Abstract
Gastroesophageal reflux disease complicated by Barrett’s esophagus (BE) is a major risk factor for esophageal adenocarcinoma (EA). However, the mechanisms of the progression from BE to EA are not fully understood. Besides acid reflux, bile acid reflux may also play an important role in the progression from BE to EA. In this study we examined the role of phosphatidylinositol-specific phospholipase C (PI-PLC) and a novel NADPH oxidase NOX5-S in bile acid-induced in cell proliferation. We found that taurodeoxycholic acid (TDCA) significantly increased NOX5-S expression, H2O2 production and cell proliferation in EA cells. The TDCA-induced increase in cell proliferation was significantly reduced by U73122, an inhibiter of PI-PLC. PI-PLCβ1, β3, β4, γ1 and γ2, but not β2 and δ1 were detectable in FLO cells by Western blot analysis. Knockdown of PI-PLCγ2 or ERK-2 MAP kinase with siRNAs significantly decreased TDCA-induced increase in NOX5-S expression, H2O2 production and cell proliferation. In contrast, knockdown of PI-PLC β1, β3, β4, γ1 or ERK-1 MAP kinase had no significant effect. TDCA significantly increased ERK-2 phosphorylation, an increase which was reduced by U73122 or PI-PLCγ2 siRNA. We conclude that TDCA-induced increase in NOX5-S expression and cell proliferation may depend on sequential activation of PI-PLCγ2 and ERK-2 MAP kinase in EA cells. It is possible that bile acid reflux present in patients with Barrett’s esophagus may increase ROS production and cell proliferation via activation of PI-PLCγ2, ERK-2 MAP kinase and NADPH oxidase NOX5-S, thereby contributing to the development of EA.
Keywords: phosphatidylinositol-specific phospholipase C, NOX5, Barrett’s esophagus, esophageal adenocarcinoma, bile acid
Introduction
The incidence of esophageal adenocarcinoma (EA) has increasedby more than 6 fold in the past three decades (1). Gastroesophageal reflux disease (GERD) complicated by Barrett’s esophagus (BE) is a major risk factor for EA (2). The mechanisms of the progression from BE to EA are not known. Reactive oxygen species (ROS) may play an important role in the development of esophageal adenocarcinoma since levels of ROS are increased in BE (3) and EA(4, 5). ROS may cause damage to DNA, RNA, lipids and proteins, which may result in increased mutation and altered functions of enzyme and proteins (e.g. activation of oncogene products and/or inhibition of tumor suppressor proteins) (4, 6). However, the sources of ROS in these conditions have not been well defined.
Low levels of ROS, seen in non-phagocytic cells, were thought to be byproducts of aerobic metabolism. More recently, however, superoxide-generating homologues of phagocytic NADPH oxidase catalytic subunit gp91phox (NOX1, NOX3-NOX5, DUOX1, DUOX2) and homologues of other subunits (p41phox or NOXO1, p51phox or NOXA1) have been found in several cell types(7, 8), suggesting that ROS generated in these cells may have distinctive cellular functions related to immunity, signal transduction and modification of the extracellular matrix. NOX5 has five isoforms: α, β, δ, γ and NOX5-S (9, 10). NOX5 α, β, δ and γ have EF-hand motifs at its N-terminal (9), whereas NOX5-S does not (11). We have shown that the NADPH oxidase isoform NOX5-S is present in EA FLO cells (12) and that levels of NOX5-S are significantly increased in Barrett’s esophageal mucosa with high-grade dysplasia (13).
Bile acids may also play an important role in the progression from BE to EA (14, 15) since 1) in animal models, diversion of duodenal contents into the lower esophagus leads to EA (16, 17); 2) Bile acids are known to induce oxidative stress and DNA damage (18, 19); 3) bile salts may induce up-regulation of cyclooxygenase-2 and c-myc expression (20, 21), and activate mitogen activated protein (MAP) kinase and NF-κB pathways (22, 23), thereby increasing cell proliferation and decreasing cell apoptosis. However, mechanisms whereby bile acids promote the development of EA are not known.
We have shown that bile acid taurodeoxycholic acid (TDCA)-induced upregulation of NOX5-S expression and increase in cell proliferation depend on activation of TGR5 receptor (a bile acid receptor) and Gαq protein in EAcells (12). PI-PLC has been reported to be activated by the Gαq protein family(24, 25). Gαq proteins are involved in TDCA-induced NOX5-S expression and H2O2 production. Whether PI-PLC plays a role in TDCA-induced NOX5 expression, H2O2 production and cell proliferation has not been established. We now show that TDCA-induced increase in cell proliferation and upregulation of NOX5-S expression is mediated by activation of phosphatidylinositol-specific phospholipase C (PI-PLC) γ2 and ERK2 MAP kinase in EAcells.
Material and Methods
Cell Culture and TDCA Treatment
Human EA cell line FLO was derived from human EA (26) and generously provided by Dr. David Beer in 2004 (University of Michigan Medical School). FLO cells matched the genotype of the progenitor tumor cells from the patient’s tumor tissue block. Cells were cultured in DMEM containing 10 % fetal bovine serum and antibiotics at 37 °C with 5 % CO2 humidified atmosphere.
Human EA cell line OE33 was cultured in DMEM containing 10 % fetal bovine serum and antibiotics. The cell lines were cultured at 37 °C in a 5 % CO2 humidified atmosphere.
For TDCA treatment, FLO cells were incubated with TDCA 10−11M for 24 h. For use of inhibitors, cells were pretreated with U73122 (10−6 M), PD98059 (10−5 M), or culture medium (control) for 1 h, and cultured in fresh medium (pH 7.2, without phenol red) without TDCA and inhibitors (control), with TDCA (10−11 M) or with TDCA and the above inhibitors for an additional 24 h. Finally, the culture medium and cells were collected for measurements.
Small Interfering RNA (siRNA) and Plasmid Transfection
24 h before transfection at 70–80 % confluence, cells were trypsinized and diluted 1:5 with fresh medium without antibiotics (1–3 ×105 cells/ml) and transferred to 12-well plates (1 ml/well). Transfection of siRNAs was carried out with Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instruction. Per well, 75 pmol of siRNA duplex of PI-PLCγ1-2, PI-PLCβ1, PI-PLCβ3-4, ERK1-2 or control siRNA formulated into liposomes were applied; the final volume was 1.2 ml/well. 48 h after transfection, cells were treated without or with TDCA in culture medium (pH 7.2, without phenol red) for 24 h, and then the culture medium and cells were collected for measurements. Transfection efficiencies were determined by fluorescence microscopy after transfection of Block-it fluorescent oligonucleotide (Invitrogen) and were about 70 % at 48 h.
Reverse Transcription-PCR
Total RNA was extracted by TRIzol reagent (Invitrogen) for the cultured cells and purified by the total RNA purification system (Invitrogen). According to the protocols of the manufacturers, 1.5 μg of total RNAs from cultured cells was reversely transcribed by using a SUPERSCRIPT™ first strand synthesis system for reverse transcription-PCR (Invitrogen).
Quantitative Real Time PCR
Quantitative real time PCR was carried out on a Stratagene Mx4000®multiplex quantitative PCR system. The primers used were: NOX5-S sense (5′-AAGACTCCATCACGGGGCTGCA-3′), NOX5-S antisense (5′-CCTTCAGCACCTTGGCCAGA -3′), GAPDH sense (5′-CATGACCACAGTC CATGCCATCAC-3′), and GAPDH antisense (5′-AGGTCCACCACCCTGTTGCTGTA-3′). All reactions were performed in triplicate in a 25 μl total volume containing a 1×concentration of Brilliant® SYBR® Green QPCR Master Mix (Stratagene), the concentration of each sense and antisense primer were 100 nM, 1 μl cDNA, and 30 nM reference dyes. Reactions were carried out in a Stratagene Mx4000®multiplex quantitative PCR system for one cycle at 94 °C for 5 min; 40 cycles at 94 °C for 30 s, 59 °C for 30 s, and 72 °C for 30 s; one cycle at 94 °C for 1 min; and one cycle at 55 °C for 30 s. Fluorescence values of SYBR Green I dye, representing the amount of product amplified at that point in the reaction, were recorded in real time at both the annealing step and the extension step of each cycle. The Ct, defined as the point at which the fluorescence signal was statistically significant above background, was calculated for each amplicon in each experimental sample using Stratagene Mx4000 software. This value was then used to determine the relative amount of amplification in each sample by interpolating from the standard curve. The transcript level of each specific gene was normalized to GAPDH amplification.
Western Blot Analysis
Cells were lysed in Triton X lysis buffer. The suspension was centrifuged at 15,000 ×g for 5 min, and the protein concentration in the supernatant was determined. Western blot was done as described previously (27). Briefly, after these supernatants were subjected to SDS-PAGE, the separated proteins were electrophoretically transferred to a nitrocellulose membrane at 100 V, 1h. The nitrocellulose membranes were blocked in 5% nonfat dry milk and then incubated with appropriate primary antibodies followed by 60-min incubation in horseradish peroxidase-conjugated secondary antibody (Amersham Biosciences). Detection was achieved with an enhanced chemiluminescence agent (Amersham Biosciences).
Primary antibodies used were as follows: PI-PLCγ1-2 antibody (1:1000), PI-PLCβ1 antibody (1:1000), PI-PLCβ3-4 antibody (1:1000), phospho-MAP kinase antibody (1:1000), ERK-2 antibody(1:1000) and GAPDH antibody (1:2000), NOX5 antibody was prepared against a mixture of unique NOX5 peptides (NH2-YESFKASDPLGRGSKRC-COOH and NH2-YRHQKRKHTCPS-COOH) and used at a dilution of 1:1000.
[3H]Thymidine Incorporation
24 h after pretreatment with U73122 or PD98059, or transfection with siRNAs of PI-PLCγ1-2, PI-PLCβ1, PI-PLCβ3-4, ERK1-2, or control, cells were treated without or with TDCA for 24 h and then incubated with methyl-[3H] thymidine (0.05 μCi/ml) for 4 h. After washing three times with PBS to remove unincorporated radioactivity, cells were collected and homogenized with a lysis buffer containing (pH 7.4): 50 mM HEPES, 50 mM NaCl, 1% Triton X-100, 1% Nonidet P-40, 0.1 mM phenylmethylsulfonyl fluoride and 1 mM dithiothreitol. Methyl-[3H] thymidine uptake was measured in a scintillation counter. The level of protein in the homogenates was also determined and the level of methyl-[3H] thymidine incorporation was normalized to protein content.
Amplex® Red Hydrogen Peroxide Fluorescent Assay
Levels of H2O2 in culture medium were determined by using the Amplex® Red H2O2 assay kit (Molecular Probes, Inc., Eugene, OR). This assay uses the Amplex Red reagent (10-acetyl-3, 7- dihydroxyphenoxazine) to detect H2O2. In the presence of peroxidase, the Amplex Red reagent reacts with H2O2 in a 1:1 stoichiometry to produce the red fluorescent oxidation product resorufin. Fluorescence is then measured with a fluorescence microplate reader using excitation at 550 nm and emission detection at 590 nm.
Protein Measurement
The amount of protein was determined by colorimetric analysis (Bio-Rad) according to the method of Bradford (28).
Materials
[3H] thymidine was purchased from PerkinElmer Life Sciences, and human phospho-MAP kinase antibody was from Cell Signal Inc. (Danvers, MA). PI-PLCγ1-2, PI-PLCβ1, PI-PLCβ3-4, ERK-2 antibody, GAPDH antibody, PI-PLC γ1-2 siRNA, PI-PLCβ1 siRNA, PI-PLCβ3-4 siRNA and ERK-1 siRNA were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA); ERK-2 siRNA was from Ambion Inc. EA cell line OE33, Triton X-100, Nonidet P-40, phenylmethylsulfonyl fluoride, DL-dithiothreitol, HEPES sodium, and other reagents were purchased from Sigma.
Statistical Analysis
Data is expressed as mean ± S.E. Statistical differences between two groups were determined by Student’s t test. Differences between multiple groups were tested using analysis of variance (ANOVA) and checked for significance using Fisher’s protected least significant difference test. All experiments were repeated for at least three times.
Results
PI-PLC Involved in TDCA-Induced NOX5-S Expression in FLO Cells
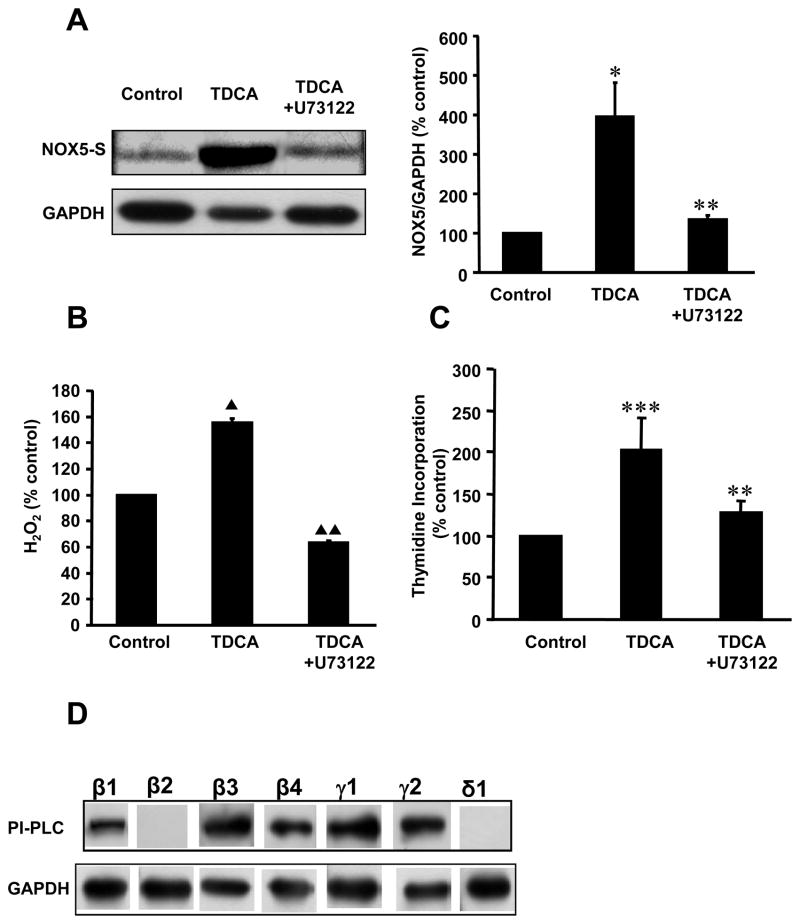
In this study, we found that low dose of TDCA (10−11 M. 24h) caused 4-fold increase in NOX5-S expression, 1.6-fold increase in hydrogen peroxide (H2O2) production and 2-fold increase in cell proliferation in FLO cells, when compared with control (Fig. 1A-C). These changes were statistically significant. We also found that the PI-PLC inhibitor U73122 (29) decreased TDCA-induced NOX5-S protein expression from 395.1±87.1 to 135.1±11.3% control (Fig. 1A, p< 0.02), H2O2 production from 155.1±3.5 to 63.2±1.9 % control (Fig. 1B, p<0.001) and thymidine incorporation from 202.4±38.0 to 127.7±13.2% control (Fig. 1C, p<0.02) in FLO cells. These data suggest that PI-PLC may be involved in TDCA-induced NOX5-S expression and H2O2 production in FLO cells.
Figure 1. Effect of U73122 on TDCA-induced NOX5-S expression.
(A) A typical example of Western blot analysis and summarized data showed PI-PLC inhibitor U73122 significantly decreased TDCA-induced NOX5-S protein expression. (B) U73122 significantly reduced TDCA-induced H2O2 production. (C) U73122 significantly reduced TDCA-induced thymidine incorporation. The data suggest that TDCA-induced increase in NOX5-S expression, H2O2 and cell proliferation may depend on activation of PI-PLC. (D) A typical example of Western blot analysis of three experiments showed that PI-PLC β1, β3, β4, γ1, and γ2, but not β2 or δ1, were detectable in human EAcells FLO. For TDCA treatment, FLO cells were pretreated with U73122 (10−6 M) or culture medium (control) for 1 h, and then treated with TDCA (10−11 M) or TDCA+U73122 for 24 h. The culture medium was collected for measurement of H2O2 and cells for measurement of mRNA expression. N=3, ANOVA, * P<0.02, compared with control, ** P<0.02, compared with TDCA alone group; *** P<0.001, compared with control group; ▲ P<0.001, compared with control, ▲ ▲ P<0.001, compared with TDCA alone group.
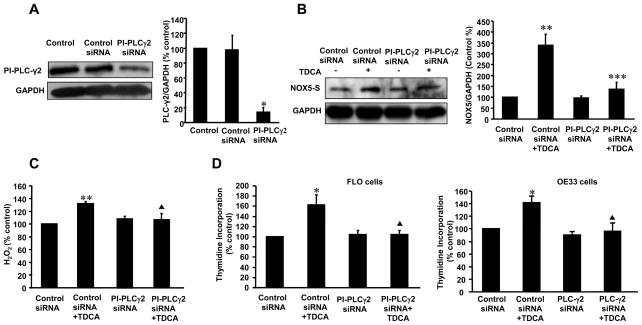
Seven major PI-PLC isoforms have been identified so far(30). To further determine which PI-PLC isoforms mediate TDCA-induced NOX5-S expression, we used Western blot analysis to examine which PI-PLC isoforms are present in FLO cells. Isoforms of PI-PLC β1, β3, β4, γ1 andγ2 (but not β2 and δ1) were detectable in FLO cells (Fig. 1D). To examine whether these isoforms of PI-PLC regulate(s) TDCA-induced NOX5-S in FLO cells, we used PI-PLC β1, β3, β4, γ1 andγ2 siRNA to knock down expression of these proteins. PI-PLC-γ2 siRNA significantly decreased its protein expression (Fig. 2A) 48 h after transfection. Knockdown of PI-PLC-γ2 protein expression with PLC-γ2 siRNA decreased TDCA-induced NOX5-S expression from 339.7±50.2 to 136.7±32.4% control (Fig. 2B, p<0.001), H2O2 production from 131.5±2.6 to 106.7±9.7% control (Fig. 2C, p<0.001) and thymidine incorporation from 162.5±19.4 to 103.6±8.8% control (Fig. 2D, p<0.001) in FLO cells. Knockdown of PI-PLCγ2 protein also remarkablely decreased TDCA-induced cell proliferation in OE33 EA cells (Fig. 2D). However, knockdown of PI-PLC-β1,β3, β4, or γ1 protein expression did not affect TDCA-induced NOX5-S expression (supplementary data Fig. 1 and Fig. 2). The data suggest that TDCA-induced NOX5-S expression may depend on activation of PI-PLC-γ2 protein, but not PI-PLC-β1, β3, β4, or γ1 protein.
Figure 2. Role of PI-PLC γ2 in TDCA-induced NOX5-S expression.
(A) A typical example of Western blot analysis and summarized data showed that transfection with PI-PLC γ2 siRNA significantly decreased PI-PLC γ2 protein expression in FLO EA cells, indicating that PI-PLC γ2 siRNA effectively knocked down PI-PLC γ2 protein expression (n=3). (B) A typical example of Western blot analysis and summarized data show that knockdown of PI-PLC γ2 protein significantly decreased TDCA-induced NOX5-S expression. (C) Knockdown of PI-PLC γ2 protein significantly decreased TDCA-induced H2O2 production. (D) Knockdown of PI-PLC γ2 protein significantly decreased TDCA-induced thymidine incorporation both in FLO and OE33 cells. The data suggest that PI-PLC γ2 protein may contribute to TDCA-induced NOX5-S expression, H2O2 production and cell proliferation in EA cells. Transfection of siRNA was carried out with Lipofectamine 2000. Per well, 75 pmol of siRNA duplex of PI-PLC γ2 or control siRNA formulated into liposomes were applied. After a 4-h transfection, the transfection medium was replaced with regular medium. 24 h after transfection, cells were treated with low dose of TDCA for 24 h, and then culture medium and cells were collected for measurements. N=3, ANOVA, * P<0.02, compared with control group and control siRNA group; ** P<0.02, compared with control siRNA group and PI-PLC γ2 siRNA group; *** P<0.001, compared with control siRNA+ TDCA group, ▲ P<0.001, compared with control siRNA+ TDCA group.
Role of ERK MAP kinases in TDCA-induced NOX5-S Expression
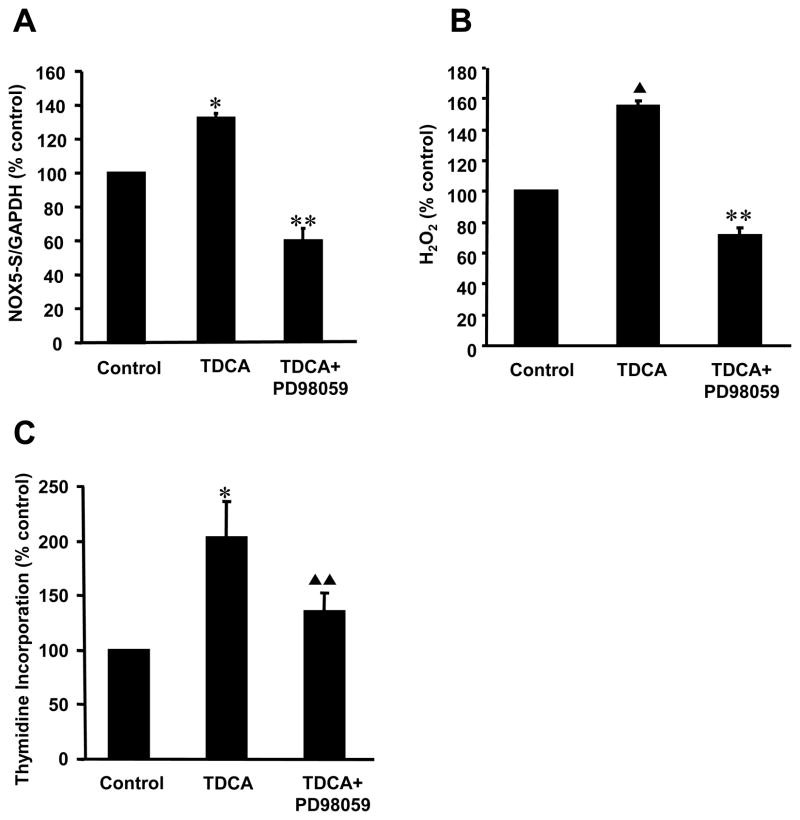
Activation of PI-PLC produces inositol triphosphate and diacylglycerol. Diacylglycerol has been shown to activate the protein kinase C-MAP kinase pathway(31).Therefore, we examined whether ERK MAP kinases mediate TDCA-induced NOX5-S expression. The MAP kinase kinase inhibitor PD98059 (32) decreased TDCA-induced NOX5-S mRNA expression from 131.4±3.9 to 59.8±7.6% control (Fig. 3A, p<0.001), H2O2 production from 155.1±3.5 to 71.1±5.2% control (Fig. 3B, p<0.001) and thymidine incorporation from 202.9±33.7 to 135.6±16.4% control (Fig. 3C, p<0.01), indicating that TDCA-induced increase in NOX5-S expression, H2O2 production and cell proliferation may depend on activation of ERK MAP kinases.
Figure 3. Effect of PD98059 on TDCA-induced NOX5-S expression.
(A) MEK1/2 kinase inhibitor PD98059 significantly decreased TDCA-induced NOX5-S expression measured by real time PCR. (B) PD98059 significantly decreased TDCA-induced H2O2 production. (C) PD98059 significantly decreased TDCA-induced thymidine incorporation. The data suggest that TDCA-induced increase in NOX5-S expression, H2O2 production and cell proliferation may depend on activation of ERK MAP kinases. N=3, ANOVA, * P<0.02, compared with control group, ** P<0.001, compared with TDCA alone group, ▲ P<0.01, compared with control group, ▲ ▲ P<0.01, compared with TDCA alone group.
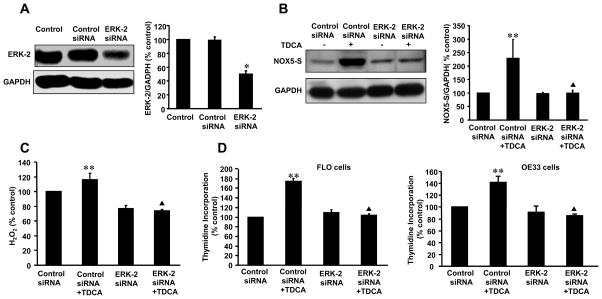
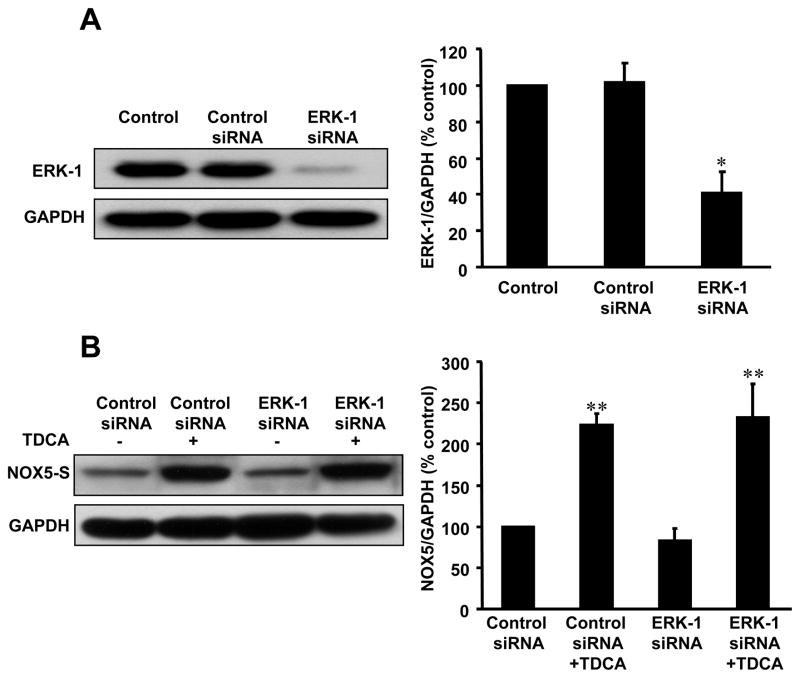
We used ERK-1 and ERK-2 siRNA to knock down ERK-1 and ERK-2 expression respectively. ERK-1 and ERK-2 siRNA significantly decreased its corresponding protein expression (Fig. 4A; Fig. 5A) 48 h after transfection. Knockdown of ERK-2 protein expression with ERK-2 siRNA decreased TDCA-induced NOX5-S expression from 228.7±70 to 89.4±11.7% control (Fig. 4B, p<0.05), H2O2 production from 116.3±8.5 to 73.6±1.7% control (Fig. 4C, p<0.05) and thymidine incorporation from 173.8±7.4 to 103.7±2.9% control (Fig. 4D) in FLO cells. Knockdown of ERK-2 protein expression with ERK-2 siRNA also significantly decreased thymidine incorporation in response to TDCA treatment in OE33 cells. Conversely, knockdown of ERK-1 protein expression had no statistically significant effect on NOX5-S expression induced by TDCA stimulation (Fig. 5B). The data suggest that TDCA-induced NOX5-S expression, H2O2 production and cell proliferation may be mediated by activation of ERK-2 MAP kinases, but not ERK-1.
Figure 4. Role of ERK-2 MAP kinase in TDCA-induced NOX5-S expression.
(A) A typical example of Western blot analysis and summarized data show that transfection with ERK-2 MAP kinase siRNA significantly decreased ERK-2 protein expression in FLO EA cells, indicating that ERK-2 siRNA effectively knocks down ERK-2 protein expression. (B) A typical example of Western blot analysis and summarized data show that knockdown of ERK-2 MAP kinase protein significantly decreased TDCA-induced NOX5-S expression. (C) Knockdown of ERK-2 MAP kinase protein significantly decreased TDCA-induced H2O2 production. (D) Knockdown of ERK-2 MAP kinase protein significantly decreased TDCA-induced thymidine incorporation both in FLO and OE33 cells. The data suggest that ERK-2 MAP kinase may mediate TDCA-induced NOX5-S expression, H2O2 production and cell proliferation in EA cells. Transfection of siRNA was carried out with Lipofectamine 2000. Per well, 75 pmol of siRNA duplex of ERK-2 MAP kinase siRNA or control siRNA formulated into liposomes were applied. After a 4-h transfection, the transfection medium was replaced with regular medium. 24 h after transfection, cells were treated with low dose of TDCA for 24 h, and then culture medium and cells were collected for measurements. N=3, ANOVA, * P<0.02, compared with control siRNA group, ** P<0.05, compared with control siRNA group; ▲ P<0.05, compared with control siRNA+TDCA group.
Figure 5. Role of ERK-1 MAP kinase in TDCA-induced NOX5-S expression.
(A) A typical example of Western blot analysis and summarized data showed that transfection with ERK-1 MAP kinase siRNA significantly decreased ERK-1 protein expression in FLO EA cells, indicating that ERK-1 siRNA effectively knocks down ERK-1 protein expression. (B) A typical example of Western blot analysis and summarized data show that knockdown of ERK-1 MAP kinase protein did not significantly affect TDCA-induced NOX5-S expression. The data suggest that TDCA-induced NOX5-S expression may not be mediated by activation of ERK-1 in FLO EA cells. Transfection of siRNA was carried out with Lipofectamine 2000. Per well, 75 pmol of siRNA duplex of ERK-1 or control siRNA formulated into liposomes were applied. After a 4-h transfection, the transfection medium was replaced with regular medium. 24 h after transfection, cells were treated with low dose of TDCA for 24 h, and then culture medium and cells were collected for measurements. N=3, ANOVA, * P<0.01, compared with control group or control siRNA group, ** P<0.01, compared with control siRNA group or ERK-1 siRNA group There no statistically significant difference between control siRNA+ TDCA group and ERK-1 siRNA+ TDCA group.
To further confirm the role of ERK-2 MAP kinases, we examined ERK-2 phosphorylation in FLO cells. We found that TDCA significantly increased ERK-2 phosphorylation by 4.5-fold (Fig. 6A), indicating that ERK-2 may be activated by TDCA in FLO cells. In addition, TDCA-induced ERK-2 phosphorylation was significantly reduced by U73122 (Fig. 6A), suggesting that activation of ERK-2 may depend on activation of PI-PLC. Knockdown of PI-PLC-γ2 expression significantly decreased TDCA-induced ERK-2 phosphorylation from 571±167.9 to 179.3±67.9% control (Fig. 6B, p<0.05). The data suggest that TDCA-induced NOX5-S expression may depend on sequential activation of PI-PLC-γ2 and ERK-2 MAP kinase.
Figure 6. ERK-2 phosphorylation in FLO EA cells.
(A) A typical example of Western blot analysis and summarized data show that TDCA significantly increased ERK-2 MAP kinase phosphorylation, an increase which was significantly reduced by U73122. The data suggest that TDCA-induced ERK-2 activation may depend on activation of PI-PLC. (B) A typical example of Western blot analysis and summarized data show that TDCA-induced ERK-2 phosphorylation was significantly reduced by knockdown of PI-PLC γ2 protein, suggesting that TDCA-induced ERK-2 activation may depend on activation of PI-PLC γ2 in FLO EA cells. Transfection of siRNA was carried out with Lipofectamine 2000. Per well, 75 pmol of siRNA duplex of ERK-2 or control siRNA formulated into liposomes were applied. After a 4-h transfection, the transfection medium was replaced with regular medium. 24 h after transfection, cells were treated with TDCA (10−9M) for 10 min, and then cells were collected for measurements. N=3, ANOVA, * P<0.001, compared with control group; ▲ P<0.001, compared with TDCA treatment group; ** P<0.05, compared with control siRNA group or PI-PLC γ2 siRNA group; ▲ ▲ P<0.05, compared with control siRNA+TDCA group.
Discussion
Bile acids have been shown to contribute to the development of EAin a rat model of Barrett’s esophagus(17). Therefore, we examined whether bile acids upregulate NOX5-S expression and increase ROS production, thereby increasing cell proliferation and contributing to the development of EA. Bile acids, a group of structurally diverse molecules that are primarily synthesized in the liver are the major components of bile. Besides their well-established roles in dietary lipid absorption and cholesterol homeostasis, it has recently emerged that bile acids are also signaling molecules in cell metabolism and signal transduction(33, 34). Taurodeoxycholic acid (TDCA) has been reported to be one of the major bile acids in the refluxate of patients with Barrett’s esophagus,(35) and is more toxic than primary bile acids, thus we used TDCA in our studies. Although reflux episodes are usually in terms of minutes or hours, particularly in long segment BE and in the supine position, per cent total time pH less than 4 ranges from 10.0 to 46.0 in BE patients,(35) i.e. esophageal mucosa is exposed to the refluxate for 2.4–11.0 hours per day. It is possible that low concentration of bile acids is present in the mucus layer that covers the surface of metaplastic cells for a much longer time after reflux episodes. In addition, it has been reported that short-term bile acid treatment does not alter cell proliferation in BAR-T cells.(36) Therefore, 24-hour treatment with TDCA was used in our studies. The concentrations of bile acids in micromolar range are commonly found in the refluxate of BE patients,(35) but metaplastic cells may be exposed to a much lower concentrations of bile acids due to the mucus layer that covers the surface of these cells. This notion is implicated by an in vivo study showing that the interstitial pH is conserved in normal rat esophagus when luminal pH is reduced to 1.0.(37) In addition, we have previously shown that 10−11M TDCA, but not higher doses, increases cell proliferation (12). Therefore, 10−11M TDCA was used in the present study. We found that TDCA increased NOX5-S expression, H2O2 production and cell proliferation.
The mechanisms of bile acids-induced increase in cell proliferation are poorly understood. We have found that TDCA-induced increase in NOX5-S expression and cell proliferation is mediated by activation of the TGR5 receptor and Gαq protein in FLO EA cells (12). It has been reported that Gαq protein family may activate PI-PLC (24, 25). Since Gαq proteins are involved in TDCA-induced NOX5-S expression and H2O2 production, we examined the role of PI-PLC in bile acid-induced NOX5-S expression in FLO cells. Based on comparison of the sequences and structural studies, PI-PLC has three kinds of eukaryotic isozymes, PI-PLCβ, PI-PLCγ and PI-PLCδ(30, 38). We found that TDCA-induced increase in NOX5-S expression and H2O2 production may depend on activation of PI-PLC-γ2 protein since 1) TDCA-induced increase in NOX5-S expression and H2O2 production was significantly reduced by the PI-PLC inhibitor U73122; 2) Knockdown of PI-PLC γ2 protein significantly reduced NOX5-S expression, H2O2 production and cell proliferation in response to TDCA treatment in FLO and/or OE33 cells; 3) knockdown of PI-PLC β1, β3, β4 and γ1 protein had no significant effect on TDCA-induced NOX5-S expression (supplementary data). The mechanisms whereby Gαq proteins activate PI-PLCγ2 are not clear. It is possible that Gαq first activates tyrosine kinase (39, 40) and then activates PI-PLCγ isoforms (41–43).
PI-PLC has been shown to regulate ERK-2 phosphorylation in osteoblasts and macrophages (44, 45). ERK MAP kinases are key kinases in cell proliferation and cell cycle regulation(46, 47). In addition, we have shown that activation of ERK MAP kinases contributed to platelet activating factor-induced NOX5-S expression (48). Therefore, we examined whether ERK MAP kinase plays a role in TDCA-induced NOX5-S expression and H2O2 production. We found that TDCA-induced increase in NOX5-S expression, H2O2 production and cell proliferation was significantly reduced by the MAP kinase inhibitor PD98059, suggesting that ERK MAP kinase is involved in TDCA-induced NOX5-S expression and H2O2 production. ERK MAP kinase has two isoforms, ERK-1 and ERK-2. These two isoforms can be activated respectively or together in different cell biological process(49, 50). To examine which isoform(s) of ERK MAP kinases participates in TDCA-induced NOX5-S expression and H2O2 production, ERK-1 and ERK-2 protein expression were down-regulated with their siRNAs, respectively. Knockdown of ERK-2 protein significantly reduced TDCA-induced NOX5-S expression, H2O2 production and cell proliferation. Conversely, knockdown of ERK-1 protein did not have significant effect. The data suggest that TDCA-induced NOX5-S expression may depend on activation of ERK-2 MAP kinase, but not ERK-1. To further confirm the role of ERK-2, we examined ERK-2 phosphorylation after TDCA simulation. We found that TDCA significantly increased ERK2 phosphorylation, which was significantly reduced by U73122 and by knockdown of PI-PLCγ2 protein, suggesting that TDCA-induced activation of ERK-2 MAP kinase may depend on activation of PI-PLC γ2. The data suggest that TDCA-induced NOX5-S expression may depend on sequential activation of PI-PLC and ERK-2 MAP kinase.
In conclusion, TDCA may induce increase in NOX5-S expression, H2O2 production and cell proliferation in EAcells. This increase may depend on sequential activation of PI-PLC γ2 and ERK-2 MAP kinase. It is possible that bile acid reflux present in patients with Barrett’s esophagus may increase ROS production and cell proliferation via activation of PI-PLCγ2, ERK-2 MAP kinase and NADPH oxidase NOX5-S, thereby causing DNA damage and gene mutation which contribute to the development of EA. Our data may provide potential targets to prevent and/or treat Barrett’s EA.
Supplementary Material
Acknowledgments
This work was supported by NIDDK R01 DK080703.
Abbreviations
- BE
Barrett’s esophagus
- EA
esophageal adenocarcinoma
- TDCA
taurodeoxycholic acid
- PI-PLC
Phosphatidylinositol-specific phospholipase C
- PPI
proton pump inhibitors
- ROS
reactive oxygen species
- NOX5-S
NAPDH oxidase short form
- siRNA
Small Interfering RNA
- H2O2
hydrogen peroxide
- MAP kinase
mitogen activated protein kinase
Footnotes
These data were presented in part at the 109th annual meeting of the American Gastroenterological Association, in San Diego CA, in May 2008.
References
- 1.Pohl H, Welch HG. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst. 2005;97:142–6. doi: 10.1093/jnci/dji024. [DOI] [PubMed] [Google Scholar]
- 2.Lagergren J, Bergstrom R, Lindgren A, Nyren O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med. 1999;340:825–831. doi: 10.1056/NEJM199903183401101. [DOI] [PubMed] [Google Scholar]
- 3.Olyaee M, Sontag S, Salman W, et al. Mucosal reactive oxygen species production in oesophagitis and Barrett’s oesophagus. Gut. 1995;37:168–73. doi: 10.1136/gut.37.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farhadi A, Fields J, Banan A, Keshavarzian A. Reactive oxygen species: are they involved in the pathogenesis of GERD, Barrett’s esophagus, and the latter’s progression toward esophageal cancer? Am J Gastroenterol. 2002;97:22–6. doi: 10.1111/j.1572-0241.2002.05444.x. [DOI] [PubMed] [Google Scholar]
- 5.Sihvo EI, Ruohtula T, Auvinen MI, Koivistoinen A, Harjula AL, Salo JA. Simultaneous progression of oxidative stress and angiogenesis in malignant transformation of Barrett esophagus. J Thorac Cardiovasc Surg. 2003;126:1952–1957. doi: 10.1016/j.jtcvs.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 6.Ohshima H, Tatemichi M, Sawa T. Chemical basis of inflammation-induced carcinogenesis. Arch Biochem Biophys. 2003;417:3–11. doi: 10.1016/s0003-9861(03)00283-2. [DOI] [PubMed] [Google Scholar]
- 7.Banfi B, Maturana A, Jaconi S, et al. A mammalian H+ channel generated through alternative splicing of the NADPH oxidase homolog NOH-1. Science. 2000;287:138–42. doi: 10.1126/science.287.5450.138. [DOI] [PubMed] [Google Scholar]
- 8.Suh YA, Arnold RS, Lassegue B, et al. Cell transformation by the superoxide-generating oxidase Mox1. Nature. 1999;401:79–82. doi: 10.1038/43459. [DOI] [PubMed] [Google Scholar]
- 9.Banfi B, Molnar G, Maturana A, et al. A Ca (2+)-activated NADPH oxidase in testis, spleen, and lymph nodes. J Biol Chem. 2001;276:37594–601. doi: 10.1074/jbc.M103034200. [DOI] [PubMed] [Google Scholar]
- 10.Vignais PV. The superoxide-generating NADPH oxidase: structural aspects and activation mechanism. Cell Mol Life Sci. 2002;59:1428–59. doi: 10.1007/s00018-002-8520-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng G, Cao Z, Xu X, van Meir EG, Lambeth JD. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene. 2001;269:131–40. doi: 10.1016/s0378-1119(01)00449-8. [DOI] [PubMed] [Google Scholar]
- 12.Hong J, Behar J, Wands J, et al. Role of a novel bile acid receptor TGR5 in the development of esophageal adenocarcinoma. GUT. 2009 doi: 10.1136/gut.2009.188375. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fu X, Beer DG, Behar J, Wands J, Lambeth D, Cao W. cAMP-response element-binding protein mediates acid-induced NADPH oxidase NOX5-S expression in Barrett esophageal adenocarcinoma cells. J Biol Chem. 2006;281:20368–82. doi: 10.1074/jbc.M603353200. [DOI] [PubMed] [Google Scholar]
- 14.Kauer WK, Stein HJ. Bile reflux in the constellation of gastroesophageal reflux disease. Thorac Surg Clin. 2005;15:335–40. doi: 10.1016/j.thorsurg.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Jankowski JA, Anderson M. Review article: management of oesophageal adenocarcinoma -- control of acid, bile and inflammation in intervention strategies for Barrett’s oesophagus. Aliment Pharmacol Ther. 2004;20(Suppl 5):71–80. doi: 10.1111/j.1365-2036.2004.02143.x. discussion 95-76. [DOI] [PubMed] [Google Scholar]
- 16.Attwood SE, Smyrk TC, DeMeester TR, Mirvish SS, Stein HJ, Hinder RA. Duodenoesophageal reflux and the development of esophageal adenocarcinoma in rats. Surgery. 1992;111:503–10. [PubMed] [Google Scholar]
- 17.Clark GW, Smyrk TC, Mirvish SS, et al. Effect of gastroduodenal juice and dietary fat on the development of Barrett’s esophagus and esophageal neoplasia: an experimental rat model. Ann Surg Oncol. 1994;1:252–61. doi: 10.1007/BF02303531. [DOI] [PubMed] [Google Scholar]
- 18.Bernstein H, Bernstein C, Payne CM, Dvorakova K, Garewal H. Bile acids as carcinogens in human gastrointestinal cancers. Mutat Res. 2005;589:47–65. doi: 10.1016/j.mrrev.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 19.Goldman A, Condon A, Adler E, et al. Protective effects of glycoursodeoxycholic acid in Barrett’s esophagus cells. Dis Esophagus. 2009 doi: 10.1111/j.1442-2050.2009.00993.x. [DOI] [PubMed] [Google Scholar]
- 20.Buskens CJ, Van Rees BP, Sivula A, et al. Prognostic significance of elevated cyclooxygenase 2 expression in patients with adenocarcinoma of the esophagus. Gastroenterology. 2002;122:1800–7. doi: 10.1053/gast.2002.33580. [DOI] [PubMed] [Google Scholar]
- 21.Tselepis C, Morris CD, Wakelin D, et al. Upregulation of the oncogene c-myc in Barrett’s adenocarcinoma: induction of c-myc by acidified bile acid in vitro. Gut. 2003;52:174–80. doi: 10.1136/gut.52.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duggan SP, Gallagher WM, Fox EJ, Abdel-Latif MM, Reynolds JV, Kelleher D. Low pH induces co-ordinate regulation of gene expression in oesophageal cells. Carcinogenesis. 2006;27:319–27. doi: 10.1093/carcin/bgi211. [DOI] [PubMed] [Google Scholar]
- 23.Souza RF, Shewmake K, Terada LS, Spechler SJ. Acid exposure activates the mitogen-activated protein kinase pathways in Barrett’s esophagus. Gastroenterology. 2002;122:299–307. doi: 10.1053/gast.2002.30993. [DOI] [PubMed] [Google Scholar]
- 24.Tobin AB. Phosphorylation of phospholipase C-coupled receptors. Pharmacol Ther. 2997;75:135–51. doi: 10.1016/s0163-7258(97)00053-3. [DOI] [PubMed] [Google Scholar]
- 25.Kawamata Y, Fujii R, Hosoya M, et al. A G protein-coupled receptor responsive to bile acids. J Biol Chem. 2003;278:9435–40. doi: 10.1074/jbc.M209706200. [DOI] [PubMed] [Google Scholar]
- 26.Hughes SJ, Nambu Y, Soldes OS, et al. Fas/APO-1 (CD95) is not translocated to the cell membrane in esophageal adenocarcinoma. Cancer Res. 1997;57:5571–8. [PubMed] [Google Scholar]
- 27.Cao W, Sohn UD, Bitar KN, Behar J, Biancani P, Harnett KM. MAPK mediates PKC-dependent contraction of cat esophageal and lower esophageal sphincter circular smooth muscle. Am J Physiol Gastrointest Liver Physiol. 2003;285:G86–95. doi: 10.1152/ajpgi.00156.2002. [DOI] [PubMed] [Google Scholar]
- 28.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 29.Bleasdale JE, Bundy GL, Bunting S, et al. Inhibition of Phospholipase C dependent processes by U-73122. In: Samuelson B, Wong PY-K, Sun FF, editors. Advances in Prostaglandin, Thromboxane and Leukotriene Research. Vol. 19. New York: Raven Press; 1989. pp. 590–3. [PubMed] [Google Scholar]
- 30.Williams RL, Katan M. Structural views of phosphoinositide-specific phospholipase C: signalling the way ahead. Structure. 1996;4:1387–94. doi: 10.1016/s0969-2126(96)00146-3. [DOI] [PubMed] [Google Scholar]
- 31.Merhi-Soussi F, Dominguez Z, Macovschi O, et al. Mechanisms involved in the stimulation of prostacyclin synthesis by human lymphocytes in human umbilical vein endothelial cells. Br J Pharmacol. 2003:321–8. doi: 10.1038/sj.bjp.0705253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD 098059 is a specific inhibitor of the activation of mitogen- activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995:27489–94. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 33.Fitzgerald RC. Barrett’s oesophagus and oesophageal adenocarcinoma: how does acid interfere with cell proliferation and differentiation? Gut. 2005;54 (Suppl 1):i21–6. doi: 10.1136/gut.2004.041558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Houten SM, Watanabe M, Auwerx J. Endocrine functions of bile acids. Embo J. 2006;25:1419–25. doi: 10.1038/sj.emboj.7601049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nehra D, Howell P, Williams CP, Pye JK, Beynon J. Toxic bile acids in gastro-oesophageal reflux disease: influence of gastric acidity. Gut. 1999;44:598–602. doi: 10.1136/gut.44.5.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bajpai M, Liu J, Geng X, Souza RF, Amenta PS, Das KM. Repeated exposure to acid and bile selectively induces colonic phenotype expression in a heterogeneous Barrett’s epithelial cell line. Lab Invest. 2008;88:643–51. doi: 10.1038/labinvest.2008.34. [DOI] [PubMed] [Google Scholar]
- 37.Tanaka S, Chu S, Hirokawa M, Montrose MH, Kaunitz JD. Direct measurement of acid permeation into rat oesophagus. Gut. 2003;52:775–83. doi: 10.1136/gut.52.6.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rhee SG. Regulation of phosphoinositide-specific phospholipase C. Annu Rev Biochem. 2001;70:281–312. doi: 10.1146/annurev.biochem.70.1.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bence K, Ma W, Kozasa T, Huang XY. Direct stimulation of Bruton’s tyrosine kinase by G(q)-protein alpha-subunit. Nature. 1997;389:296–9. doi: 10.1038/38520. [DOI] [PubMed] [Google Scholar]
- 40.Ohtsu H, Higuchi S, Shirai H, et al. Central role of Gq in the hypertrophic signal transduction of angiotensin II in vascular smooth muscle cells. Endocrinology. 2008;149:3569–75. doi: 10.1210/en.2007-1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Todt JC, Hu B, Curtis JL. The receptor tyrosine kinase MerTK activates phospholipase C gamma2 during recognition of apoptotic thymocytes by murine macrophages. J Leukoc Biol. 2004;75:705–13. doi: 10.1189/jlb.0903439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ye K, Snyder SH. PIKE GTPase: a novel mediator of phosphoinositide signaling. J Cell Sci. 2004;117:155–61. doi: 10.1242/jcs.00924. [DOI] [PubMed] [Google Scholar]
- 43.Goldschmidt-Clermont PJ, Kim JW, Machesky LM, Rhee SG, Pollard TD. Regulation of phospholipase C-gamma 1 by profilin and tyrosine phosphorylation. Science. 1991;251:1231–3. doi: 10.1126/science.1848725. [DOI] [PubMed] [Google Scholar]
- 44.Yadav M, Clark L, Schorey JS. Macrophage’s proinflammatory response to a mycobacterial infection is dependent on sphingosine kinase-mediated activation of phosphatidylinositol phospholipase C, protein kinase C, ERK1/2, and phosphatidylinositol 3-kinase. J Immunol. 2006;176:5494–503. doi: 10.4049/jimmunol.176.9.5494. [DOI] [PubMed] [Google Scholar]
- 45.Katz S, Boland R, Santillan G. Modulation of ERK 1/2 and p38 MAPK signaling pathways by ATP in osteoblasts: involvement of mechanical stress-activated calcium influx, PKC and Src activation. Int J Biochem Cell Biol. 2006;38:2082–91. doi: 10.1016/j.biocel.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 46.McCubrey JA, Steelman LS, Chappell WH, et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta. 2007;1773:1263–84. doi: 10.1016/j.bbamcr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Torii S, Yamamoto T, Tsuchiya Y, Nishida E. ERK MAP kinase in G cell cycle progression and cancer. Cancer Sci. 2006;97:697–702. doi: 10.1111/j.1349-7006.2006.00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Si J, Behar J, Wands J, et al. STAT5 mediates PAF-induced NADPH oxidase NOX5-S expression in Barrett’s esophageal adenocarcinoma cells. Am J Physiol Gastrointest Liver Physiol. 2008;294:G174–83. doi: 10.1152/ajpgi.00291.2007. [DOI] [PubMed] [Google Scholar]
- 49.Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–2. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- 50.Otis M, Gallo-Payet N. Role of MAPKs in angiotensin II-induced steroidogenesis in rat glomerulosa cells. Mol Cell Endocrinol. 2007;265–266:126–30. doi: 10.1016/j.mce.2006.12.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.