Abstract
Aim
In children with bilateral spastic cerebral palsy (CP), periventricular leukomalacia (PVL) is commonly identified on magnetic resonance imaging. We characterized this white matter condition by examining callosal microstructure, interhemispheric inhibitory competence (IIC), and mirror movements.
Method
We examined 7 children (age range 11y 9mo–17y 9mo, median age 15y 10mo, 4 females) with bilateral spastic CP/PVL (Gross Motor Function Classification System level I or II, Manual Ability Classification System level I) and 12 age-matched controls (age range 11y 7mo–17y 1mo, median age 15y 6mo, 7 females). Fractional anisotropy of the transcallosal motor fibers (TCMF) and the corticospinal tract (CST) of both sides were calculated. The parameters of IIC (transcranial magnetic stimulation) and mirror movements were measured using standardized clinical examination and a computer-based hand motor test.
Results
Fractional anisotropy was lower in children with bilateral spastic CP/PVL regarding the TCMF, but not the left or right CST. Resting motor threshold was elevated in children with bilateral spastic CP/PVL whereas measures of IIC tended to be lower. Mirror movements were markedly elevated in bilateral spastic CP/PVL.
Interpretation
This study provides new information on different aspects of motor function in children with bilateral spastic CP/PVL. Decreased fractional anisotropy of TCMF is consistent with impairment of hand motor function in children with bilateral spastic CP/PVL. The previously overlooked microstructure of the TCMF may serve as a potential indicator for hand motor function in patients with bilateral spastic CP/PVL.
Periventricular leukomalacia (PVL) is caused by a hypoxic-ischemic event resulting in encephalopathy in the developing brain, predominantly between 24 to 34 weeks’ gestation.1–2 Histologically, PVL is characterized by focal necrosis and associated gliosis in the periventricular white matter. PVL is highly associated with preterm birth and is the leading cause of bilateral spastic cerebral palsy (CP) and cognitive abnormalities in these children.3 Impaired motor control in children with PVL has traditionally been attributed to damage to the descending pyramidal corticospinal tracts (CST).4 However, much less consideration has been given to alterations in transcallosal pathways, even though they are a major contributor to motor development in children.5–6
Transcallosal motor fibers (TCMF) can be evaluated by magnetic resonance diffusion tensor imaging (DTI) which provides information about the passive diffusion of water molecules reflecting coherence, organization, and density of the fiber bundles of the brain.7 Fractional anisotropy indicates how directional the diffusion of the water molecules is in a specific voxel. In this context a high fractional anisotropy value means a more unidirectional flow whereas a low fractional anisotropy means equal water movement in all directions. Trace (given in mm2/sec) measures the total diffusion in all directions. A high trace indicates that there is a large amount of water diffusion in virtually any direction. Tractography further provides a non-invasive visualization of white matter pathways based on DTI maps. These maps provide a scalar index for each voxel that estimates the degree of connection to a particular starting voxel and provides information about how fibers are connected between neighbouring voxels. Of note, DTI has been proven useful for evaluating brain development and white matter injury.8 Moreover, the corpus callosum has been a special research focus using tractography due to the number of tracts that pass through it.9–10 Further, the fractional anisotropy of the TCMF has been shown to correlate with measurements of interhemispheric inhibition indicating that the TCMF plays an important role in the interhemispheric communication.10–11
Importantly, interhemispheric inhibition can be examined with transcranial magnetic stimulation (TMS).5 Here, motor cortex stimulation leads to a suppression of voluntary electromyography (EMG) activity of the ipsilateral target muscle, known as the ipsilateral silent period (iSP).12 Although the pathways mediating this inhibitory effect have not been completely defined, there is evidence that transcallosal neurons connecting the motor cortices (M1) are the main connections.13 Therefore ISP has been proposed as a diagnostic tool for transcallosal function.5
Mirror movements are considered a transient phenomenon during motor development. Their decrease in intensity during the second decade of life is thought to depend on the maturation of interhemispheric, inhibitory competence resulting in the ability to restrict movement to one side of the body.14 Increased mirror movements are regarded as a clinical indicator of disturbed neurological function and are therefore frequently assessed to appraise a child’s overall motor function.
We aimed to characterize the motor deficits of children with bilateral spastic CP/PVL as they pertain to white matter integrity, interhemispheric inhibitory competence and hand motor function. We intended to investigate if changes in TCMF could be a potential indicator of motor function in children with bilateral spastic CP/PVL.
METHOD
Participants
Fifty-four consecutive patients were screened for potential inclusion in the study. Out of these, 36 had to be excluded due to the presence of exclusion criteria. Of the remaining 18 patients, seven families agreed to take part in the study and provided written informed consent. Seven patients (mean age 15y 2mo, 4 females) with bilateral spastic CP without any pronounced side dominance of their hand motor impairment were included. Inclusion criteria were 10 to 18 years of age and Gross Motor Function Classification System (GMFCS)15 level I (5 participants) or II (2 participants) and proven PVL on previous magnetic resonance imaging (MRI). Exclusion criteria were TMS-and MRI-related contraindications such as ventriculoperitoneal shunt, metal implants, and history of seizures or neurosurgery. We compared these patients to 12 age-matched right-handed controls (mean age 15y 3mo, 7 females) without any history of neurological disease. Age differed between the matching partners from 0 to 9 months (median difference 2mo). We initially intended to have a two-to-one matching, which was possible in three of the participants (PVL_1, PVL_4 and PVL_5). But for each of the other four participants we were only able to find a single control.
Approval of the study design by the local institutional review board was obtained prior to the commencement of the study.
MR Diffusion Tensor Imaging
MRI was performed on a 1.5-Tesla MR scanner (Magnetom Sonata Maestro Class, Siemens Healthcare, Erlangen, Germany) using an 8-element phased-array head coil. Three-dimensional magnetization-prepared rapid-acquisition with gradient echo (3D MPRAGE) imaging was obtained prior to DTI. Single shot echo planar imaging (EPI) DTI was acquired in 6 non-collinear directions using the following parameters: TR 8000ms, TE 79ms, FOV 230mm, matrix 128×128 (voxel size=1.8×1.8×3.6mm3), parallel acquisition technique (iPAT), acceleration factor 2, b value=0 and 1000s/mm2. Thirty-six axial slices parallel to the anterior–posterior commissure line were acquired, covering the entire brain.
The DTI sets were examined for image quality and head movement. Fractional anisotropy, vector, and color-by-orientation maps were generated using 3DSlicer Version 3.6 (www.slicer.org). Regions of interest (ROI) were drawn individually on the fractional anisotropy maps with respect to the T1w images and the color-coded maps. Tractography of the CST was performed in 3DSlicer by placing a ROI in the M1 and another in the ipsilateral, anterior part of the pons. To create the CST we seeded from the M1 and filtered through the anterior pons then did the reverse by seeding from the anterior pons and filtering through the M1. This generated two tract files which we combined to create one comprehensive CST tract for each hemisphere without altering the measurement per se. In order to identify and track the TCMF, an additional ROI was drawn which covered the entire corpus callosum on the mid-sagittal slice. Tractography was then performed by seeding from the right and the left M1 and filtering through the corpus callosum and then seeding from the corpus callosum and filtering through each M1 separately, in order to ascertain the correct identification of the TCMF. This gave us a total of four tract files which we combined to create one comprehensive TCMF tract for each participant. Tracking was terminated when a voxel with low fractional anisotropy and/or a predetermined trajectory curvature between 2 contiguous vectors was reached (stop criterion of fractional anisotropy and track curvature were 0.1 and 0.8 respectively). The tracts were analyzed based on the DTI measures of fractional anisotropy and trace. The DTI analysis was performed by the primary study rater (IK) and an experienced investigator (PP), who was blind to the participant’s clinical status.
Resting motor threshold and interhemispheric inhibitory competence
Neuronavigated TMS (Brainview Neuronavigation, Fraunhofer Institute, Stuttgart, Germany) was performed using a figure-of-8-coil (MagproX100 Magnetic Stimulator, Medtronic, Minneapolis, USA). The hand area of the left M1 was identified according to anatomical landmarks in the individual MRI.16 The maximum motor evoked potential amplitude of the first dorsal interosseous muscle defined the exact localization of the hotspot. This site was digitally marked to reassure correct positioning of the coil throughout the experiment. Thereafter the stimulator output was adjusted using the maximum-likelihood threshold-hunting method according to Awiszus et al.17 to obtain the resting motor threshold (RMT) of the relaxed first dorsal interosseous muscle. Resting motor threshold was defined as the percentage of stimulator output necessary to obtain a MEP response greater than 50μV in five out of ten consecutive trials in the relaxed muscle.
Participants were then asked to press a softball between thumb and index finger to provide continuous isometric activation of the first dorsal interosseus muscle. Visual feedback was provided by EMG-amplitude using surface electrodes in belly tendon montage. Ipsilateral silent period was elicited at 80% of stimulator output. An EMG signal of the first dorsal interosseous muscle from both hands was recorded and sampled with a D360 amplifier (Digitimer Ltd, Hertfordshire, UK) at a 2kHz analog-to-digital conversion rate through a CED-1401-laboratory interface (Signal 3 for Windows software and Micro 1401, Cambridge Electronic Device, Cambridge, UK) and stored for off-line analysis on a personal computer (Signal3, Cambridge Electronic Device, Cambridge, UK). Onset and duration of iSP were defined on 10 rectified and averaged trials adapted from the graphical method by Garvey et al.12 The extent of the EMG-suppression was determined by calculating the normalized area of iSP (nArea-iSP).18
Clinical examination of mirror movements
Participants unimanually performed four different well-established routine clinical tests:19 (1) serial thumb-finger opposition (2) flicking of the index finger, (3) opening the hand, and (4) spreading the index and middle finger against the examiner’s resistance. Involuntary mirror activity of the resting hand was rated by one of the authors (BK) with (0) no mirror movements, (1) slight mirror movements and (2) pronounced mirror movements. The rater of the clinical hand motor test was blinded to the TMS and MRI results.
Computer-based hand motor function test
Participants were asked to hold a force transducer in each hand between thumb and index finger. First, the individual grip force maximum was determined for each hand. Then, the participants had to perform oscillatory grip force changes with one hand for 15s. Participants performed the test at two different frequencies: (a) low frequency condition, oscillating unimanual grip force changes (1/s), and (b) high frequency condition, oscillating unimanual grip force changes at individual maximum frequency.
Participants were generally informed that grip force was being tested in the active hand while the other hand only had to prevent the force transducer from dropping. To prevent systematic error, participants were asked to begin with either the right or the left hand in an alternating sequence. A repetition of each task was performed to gain a more comprehensive sample of data points.
For a detailed description of the apparatus as well as the analysis see Hermsdörfer et al.20
Statistical Analysis
Statistical analyses were performed with SPSS 17.0 for Windows (SPSS Inc., Chicago, IL, USA) and R 2.10.0 software package (The R Foundation for Statistical Computing, Vienna, Austria). To account for the small group size, a non-parametric test (Mann–Whitney U) was used to test for group differences. Concerning the DTI parameters the mean values of the two raters were used for further calculations. Interrater reliability was evaluated using the approach of Bland and Altman.21 Further, we tested the independence between the difference and the average of the measurements of the two raters for each DTI-parameter using correlation coefficients according to Spearman. Due to the small sample size, an ordinal mixed effects model in order to evaluate discrepancies between bilateral spastic CP/PVL and the controls for the combination of each clinical routine test and both hands was not possible. In order to evaluate the group differences in the computer-based hand motor function test, we used a linear mixed effects regression model with random intercepts, which allows taking into account the correlated nature of the observations with regard to the voluntarily active hand and to relative frequency. For judging the model fit and assumptions of all regression analyses we performed graphically based residual analyses. Due to the large number of evaluated hypotheses relative to the small sample size, numerical p-values are reported rather than tests performed at the 0.05 level of statistical significance.
RESULTS
MRI and DTI tractography
All participants with bilateral spastic CP/PVL presented characteristic imaging findings of PVL, including almost symmetric reduction of the peri-trigonal white matter volume with a consecutive enlargement of the posterior horns, scalloped ventricular contours, periventricular gliosis and secondary atrophy of the posterior part of the body, and the splenium of the corpus callosum. No other pathologies were found. One child in the control group was excluded from analyses because the DTI sequences were degraded by artifact due to an orthodontic device, which precluded identification of the tracts of interest. The remainder of the control group showed normal appearance of the white matter on conventional MRI sequences and was included into the analysis. The CST of both sides and the TCMF could be identified in all investigated participants.
Mann–Whitney U test revealed a lower fractional anisotropy (p=0.01) and trace (p=0.001) of the TCMF in children with bilateral spastic CP/PVL compared to the controls. No pronounced differences were found between the two groups regarding the fractional anisotropy of the CST on either side (CST left p=0.739; CST right p=0.571). However, trace was increased in the patient group (CST left p=0.036; CST right p=0.028). Table I summarizes the results. Figures 1 and 2 show an example of the TCMF and the CST in a child with BS-CP/PVL compared to a subject of the control group.
Table I.
Values for fractional anisotropy and trace in mm2/sec for the two examined groups
| Tract | Values (SD) | p-value | Difference between groups | |
|---|---|---|---|---|
| Controls | BS-CP/PVL | |||
| CST left | ||||
| FA | 478.47 (34.05) | 486.10 (62.04) | 0.739 | 7.63 |
| Trace | 2.31 (0.10) | 2.47 (0.20) | 0.036 | 0.16 |
| CST right | ||||
| FA | 484.06 (35.98) | 493.95 (34.33) | 0.571 | 9.89 |
| Trace | 2.27 (0.08) | 2.43 (0.20) | 0.028 | 0.16 |
| TCMF | ||||
| FA | 429.49 (21.04) | 372.22 (60.74) | 0.010 | 57.27 |
| Trace | 2.55 (0.13) | 2.95 (0.29) | 0.001 | 0.4 |
The table displays the mean of both readers followed by the standard deviation. The difference of each measure between the two groups is listed on the right. CST, corticospinal tract; FA, fractional anisotropy; TCMF, transcallosal motor fibers.
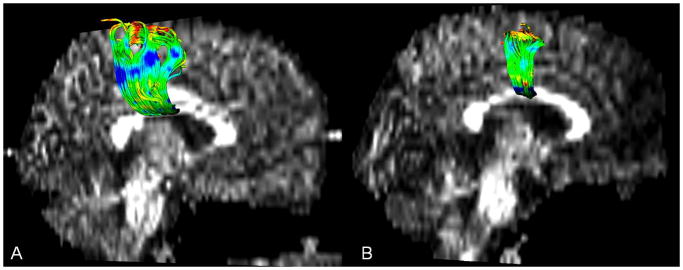
Figure 1.
Example of transcallosal motor fibers (TCMF) in (a) healthy control and (b) child with bilateral spastic CP/PVL. Note the decreased number of TCMF in the patient brain image (right).
Figure 2.
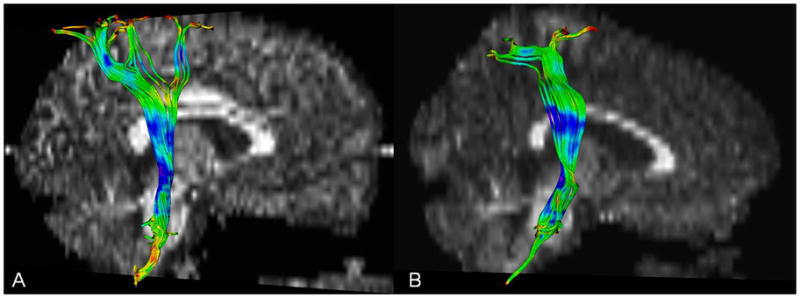
Example of corticospinal tracts (CST) in (a) healthy control and (b) child with bilateral spastic CP/PVL. Note the lack of difference between the CST of the patient and healthy control.
As indicated by Bland-Altman plots the two raters showed close agreement on fractional anisotropy and trace for the TCMF, and the CST of both sides. The test of the correlation coefficients did not reveal any statistically significant results so that the assumption of independence between the ‘differences’ and ‘averages’ of the measurements seems not to be violated. Figure 3 displays the according Bland-Altman plots.
Figure 3.
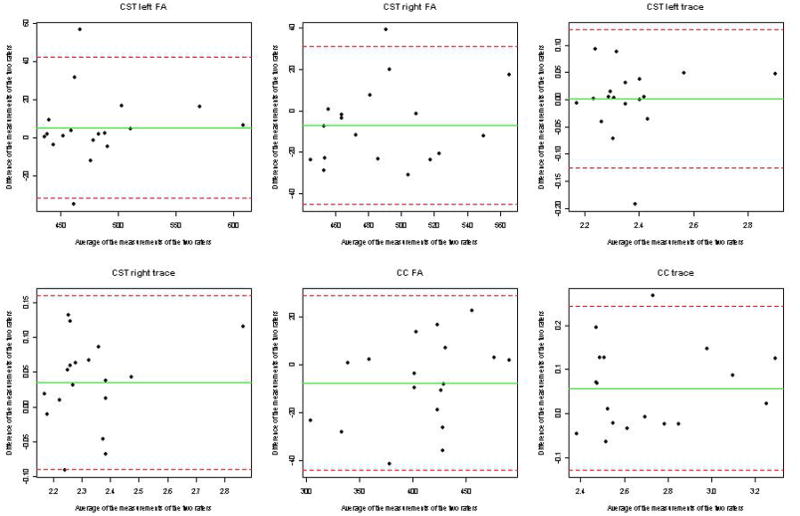
Plot of difference versus mean (Bland-Altman plot) for data on agreement between the two raters for the diffusion tensor imaging variables fractional anisotropy, Trace for the corticospinal tracts (CST) of both sides and the corpus callosum. According to the Bland-Altman approach it is expected that one observation will fall outside the ‘limits of agreement’ in case of a sample size of n=18. Our study results reveal two observations outside the limits of agreement for the fractional anisotropy of the left CST. For the measures CST right fractional anisotropy, CST left trace, CST right trace, and corpus callosum trace, only one observation, and for corpus callosum fractional anisotropy no observation is outside these limits. In addition, the plots show that the within-repeatability is not associated with the size of the measurement.
Resting motor threshold and ipsilateral silent period
A higher resting motor threshold was found in children with bilateral spastic CP/PVL (median 42 vs 39% of stimulator output; p=0.045). When stimulating the left motor cortex with 80% of the stimulator output maximum iSP could be elicited in all healthy controls but only in six of the seven children with bilateral spastic CP/PVL. The time between applied stimulus over the left motor cortex and the appearance of the iSP tended to be longer in children with bilateral spastic CP/PVL (median 47.0 vs 42.3ms after stimulus; p=0.29). Children with bilateral spastic CP/PVL had a slightly shorter duration of the iSP (19.8 vs. 20.3ms; p=0.64) and tended to have a lower extent of iSP (8.6 vs 9.8qmm; p=0.51; Table II).
Table II.
Analyses of the ipsilateral silent period (iSP)
| ID | Age (y, mo) | Sex | Resting motor threshold % | latency ms | Ipsilateral silent period duration ms | extent qmm |
|---|---|---|---|---|---|---|
| PVL_01 | 17, 9 | F | 43 | 34.0 | 22.5 | 8.1 |
| PVL_02 | 16, 7 | M | 38 | 48.5 | 32.0 | 12.6 |
| PVL_03 | 16, 6 | F | 60 | 45.0 | 12.5 | 4.4 |
| PVL_04 | 15, 10 | M | 42 | 49.5 | 30.0 | 16.2 |
| PVL_05 | 15, 2 | F | 40 | 53.0 | 8.5 | 2.9 |
| PVL_06 | 12, 3 | M | 53 | 45.5 | 17.0 | 9.1 |
| PVL_07 | 11, 9 | F | 40 | – | – | – |
| CTR_01 | 17, 1 | M | 33 | 41.0 | 51.0 | 22.2 |
| CTR_02 | 17, 1 | M | 40 | 39.0 | 22.5 | 9.6 |
| CTR_03 | 16, 5 | M | 41 | 42.5 | 24.5 | 11.1 |
| CTR_04 | 16, 4 | M | 42 | 52.5 | 18.0 | 9.9 |
| CTR_05 | 15, 9 | F | 37 | 33.5 | 34.0 | 17.0 |
| CTR_06 | 15, 8 | F | 29 | 40.5 | 33.0 | 16.1 |
| CTR_07 | 15, 4 | F | 36 | 42.0 | 14.5 | 6.8 |
| CTR_08 | 15, 2 | F | 42 | 53.0 | 15.5 | 7.5 |
| CTR_09 | 15, 2 | M | 32 | 41.0 | 36.0 | 18.5 |
| CTR_10 | 15, 2 | F | 36 | 50.5 | 10.5 | 4.5 |
| CTR_11 | 12, 5 | F | 43 | 42.5 | 8.5 | 3.3 |
| CTR_12 | 11, 7 | F | 40 | 54.5 | 12.5 | 2.9 |
| Mean | Median | |||||
| PVL | 15, 2 | 4F: 3M | 42.0 | 47.0 | 19.8 | 8.6 |
| Controls | 15, 3 | 7F: 5M | 38.5 | 42.3 | 20.3 | 9.8 |
| p-value | 0.045 | 0.29 | 0.64 | 0.51 |
The table displays the individual values of the iSP measures for each participant. The status is indicated by the participant’s ID (PVL= participant with bilateral spastic CP/PVL; CTR = control). The second column gives the individual’s age in years and additional months. Age differed between the matching partners from 0 to 9 months (median difference was 2mo). Resting motor threshold is given as the percentage of stimulator output. The latency and the duration of iSP are given in milliseconds (MS), the extent of iSP in qmm. – indicates that iSP could not be elicited. p-values are derived by calculating the Mann-Whitney U test. F=female, M=male.
Clinical and computer-based tests for mirror movements
Patients with bilateral spastic CP/PVL showed more mirror activity of both hands than the controls (Figure 4). The results of the tests 1 to 3 had consistent degree and distribution of mirror movements. In comparison test 4 elicited more often ‘pronounced’ mirror movements in both groups and showed no difference between the two groups on the right hand.
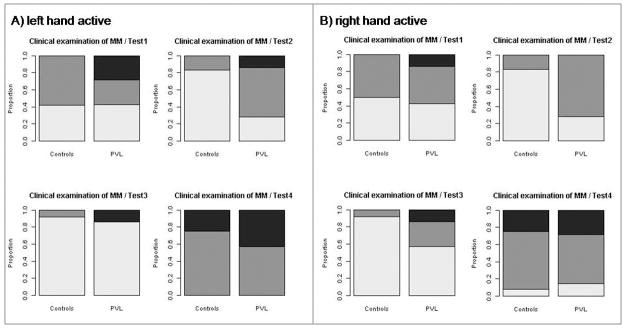
Figure 4.
Clinical examination of mirror movements in the control group and in children with bilateral spastic CP/PVL. (a) Left hand actively performed the tests, the mirroring of the right hand is displayed; (b) right hand actively performed the tests, the mirroring of the left hand is displayed. No mirror movements (light grey), slight mirror movements (dark grey), pronounced mirror movements (black). Children with bilateral spastic CP/PVL showed more mirror movements in all four tests. Test 4 elicited more mirror movements in both investigated groups.
Children with bilateral spastic CP/PVL were found to have increased mirror movements with a wider range of variability compared to the controls. Therefore, we transformed the mirror movements’ ratios to the logarithmic scale. This difference between the PVL participants and control participants was pronounced (p=0.042) adjusted for all four conditions (high and low frequency; active left and right hand) in the linear mixed effects model. In addition, this mixed effects model revealed a difference in both groups for ‘hand’ (p=0.041) where the condition ‘left hand active’ shows increased mirror movements while performing both the low and high frequency condition. There was no difference between the low and high frequency condition (p=0.968). The results of the mixed effects model is visually summarized in Figure 5 which shows the mirror movements ratios on a logarithmic scale for each frequency condition stratified by group. Children with bilateral spastic CP/PVL performed the high frequency condition on both sides with a lower absolute frequency of grip force changes.
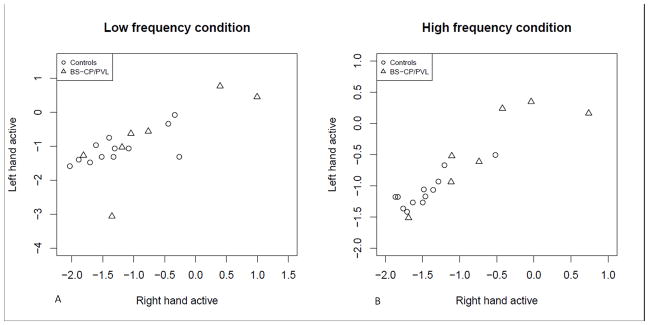
Figure 5.
The figure displays the mirror movements ratios (logarithmic scale) of the right versus the left hand. The plots are stratified by group (circles represent controls; triangles represent children with bilateral spastic CP/PVL) and by the frequency condition.
DISCUSSION
This study provides new information on different aspects of motor function in children with bilateral spastic CP/PVL compared to healthy controls. We were able to demonstrate coherent alterations in the various motor function parameters in children with bilateral spastic CP/PVL, with a lower anisotropy of TCMF, altered mirror movements and a coherent tendency towards decreased interhemispheric inhibitory competence.
The clinical assessment and the computer-based test of mirror movements showed higher values for these in children with bilateral spastic CP/PVL. This is consistent with the findings of previous studies.22–23 The higher variability in the degree of mirroring in children with PVL might be caused by a lack of motor control and coordination due to immaturity of the motor system and spasticity. This could also be a reason for the lower absolute frequency in the high-frequency condition. Increased mirror movements reflect decreased interhemispheric inhibitory competence. Thus the ability to lateralize movements to one side of the body integrates primary and supplementary motor areas of both hemispheres and is especially needed while performing higher order motor functions.24
Resting motor threshold was higher in children with bilateral spastic CP/PVL indicating a reduced cortical excitability. This was previously found for the affected hemisphere in children with congenital stroke.25 In this regard it is assumed that reduced cortical excitability is a consequence of structural damage to the interneurons in the primary motor cortex. In children with bilateral spastic CP/PVL this might be caused by injury to the developing oligodendrocytes leading to necrosis and gliosis of the surrounding white matter.26 Measurements of interhemispheric inhibitory competence as evaluated with TMS tended to be decreased in children with bilateral spastic CP/PVL. Despite the relatively higher heterogeneity of the affected group compared to the controls it has to be taken into account that only children with a mild form of bilateral spastic CP/PVL (GMFCS level I and II) were included.
Previous studies attributed the impaired motor function in children with bilateral spastic CP/PVL to the injury of the descending CST.4,27–28 A greater degree of diffusion was found in children with bilateral spastic CP/PVL as shown by a higher trace. This may indicate impairment in the integrity of the surrounding white matter. However, no difference was found between the two groups for the fractional anisotropy value of the CST; these findings are consistent with recently published results.29 The inconsistency in reported results for the integrity of the CST might be related to the severity of the disease. It is reasonable to assume that patients with a higher degree of motor impairment (GMFCS levels III–V) and more pronounced periventricular white matter loss will have more pronounced damage to the nearby localized CST. Fan et al. found the CST at the level of the ventricles attenuated in size while a relatively normal appearance of the CST at the level of the brainstem was observed.30
Although patients with milder forms of bilateral spastic CP/PVL (GMFCS level I and II) might have no or only slight impairment of descending pyramidal fibers, injuries to other parts of the neuronal network involved in motor function (such as the corpus callosum) may have an impact on coordination and movement.29,31
The most pronounced difference between the two investigated groups was the lower fractional anisotropy value of TCMFs in children with bilateral spastic CP/PVL, indicating diminished communication between the motor cortices of the two hemispheres. Thinning of the corpus callosum especially in the posterior part of the body is commonly seen in bilateral spastic CP/PVL. This is thought to be secondary to white matter loss of the hemispheres but direct involvement of the corpus callosum may also be possible. The most probable correlate of the impaired acquisition of anisotropic diffusion is the bilateral white matter injury leading to deficits in development of the axonal-oligodendroglial unit, limitation of axon diameter growth, and even overt axonal loss. Therefore, a low fractional anisotropy value reflects impaired microstructure of white matter tracts, however, DTI is not able to distinguish among the possible causes.32
The relevance of the corpus callosum regarding motor performance is documented by studies investigating its macrostructure along with the child’s neurological development until the second decade of life. Especially in preterm infants, the size of the corpus callosum as well as its growth rate correlates closely with neurological development and outcome.33
The impaired microstructure of the TCMF is congruent with the tendency of decreased interhemispheric inhibitory competence which is known to be mainly processed via the corpus callosum. Both findings reflect the markedly higher degree of mirror movements in children with bilateral spastic CP/PVL compared to controls.
There are limitations to this study that need to be taken into account when interpreting the results. First, the sample sizes were small. The comparatively low number of investigated participants was, however, due to a highly selective recruitment because of the necessary capability to perform the challenging hand motor tests. Additionally, the TMS method required particularly strict exclusion criteria. Second, the tractography methods used to generate tracts do not necessarily correspond to an exact anatomical structure, that is, an axon. The periventricular white matter alterations in children with bilateral spastic CP/PVL may potentially alter the tractography algorithm thereby leading to a reduced detection of intact fibers. Furthermore, while we tried to compensate by using a small step size, tracts are nonetheless sensitive to crossing fibers and data noise, particularly in 1.5T scans which have a larger slice size.
Our findings indicate that the microstructure of TCMF, as evaluated with DTI, reflects the neuropsychological and clinical function of TCMF, as evaluated with TMS. Furthermore, taken together, these two measures provide new insight into the interaction of the central neuroanatomical structures and their impact on motor function in children with bilateral spastic CP/PVL. These results suggest that the fractional anisotropy of TCMF may be a potential indicator for motor performance and developmental capacities in children with bilateral spastic CP/PVL.
What this paper adds.
This study provides new information on alterations in motor functions in bilateral spastic CP/PVL, with a lower anisotropy of TCMF, decreased interhemispheric inhibitory competence, and increased mirror movements.
Impaired motor control in children with PVL has been attributed to damage to the pyramidal corticospinal tracts. However, less consideration has been given to alterations in transcallosal pathways, even though they are a major contributor to motor development in children.
TCMF may be a potential indicator of motor developmental capacities in bilateral spastic CP/PVL.
Acknowledgments
The authors wish to thank Professor Joachim Hermsdoerfer and Professor Peter Winkler for their expert neurological opinion. Additionally, we gratefully acknowledge the support of the Society for Neuropediatrics, Europe (IK), the United States National Institutes of Health (U54EB005149, R01MH50740, K05MH070047, P50MH080272 to MES, and U54EB005149 to MK and MES), the Department of Veteran Affairs Merit Awards (MES), and the VA Schizophrenia Center Grant (MES).
LIST OF ABBREVIATIONS
- CST
Corticospinal tract
- DTI
Diffusion tensor imaging
- iSP
Ipsilateral silent period
- PVL
Periventricular leukomalacia
- TCMF
Transcallosal motor fibers
- TMS
Transcranial magnetic stimulation
References
- 1.Krageloh-Mann I, Toft P, Lunding J, Andresen J, Pryds O, Lou HC. Brain lesions in preterms: origin, consequences and compensation. Acta Paediatr. 1999;88:897–908. doi: 10.1080/08035259950168856. [DOI] [PubMed] [Google Scholar]
- 2.Volpe JJ. Neurobiology of periventricular leukomalacia in the premature infant. Pediatr Res. 2001;50:553–62. doi: 10.1203/00006450-200111000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Volpe JJ. Brain injury in the premature infant – from pathogenesis to prevention. Brain Dev. 1997;19:519–34. doi: 10.1016/s0387-7604(97)00078-8. [DOI] [PubMed] [Google Scholar]
- 4.Kuban KC, Leviton A. Cerebral palsy. N Engl J Med. 1994;330:188–95. doi: 10.1056/NEJM199401203300308. [DOI] [PubMed] [Google Scholar]
- 5.Heinen F, Glocker FX, Fietzek U, Meyer BU, Lucking CH, Korinthenberg R. Absence of transcallosal inhibition following focal magnetic stimulation in preschool children. Ann Neurol. 1998;43:608–12. doi: 10.1002/ana.410430508. [DOI] [PubMed] [Google Scholar]
- 6.Heinen F, Kirschner J, Fietzek U, Glocker FX, Mall V, Korinthenberg R. Absence of transcallosal inhibition in adolescents with diplegic cerebral palsy. Muscle Nerve. 1999;22:255–7. doi: 10.1002/(sici)1097-4598(199902)22:2<255::aid-mus14>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 7.Basser PJ, Jones DK. Diffusion-tensor MRI: theory, experimental design and data analysis – a technical review. NMR Biomed. 2002;15:456–67. doi: 10.1002/nbm.783. [DOI] [PubMed] [Google Scholar]
- 8.Neil J, Miller J, Mukherjee P, Huppi PS. Diffusion tensor imaging of normal and injured developing human brain - a technical review. NMR Biomed. 2002;15:543–52. doi: 10.1002/nbm.784. [DOI] [PubMed] [Google Scholar]
- 9.Hofer S, Frahm J. Topography of the human corpus callosum revisited--comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. NeuroImage. 2006;32:989–94. doi: 10.1016/j.neuroimage.2006.05.044. [DOI] [PubMed] [Google Scholar]
- 10.Wahl M, Lauterbach-Soon B, Hattingen E, et al. Human motor corpus callosum: topography, somatotopy, and link between microstructure and function. J Neurosci. 2007;27:12132–8. doi: 10.1523/JNEUROSCI.2320-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koerte I, Heinen F, Fuchs T, et al. Anisotropy of callosal motor fibers in combination with transcranial magnetic stimulation in the course of motor development. Invest Radiol. 2009;44:279–84. doi: 10.1097/RLI.0b013e31819e9362. [DOI] [PubMed] [Google Scholar]
- 12.Garvey MA, Ziemann U, Becker DA, Barker CA, Bartko JJ. New graphical method to measure silent periods evoked by transcranial magnetic stimulation. Clin Neurophysiol. 2001;112:1451–60. doi: 10.1016/s1388-2457(01)00581-8. [DOI] [PubMed] [Google Scholar]
- 13.Avanzino L, Teo JT, Rothwell JC. Intracortical circuits modulate transcallosal inhibition in humans. J Physiol. 2007;583:99–114. doi: 10.1113/jphysiol.2007.134510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hubers A, Orekhov Y, Ziemann U. Interhemispheric motor inhibition: its role in controlling electromyographic mirror activity. Eur J Neurosci. 2008;28:364–71. doi: 10.1111/j.1460-9568.2008.06335.x. [DOI] [PubMed] [Google Scholar]
- 15.Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39:214–23. doi: 10.1111/j.1469-8749.1997.tb07414.x. [DOI] [PubMed] [Google Scholar]
- 16.Yousry TA, Schmid UD, Alkadhi H, et al. Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain. 1997;120:141–57. doi: 10.1093/brain/120.1.141. [DOI] [PubMed] [Google Scholar]
- 17.Awiszus F. TMS and threshold hunting. Suppl Clin Neurophysiol. 2003;56:13–23. doi: 10.1016/s1567-424x(09)70205-3. [DOI] [PubMed] [Google Scholar]
- 18.Trompetto C, Bove M, Marinelli L, Avanzino L, Buccolieri A, Abbruzzese G. Suppression of the transcallosal motor output: a transcranial magnetic stimulation study in healthy subjects. Exp Brain Res. 2004;158:133–40. doi: 10.1007/s00221-004-1881-6. [DOI] [PubMed] [Google Scholar]
- 19.Woods BT, Teuber HL. Mirros movements after childhood hemiparesis. Neurology. 1978;28:1152–57. doi: 10.1212/wnl.28.11.1152. [DOI] [PubMed] [Google Scholar]
- 20.Uttner I, Mai N, Esslinger O, Danek A. Quantitative evaluation of mirror movements in adults with focal brain lesions. Eur J Neurol. 2005;12:964–75. doi: 10.1111/j.1468-1331.2005.01098.x. [DOI] [PubMed] [Google Scholar]
- 21.Altman DG, Bland JM. Measurement in medicine: The analysis of method comparison studies. The Statistician. 1983;32:307–17. [Google Scholar]
- 22.Peralta-Carcelen M, Moses M, Adams-Chapman I, Gantz M, Vohr BR. Stability of neuromotor outcomes at 18 and 30 months of age after extremely low birth weight status. Pediatrics. 2009;123:e887–95. doi: 10.1542/peds.2008-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuhtz-Buschbeck JP, Sundholm LK, Eliasson AC, Forssberg H. Quantitative assessment of mirror movements in children and adolescents with hemiplegic cerebral palsy. Dev Med Child Neurol. 2000;42:728–36. doi: 10.1017/s0012162200001353. [DOI] [PubMed] [Google Scholar]
- 24.Jancke L, Peters M, Himmelbach M, Nosselt T, Shah J, Steinmetz H. fMRI study of bimanual coordination. Neuropsychologia. 2000;38:164–74. doi: 10.1016/s0028-3932(99)00062-7. [DOI] [PubMed] [Google Scholar]
- 25.Berweck S, Walther M, Brodbeck V, et al. Abnormal motor cortex excitability in congenital stroke. Pediatr Res. 2008;63:84–8. doi: 10.1203/PDR.0b013e31815b88f1. [DOI] [PubMed] [Google Scholar]
- 26.Takashima S, Itoh M, Oka A. A history of our understanding of cerebral vascular development and pathogenesis of perinatal brain damage over the past 30 years. Semin Pediatr Neurol. 2009;16:226–36. doi: 10.1016/j.spen.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 27.Staudt M, Pavlova M, Bohm S, Grodd W, Krageloh-Mann I. Pyramidal tract damage correlates with motor dysfunction in bilateral periventricular leukomalacia (PVL) Neuropediatrics. 2003;34:182–8. doi: 10.1055/s-2003-42206. [DOI] [PubMed] [Google Scholar]
- 28.Volpe JJ, Edward B. Neuhauser lecture. Current concepts of brain injury in the premature infant. AJR Am J Roentgenol. 1989;153:243–51. doi: 10.2214/ajr.153.2.243. [DOI] [PubMed] [Google Scholar]
- 29.Hoon AH, Jr, Stashinko EE, Nagae LM, et al. Sensory and motor deficits in children with cerebral palsy born preterm correlate with diffusion tensor imaging abnormalities in thalamocortical pathways. Dev Med Child Neurol. 2009;51:697–704. doi: 10.1111/j.1469-8749.2009.03306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fan GG, Yu B, Quan SM, Sun BH, Guo QY. Potential of diffusion tensor MRI in the assessment of periventricular leukomalacia. Clin Radiol. 2006;61:358–64. doi: 10.1016/j.crad.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 31.Nagae LM, Hoon AH, Jr, Stashinko E, et al. Diffusion tensor imaging in children with periventricular leukomalacia: variability of injuries to white matter tracts. Am J Neuroradiol. 2007;28:1213–22. doi: 10.3174/ajnr.A0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huppi PS, Murphy B, Maier SE, et al. Microstructural brain development after perinatal cerebral white matter injury assessed by diffusion tensor magnetic resonance imaging. Pediatrics. 2001;107:455–60. doi: 10.1542/peds.107.3.455. [DOI] [PubMed] [Google Scholar]
- 33.Anderson NG, Laurent I, Woodward LJ, Inder TE. Detection of impaired growth of the corpus callosum in premature infants. Pediatrics. 2006;118:951–60. doi: 10.1542/peds.2006-0553. [DOI] [PubMed] [Google Scholar]