated, autologous melanoma cells engineered to secrete granulocyte-macrophage colony-stimulating factor by adenoviral-mediated gene transfer augments antitumor immunity in patients with metastatic melanoma. J Clin Oncol. 2003;2117:3343–50. doi: 10.1200/JCO.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 16.Soiffer R, Lynch T, Mihm M, et al. Vaccination with irradiated, autologous melanoma cells engineered to secrete human granulocyte-macrophage colony stimulating factor generates potent anti-tumor immunity in patients with metastatic melanoma. Proc Natl Acad Sci USA. 1998;95:13141–6. doi: 10.1073/pnas.95.22.13141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hodi FS, Mihm MC, Soiffer RJ, et al. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci USA. 2003;1008:4712–7. doi: 10.1073/pnas.0830997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hodi FS, Butler M, Oble DA, et al. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci U S A. 2008;1058:3005–10. doi: 10.1073/pnas.0712237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salgia R, Lynch T, Skarin A, et al. Vaccination with irradiated autologous tumor cells engineered to secrete granulocyte-macrophage colony-stimulating factor augments antitumor immunity in some patients with metastatic non-small-cell lung carcinoma. J Clin Oncol. 2003;214:624–30. doi: 10.1200/JCO.2003.03.091. [DOI] [PubMed] [Google Scholar]
- 20.Hodi FS, Schmollinger JC, Soiffer RJ, et al. ATP6S1 elicits potent humoral responses associated with immune mediated tumor destruction. Proc Natl Acad Sci USA. 2002;9910:6919–24. doi: 10.1073/pnas.102025999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmollinger JC, Vonderheide RH, Hoar KM, et al. Melanoma inhibitor of apoptosis protein (ML-IAP) is a target for immune-mediated tumor destruction. Proc Natl Acad Sci USA. 2003;1006:3398–403. doi: 10.1073/pnas.0530311100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mollick JA, Hodi FS, Soiffer RJ, Nadler LM, Dranoff G. MUC1-like tandem repeat proteins are broadly immunogenic in cancer patients. Cancer Immunity. 2003;3:3–20. [PubMed] [Google Scholar]
- 23.Jinushi M, Hodi FS, Dranoff G. Therapy-induced antibodies to MHC class I chain-related protein A antagonize immune suppression and stimulate antitumor cytotoxicity. Proc Natl Acad Sci U S A. 2006;10324:9190–5. doi: 10.1073/pnas.0603503103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sittler T, Zhou J, Park J, et al. Concerted potent humoral immune responses to autoantigens are associated with tumor destruction and favorable clinical outcomes without autoimmunity. Clin Cancer Res. 2008;1412:3896–905. doi: 10.1158/1078-0432.CCR-07-4782. [DOI] [PubMed] [Google Scholar]
- 25.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;254:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 26.Thurston G. Role of Angiopoietins and Tie receptor tyrosine kinases in angiogenesis and lymphangiogenesis. Cell Tissue Res. 2003;3141:61–8. doi: 10.1007/s00441-003-0749-6. [DOI] [PubMed] [Google Scholar]
- 27.Holash J, Maisonpierre PC, Compton D, et al. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science. 1999;2845422:1994–8. doi: 10.1126/science.284.5422.1994. [DOI] [PubMed] [Google Scholar]
- 28.Silvestre JS, Thery C, Hamard G, et al. Lactadherin promotes VEGF-dependent neovascularization. Nat Med. 2005;115:499–506. doi: 10.1038/nm1233. [DOI] [PubMed] [Google Scholar]
- 29.Oliner J, Min H, Leal J, et al. Suppression of angiogenesis and tumor growth by selective inhibition of angiopoietin-2. Cancer Cell. 2004;65:507–16. doi: 10.1016/j.ccr.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 30.Melani C, Stoppacciaro A, Foroni C, Felicetti F, Care A, Colombo MP. Angiopoietin decoy secreted at tumor site impairs tumor growth and metastases by inducing local inflammation and altering neoangiogenesis. Cancer Immunol Immunother. 2004;537:600–8. doi: 10.1007/s00262-004-0500-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lewis CE, De Palma M, Naldini L. Tie2-expressing monocytes and tumor angiogenesis: regulation by hypoxia and angiopoietin-2. Cancer Res. 2007;6718:8429–32. doi: 10.1158/0008-5472.CAN-07-1684. [DOI] [PubMed] [Google Scholar]
- 32.De Palma M, Venneri MA, Galli R, et al. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell. 2005;83:211–26. doi: 10.1016/j.ccr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 33.Murdoch C, Tazzyman S, Webster S, Lewis CE. Expression of Tie-2 by human monocytes and their responses to angiopoietin-2. J Immunol. 2007;17811:7405–11. doi: 10.4049/jimmunol.178.11.7405. [DOI] [PubMed] [Google Scholar]
- 34.Wilson JM, Coletta PL, Cuthbert RJ, et al. Macrophage migration inhibitory factor promotes intestinal tumorigenesis. Gastroenterology. 2005;1295:1485–503. doi: 10.1053/j.gastro.2005.07.061. [DOI] [PubMed] [Google Scholar]
- 35.Yu X, Lin SG, Huang XR, et al. Macrophage migration inhibitory factor induces MMP-9 expression in macrophages via the MEK-ERK MAP kinase pathway. J Interferon Cytokine Res. 2007;272:103–9. doi: 10.1089/jir.2006.0054. [DOI] [PubMed] [Google Scholar]
- 36.Jinushi M, Hodi FS, Dranoff G. Enhancing the clinical activity of granulocyte-macrophage colony-stimulating factor-secreting tumor cell vaccines. Immunol Rev. 2008;222:287–98. doi: 10.1111/j.1600-065X.2008.00618.x. [DOI] [PubMed] [Google Scholar]
- 37.Brown JL, Cao ZA, Pinzon-Ortiz M, et al. A human monoclonal anti-ANG2 antibody leads to broad antitumor activity in combination with VEGF inhibitors and chemotherapy agents in preclinical models. Mol Cancer Ther. 2010;91:145–56. doi: 10.1158/1535-7163.MCT-09-0554. [DOI] [PubMed] [Google Scholar]
- 38.Herbst RS, Hong D, Chap L, et al. Safety, pharmacokinetics, and antitumor activity of AMG 386, a selective angiopoietin inhibitor, in adult patients with advanced solid tumors. J Clin Oncol. 2009;2721:3557–65. doi: 10.1200/JCO.2008.19.6683. [DOI] [PubMed] [Google Scholar]
- 39.Koh YJ, Kim HZ, Hwang SI, et al. Double antiangiogenic protein, DAAP, targeting VEGF-A and angiopoietins in tumor angiogenesis, metastasis, and vascular leakage. Cancer Cell. 2010;182:171–84. doi: 10.1016/j.ccr.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 40.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive Strategies that are Mediated by Tumor Cells. Annu Rev Immunol. 2007;25:267–96. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.