Abstract
Arp2/3 complex is an actin polymerization nucleator and localized in the leading protrusions of migrating cells. It has been unclear how this complex is targeted to the protrusions and whether its localization is functionally important. We previously demonstrated that mRNAs encoding for the subunits of the complex were localized in the protrusions of fibroblasts, suggesting a mechanism to target the complex to the protrusions. We here present data demonstrating the importance of Arp2/3 complex mRNA localization in directional cell migration. Using a novel mechanism by which Dia1 mRNA is targeted to the perinuclear endoplasmic reticulum, we re-directed the mRNA encoding Arp2, a subunit of the Arp2/3 complex, to the perinuclear region in fibroblasts. Knockdown of Arp2 alone caused dramatic reduction of the complex and resulted in narrow protrusions, increased random cell migration speed and loss of directionality. Rescue with a protrusion-localizing Arp2 mRNA restored normal cell migration behavior, whereas rescue with a mis-localizing Arp2 mRNA failed to restore speed and directionality. These results demonstrate that localization of Arp2/3 complex mRNAs in the leading protrusions is functionally important for directional cell migration.
Keywords: Arp2/3, formin, cytoskeleton, actin, fibroblast, translation
Introduction
Directional cell migration plays an important role in many biological processes such as development, immune defense and wound healing. A hallmark of directional cell migration is the localized actin polymerization at the leading protrusions of the cell [1]. Arp2/3, a seven-protein complex, is a critical nucleator that promotes actin polymerization [2]. Despite intensive studies on the Arp2/3 complex, its role in directional cell migration is not well understood. Numerous studies have indicated that Arp2/3 complex mediates the formation of branched actin network which is predicted to be critical for cell motility [1, 3]. However, depletion of Arp2/3 complex did not inhibit formation of lamellipodia and had little effect on cell migration in platelets and mouse embryo fibroblasts [4]. Furthermore, little branched actin was observed in the leading edges of several cell types [5]. In a variety of cell types, Arp2/3 complex is localized at the leading edge of migrating cells and such localization has been proposed to be important for directional cell migration [6–8]. However, little is known as to what governs its localization to the protrusions and whether its localization is important for cellular function.
In addition to protein transport, mRNA localization is an effective way to target proteins to the site of function via local translation [9–12]. In many cases, such localized translation could not be functionally substituted by synthesizing equivalent amount proteins elsewhere. For example, loss of leading edge β-actin mRNA localization did not alter β-actin protein level but resulted in slower cell movement and reduction of cell migration directionality [13–15]. Redirection of apically localized Wingless mRNAs to the basolateral compartment in epithelial cells led to reduced protein activity even though the protein was sufficiently translated [16]. As at least 70% of the mRNAs have the potential to be localized [10], the significance of mRNA localization in protein targeting should not be underestimated. To understand why and how the Arp2/3 complex is targeted to the leading protrusions, we examined Arp2/3 complex mRNA distribution in fibroblasts. Interestingly, all the mRNAs encoding the seven subunits of the Arp2/3 complex are localized at the cell protrusions, suggesting that mRNA localization is a mechanism to target the complex to its site of function via local translation and complex assembly [17]. However, it is not known whether Arp2/3 complex mRNA localization is functionally important for cell migration. This prompted us to investigate the role of Arp2/3 complex mRNA localization in fibroblast migration using Arp2 subunit of the complex as the testing subject.
Materials and Methods
Materials
Diethyl-pyrocarbonate (DEPC), Escherichia coli tRNA, heparin, were from Sigma (St Louis, MO). Bovine serum albumin (BSA, protease and nuclease free), digoxigenin-11-dUTP (DIG-11-dUTP) and sheep anti-DIG antibody (peroxidase conjugated) were from Roche (Indianapolis, IN). Antibodies against the Arp2/3 complex were from Santa Cruz (Santa Cruz, CA) and their catalog numbers are: sc-15389 (for Arp2); sc-15390 (for Arp3); sc-32196 (for p34) and sc-68396 (for p21). Rabbit anti-Dia1 was generated via a contract with Biosynthesis (Lewisville, Texas) using chicken Dia1 C-terminus peptide (residues 1238–1253: CLEDSRTRHSGTRPAL) as an antigen. Superasin RNase inhibitor and mouse anti-GAPDH antibody were from Ambion (Austin, TX). RNasin was from Promega (Madison, WI). Eagle's minimum essential medium (MEM), fetal bovine serum (FBS) and trypsin/EDTA were from Mediatech (Herndon, VA). Tyramide signal amplification (TSA) reagents were purchased from Perkin Elmer (Boston, MA). Other general chemicals were from Sigma and Fisher (Pittsburgh, PA).
Construction of expression plasmids
Standard molecular biology techniques were used for cloning and sub-cloning. Full length chicken Dia1 cDNA (accession NM_204210) was obtained by RT-PCR. Unless indicated otherwise all the expression plasmids were constructed using the pNE plasmid backbone which is under the control of a chicken β-actin promoter (courtesy of Dr. Stefan Kindler, Hamburg). In order to detect exogenous Arp2 protein and mRNA, we first made an HA-LacZ cassette to replace the GFP in the pNE plasmid. The cassette contains an HA-tag at the end of the coding region for protein detection and a 299 base-pair (bp) fragment of LacZ in the 3'-UTR for mRNA detection. cDNA sequences of interest were then cloned into this modified plasmid upstream of the cassette. For cArp2-siRNA resistant expression of rescue Arp2, three silent mutations were created in the cArp2 mRNA target sequence 5'-GCAAGUGAAUUACGUUCAA-3' (bold nucleotides have been mutated to C, C and T, respectively). All the constructs were confirmed by DNA sequencing.
Cell Culture and transfection
Primary chicken embryo fibroblasts (CEFs) were prepared and cultured as described previously [17]. For immunofluorescence staining (IF) or fluorescence in situ hybridization (FISH), the cells were plated on glass cover slips coated with 0.5% gelatin for ~50–70% of confluence 24 hours later. The cells on each cover slip were incubated with 0.3 μg DNA of each construct and Lipofectamine-PLUS (Invitrogen) for 2 hours before change of medium then cultured for 22 hours. The cells were then fixed and subjected to FISH combined with immunostaining for the detection of mRNA and the HA-tag, respectively. For siRNA-mediated knockdown, the cells were plated into 6-well plate for 24 hours followed by transfection using Lipofectamine 2000 (Invitrogen). For rescue experiments, the cells were firstly transfected with siRNA using Lipofectamine 2000 for 24 hours then further transfected with rescue expression plasmids using Lipofectamine-PLUS.
Probe preparation, fluorescence in situ hybridization (FISH) and immunofluorescence staining (IF)
The preparation of RNA probe for chicken Arp2 mRNA was previously described in Mingles et al. [17]. The RNA probes for chicken Dia1 and bacterial lacZ fragment were prepared similarly as for the Arp2 probe. Briefly, nucleotides 62-1470 of chicken Dia1 (accession AB025226) and nucleotides 1599-1370 of LacZ (accession U73857) were cloned into pGEM-T Easy plasmids (Promega). These plasmids were linearized and then transcribed in vitro in the presence of DIG- or fluorescein-labelled dUTP for RNA probes using a Maxiscript transcription kit (Ambion, Austin, TX). The RNA probes were quantified by comparison with standard RNA for ethidium-bromide fluorescence intensity. Corresponding sense probes were also prepared in the same way and used for specificity control. FISH was performed essentially the same as previously described [17]. Briefly, labeled RNA probe(s) was incubated with the fixed and permeabilized cells overnight then washed extensively. Peroxidase-conjugated sheep anti-DIG (or anti-fluoreiscein) antibody was used and the fluorescence signal was amplified with tyramide signal amplification (TSA). For the detection of two types of mRNA or mRNA and protein in the same cells, after FISH, the samples were treated with HCl to remove the antibody then washed with PBS as described previously [18]. The samples were then processed similarly for another mRNA or incubated with primary antibodies for the target proteins followed by fluorescence-conjugated secondary antibodies using normal IF procedures.
Quantification of mRNA Localization
The localization of mRNA was scored visually as described previously [17, 19]. The vast majority of these fibroblasts are polarized and we based on the apparent cell morphology to judge leading lamellae/protrusion and trailing edge. A cell was determined to have localized mRNA if the majority (>60%) of the fluorescence signal of the mRNA was found at the leading lamellae. For a cell without apparent polarization, if more than 60% of the fluorescence signal is concentrated at one side of the cell edge, it would be score as localized. In some cases, in addition to being localized to the leading protrusions, a small portion of the mRNA is concentrated at the trailing edge. Such a cell would be scored as localized if the mRNA at the leading and trailing edge combined is >70% of the total mRNA in the cell. A cell that failed to meet the above criteria was scored as not localized. All the cells in each given microscopic field were scored without selection. This method was previously verified with fluorescence quantification [17]. All the samples were scored blindly with their identity concealed. At least 300–500 cells from three independent experiments were scored for each mRNA. Statistical analysis was performed using the Student's t-test.
SDS-PAGE and Western Blotting
The protein components were separated with electrophoresis using 10% SDS-polyacrylamide gel then transferred to nitrocellulose membranes. Protein signal on Western blots was detected with corresponding first antibodies then followed by peroxidase conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA) and Super-Signal enhanced chemiluminescence substrate (Pierce, Rockford, IL). The chemiluminescent signal was recorded and quantified with a Fuji LAS 3000 gel documentation system.
Fluorescence microscopy
Samples were viewed and imaged with an Olympus microscope BX61 using a 40× oil objective (NA 1.0), a cooled CCD camera (SensiCam from Cooke) and IPLab software (version 3.6.5, Scanalytics Inc. Fairfax, VA). Additional image processing was performed using Adobe Photoshop (version 7.0, Adobe Systems, Mountain View, CA).
Time-lapse microscopy and cell motility analysis
Cells were firstly treated with siRNA for two days then plated into glass-bottom 35mm dish (MatTek) at low density to minimize cell-cell contact. About 20 hours after plating, the cells were transferred into the environmental chamber on an Olympus IX70 inverted microscope controlled at 37 °C and 5% balanced CO2. For rescue samples, 24 hours after first transfection with siRNA, the cells were further transfected with rescue plasmids and incubated for about 24 hours before plated into the glass-bottom 35 mm dish. In the rescue plasmids used for the live cell migration analysis, mCherry replaced GFP as marker for the transfected cells. The rescued cells were identified based on their fluorescence marker (red for rescue Arp2 and green for co-transfected Dia1 expression). Identical microscopic parameters were used for fluorescence and phase contrast images. Time lapse movies were recorded at 5 min/frame for 2 hours. Cell migration was tracked using Image-J and cell location was defined as the centroid of a cell's contour. Even though the cells were plated at low density, some of them ran into each other, which might interfere with their migration behavior. Some cells divided during the 2-hour time lapse and some moved out of the field. These cells were excluded from analysis. Four parameters of cell migration were used for analysis. Speed (velocity) was presented as μm/min; total path is the accumulated total travel distance; net path is the linear distance between the starting point and the ending point, and directionality is the ratio of net-path/total-path. Because the numbers of cells analyzed from each condition were not the same, statistics for cell migration was performed using Kruskal-Wallis test.
Determination of HA-tagged Arp2 level in live cells using standard curves
Because it is impossible to directly determine the level of HA-tagged exogenous Arp2 protein in the live cells, we chose to determine the HA-Arp2 level in the cells indirectly using mCherry fluorescence as a reference. This is based on that both mCherry and HA-Arp2 are in the same plasmid and there is a positive correlation between their protein levels. We first established standard curves for mCherry and HA-Arp2 (localizing rescue) and mCherry-Dia1 fusion and HA-Arp2 (mis-localizing rescue), respectively. Normally cultured CEFs were transfected with rescue plasmids to express either localizing Arp2 mRNA or mis-localizing Arp2 mRNA. 22 hours post transfection, the cells were fixed and processed for immunofluorescence staining (Cy2) for detection of HA-Arp2. Using identical microscopic parameters for acquisition of fluorescence images of the live cells that were used for single cell migration analysis, the fluorescence intensity of mCherry and HA-Arp2 in these fixed cells were acquired and quantified. From these quantitative data, two standard curves were established (Supplementary Figure 1) and used for selection of rescued cells with comparable HA-Arp2 protein level (Figure 5A).
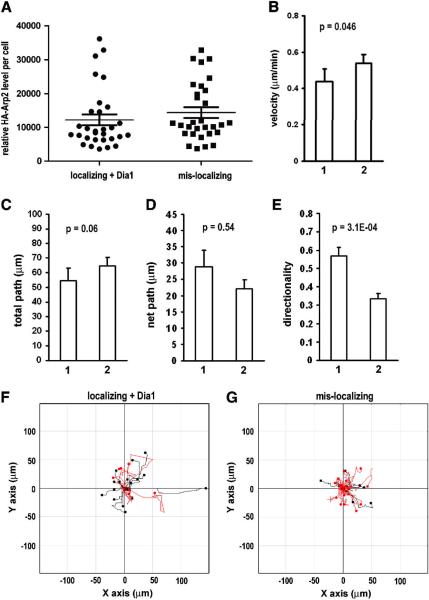
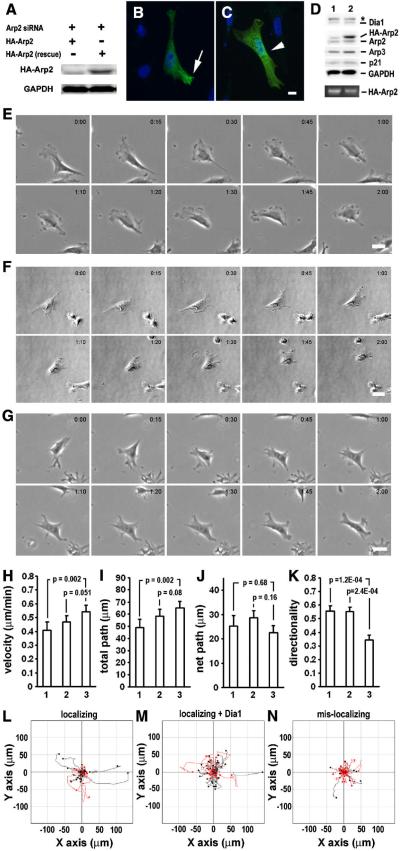
Figure 5. With comparable Arp2 protein level to the wild-type rescue, cells rescued with mis-localizing Arp2 mRNA failed to restore normal cell migration speed and directionality.
A. Selected Arp2 rescue cells with comparable HA-Arp2 levels. 30 cells of each group were selected from the cells analyzed in Figure 4 using standard curves (Supplementary Figure 1). B–E. Results of single cell migration analysis of the selected cells rescued with 1. localizing Arp2 mRNA plus Dia1 or 2. mis-localizing Arp2 mRNA. Error bar: s.e.m. F & G. Cell tracks of the selected cells. Red track indicate directionality score < 0.5.
Results and Discussion
Mis-localization of Arp2 mRNA to the perinuclear region
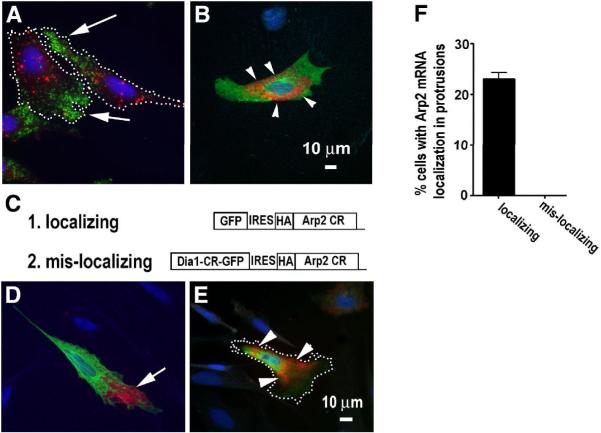
To test the functional importance of localization of an mRNA, a common strategy is to alter the localization of the mRNA then perform a functional analysis. Because mRNA localization is generally determined by localization signal sequence (zipcode) on the RNA, a traditional approach is to first identify the zipcode(s) on a RNA and then to mutate the zipcode in order to alter the localization of the RNA. Because the Arp2/3 complex requires all the seven subunits for its normal activity, manipulation of one subunit is probably sufficient to affect the assembly and function of the intact complex. To accomplish this, we chose the Arp2 subunit as the subject of this study due to its importance in maintaining the stability of the complex [20]. During the time when we worked on Arp2 mRNA using the conventional approach, we also developed a new approach to target mRNAs to the perinuclear region without a prerequisite for the zipcode. This approach is based on a novel mechanism by which mRNA encoding for Dia1 (a formin family member that nucleates actin polymerization) is localized to the perinuclear endoplasmic reticulum (ER) (Fig. 1A, B) [21]. Importantly, the Dia1 coding region itself was sufficient to override β-actin mRNA zipcode and targeted β-actin mRNA to the perinuclear region instead of its normal destination in the protrusions [21]. Using this strategy to express a bi-cistronic transcript containing the Dia1 and Arp2, (see Fig. 1C), we were able to redirect the exogenously expressed Arp2 mRNA from protrusions to the perinuclear region (Fig. 1E–F).
Figure 1. Mis-localization of Arp2 mRNA to the perinuclear region in the CEFs.
A. Endogenous Arp2 mRNA (green) and Dia1 mRNA (red) are localized in the protrusions (indicated by arrows) and perinuclear region in the CEFs, respectively. Note that protrusions in this report are defined broadly to include lamellae and lamellipodia. Dotted lines indicate cell border. B. A fibroblast cell expressing exogenous full-length (coding region and 3'-UTR) Dia1 mRNA (red). HA tagged exogenous Dia1 (green) indicates a transfected cell and arrow heads indicate exogenous Dia1 mRNA in the perinuclear region. A & B are at the same scale and blue indicates nucleus. C. Illustration of expression constructs for expression of localizing or mis-localizing Arp2 mRNA. D & E show tranfected CEFs expressing (D) exogenous localizing Arp2 mRNA (red, indicated with an arrow) and (E) exogenous mis-localizing Arp2 mRNA (red, indicated by arrow heads), respectively. Dotted line in E indicates cell border. Green: HA-tagged exogenous Arp2 protein and Blue: nucleus. Note that the exogenous proteins tend to be more diffuse than their corresponding mRNAs. This may be caused by over-expression and competition with the endogenous proteins for binding partners or complex incorporation. F. Quantitative results of normal localization of exogenous Arp2 mRNA to protrusions in transfected CEFs (see Materials and Methods for details of quantification). Data represent analysis of more than 300 cells in three independent experiments.
Knockdown of Arp2 altered normal cell migration behavior
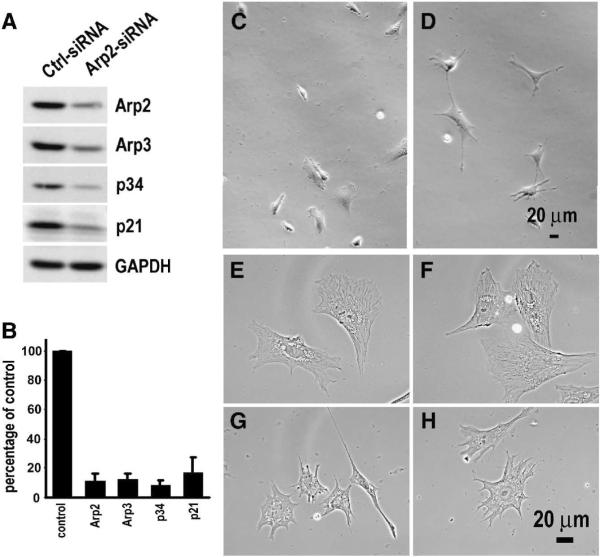
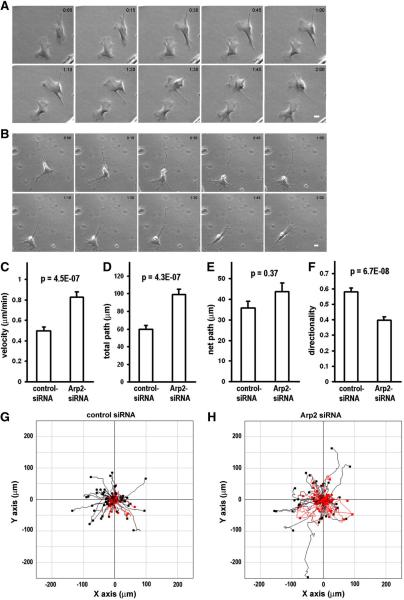
Being able to alter the Arp2 mRNA localization, we went on to test whether there would be any impact on cell migration if the Arp2 mRNA was mis-localized to the perinuclear region. However, there was a concern that simply expressing mis-localizing Arp2 in wild-type cells might produce minimal effect because the endogenous Arp2 mRNA would be maintained at the normal location and its protein products would be assembled normally into the Arp2/3 complex. To minimize the competition of correctly localized endogenous Arp2, we knocked-down the endogenous Arp2 with siRNA so the effects of mis-localization of Arp2 mRNA could be evaluated at a lower background. As shown in Figure 2A,B, Arp2 protein was significantly knocked down after treatment with Arp2-siRNA for three days. In addition to Arp2, the level of several subunits of the Arp2/3 was also significantly reduced in these cells (Fig. 2). This result is consistent with previous report in yeasts that depletion of one subunit caused degradation of the Arp2/3 complex [20]. Before studying the effects of mis-localization of Arp2 mRNA in the Arp2-KD cells, we characterized the effects of Arp2 KD alone on cell morphology and single cell migration in the CEFs. The characterization was done for three reasons. Firstly, the role of Arp2/3 complex in cell protrusion formation has been controversial. Di Nardo et al. [4] reported that depletion of Arp3 in mouse embryo fibroblasts had little effect on cell protrusions while others showed that the Arp2/3 complex was required for normal cell protrusion formation [22, 23]. Secondly, we must establish a baseline of single cell migration in wild-type and KD cells for subsequent rescue studies. Finally, there has been little investigation on the function of the Arp2/3 complex in single cell migration behavior even though Arp2/3 complex has been intensively studied for actin polymerization in cell-free systems, and in vivo for pathogenic bacterial motility, protrusion dynamics and actin network structures. Results of our morphological characterization showed that most of the KD cells exhibited multiple narrow protrusions while the control cells showed normal lamellipodial protrusions (Fig 2C–H). Our observation suggests that Arp2/3 complex is required for the formation of normal lamellipodial protrusion, which is consistent with a previous report that depletion of Arp2 inhibited sheet-like protrusions but not linear protrusions [23]. For single cell migration, we used time lapse microscopy to record and analyze individual cells that were cultured in uniform medium without a chemo-attractant gradient. First, we chose two hours as the time for time-lapse microscopy. This was based on our preliminary experiments which indicated that a wild-type cell could maintain persistent directional migration during this time period (within which a wild-type cell underwent two cycles of extension-contraction, see Suppl. movie 1). The capability of our fibroblast cells migrating persistently in a direction in uniform medium is in agreement with a previous report on Dictystelium cells which could maintain persistent directional migration for certain distant and time independent of chemo-attractant gradient [24]. The comparison of the migration of CEFs transfected with control siRNA or Arp2-siRNA provided surprising results as KD of Arp2 did not reduce cell motility as we expected. Rather, cell migration speed and total path (accumulated total travel distance) were increased in KD cells as compared to the control (Fig. 3A–D, Suppl. Movies 2–3). The KD cells also showed an increase in net path (the linear distance between the starting point and finishing point) although this was not statistically significant (Fig. 3E, G,H). Interestingly, even though the KD cells moved faster, their movement appeared less persistent in direction as shown by the significantly lower score of directionality (Fig. 3FH). These data indicate that the Arp2/3 complex plays a significant role in controlling normal migration speed and directional cell migration in the CEFs.
Figure 2. Knockdown of Arp2 changes cell morphology.
A. A representative Western blot showing knockdown of endogenous Arp2 in CEFs by RNA interference. 40 nM of siRNA (control or Arp2-specific) was used to transfect the CEFs. Three other subunits of the Arp2/3 complex were also analyzed and showed significant reduction. B. Quantitative results of Arp2, Arp3, p34 and p21 subunits of the Arp2/3 complex in Arp2-KD cells (n = 3, error bar; s.e.m). C & D. Representative phase contrast images of live CEFs transfected with control siRNA (C) or Arp2-siRNA (D). E–H: higher magnification phase contrast images of fixed cells transfected with control siRNA (E, F) or Arp2-siRNA (G, H).
Figure 3. Effects of knockdown Arp2 in cell migration.
A. Montage of still images of CEFs treated with control siRNA. B. Montage of still images of CEFs treated with Arp2-siRNA. (for corresponding movies of A & B, see Supplementary movies 2 & 3, respectively). Scale bar: 20 μm. Note that the control cells showed normal lamellipodia-like protrusions whereas the KD cells showed relatively narrower protrusions. The control cells moved more persistently in direction while the KD cell moved faster but less persistently in direction. C–F: Quantitative comparison of single cell migration parameters (see Materials and Methods for details). Results of analysis of 2-hour time lapse movies of 96 cells (control siRNA) and 105 cells (Arp2-siRNA) from at least four independent experiments. Error bars: s.e.m. G & H. cell migration tracks of all the analyzed cells in C–F. All the original X–Y coordinates were normalized as such that the migration starting points of the cells were set at 0,0. Black (or red) circles represent the end points. The migration tracks of cells with directionality score < 0.5 are highlighted in red. Note that there are proportionally more KD cells highlighted in red than the control cells.
Mis-localization of Arp2 mRNA caused reduction in persistence of directional cell migration
Having developed a novel method to mis-localize Arp2 mRNA and evaluated the effects of Arp2 KD in single cell migration, we then addressed the central question of whether there is a functional role for Arp2/3 complex mRNA localization in cell migration. To this end, endogenous Arp2 was firstly KD with siRNA and the cells were rescued by transfection of constructs expressing either localizing or mis-localizing Arp2 mRNA. The expression of the rescue Arp2 protein and their corresponding intracellular localization at the protrusions or perinuclear region were confirmed by Western blotting (Fig. 4A) and immunofluorescence staining (Fig. 4B,C), respectively. The migration behavior of the KD and rescued cells was recorded using time lapse microscopy and the movies were analyzed similarly as for the Arp2 KD cells. Two controls were set up for the evaluation of the impact of mis-localization of Arp2 mRNA on cell migration. The first one only involved rescue expression of localizing Arp2 mRNA in cells treated with Arp2-siRNA. In the second control, a Dia1-expression plasmid was co-transfected with the localizing rescue Arp2 construct to ensure adequate comparison between cells rescued with localizing and mis-localizing Arp2 as the latter expresses exogenous Dia1. As shown in Figure 4D, the rescued cells expressed comparable amount of exogenous Dia1. Interestingly, even though equal molar rescue plasmids were used and there was slightly higher level of exogenous localizing Arp2 mRNA than the mis-localizing Arp2 mRNA, the exogenous Arp2 protein level was much higher in the samples from cells rescued with mis-localizing Arp2 than the localizing Arp2. This is likely caused by the Dia1-mediated localization of the bi-cistronic mRNA on the perinuclear endoplasmic reticulum (ER), and is consistent with a previous report that translation on the ER is more efficient than in the cytoplasm for the same mRNA species [25]. Morphologically, rescued cells showed apparently normal lamellipodial protrusions regardless whether localizing or mis-localizing Arp2 was expressed (Fig. 4E–G, suppl. Movies 4–6). Although both localizing rescue controls were able to restore the altered cell migration parameters caused by KD of Arp2, rescue with mis-localizing Arp2 mRNA could only restore partial parameters. Specifically, rescue expression of mis-localizing Arp2 showed almost normal speed and total path as compared to the co-transfectional rescue control (Fig. 4G & I). However, in contrary to rescue expression of localizing Arp2, expression of mis-localizing Arp2 could not restore directionality (Fig. 4K–N).
Figure 4. Effects of mis-localization of Arp2 mRNA in cell migration.
A. Expression of exogenous Arp2 in Arp2-siRNA treated CEFs. Western blotting shows expression of siRNA-resistant rescue localizing Arp2 (HA-tagged, right lane) in comparison to localizing wild-type Arp2 (also HA-tagged, not resistant to Arp2-siRNA, left lane). B & C. Expression of exogenous Arp2 proteins in CEFs transfected with localizing Arp2 (B) or mis-localizing Arp2 (C) rescue construct. Note that to better show the localization of the exogenous Arp2 protein, these cells were fixed at 16 hours after transfection. HA-tagged exogenous Arp2 proteins were detected with immunofluorescence staining (green). Arrow points to protrusion-localized protein whereas arrow head points to perinuclearly localized protein. Scale bar: 10 μm. D. Protein and mRNA levels in Arp2-rescue cells detected by Western blotting and RT-PCR, respectively. 1. localizing rescue, 2. mis-localizing rescue. * exogenous Dia1 (fused with GFP or mCherry). Bottom panel shows exogenous HA-Arp2 mRNA (20 cycles of PCR). E–G. Montage of still images of representative Arp2-KD cell rescued with localizing Arp2 (E), localizing Arp2 plus Dia1 (F) and mis-localizing Arp2 (G) (also see corresponding supplementary movies 4–6). H–K, quantitative comparison of single cell migration parameters (see Materials and Methods for details). Results of analysis of 2-hour time-lapse movies of Arp2-KD cells rescued with 1. localizing (44 cells), 2. localizing plus Dia1 (58 cells) or 3. mis-localizing (36 cells) from three independent experiments. Similar to Figure 2, L–N show cell migration tracks of all the analyzed cells in H–K and tracks highlighted in red represent cells with directionality value < 0.5. Note that for live cell migration experiments, mCherry replaced GFP in the rescue plasmids as marker for the transfected cells.
Because the cells rescued with mis-localizing Arp2 exhibited higher exogenous Arp2 protein than those rescued with localizing Arp2, there was a concern that this higher level of Arp2 protein contributed to the failed rescue in directional migration. To address this issue, we selected cells with comparable Arp2 expression levels for migration analysis (Fig. 5A). This selection is possible because identical microscopic parameters were used to acquire fluorescence images of the rescue cells and our cell migration analysis is single cell based. The results of single cell migration analysis from these selected cells are in general similar to those unselected (comparing Figures 4 and 5). The only exception is that between the selected groups, there is a statistically significant difference in the category of velocity, indicating that cells rescued with mis-localizing Arp2 could not restore the defect in speed even though the same amount of rescue Arp2 protein was expressed. Consistently shown in both unselected and selected groups of cells, rescue with mis-localizing Arp2 mRNA failed to restore normal directionality. These results are reminiscent of Wingless mRNAs whose mis-localization to the basolateral compartments in epithelial cells led to reduced wingless protein activity even though the protein was efficiently translated [16]. Our results clearly indicate that Arp2 mRNA localization is critical for directional cell migration.
A role of Arp2/3 complex mRNA localization in directional cell migration
As localized actin polymerization plays a critical role in directional cell migration, it is not surprising to find that many proteins that regulate actin dynamics are enriched at the cell protrusions because this is where these proteins exert their function. The importance of such spatial regulation is generally recognized and studies on protrusional enrichment of β-actin mRNA demonstrated the role of mRNA localization in targeting cytoskeletal proteins to their site of function [26]. However, the diverse mechanisms that target actin-associated proteins to cell protrusions are poorly understood. We previously reported that all the mRNAs encoding for the seven subunits of the Arp2/3 complex were localized in the protrusions in fibroblasts [17]. We here provide evidence demonstrating that Arp2/3 complex mRNA localization is important for directional cell migration. This mRNA localization-mediated local synthesis of the complex components serves two related but different functions: facilitating complex assembly and targeting the complex to the site of function. By concentrating the Arp2/3 complex mRNAs to a confined compartment of the cell, localized translation likely increases the local concentration of the subunits there hence greatly enhances the rate of complex assembly. Furthermore, a possible mechanism of co-translational assembly could push this process to even higher efficiency [17, 27]. Arp2 protein synthesized in the perinuclear region could reach the protrusions by diffusion albeit expected at a lower concentration, leading to lower rate of complex assembly. It is also possible that these Arp2 protein molecules might be less assembly-competent if they were post-translationally modified en route to the protrusions, which could be another advantage of local synthesis of all the subunits of the Arp2/3 complex in the same compartment. In addition to efficient complex assembly by translating all the subunit mRNAs at the same site, like most of other localizing mRNAs, the location of Arp2/3 complex protein synthesis may also play a role in its normal function. It is of interest to notice that in the cells rescued with mis-localizing Arp2 mRNA, besides higher level of the exogenous Arp2 protein, there was also somewhat higher level of the other Arp2/3 complex proteins compared to the cells rescued with localizing Arp2 (Fig. 4D). However, this higher level of Arp2/3 complex in these cells only partially restored the defects caused by Arp2 KD, implying that the role of Arp2/3 complex mRNA localization is not limited to facilitating complex assembly although it is a critical function. Regardless of the details, our data suggest that a localized and functional Arp2/3 complex is critical for persistent directional cell migration. In a wild type cell, the signaling pathways likely orchestrate the localization of Arp2/3 complex mRNAs, their local translation and the activities of other cytoskeletal proteins in the leading protrusions thereby promoting the formation of dominant protrusion and its extension for directional cell migration. In a scenario where Arp2 mRNA is mis-localized, even though the signaling pathways and other cytoskeletal proteins still act normally in a potential site for protrusion formation and/or extension, the lack of a local high concentration of Arp2/3 complex may prevent this site from becoming a dominant protrusion and/or its extension. Consequently this may result in less persistent directional migration as observed in the Arp2 mutants.
Comparison of cell migration affected by altered localization of mRNAs encoding for β-actin and Arp2 in the CEFs
β-actin mRNA was found to localize in the protrusions of CEFs and treatment of the cells with antisense oligonucleotide against β-actin RNA zipcode in its 3'-UTR reduced β-actin mRNA localization in the protrusions and caused significant reduction in the migration parameters of net path, speed and directionality [14, 15]. Although altered localization of mRNAs encoding for β-actin or Arp2 results in a reduction in directionality, there are differences in other single cell migration parameters. Unlike β-actin mRNA, mis-localization of Arp2 mRNA to the perinuclear region did not cause reduction in speed and net path. These differences could be derived from the different roles of actin and the Arp2/3 complex in actin dynamics. Because β-actin is the fundamental building block for actin network that is essential for protrusion formation and cell motility, a reduction of localized β-actin protein concentration in the leading protrusions may limit the continuous supply of actin for directional migration. In contrast to the fundamental role of β-actin, the Arp2/3 complex is only one of the several actin polymerization nucleators, and other nucleators (such as the formins) could have overlapping functions for basal cell motility. Nevertheless, a common defect in directionality by altering the localization of mRNA for β-actin or Arp2 suggests that Arp2/3 complex activated, β-actin-dependent local actin polymerization in the protrusions is critical for directional cell migration and such localized actin dynamics is supported by mRNA localization mediated local protein synthesis.
A role of Arp2/3 complex in maintenance of normal cell migration speed
It was somewhat unexpected that KD of Arp2 did not inhibit cell motility in the CEFs given that the Arp2/3 complex was significantly degraded (Fig. 2). Numerous studies have indicated that the Arp2/3 complex mediates the formation of branched actin network which is predicted to push the cell membrane for cell motility [1, 3]. On the other hand, depletion of Arp2/3 complex did not inhibit formation of lamellipodia in platelets and mouse embryo fibroblasts [4], and little branched actin was observed in the leading edge [5], suggesting other actin polymerization nucleators may be sufficient for basal cell motility. It was intriguing that cell migration speed was increased in Arp2-KD cells. Compared to control cells, these KD cells appeared less spread and exhibited narrow protrusions (Fig. 2C–H and Fig. 3AB), suggesting a possible defect in cell adhesion. So far, there have been several reports on a possible role of the Arp2/3 complex in cell adhesion and spreading [28–30]. While this report focuses on the role of Arp2/3 complex mRNA localization in directional cell migration, whether the altered cell migration speed in our cell model is due to defective adhesion and/or spreading remains an interesting issue to be addressed in future studies.
This report provides evidence to support a physiological role of Arp2/3 complex mRNA localization in directional cell migration. It also demonstrates the application of the mechanism for Dia1 mRNA localization as a useful tool in manipulation of intracellular localization of mRNAs. Like any study, our results also raise a few new questions: does mis-localization of the Arp2 mRNA affect the assembly and stability of the Arp2/3 complex, and the actin network in the leading protrusions, and how do these dictate the protrusion dynamics? Future investigations are required to address these questions to provide critical insights for the mechanism and functionality of mRNA localization mediated local synthesis of the Arp2/3 complex in directional cell migration.
Supplementary Material
Acknowledgment
We thank Dr. Livingston Van De Water for critical reading. This work is supported by NIH grant GM70560.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pollard TD, Cooper JA. Actin, a central player in cell shape and movement. Science. 2009;326:1208–12. doi: 10.1126/science.1175862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pollard TD. Regulation of actin filament assembly by Arp2/3 complex and formins. Annu Rev Biophys Biomol Struct. 2007;36:451–77. doi: 10.1146/annurev.biophys.35.040405.101936. [DOI] [PubMed] [Google Scholar]
- 3.Goley ED, Welch MD. The ARP2/3 complex: an actin nucleator comes of age. Nat Rev Mol Cell Biol. 2006;7:713–26. doi: 10.1038/nrm2026. [DOI] [PubMed] [Google Scholar]
- 4.Di Nardo A, Cicchetti G, Falet H, Hartwig JH, Stossel TP, Kwiatkowski DJ. Arp2/3 complex-deficient mouse fibroblasts are viable and have normal leading-edge actin structure and function. Proc Natl Acad Sci U S A. 2005;102:16263–8. doi: 10.1073/pnas.0508228102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Urban E, Jacob S, Nemethova M, Resch GP, Small JV. Electron tomography reveals unbranched networks of actin filaments in lamellipodia. Nat Cell Biol. 2010;12:429–35. doi: 10.1038/ncb2044. [DOI] [PubMed] [Google Scholar]
- 6.Machesky LM, Insall RH. Signaling to actin dynamics. J Cell Biol. 1999;146:267–72. doi: 10.1083/jcb.146.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mullins RD, Pollard TD. Structure and function of the Arp2/3 complex. Curr Opin Struct Biol. 1999;9:244–9. doi: 10.1016/s0959-440x(99)80034-7. [DOI] [PubMed] [Google Scholar]
- 8.Cooper JA, Wear MA, Weaver AM. Arp2/3 complex: advances on the inner workings of a molecular machine. Cell. 2001;107:703–5. doi: 10.1016/s0092-8674(01)00605-5. [DOI] [PubMed] [Google Scholar]
- 9.St Johnston D. Moving messages: the intracellular localization of mRNAs. Nat Rev Mol Cell Biol. 2005;6:363–75. doi: 10.1038/nrm1643. [DOI] [PubMed] [Google Scholar]
- 10.Lecuyer E, Yoshida H, Parthasarathy N, Alm C, Babak T, Cerovina T, Hughes TR, Tomancak P, Krause HM. Global analysis of mRNA localization reveals a prominent role in organizing cellular architecture and function. Cell. 2007;131:174–87. doi: 10.1016/j.cell.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Holt CE, Bullock SL. Subcellular mRNA localization in animal cells and why it matters. Science. 2009;326:1212–6. doi: 10.1126/science.1176488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meignin C, Davis I. Transmitting the message: intracellular mRNA localization. Curr Opin Cell Biol. 2010;22:112–9. doi: 10.1016/j.ceb.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 13.Kislauskis EH, Zhu X, Singer RH. Sequences responsible for intracellular localization of beta-actin messenger RNA also affect cell phenotype. J Cell Biol. 1994;127:441–51. doi: 10.1083/jcb.127.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kislauskis EH, Zhu X, Singer RH. beta-Actin messenger RNA localization and protein synthesis augment cell motility. J Cell Biol. 1997;136:1263–70. doi: 10.1083/jcb.136.6.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shestakova EA, Singer RH, Condeelis J. The physiological significance of beta -actin mRNA localization in determining cell polarity and directional motility. Proc Natl Acad Sci U S A. 2001;98:7045–50. doi: 10.1073/pnas.121146098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simmonds AJ, dosSantos G, Livne-Bar I, Krause HM. Apical localization of wingless transcripts is required for wingless signaling. Cell. 2001;105:197–207. doi: 10.1016/s0092-8674(01)00311-7. [DOI] [PubMed] [Google Scholar]
- 17.Mingle LA, Okuhama NN, Shi J, Singer RH, Condeelis J, Liu G. Localization of all seven messenger RNAs for the actin-polymerization nucleator Arp2/3 complex in the protrusions of fibroblasts. J Cell Sci. 2005;118:2425–33. doi: 10.1242/jcs.02371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu G, Amin S, Okuhama NN, Liao G, Mingle LA. A quantitative evaluation of peroxidase inhibitors for tyramide signal amplification mediated cytochemistry and histochemistry. Histochem Cell Biol. 2006;126:283–91. doi: 10.1007/s00418-006-0161-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mingle LA, Bonamy G, Barroso M, Liao G, Liu G. LPA-induced mutually exclusive subcellular localization of active RhoA and Arp2 mRNA revealed by sequential FRET and FISH. Histochem Cell Biol. 2009;132:47–58. doi: 10.1007/s00418-009-0589-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morrell JL, Morphew M, Gould KL. A mutant of Arp2p causes partial disassembly of the Arp2/3 complex and loss of cortical actin function in fission yeast. Mol Biol Cell. 1999;10:4201–15. doi: 10.1091/mbc.10.12.4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liao G, Ma X, Liu G. An RNA-zipcode-independent mechanism that localizes Dia1 mRNA to the perinuclear ER through interactions between Dia1 nascent peptide and Rho-GTP. J Cell Sci. 2011;124:589–599. doi: 10.1242/jcs.072421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bailly M, Ichetovkin I, Grant W, Zebda N, Machesky LM, Segall JE, Condeelis J. The F-actin side binding activity of the Arp2/3 complex is essential for actin nucleation and lamellipod extension. Curr Biol. 2001;11:620–5. doi: 10.1016/s0960-9822(01)00152-x. [DOI] [PubMed] [Google Scholar]
- 23.Nicholson-Dykstra SM, Higgs HN. Arp2 depletion inhibits sheet-like protrusions but not linear protrusions of fibroblasts and lymphocytes. Cell Motil Cytoskeleton. 2008;65:904–22. doi: 10.1002/cm.20312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCann CP, Kriebel PW, Parent CA, Losert W. Cell speed, persistence and information transmission during signal relay and collective migration. J Cell Sci. 2010;123:1724–31. doi: 10.1242/jcs.060137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stephens SB, Nicchitta CV. Divergent Regulation of Protein Synthesis in the Cytosol and Endoplasmic Reticulum Compartments of Mammalian Cells. Mol Biol Cell. 2008;19:623–632. doi: 10.1091/mbc.E07-07-0677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Condeelis J, Singer RH. How and why does beta-actin mRNA target? Biol Cell. 2005;97:97–110. doi: 10.1042/BC20040063. [DOI] [PubMed] [Google Scholar]
- 27.Moore MJ. From birth to death: the complex lives of eukaryotic mRNAs. Science. 2005;309:1514–8. doi: 10.1126/science.1111443. [DOI] [PubMed] [Google Scholar]
- 28.DeMali KA, Barlow CA, Burridge K. Recruitment of the Arp2/3 complex to vinculin: coupling membrane protrusion to matrix adhesion. J Cell Biol. 2002;159:881–91. doi: 10.1083/jcb.200206043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Serrels B, Serrels A, Brunton VG, Holt M, McLean GW, Gray CH, Jones GE, Frame MC. Focal adhesion kinase controls actin assembly via a FERM-mediated interaction with the Arp2/3 complex. Nat Cell Biol. 2007;9:1046–56. doi: 10.1038/ncb1626. [DOI] [PubMed] [Google Scholar]
- 30.Butler B, Cooper JA. Distinct roles for the actin nucleators Arp2/3 and hDia1 during NK-mediated cytotoxicity. Curr Biol. 2009;19:1886–1896. doi: 10.1016/j.cub.2009.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.