Abstract
Fluid flow due to loading in bone is a potent mechanical signal that may play an important role in bone adaptation to its mechanical environment. Previous in vitro studies of osteoblastic cells revealed that the upregulation of cyclooxygenase-2 (COX-2) and c-fos induced by steady fluid flow depends on a change in actin polymerization dynamics and the formation of actin stress fibers. Exposing cells to dynamic oscillatory fluid flow, the temporal flow pattern that results from normal physical activity, is also known to result in increased COX-2 expression and PGE2 release. The purpose of this study was to determine whether dynamic fluid flow results in changes in actin dynamics similar to steady flow and to determine whether alterations in actin dynamics are required for PGE2 release. We found that exposure to oscillatory fluid flow did not result in the development of F-actin stress fibers in MC3T3-E1 osteoblastic cells and that inhibition of actin polymerization with cytochalasin D did not inhibit intracellular calcium mobilization or PGE2 release. In fact, PGE2 release was increased threefold in the polymerization inhibited cells and this PGE2 release was dependent on calcium release from the endoplasmic reticulum. This was in contrast to the PGE2 release that occurs in normal cells, which is independent of calcium flux from endoplasmic reticulum stores. We suggest that this increased PGE2 release involves a different molecular mechanism perhaps involving increased deformation due to the compromised cytoskeleton.
Keywords: mechanotransduction, cell mechanics
Mechanotransduction is the conversion of external mechanical stimuli into intracellular biochemical signals. Mechanical signals have been found to regulate many aspects of bone cell function, including differentiation, gene expression, protein synthesis, and apoptosis. The mechanosensitive signaling pathways of cells may depend on the interactions that exist between the cell's cytoskeleton and sites of transduction of mechanical signals (24, 30).
In vitro experiments conducted with bone cells suggest that loading-induced fluid flow is an important extracellular signal in mechanotransduction (1, 2, 14, 19, 20, 26). Pavalko et al. (18) found that the upregulation of COX-2 and the transcription factor c-fos was induced by a mechanism that involves the reorganization of the actin cytoskeleton under unidirectional steady fluid flow. Furthermore, disruption of the α-actinin mediated linkage between actin cytoskeletal filaments and plasma membrane bound integrins also inhibited the effect of fluid flow on both COX-2 and c-fos. COX-2 (also known as prostaglandin synthase 2) is an enzyme that is essential for the production of prostaglandins and may promote bone formation by increasing proliferation and differentiation of osteoprogenitor cells (6, 11). C-fos, an early response gene product that directly affects DNA transcription, is also essential for normal bone physiology. Thus the finding that the mechanical regulation of both of these proteins requires an intact actin-membrane connection suggests that a mechanically loaded cytoskeleton with functional linkages to integrins may play an essential role in mechanotransduction.
In bone, locomotion and postural control induce dynamic oscillatory fluid flow in the lacunar-canalicular network (26). During a loading event, deformation in the mineralized matrix causes a heterogeneous pressure gradient that drives fluid flow in the lacunar-canalicular network (15, 19, 26). When bone is subsequently unloaded, the fluid flow direction is reversed due to the reversed pressure gradient. The dynamic nature of this pressure gradient results in the fluid flow induced by loading being oscillatory in nature. You et al. (29) and Donahue et al. (9) applied oscillatory fluid flow to osteoblasts and found that COX-2 and PGE2 were upregulated. Although these studies further implicated fluid flow as an important cellular physical signal in bone cell mechanotransduction, it was not determined whether reorganization of the actin cytoskeleton is required for this observed upregulation.
In this study, we investigated the ability of oscillatory fluid flow to induce F-actin cytoskeletal reorganization in osteoblasts. We examined how altering actin polymerization dynamics affects intracellular calcium mobilization and PGE2 release in osteoblasts exposed to oscillatory flow. We postulated that the formation of stress fibers in osteoblasts requires actin filaments to experience a prolonged tension. Although steady flow would provide the necessary chronic tension to actin filaments, oscillatory flow would not. This would suggest that oscillatory fluid flow will not induce the reorganization of actin fibrils into stress fibers. We utilized low-dose cytochalasin D, which blocks actin polymerization at the barbed end, to determine the role of actin polymerization dynamics in intracellular calcium mobilization and PGE2 release of cells exposed to a physiological flow profile.
METHODS
Cell culture
MC3T3-E1 osteoblastic cells (subclone 14; ATCC, Manassas, VA) were cultured in α-modified minimal essential medium (αMEM; Invitrogen, Carlsbad, CA) containing 10% fetal bovine serum (FBS; Hyclone, Logan, UT), 1% penicillin and streptomycin (Invitrogen), and maintained at 37°C and 5% CO2 in a humidified incubator. All experiments were conducted on passage 18 cells. For calcium imaging experiments, cells were subcultured on fibronectin-coated (10 μg/ml; Sigma, St. Louis, MO) UV-transparent quartz slides (76 × 26 × 1.6 mm) at 100,000 cells/slide. For PGE2 experiments, cells were subcultured on fibronectin coated glass slides (76 × 48 × 1 mm) at 300,000 cells/slide. Fluid flow was applied 48 h after subculture such that cells were 80–90% confluent at the time of experimentation.
Fluid flow
Steady flow experiments were conducted by applying a unidirectional steady flow to cells using a multislide parallel plate flow chamber (Flexcell Streamer, Hillsborough, NC). A previously described fluid flow device was used to deliver oscillatory fluid flow to MC3T3-E1 osteoblastic cells (14). In brief, oscillatory flow was driven by a Hamilton glass syringe in series with rigid walled tubing and a parallel plate flow chamber. The syringe was mounted in and driven by an electromechanical loading device (EnduraTec, Eden Prarie, MN). The flow rate was monitored with an ultrasonic flow meter (Transonic Systems, Ithaca, NY). In both the steady and oscillatory flow cases, the flow rate was selected to yield peak shear stresses of 1.2 Pa (12 dyn/cm2). For dynamic oscillatory flow, the flow profile was sinusoidal at a frequency of 1 Hz. Flow media for all experiments consisted of αMEM, 1% FBS, and 1% penicillin and streptomycin.
Fluorescence microscopy
Following fluid flow, cells were fixed in formaldehyde and incubated with 0.5 μM Alexa Fluor 488-conjugated phalloidin (Molecular Probes, Eugene, OR) for 20 min. The cells were rinsed twice with PBS before a 1:1 solution of glycerol and PBS was applied to avoid evaporation. A glass coverslip was placed on the slide before observation with an epifluorescent microscope (Eclipse TE-300; Nikon, Melville, NY). To quantify the level of polymerized F-actin in cells exposed to each flow profile, pixel intensity analysis was performed following the subtraction of background values (MATLAB; The MathWorks, Natick, MA).
PGE2 release
Immediately following 1 h of fluid flow exposure, cell seeded slides were removed from the flow chambers, placed in sterile petri dishes with 1 ml of fresh media, and returned to the incubator for 1 h. Media samples were then collected and centrifuged at 12,000 g to remove any particulate matter. Supernatant PGE2 levels were measured using an enzyme immunoassay kit (Amersham Biosciences, Piscataway, NJ). PGE2 levels were normalized to total protein as determined by a BCA assay (Pierce, Rockford, IL). Pharmacological agents (when utilized) were applied before and for the duration of flow.
Calcium imaging
Real time intracellular levels of calcium were quantified using a ratiometric imaging technique. Before flow exposure, cells were incubated with 10 μM fura 2-AM (Molecular Probes) for 30 min at 31°C (to reduce dye compartmentalization) then washed with fresh αMEM and 1% FBS. Following fura 2-AM loading, the cell seeded quartz slides were mounted in a parallel plate flow chamber and fixed to the stage of a fluorescent microscope. For 30 min, the cells were left undisturbed. Fluorescent images were collected for 3 min before flow began and for 3 min after flow was initiated. All experiments were performed at room temperature to reduce fura 2-AM dye compartmentalization. A responding cell was defined by a transient increase in [Ca2+]i of at least four times the maximum oscillation recorded during the 3-min preflow baseline period. The percentage of responsive cells was expressed as the fraction of the total number of cells observed that were classified as having responded.
Pharmacological agents
Cytochalasin D (Sigma) was used to inhibit actin polymerization. At low doses, cytochalasin D inhibits actin polymerization by capping the barbed end of F-actin polymers, while at high doses it will remove existing F-actin filaments (7). A dose-response experiment was conducted to find a concentration where actin polymerization was blocked, but cell adhesion, morphology, and mechanical compliance were not drastically altered. Cells were exposed to 5, 2, 0.5, and 0.1 μM cytochalasin D for 30 min. After exposure, cells were either visually observed under flow or stained for F-actin with Alexa Fluor 488 phalloidin as described above. At concentrations >0.5 μM the cells were found to be too compliant to tolerate flow without exhibiting gross deformation or detaching. However, at 0.5 μM, we did not observe visible changes in cell morphology; therefore, this concentration was used for subsequent experiments. Cells were exposed to cytochalasin D for 30 min before flow and for the duration of flow exposure. Ninety minutes of exposure (as occurs with the PGE2 experiments) at 0.5 μM did not result in noticeable changes in morphology (data not shown). Thapsigargin (EMD Biosciences, San Diego, CA) was used to examine the effects of emptying endoplasmic reticulum (ER) stores of Ca2+ (5). Cells were exposed to 50 nM thapsigargin for 30 min before and for the duration of flow exposure. We found this protocol results in a transient increase in intracellular Ca2+, which peaks at 7–10 min after exposure, consistent with release from the endoplasmic reticulum. After 30 min of thapsigargin exposure, intracellular calcium levels returned to within 4.8 ± 4.3% of normal levels (data not shown). In other published reports, thapsigargin has been used to induce capacitative calcium entry and apoptosis. However, it should be noted that these studies utilize much higher doses of the drug for longer periods of time (8, 27). No evidence of cell death was observed with as much as 2 h of exposure at the concentration utilized in this study. To examine whether there was some effect of intracellular calcium that did not originate from ER stores, we utilized BAPTA-AM, which is taken up by cells and chelates free calcium ions. Cells were exposed to 50 μM BAPTA-AM for 30 min before and for the duration of flow exposure (28). In the case of multiple treatments, both protocols were applied simultaneously.
Data analysis
For calcium imaging experiments, we quantified the peak magnitude of response and the percentage of cells responding. PGE2 data were normalized to total protein. Data are expressed as means ± SE. To compare observations from the calcium and PGE2 experiments, statistical analysis using one-way ANOVA and Fisher's protected least-significant differences test was utilized (Statview, SAS Institute, San Francisco, CA). A significance level of 0.05 was employed for all statistical analyses.
RESULTS
Oscillatory fluid flow does not induce stress fiber formation
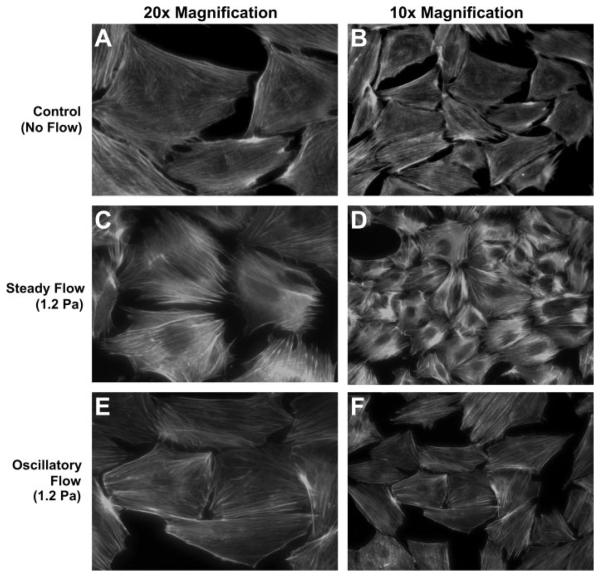
Application of oscillatory flow did not induce a large increase in F-actin density compared with no flow controls (Fig. 1, A and B). In contrast, the development of thick actin fibers, consistent with the formation of stress fibers, was apparent in cells exposed to unidirectional steady flow of the same peak shear stress and duration (Fig. 1, C and D). In steady flow cells, actin fibers were orientated parallel to the long axis of the cell and present around the nucleus of the cells. However, no observable relationship was found between the direction of fluid flow and the orientation of actin fibers. For all cells shown in Fig. 1, fluid flow was applied in the right-left direction. To analytically determine if there was a difference in actin stress fiber formation between no flow, oscillatory flow, and steady flow groups, florescent pixel intensity measurements were done on individual cells in each of these groups. Analysis of image pixel intensity revealed that cells exposed to steady flow had 1.4× the average pixel intensity of no-flow controls (P < 0.05), whereas cells exposed to oscillatory flow exhibited no difference in the pixel intensity compared with no-flow controls (no flow = 65.5 ± 1.2, oscillatory flow = 66.1 ± 2.5, steady flow = 98.9 ± 2.4; n = 3 slides for all groups).
Fig. 1.
Change in F-actin organization of MC3T3-E1 osteoblastic cells in response to no flow control (A and B), unidirectional steady flow (C and D), and oscillatory flow (E and F) for 1 h. Only application of steady unidirectional flow (C and D) resulted in the reorganization of the actin cytoskeleton into distinct stress fibers.
Inhibition of actin polymerization does not inhibit intracellular calcium mobilization due to oscillatory fluid flow
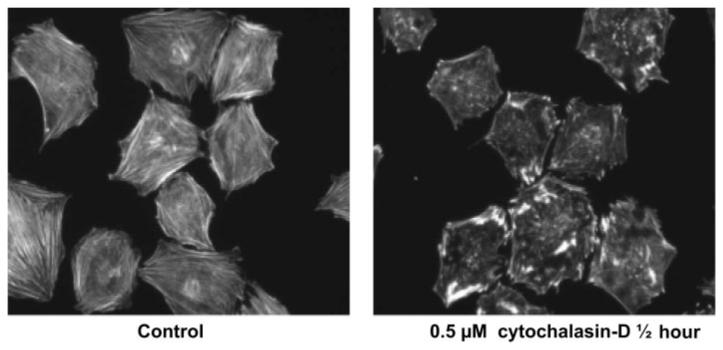
To determine a dose of cytochalasin D, which would inhibit actin polymerization at the barbed end without causing depolymerization of the entire actin cytoskeleton, a dose-response experiment was conducted. When these cells were exposed to oscillatory fluid flow, those treated with cytochalasin D concentrations in excess of 0.5 μM exhibited reduced adhesion, abnormal cell morphology, and a tendency to undergo large visible shear deformations with flow. At 0.5 μM cells retained morphology, adhesion, and could withstand flow without large deformation. However, actin staining demonstrated a clear inhibition of polymerization, and after 30 min, compromise of the actin cytoskeleton was evident (Fig. 2). When these cells were exposed to oscillatory fluid flow, cells with impaired actin polymerization exhibited increased calcium responses both in terms of a greater average magnitude of intracellular calcium concentration (Fig. 3) and a greater overall number of cells exhibiting an intracellular calcium response (Fig. 4). Emptying the cell's ER-stored calcium with exposure to thapsigargin completely blocked the calcium response to oscillatory fluid flow, consistent with our previous work (29). When thapsigargin and cytochalasin D were applied simultaneously, the Ca2+ response was again completely abrogated. Chelating intracellular calcium using BAPTA-AM caused a significant decrease in the percent of cells responding to fluid flow; however, the inhibition was less than that observed with thapsigargin. When BAPTA-AM and cytochalasin were applied simultaneously, the calcium response was significantly less than the no drug controls or cytochalasin alone but did not completely eliminate the response (Fig. 4). The magnitude of the BAPTA-AM alone and BAPTA-AM with cytochalasin responses were similar to the no drug group and significantly less than the cytochalasin alone group (Fig. 3).
Fig. 2.
Treatment of cells with 0.5 μM cytochalasin D for 30 min resulted in inhibited actin polymerization and a compromised actin cytoskeleton.
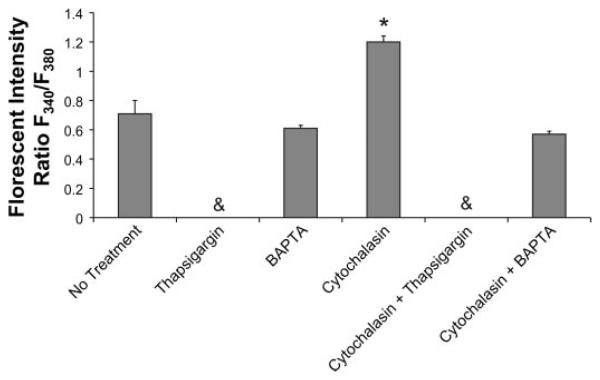
Fig. 3.
Cells treated with cytochalasin D had larger increases in intracellular calcium concentration as measured by florescent intensity when exposed to flow than untreated control cells or cells that had been pretreated with BAPTA-AM (in the presence or absence of cytochalasin D) (ratio F340/380 = 0.71 ± 0.09 for control, 1.2 ± 0.04 for cytochalasin D treatment, 0.61 ± 0.02 for BAPTA-AM treatment, 0.57 ± 0.02 for BAPTA-AM plus cytochalasin treatment). &P < 0.05, Treatment with thapsigargin completely blocked the calcium increase both in the case of thapsigargin alone and thapsigargin applied simultaneously with cytochalasin D. *P < 0.05, Significant difference from untreated flow exposed cells (n ≥ 3 slides for all groups).
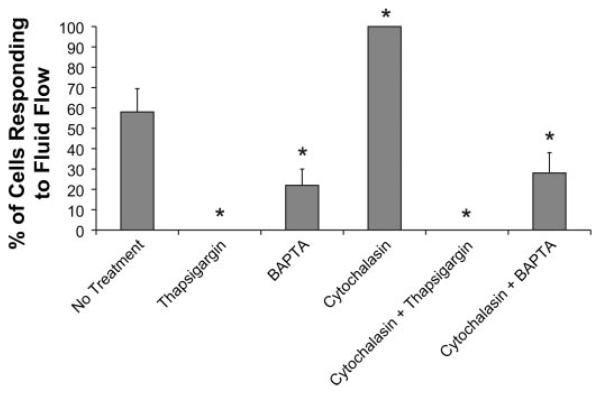
Fig. 4.
The percentage of cells responding to flow was greater in cells with cytochalasin D treatment than untreated control cells (P < 0.05; 58 ± 11.5% of control cells responded, 100 ± 0% of cytochalasin D-treated cells responded; n = 3 slides). The percentage of cells responding to flow was significantly less in cells with BAPTA-AM treatment (both with and without cytochalasin) than untreated control cells (P < 0.05; 22 ± 8% of BAPTA-AM treated cells responded, 28 ± 10% of BAPTA-AM plus cytochalasin-treated cell responded). No cells were found to exhibit a calcium response to flow both in the case of thapsigargin alone and thapsigargin applied simultaneously with cytochalasin D. *P < 0.05, significant difference from untreated flow-exposed cells (n ≥ 3 slides for all groups).
Inhibition of actin polymerization does not inhibit PGE2 release
Cells with inhibited actin polymerization exposed to 1 h of oscillatory fluid flow also exhibited a threefold increase in release of PGE2 compared with untreated cells (Fig. 5). The addition of thapsigargin to the cytochalasin D treatment reduced the levels of PGE2 release to that of untreated cells exposed to flow, whereas the addition of BAPTA-AM to the cytochalasin treatment completely eliminated the flow-induced increase in PGE2 (Fig. 5). Thapsigargin alone slightly reduced PGE2 release compared with untreated cells while BAPTA-AM completely eliminated the increase in PGE2. This suggests that flow induced PGE2 release is independent of ER stores of calcium in untreated cells but is dependent on some other cellular or extracellular source of calcium, which is blocked with BAPTA-AM. However, when the cell's cytoskeleton is inhibited using cytochalasin, the observed dramatic increase in PGE2 release is dependent on ER stores of calcium. The discrepancy between the two calcium inhibitors suggests that PGE2 release in cells with intact cytoskeletons is independent of ER stores of calcium, yet does depend on some calcium mobilization, while the actin disrupted cells produce PGE2 through a distinct molecular mechanism that requires the release of ER calcium.
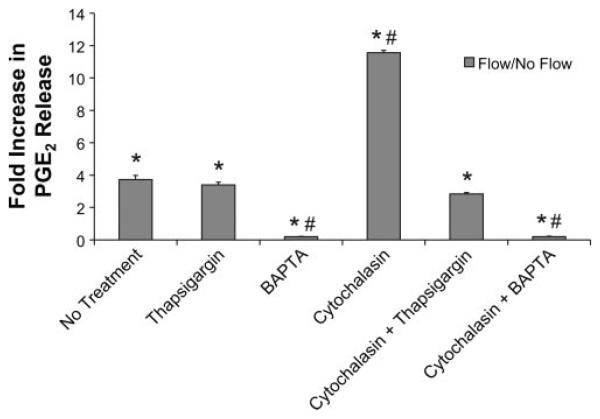
Fig. 5.
Untreated cells that were exposed to 1 h of oscillatory flow had a 3.7 ± 0.3 fold increase in PGE2 compared with untreated cells exposed to flow (P < 0.05). Cytochalasin D-treated cells had a 11.6 ± 0.2 fold increase in PGE2 release compared with untreated no flow control cells (P < 0.05). The addition of thapsigargin to the cytochalasin D treatment reduced the fold increase of PGE2 release in response to flow to that slightly less than untreated cells exposed to flow (2.8 ± 0.1 fold increase for thapsigargin + cytochalasin D-treated cells compared with untreated no-flow control cells; P < 0.05). Thapsigargin alone had similar effects as no treatment (3.4 ± 0.2 fold increase over untreated no flow control cells; P < 0.05). BAPTA-AM cause a significant decrease in PGE2 release compared with no flow control cells regardless of treatment with cytochalasin [0.2 ± 0.02 fold increase (5-fold decrease) for BAPTA-AM alone and 0.2 ± 0.03 fold increase for BAPTA-AM + cytochalasin (P < 0.05)]. *Significant difference from no flow. #Significant difference from untreated flow exposed cells (n ≥ 3 slides for all groups).
DISCUSSION
The purpose of this project was to examine the effect of loading-induced oscillatory fluid flow on the actin cytoskeleton of bone cells as well as to determine the potential role of the actin cytoskeleton in the release of PGE2 in response to flow. It is generally accepted that the dynamic nature of activities of daily living leads to oscillatory flow profiles in the pericellular fluid. Furthermore, although it has been shown that steady flow applied for a sufficient period of time results in densification of the actin fiber network, we wanted to compare the effects of steady flow vs. dynamic flow on actin dynamics. Because adaptations in the actin network occur over a relatively long time scale (greater than seconds), we felt that dynamic flow might have quite different effects.
Indeed, we found that exposing cells to 1 h of oscillatory fluid flow did not induce a dramatic densification of the actin cytoskeleton. Although the same magnitude peak shear stress (1.2 Pa) was applied in both the oscillatory and steady flow experiments, only in the steady flow experiments did we observe an increase in F-actin. Image intensity analysis confirmed this actin densification with steady flow and revealed that steady flow resulted in 40% increase in florescent intensity, which we believe corresponds to more total polymerized F-actin. Previous studies (17, 19) have shown that unidirectional steady flow induced actin stress fiber formation. Norvell et al. (17) showed by quantifying insoluble actin levels that the observed increase in stress fibers was not due to an increase in total actin or a decrease in unbound actin and they concluded that the increase in stress fibers was solely due to fiber reorganization. These results appear contradictory to our finding that image intensity is increased with steady flow. However, fluorescent image intensity analysis is potentially less accurate than measurement of insoluble actin and direct comparison of these finding may not be appropriate. It is also possible that our slightly higher flow rate resulted in increased actin production or decreased levels of free cellular actin. In terms of the effect of oscillatory flow on actin dynamics, the dramatic increase in actin intensity observed with steady flow was clearly absent. Qualitatively, the cells that were exposed to oscillatory fluid flow appear visually similar to no flow controls and quantitatively they had no significant increase in polymerized F-actin as measured by individual cells' florescent intensity. It is possible that in cells exposed to oscillatory fluid flow actin filament diameter was increased without an increase in overall F-actin levels, which would not affect net intensity. However, this could not be determined because fluorescent images do not have sufficient resolution to distinguish changes in filament number or diameter.
The F-actin reorganization observed in cells exposed to steady flow may result from a cellular response that reinforces the cell's mechanical structure. That is, bone cells may produce stress fibers to maintain morphology in response to a chronic applied tension. However, for stress fiber formation to occur, osteoblasts may need to experience a sustained deformation for a greater duration than that experienced when exposed to 1-Hz oscillatory flow. The viscoelastic properties of osteoblastic cells have not yet been determined; however, the relaxation time constant of 3T3 mouse fibroblasts cultured in monolayer is ~0.1 s (3, 4), while the time constant of isolated chondrocytes has been reported to be 20–30 s (12, 25). For cells to fully deform in response to flow, a force in one direction needs to be applied for a substantially greater time period than the cell's relaxation time constant. If the flow reversal frequency used (1 Hz) is close to or shorter than the viscoelastic time constant for MC3T3-E1 cells, then cell reinforcement via cytoskeletal reorganization may not be required to maintain cell shape and deformations sufficiently large enough to initiate cytoskeletal reorganization may not occur. Since we did not observe F-actin reorganization in osteoblasts exposed to oscillatory flow, the observed stress fiber formation under unidirectional steady flow may serve only to reinforce existing cytoskeletal elements involved in maintaining cell morphology.
Although we did not observe the dramatic increase in filamentous actin with oscillatory flow exposure as was seen with steady flow, the second goal of this work was to determine whether altered actin polymerization dynamics play any role in oscillatory flow-induced PGE2 release. Previous studies (10, 13, 16, 21) indicate that prostaglandins may play an essential role in in vivo bone formation in response to mechanical loading. The synthesis of PGE2 and its release in response to mechanical loading by osteoblasts are dependent on the activity of the enzyme cyclooxygenase-2 (COX-2) (11). Both PGE2 release and COX-2 induction have been shown to increase with the application of fluid flow to osteoblasts in vitro (9, 17). We hypothesized that even though we did not observe F-actin densification, changes in actin polymerization dynamics may be involved in the PGE2 release of bone cells in response to oscillatory fluid flow. To test this hypothesis we utilized a concentration of cytochalasin D that inhibits actin polymerization but does not result in changes in cell morphology and gross cell deformation with flow. Interestingly, we found that PGE2 release under these conditions was actually increased, and furthermore, intracellular calcium mobilization was also increased. This PGE2 data is consistent with Norvell et al. (17) who reported that both PGE2 release and increased COX-2 expression due to steady flow do not require an intact cytoskeleton. In contrast, a different study reported that increased COX-2 expression due to steady flow was blocked by disrupting the cytoskeleton D (18). While there is some discrepancy in the literature, it appears that an intact microfilament network is not necessary for PGE2 release. Nonetheless, our findings do not eliminate the role of actin as a structural element that transmits intracellular forces to the site of molecular transduction. Indeed, our results suggest that the flow-induced release of PGE2 from cells with disrupted cytoskeletons occurs via a distinct mechanism not activated in cells with intact cytoskeletons.
It is interesting to note that we found PGE2 release occurred independently of the release of calcium from the ER but did depend on intracellular calcium signaling since it was blocked with BAPTA-AM. Furthermore, unlike cells with intact cytoskeletons, the increase in PGE2 release we observed in cytochalasin D-treated cells was dependent on the release of ER stores of calcium. When this calcium increase was blocked with thapsigargin, PGE2 release with flow returned to that observed in noncytochalasin D-treated cells. BAPTA-AM completely abolished the flow-induced increase in PGE2 release with and without cytochalasin D treatment. Prior work concluded that PGE2 release in response to oscillatory flow occurs via a calcium-independent mechanism (22, 23) in cells with intact cytoskeletons. Saunders et al. (22) showed that inhibiting gap junctions reduced PGE2 release but had no effect on intracellular calcium release. They also showed that ROS 17/2.8 cells, which do not experience a flow induced calcium response, still exhibit flow induced PGE2 release (23). However, neither of these studies used BAPTA-AM to eliminate all sources of intracellular calcium. The level of intracelluar calcium needed for PGE2 release in our study was below the sensitivity of fura 2 imaging. Thus, it is possible that in these prior studies the cells also underwent calcium flux at levels undetectable by fura 2. In summary, PGE2 release due to oscillatory fluid flow appears to be dependent on calcium flux but is independent of the dramatic increase in intracellular calcium from the ER stores. However, when the actin polymerization in MC3T3-E1 cells is disrupted by cytochalasin D treatment, the dramatic increase in flow-induced PGE2 release does involve the release of ER stores of calcium. This indicates that distinct molecular mechanisms may be responsible for these two types of PGE2 release.
We did not observe dramatic motion of the cells with flow at the 0.5 μM concentration of cytochalasin D we used in this study. Nonetheless, it is possible that although we did not see a significant change in the cell morphology with cytochalasin D treatment or gross deformation of the treated cells with flow, blocking of actin polymerization for 30 min may have indeed increased cellular compliance. Figure 2 demonstrates that the cytochalasin D-treated cells did have a compromised actin cytoskeleton and suggests cellular deformation was increased. This increased deformation may have stimulated mechanotransduction pathways not activated in cells with physiological stiffness. The increased PGE2 release observed with cytochalasin D treatment may involve influx of extracellular calcium through stretch activated transmembrane ion channels causing an increase in calcium-dependent PGE2 release. However, we did not quantify cellular deformation with flow, which would be required to definitively demonstrate that the cytochalasin D treatment increased cellular compliance.
In summary, we found that while steady flow did induce significant cytoskeletal reorganization and the formation of stress fibers, exposing osteoblastic cells to 1 h of dynamic oscillatory flow (1.2 Pa) did not. Blocking actin polymerization did not inhibit the ability of the cells to respond to oscillatory fluid flow with intracellular calcium mobilization or release of PGE2. In fact PGE2 release was increased threefold in cells with inhibited actin polymerization. We also found that this increase in PGE2 release that occurs in cells with blocked actin polymerization involved release of calcium from ER stores. Therefore, we suggest that this increased PGE2 release involves a distinct molecular mechanism perhaps involving increased deformation that may occur in response to flow in the actin inhibited cells.
ACKNOWLEDGMENTS
We gratefully acknowledge the technical support of Lauren McKormick.
GRANTS
This study was supported by National Institutes of Health Grant AR45989, US Army Medical Research Award DAMD 17-98-1-8509, and by NASA Grant NAG2-1601.
REFERENCES
- 1.Ajubi NE, Klein-Nulend J, Nijweide PJ, Vrijheid-Lammers T, Alblas MJ, Burger EH. Pulsating fluid flow increases prostaglandin production by cultured chicken osteocytes–a cytoskeleton-dependent process. Biochem Biophys Res Commun. 1996;225:62–68. doi: 10.1006/bbrc.1996.1131. [DOI] [PubMed] [Google Scholar]
- 2.Bakker AD, Soejima K, Klein-Nulend J, Burger EH. The production of nitric oxide and prostaglandin E2 by primary bone cells is shear stress dependent. J Biomech. 2001;34:671–677. doi: 10.1016/s0021-9290(00)00231-1. [DOI] [PubMed] [Google Scholar]
- 3.Bausch AR, Moller W, Sackmann E. Measurement of local viscoelasticity and forces in living cells by magnetic tweezers. Biophys J. 1999;76:573–579. doi: 10.1016/S0006-3495(99)77225-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bausch AR, Ziemann F, Boulbitch AA, Jacobson K, Sackmann E. Local measurements of viscoelastic parameters of adherent cell surfaces by magnetic bead microrheometry. Biophys J. 1998;75:2038–2049. doi: 10.1016/S0006-3495(98)77646-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen NX, Ryder KD, Pavalko FM, Turner CH, Burr DB, Qiu J, Duncan RL. Ca2+ regulates fluid shear-induced cytoskeletal reorganization and gene expression in osteoblasts. Am J Physiol Cell Physiol. 2000;278:C989–C997. doi: 10.1152/ajpcell.2000.278.5.C989. [DOI] [PubMed] [Google Scholar]
- 6.Chow JW, Chambers TJ. Indomethacin has distinct early and late actions on bone formation induced by mechanical stimulation. Am J Physiol Endocrinol Metab. 1994;267:E287–E292. doi: 10.1152/ajpendo.1994.267.2.E287. [DOI] [PubMed] [Google Scholar]
- 7.Cooper JA. Effects of cytochalasin and phalloidin on actin. J Cell Biol. 1987;105:1473–1478. doi: 10.1083/jcb.105.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Copanaki E, Schurmann T, Eckert A, Leuner K, Muller WE, Prehn JH, Kogel D. The amyloid precursor protein potentiates CHOP induction and cell death in response to ER Ca2+ depletion. Biochim Biophys Acta. 2007;1773:157–165. doi: 10.1016/j.bbamcr.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Donahue TL, Haut TR, Yellowley CE, Donahue HJ, Jacobs CR. Mechanosensitivity of bone cells to oscillating fluid flow induced shear stress may be modulated by chemotransport. J Biomech. 2003;36:1363–1371. doi: 10.1016/s0021-9290(03)00118-0. [DOI] [PubMed] [Google Scholar]
- 10.Feyen JH, van der Wilt G, Moonen P, Di Bon A, Nijweide PJ. Stimulation of arachidonic acid metabolism in primary cultures of osteoblast-like cells by hormones and drugs. Prostaglandins. 1984;28:769–781. doi: 10.1016/0090-6980(84)90034-0. [DOI] [PubMed] [Google Scholar]
- 11.Forwood MR. Inducible cyclo-oxygenase (COX-2) mediates the induction of bone formation by mechanical loading in vivo. J Bone Miner Res. 1996;11:1688–1693. doi: 10.1002/jbmr.5650111112. [DOI] [PubMed] [Google Scholar]
- 12.Guilak F. The deformation behavior and viscoelastic properties of chondrocytes in articular cartilage. Biorheology. 2000;37:27–44. [PubMed] [Google Scholar]
- 13.Imamura K, Ozawa H, Hiraide T, Takahashi N, Shibasaki Y, Fukuhara T, Suda T. Continuously applied compressive pressure induces bone resorption by a mechanism involving prostaglandin E2 synthesis. J Cell Physiol. 1990;144:222–228. doi: 10.1002/jcp.1041440207. [DOI] [PubMed] [Google Scholar]
- 14.Jacobs CR, Yellowley CE, Davis BR, Zhou Z, Cimbala JM, Donahue HJ. Differential effect of steady versus oscillating flow on bone cells. J Biomech. 1998;31:969–976. doi: 10.1016/s0021-9290(98)00114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knothe Tate ML, Steck R, Forwood MR, Niederer P. In vivo demonstration of load-induced fluid flow in the rat tibia and its potential implications for processes associated with functional adaptation. J Exp Biol. 2000;203:2737–2745. doi: 10.1242/jeb.203.18.2737. [DOI] [PubMed] [Google Scholar]
- 16.Nolan RD, Partridge NC, Godfrey HM, Martin TJ. Cyclo-oxygenase products of arachidonic acid metabolism in rat osteoblasts in culture. Calcif Tissue Int. 1983;35:294–297. doi: 10.1007/BF02405049. [DOI] [PubMed] [Google Scholar]
- 17.Norvell SM, Ponik SM, Bowen DK, Gerard R, Pavalko FM. Fluid shear stress induction of COX-2 protein and prostaglandin release in cultured MC3T3-E1 osteoblasts does not require intact microfilaments or microtubules. J Appl Physiol. 2004;96:957–966. doi: 10.1152/japplphysiol.00869.2003. [DOI] [PubMed] [Google Scholar]
- 18.Pavalko FM, Chen NX, Turner CH, Burr DB, Atkinson S, Hsieh YF, Qiu J, Duncan RL. Fluid shear-induced mechanical signaling in MC3T3-E1 osteoblasts requires cytoskeleton-integrin interactions. Am J Physiol Cell Physiol. 1998;275:C1591–C1601. [PubMed] [Google Scholar]
- 19.Piekarski K, Munro M. Transport mechanism operating between blood supply and osteocytes in long bones. Nature. 1977;269:80–82. doi: 10.1038/269080a0. [DOI] [PubMed] [Google Scholar]
- 20.Reich KM, Frangos JA. Effect of flow on prostaglandin E2 and inositol trisphosphate levels in osteoblasts. Am J Physiol Cell Physiol. 1991;261:C428–C432. doi: 10.1152/ajpcell.1991.261.3.C428. [DOI] [PubMed] [Google Scholar]
- 21.Rodan SB, Wesolowski G, Rodan GA. Clonal differences in prostaglandin synthesis among osteosarcoma cell lines. J Bone Miner Res. 1986;1:213–220. doi: 10.1002/jbmr.5650010208. [DOI] [PubMed] [Google Scholar]
- 22.Saunders MM, You J, Trosko JE, Yamasaki H, Li Z, Donahue HJ, Jacobs CR. Gap junctions and fluid flow response in MC3T3-E1 cells. Am J Physiol Cell Physiol. 2001;281:C1917–C1925. doi: 10.1152/ajpcell.2001.281.6.C1917. [DOI] [PubMed] [Google Scholar]
- 23.Saunders MM, You J, Zhou Z, Li Z, Yellowley CE, Kunze EL, Jacobs CR, Donahue HJ. Fluid flow-induced prostaglandin E2 response of osteoblastic ROS 17/2.8 cells is gap junction-mediated and independent of cytosolic calcium. Bone. 2003;32:350–356. doi: 10.1016/s8756-3282(03)00025-5. [DOI] [PubMed] [Google Scholar]
- 24.Sukharev SI, Blount P, Martinac B, Kung C. Mechanosensitive channels of Escherichia coli: the MscL gene, protein, and activities. Annu Rev Physiol. 1997;59:633–657. doi: 10.1146/annurev.physiol.59.1.633. [DOI] [PubMed] [Google Scholar]
- 25.Trickey WR, Lee GM, Guilak F. Viscoelastic properties of chondrocytes from normal and osteoarthritic human cartilage. J Orthop Res. 2000;18:891–898. doi: 10.1002/jor.1100180607. [DOI] [PubMed] [Google Scholar]
- 26.Weinbaum S, Cowin SC, Zeng Y. A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses. J Biomech. 1994;27:339–360. doi: 10.1016/0021-9290(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 27.Yao Y, Tsien RY. Calcium current activated by depletion of calcium stores in Xenopus oocytes. J Gen Physiol. 1997;109:703–715. doi: 10.1085/jgp.109.6.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshida I, Monji A, Tashiro K, Nakamura K, Inoue R, Kanba S. Depletion of intracellular Ca2+ store itself may be a major factor in thapsigargin-induced ER stress and apoptosis in PC12 cells. Neurochem Int. 2006;48:696–702. doi: 10.1016/j.neuint.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 29.You J, Reilly GC, Zhen X, Yellowley CE, Chen Q, Donahue HJ, Jacobs CR. Osteopontin gene regulation by oscillatory fluid flow via intracellular calcium mobilization and activation of mitogen-activated protein kinase in MC3T3-E1 osteoblasts. J Biol Chem. 2001;276:13365–13371. doi: 10.1074/jbc.M009846200. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Hamill OP. On the discrepancy between whole-cell and membrane patch mechanosensitivity in Xenopus oocytes. J Physiol. 2000;523:101–115. doi: 10.1111/j.1469-7793.2000.00101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]