Abstract
In plants, accumulation in specific compartments and huge structural diversity of secondary metabolites is one trait that is not understood yet. By exploring the diverse abiotic and biotic interactions of plants above- and belowground, we provide examples that are characterized by nonlinear effects of the secondary metabolites. We propose that redox chemistry, specifically the reduction of reactive oxygen species (ROS) and, in their absence, reduction of molecular oxygen by the identical secondary metabolite, is an important component of the hormetic effects caused by these compounds. This is illustrated for selected phenols, terpenoids, and alkaloids. The redox reactions are modulated by the variable availability of transition metals that serve as donors of electrons in a Fenton reaction mode. Low levels of ROS stimulate growth, cell differentiation, and stress resistance; high levels induce programmed cell death. We propose that provision of molecules that can participate in this redox chemistry is the raison d’être for secondary metabolites. In this context, the presence or absence of functional groups in the molecule is more essential than the whole structure. Accordingly, there exist no constraints that limit structural diversity. Redox chemistry is ubiquitous, from the atmosphere to the soil.
Keywords: Reactive oxygen species, Fenton reaction, abiotic interactions, biotic interactions, litter decomposition, phenols, terpenoids, alkaloids
INTRODUCTION
Hormesis is the stimulatory effect of toxic agents at low concentrations. The phenomenon itself, however, was known well long ago before the term hormesis was coined (Southam and Ehrlich, 1943), but largely ignored for a long time as nobody could really explain it (Stebbing, 1982). Southam and Ehrlich (1943) isolated compounds that they called “antibiotics” from the heartwood of red cedar (Thuja plicata) and, contrary to their expectations, noted that low dosages were not antimicrobial, but stimulated the growth of the assayed microbes. Later, the structures of tropolone derivatives (Gripenberg, 1951) and lignans (Swan et al., 1969), characteristic secondary plant metabolites or natural products—a term more often used by pharmacists—were identified as constituents that were responsible for the observed effect. The term “secondary metabolite” started to appear in the text books from the 1950s onwards (Hadacek, 2002), and is ascribed to the German physiological chemist Albrecht Kossel (Nobel award laureate of medicine 1910) who, unintentionally, initiated the schism of primary and secondary metabolism (Hartmann, 2007). Kossel proposed that primary metabolites are essential for all life-sustaining processes and common to all organisms whereas, by contrast, secondary metabolites arise from distinct pathways that, as a rule, are limited only to a few organisms. Consequently, he classified them as less essential and of “secondary” relevance.
Today, the distinction into primary and secondary metabolism is coming increasingly under criticism (Firn and Jones, 2009). One reason is that some important metabolites, among which we find lipids, polysaccharides, carotenoids and epicuticular waxes, are known to possess distinct chemical functions, but do not fit into either primary or secondary metabolites. Firn and Jones (2009) suggested to classify them as supporting metabolites, in contrast to the larger portion of secondary metabolites that are to be included into the speculative metabolites. The latter comprise structures that may act either as hormones in low or toxins in high concentration in a non-linear hormetic mode of action.
Today, the widely accepted raison d’être of secondary metabolites is to contribute to the chemical defense of sessile organisms that lack an immune system (Hadacek, 2002; Hartmann, 2007; Berenbaum and Zangerl, 2008; van Dam, 2009). In this context, the term “biological function” is used often. Recently, we commemorated Charles Darwin. In reference to Darwin, we have to be aware that the usage of the term “biological function” is ambiguous and may suggest that the evolution of secondary metabolites is a teleological process. In contrast to Jean-Baptiste de Lamarck, who also developed an evolutionary theory, this was never stated explicitly by Darwin (Selmar, 2009). The entomologist Gottfried Fraenkel was the first who took up ideas that were voiced earlier by the botanists Ernst Stahl and Anton Kerner von Marilaun, who suggested that secondary plant metabolites are not waste chemicals, but produced for chemical defense against herbivores (Fraenkel, 1959). This hypothesis was not well received first, but later stimulated research on plant–herbivore interactions tremendously (Hartmann, 2008). The same compounds, however, that serve as defense against generalists may also be utilized as signal cues by specialized parasites and predators. To accommodate this fact, the terminology of allomones and kairomones was introduced (Brown et al., 1970).
Despite the suggestion of Firn and Jones (2009) to apply an alternative nomenclature for secondary metabolites, we will continue to use this term for convenience. The chemistry of secondary metabolites that we intend to point out blurs the boundaries to primary metabolism anyhow (Hartmann, 1985). Historically, interest in plant secondary metabolites was directed by well-defined goals: in traditional medicine, an extensive knowledge of healing properties of plants accumulated that is tapped by modern pharmacology (McChesney et al., 2007; Newman and Cragg, 2007). Recently, the notion that many pharmaceuticals are characterized by a hormetic dose–response behavior was voiced (Calabrese, 2008). More than 20 years earlier, Langenheim and Thimann (1982) already pointed out that many alkaloids, which are used for medicinal purposes, loose their beneficial effect with increasing dosage. Among those alkaloids, compounds such as atropine, cocaine, morphine, nicotine, quinine, and strychnine were listed. The famous physician Philippus Aureolus Theophrast Bombastus von Hohenheim, better known as Paracelsus, was the first to report this phenomenon in the 16th century: “All things are poison and nothing is without poison, only the dose permits something not to be poisonous” (Paracelsus [1538] Third Defensio).
Hans Molisch, a professor of plant physiology, first in Prague and later in Vienna, coined the term allelopathy (Molisch, 1937). In recent years, interest in allelopathy again has increased in context with concerns about invasive plants (Callaway and Ridenour, 2004). Amongst others, Molisch writes: “If seedlings only are exposed to the gas emitted by apples (ethylene) a relatively short time, then it becomes evident that not an inhibition of longitudinal growth occurs but a very pronounced stimulation. Exposure to apple odor for one to five hours caused an unambiguous stimulation, whereas 24 hours caused a slight inhibition that became more pronounced with ongoing exposure” (our translation). In the ongoing text, he makes a comment on this observation: “Here, the often observed rule is confirmed that poisons and irritating compounds are harmful in higher concentrations but stimulate in diluted form” (our translation). This suggests that Molisch (1937) was aware of the phenomenon that a couple of years later was described as hormesis (Southam and Ehrlich, 1943).
The aim of this paper is to discuss hormesis in the context of plant biology and it will focus specifically on secondary metabolites. The existing literature provides numerous hints that hormesis occurs widely in plants (Calabrese and Blain, 2009). Not only that this phenomenon is caused by bio-organic chemicals, but inorganic chemicals and abiotic stress also are known to cause hormetic effects (Calabrese and Blain, 2009). As primary producers, plants are regarded as pillars of terrestrial ecosystems and, thus, are associated with a wide range of organisms that depend on the carbon that is fixed by photosynthesis. In order to understand benefits of secondary metabolites for the producing plant, we have to refrain from limiting this discussion to the current paradigm of chemical defense, but have to pay attention to the complex redox chemistry of secondary metabolites. We argue that this chemistry represents a more plausible raison d’être for secondary metabolites than the need for chemical weapons. The latter paradigm, however is firmly established in the literature and nearly indisputable in the ecological research community (Firn and Jones, 2003). Secondary metabolites, however, have not first evolved in plants but in microbes. This will be the focus of the first section.
SECONDARY METABOLITES HAVE NOT BEEN INVENTED BY PLANTS
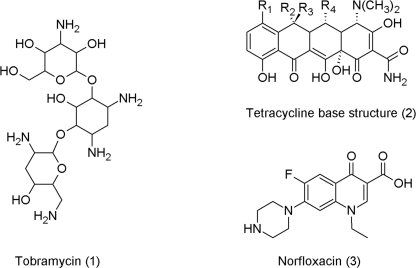
Soil-dwelling bacteria and fungi also produce secondary metabolites that are commonly called antibiotics regardless of their specific activity. In terms of evolutionary age, microbes are much older than plants: Anaerobic microbes existed even when the atmosphere of our planet contained nearly no oxygen. Today, much can be read about microbial warfare in the soil, but there exists only scant evidence that bacteria actually deploy antibiotics to kill or ward off other microbes that compete for the same resource (Mlot, 2009). Despite their success story in controlling microbial-induced human diseases, recent studies suggest that antibiotics may possess other activities besides that of chemical weapons against bacteria: specific antibiotics, such as tobramycin (1), tetracycline (2), and even the synthetic norfloxacin (3), were shown to stimulate biofilm formation of Pseudomonas aeruginosa at sub-inhibitory concentrations—the application of this drug is to inhibit the same bacterium (Linares et al., 2006). Accordingly, at lower concentrations, antibiotics serve as signal molecules that, amongst others, facilitate adaptation of the producing microbe to its environment.
The wide-spread occurrence of antibiotics in prokaryotes implies that antibiotics are ancient molecules. They exert their biological activities (e.g. as antibiotics) through interactions with ancient and conserved sites in macromolecules (Davies, 1990). In periods, when proteins were not available yet—Davies (1990) suggests—many biosynthetic reactions were catalyzed or “effected” by low-molecular-weight compounds. Later, polypeptides (enzymes) with specific ligand sites for these molecules evolved that took over the catalysis of life-sustaining reactions.
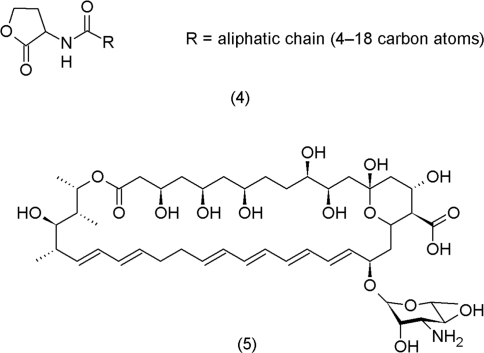
During the last decade, quorum sensing has been identified as an essential biochemical mechanism that allows populations of bacteria to coordinate their behavior in biofilm formation, symbiosis and virulence. Low-molecular compounds such as N-acyl homoserine lactones (AHL) (4) in gram-negative bacteria and small peptides in gram-positive bacteria were shown to induce expression of specific genes in bacterial populations in a concentration-dependent fashion (Taga and Bassler, 2003).
Nystatin (5) is a well-known antifungal drug. A recent study showed that low dosages of this compound trigger multicellularity in Bacillus subtilis by initiating potassium leakage of the cell wall (Lopez et al., 2009). These findings provide additional evidence that many bacterial metabolites with antagonistic effects in higher concentrations may act non-antagonistically in lower concentrations (Shank and Kolter, 2009).
CHEMICAL CONSTRAINTS FOR THE EVOLUTION OF SECONDARY METABOLITES
Low concentrations of microbial secondary metabolites may act as signals and not as weapons. This agrees with a hormetic mode of action. Does this also apply for plants? Today, the structural diversity of secondary metabolites in plants still remains unexplained (Berenbaum and Zangerl, 2008). According to the principles of evolution as outlined by Darwin and Wallace, traits that do not confer benefits are prone to elimination by negative selection. Screenings for inhibitory effects of secondary metabolites (toxicity to other organisms) have procured generally low numbers of hits. In response to this observation, the Screening Hypothesis was developed (Jones and Firn, 1991). It predicts large numbers of inactive compounds in plants. Probably, one portion was aimed at extinct predators, and the other portion represents a required pool of structural diversity out of which new active compounds may arise. The Screening Hypothesis was developed to provide a null model for the plant defense hypotheses (Firn and Jones, 2003).
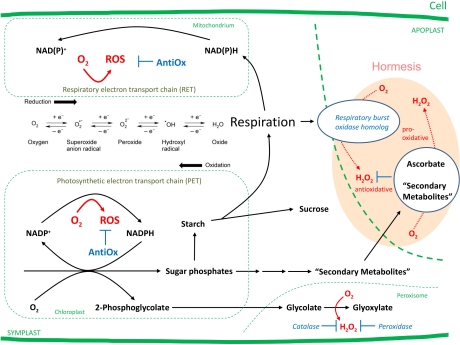
To find a scenario for the evolution of secondary metabolites we have to go back to the early stages of evolution of life. If redox chemistry is to constitute an important issue, we have to look for chemical constraints (Williams and Fraústo da Silva, 2006; Morowitz and Smith, 2007). Most probably, the rise in the concentration of molecular oxygen caused one of the first mass extinctions. It affected those prokaryotes which took their energy from the oxidation of hydrogen sulphide or iron salts instead from water (Hartman, 1996). The rising concentrations of oxygen led to the evolution of pro- and eukaryotes that tolerated substantial concentrations of oxygen in their environment. Enzymes, such as superoxide dismutase and catalase, evolved which efficiently remove reactive oxygen species (ROS). Superoxide anion radicals ( ) and hydrogen peroxide (H2O2) are formed following the reduction of molecular oxygen. During the anoxic period of evolution, some anaerobic prokaryotes already were exposed to H2O2 that was formed by the UV-induced photolysis of water. This constraint may have led to the evolution of superoxide dismutase and the oxygen-releasing photosystem II; some bacteria are still known to utilize H2O2 as an electron source (McKay and Hartman, 1991; Blankenship and Hartman, 1998). In anoxic times, when the surface of our globe was reducing, the metals formed sulphides which were water soluble in the following order: Mn2+ > Fe2+ > Co2+ > Ni2+ >> Cu2+ > Zn2+. This led to a high availability of Mn2+ and Fe2+ that evolved as co-factors of enzymes. The newly formed oxygen was initially consumed by oxidation of the reducing surface; only after this, the oxygen concentration in the air rose. HS– and Fe2+ were oxidized into and Fe3+. The new oxidizing environment forced life to develop compartmental forms that were capable to utilize the new conditions more efficiently. Cu2+ and Zn2+ became available as co-factors for enzymes; this facilitated the evolution of more complex enzymes that are characteristic for eukaryotes (Williams and Fraústo da Silva, 2006).
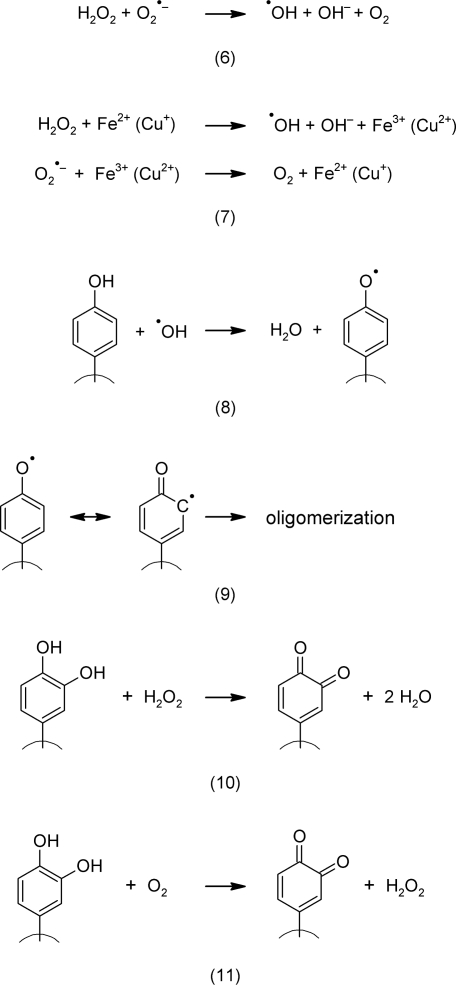
ROS are recognized as important cellular messengers (Gechev et al., 2006). The hydroxyl radical is the most reactive and destructive species of all ROS and is formed by the Haber-Weiss reaction between H2O2 and (6).
This reaction (6), however, proceeds rather slowly under conditions that normally are found in living cells. Only the presence of transition metals, such as iron and copper, causes the formation of significant levels of •OH in a reaction that is known as the Fenton reaction (7). Iron and copper are catalysts. Their local availability most probably determines the rate of formation of •OH in the tissue (Wojtaszek, 1997).
Secondary metabolites, especially phenols, interact with cellular ROS levels in various ways; they can either increase or decrease their concentrations as pro- or antioxidants (Grace, 2005). These reactions depend on the presence or absence of specific functional groups. Many phenols and alkaloids are known to possess pro- or antioxidant activities (Larson, 1988; 1997). By transfer of one electron, •OH can be reduced to H2O (8). The phenolic hydroxyl group, however, is oxidized into a radical that may oligomerize to form proanthocyanidins or phlobaphens (9). By transfer of two electrons, catechol, a phenol with two o-hydroxyl groups, can reduce H2O2 to H2O. The catechol is oxidized to the more or less stable quinone (10). In the absence of ROS, however, the catechol moiety can reduce O2 into H2O2 (11).
The last two reactions not only depend on the concentration of the catechol but also on the redox milieu at the site. If ROS are present, then the catechol scavenges them until an equilibrium is reached. As a part of this equilibrium, additional non-oxidized catechol molecules (present with increasing concentration) then start to reduce O2 to ROS. As a result, we obtain a non-linear and hormetic effect that, at low concentrations, is antioxidative, and at higher concentrations, pro-oxidative.
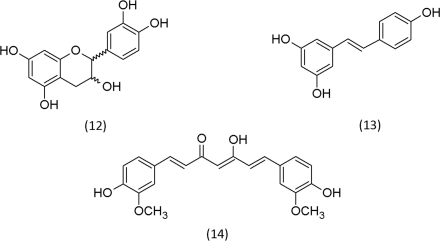
ROS are recognized to be involved in the development of many human diseases, especially in relation with aging, such as cancer, cardiovascular diseases, atherosclerosis, hypertension, ischemia/reperfusion injury, diabetes mellitus, Alzheimer’s disease, Parkinson’s disease, and rheumatoid arthritis (Valko et al., 2007; Kell, 2009). As long as the administered drugs help to restore the redox homeostasis, they control the disease; too high dosages, by contrast, may cause a pro-oxidative and detrimental effect, the recognized side-effects of many drugs. This universal and, in terms of target specificity, rather unspecific mode of action may be responsible why plant secondary metabolites have gained importance in traditional medicine. If toxic plants are consumed in low portions, they can restore health. Diets that are rich in fruit and vegetables were suggested to help to prevent cancer (Block et al., 1992). Hormesis may be involved. At low concentrations, the plant secondary metabolites precondition against disease development (Son et al., 2008). Among those secondary metabolites, which are especially regarded as beneficial for health, (±)-catechin (12), resveratrol (13), and curcumin (14) are found, all phenolic compounds.
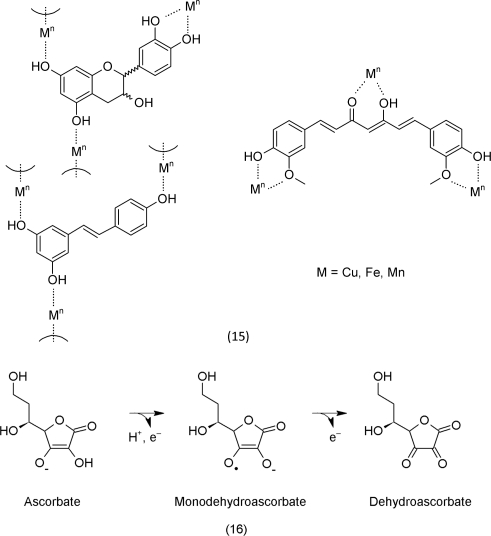
The phenolic hydroxyl groups can reduce ROS that are present in elevated levels. This explains the antioxidative mode of action of the compounds 12–14. The chemistry of these reactions, however, is more complex and cannot be elucidated alone on the basis of the redox chemistry of the phenolic moieties. Reduced transition metals, such as iron (II) and copper (I), represent important electron donors that reduce H2O2 to •OH (Groves, 2006). Phenolic organic compounds, such as (±)-catechin, resveratrol and curcumin (15), also serve as ligands for transition metals. In the complex, they maintain their catalytic properties. The ligand, however, determines their efficacy as catalysts (Mahal et al., 2005).
The deoxyribose assay was originally developed to detect degradation of organic compounds by •OH and allow the determination of the auto-oxidative properties of the test compound in complex with a transition metal and in free form (Halliwell et al., 1987). Modified versions of this assay showed that the pro-oxidative effect of certain secondary metabolites may be enhanced in complex with iron whilst complexes of iron with other compounds caused the opposite effect (Chobot and Hadacek, 2009; Chobot et al., 2009; Chobot, 2010).
The dependance of •OH formation on the availability and reactivity of transition metals (caused by complexation with variable ligands) facilitates fine-tuning of signaling in the cell. Conversely, failure to regulate the levels of ROS initiates programmed cell death (Halliwell, 2006). Healthy cells are characterized by a reduced state with slightly elevated levels of ascorbate and glutathione. Ascorbate can provide two electrons for reduction (16). These electrons, however, may also be transferred to O2, and then H2O2 is formed (Vreeburg and Fry, 2005). Dehydroascorbate is reduced back to ascorbate by a two-electron transfer from reduced glutathione (GSH) within the glutathione-ascorbate cycle that detoxifies H2O2 to H2O. The oxidized dimeric glutathione (GSSG) is reduced back to the monomer by NADPH (Foyer and Noctor, 2009).
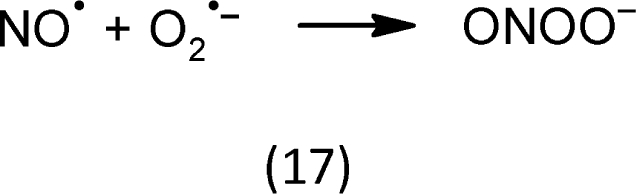
Our ideas about the development of oxidative stress are as follows (Neill et al., 2003; Halliwell, 2006): Mild oxidative stress stimulates cell growth and proliferation. Amongst others, ascorbate can reduce nitrite ( ) to nitric oxide (NO) which occurs as radical NO•. Nitrate reductase catalyzes the reduction of nitrate ( ) to and can also reduce to NO•. Not only that NO• can arise by catalysis of various enzymes and by non-enzymatic reactions, it is a very reactive molecule itself. It easily reacts with to peroxynitrite (17). Peroxynitrite is a strong oxidant that damages many bio-molecules. The primary targets of NO include mitogen-activated protein kinases (MAPK) and Ca2+-permeable ion channels (Neill et al., 2003).
As a consequence, the concentration of Ca2+ in the cytoplasm rises. Increasing oxidative stress is accompanied by the release of transition metals that catalyze reduction of H2O2 to •OH; hydroxyl radicals oxidize a wide range of bio-molecules, among them also DNA (Elstner, 1990; Dizdaroglu, 1998; Guo and Tullius, 2003). The peroxidation of unsaturated lipids also is started by •OH. The formed radicals trigger a chain reaction in the course of which peroxides are formed that enter further reactions depending on the position of the peroxide in the chain of the lipid (Marnett, 1999). One lipid peroxidation product is malonyldialdehyde (MDA), a reactive electrophilic species (RES). It becomes especially active in its protonated form when the symplastic pH decreases as a result of stress. This effect is regarded as a kind of repair mechanism that is aimed at helping the plant to prevent excessive DNA damage when the levels of the precursor, linoleic acid, increase during stress and the pH in the cytoplasm decreases (Farmer and Davoine, 2007).
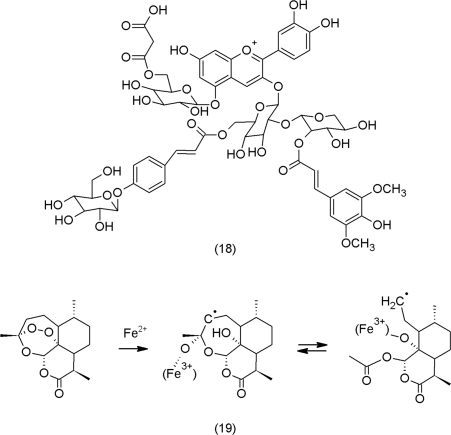
Production by NADPH oxidases and physiological processes that include electron transport chains, photosynthesis and respiration, are the main sources of ROS in the cell (Figure 1). Further, insufficient catalase and peroxidase activity in the peroxysomes also increase ROS levels (Foyer and Noctor, 2009). Anthocyanins are good examples for phenolic secondary metabolites that act as antioxidants (Figure 2). They occur as oligomers of structures containing all the required combination of functional groups to scavenge ROS and chelate iron; a characteristic example has even been described from Arabidopsis thaliana (18) (Bloor and Abrahams, 2002).
FIGURE 1.
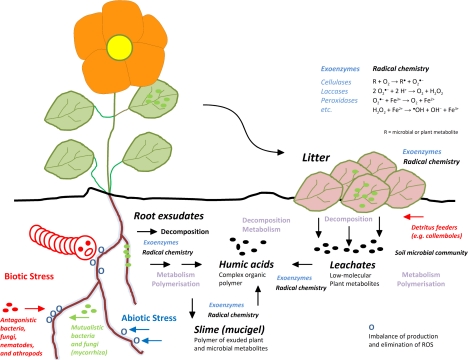
Location of major pathways of ROS production in plant cells following Foyer and Noctor (2009), modified (for detailed explanation see text), and proposed hormetic redox chemistry in the apoplast (AntiOx, reducing activities of low-molecular-weight compounds and enzymes).
FIGURE 2.
Above ground, abiotic and biotic stress factors trigger ROS-mediated signal cascades that facilitate the survival of the plant (for detailed explanation see text).
Further physiological processes that can restore the redox homeostasis include the activation of redox-sensitive transcription factors for expression of stress-related genes (Wormuth et al., 2006) and the initiation of protective mechanisms, such as chaperones, antioxidant enzymes, and elevated levels of ferritin to sequester iron (Wojtaszek, 1997; Foyer and Noctor, 2009). If this complex set of measures fails, oxidative stress increases further. As a result, the cell cycle is stopped and apoptosis, programmed cell death (PCD, see Figure 2), starts. The visible signs of this process are necrotic lesions in the tissue (Halliwell, 2006).
Reduced transition metals not only participate in the generation of •OH in the Fenton reaction, they may also reduce secondary metabolites into radicals. One illustrative example is the iron-dependent activation of artemisinin (19), a non-phenolic sesquiterpene lactone that has proven as an efficient anti-malaria drug (Ridley, 2003). At present, it is assumed that the formed radicals inhibit a calcium-dependent ATPase located in the endoplasmic reticulum in the cells of the protozoan parasite (Eckstein-Ludwig et al., 2003).
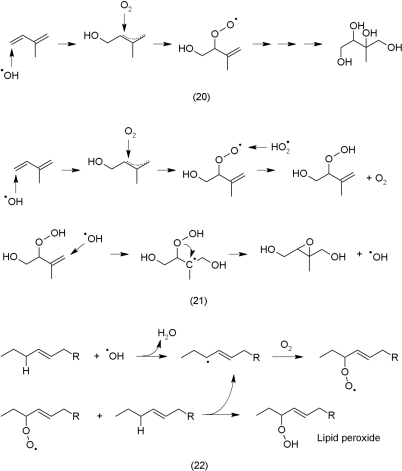
Recent insights into atmosphere chemistry provide ideas about potential reactions between ROS and non-phenolic secondary metabolites. Isoprene is a volatile plant compound that is emitted in considerable amounts and reacts with •OH and O2 in the atmosphere to form secondary organic aerosols, 2-methyltetrols (20), that induce cloud formation (Claeys et al., 2004).
It is feasible that the illustrated oxidation of isoprene (20) decreases levels of free radicals analogously as phenolic compounds do by reduction. The rate constants of these reactions are most likely determined by the presence, number, and position of the double bonds in the molecule as well as other functional groups in the molecule. Recently, it was shown that the oxidation of isoprene may yield hydroxyhydroperoxides that are further oxidized into dihydroepoxides (Paulot et al., 2009). During the second oxidation reaction •OH is even reformed (21).
The illustrated reactions (20, 21) also may occur in plant tissues. It is known that the emission of isoprene confers protection against oxidative stress, for instance isoprene protects against ozone damage of the photosynthetic apparatus (Loreto and Velikova, 2001). Oxidized terpenoids, however, as shown above (21), may undergo reactions with •OH that result in the reformation of •OH. Hydroxyl radicals also initiate the peroxidation of lipids and especially target isolated double bonds (22) (Marnett, 1999). Most probably, 21 and 22 provide just a glimpse into reactions that are possible.
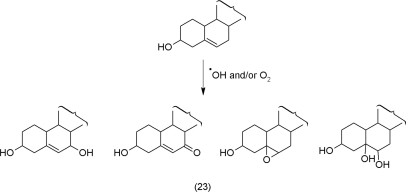
The peroxidation of cholesterol (23), a steroid triterpene that contributes to membrane fluidity in animals, has been the object of intensive research in context with skin cancer (Sevanian and Peterson, 1986). β-Sitosterol that substitutes for cholesterol in plants becomes oxidized similarly (Meyer and Spiteller, 1997).
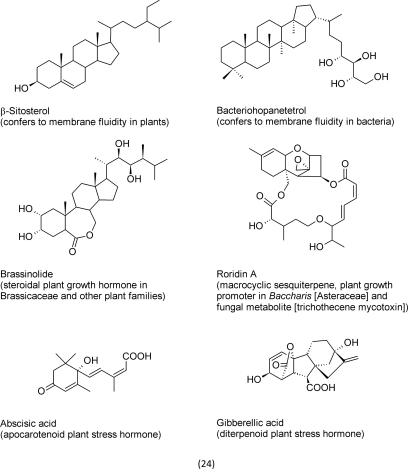
If we look at terpenoid structures that have evolved to possess signal functions in plants, the increased level of oxidation of the carbon skeleton (compared to membrane steroids) becomes evident. Examples include abscisic acid, gibberellic acid, brassinolide, and roridin A (24).
Plant hormones, such as abscisic acid, gibberellic acid and brassinolide, occur in low concentrations in the tissue and low levels are also required for their signaling effects. The few investigations that explore effects caused by higher concentrations indicated toxic effects in accordance with a hormetic dose–response behavior (Bajguz and Czerpak, 1998; Celik et al., 2007). At present, the general notion is that abscisic acid and gibberellic acid act by binding to specific receptors. In the case of abscisic acid, several were suggested. First, a nuclear protein, FCA, was pointed out to act as receptor for abscisic acid (Razem et al., 2006). This study, however, was retracted two years later due to the failure of repeatability by the reporting and other labs (Pennisi, 2009). Other groups suggested other receptors (Pandey et al., 2006; Liu et al., 2007; Pandey et al., 2009). Recently, the plasma membrane bound receptor linked to a G-protein was also questioned (Risk et al., 2009). Most currently, several papers that were published in highly ranked journals point to a group of proteins designated as PYR/PYL/RCAR that specifically inhibit protein phosphatases as receptors for abscisic acid (Sheard and Zheng, 2009). For gibberellic acid, a cytosolic protein was suggested as target site that induces DELLA protein degradation (Ueguchi-Tanaka et al., 2005). Besides, other hitherto unknown mechanisms are assumed to exist that control this pathway (Schwechheimer, 2008).
Roridin A (24), a sesquiterpene, is a trichothecene derivative that is classified as toxic mycotoxin found in cereals. Mycotoxins are characteristic secondary metabolites of mould fungi that infect cereals. Interestingly, roridin A also was detected in the higher plant genus Baccharis (Asteraceae). Roridin A showed only in seedlings and was identified to act as growth hormone (Jarvis and Miller, 1996). The results that were obtained from further studies suggested that roridin A (24) was synthesized by the plant itself instead, as originally assumed, by an endophytic fungus. The responsible genes, however, were never identified in the genome of Baccharis sp., and, thus, the hypotheses that an endophytic fungus synthesizes roridin A is still valid (Rosso et al., 2000). The contrasting activities, however, provide a further example for hormetic secondary metabolites.
Understanding the mode of action of phytohormones represents one of the greatest challenges for plant science today and genomic studies have provided valuable information so far. Redox homeostasis and antioxidant pools were suggested to act as a metabolic interface that adjusts responses to various environmental cues (Foyer and Noctor, 2005a; Foyer and Noctor, 2005b; Foyer and Noctor, 2009). A metabolomic study of abscisic acid-treated Arabidopsis seedlings revealed an increase of compatible solutes, ascorbic acid and α-tocopherol. This was interpreted as relationship between the biosynthesis of abscisic acid and the xanthophyll cycle and ascorbic acid recycling (Ghassemian et al., 2008). An exploration of this phenomenon might not only contribute to improved insights into the crosstalk between stress signaling pathways but also into a possible raison d’être for secondary metabolites that are specifically synthesized as response to imbalances in these signaling pathways.
The redox chemistry of terpenoids is more complex than that of phenolic compounds. The latter are strongly reducing and strongly competitive ligands of transition metals. These chemical characteristics are less pronounced for terpenoids. Similar to phenolic compounds, specific combinations of unsaturated bonds also may allow their participation in redox chemical reactions. The exploration of these reactions will prove much more difficult due to the fact that the follow-up reactions are more complex by several order magnitudes. The complexity of reactions of •OH in the atmosphere that was previously outlined provides some hints at least. Despite this, data from biological assays that indicate a non-linear, hormetic mode of action provide compelling evidence for the existence of this chemistry.
So far, we have only explored the chemistry of secondary metabolites with oxygen functions. In the introduction, alkaloids were mentioned to be characterized by a hormetic mode of action (Langenheim and Thimann, 1982).
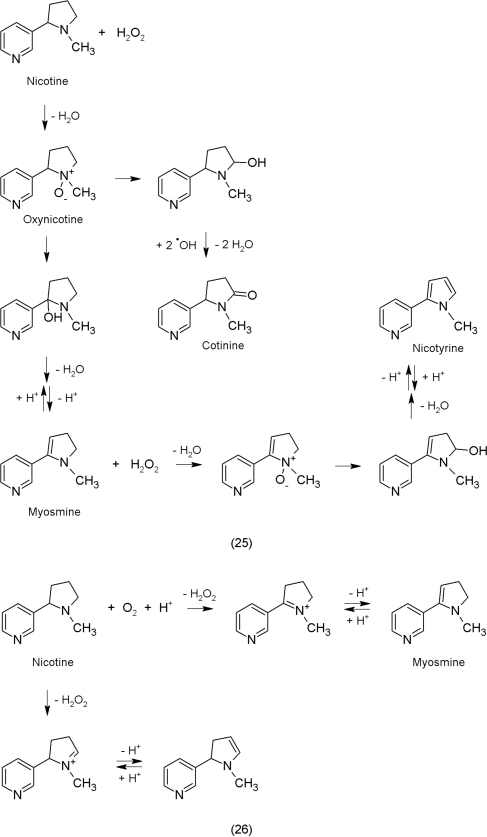
To illustrate the possible chemistry we chose nicotine as an example because this alkaloid is well investigated due to its presence in tobacco and also known to show a hormetic mode of action against brine shrimp (Calabrese, 2005). Furthermore, epidemiological studies revealed protective effects of nicotine in the development of some neurodegenerative diseases (Cormier et al., 2001). Nicotine is able to decrease the levels of ROS. Possible reactions with ROS are shown (25). Their probability is corroborated by various chemical studies on nicotine (Hucker et al., 1959; Taylor and Boyer, 1959; Wada et al., 1959; Craig et al., 1964; Mohrle and Berlitz, 2008; Mohrle and Berlitz, 2009).
Conversely, higher concentrations of nicotine may lead to the formation of ROS that can be alleviated by antioxidants (Sudheer et al., 2008; Das et al., 2009). The following reactions (26) that reduce oxygen to hydrogen peroxide also are possible in plant tissues. Most alkaloids that possess tertiary amine functions may potentially react in a similar fashion to that shown for nicotine.
The illustrated redox chemical reaction of phenolic, terpenoid and alkaloid secondary compounds document their participation in electron transfer reactions, either as donors or as acceptors. The high structural diversity of those secondary metabolites that undergo similar reactions suggests that introduction of specific combinations of functional groups on the carbon skeleton may be more important for this kind of interaction than the three-dimensional structure of the compound itself. Evolution may work more on this trait than on the quality of the carbon skeleton. This fact may also add to the fact that the biosynthesis of secondary metabolites is promiscuous. The Screening Hypothesis (Firn and Jones, 2003) argues that low substrate specificity and generation of multiple products generate this effect. We think that the inevitable redox chemistry also may substantially contribute to the observed promiscuity. Redox chemistry runs very fast and generates radicals; oxidation and oligomerization may occur even faster before the compound interacts with a potential receptor. Radical attacks are highly unspecific; they target the hydrogen atoms which are numerous in molecules close by. These chemical characteristics may contribute to the fact that identification of ligand sites of plant hormones and secondary metabolites may prove difficult sometimes.
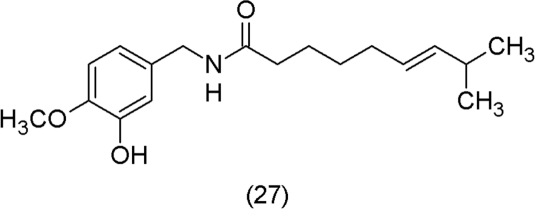
Plants feel no pain. Pain, however, is known to get worse with oxidative stress (Kim et al., 2004) and compounds such as capsaicin (27) are known to interact with pain receptors. The capsaicin receptor TRPV1 has evolved to as an efficient and highly sensitive detector of oxidative stress (Chuang and Lin, 2009).
Capsaicin (27) is a secondary plant metabolite. What is its function in the plant tissue? Many secondary metabolites are lipophilic (as capsaicin) and stored in specific compartments, e.g. idioblasts, glandular hairs, resin or latex canals, or included as glycosides in the vacuole (Hadacek, 2002; Langenheim, 2003).
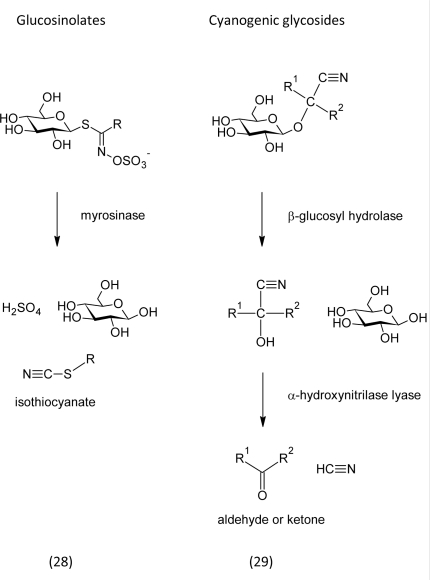
Damage of the tissue mixes formerly compartmented secondary metabolites and enzymes. In some cases, the masking sugars are cleaved by enzymes that were originally stored in separately in intact plants. This was shown to be the case for glucosinolates (28) (Grubb and Abel, 2006) and cyanogenic glycosides (29) (Zagrobelny et al., 2008).
It is quite possible that the main function of constitutive secondary metabolites is to signal tissue damage. Disturbance of the redox homeostasis in the tissue then facilitates local and systemic responses that are monitored by expression of hitherto silent genes. This is related to the preconditioning effect that secondary metabolites can exert when they are constituents of our diet (Son et al., 2008). For these actions, already low dosages are sufficient. Most of the secondary metabolites are poorly soluble in the aqueous milieu of the sym- and apoplast. The development of specialized compartments for accumulation and storage of secondary metabolites may have spurred diversification of evolutionary modern plant groups that invested in this trait compared to their ancestors lacking it (Farrell et al., 1991). The authors of this study interpreted the efficient accumulation of secondary metabolites as advantage for defense against predators. In modification of this and in accordance with the outlined preconditioning effect that represents an important component of hormesis, we suggest that the main benefit of secondary metabolites in plants is preconditioning for stress.
In this section we presented a selection of examples for chemical reactions that support the notion that phenolic, alkaloid and terpenoid secondary plant metabolites interact with the redox homeostasis of the cell. The presence or absence of specific functional groups, oxygen and/or nitrogen functions, determines the redox chemistry of the particular secondary metabolite. The wide-spread occurrence of these functions in the structures of secondary metabolites also suggests that most secondary metabolites may be biologically active in specific situations. Depending on the presence and absence of ROS, the secondary metabolite either reduces ROS or molecular oxygen, directly or indirectly via transition metals (Figure 1). We suggest that this particular reaction mechanism offers an explanation for the nonlinear, hormetic mode of action of many secondary metabolites. For example, a strongly reducing compound may reduce ROS such as H2O2 or •OH to water. Conversely, in absence of ROS, the same compound reduces oxygen to ROS (Figure 1). The outcome of the redox reactions both depends on the concentration of the secondary metabolite itself and the milieu at the site of the reaction—in simple terminology, on which additional compounds are either present or absent. To make things even more complicated, the availability as well as the complexation state of transition metals, important catalysts for these reactions, additionally affect this chemistry (Kell, 2009; Chobot and Hadacek, 2010). The cytosolic pH of 7.4 favors the above described chemistry; the acid pH of 3.6 of the vacuole rather acts against it.
The next section will explore if our current knowledge supports the existence of potential benefits of secondary metabolites for the survival of plants in the ecosystem, but not in terms of defense efficacy but in terms of stress survival. We do not question the prediction that secondary metabolites help plants to survive in the ecosystem (Hartmann, 1985), but the way they do it.
HORMESIS AND PLANT ECOLOGY
Plants occur in distinct communities. Some authors suggest that abiotic and biotic factors determine their composition (Tilman, 1982; Bever, 2003; Wardle et al., 2004). Others think that stochastic processes are responsible (Hubbell, 2001). Plant secondary metabolites have been proposed to be components of a co-evolutionary arms race between plants and their predators (Fraenkel, 1959; Ehrlich and Raven, 1964; Flor, 1971; Berenbaum, 1983). This is still the main paradigm in research on plant–herbivore interactions (Berenbaum and Zangerl, 2008).
The benefit of efficient chemical defense cannot be denied if we examine those plants that survive on range lands. Morphological defenses, spines and thorns, and unpalatable primary metabolites, e.g. oxalic acid in common sorrel, and/or secondary metabolites with reputation for toxic effects, e.g. cardenolides (milkweed) or terpene alkaloids (false heleborine), are often found in plants that resist herbivore pressure. Mammal herbivores exert strong effects on plant communities, and with increasing intensity a shift to “well defended species” occurs (Wardle and Bardgett, 2004). Is there a hormetic component hidden in the herbivore pasture with the “well-defended” plant? Let’s look from another perspective: If you are plant in a pasture, your tissue gets often damaged by foraging mammals. In order to survive this, plants need an efficient signaling system to counter the damage by local and systemic physiological responses. The textbook notion is that plants lack, in contrast to animals, a nervous system. Recently, this notion was challenged (Brenner et al., 2006). The authors argue that plants possess something of an equivalent to a nervous system; this is supported by recent insights into plant hormone activities, ROS functions, quorum sensing and quenching in bacteria as well as ionic imbalances that are caused by membrane-depolarizing activities of secondary metabolites. Amongst others, the transmembranic vesicular transport of auxin is regarded as a possible component of a nerve system analogue in plants. Not unexpectedly, these suggestions were rejected by other researchers in this field (Alpi et al., 2007). Despite this, the ideas of Brenner et al. (2006) are quite compelling in context with the redox chemistry of secondary metabolites that we suggest to be also an integral part of signaling in plant tissues. ROS bursts are usually accompanied by increased levels of ethylene, a signal molecule that not only easily passes membranes but also can cause ionic imbalances and modulation of ion channel activities (Laohavisit and Davies, 2007). The hormetic component in this proposed scenario is that low dosages of secondary metabolites signal tissue damage for the plant and higher dosages that are released by chewing repel the herbivore. The initial signaling events at the plant–herbivore interface include rapid changes in the Ca2+ concentration, membrane potential and phosphorylation status. Although these processes are poorly understood, their importance for plant survival is established (Howe and Jander, 2008). Animals identify their food plants on the basis of cues such as odor and taste. In addition, post-ingestive effects help the animal to learn to avoid plants with too high concentrations of secondary metabolites that are toxic on a daily basis and to identify plants with appropriate concentrations of secondary metabolites that may act beneficially as medicine (Villalba and Provenza, 2009). Insects identify their food plants on the basis of signals obtained from their sensory system; in this context, stimulation appears to be more important than deterrence (Jermy, 1993; Bernays and Chapman, 1994; Schoonhoven and van Loon, 2002).
The larvae of some herbivorous insects are capable to sequester secondary metabolites from their food plant by developing highly specific transport systems (Kuhn et al., 2004). Does this behavior contribute to improved defense or to increased fitness? Flavonoids are sequestered by larvae of blue wing butterflies from their food plants and later deposited in the wings of the adults (Knüttel and Fiedler, 2001). The authors of this study suggest that the sequestered flavonoids serve as visual cues for mate attraction. From our point of view, the flavonoids also might contribute to the fitness of the butterfly in the first place, and in the second place to mate attraction. The larvae of many insects that sequester secondary metabolites are often conspicuously colored (aposematic warning colorization). The question is: Are the pigments signals or do they just help to maintain the redox homeostasis in the skin of the larvae or in the wings of the butterfly? So far, two studies also support our view: Cold stress (Otaki, 2008) and injection of sulfinated polysaccharides (Serfas and Carroll, 2005) triggered changes in the color pattern of butterfly wings.
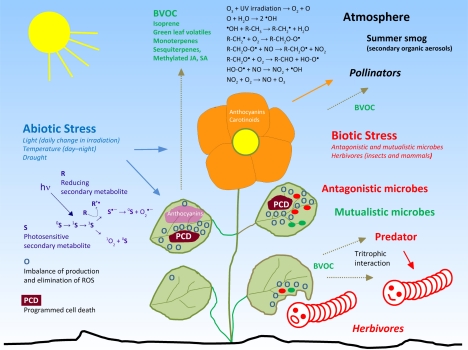
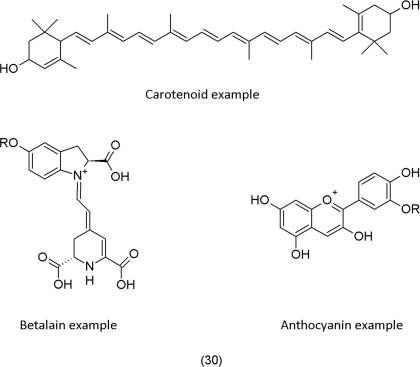
The fruits and flowers of plants contain pigments. Carotenoids mostly are responsible for yellow, betalains for pink in Caryophyllaceae and related families, and anthocyanins for blue, pink, and purple in other families (for example structures see 30). The general notion is that pigmentation helps to attract insects and animals that pollinate flowers and distribute fruits (Grotewold, 2006). Carotenoids also accumulate in the chloroplast where they protect the photosynthetic apparatus against deleterious effects of singlet oxygen (1O2) that is formed during photosynthesis, specifically by energy transfer of the relaxing pigments to oxygen during the light-harvesting process (Smirnoff, 2005).
Anthocyanins accumulate in leaves that are exposed to high light conditions and other abiotic stress (Figure 2). In autumn, the leaves of some trees show impressive autumn colorization. The red color is caused by the massive accumulation of anthocyanins. A recent review (Archetti, 2009) lists several hypotheses that try to explain the phenomenon of autumn colorization. The phenomenon itself is even more puzzling because a visible accumulation of anthocyanins sometimes occurs in young leaves too. Hatier and Gould (2008) argue that anthocyanins are antioxidants that contribute to stress regulation in the tissue. Similarly, the accumulation of pigments in flower petals may have evolved to confer tolerance to abiotic stress such as high light and temperature (difference day–night, especially in spring). Hence, attraction of pollinators by flower pigments only may constitute an indirect benefit of this trait.
The mechanisms involved in signaling and tolerating abiotic and biotic stress have been investigated independently until recently. Presently, the recognition grows that abiotic and biotic stress are regulated by identical signal cascades involving ROS and phytohormones (Fujita et al., 2006). Likewise, attack of microbes and herbivores trigger similar signals cascades, including a ROS burst that is accompanied by high phytohormone levels that cause ionic imbalances by modulation of ion channel activities (Wojtaszek, 1997; Maffei et al., 2007). Specific combinations of these common molecular processes reflect different types of stress and cause a specifically orchestrated stress-induced morphogenic response in the growth of the affected tissue such as inhibition of cell elongation, localized stimulation of cell division and alterations in the cell differentiation status (Potters et al., 2007; Potters et al., 2009). As already pointed out, the redox homeostasis may be viewed as an interface that allows plants to react to a broad range of environmental stress (Foyer and Noctor, 2005b; 2005a; 2009).
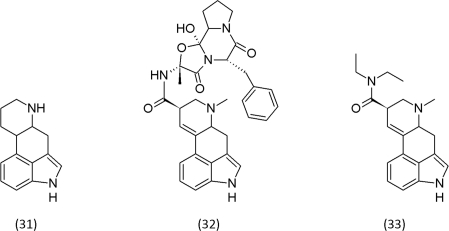
Various bacteria and fungi, colonize above- and belowground parts of plants. If they cause disease symptoms, they are classified as pathogens, in case of a symptomless presence, they are designated as endophytes (Wilson, 1995; Rodriguez et al., 2009). Mycorrhizal fungi are not classified as endophytic fungi but are considered as a special type of symbiosis. Conventionally, endophytes are classified into clavicipitaceous and non-clavicipitaceous types. The first is limited to grasses, the second has a wide distribution among plant families (Schardl et al., 2006). Some clavicipitaceous fungi are known to produce ergot alkaloids, derivatives of ergoline (31). Ergotamine (32) is one of the alkaloids that are produced by the fungus Claviceps purpurea and was shown to be responsible for long-term ergot poisoning. Originally, the clavicipitaceous endophytes were regarded as beneficial mutualists that protected the grass hosts with their ergot alkaloids against herbivores. Today, the notion prevails that the endophytic fungi contribute to the fitness of the grass host (Clay and Schardl, 2002).
In the Middle Ages, ergot poisoning was known as ignis sacer or “Saint Anthony’s fire”. Ergotamine (32) is also a precursor of LSD (33), a semi-synthetic psychedelic drug. Ergotamine shares structural similarities with neurotransmitters such as serotonin, adrenaline and dopamine and can thus act as antagonist or partial agonist (Filipov et al., 1999); further, ergotamine is also known for its anti-migraine effects (Tfelt-Hansen et al., 2000). The effects that were caused by variable concentrations of ergot alkaloids on humans indicate a hormetic mode action that probably affects all organism that come into contact with them. Consumed in large concentrations, ergot alkaloids are toxic, in low concentrations, they may be beneficial and improve growth and drought tolerance of the grass host. The geothermal grass Dichanthelium lanuginosum is colonized by a non-clavicipitaceous endophytic fungus, a Curvularia sp., that provides protection against high temperatures. Again, we are confronted with a preconditioning effect. Colonization by this endophytic fungus triggers a low-stress response that helps the grass to tolerate the high temperatures (Redman et al., 2002). Sub-lethal biotic stress may improve resistance against an abiotic stress and, of course, vice versa. Similar beneficial effects are also known for mycorrhizal fungi (Davies et al., 1992).
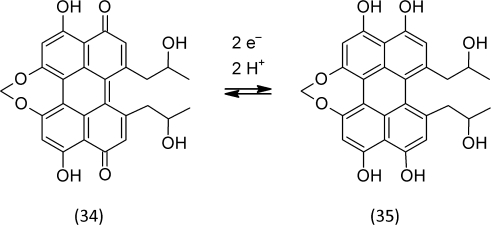
Many microbial plant pathogens are classified as necrotrophs, i.e. the bacterium or fungus colonizes dead plant tissue. Necrotrophs produce secondary metabolites in high amounts that kill the cells of the host plant, often by triggering programmed cell death (Howlett, 2006). As a consequence, the necrotrophic microbes benefit from the nutrients stored in this cell. Cercosporin (34) is a phenolic “toxin” that is produced by the phytopathogenic fungus Cercospora (Daub and Ehrenshaft, 2000). Its chemical reactions illustrate a possibly wide-spread mode of action of microbial-produced redox active secondary metabolites. The fungus secretes a reduced form of cercosporin (35) into the plant tissue. There, it is oxidized by ROS that are generated by the oxidative burst triggered during the infection process. Reduced cercosporin molecules (35) scavenge ROS and are oxidized in this process. Cercosporin (34) is a photosensitizer that generates superoxide anion radicals and singlet oxygen (Figure 2). Singlet oxygen attacks double bonds and sulfur in various bio-molecules (Chobot et al., 2004; Triantaphylidès and Havaux, 2009). Cercosporin (34) is a nice example that illustrates how a changing redox milieu may turn an initially reducing compound into an oxidizing one. A change of the milieu modifies the activity of a redox compound much more dramatically than a change of concentration would do. Higher dosages, however, are always pro-oxidative due to the unequal ratio of the reactants. Toxicity at high dosages is the principle of a hormetic mode of action of a compound.
The production of secondary metabolites is not restricted to phytopathogenic bacteria and fungi. Many saprophytic soil bacteria and fungi are also known to be efficient producers. Among bacteria, actinobacteria especially represent an especially rich source for lead structures of antibiotics and other drugs (Omura, 1986). So far, the most often expressed notion is that these secondary metabolites aid the producing soil microbes in biological warfare against competitors for the same resources; however in recent times, and also earlier, the potential signaling roles for antibiotics and related compounds have been pointed out, especially in cell differentiation (Campbell, 1984; Davies, 1990; Price-Whelan et al., 2006; Lopez et al., 2009; Mlot, 2009). In contrast to plants, microbes secrete their secondary metabolites they produce as fast as possible. This behavior merits more attention.
We think that plants use secondary metabolites to maintain the redox homeostasis for efficient signaling. One of the main modes of interactions with signal cascades depend on the ability of secondary metabolites to participate in electron transfer reactions. Upon tissue damage, the compartment of the secondary metabolite is destroyed and it can undergo various redox reactions. Bad solubility in the cytoplasm limits the amount of the compound that actually can react. The amounts, however, are sufficient to disturb the redox homeostasis in adjacent cells. This facilitates a local and, if appropriate, a systemic response that helps the plant to survive the damage of the tissue. Compared to microbes, vascular plants are a recent innovation of evolution. The phenomenon that we observe in plants is the end of the story, not the beginning. One component that characterizes this story inherently is hormesis. Put in a simple way, plants use the same chemistry for signaling that microbes use for the decomposition of polymers but in a much more modulated fashion. The oxidative degradation chemistry also involves the formation of hydroxyl radicals that are generated by similar reactions that include transition metal-catalyzed electron transfer reactions onto oxygen that result in the formation of hydroxyl radicals (36); phenolic microbial metabolites function as redox cyclers (compare 6–11) (Jensen et al., 2001; Suzuki et al., 2006; Valenzuela et al., 2008).
In the soil, carbon is deposited as soil organic matter. Plants contribute to this carbon pool through their litter that contains cellulose and lignin and root exudation of soluble metabolites, such as sugars, organic and amino acids as well as secondary metabolites (see Figure 3). This carbon represents a major nutrient source for diverse soil microbes (Berg and Laskowski, 2006; Gregory, 2006). At present, wood-decomposing basidiomycetes belong to the best investigated group of organisms that contribute to decomposition of soil organic matter (Baldrian and Valaskova, 2008).
FIGURE 3.
Below ground, plants try to survive by root exsudation and formation of mucigels to protect roots from the oxidative milieu caused by litter decomposition that also utilizes redox chemistry as an important component (for detailed explanation see text).
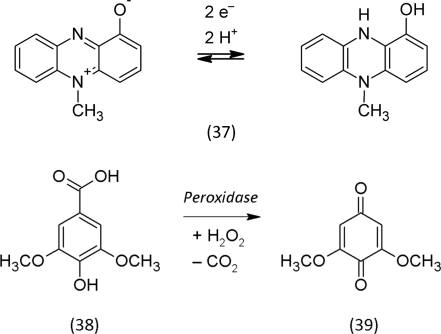
Forty years ago, Halliwell (1965) proposed that hydroxyl radicals contribute to the degradation of cellulose. Brown-rot (Gomez-Toribio et al., 2009) and white-rot fungi (Wang and Newman, 2008) were shown to utilize this chemistry. Similarly, bacteria also produce redox-active secondary metabolites that are capable to reduce iron, e.g. phenazines such as procyanin (37). Such low-molecular phenolic metabolites represent ideal electron shuttles that diffuse into the substrate to be decomposed (Newman, 2008). If the hydroxyl radical-generating chemistry occurs to close to the microbe, then it would be harmful to the initiator of this chemistry. Radicals of oxidized decomposition products of lignin, hemi-cellulose, cellulose and chitin, secondary organic compounds in soil, lead to the formation humic and fulvic acids and the larger polymer humin, the major portion of soil organic matter (Stevenson, 1994). Some authors even propose that the strongly oxidized phenolic structures of humic and fulvic acids catalyze the oxidative decomposition chemistry (Kang and Choi, 2009).
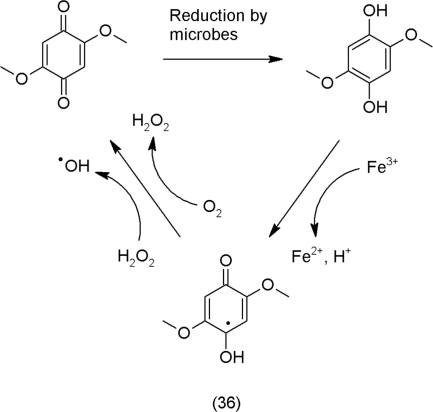
The strong hormetic component in the redox chemistry of secondary metabolites is that it is involved both in regulatory and destructive processes and a clear line between both is difficult to draw. The actual effect does not depend on a specific molecule alone but also on the chemical environment. We just want to present one further intriguing example for a regulation: In the infection process of the parasitic plant Striga, quinones signal host recognition and haustorium formation (Keyes et al., 2000). The root tip of Striga produces H2O2 that is reduced to water by the peroxidases in the plant tissues. The electrons required for these reactions are supplied by syringic acid (38), a precursor for lignin that is oxidized to a quinone, dimethoxybenzoquinone (39); the quinone then activates the expression of genes in Striga that again regulate the development of the haustorium, a special structure of the Striga root for successful establishment of a parasitic relationship with the host plant.
Complex interactions between extracellular enzymes and redox active secondary metabolites are required both for growth of plants in soil as well as recycling for soil organic matter (Figures 2 and 3). Within plant tissues, the cytoplasm and the apoplast provide the required milieu and compartmentalization for this chemistry. At the first glance, the soil is a very hostile environment. The root cap, however, produces mucilage which consist of a complex mixture of free and polymerized sugars, rhamnose, fucose, xylose, arabinose, galactose, glucose, and mannose (Gregory, 2006). As the root grows, mucilage and the associated cap cells are left behind and, after mixing with water, penetrate among soil particles. Further, mucilage is also produced by bacteria. This boundary between the plant root and the soil creates an environment in which the redox chemistry of secondary metabolites can work: (1) colloidal properties of mucilage enable tubular aggregates for molecule transport; (2) electromagnetic fields may change the surface properties of mucilage; (3) mucilage may form thin-layer diffusion barriers, and (4) mucilage serves as depot storage for exuded plant and microbial metabolites (Claus et al., 1958; Gutierrez et al., 1961; Lai et al., 2007; Cone, 2009). Matrices with colloidal properties constitute a major prerequisite for buffering processes in soils. Another colloidal structure, the humic acids polymers, may have one important function: they complex iron and other transition metals. This characteristic may either add to the uptake of these minerals by plants (Varanini and Pinton, 2001) or provide catalysts for a Fenton-type non-enzymatic decomposition of soil organic matter. The latter aspect certainly deserves more attention in future research.
JUSTIFICATION FOR A CHEMICAL RAISON D’ÊTRE OF SECONDARY METABOLITES
Oxygen forms various reactive oxygen species (ROS) that can be found from soil environments to the atmosphere where they participate in chemical reactions. In soil environments, •OH is involved in the decomposition of lignin and cellulose, in the atmosphere in the “decomposition” of emitted volatile organic compounds from natural and human sources that leads to cloud formation (Figures 2 and 3). In plant tissues, functional electron transport chains guarantee the smooth action of photosynthesis and respiration. On disturbance, the electrons are transferred to oxygen instead and this causes the formation of superoxide anion radicals ( ) and hydrogen peroxide (H2O2). In living tissues, these ROS are either reduced by ascorbate and glutathione in concert with enzymes such as superoxide dismutase and catalase.
Accidental generation of ROS by disturbed electron transfers has evolved as important signaling system for stress tolerance in all living organism, both unicellular and multi-cellular. One compelling argument for the contribution of secondary metabolites to signaling is that the evolution of a nerve system selected against the accumulation of constitutive secondary metabolites. This becomes especially evident in the vertebrate animals (Rubinstein, 1992). Secondary metabolites can reduce ROS into less harmful oxygen compounds. The more efficient of them are classified as antioxidants. In absence of ROS, however, the same compound reduces molecular oxygen. The rate constants of these reactions may be determined by the variable availability of transition metals (Kell, 2009; Chobot and Hadacek, 2010). Transition metals are efficient catalysts for many redox reactions, both enzymatically and non-enzymatically. In the Fenton reaction, H2O2 is reduced to •OH, the most reactive of all ROS. Here we propose that this milieu-dependent redox chemistry also may explain the non-linear hormetic dose–response response of many secondary metabolites to some extent. In this context, it is plausible to assume that redox chemistry acted and acts as important constraint for the evolution of secondary metabolites. Essentially, this chemistry depends on the presence and absence of functional groups in the molecule. As a consequence, structures may vary but still participate in similar chemical reactions. A portion of the structural diversity of secondary metabolites that we find today is most probably generated by the redox chemistry or by follow-up reactions of oxidized original structures. System and chance is closely linked in the chemistry of living organisms as, for example, in the codon structure of amino acids (Copley et al., 2005). The first letter codes for the α-ketoacid that identifies the biosynthetic precursor, the second letter denotes hydrophilicity, and the third letter is random. The system in the evolution of secondary metabolites may be viewed analogously.
To illustrate why a raison d’être of secondary metabolites should be sought in the exploration of their redox chemistry we have provided reactions of phenolic, terpenoid and alkaloid secondary metabolites. Compared to terpenoids and alkaloids, the redox chemistry of phenols is more straightforward and, consequently, much better explored. All classes of secondary metabolites comprise compounds that either were used in traditional medicine or offered lead structures for drug development. In the tissues of living organisms, secondary metabolites are involved in signaling and maintaining of the redox homeostasis. In cells, only low dosages are required, high dosages are detrimental. In intact tissues of the plant, secondary metabolites are either compartmented (constitutive) or synthesized when required (induced). In our opinion, in both cases, secondary metabolites signal tissue damage and facilitate a systemic response that reinstates the redox homeostasis. Often, various abiotic and biotic stress factors induce the identical compounds.
Secondary metabolites help the plant to tolerate stress and to survive in the ecosystem. Our hypothesis regards survival as more important than defense. The classic defense hypotheses rate the biosynthetic costs as major constraint for the accumulation of secondary metabolites. The dilemma of plants is to grow or to defend (Herms and Mattson, 1992). We think that the main dilemma of plants is to survive and grow (Potters et al., 2009). In the literature, especially in ecology, the defense hypotheses, ideally in a co-evolutionary framework, still prevail despite the fact that a thorough analysis of the literature found few studies that unambiguously support them (Stamp, 2003). Our suggestions of a chemical raison d’être hypothesis also aims to revive the discussion about the issue that should not be reduced to the comfortable dogma of chemical defense as in recent reviews (van Dam, 2009). Even if the notion that we present appears to be heretical at the first glance we hope that it will prove as Copernican in the end.
One objection that our chemical hypothesis will be confronted with is that molecular targets (receptors) are known for many secondary metabolites. The reactions that we pointed out, the reduction of oxygen to produce ROS is accepted as mode of action of quinone redox cyclers that are used as antitumor drugs (Butler, 1998). The question that has to be answered in the future is: how widespread is this mechanism? A strong redox cycler that we investigated in our lab is juglone (Chobot and Hadacek, 2009). In the plant, juglone is known to occur mainly in glycosylated form (Duroux et al., 1998). With good reason, it is too reactive, a chemical bomb. Redox cycling secondary metabolites form radicals. This phenomenon explains the instability of many reactive compounds. Still, however, it is possible that secondary metabolites bind to receptors. In this case, it has to be established that the signaling is exclusively triggered by this binding reaction. Conversely, radicals of secondary metabolites that are formed during redox chemical interactions may bind to any protein that happens to be close by.
In the mid-20th century, Dimitri Nasonov and his colleagues described the universal cell response (UCR), a biphasic, hormetic response of cells to external stimuli (Agutter, 2007). Agutter (2007) suggests that hormesis represents an aspect or alternative manifestation of the UCR. Attempts to identify the mechanistic basis of hormesis include (1) two membrane receptor subtypes with different ligand affinities and contrasting downstream effects, (2) two intracellular steroid receptor subtypes that activate responsive genes in homodimeric but not in heterodimeric form, (3) DNA repair by enzymes that are activated by forming adducts with the xenobiotic, and (4) a combination of rates of DNA damage and cell division that affect mutation (Conolly and Lutz, 2004). Agutter (2007) regards these mechanisms as intrinsically plausible, but criticizes their low general applicability. The redox homeostasis of the cell is affected by biotic and synthetic chemicals as well as by a wide range of biotic and abiotic stress factors. Thus, the inherent redox chemistry might explain hormetic phenomena. One challenge will be to understand the complex role of iron and other transition metals that, depending on their ligands, specifically catalyze the redox chemistry in the cell (Kell, 2009).
If one compares the structures of currently used drugs—unfortunately, most of them interact with more receptors than desired (Keiser et al., 2009)—with proposed structures of humic acids (Stevenson, 1994) (40), the striking structural similarities are evident. Of course, humic acids are more oxidized since the environment of their formation is a more oxidizing one. In living tissues, the milieu is reducing. If this changes to be oxidative, stress develops. A simple example is the browning of damaged tissue of plants that contain phenolic secondary metabolites. If the redox homeostasis cannot be maintained, disease develops that, ultimately, leads to death. Put in a simple way: decomposition of the tissue cannot be prevented any more. The oxidative chemistry that starts to run in the tissue is intriguingly similar to that of litter decomposition in soil. Better insights into the raison d’être of secondary metabolites will not only advance our understanding of plant biology but many related disciplines might benefit including those that focus of human health. For this, revised hypotheses are needed. Gottfried Fraenkel is to be recognized for pointing out that secondary metabolites are not waste products (Fraenkel, 1959). Fifty years later, substantial progress to understand their raison d’être has been made but we have not arrived at the end of the road. The chemistry that defines secondary metabolism is more ancient than the trait itself. The insight that it highly depends on its chemical environment that, in the extreme case, may revert the reaction itself, additionally complicates further exploration. These facts only provide scant support for the proposed function of chemical weapons. An extension of this hypothesis is inevitable to obtain a hypothesis that predicts all effects of secondary metabolites including that of hormesis.
Acknowledgments
DE is supported by the Federal Ministry of Agriculture, Forestry, Environment and Water Management (100127) and VC by the FWF (M920-B03, P20923-B03). The research of FH and GB is supported by the Federal Ministry of Agriculture, Forestry, Environment and the FWF. The authors acknowledge the stimulating and insightful comments of two anonymous reviewers.
REFERENCES
- Agutter PS. Cell mechanics and stress: from molecular details to the ‘universal cell reaction’ and hormesis. Bioessays. 2007;29:324–333. doi: 10.1002/bies.20550. [DOI] [PubMed] [Google Scholar]
- Alpi A, Amrhein N, Bertl A, Blatt MR, Blumwald E, Cervone F, Dainty J, De Michelis MI, Epstein E, Galston AW, Goldsmith MHM, Hawes C, Hell R, Hetherington A, Höfte H, Juergens G, Leaver CJ, Moroni A, Murphy A, Oparka K, Perata P, Quader H, Rausch T, Ritzenthaler C, Rivetta A, Robinson DG, Sanders D, Scheres B, Schumacher K, Sentenac H, Slayman CL, Soave C, Somerville C, Taiz L, Thiel G, Wagner R. Plant neurobiology: no brain, no gain? Trends Plant Sci. 2007;12:135–136. doi: 10.1016/j.tplants.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Archetti M. Classification of hypotheses on the evolution of autumn colours. Oikos. 2009;118:328–333. [Google Scholar]
- Bajguz A, Czerpak R. Physiological and biochemical tole of brassinosteroids and their structure-activity relationship in the green alga Chlorella vulgaris Beijerinck (Chlorophyceae) J Plant Growth Regul. 1998;17:131–139. [Google Scholar]
- Baldrian P, Valaskova V. Degradation of cellulose by basidiomycetous fungi. FEMS Microbiol Rev. 2008;32:501–521. doi: 10.1111/j.1574-6976.2008.00106.x. [DOI] [PubMed] [Google Scholar]
- Berenbaum MR. Coumarins and caterpillars - a case for coevolution. Evolution. 1983;37:163–179. doi: 10.1111/j.1558-5646.1983.tb05524.x. [DOI] [PubMed] [Google Scholar]
- Berenbaum MR, Zangerl AR. Facing the future of plant-insect interaction research: Le Retour a la “Raison d’Etre”. Plant Physiol. 2008;146:804–811. doi: 10.1104/pp.107.113472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg B, Laskowski R. Litter Decomposition: A Guide to Carbon and Nutrient Turnover. Academic Press; Burlington, MA, USA: 2006. [Google Scholar]
- Bernays EA, Chapman RF. Host-Plant Selection by Phytophagous Insects. Chapman & Hall; New York: 1994. [Google Scholar]
- Bever JD. Soil community feedback and the coexistence of competitors: conceptual frameworks and empirical tests. New Phytol. 2003;157:465–473. doi: 10.1046/j.1469-8137.2003.00714.x. [DOI] [PubMed] [Google Scholar]
- Blankenship RE, Hartman H. The origin and evolution of oxygenic photosynthesis. Trends Biochem Sci. 1998;23:94–97. doi: 10.1016/s0968-0004(98)01186-4. [DOI] [PubMed] [Google Scholar]
- Block G, Patterson B, Subar A. Fruit, vegetables, and cancer prevention. A review of epidemiological evidence. Nutr Cancer. 1992;18:1–29. doi: 10.1080/01635589209514201. [DOI] [PubMed] [Google Scholar]
- Bloor SJ, Abrahams S. The structure of the major anthocyanin in Arabidopsis thaliana. Phytochemistry. 2002;59:343–346. doi: 10.1016/s0031-9422(01)00460-5. [DOI] [PubMed] [Google Scholar]
- Brenner ED, Stahlberg R, Mancuso S, Vivanco J, Baluska F, Van Volkenburgh E. Plant neurobiology: an integrated view of plant signaling. Trends Plant Sci. 2006;11:413–419. doi: 10.1016/j.tplants.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Brown WL, Eisner T, Whittaker H. Allomones and kairomones: transspecific chemical messengers. Bioscience. 1970;20:21–22. [Google Scholar]
- Butler J. Redox cycling antitumor drugs. In: Aruoma OI, Halliwell B, editors. DNA and Free Radicals: Techniques, Mechanisms & Applications. OICA International; London: 1998. pp. 131–159. [Google Scholar]
- Calabrese EJ. Paradigm lost, paradigm found: The re-emergence of hormesis as a fundamental dose response model in the toxicological sciences. Environ Pollut. 2005;138:378–411. doi: 10.1016/j.envpol.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ. Hormesis and medicine. Br J Clin Pharmacol. 2008;66:594–617. doi: 10.1111/j.1365-2125.2008.03243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese EJ, Blain RB. Hormesis and plant biology. Environ Pollut. 2009;157:42–48. doi: 10.1016/j.envpol.2008.07.028. [DOI] [PubMed] [Google Scholar]
- Callaway RM, Ridenour WM. Novel weapons: invasive success and the evolution of increased competitive ability. Front Ecol Environ. 2004;2:436–443. [Google Scholar]
- Campbell IM. Secondary metabolism and microbial physiology. Adv Microb Physiol. 1984;25:1–60. doi: 10.1016/s0065-2911(08)60290-8. [DOI] [PubMed] [Google Scholar]
- Celik I, Tuluce Y, Isik I. Evalution of toxicity of abscisic acid and gibberellic acid in rats: 50 days drinking water study. J Enzym Inhib Med Chem. 2007;22:219–226. doi: 10.1080/14756360600988955. [DOI] [PubMed] [Google Scholar]
- Chobot V. Simultaneous detection of pro- and antioxidative effects in the variants of the deoxyribose degradation assay. J Agric Food Chem. 2010;58:2088–2094. doi: 10.1021/jf902395k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chobot V, Hadacek F. Milieu-dependent pro- and antioxidant activity of juglone may explain linear and nonlinear effects on seedling development. J Chem Ecol. 2009;35:383–390. doi: 10.1007/s10886-009-9609-5. [DOI] [PubMed] [Google Scholar]
- Chobot V, Hadacek F. Iron and its complexation by phenolic cellular metabolites. Plant Signal Behav. 2010;5:4–8. doi: 10.4161/psb.5.1.10197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chobot V, Vytlacilova J, Jahodar L. Phototoxic activity and the possibilities of its testing. Cent Eur J Publ Health. 2004;12:S31–S33. [PubMed] [Google Scholar]
- Chobot V, Huber C, Trettenhahn G, Hadacek F. (±)-Catechin: Chemical weapon, antioxidant, or stress regulator. J Chem Ecol. 2009;35:980–996. doi: 10.1007/s10886-009-9681-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang H, Lin S. Oxidative challenges sensitize the capsaicin receptor by covalent cysteine modification. Proc Natl Acad Sci USA. 2009;106:20097–20102. doi: 10.1073/pnas.0902675106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claeys M, Graham B, Vas G, Wang W, Vermeylen R, Pashynska V, Cafmeyer J, Guyon P, Andreae MO, Artaxo P, Maenhaut W. Formation of secondary organic aerosols through photooxidation of isoprene. Science. 2004;303:1173–1176. doi: 10.1126/science.1092805. [DOI] [PubMed] [Google Scholar]
- Claus D, Wittmann H, Rippelbaldes A. Untersuchungen über die Zusammensetzung von Bakterienschleimen und deren Lösungsvermögen gegenüber schwer löslichen anorganischen Verbindungen. Arch Mikrobiol. 1958;29:169–178. [PubMed] [Google Scholar]
- Clay K, Schardl C. Evolutionary origins and ecological consequences of endophyte symbiosis with grasses. Am Nat. 2002;160:S99–S127. doi: 10.1086/342161. [DOI] [PubMed] [Google Scholar]
- Cone RA. Barrier properties of mucus. Adv Drug Deliv Rev. 2009;61:75–85. doi: 10.1016/j.addr.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Conolly RB, Lutz WK. Nonmonotonic dose-response relationships: Mechanistic basis, kinetic modeling, and implications for risk assessment. Toxicol Sci. 2004;77:151–157. doi: 10.1093/toxsci/kfh007. [DOI] [PubMed] [Google Scholar]
- Copley SD, Smith E, Morowitz HJ. A mechanism for the association of amino acids with their codons and the origin of the genetic code. Proc Natl Acad Sci USA. 2005;102:4442–4447. doi: 10.1073/pnas.0501049102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cormier A, Morin C, Zini R, Tillement JP, Lagrue G. In vitro effects of nicotine on mitochondrial respiration and superoxide anion generation. Brain Res. 2001;900:72–79. doi: 10.1016/s0006-8993(01)02254-5. [DOI] [PubMed] [Google Scholar]
- Craig JC, Mary NY, Goldman NL, Wolf L. Tertiary amine oxide rearrangements 3. Mechanism of demethylation of nicotine. J Am Chem Soc. 1964;86:3866–3869. [Google Scholar]
- Das S, Neogy S, Gautam N, Roy S. In vitro nicotine induced superoxide mediated DNA fragmentation in lymphocytes: Protective role of Andrographis paniculata Nees. Toxicology in Vitro. 2009;23:90–98. doi: 10.1016/j.tiv.2008.10.012. [DOI] [PubMed] [Google Scholar]
- Daub ME, Ehrenshaft M. The photoactivated Cercospora toxin cercosporin: Contributions to plant disease and fundamental biology. Annu Rev Phytopathol. 2000;38:461–490. doi: 10.1146/annurev.phyto.38.1.461. [DOI] [PubMed] [Google Scholar]
- Davies FT, Potter JR, Linderman RG. Mycorrhiza and repeated drought exposure affect drought resistance and extraradical hyphae development independent of plant size and nutrient content. J Plant Physiol. 1992;139:289–294. [Google Scholar]
- Davies J. What are antibiotics - archaic functions for modern activities. Mol Microbiol. 1990;4:1227–1232. doi: 10.1111/j.1365-2958.1990.tb00701.x. [DOI] [PubMed] [Google Scholar]
- Dizdaroglu M. Mechanisms of free radical damage to DNA. In: Aruoma OI, Halliwell B, editors. DNA and Free Radicals: Techniques, Mechanisms and Applications. OICA International; London: 1998. pp. 3–26. [Google Scholar]
- Duroux L, Delmotte FM, Lancelin JM, Keravis G, Jay-Allemand C. Insight into naphthoquinone metabolism: beta-glucosidase-catalysed hydrolysis of hydrojuglone beta-D-glucopyranoside. Biochem J. 1998;333:275–283. doi: 10.1042/bj3330275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckstein-Ludwig U, Webb RJ, van Goethem IDA, East JM, Lee AG, Kimura M, O’Neill PM, Bray PG, Ward SA, Krishna S. Artemisinins target the SERCA of Plasmodium falciparum. Nature. 2003;424:957–961. doi: 10.1038/nature01813. [DOI] [PubMed] [Google Scholar]
- Ehrlich PR, Raven PH. Butterflies and plants - a study in coevolution. Evolution. 1964;18:586–608. [Google Scholar]
- Elstner EF. Der Sauerstoff. BI Wissenschaftsverlag; Mannheim: 1990. [Google Scholar]
- Farmer EE, Davoine C. Reactive electrophile species. Curr Op Plant Biol. 2007;10:380–386. doi: 10.1016/j.pbi.2007.04.019. [DOI] [PubMed] [Google Scholar]
- Farrell BD, Dussourd DE, Mitter C. Escalation of plant defense - do latex and resin canals spur plant diversification. Am Nat. 1991;138:881–900. [Google Scholar]
- Filipov NM, Thompson FN, Tsunoda M, Sharma RP. Region-specific decrease of dopamine and its metabolites in brains of mice given ergotamine. J Toxicol Env Health Pt A. 1999;56:47–58. doi: 10.1080/009841099158222. [DOI] [PubMed] [Google Scholar]
- Firn RD, Jones CG. Natural products - a simple model to explain chemical diversity. Nat Prod Rep. 2003;20:382–391. doi: 10.1039/b208815k. [DOI] [PubMed] [Google Scholar]
- Firn RD, Jones CG. A Darwinian view of metabolism: molecular properties determine fitness. J Exp Bot. 2009;60:719–726. doi: 10.1093/jxb/erp002. [DOI] [PubMed] [Google Scholar]
- Flor HH. Current status of the gene-for gene concept. Annu Rev Phytopathol. 1971;9:275–296. [Google Scholar]
- Foyer CH, Noctor G. Redox homeostasis and antioxidant signaling: A metabolic interface between stress perception and physiological responses. Plant Cell. 2005a;17:1866–1875. doi: 10.1105/tpc.105.033589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer CH, Noctor G. Oxidant and antioxidant signalling in plants: a re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ. 2005b;28:1056–1071. [Google Scholar]
- Foyer CH, Noctor G. Redox regulation in photosynthetic organisms: signaling, acclimation, and practical implications. Antioxid Redox Signal. 2009;11:861–905. doi: 10.1089/ars.2008.2177. [DOI] [PubMed] [Google Scholar]
- Fraenkel GS. The raison d’être of secondary plant substances. Science. 1959;129:1466–1470. doi: 10.1126/science.129.3361.1466. [DOI] [PubMed] [Google Scholar]
- Fujita M, Fujita Y, Noutoshi Y, Takahashi F, Narusaka Y, Yamaguchi-Shinozaki K, Shinozaki K. Crosstalk between abiotic and biotic stress responses: a current view from the points of convergence in the stress signaling networks. Curr Op Plant Biol. 2006;9:436–442. doi: 10.1016/j.pbi.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Gechev TS, Van Breusegem F, Stone JM, Denev I, Laloi C. Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. Bioessays. 2006;28:1091–1101. doi: 10.1002/bies.20493. [DOI] [PubMed] [Google Scholar]
- Ghassemian M, Lutes J, Chang HS, Lange I, Chen W, Zhu T, Wang X, Lange BM. Abscisic acid-induced modulation of metabolic and redox control pathways in Arabidopsis thaliana. Phytochemistry. 2008;69:2899–2911. doi: 10.1016/j.phytochem.2008.09.020. [DOI] [PubMed] [Google Scholar]
- Gomez-Toribio V, Garcia-Martin AB, Martinez MJ, Martinez AT, Guillen F. Induction of extracellular hydroxyl radical production by white-rot fungi through quinone redox cycling. Appl Environ Microbiol. 2009;75:3944–3953. doi: 10.1128/AEM.02137-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace SC. Phenolics as antioxidants. In: Smirnoff N, editor. Antioxidants and Reactive Oxygen Species in Plants. Blackwell Publishing; Oxford: 2005. pp. 141–168. [Google Scholar]
- Gregory P. Plant Roots: Growth, Activity and Interactions with Soil. Blackwell Publishing Inc; Oxford, UK: 2006. [Google Scholar]
- Gripenberg J. Antibiotic substances from the heartwood of Thuja plicata. VII. A partial synthesis of thujic acid. Acta Chem Scand B. 1951;5:995–1002. [Google Scholar]
- Grotewold E. The genetics and biochemistry of floral pigments. Ann Rev Plant Biol. 2006;57:761–780. doi: 10.1146/annurev.arplant.57.032905.105248. [DOI] [PubMed] [Google Scholar]
- Groves JT. High-valent iron in chemical and biological oxidations. J Inorg Biochem. 2006;100:434–447. doi: 10.1016/j.jinorgbio.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Grubb CD, Abel S. Glucosinolate metabolism and its control. Trends Plant Sci. 2006;11:89–100. doi: 10.1016/j.tplants.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Guo H, Tullius TD. Gapped DNA is anisotropically bent. Proc Natl Acad Sci USA. 2003;100:3743–3747. doi: 10.1073/pnas.0737062100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez J, Lindahl IL, Davis RE. Some chemical and physical properties of a slime from rumen of cattle. Appl Microbiol. 1961;9:209–212. doi: 10.1128/am.9.3.209-212.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadacek F. Secondary metabolites as plant traits: Current assessment and future perspectives. Crit Rev Plant Sci. 2002;21:273–322. [Google Scholar]
- Halliwell B. Catalytic decomposition of cellulose under biological conditions. Biochem J. 1965;95:35–40. doi: 10.1042/bj0950035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol. 2006;141:312–322. doi: 10.1104/pp.106.077073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JMC, Aruoma OI. The deoxyribose method - a simple test-tube assay for determination of rate constants for reactions of hydroxyl radicals. Anal Biochem. 1987;165:215–219. doi: 10.1016/0003-2697(87)90222-3. [DOI] [PubMed] [Google Scholar]
- Hartman H. Photosynthesis and the origin of life. Orig Life Evol Biosph. 1996;28:515–521. doi: 10.1023/a:1006548904157. [DOI] [PubMed] [Google Scholar]
- Hartmann T. Principles of plant secondary metabolism. Plant Syst Evol. 1985;150:15–34. [Google Scholar]
- Hartmann T. From waste products to ecochemicals: Fifty years research of plant secondary metabolism. Phytochemistry. 2007;68:2831–2846. doi: 10.1016/j.phytochem.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Hartmann T. The lost origin of chemical ecology in the late 19th century. Proc Natl Acad Sci USA. 2008;105:4541–4546. doi: 10.1073/pnas.0709231105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatier JHB, Gould KS. Foliar anthocyanins as modulators of stress signals. J Theor Biol. 2008;253:625–627. doi: 10.1016/j.jtbi.2008.04.018. [DOI] [PubMed] [Google Scholar]
- Herms DA, Mattson WJ. The dilemma of plants: To grow or defend. Q Rev Biol. 1992;67:283–335. [Google Scholar]
- Howe GA, Jander G. Plant immunity to insect herbivores. Ann Rev Plant Biol. 2008;59:41–66. doi: 10.1146/annurev.arplant.59.032607.092825. [DOI] [PubMed] [Google Scholar]
- Howlett BJ. Secondary metabolite toxins and nutrition of plant pathogenic fungi. Curr Op Plant Biol. 2006;9:371–375. doi: 10.1016/j.pbi.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Hubbell SP. The Unified Neutral Theory of Biodiversity and Biogeography. Princeton University Press; Princeton: 2001. [Google Scholar]
- Hucker HB, Gillette JR, Brodie BB. Cotinine: an oxidation product of nicotine formed by rabbit liver. Nature. 1959;183:47. doi: 10.1038/183047a0. [DOI] [PubMed] [Google Scholar]
- Jarvis BB, Miller JD. Natural products, complexity and evolution. In: Romeo JT, Saunders JA, Barbosa P, editors. Phytochemical Diversity and Redundancy in Ecological Interactions. Plenum Press; New York: 1996. pp. 265–294. [Google Scholar]
- Jensen KA, Houtman CJ, Ryan ZC, Hammel KE. Pathways for extracellular fenton chemistry in the brown rot basidiomycete Gloeophyllum trabeum. Appl Environ Microbiol. 2001;67:2705–2711. doi: 10.1128/AEM.67.6.2705-2711.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jermy T. Evolution of insect-plant relationships - A devils advocate approach. Entomol Exp Appl. 1993;66:3–12. [Google Scholar]
- Jones CG, Firn RD. On the evolution of plant secondary diversity. Philos Trans R Soc Lond Ser B. 1991;333:273–280. [Google Scholar]
- Kang SH, Choi W. Oxidative degradation of organic compounds using zero-valent iron in the presence of natural organic matter serving as an electron shuttle. Environ Sci Technol. 2009;43:878–883. doi: 10.1021/es801705f. [DOI] [PubMed] [Google Scholar]
- Keiser MJ, Setola V, Irwin JJ, Laggner C, Abbas AI, Hufeisen SJ, Jensen NH, Kuijer MB, Matos RC, Tran TB, Whaley R, Glennon RA, Hert J, Thomas KLH, Edwards DD, Shoichet BK, Roth BL. Predicting new molecular targets for known drugs. Nature. 2009;462:175–181. doi: 10.1038/nature08506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kell DB. Iron behaving badly: Inappropriate iron chelation as a major contributor to the aetiology of vascular and other progressive inflammatory and degenerative diseases. BMC Med Genom. 2009;2:2. doi: 10.1186/1755-8794-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes WJ, O’Malley RC, Kim D, Lynn DG. Signaling organogenesis in parasitic angiosperms: Xenognosin generation, perception, and response. J Plant Growth Regul. 2000;19:217–231. doi: 10.1007/s003440000024. [DOI] [PubMed] [Google Scholar]
- Kim HK, Park SK, Zhou JL, Taglialatela G, Chung K, Coggeshall RE, Chung JM. Reactive oxygen species (ROS) play an important role in a rat model of neuropathic pain. Pain. 2004;111:116–124. doi: 10.1016/j.pain.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Knüttel H, Fiedler K. Host-plant-derived variation in ultraviolet wing patterns influences mate selection by male butterflies. J Exper Biol. 2001;204:2447–2459. doi: 10.1242/jeb.204.14.2447. [DOI] [PubMed] [Google Scholar]
- Kuhn J, Pettersson EM, Feld BK, Burse A, Termonia A, Pasteels JM, Boland W. Selective transport systems mediate sequestration of plant glucosides in leaf beetles: A molecular basis for adaptation and evolution. Proc Natl Acad Sci USA. 2004;101:13808–13813. doi: 10.1073/pnas.0402576101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai SK, O’Hanlon DE, Harrold S, Man ST, Wang YY, Cone R, Hanes J. Rapid transport of large polymeric nanoparticles in fresh undiluted human mucus. Proc Natl Acad Sci USA. 2007;104:1482–1487. doi: 10.1073/pnas.0608611104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenheim J. Plant Resins. Timber Press Inc; Portland, OR, USA: 2003. [Google Scholar]
- Langenheim J, Thimann K. Botany: Plant Biology and its Relation to Human Affairs. John Wiley & Sons; New York: 1982. [Google Scholar]
- Laohavisit A, Davies JM. The gas that opens gates: calcium channel activation by ethylene. New Phytol. 2007;174:470–473. doi: 10.1111/j.1469-8137.2007.02081.x. [DOI] [PubMed] [Google Scholar]
- Larson RA. The antioxidants of higher plants. Phytochemistry. 1988;27:969–978. [Google Scholar]
- Larson RA. Naturally Occurring Antioxidants. CRC Press; Boca Raton: 1997. [Google Scholar]
- Linares JF, Gustafsson I, Baquero F, Martinez JL. Antibiotics as intermicrobial signaling agents instead of weapons. Proc Natl Acad Sci USA. 2006;103:19484–19489. doi: 10.1073/pnas.0608949103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Yue Y, Li B, Nie Y, Li W, Wu WH, Ma L. A G protein-coupled receptor is a plasma membrane receptor for the plant hormone abscisic acid. Science. 2007;315:1712–1716. doi: 10.1126/science.1135882. [DOI] [PubMed] [Google Scholar]
- Lopez D, Fischbach MA, Chu F, Losick R, Kolter R. Structurally diverse natural products that cause potassium leakage trigger multicellularity in Bacillus subtilis. Proc Natl Acad Sci USA. 2009;106:280–285. doi: 10.1073/pnas.0810940106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreto F, Velikova V. Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol. 2001;127:1781–1787. [PMC free article] [PubMed] [Google Scholar]
- Maffei ME, Mithofer A, Boland W. Before gene expression: early events in plant-insect interaction. Trends Plant Sci. 2007;12:310–316. doi: 10.1016/j.tplants.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Mahal HS, Kapoor S, Satpati AK, Mukherjee T. Radical scavenging and catalytic activity of metal-phenolic complexes. J Phys Chem B. 2005;109:24197–24202. doi: 10.1021/jp0549430. [DOI] [PubMed] [Google Scholar]
- Marnett LJ. Lipid peroxidation—DNA damage by malondialdehyde. Mut Res. 1999;424:83–95. doi: 10.1016/s0027-5107(99)00010-x. [DOI] [PubMed] [Google Scholar]
- McChesney JD, Venkataraman SK, Henri JT. Plant natural products: Back to the future or into extinction? Phytochemistry. 2007;68:2015–2022. doi: 10.1016/j.phytochem.2007.04.032. [DOI] [PubMed] [Google Scholar]
- McKay CP, Hartman H. Hydrogen peroxide and the evolution of oxygenic photosynthesis. Orig Life Evol Biosph. 1991;21:157–163. doi: 10.1007/BF01809444. [DOI] [PubMed] [Google Scholar]
- Meyer W, Spiteller G. Oxidized phytosterols increase by ageing in photoautotrophic cell cultures of Chenopodium rubrum. Phytochemistry. 1997;45:297–302. [Google Scholar]
- Mlot C. Antibiotics in nature: beyond biological warfare. Science. 2009;324:1637–1639. doi: 10.1126/science.324_1637. [DOI] [PubMed] [Google Scholar]
- Mohrle H, Berlitz J. Oxidation of nicotine and chelating agent by mercury(II)-compounds. Pharmazie. 2008;63:7–13. [PubMed] [Google Scholar]
- Mohrle H, Berlitz J. Enamine trapping with the oxidation of nicotine and analogues. Pharmazie. 2009;64:219–226. [PubMed] [Google Scholar]
- Molisch H. Gustav Fischer; Jena: 1937. Der Einfluß der einen Pflanze auf die andere: Allelopathie. [Google Scholar]
- Morowitz H, Smith E. Energy flow and the organization of life. Complexity. 2007;13:51–59. [Google Scholar]
- Neill SJ, Desikan R, Hancock JT. Nitric oxide signalling in plants. New Phytol. 2003;159:11–35. doi: 10.1046/j.1469-8137.2003.00804.x. [DOI] [PubMed] [Google Scholar]
- Newman DJ, Cragg GM. Natural products as sources of new drugs over the last 25 years. J Nat Prod. 2007;70:461–477. doi: 10.1021/np068054v. [DOI] [PubMed] [Google Scholar]
- Newman DK. From iron oxides to infections. Geobiology. 2008;6:196–200. doi: 10.1111/j.1472-4669.2008.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omura S. Philosophy of new drug discovery. Microbiol Rev. 1986;50:259–279. doi: 10.1128/mr.50.3.259-279.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otaki JM. Physiologically induced color-pattern changes in butterfly wings: Mechanistic and evolutionary implications. J Insect Physiol. 2008;54:1099–1112. doi: 10.1016/j.jinsphys.2008.05.006. [DOI] [PubMed] [Google Scholar]
- Pandey S, Nelson DC, Assmann SM. Two novel GPCR-Type G proteins are abscisic acid receptors in Arabidopsis. Cell. 2009;136:136–148. doi: 10.1016/j.cell.2008.12.026. [DOI] [PubMed] [Google Scholar]
- Pandey S, Chen JG, Jones AM, Assmann SM. G-protein complex mutants are hypersensitive to abscisic acid regulation of germination and postgermination development. Plant Physiol. 2006;141:243–256. doi: 10.1104/pp.106.079038. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Paulot F, Crounse JD, Kjaergaard HG, Kurten A, St Clair JM, Seinfeld JH, Wennberg PO. Unexpected epoxide formation in the gas-phase photooxidation of isoprene. Science. 2009;325:730–733. doi: 10.1126/science.1172910. [DOI] [PubMed] [Google Scholar]
- Pennisi E. Stressed out over a stress hormone. Science. 2009;324:1012–1013. doi: 10.1126/science.324_1012. [DOI] [PubMed] [Google Scholar]
- Potters G, Pasternak TP, Guisez Y, Jansen MAK. Different stresses, similar morphogenic responses: integrating a plethora of pathways. Plant Cell Environ. 2009;32:158–169. doi: 10.1111/j.1365-3040.2008.01908.x. [DOI] [PubMed] [Google Scholar]
- Potters G, Pasternak TP, Guisez Y, Palme KJ, Jansen MAK. Stress-induced morphogenic responses: growing out of trouble? Trends Plant Sci. 2007;12:98–105. doi: 10.1016/j.tplants.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Price-Whelan A, Dietrich LEP, Newman DK. Rethinking ‘secondary’ metabolism: physiological roles for phenazine antibiotics. Nature Chem Biol. 2006;2:71–78. doi: 10.1038/nchembio764. [DOI] [PubMed] [Google Scholar]
- Razem FA, El-Kereamy A, Abrams SR, Hill RD. The RNA-binding protein FCA is an abscisic acid receptor. Nature. 2006;439:290–294. doi: 10.1038/nature04373. [DOI] [PubMed] [Google Scholar]
- Redman RS, Sheehan KB, Stout RG, Rodriguez RJ, Henson JM. Thermotolerance generated by plant/fungal symbiosis. Science. 2002;298:1581. doi: 10.1126/science.1072191. [DOI] [PubMed] [Google Scholar]
- Ridley RG. Malaria - To kill a parasite. Nature. 2003;424:887–889. doi: 10.1038/424887a. [DOI] [PubMed] [Google Scholar]
- Risk JM, Day CL, MacKnight RC. Reevaluation of abscisic acid-binding assays shows that G-protein-coupled receptor2 does not bind abscisic acid. Plant Physiol. 2009;150:6–11. doi: 10.1104/pp.109.135749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez RJ, White JF, Arnold AE, Redman RS. Fungal endophytes: diversity and functional roles. New Phytol. 2009;182:314–330. doi: 10.1111/j.1469-8137.2009.02773.x. [DOI] [PubMed] [Google Scholar]
- Rosso L, Maier MS, Bertoni MD. Trichothecenes production by the Hypocrealean epibiont of Baccharis coridifolia. Plant Biol. 2000;2:684–686. doi: 10.1055/s-2002-23134. [DOI] [PubMed] [Google Scholar]
- Rubinstein B. Similarities between plants and animals for avoiding predation and disease. Physiol Zool. 1992;65:473–492. [Google Scholar]
- Schardl CL, Panaccione DG, Tudzynski P. Ergot alkaloids - biology and molecular biology. In: Cordell GA, editor. The Alkaloids: Chemistry and Biology. Academic Press; London: 2006. pp. 45–86. [DOI] [PubMed] [Google Scholar]
- Schoonhoven LM, van Loon JJA. An inventory of taste in caterpillars: Each species its own key. Acta Zool Acad Sci Hung. 2002;48:215–263. [Google Scholar]
- Schwechheimer C. Understanding gibberellic acid signaling-are we there yet? Curr Op Plant Biol. 2008;11:9–15. doi: 10.1016/j.pbi.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Selmar D. Increased understanding of metabolism of secondary plant products frequently generates sketchy and incorrect statements concerning their evolution and function. Plant Biol. 2009;11:259–261. doi: 10.1111/j.1438-8677.2009.00190.x. [DOI] [PubMed] [Google Scholar]
- Serfas MS, Carroll SB. Pharmacologic approaches to butterfly wing patterning: Sulfated polysaccharides mimic or antagonize cold shock and alter the interpretation of gradients of positional information. Developm Biol. 2005;287:416–424. doi: 10.1016/j.ydbio.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Sevanian A, Peterson AR. The cytotoxic and mutagenic properties of cholesterol oxidation products. Food Chem Toxicol. 1986;24:1103–1110. doi: 10.1016/0278-6915(86)90295-4. [DOI] [PubMed] [Google Scholar]
- Shank EA, Kolter R. New developments in microbial interspecies signaling. Curr Opin Microbiol. 2009;12:205–214. doi: 10.1016/j.mib.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheard LB, Zheng N. Plant biology: Signal advance for abscisic acid. Nature. 2009;462:575–576. doi: 10.1038/462575a. [DOI] [PubMed] [Google Scholar]
- Smirnoff N. Ascorbate, tocopherol and carotenoids: metabolism, pathway engineering and functions. In: Smirnoff N, editor. Antioxidants and Reactive Oxygen Species in Plants. Blackwell Publishing Inc; Oxford: 2005. pp. 53–86. [Google Scholar]
- Son TG, Camandola S, Mattson MP. Hormetic dietary phytochemicals. Neuromol Med. 2008;10:236–246. doi: 10.1007/s12017-008-8037-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southam CM, Ehrlich J. Effects of extract of western red-cedar heartwood on certain wood-decaying fungi in culture. Phytopathology. 1943;33:517–524. [Google Scholar]
- Stamp N. Out of the quagmire of plant defense hypotheses. Q Rev Biol. 2003;78:23–55. doi: 10.1086/367580. [DOI] [PubMed] [Google Scholar]
- Stebbing ARD. Hormesis - The stimulation of growth by low-levels of inhibitors. Sci Total Environ. 1982;22:213–234. doi: 10.1016/0048-9697(82)90066-3. [DOI] [PubMed] [Google Scholar]
- Stevenson FJ. Humus Chemistry Genesis, Composition, Reaction. Wiley; New York: 1994. [Google Scholar]
- Sudheer AR, Muthukumaran S, Devipriya N, Devaraj H, Menon VP. Influence of ferulic acid on nicotine-induced lipid peroxidation, DNA damage and inflammation in experimental rats as compared to N-acetylcysteine. Toxicology. 2008;243:317–329. doi: 10.1016/j.tox.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Suzuki MR, Hunt CG, Houtman CJ, Dalebroux ZD, Hammel KE. Fungal hydroquinones contribute to brown rot of wood. Environ Microbiol. 2006;8:2214–2223. doi: 10.1111/j.1462-2920.2006.01160.x. [DOI] [PubMed] [Google Scholar]
- Swan EP, Jiang KS, Gardner JAF. Lignans of Thuja plicata and the sapwood-heartwood transformation. Phytochemistry. 1969;8:345–351. [Google Scholar]
- Taga ME, Bassler BL. Chemical communication among bacteria. Proc Natl Acad Sci USA. 2003;100:14549–14554. doi: 10.1073/pnas.1934514100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor EC, Boyer NE. Pyridine-1-oxides 4. Nicotine-1-oxide, Nicotine-1’-oxide, and nicotine-1,1’-dioxide1,2. J Org Chem. 1959;24:275–277. [Google Scholar]
- Tfelt-Hansen P, Saxena PR, Dahlof C, Pascual J, Lainez M, Henry P, Diener HC, Schoenen J, Ferrari MD, Goadsby PJ. Ergotamine in the acute treatment of migraine - A review and European consensus. Brain. 2000;123:9–18. doi: 10.1093/brain/123.1.9. [DOI] [PubMed] [Google Scholar]
- Tilman D. Resource Competiton and Community Structure. Princetown University Press; Princetown: 1982. [Google Scholar]
- Triantaphylidès C, Havaux M. Singlet oxygen in plants: production, detoxification and signaling. Trends Plant Sci. 2009;14:219–228. doi: 10.1016/j.tplants.2009.01.008. [DOI] [PubMed] [Google Scholar]
- Ueguchi-Tanaka M, Ashikari M, Nakajima M, Itoh H, Katoh E, Kobayashi M, Chow TY, Hsing YIC, Kitano H, Yamaguchi I, Matsuoka M. GIBBERELLIN INSENSITIVE DWARF1 encodes a soluble receptor for gibberellin. Nature. 2005;437:693–698. doi: 10.1038/nature04028. [DOI] [PubMed] [Google Scholar]
- Valenzuela R, Contreras D, Oviedo C, Freer J, Rodríguez J. Copper catechol-driven Fenton reactions and their potential role in wood degradation. Int Biodeter Biodegrad. 2008;61:345–350. [Google Scholar]
- Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- van Dam NM. Belowground herbivory and plant defenses. Annu Rev Ecol Evol Syst. 2009;40:373–391. [Google Scholar]
- Varanini Z, Pinton R. Direct versus indirect effects od soil humic substances in plant growth and nutrition. In: Pinton R, Varanini Z, Nannipieri P, editors. The Rhizosphere. Marcel Dekker Inc; New York: 2001. pp. 141–157. [Google Scholar]
- Villalba JJ, Provenza FD. Learning and dietary choice in herbivores. Rangel Ecol Manag. 2009;62:399–406. [Google Scholar]
- Vreeburg AM, Fry SC. Reactive oxygen species in cell walls. In: Smirnoff N, editor. Antioxidants and Reactive Oxygen Species in Plants. Blackwell Publishing; Oxfork, UK: 2005. pp. 215–249. [Google Scholar]
- Wada E, Kisaki T, Saito K. Autooxidation of nicotine. Arch Biochem Biophys. 1959;79:124–130. [Google Scholar]
- Wang Y, Newman DK. Redox reactions of phenazine antibiotics with ferric (hydr)oxides and molecular oxygen. Environ Sci Technol. 2008;42:2380–2386. doi: 10.1021/es702290a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle DA, Bardgett RD. Human-induced changes in large herbivorous mammal density: the consequences for decomposers. Front Ecol Environ. 2004;2:145–153. [Google Scholar]
- Wardle DA, Bardgett RD, Klironomos JN, Setala H, van der Putten WH, Wall DH. Ecological linkages between aboveground and belowground biota. Science. 2004;304:1629–1633. doi: 10.1126/science.1094875. [DOI] [PubMed] [Google Scholar]
- Williams RJP, Fraústo da Silva JJR. The Chemistry of Evolution. Elsevier; Amsterdam: 2006. [Google Scholar]
- Wilson D. Endophyte - The evolution of a term, and clarification of its use and definition Oikos. 1995;73:274–276. [Google Scholar]
- Wojtaszek P. Oxidative burst: An early plant response to pathogen infection. Biochem J. 1997;322:681–692. doi: 10.1042/bj3220681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wormuth D, Heiber I, Shaikali J, Kandlbinder A, Baier M, Dietz KJ. Redox regulation and antioxidative defence in Arabidopsis leaves viewed from a systems biology perspective. J Biotechnol. 2006;129:229–248. doi: 10.1016/j.jbiotec.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Zagrobelny M, Bak S, Moller BL. Cyanogenesis in plants and arthropods. Phytochemistry. 2008;69:1457–1468. doi: 10.1016/j.phytochem.2008.02.019. [DOI] [PubMed] [Google Scholar]