Abstract
Smooth pursuit (SP) eye movements are used to maintain the image of a moving object relatively stable on the fovea. Even when tracking a single target over a dark background, multiple areas including frontal eye fields (FEF), middle temporal (MT) and medial superior temporal (MST) cortex contribute to converting visual signals into initial commands for SP. Signals in the cortical pursuit system reach the oculomotor cerebellum through brainstem centers including the dorsolateral pontine nucleus (DLPN), nucleus reticularis tegmenti pontis (NRTP) and pretectal nucleus of the optic tract (NOT). The relative information carried in these parallel pathways remains to be fully defined. We used multiple linear-regression modeling to estimate the relative sensitivities of cortical (MST, FEF), pontine (NRTP, DLPN) and NOT neurons to eye- and retinal-error parameters (position, velocity and acceleration) during step-ramp SP of macaques (Macaca mulatta). We found that a large proportion of pursuit-related MST and DLPN neurons were most sensitive to eye-velocity or retinal error velocity. In contrast, a large proportion of FEF and rostral NRTP neurons were most sensitive to eye-acceleration. Visual neurons in MST, DLPN and NOT neurons were most sensitive to retinal image velocity.
Keywords: cerebral cortex, eye movements, macaque, pontine, pretectum
INTRODUCTION
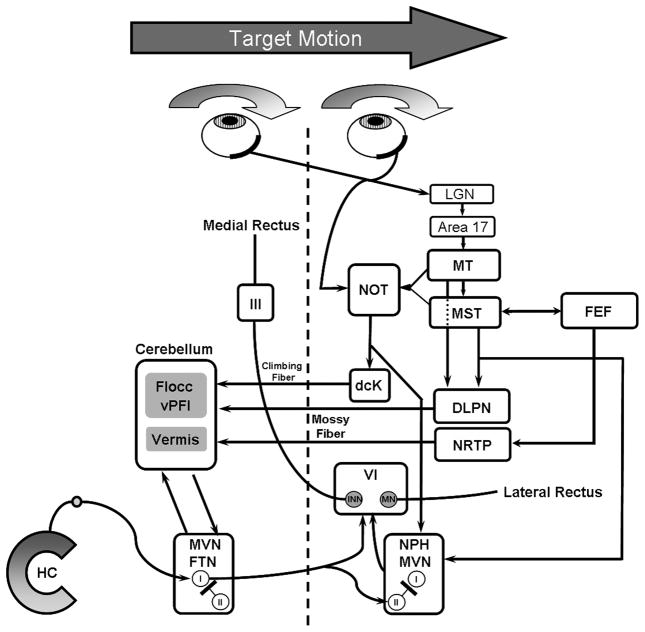
Smooth pursuit eye movements are used to maintain the image of a moving object relatively stable on or near the fovea [1]. Our understanding of neural mechanisms associated with different aspects of smooth pursuit has advanced significantly in the last decade [2]. Figure 1 provides a simplified diagram of some of the feed-forward connections essential for smooth pursuit. Briefly, retinal input to the accessory optic system (e.g., NOT) and geniculo-striate system comprise the afferent limbs for smooth pursuit. Especially important are the dorsal-stream pathways involving MT and MST and their connections with the frontal lobe regions (e.g., FEF). All of these cortical areas have direct projections to brainstem areas (e.g., rNRTP, DLPN and NOT) critical for the generation of smooth pursuit. These brainstem centers project to different regions of the cerebellum (e.g., flocculus, paraflooculus and vermis), which play complimentary roles in smooth pursuit [3, 1].
Figure 1.
Major feed-forward pathways for smooth pursuit (SP). Visual motion is decoded in retinal-genciulo-striate pathways to dorsal stream areas (e.g., MT). Direct retinal and indirect cortical input to the accessory optic system and NOT also provide visual signals for SP. Area MT projects to MST and FEF where different aspects of SP are supported. The cortical SP areas (MT, MST and FEF) project to the pontine nuclei (DLPN and NRTP), which provide parellel channels of information to different cerebellar areas including, flocculus, ventral paraflocculus and vermis. These cerebellar areas enable SP via connections with deep cerebellar nuclei and distal brainstem motor centers. Abbreviations; lateral geniculate nucleus (LGN), dCK (dorsal cap of Kooy inferior olive), vPFloc (ventral paraflocculus), FTN (floccular target neuron), III (oculomotor nucleus), VI (abducens nucleus), MVN (medial vestibular nucleus), NPH (nucleus prepositus hypoglossi), HC, (horizontal semicircular canal).
Discrete lesions placed in the cortical motion processing areas MT and MST produce retinotopic and directional deficits in smooth pursuit, respectively [4, 5]. These deficits could be related to the role played by MT in motion perception and MST in beginning the process of translating motion information into commands for movement. Neurons in the lateral aspect of MST (MSTl) prefer small-sized moving stimuli, while cells in the dorsal part (MSTd) respond best to large-field stimuli [6]. SP related signals in MSTd often follow the onset of the eye movements by 50 to 100 ms suggesting a role other then initiating smooth pursuit [6,7,8].
Both MT and MST have reciprocal connections with the FEF region [9]. Lesions of FEF are associated with defective predictive and visually guided smooth pursuit [10,11,12]. Electrical stimulation of fundus of the FEF, where smooth pursuit related neurons are found alters ongoing pursuit [13]. The majority of FEF neurons begin their response before the onset of pursuit, and they contribute to the initiation of pursuit, which is characterized by high retinal slip and eye acceleration [14].
Neurons in the cortical smooth pursuit system (MT, MST, FEF, SEF) project to the basilar pontine nuclei, DLPN, NRTP [15,16], and pretectal nucleus of the optic tract (NOT), which provide essential signals for smooth pursuit commands, adaptation and plasticity to the cerebellum (see below). There is considerable specificity in the cortical projections to DLPN, rNRTP and NOT [15,16]. In this review, we compare smooth pursuit related information carried by individual neurons at different nodes of the cortical pursuit system and related brainstem targets.
METHODS
Surgical procedures
A detailed description of our surgical procedures can be found in recent publications [8]. All surgical procedures conform to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of Emory University. Surgery was performed in a dedicated facility using aseptic techniques and inhalation anesthesia (isoflurane, 1.25–2.5%). Post-surgical analgesia (Buprenorphine. 0.01 mg/kg, I.M.) and anti-inflammatory (Banamine 1.0 mg/kg, I.M.) treatment were provided. We used stereotaxic methods to implant a titanium head stabilization post and titanium recording chambers (FEF, A22, L20, tilted 15° lateral; MT/MST, P5, L15; brainstem, A3, L1, tilted 20° lateral). We verified that our recording locations were in specified brain regions by using magnetic resonance imaging (T1- weighted, fast spin-echo; Siemens, 3T magnet), microdrive readings and Nissl stained sections [17].
Behavioral paradigms
During all single-unit recording experiments, monkeys (Macaca mulatta; 3–5 years old) were seated in a primate chair (Crist Instruments, MD) with the head stabilized in the horizontal stereotaxic plane. Eye movements were measured with electromagnetic methods employing scleral search coils [8]. Animals worked for a juice reward for accurately maintaining their eye position in a reward window around the target. Visual stimuli (small spot or large-field) were rear projected on a tangent screen 57cm distant from the monkey using optic bench hardware and computer controlled two-axis mirror galvanometers (General Scanning, Watertown, MA). All SP neurons were tested during step-ramp conditions where a small diameter (0.1°) target moved over a dark background. We also included step-ramp trials where the target was briefly extinguished eliminating visual stimulation and revealing extra-retinal signals, if present.
Data Collection
Eye and target position feedback signals were sampled at 1 KHz with 16-bit precision. Single unit activity was recorded using epoxy-coated tungsten electrodes (1–5 MΩ) and standard hardware (Bak Electronics, Mount Airy, MD) or software discriminators (Alpha-Omega, Israel). Isolated spikes were represented by a TTL pulse, which was sampled at high precision as an event mark in our data acquisition system (CED Power1401, Cambridge, England). To facilitate modeling, neuronal response was represented as a spike-density function generated by convolving spike times with a 5ms Gaussian function.
Model fitting and optimization
We used a model estimation procedure to identify smooth pursuit related signals in FEF, MST, DLPN, NRTP and NOT during step-ramp tracking. We attempted to reconstruct the individual response profiles of smooth pursuit-related neurons by using combinations of position, velocity and acceleration of eye- and retinal error-motion (e.g., Das et al., 2001; Ono et al., 2004). Averaged data taken from at least ten smooth pursuit trials were used to identify coefficients in the following model.
In the equation described above, E(t) denotes the eye position at time “t”, R(t) denotes the retinal error position at time “t” and FR(t) is the estimated value of the unit spike density function at time “t”. Coefficients in the models are defined by terms A-G. This model attempts to relate unit response to a combination of eye- and retinal error-motion parameters. Goodness of fit was determined by calculating a coefficient of determination (CD) or the square of the cross-correlation coefficient between experimentally observed unit data and model estimated fit. T1 represents eye latency with respect to unit response and T2 signifies visual latency re target motion onset. We calculated a set of coefficients (A-G) and estimated coefficients of determination (CD) for a series of T1 and T2 latencies. In our final models, we used coefficients that yielded a maximum CD for specific latency values [18, 19]. Retinal error parameters were calculated as the difference between target and eye motion parameters. We also calculated partial r2 values for each component to estimate the relative contribution of eye- and retinal error-(position, velocity and acceleration) to the firing rate of the neurons.
RESULTS
Smooth pursuit related responses of brainstem neurons
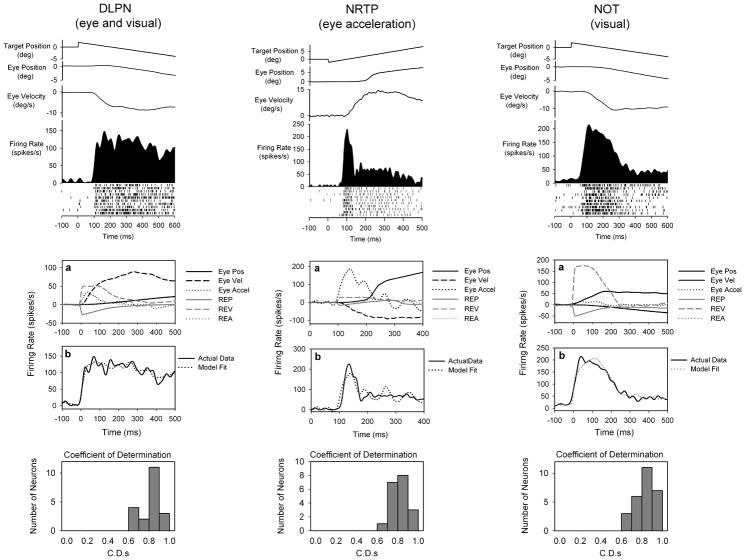
Multiple-linear regression modeling allows us to readily compare smooth pursuit related contributions of neurons in different areas. In this review, we only consider neurons that were modulated during smooth pursuit. Figure 2 shows smooth pursuit related responses of DLPN (left column), NRTP (middle column) and NOT (right column) neurons. In figure 2, we show the sensitivity of each neuron to components of eye- and retinal error-motion. Eye velocity makes the largest contribution to DLPN neuronal response. A 6-component model that included both eye- and retinal-error terms produced the best fits to observed firing rate data (Fig. 2, bottom). Target blink testing of DLPN smooth pursuit neurons reveals some neurons are visually contingent and others carry both retinal-error and eye-motion sensitivity.
Figure 2.
Examples of smooth pursuit related brainstem neurons. Top panel shows neuronal response of DLPN (left), rNRTP (middle) and NOT (right) neurons during step-ramp SP. Traces show horizontal target and eye position, eye velocity, and neuronal activity (spike density and rasters). (A), Curve fitting with individual traces showing the dynamic values of the components that make up the model. (B), Model fits to neuronal data using 6- or 3-component models. SP neurons in DLPN, rNRTP and NOT are most sensitive to eye velocity, eye acceleration and retinal image velocity, respectively. (C), Histograms of C.D.s for neurons in different regions.
Smooth pursuit neurons in rNRTP typically have different balances of eye- and retinal-error sensitivities than DLPN neurons. Most smooth pursuit related rNRTP neurons evince strong eye-motion sensitivity (Fig. 2, middle column), with a pulse of activity starting just before smooth pursuit initiation. Modeling studies confirm that this activity is often most strongly associated with eye acceleration [19]. Retinal-error velocity also makes a contribution to NRTP neuronal response during smooth pursuit. Target blink testing of rNRTP neurons during smooth pursuit shows that most neurons have extraretinal sensitivity.
Smooth pursuit related NOT neurons are sensitive to visual motion near the fovea. During step-ramp smooth pursuit, retinal-error is initially high and NOT neurons show strong modulation as long as retinal-error continues in an ipsiversive direction. When the target spot is briefly extinguished during pursuit, NOT neuronal firing abruptly drops, even if eye motion continues. Modeling results show that NOT pursuit neurons are most sensitive to retinal-error velocity [18]. Three-component retinal-error models provide excellent fits to empirical data (Fig. 2, right).
Smooth pursuit related responses of MST and FEF neurons
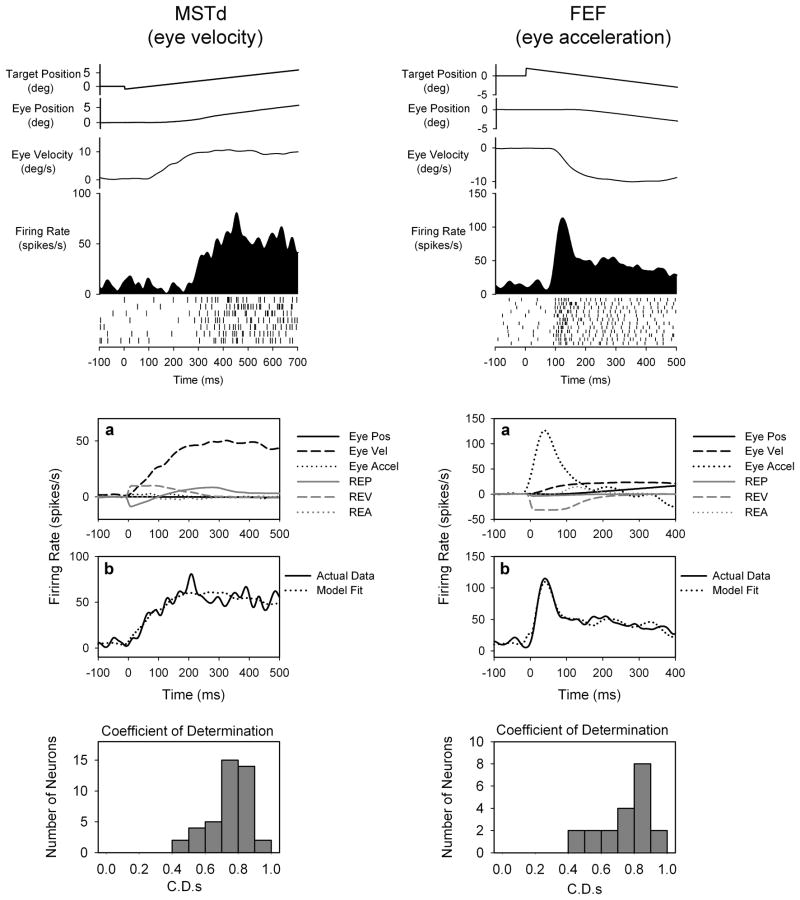
DLPN and NRTP neurons receive strong inputs from MST and FEF, respectively. In figure 3 we illustrate typical responses of MSTd and FEF neurons during step-ramp smooth pursuit. MSTd neurons show strikingly different response properties compared to those of FEF neurons. MSTd neurons are most sensitive to eye velocity but also have response onset times that occur after pursuit initiation [20]. Modeling results show that MSTd neurons are most sensitive to eye velocity. In contrast, FEF neurons have responses that lead smooth pursuit initiation. Many FEF neurons show maximum sensitivity to eye-acceleration or eye velocity. Retinal-error makes relatively small contributions to FEF neuronal response during smooth pursuit. Target blink testing of MSTd and FEF neurons demonstrates extraretinal sensitivity. Therefore, models including eye- and retinal error-motion provide the best fits to observed neuronal response.
Figure 3.
Examples of SP related neurons in MSTd and FEF. MSTd and FEF neurons are most sensitive to eye velocity and eye acceleration, respectively. (conventions as in figure 2).
Smooth pursuit related responses of identified cortical projection neurons
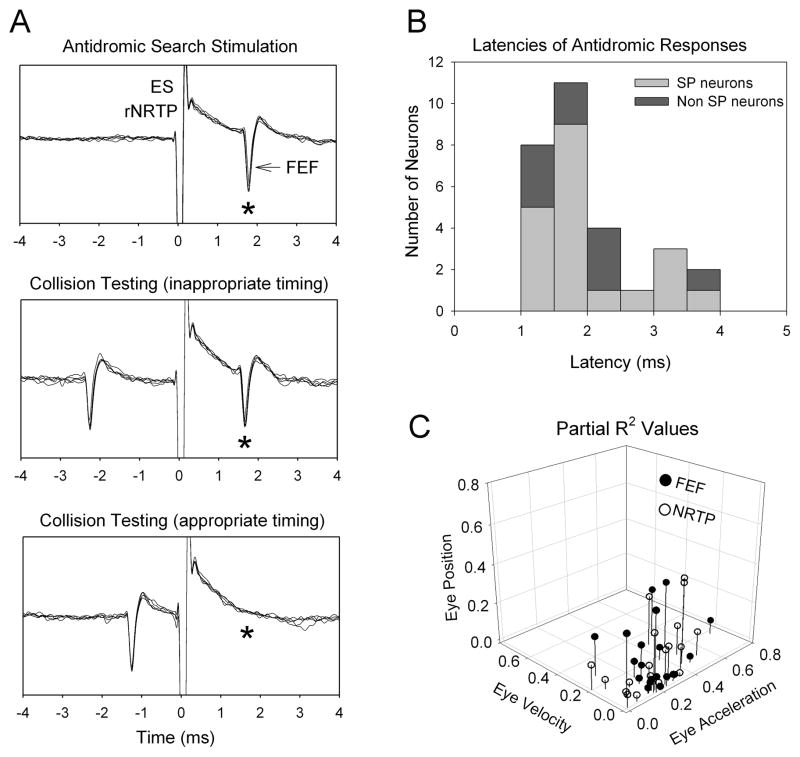
Antidromic activation provides the best method for identifying projection neurons in a given cortical area. We delivered biphasic, electrical stimulation (ES) pulses (200μs, 20–100μA) in the rNRTP, to activate FEF neurons (Fig. 4. left). We were successful in activating 29 well-isolated FEF neurons. Proof of antidromic activation was achieved using collision testing [17], where a naturally occurring FEF spike triggers a single pulse of ES (Fig. 4B, middle). When the delay between the naturally occurring spike and the ES pulse is appropriate spike collision occurs (Fig. 4A, bottom). We found that FEF smooth pursuit neurons projecting to rNRTP were activated at short latencies (median 1.69 ms; Fig. 4B) and were sensitive to eye-acceleration sensitivity. We found considerable overlap in the parital-r2 derived from models of antidromically activated FEF and rNRTP smooth pursuit neurons in (Fig. 4C). This result supports the suggestion that FEF signals are faithfully relayed by rNRTP neurons to distal sites. In contrast, MSTd have less overlap with NRTP and FEF neurons (not illustrated).
Figure 4.
Characterization of signals in FEF-NRTP pathway. Example of FEF that was antidromically activated following stimulation of rNRTP. Left column (A) shows five successive antidromic trials for each testing condition aligned on the electrical stimulation artifact. Top, search mode reveals antidromic neuron elicited at constant latency. Middle: Antidromic spikes (*) continued to be elicited when inappropriate timing was used between a naturally occurring FEF spike and the stimulus pulse. Bottom panel: when appropriate timing was used between the naturally occurring spike and the stimulus pulse, collision occurs (i.e., no evoked FEF at expected time (*). Antidromic latencies for smooth pursuit and visual neurons in FEF (B). partial-r2 values for FEF and NRTP neurons (C).
DISCUSSION
Smooth pursuit depends on the activity of neurons in multiple cortical, brainstem and cerebellar areas [2, 21]. A wealth of anatomical data shows that there is specificity in cortical projections to brainstem targets with known roles in smooth pursuit [16]. DLPN, NRTP and NOT all provide different regions of the cerebellum with signals appropriate for initiation, maintenance and plasticity of smooth pursuit. For example, the projection of NRTP to the vermis and DLPN to the floccular complex may preserve complimentary channels of information originating in parietal and frontal cortex [16]. In addition to their role in driving smooth pursuit, these pathways may have differential contributions to gain control [13] and adaptive capability [22].
The properties of neurons in NOT, DLPN and NRTP could be largely determined by their cortical inputs. One way to test this possibility is to use antidromic activation of cortical neurons from specific brainstem areas and then compare the properties of neurons in the connected regions. Our initial work using this approach indicates that at least some rNRTP neurons have overlapping smooth pursuit sensitivities with FEF neurons. Further studies are needed to determine the degree of signal integration in other basilar pontine centers.
Using an approach like that described here has some important limitations and advantages. First, only layer-5 neurons project to the brainstem, so most recorded neurons will not be antidromically activated following brainstem stimulation. We know relatively little about laminar specialization of function in smooth pursuit related cortical areas, so positively identifying and characterizing layer-5 neurons is important. Second, in our current studies we have focused on smooth pursuit metrics and neuronal response related to motion components. Other influences including attention [23], prediction [14] and target selection [2] may influence brainstem neurons in ways not tested in the present work.
In conclusion, by using the same multiple linear regression modeling approach in rNRTP, DLPN, NOT, MST and FEF, we have been able to compare and contrast eye- and retinal error-motion sensitivities in all of these areas. Converging evidence from neuroanatomical and neurophysiological studies indicate that parallel FEF-rNRTP, MST-DLPN and MT/MST-NOT channels carry different signals to the floccular complex and vermis of the cerebellum appropriate for initiation, maintenance and plastic adjustment of smooth pursuit.
Acknowledgments
We wish to acknowledge the expert assistance provided by Mrs. Tracey Fountain and Dr. Katia C. Vitorello. This work was supported by grants from the National Institutes of Health Grants. (EY13308; EY06069; RR00165).
References
- 1.Leigh RJ, Zee DS. The neurology of eye movements. 4. New York: Oxford Univ. Press; 2006. [Google Scholar]
- 2.Krauzlis RJ. Recasting the smooth pursuit eye movement system. J Neurophysiol. 2004;91:591–603. doi: 10.1152/jn.00801.2003. [DOI] [PubMed] [Google Scholar]
- 3.Voogd J, Barmack NH. Oculomotor Cerebellum. Prog Brain Res. 2006;151:231–268. doi: 10.1016/S0079-6123(05)51008-2. [DOI] [PubMed] [Google Scholar]
- 4.Newsome WT, Wurtz RH, Dursteler MR, Mikami A. Deficits in visual motion processing following ibotenic acid lesions of the middle temporal visual area of the macaque monkey. J Neurosci. 1985;5:825–840. doi: 10.1523/JNEUROSCI.05-03-00825.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dursteler MR, Wurtz RH, Newsome WT. Directional pursuit deficits following lesions of the foveal representation within the superior temporal sulcus of the macaque monkey. J Neurophysiol. 1987;57:1262–1287. doi: 10.1152/jn.1987.57.5.1262. [DOI] [PubMed] [Google Scholar]
- 6.Newsome WT, Wurtz RH, Komatsu H. Relation of cortical areas MT and MST to pursuit eye movements. II. Differentiation of retinal from extraretinal inputs. J Neurophysiol. 1988;60:604–620. doi: 10.1152/jn.1988.60.2.604. [DOI] [PubMed] [Google Scholar]
- 7.Squatrito S, Maioli MG. Gaze field properties of eye position neurones in areas MST and 7a of the macaque monkey. Visual Neuroscience. 1996;13:385–398. doi: 10.1017/s0952523800007628. [DOI] [PubMed] [Google Scholar]
- 8.Ono S, Mustari MJ. Horizontal smooth pursuit adaptation in macaques after muscimol inactivation of the dorsolateral pontine nucleus (DLPN) J Neurophysiol. 2007;98:2918–2932. doi: 10.1152/jn.00115.2007. [DOI] [PubMed] [Google Scholar]
- 9.Lynch JC, Tian JR. Cortico-cortical networks and cortico-subcortical loops for the higher control of eye movements. Prog Brain Res. 2006;151:461–501. doi: 10.1016/S0079-6123(05)51015-X. [DOI] [PubMed] [Google Scholar]
- 10.Keating EG. Frontal eye field lesions impair predictive and visually-guided pursuit eye movements. Exp Brain Res. 1991;86:311–323. doi: 10.1007/BF00228954. [DOI] [PubMed] [Google Scholar]
- 11.MacAvoy MG, Gottlieb JP, Bruce CJ. Smooth-pursuit eye movement representation in the primate frontal eye field. Cerebral Cortex. 1991;1:95–102. doi: 10.1093/cercor/1.1.95. [DOI] [PubMed] [Google Scholar]
- 12.Shi D, Friedman HR, Bruce CJ. Deficits in smooth-pursuit eye movements after muscimol inactivation within the primate’s frontal eye field. J Neurophysiol. 1998;80:458–464. doi: 10.1152/jn.1998.80.1.458. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka M, Lisberger SG. Regulation of the gain of visually guided smooth-pursuit eye movements by frontal cortex. Nature. 2001;409:191–194. doi: 10.1038/35051582. [DOI] [PubMed] [Google Scholar]
- 14.Fukushima K. Frontal cortical control of smooth-pursuit. Current Opinion in Neurobiology. 2003;13:647–654. doi: 10.1016/j.conb.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 15.Distler C, Mustari MJ, Hoffmann KP. Cortical projections to the nucleus of the optic tract and dorsal terminal nucleus and to the dorsolateral pontine nucleus in macaques: a dual retrograde tracing study. J Comp Neurol. 2002;444:144–158. doi: 10.1002/cne.10127. [DOI] [PubMed] [Google Scholar]
- 16.Their P, Möck M. The oculomotor role of the pontine nuclei and the nucleus reticularis tegmenti pontis. Prog Brain Res. 2006;151:293–320. doi: 10.1016/S0079-6123(05)51010-0. [DOI] [PubMed] [Google Scholar]
- 17.Ono S, Mustari MJ. Smooth Pursuit Related Information Processing in Frontal Eye Field (FEF) Neurons that Project to the NRTP. Cerebral Cortex. 2008 doi: 10.1093/cercor/bhn166. in-press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Das VE, Economides JR, Ono S, Mustari MJ. Information processing by parafoveal cells in the primate nucleus of the optic tract. Exp Brain Res. 2001;140:301–310. doi: 10.1007/s002210100816. [DOI] [PubMed] [Google Scholar]
- 19.Ono S, Das VE, Mustari MJ. Gaze-related response properties of DLPN and NRTP neurons in the rhesus macaque. J Neurophysiol. 2004;91:2484–2500. doi: 10.1152/jn.01005.2003. [DOI] [PubMed] [Google Scholar]
- 20.Ono S, Mustari MJ. Extraretinal signals in MSTd neurons related to volitional smooth pursuit. J Neurophysiol. 2006;96:2819–2825. doi: 10.1152/jn.00538.2006. [DOI] [PubMed] [Google Scholar]
- 21.Fukushima K, Kasahara S, Akao T, Kurkin S, Fukushima J, Peterson BW. Eye-Pursuit and Reafferent Head Movement Signals Carried by Pursuit Neurons in the Caudal Part of the Frontal Eye Fields during Head-Free Pursuit. Cereb Cortex. 2008 doi: 10.1093/cercor/bhn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takagi M, Zee DS, Tamargo RJ. Effects of lesions of the oculomotor cerebellar vermis on eye movements in primate: smooth pursuit. J Neurophysiol. 2000;83:2047–2062. doi: 10.1152/jn.2000.83.4.2047. [DOI] [PubMed] [Google Scholar]
- 23.Schall JD. On the role of frontal eye field in guiding attention and saccades. Vision Res. 2004;44:1453–1467. doi: 10.1016/j.visres.2003.10.025. [DOI] [PubMed] [Google Scholar]