Abstract
Background
Complement system, an innate immunity, has been well documented to play a critical role in many inflammatory diseases. However, the role of complement in pathogenesis of abdominal aortic aneurysm (AAA), which is considered as an immune and inflammatory disease, remains obscure.
Methods and Results
Here, we evaluated the pathogenic roles of complement membrane attack complex (MAC) and CD59, a key regulator that inhibits MAC, in the development of AAA. We demonstrated that in the angiotensin II-induced AAA model, deficiency of MAC regulator CD59 in ApoE-null mice (mCd59ab−/−/ApoE−/−) accelerated the disease development, while transgenic over-expression of human CD59 (hCD59ICAM-2+/−/ApoE−/−) in this model attenuated progression of AAA. The severity of aneurysm among these three groups positively correlates with C9 deposition, and/or the activities of MMP2 and MMP9, and/or the levels of phosphor (p)-c-Jun, p-c-Fos, p-IKK-α/β, and p-65. Furthermore, we demonstrated that MAC directly induced gene expression of MMP2 and MMP9 in vitro, which required activation of AP-1 and NF-κB signaling pathways.
Conclusions
Together, these results defined the protective role of CD59 and shed light on the important pathogenic role of MAC in AAA.
Keywords: CD59, complement, complement regulation, MMP2 and MMP9, abdominal aortic aneurysm, signaling pathway
Abdominal aortic aneurysm (AAA) is characterized by chronic aortic wall inflammation and destructive connective tissue remodeling, including depletion of aortic elastin and fragmentation of medial elastic fibers. AAA is a leading cause of sudden death in aging (> 55 years) men1. Rupture of AAA accounts for 4% of all deaths in people over the age of 65. Risk factors for AAA include age, male gender, atherosclerosis, hypertension, and genetic predisposition though atherosclerosis is considered to be a main cause of AAA2. Enzymatic degradation of elastic lamellae and extracellular matrix (ECM) proteins are underlying characteristics of AAA3. It is well-established that abnormally increased protease activity in aortic tissue plays a critical role in the pathogenesis of aortic dissection and aneurysm. Matrix metalloproteinases (MMPs) such as MMP2 and MMP9 are the predominant proteinases in AAA wall2. In animal models, the development of aneurysms can be suppressed by pharmacologic inhibition (i.e. by tetracycline derivatives) of MMP2 and MMP9, or genetic alterations that eliminate the expression of either proteinase4. Elastolytic cysteine proteases including cathepsins also play a critical role in the development of aneurysm5. AAA is also considered to be an immune and inflammatory disease and macrophages, lymphocytes, and mast cells participate in its development1. However, the role of complement, a main effector of immunity and inflammation, remains unclear.
The complement system consists of about 30 soluble and membrane-bound proteins, and is activated by different sequential activation cascades (the classical, alternative, and lectin pathways) either on the pathogens or in plasma. These pathways converge in one terminal cascade that leads to formation of the membrane attack complex (MAC), a macromolecular pore capable of inserting itself into cell membranes and lysing heterologous cells and bacteria. To protect autologous cells from MAC-mediated attack, an array of complement regulators including CD59 have evolved to restrict complement activation. CD59, a glycosylphosphatidyl inositol (GPI)-linked membrane protein, strongly restricts MAC formation by binding to complement proteins C8 and C9 and preventing C9 incorporation and polymerization.
MAC is also an important mediator of cellular signals that trigger mitogenic effects. Insertion of the MAC allows the release of growth factors such as bFGF and PDGF, and cytokines such as IL-1 and MCP-1, which autocrinally and paracrinically- stimulate proliferation, inflammation, and thrombosis6, 7. In vitro studies indicate that sublytic MAC activates several cellular signaling pathways such as PI3/Akt (serine/threonine) kinase pathway8, 9, the nuclear factor NF-κB, and the activator protein-1 (AP-1)10, 11.
Extensive evidence from human and animal studies indicates a protective role of CD5912,13 and an atherogenic role of MAC in the pathogenesis of atherosclerosis14. However, only a few human studies (and no animal studies) have examined the role of MAC in the pathogenesis of aneurysm15, 16. Here we investigate the role of CD59 and MAC in the pathogenesis of the AAA induced by angiotensin (Ang II) in ApoE−/− mice, either deficient in mouse CD59a and mouse CD59b (mCd59ab−/−/ApoE−/− described in14,17 ) or transgenically over-expressing human CD59 (hCD59) in endothelial and circulating cells (hCD59ICAM-2+/−/ApoE−/− as described in14,18).
METHODS
Animal studies were approved by the Harvard Medical School Institutional Animal Care and Use Committee.
Animal models and AAA production and quantification
Both mCd59ab−/− and hCD59ICAM-2+/− mice were in C57BL/6 (B6) genetic background as described in17, 18. The hCD59 in hCD59ICAM-2+/− mice is selectively expressed in endothelial cells, macrophages, and platelets19. In these transgenic strains, over-expression of hCD59 is effective in providing additional protection against mouse complement18, 19. We crossed each of the two strains with ApoE−/− mice (B6 background, Jackson Laboratory) to produce mCd59ab−/−/ApoE−/− and hCD59ICAM-2+/−/ApoE−/− mice, respectively. The successful generation of these compound mice was determined by both PCR methods (for genotyping as described in Supplemental Figure 1A–C), and FACS analysis of mCd59a and mCd59b proteins in mCd59ab−/− /ApoE−/−‘s erythrocytes (for confirming the physical absence of mCd59a and mCd59b) or hCD59 in the hCD59ICAM-2+/−/ApoE−/−‘s platelets [for confirming the over-expression of hCD59 (Data not shown)].
It has been established that the low dose of Ang II used is associated with a significant (30–40%) increase in blood pressure20. The high blood pressure may participate in the pathogenesis of Ang II-induced AAA20 and Ang II may contribute to medial macrophage accumulation associated with elastin degradation21.
Since in hyperlipidemic mice, the propensity for the development of AAA is much more common in males than females (as is the case in humans), we selected males as experimental animals. To induce AAA, three-month-old male mice were infused with 1000 ng/kg/min Ang II (Calbiochem, San Diego, California, USA) subcutaneously delivered by Alzet model 2004 osmotic minipumps (DURECT Corp., Cupertino, California, USA) as described in22, and simultaneously fed a high fat diet (C12108; Research Diets Inc.) for one month23, 24. After fasting the mice overnight, we sacrificed them by CO2 asphyxiation, drew blood by heart puncture, and stored the sera at −80°C. The aorta was viewed with a dissection microscope. The per adventitial tissue was carefully removed from the aortic wall as described in22. The maximal diameter of each aneurysm was measured with a digital caliper. The segment of the aorta from the aortic arch to renal artery was weighed, and one half of the AAA lesion was preserved with OTC and stored at −80°C (for immunoassays). The other half was stored at −80°C for extraction of proteins for western analysis and gelatin zymography. For the detailed procedures of immunofluorescence, histology and gelatin zymography, please see the online-only Data Supplement.
Reporter Assays
pGL2-AP-1-Luc and pGL2- NF-κB-Luc luciferase reporters, with AP-1 and NF-κB response elements cloned into the pGL2-Luc plasmid, respectively, were generous gifts from Drs. Zhu and Rosenfeld25. Mouse endothelial cells (2×104 each well) were transfected by Lipofectamine 2000 (Invitrogen) with pGL2-AP-1-Luc or pGL2- NF-κB-Luc luciferase reporter plasmid (see details in the online-only Data Supplement).
RNA Interference
Endothelial cells (2.5×105 per well, 6-well plates) were transiently transfected using Lipofectamine RNAiMA X (Invitrogen) 5µl/each well with 50nM of mouse p50, mouse c-jun, and siGENOME non-targeting siRNA Pool™ (Dharmacon). The sequences of these siRNA are listed in Supplemental Table 1. After 36 hrs incubation, cells were stimulated with MAC (C5b6: 24 µg/ml and + for C7, C8 or C9: 24 µg/ml) for an additional 12 hrs, and proteins or RNA were isolated and subjected to western blot and Zymography, or real-time RT-PCR analysis respectively.
Quantitative real-time PCR analysis
Total RNA was isolated from the cultured mouse endothelial cells C166 (ATCC) treated with C5b-9 assembled and siRNA for 12 hours with Trizol reagent (Invitrogen). Real-time PCR was performed using SYBR Green in a real-time PCR machine (iCycler; Bio-Rad). We used the primers (listed in Supplemental Table 2) for detecting p50, c-Jun, MMP2, and MMP9 RNA levels: To normalize expression data, 18s rRNA was used as an internal control gene.
Western Blotting
The proteins were isolated from the mouse AAA or mouse endothelial cells treated with MAC. Primary antibodies for Western blotting including rabbit Phospho-p65 antibody (Cell Signaling), rabbit Phospho-IKK-α/β antibody (Cell Signaling), rabbit Phospho-c-Jun antibody (Cell Signaling), rabbit Phospho-c-Fos antibody ( Abcam ), rabbit c-Jun antibody (Santa Cruz Biotechology), rabbit p65 monoclonal antibody (Cell Signaling), rabbit IKK-α/β antibody (Santa Cruz Biotechology), rabbit p50 antibody (Santa Cruz Biotechology), rabbit c-Fos monoclonal antibody (Cell Signaling), and mouse anti-mouse β-actin antibody (Santa Cruz Biotechology), which were diluted in blocking buffer, were incubated with the membranes. The final concentrations for these antibodies are listed in Supplemental Table 3 (see details in the online-only Data Supplement).
Statistical analysis
Experimental results are shown as the mean ± SEM. The comparison between two groups or three groups was examined with a nonparametric Mann-Whitney test or nonparametric Kruskal-Wallis test respectively. The details of real-time PCR result analysis are in the supplement materials. P values of less than 0.05 were considered significant. The difference in the mortality rate was analyzed by Chi-Square test. All statistical tests with P< 0.05 were considered significant.
RESULTS
Protective role of CD59 against AAA development
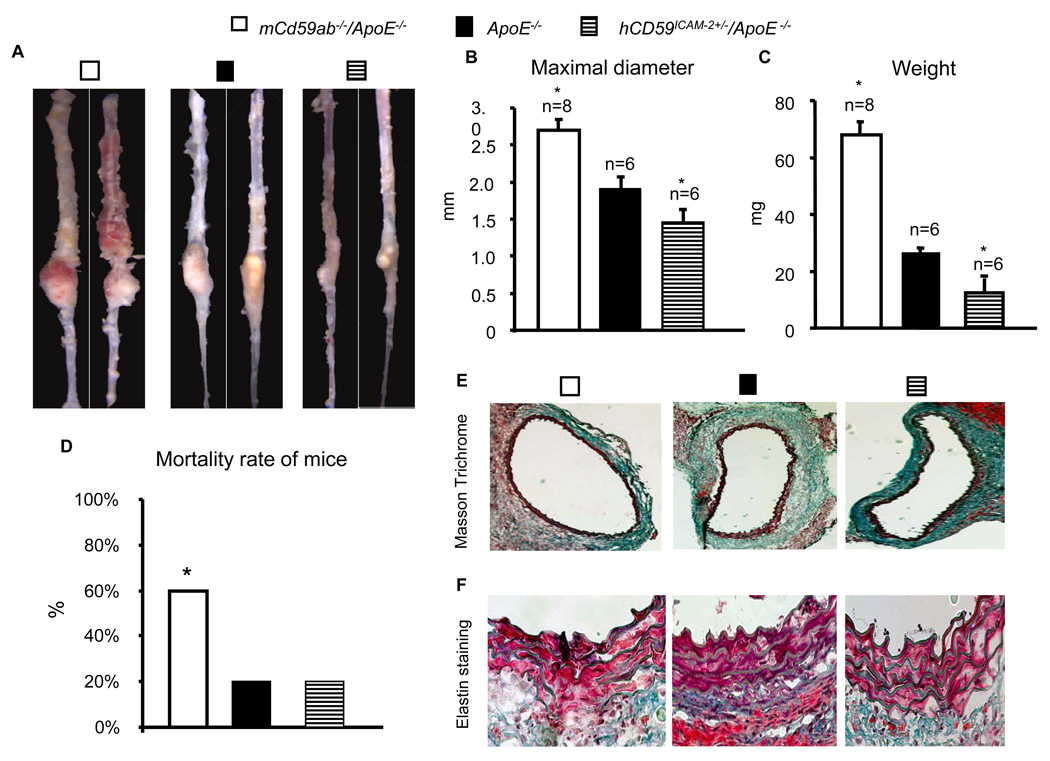
We used the ApoE−/− either deficient in mCd59a and mCd59b (mCd59ab−/−/ApoE−/− ) or over-expressing hCD59 in the endothelial cells, macrophages and platelets (hCD59ICAM-2+/−/ApoE−/−) to investigate the role of CD59 and MAC in the AAA. Here, we induced the AAA by the infusion of Ang II to these models for the study of the role of CD59 in the pathogenesis of AAA. mCd59ab−/−/ApoE−/− developed significantly more severe AAA assessed by diameter and weight of the aneurysms (Figs. 1A–C, and supplemental figure 2) and associated with higher mortality rate than ApoE−/− (Fig. 1D). In contrast, the mice transgenic for hCD59 in the endothelial and hematopoietic cells (hCD59ICAM-2+/−/ApoE−/−) developed significantly less severe AAA than ApoE−/− mice (Figs. 1A–D). The incidence rates of the development of AAA in mCd59ab−/−/ApoE−/−, ApoE−/−, and hCD59ICAM-2+/−/ApoE−/− mice are 100%, 75% and 75% among the survival mice, respectively. Also, mCd59ab−/−/ApoE−/− mice in the aortas of the AAA had much lower collagen content than ApoeE−/− mice, while hCD59ICAM-2+/−/ApoE−/− mice had higher collagen content than ApoE−/− mice (Fig. 1E and supplemental figure 3A). An additional difference between mCd59ab−/−/ApoE−/− aneurysms and the others was that mCd59ab−/−/ApoE−/− had more disrupted elastic lamellae than other two group mice (Fig. 1F). In addition, the cholesterol and triglyceride levels among these groups were not significantly different (Supplemental figure 3B and 3C). The systolic blood pressure among three different group mice after Ang II infusion was not significantly different (Supplemental figure 4), which indicates that either CD59 deficiency or hCD59 over-expression in ApoE−/− background mice does not change blood pressure response to Ang II and HFD treatments compared to that in ApoE−/−. Together, these results suggest that CD59 protects the mice against AAA development.
Figure 1. Protective role of CD59 in AAA.
A. Representative aneurysm lesions from mCd59ab−/−/ApoE−/−, ApoE−/− and hCD59ICAM-2+/−/ApoE−/− (see supplemental figure 2 for full presentation of aneurysm lesions). B–D.: The difference in the maximal diameters (mm) (b), the weight of aneurysm (c) and mortality rates among the experimental mice infused by Ang II and simultaneously fed a HFD for one month. The data are displayed by mean ± sem. *P<0.05 vs ApoE−/−. The difference in the mortality rate among the groups after one month Ang II and HFD treatment was analyzed by X2 test. E. The representation of Massons trichrome stain demonstrated that lower collagen content in the aneurysm lesions from mCd59ab−/−/ApoE−/− and higher collagen contents in aneurysm lesion of hCD59ICAM-2+/−/ApoE−/− compared to those from ApoE−/− (see supplemental figure 3A for quantitative analysis of the result). (F). The representative picture of elastin staining demonstrated that the medial elastin was more degraded in the aneurysm lesions of mCd59ab−/−/ApoE−/− mice, and less degraded in the aneurysm lesions of hCD59ICAM-2+/−/ApoE−/− and ApoE−/− mice.
MAC deposition in the AAA lesions
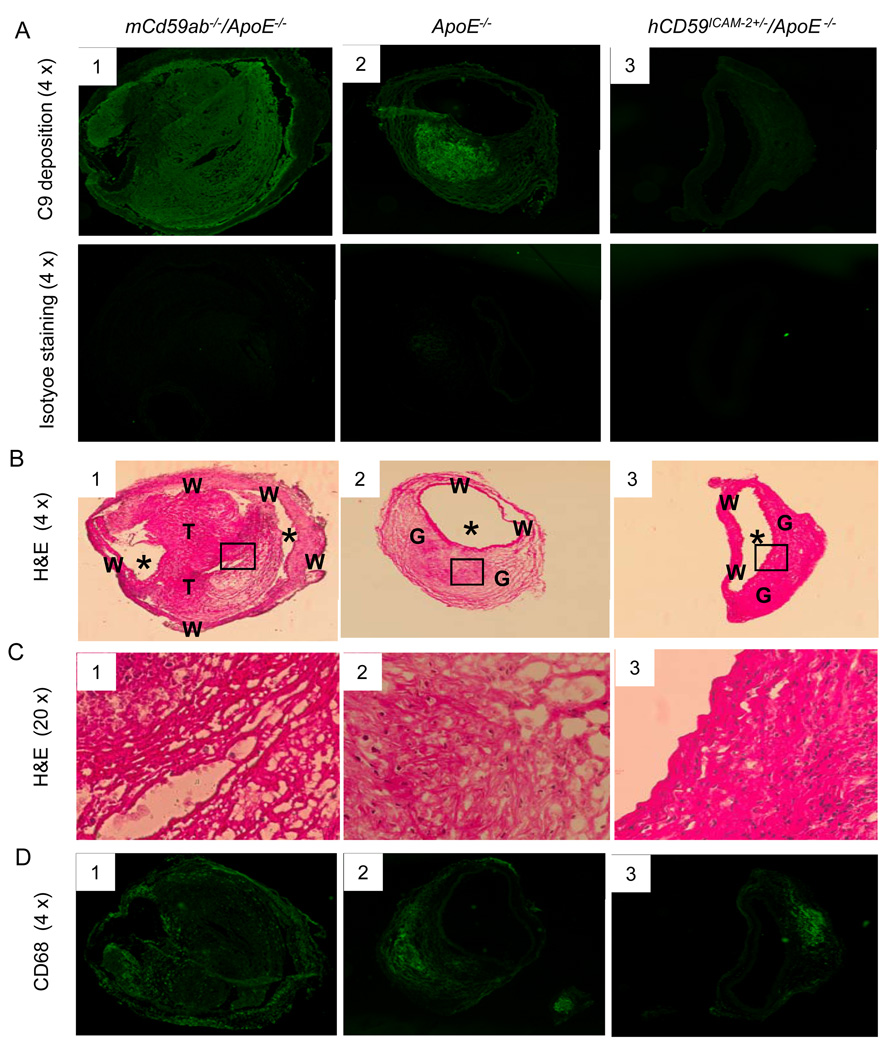
CD59 is well known as a key inhibitor of MAC formation. To further investigate the underlying mechanisms, by which CD59 protects against the development of AAA, we analyzed MAC deposition in AAA lesions. Staining of the aneurysm sections with anti-C9-specific antibodies revealed that mCd59ab−/−/ApoE−/− mice had significantly more extensive deposits of C9 than ApoE−/− mice, and ApoE−/− mice has significantly more deposits of C9 than hCD59ICAM-2+/−/ApoE−/− mice (Fig. 2A and supplemental figure 5A). Analysis of adjacent sections with H&E staining demonstrated that the C9 deposition was both in thrombi (Fig 2B1 and 2C1) and granulation tissue (Fig. 2B2, 2B3, 2C2, and Fig. 3C3). Immunofluorescence staining of AAA showed that mCd59ab−/−/ApoE−/− mice had significantly more macrophages than ApoE−/− mice, which in turn had significantly more macrophage cells than hCD59ICAM-2+/−/ApoE−/− mice (Fig. 2D and supplemental figure 5B). Together, these results are consistent with a pathogenic role of the MAC in the development of AAA in our experimental mice.
Figure 2. Characterization of aneurysms.
A. C9 deposition in the AAA lesions of mice (see supplemental figure 5A for quantitative analysis of the result). B and C: Representative image of AAA H&E staining. B1. The wall (W) of the aorta is severely diluted. Most of lumen (*) has been occluded by a thrombus (T) shown in high magnification (X20) in Fig. C1. It is within the thrombus that C9 staining (Fig. 2A1) is most intense. A2. The much of wall (W) of the aorta has been thickened by proliferative granulation tissue (G), fibroblasts, and collagen, some of inflammatory cells and edema fluid shown in higher magnification (X20) Fig. C2. A3. Much of the wall (W) has been thickened by granulation tissue (G), which in this case is less edematous than in figure C3, shown in higher magnification (X20). D. CD68 for the detection of macrophage content in lesion areas (see supplemental figure 5B for quantitative analysis of the result).
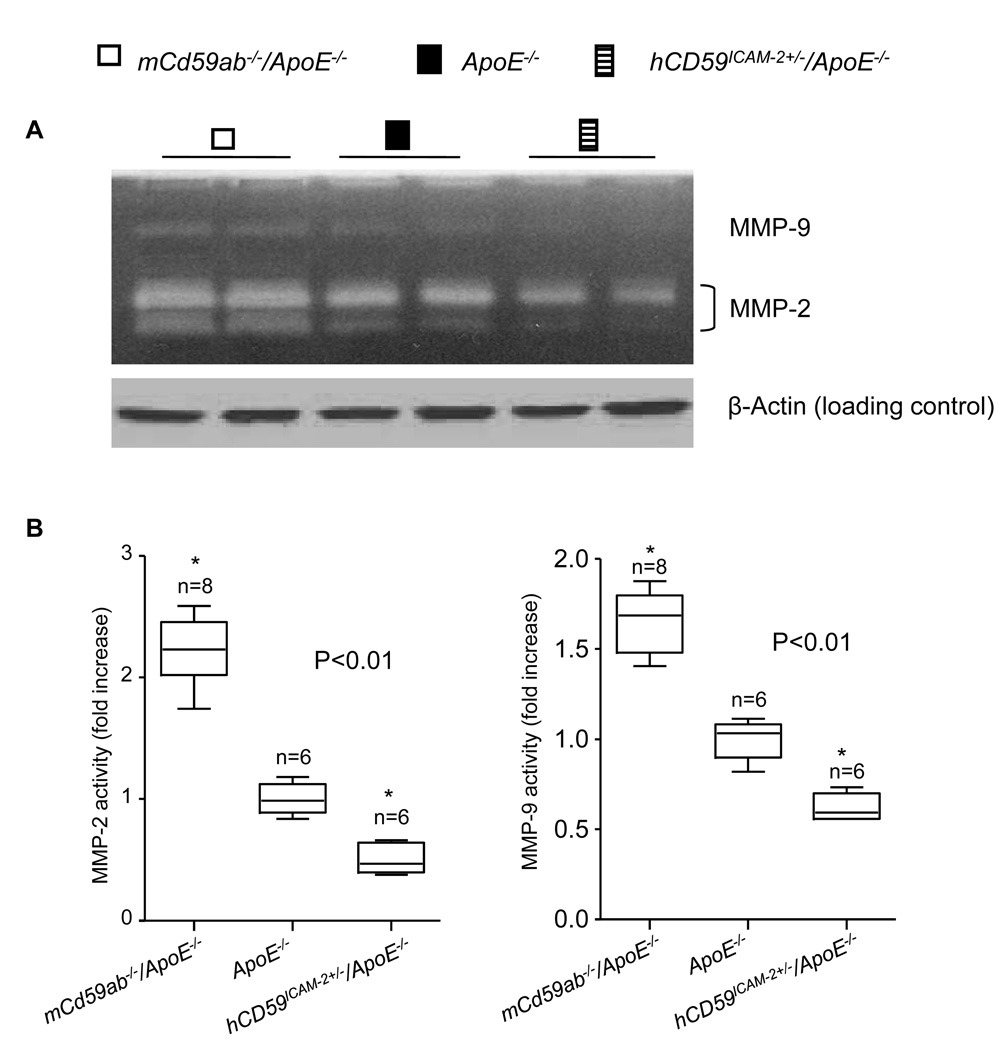
Figure 3. MMP2 and MMP9 in the mouse AAA wall.
A. Gelatin gel zymography to detect MMP activity in aortic tissue extracts from different groups of mice (Top panel). The same amount of total proteins from each group was demonstrated by β-actin detection (Bottom panel). B. Densitometric analysis of MMP activity changes compared to the density of MMP activity in ApoE−/− mice. The data from the different mice (n) are represented by boxplots. *P<0.01 vs ApoE−/−.
MAC up-regulates MMP2 and MMP9 activities
MMP2 and MMP9 play essential roles in the pathogenesis of AAA. Inhibition of and deficiency for MMP2 and MMP9 have been shown to attenuate AAA development in humans and animals 26. Increased MMP2 and MMP9 activities contribute to degradation of elastin and type IV collagen in tissues including arteries27, which could explain the greater disruption in elastic lamellae and the lower collagen content in AAA lesions of mCd59ab−/−/ApoE−/− mice (Fig. 1F). For these reasons, we assessed the MMP2 and MMP9 activities in AAA lesions, by gelatin zymography28. AAA aortic extracts from mCd59ab−/−/ApoE−/− mice exhibited significantly higher MMP2 and MMP9 activities, while extracts from hCD59ICAM-2+/−/ApoE−/− mice showed lower activities of than ApoE−/− mice (Figs. 3A and 3B).
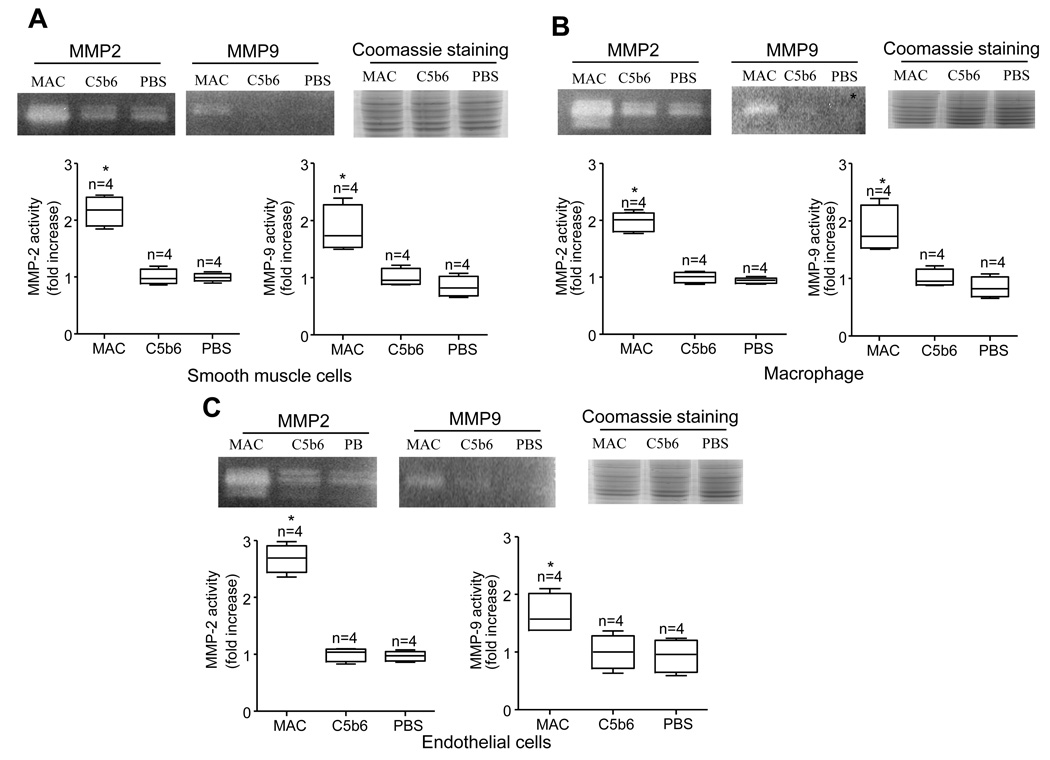
The fact that MAC deposition and MMP activity correlated with the severity of AAA in our animal models prompted us to investigate whether MAC up-regulates MMP2 and MMP9 activities in vitro. MAC treatment increased MMP2 and MMP9 activities in the mouse vascular smooth muscle (VSMC), endothelial and macrophage cells compared to C5b6 alone, which does not lead to MAC assemble, or PBS treatment (Figs. 4A–C). These results indicate that MAC may stimulate the cells and increase MMP2 and MMP9 activities, which may contribute to the development of accelerated AAA lesions in mCd59ab−/−/ApoE−/− mice.
Figure 4. MAC treatment increases MMP activities in mouse cell lines.
A–C. Activities of MMP2 or MMP9 from the cell extracts of the mouse smooth muscle (A), macrophage (B), and endothelial cells (C) 12 hours after MAC treatment (assembled by C5b6: 24 µg/ml and + for C7, C8 or C9: 24 µg/ml) on the cells. The MMP activities were examined by gelatin zymography. Coomassie staining of SDS-PAGE showed equal protein loading. Fold changes in the MMP activities treated by MAC assemble compared to that treated with C5b6 alone. The data from the independent experiments (n) are displayed by boxplots. *P<0.05 vs C5b6.
MAC up-regulates MMP2 and MMP9 activities through AP-1 and NF-κB signaling pathways
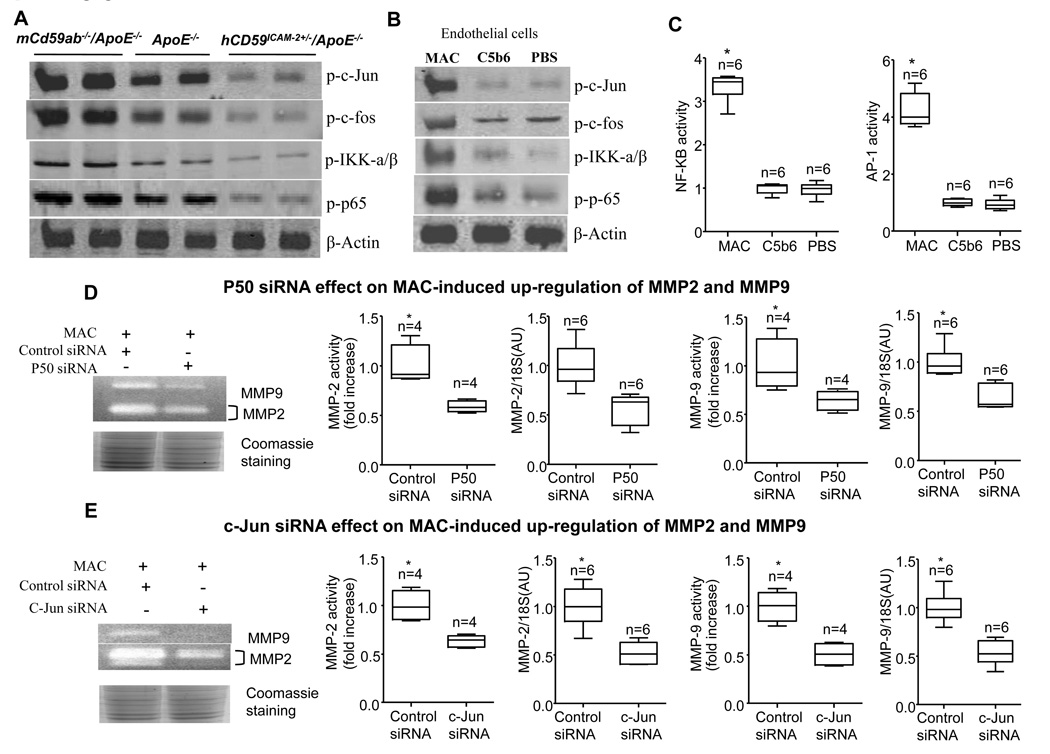
It has been demonstrated that sublytic MAC induces increased activity of c-Jun NH2-terminal kinase JNK1 in many cells (including endothelial cells and smooth muscle cells29) and activation of NF-κB and AP-110, 11. In order to assess the effect of MAC on these signaling pathways, we analyzed the expression of the phosphorylated c-Jun (p-c-Jun), c-Fos (AP-1 components), p65 (an NF-κB component), and IKK-α/β (a NF-κB regulator) in the AAA lesions of three groups of mice or in the endothelial cell cultures challenged by MAC in vitro. Western blot assay revealed that the AAA in mCd59ab−/−ApoE−/− mice had significantly higher levels of p-c-Jun, p-c-Fos, p-IKK-α/β, and p-p65, while AAA from hCD59ICAM-2+/−/ApoE−/− mice had significantly lower levels of than Apoe−/− mice (Fig. 5A). In contrast, total levels of c-Jun, IKK-α/β and p65 did not differ among the three groups (Supplemental figure 6A). This indicates that the activation of these signaling pathways may play a critical role in the development of AAA in our models. Consistent with these results, we also demonstrated that MAC assembly in mouse endothelial cells directly induced significantly higher levels of p-c-Jun, p-c-Fos, p-IKK-α/β, and p-p65 than C5b6 alone in vitro (Fig 5B and supplemental figure 6B), confirming the previous finding that MAC activates these signaling pathways in vitro 11, 30, 31, These results, together with the facts that AP-1 is composed of c-Jun and c-Fos, and p65 is a major component of NF-κB 32, 33, indicate that MAC is capable of triggering signaling pathways upstream of AP-1 and NF-κB.
Figure 5. MAC induces MMP2 and MMP9 activities by activating AP-1 and NF-κB transcription factors.
A. Representative western blot shows the levels of phosphorylated c-Jun, c-Fos, p65, and IKK-α/β in three groups of mice treated with angiotension-II and fed HFD for 28 days. This experiment was repeated from the AAA lesions of five mice from each group. B. Western blot analysis of MAC induced the levels of phosphorylated c-Jun, c-Fos, p65, and IKK-α/β levels in mouse endothelial cell lines (western blot). C. MAC induced AP-1 and NF-κB transcription factor activities in mouse endothelial cell lines (reporter assay). Fold changes in these transcriptional factor activities treated by MAC assemble compared to that treated with C5b6 alone. The data from the independent experiments (n) are displayed by boxplots. *P<0.01 vs. C5b6. D and E. Effects of p50 (D) and c-Jun’s (E) siRNA on MAC-induced up-regulation of MMP2 and MMP9. Coomassie staining of SDS-PAGE showed equal protein loading. MMPs activities were examined by gelatin zymography and were quantified by densitometric analysis. MMPs mRNA levels were detected by real time-PCR. Specific primers detected for MMP2 or MMP9 were listed in supplemental table 2. Fold changes in the MMP activities or levels with specific siRNAs treatment compared to these treated with control siRNA. The data from the independent experiments (n) are displayed by boxplots. *P<0.05 vs control siRNA.
To directly evaluate whether MAC up-regulates the activity of AP-1 or NF-κB, we used a firefly luciferase reporter gene assay to measure their transcriptional activity upon MAC assembly on endothelial cells. The reporter gene activity is directly under the control of AP-1 and NF-κB response elements. As shown in Figure 5C, MAC induced significantly higher levels of AP-1, and NF-κB transcriptional activity than C5b6 alone or PBS treatment (Fig. 5C). These results indicate that MAC assembly on the cells transcriptionally up-regulates AP-1 and NF-κB.
Furthermore, to investigate whether AP-1 and NF-κB signaling pathway activations are involved in the up-regulation of MMP2 and MMP9 mediated by MAC in vitro, we used RNA interference against c-Jun, and p50, another component of NF-κB, to suppress these pathways. The up-regulation of MMP2 and MMP9 mRNA and proteins mediated by MAC was attenuated by both p50 (Fig. 5D) and c-Jun RNAi (Fig. 5E). RNAi efficacy was validated at the level of both mRNA and protein (Supplemental figures 7A and 7B). Together, these results suggest that MAC-activated c-Jun and NF-κB signaling pathways participate in the up-regulation of MMP2 and MMP9, which may in turn contribute to the pathogenesis of AAA in our animal models.
DISCUSSION
These results we reported here provide strong support for a protective role of CD59 against the development of AAA and indicate that MAC may indeed play a critical role in the pathogenesis of AAA. The role of complement and MAC in AAA has only been suggested in human studies and has not extensively been studied in experimental models. Patients with vasospasm showed a twofold increase in plasma C3d levels when the spasm occurred, whereas no significant changes in C3d concentration could be detected in aneurysm patients without spasm or in patients with hematoma unrelated to aneurysm rupture34. Specific gene array analysis revealed that increased gene expression was associated with proteinase, reactive oxygen species, growth factors, chemokines, complement, adhesion molecules, and apoptosis in both the intima and the media of aneurysmal walls35. Moreover, immunohistochemistry studies of human saccular cerebral artery aneurysm (SCAA) demonstrated that MAC localized consistently in a decellularized layer in the outer wall, and were found in all SCAA samples. The percentage of MAC-positive area relative to the total SCAA wall surface area was greater in ruptured than in un-ruptured SCAAs16.
Moreover, a recent study demonstrates that C3a and C5a play a critical role in the development of elastase-induced abdominal aortic aneurysm36. Together, these data suggest that complement activation and MAC formation are involved in aneurysm wall degeneration and rupture. We used genetic strategies to investigate the role of MAC in AAA. We identified a critical role of CD59 in protecting against AAA and demonstrated that MAC may play a critical pathogenic role in AAA. It is notable that the deficiency of CD59 results in mild complement-mediated hemolytic anemia and platelet activation17, 37, 38. The hCD59 in hCD59ICAM-2 is expressed not only in endothelial18 but also in circulating cells including macrophages (see supplemental figure 8) and platelets14 because the hCD59 transgenic in hCD59ICAM-2 mice is under the control of ICAM-II19. The protective effects in hCD59ICAM-2+/−/ApoE−/− mice against the development of AAA are contributed by the expression of hCD59 in these cells. However, the cellular mechanism of hCD59 protective role remains unclear. In addition, CD59 also has a complement-independent function in regulating NK, B and T cell activities39, 40, which may also contribute to the AAA. Thus, the mechanisms underlying CD59 complement independent function and MAC-mediated cellular dysfunction in the development of AAA require further investigation.
Cellular response to MAC formation can be classified into two groups (lytic and sublytic) along a response continuum. Lytic MAC formation results in colloidoosmotic swelling and lysis of the target41. Sublytic MAC formation results in release of growth factors and cytokines, activation of cellular signaling, and and/or reprogramming of gene expression. When the pore opens transiently, it can generate significant changes in membrane permeability and internal composition of autologous cells without compromising their viability. Thus, lytic MAC can induce lethal colloido-osmotic swelling in cells such as gram-negative bacteria and heterologous erythrocytes. On the other hand, sublytic MAC mediates non-lethal physiological and/or pathological responses and activates many signaling pathways in autologous cells7. These cellular signaling pathways include 1) elevated Ca2+ via Ca2+ influx through transient pore in cells, which partially activates PKC and other cellular signaling pathways42; 2) G protein coupled-activation of Ras, Raf-1, MEK, ERK-1 pathway and increased activities of ERK-1, c-jun NH2-terminal kinase JNK1 and p38 MAPK in many cells including endothelial cells and smooth muscle cells30; 3) activation of the PI3/Akt kinase pathway9, 30; 4) activation of NF-κB and AP-1 signaling pathways11, 31; and 5) activation of the JAK1/STAT3 pathway29. Among these signaling pathways, c-Jun and NF-κB activation pathways have been demonstrated to play a critical role in the AAA in vivo43, 44. Here, we demonstrated that AAA lesions in mCd59ab−/−/ApoE−/− had significantly higher, while that of hCD59ICAM-2+/−/ApoE−/− had significantly lower levels of p-c-Jun p-c-Fos, p-IKK-α/β, and p-65 than those in ApoE−/−. The fact that the activation of AP-1 and NF-κB signaling pathways positively correlated with the severity of the AAA among our experimental mice confirms some of these findings and further suggests that MAC is an important of signaling activator and may play a critical role in the pathogenesis of chronic inflammatory diseases such as AAA.
Whether MAC induces up-regulation of MMP2 and MMP9, and which MAC-triggered signaling pathways are involved in the pathogenesis of AAA have not been examined up to now. Here, we demonstrate that 1) MAC induces the production of MMP2 and MMP9, 2) MMP2 and MMP9 activities were correlated with the severity of AAA, 3) MAC-induced up-regulation of MMP2 and MMP9 activities is related to the activation of AP-1 and NF-κB signaling pathways. Together, these results shed light on the important pathogenic role of MAC in the AAA, and point towards the molecular mechanism of MAC-activated signaling pathways in the development of aneurysm and atherosclerosis.
Extensive evidence indicates that MMP2 and MMP9 play essential roles in the pathogenesis of AAA26 and the primary source of MMP-9 production is the macrophages in human and mouse AAA tissues45, 46. In the AAA lesions of mCd59ab−/−/Apoe−/− mice, the macrophage content is significantly increased as shown in Figure 2D and Supplemental figure 5B. This is consistent with the severity of AAA and higher levels of MMP2 and MMP9 in these lesions. We previously demonstrated that MAC induced endothelial dysfunction in mCd59ab−/− /ApoE−/−, which contributes the atherogenesis14. MAC also mediates the endothelial cell apoptosis in vitro 47. Furthermore, we document that the supernatant obtained from MAC-treated endothelial cells triggers the migration of both MAC-treated and non-MAC treated macrophage in vitro (Supplemental figure 9A). The increase of macrophage migration may result from the releases of growth factors and cytokines induced by sublytic MAC assemble in endothelial cells, as we reported previously6, 7. Of note, the deficiency of CD59 in mice does not result in the decreased number of monocytes (Supplement figure 9B). Together, these findings let us to conclude that increased MMP expression in the aorta with AAA could be caused by 1) increased macrophage infiltration, 2) up-regulation by increased MAC tissue levels or some combination of these two mechanisms.
CLINICAL PERSPECTIVE
Aneurysm including abdominal aortic aneurysm (AAA) is also considered to be an immune and inflammatory disease. The complement is a main effector of the immune response and inflammation. However, the role of complement in the aneurysm pathogenesis has not been extensively investigated. The complement system is activated by three activation cascades, which lead to formation of the membrane attack complex (MAC). MAC is a macromolecular pore capable of inserting itself into cell membranes and lysing heterologous cells and bacteria, and an important mediator of cellular signals including the nuclear factor NF-κB, and the activator protein-1 (AP-1) that trigger mitogenic effects. To protect autologous cells from MAC, an array of complement regulators including CD59 have evolved to restrict complement activation. CD59 strongly restricts MAC formation. Here, we demonstrated that in the angiotensin (Ang) II-induced AAA model, deficiency of CD59 in Apoe-null mice accelerated the AAA development, while transgenic over-expression of CD59 attenuated the AAA progression. The severity of aneurysm positively correlates with C9 deposition, the activities of MMP2 and MMP9, and the levels of phosphorylated (p)-c-Jun, p-c-Fos, p-IKK-α/β and p-p65. Furthermore, we demonstrated that MAC directly induced gene expression of MMP2 and MMP9 in vitro, which depended on the activation of AP-1 and NF-κB signaling pathways. Together, these results shed light on the important pathogenic role of MAC in aneurysm, point towards the molecular mechanism of MAC-activated signaling pathways in aneurysm, and suggest inhibition of MAC may provide a novel approach for the treatment/prevention of aneurysm.
Supplementary Material
ACKNOWLEDGEMENTS
This article is dedicated to the memory of Dr. Daniel.C. Tosteson, Dean Emeritus of Harvard Medical School and an untiring mentor and role model to authors. We are grateful to Dr. P. Zhu for critical comments and help and Dr. Lijuan Deng, a biostatistician for our statistical data analysis at Harvard University School of Public Health.
Sources of Funding
This work was supported by US NIH grants RO1 AI061174 (XQ) and by a Scientist Development grant from the American Heart Association 0435483N (XQ).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures
Gongxiong Wu: None
Ting Chen: None
Aliakbar Shahsafaei: None
Weiguo Hu: None
Rod T Bronson: None
Guo-ping Shi: None
Jose A Halperin: None
Huseyin Aktas: None
Xuebin Qin: None
References
- 1.Thompson RW. Detection and management of small aortic aneurysms. N Engl J Med. 2002;346:1484–1486. doi: 10.1056/NEJM200205093461910. [DOI] [PubMed] [Google Scholar]
- 2.Daugherty A, Cassis LA. Mouse models of abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2004;24:429–434. doi: 10.1161/01.ATV.0000118013.72016.ea. [DOI] [PubMed] [Google Scholar]
- 3.Kadoglou NP, Liapis CD. Matrix metalloproteinases: contribution to pathogenesis, diagnosis, surveillance and treatment of abdominal aortic aneurysms. Curr Med Res Opin. 2004;20:419–432. doi: 10.1185/030079904125003143. [DOI] [PubMed] [Google Scholar]
- 4.Mosorin M, Juvonen J, Biancari F, Satta J, Surcel HM, Leinonen M, Saikku P, Juvonen T. Use of doxycycline to decrease the growth rate of abdominal aortic aneurysms: a randomized, double-blind, placebo-controlled pilot study. J Vasc Surg. 2001;34:606–610. doi: 10.1067/mva.2001.117891. [DOI] [PubMed] [Google Scholar]
- 5.Shi GP, Sukhova GK, Grubb A, Ducharme A, Rhode LH, Lee RT, Ridker PM, Libby P, Chapman HA. Cystatin C deficiency in human atherosclerosis and aortic aneurysms. J Clin Invest. 1999;104:1191–1197. doi: 10.1172/JCI7709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benzaquen LR, Nicholson-Weller A, Halperin JA. Terminal complement proteins C5b-9 release basic fibroblast growth factor and platelet-derived growth factor from endothelial cells. J Exp Med. 1994;179:985–992. doi: 10.1084/jem.179.3.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nicholson-Weller A, Halperin JA. Membrane signaling by complement C5b-9, the membrane attack complex. Immunol Res. 1993;12:244–257. doi: 10.1007/BF02918256. [DOI] [PubMed] [Google Scholar]
- 8.Niculescu F, Rus H. The role of complement activation in atherosclerosis. Immunol Res. 2004;30:73–80. doi: 10.1385/IR:30:1:073. [DOI] [PubMed] [Google Scholar]
- 9.Fosbrink M, Niculescu F, Rus V, Shin ML, Rus H. C5b-9-induced endothelial cell proliferation and migration are dependent on Akt inactivation of forkhead transcription factor FOXO1. J Biol Chem. 2006;281:19009–19018. doi: 10.1074/jbc.M602055200. [DOI] [PubMed] [Google Scholar]
- 10.Viedt C, Hansch GM, Brandes RP, Kubler W, Kreuzer J. The terminal complement complex C5b-9 stimulates interleukin-6 production in human smooth muscle cells through activation of transcription factors NF-kappaB and AP-1 [In Process Citation] Faseb J. 2000;14:2370–2372. doi: 10.1096/fj.00-0468fje. [DOI] [PubMed] [Google Scholar]
- 11.Kilgore KS, Schmid E, Shanley TP, Flory CM, Maheswari V, Tramontini NL, Cohen H, Ward PA, Friedl HP, Warren JS. Sublytic concentrations of the membrane attack complex of complement induce endothelial interleukin-8 and monocyte chemoattractant protein-1 through nuclear factor-kB activation. Am. J. Pathol. 1997;150:2019–2031. [PMC free article] [PubMed] [Google Scholar]
- 12.Yun S, Leung VW, Botto M, Boyle JJ, Haskard DO. Brief report: accelerated atherosclerosis in low-density lipoprotein receptor-deficient mice lacking the membrane-bound complement regulator CD59. Arterioscler Thromb Vasc Biol. 2008;28:1714–1716. doi: 10.1161/ATVBAHA.108.169912. [DOI] [PubMed] [Google Scholar]
- 13.An G, Miwa T, Song WL, Lawson JA, Rader DJ, Zhang Y, Song WC. CD59 but not DAF deficiency accelerates atherosclerosis in female ApoE knockout mice. Mol Immunol. 2009;46:1702–1709. doi: 10.1016/j.molimm.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu G, Hu W, Shahsafaei A, Song W, Dobarro M, Sukhova GK, Bronson RR, Shi GP, Rother RP, Halperin JA, Qin X. Complement regulator CD59 protects against atherosclerosis by restricting the formation of complement membrane attack complex. Circ Res. 2009;104:550–558. doi: 10.1161/CIRCRESAHA.108.191361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fiane AE, Videm V, Lingaas PS, Heggelund L, Nielsen EW, Geiran OR, Fung M, Mollnes TE. Mechanism of complement activation and its role in the inflammatory response after thoracoabdominal aortic aneurysm repair. Circulation. 2003;108:849–856. doi: 10.1161/01.CIR.0000084550.16565.01. [DOI] [PubMed] [Google Scholar]
- 16.Tulamo R, Frosen J, Junnikkala S, Paetau A, Pitkaniemi J, Kangasniemi M, Niemela M, Jaaskelainen J, Jokitalo E, Karatas A, Hernesniemi J, Meri S. Complement activation associates with saccular cerebral artery aneurysm wall degeneration and rupture. Neurosurgery. 2006;59:1069–1076. doi: 10.1227/01.NEU.0000245598.84698.26. discussion 1076-1067. [DOI] [PubMed] [Google Scholar]
- 17.Qin X, Hu W, Song W, Grubissich L, Hu X, Wu G, Ferris S, Dobarro M, Halperin JA. Generation and phenotyping of mCd59a and mCd59b double-knockout mice. Am J Hematol. 2009;84:65–70. doi: 10.1002/ajh.21319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu W, Ferris SP, Tweten RK, Wu G, Radaeva S, Gao B, Bronson RT, Halperin JA, Qin X. Rapid conditional targeted ablation of cells expressing human CD59 in transgenic mice by intermedilysin. Nat Med. 2008;14:98–103. doi: 10.1038/nm1674. [DOI] [PubMed] [Google Scholar]
- 19.Cowan PJ, Somerville CA, Shinkel TA, Katerelos M, Aminian A, Romanella M, Tange MJ, Pearse MJ, d'Apice AJ. High-level endothelial expression of human CD59 prolongs heart function in an ex vivo model of xenograft rejection. Transplantation. 1998;65:826–831. doi: 10.1097/00007890-199803270-00010. [DOI] [PubMed] [Google Scholar]
- 20.Cassis LA, Helton MJ, Howatt DA, King VL, Daugherty A. Aldosterone does not mediate angiotensin II-induced atherosclerosis and abdominal aortic aneurysms. Br J Pharmacol. 2005;144:443–448. doi: 10.1038/sj.bjp.0706098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daugherty A, Manning MW, Cassis LA. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J Clin Invest. 2000;105:1605–1612. doi: 10.1172/JCI7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomas M, Gavrila D, McCormick ML, Miller FJ, Jr., Daugherty A, Cassis LA, Dellsperger KC, Weintraub NL. Deletion of p47phox attenuates angiotensin II-induced abdominal aortic aneurysm formation in apolipoprotein E-deficient mice. Circulation. 2006;114:404–413. doi: 10.1161/CIRCULATIONAHA.105.607168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manning MW, Cassis LA, Daugherty A. Differential effects of doxycycline, a broad-spectrum matrix metalloproteinase inhibitor, on angiotensin II-induced atherosclerosis and abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2003;23:483–488. doi: 10.1161/01.ATV.0000058404.92759.32. [DOI] [PubMed] [Google Scholar]
- 24.Wen J, Wang P, Smith SV, Haller CA, Chaikof EL. Syndecans are differentially expressed during the course of aortic aneurysm formation. J Vasc Surg. 2007;46:1014–1025. doi: 10.1016/j.jvs.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 25.Perissi V, Aggarwal A, Glass CK, Rose DW, Rosenfeld MG. A corepressor/coactivator exchange complex required for transcriptional activation by nuclear receptors and other regulated transcription factors. Cell. 2004;116:511–526. doi: 10.1016/s0092-8674(04)00133-3. [DOI] [PubMed] [Google Scholar]
- 26.Caird J, Napoli C, Taggart C, Farrell M, Bouchier-Hayes D. Matrix metalloproteinases 2 and 9 in human atherosclerotic and non-atherosclerotic cerebral aneurysms. Eur J Neurol. 2006;13:1098–1105. doi: 10.1111/j.1468-1331.2006.01469.x. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka K, Iwamoto Y, Ito Y, Ishibashi T, Nakabeppu Y, Sekiguchi M, Sugioka Y. Cyclic AMP-regulated synthesis of the tissue inhibitors of metalloproteinases suppresses the invasive potential of the human fibrosarcoma cell line HT1080. Cancer Res. 1995;55:2927–2935. [PubMed] [Google Scholar]
- 28.Sun J, Sukhova GK, Yang M, Wolters PJ, MacFarlane LA, Libby P, Sun C, Zhang Y, Liu J, Ennis TL, Knispel R, Xiong W, Thompson RW, Baxter BT, Shi GP. Mast cells modulate the pathogenesis of elastase-induced abdominal aortic aneurysms in mice. J Clin Invest. 2007;117:3359–3368. doi: 10.1172/JCI31311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niculescu F, Rus H. Complement activation and atherosclerosis. Mol Immunol. 1999;36:949–955. doi: 10.1016/s0161-5890(99)00117-0. [DOI] [PubMed] [Google Scholar]
- 30.Niculescu F, Badea T, Rus H. Sublytic C5b-9 induces proliferation of human aortic smooth muscle cells: role of mitogen activated protein kinase and phosphatidylinositol 3-kinase. Atherosclerosis. 1999;142:47–56. doi: 10.1016/s0021-9150(98)00185-3. [DOI] [PubMed] [Google Scholar]
- 31.Kilgore KS, Flory CM, Miller BF, Evans VM, Warren JS. The membrane attack complex of complement induces interleukin-8 and monocyte chemoattractant protein-1 secretion from human umbilical vein endothelial cells. Am J Pathol. 1996;149:953–961. [PMC free article] [PubMed] [Google Scholar]
- 32.Simmonds RE, Foxwell BM. Signalling, inflammation and arthritis: NFkappaB and its relevance to arthritis and inflammation. Rheumatology (Oxford) 2008;47:584–590. doi: 10.1093/rheumatology/kem298. [DOI] [PubMed] [Google Scholar]
- 33.Zenz R, Eferl R, Scheinecker C, Redlich K, Smolen J, Schonthaler HB, Kenner L, Tschachler E, Wagner EF. Activator protein 1 (Fos/Jun) functions in inflammatory bone and skin disease. Arthritis Res Ther. 2008;10:201. doi: 10.1186/ar2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ostergaard JR, Kristensen BO, Svehag SE, Teisner B, Miletic T. Immune complexes and complement activation following rupture of intracranial saccular aneurysms. J Neurosurg. 1987;66:891–897. doi: 10.3171/jns.1987.66.6.0891. [DOI] [PubMed] [Google Scholar]
- 35.Aoki T, Kataoka H, Ishibashi R, Nozaki K, Hashimoto N. Gene expression profile of the intima and media of experimentally induced cerebral aneurysms in rats by laser-microdissection and microarray techniques. Int J Mol Med. 2008;22:595–603. [PubMed] [Google Scholar]
- 36.Pagano MB, Zhou HF, Ennis TL, Wu X, Lambris JD, Atkinson JP, Thompson RW, Hourcade DE, Pham CT. Complement-dependent neutrophil recruitment is critical for the development of elastase-induced abdominal aortic aneurysm. Circulation. 2009;119:1805–1813. doi: 10.1161/CIRCULATIONAHA.108.832972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holt DS, Botto M, Bygrave AE, Hanna SM, Walport MJ, Morgan BP. Targeted deletion of the CD59 gene causes spontaneous intravascular hemolysis and hemoglobinuria. Blood. 2001;98:442–449. doi: 10.1182/blood.v98.2.442. [DOI] [PubMed] [Google Scholar]
- 38.Qin X, Krumrei N, Grubissich L, Dobarro M, Aktas H, Perez G, Halperin JA. Deficiency of the mouse complement regulatory protein mCd59b results in spontaneous hemolytic anemia with platelet activation and progressive male infertility. Immunity. 2003;18:217–227. doi: 10.1016/s1074-7613(03)00022-0. [DOI] [PubMed] [Google Scholar]
- 39.Longhi MP, Williams A, Wise M, Morgan BP, Gallimore A. CD59a deficiency exacerbates influenza-induced lung inflammation through complement-dependent and -independent mechanisms. Eur J Immunol. 2007;37:1266–1274. doi: 10.1002/eji.200636755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sivasankar B, Donev RM, Longhi MP, Hughes TR, Davies R, Cole DS, Morgan BP, Marchbank KJ. CD59a deficient mice display reduced B cell activity and antibody production in response to T-dependent antigens. Mol Immunol. 2007;44:2978–2987. doi: 10.1016/j.molimm.2006.12.025. [DOI] [PubMed] [Google Scholar]
- 41.Mayer MM. Complement. Historical perspectives and some current issues. Complement. 1984;1:2–26. [PubMed] [Google Scholar]
- 42.Papadimitriou JC, Ramm LE, Drachenberg CB, Trump BF, Shin ML. Quantitative analysis of adenine nucleotides during the prelytic phase of cell death mediated by C5b-9. J Immunol. 1991;147:212–217. [PubMed] [Google Scholar]
- 43.Yoshimura K, Aoki H, Ikeda Y, Fujii K, Akiyama N, Furutani A, Hoshii Y, Tanaka N, Ricci R, Ishihara T, Esato K, Hamano K, Matsuzaki M. Regression of abdominal aortic aneurysm by inhibition of c-Jun N-terminal kinase. Nat Med. 2005;11:1330–1338. doi: 10.1038/nm1335. [DOI] [PubMed] [Google Scholar]
- 44.Aoki T, Kataoka H, Shimamura M, Nakagami H, Wakayama K, Moriwaki T, Ishibashi R, Nozaki K, Morishita R, Hashimoto N. NF-kappaB is a key mediator of cerebral aneurysm formation. Circulation. 2007;116:2830–2840. doi: 10.1161/CIRCULATIONAHA.107.728303. [DOI] [PubMed] [Google Scholar]
- 45.Thompson RW, Holmes DR, Mertens RA, Liao S, Botney MD, Mecham RP, Welgus HG, Parks WC. Production and localization of 92-kilodalton gelatinase in abdominal aortic aneurysms. An elastolytic metalloproteinase expressed by aneurysm-infiltrating macrophages. J Clin Invest. 1995;96:318–326. doi: 10.1172/JCI118037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Longo GM, Xiong W, Greiner TC, Zhao Y, Fiotti N, Baxter BT. Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J Clin Invest. 2002;110:625–632. doi: 10.1172/JCI15334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hughes J, Nangaku M, Alpers CE, Shankland SJ, Couser WG, Johnson RJ. C5b-9 membrane attack complex mediates endothelial cell apoptosis in experimental glomerulonephritis. Am J Physiol Renal Physiol. 2000;278:F747–F757. doi: 10.1152/ajprenal.2000.278.5.F747. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.