Abstract
Background
Over one third of ischemic strokes occur in the posterior circulation, a leading cause of which is atherosclerotic vertebrobasilar disease (VBD). Symptomatic VBD carries a high annual recurrent stroke risk, averaging 10–15% per year. Endovascular angioplasty and stenting are increasingly employed, but carry risks, and the benefit remains unproven. Determining stroke predictors in this population is critical to identifying high risk patients for future trials of intervention. Preliminary studies indicate that stroke risk in VBD is strongly related to hemodynamic compromise, which can be measured noninvasively using quantitative magnetic resonance angiography (QMRA).
Methods/Study Design
The VERiTAS Study, a prospective multi-center NIH funded observational study of symptomatic vertebrobasilar stenosis (≥ 50%) or occlusion, is designed to test the hypothesis that patients demonstrating compromised blood flow as assessed by QMRA are at higher stroke risk. The study will recruit 80 patients at 6 sites in North America over four years. Upon enrollment, subjects will undergo hemodynamic assessment with blinded QMRA to assess large vessel flow in the vertebrobasilar territory, and be prospectively designated as compromised or normal flow. Patients will be reimaged with QMRA at 6, 12 and 24 months, and followed for 12 to 24 months for the primary endpoint of stroke in the vertebrobasilar territory.
Conclusion
VERiTAS is the first prospective study of hemodynamics and stroke risk in the posterior circulation. The results may impact the selection criteria for interventional candidates and also define a low risk population in whom the risks of invasive interventions would be unnecessary.
Keywords: stroke, vertebrobasilar ischemia, magnetic resonance imaging, quantitative magnetic resonance angiography, blood flow
INTRODUCTION
Posterior circulation strokes account for up to 30–40% of all ischemic events (1), an important etiology of which is atherosclerotic vertebrobasilar disease (VBD). Symptomatic VBD carries a high annual risk of recurrent stroke, averaging 10–15% per year despite medical therapy. Vertebrobasilar stroke is particularly prone to devastating consequences due to the ‘eloquence’ of the regional brain tissue (2), and is associated with high rates of death and disability.
Vertebrobasilar Disease and Stroke
Large vessel atherosclerotic VBD accounts for approximately one third of ischemic events in the posterior circulation territory (3, 4). An important stroke mechanism in vertebrobasilar atherostenosis is regional hypoperfusion (5). Furthermore, both embolic and flow processes can synergize to increase stroke risk; a proposed mechanism is the reduced wash-out of emboli from the distal circulation in hypoperfused regions (6, 7). Low flow may also promote local thrombus formation at the site of disease, with resultant stroke (3). In the setting of hypoperfusion, however, posterior circulation collateral channels such as the posterior communicating arteries may maintain adequate distal flow (8). The existence and extent of these compensatory blood flow pathways may, therefore, influence the risk of stroke. Hemodynamic impairment has been linked to stroke in the anterior circulation: in carotid occlusion, severe hemodynamic compromise measured by positron emission tomography (PET) and other perfusion modalities predicts subsequent stroke risk (7, 9). The same role for hemodynamic impairment may be relevant to the posterior circulation, but has not been well examined to date.
Several studies have estimated the recurrent stroke risk associated with vertebrobasilar stenosis (50–99%) to be 10–15% per year (10–13). Current medical therapies for cerebrovascular occlusive disease are directed primarily at curtailing the risk of thrombo-embolism (antithrombotic agents) and stabilizing plaque (statins, antihypertensives), but do not address an underlying low flow state. Recently, therapies aimed at augmenting blood flow have emerged, primarily in the endovascular arena. Endovascular angioplasty and stenting offer the potential to restore vessel flow and improve hemodynamic compromise. However, these interventions carry significant procedural risk, with periprocedural and one year stroke rates ranging from 8% up to 26% in recent series (14–18). The benefit from these interventions has not yet been established. Therefore, identification of a high risk subgroup, owing to hemodynamic impairment, would be important for future trials of intervention.
Hemodynamic Assessment in the Posterior Circulation
Various imaging techniques exist for assessing cerebrovascular hemodynamic compromise, including single photon emission computed tomography (SPECT,) PET, Xenon computed tomography (CT), perfusion magnetic resonance (MR) and CT imaging, and transcranial Doppler (TCD). These modalities primarily assess cerebral tissue perfusion, and have proven useful in evaluating anterior circulation flow compromise (7, 9). However, factors such as inadequate spatial resolution and bony skull base artifacts have rendered them less useful in the posterior circulation, necessitating an alternative strategy. Measurement of large vessel flow in affected and major collateral vessels is one such strategy, and can be obtained non-invasively using the technique of quantitative magnetic resonance angiography (QMRA). QMRA involves anatomic visualization of cerebral vessels using time of flight (TOF) imaging, and direct measurement of flow velocity and volume flow rate using phase contrast (PC) imaging. Flow measurement with this technique has demonstrated accuracy in both in vitro and in vivo settings (19, 20). Commercially available software, Noninvasive Optimal Vessel Analysis (NOVA, VasSol, Inc.) uses a standardized TOF and PC MRA protocol to perform cerebrovascular QMRA (21–24). In comparison to QMRA, MR perfusion imaging provides tissue level perfusion data from the brain region of interest. Tissue perfusion has not been well studied in the posterior circulation territory, particularly the brainstem and cerebellum, but may provide important correlative and predictive data for the large vessel conductance flow measured by QMRA. The two imaging modalities can be readily combined to provide both large vessel and tissue level hemodynamic assessment in VBD.
Rationale for the VERiTAS study
Retrospective data suggest that QMRA flow measurements have predictive value for subsequent stroke (22): 48 patients with symptomatic VBD (>50% stenosis or occlusion, extracranial or intracranial vertebral or basilar artery) were evaluated based on an algorithm defining flow compromise as >20% reduction below normative lower limits of posterior circulation vessel-specific flow. One third of patients were characterized as ‘low’ flow based upon the algorithm, and demonstrated a significantly worse stroke free survival of 71% (95% CI 23–92%) at 24 months compared to 100% stroke free survival in the ‘normal’ flow group. Flow status remained an independent predictor of recurrent stroke after adjusting for stenosis severity and disease location. These data strongly suggest that stroke risk in VBD is determined by the extent of intracranial blood flow compromise, as assessed by QMRA.
Although compelling, retrospective studies have limitations, including the potential for selection bias, recall bias, and ascertainment bias. These can be improved upon with a well designed and conducted prospective study. Furthermore it is important to establish that flow measurement QMRA technology is generalizable to a broad range of sites. Therefore, the Vertebrobasilar Flow Evaluation and Risk of Transient Ischemic Attack and Stroke (VERiTAS) study is designed as a prospective blinded multi-center observational study to provide high quality evidence assessing flow status as a stroke predictor in patients with symptomatic VBD.
STUDY DESIGN
Overview
VERiTAS is a multi-center prospective cohort study. Eligible patients undergo MR imaging to assess their cerebrovascular hemodynamic status, the results of which are interpreted centrally and kept blinded from the treating clinicians. The patients are prospectively followed for a minimum of one year on current standard medical regimen including vascular risk factor modification, statins and antithrombotic therapy, and evaluated for recurrent ischemic events.
Study hypotheses
The primary hypothesis is that among patients with symptomatic VBD, those with distal blood flow compromise are at higher risk of subsequent posterior circulation stroke than those with normal flow. Secondary aims of the study are to determine the correlation between large vessel flow and tissue level perfusion in the posterior circulation, determine other predictive factors for stroke in the target population, evaluate the hemodynamic effects of varying degrees of vertebrobasilar stenosis, examine changes in hemodynamic status of patients on medical therapy over time, and determine the utility of QMRA as a non-invasive screening and monitoring tool for VBD.
Eligibility Criteria
Patients presenting with vertebrobasilar distribution TIA or stroke are the source group. Symptoms and signs indicative of vertebrobasilar ischemia are defined as outlined by the Special Report from the National Institute of Neurological Disorders and Stroke (25). Diagnostic evaluation is performed at the discretion of the treating physicians, but commonly includes: MRA, CT angiography (CTA), TCD or conventional angiography. Patients with evidence of ≥ 50% extracranial or intracranial vertebrobasilar stenosis or occlusion are screened for final eligibility based upon the inclusion/exclusion criteria (Table). Final eligibility requires either CTA or conventional angiographic confirmation; if not performed as standard of care, subjects are asked to consent to craniocervical CTA or two vessel vertebral catheter angiography as part of the research protocol.
TABLE.
Inclusion Criteria:
|
Exclusion Criteria:
|
Note: Renal dysfunction exclusionary if it precludes angiography. Renal dysfunction is not exclusionary if it precludes gadolinium administration; such patients are enrolled but the MR perfusion portion of the study imaging protocol is omitted.
Baseline Study Assessments
Clinical evaluation
The nature and frequency of cerebral ischemic events is recorded Medications at the time of enrollment and specific data regarding vascular risk factors are gathered including age, gender, race, hypertension, diabetes mellitus, lipid disorder, coronary disease, smoking, alcohol consumption, and parental death from stroke. A physical examination and neurological evaluation is performed by a study physician. The National Institute of Health Stroke Scale (NIHSS) is administered, and functional status assessed using the Barthel Index, modified Rankin Scales and modified Glasgow Outcome Score. Available laboratory data including hemoglobin, serum creatinine, lipid profile, homocysteine, high sensitivity C-reactive protein, hemoglobin-A1c, and casual or fasting plasma glucose is collected, in addition to results of cerebral imaging studies and ancillary testing such as echocardiography and electrocardiography.
MR imaging
A standardized MR imaging protocol consisting of QMRA and MR perfusion is performed within 7–14 days of enrollment. The QMRA portion of the study is performed using NOVA (Noninvasive Optimal Vessel Analysis, VasSol, Inc.) software, and MR perfusion is performed using dynamic susceptibility contrast (DSC) technique. Imaging consists of axial 3D TOF imaging of the cranial vasculature, from which a 3-D image of the vessels is created. Standard sites for flow measurement are chosen from this image in 15 vessels (Figure 1); the locations have been pre-specified for consistency between scans and between patients. To minimize potential effects of turbulent flow, the site for flow measurement is placed at the furthest distance from any existing stenosis within the designated segment. Quantitative flow measurements are then obtained in the chosen vessels using PC MR technique (24). Finally, DSC MR perfusion scanning is performed using the magnetic susceptibility contrast bolus tracking method with gradient echo, echo-planar imaging during first passage through the brain of intravenously administered gadolinium contrast (Omniscan, 0.1mmol/kg, via 20 gauge angiocath in antecubital vein using mechanical injector). Equivalent protocols for each MR vendor (GE, Siemens, Phillips) have been specified, with all imaging performed on 3 Tesla machines. The QMRA portion of the study is remotely supervised at the time of imaging by a certified NOVA technician to ensure correct parameters and vessel placement for flow measurement, and the data is automatically transferred via secure internet based transfer to the clinical coordinating center (CCC) at University of Illinois at Chicago (UIC) for central review. The MR perfusion portion of the study is similarly sent for review to the CCC. The imaging results remain blinded to the patient and all participating site personnel. To ensure blinding, the QMRA imaging is permanently removed from the local site workstation after central transfer.
Figure 1.
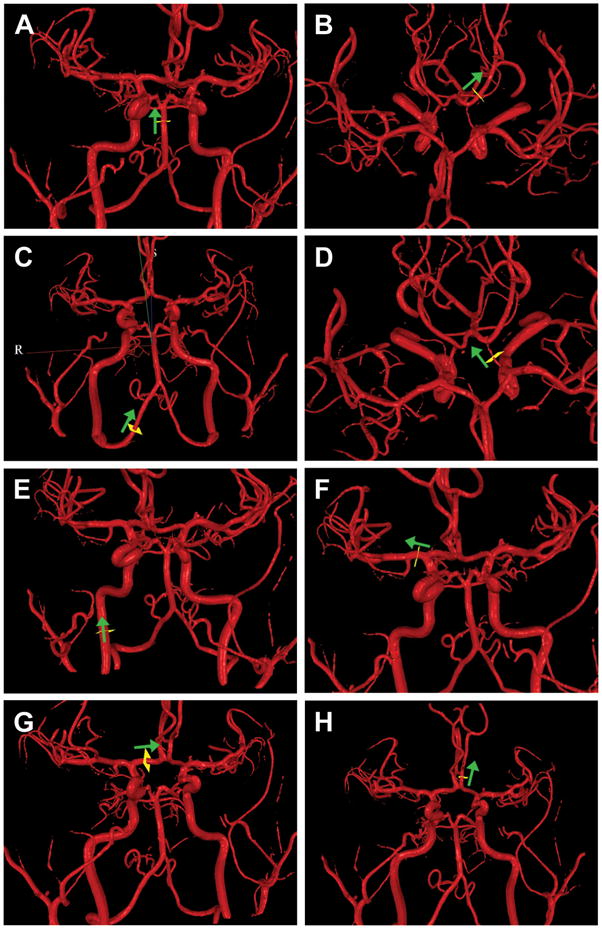
QMRA 3- dimensional surface rendering of the cerebral vasculature based on time-of-flight MRA created by NOVA software. The standardized locations for placing the scan line (yellow square) for phase contrast imaging to obtain flow measurements are indicated. Arrow indicated direction of flow.
A. Basilar artery – scan plane on straight segment proximal to SCA (distal to stenosis if present)
B. Posterior cerebral arteries (left depicted) – scan plane on straight portion of the P2 segment, distal to the posterior communicating artery origin.
C. Vertebral arteries (right depicted) – scan plane on straight segment of vertebral artery proximal to posterior inferior cerebellar artery.
D. Posterior communicating artery (left depicted) – scan plane on straight mid segment
E. Internal carotid artery (right depicted) – scan plane on straight portion below the petrous segment.
F. Middle cerebral artery (right depicted) – scan plane on straight portion of M1 segment.
G. Anterior cerebral artery, A1 segment (right depicted) – scan plane on straight mid segment of A1.
H. Anterior cerebral artery, A2 segment (left depicted) – scan plane on straight proximal segment of A2 prior to branch points.
Follow-up Study Assessments and Schedule of Visits
Risk factor modifications
Treating physicians are encouraged to adhere to published guidelines and employ antithrombotic agents, antihypertensive agents, statins and vascular risk factor control strategies as per published guidelines (26–29). Risk factor modification is managed by the study physician in conjunction with the patient’s primary care physician or neurologist.
Clinical follow-up evaluations
The patient is contacted monthly by telephone until 12 months for determination of new events using a standardized telephone questionnaire. In-person follow-up is performed at 6 and 12 months at a minimum, and every 6 months thereafter up until completion of the study or 2 years maximum. A detailed neurological evaluation including the standardized assessments (such as NIHSS, Barthel Index etc.) is performed by the blinded study physician, and interval occurrence of any cerebral ischemic symptoms/signs are noted.
Imaging follow-up
The MR imaging protocol is repeated at 6 and 12 months, and at 24 months if prior to the completion of the study. Results are centrally reviewed and remain blinded from the participating center.
Endpoints
The primary endpoint is fatal and nonfatal ischemic stroke in the vertebrobasilar territory within 12 months. Ischemic stroke is defined as new neurological symptoms or signs lasting at least 24 hours or lasting < 24 hours but associated with new infarct on CT or MRI. Symptoms and signs indicative of vertebrobasilar ischemia are defined as outlined previously (25). Secondary endpoints are: TIA and ischemic stroke in the vertebrobasilar territory; ischemic stroke in any vascular territory; all ischemic stroke and vascular death (death from a vascular etiology or sudden death not explained by a known nonvascular process); aggregate of any ischemic stroke, myocardial infarction and vascular death; all stroke (ischemic and hemorrhagic); all death; neurological impairment as determined by the NIHSS; neurological disability as determined by the modified Barthel Index; and handicap as determined by the modified Rankin Scale.
When a potential endpoint is identified, study personnel arrange for the patient to be evaluated by the blinded study physician within 72 hours. Initial endpoint determinations are made by the participating site but the relevant information is submitted to the CCC for final adjudication by an independent panel of two vascular neurologists, blinded to the patient’s study protocol imaging results. If the opinions of the two adjudicators differ, a third adjudicator is consulted for majority opinion.
Data Management
All clinical data is submitted to the Data Management Center (DMC) at UIC via secure web-based data entry system. All study imaging data is centrally reviewed to confirm adequate imaging quality and completeness. Data from the MR perfusion portion of the imaging is analyzed for calculation of regional perfusion parameters (tissue transit time, time to arrival, and cerebral blood volume). Initial determination of vertebral or basilar stenosis for eligibility is made locally at the participating center using the WASID technique (30). Relevant angiographic images are sent to the CCC, and reviewed by a blinded neuroradiologist for final determination of degree of stenosis.
Sample Size Estimates
Event rates were estimated from published preliminary data (22), which indicated a 71% one year stroke free survival in symptomatic VBD patients designated as low flow, translating into an annual event rate of 29%. There was a 0% stroke and 4% TIA rate in the normal flow group; consequently, a conservative estimate of 2% stroke event rate was used for this group. The data also indicated that recruitment into the normal vs. low flow group would occur at a 2:1 ratio. Sample sizes were calculated for a range of possible event rates in the low flow group from 18% to 28%, assuming a 2:1 proportion of normal: low flow. 10% attrition rate was included. Based upon these assumptions, a sample size of 80 patients achieves 80% power in detecting a significant difference between an annual event rate of 22% in the low flow group and 2% in the normal flow group, and at least 50% power for detecting a difference even if the event rate in the low flow group is as low as 13%.
Statistical Analysis
Analysis of the primary endpoint (ischemic stroke in the vertebrobasilar territory) will be a time-to-event comparison using the log-rank test between subjects designated as low vs. normal flow, based upon the subject’s initial enrollment QMRA study (using parameters defined in our preliminary studies) (22). Subjects will be censored at the time of any endovascular or surgical revascularization procedures, or at the end of the follow-up period. Exploration for risk factors and adjustment for covariates will be performed with Cox proportional hazards analysis. Post-hoc sensitivity analysis will examine alternative cut-points for designation of low or normal flow status, to determine if an ‘optimized’ flow algorithm can be generated with greater sensitivity/specificity in stroke prediction. Correlation analyses will be performed to assess the linear relationships between perfusion parameters and large vessel flow measurements. The sensitivity analysis performed to examine varying cut-points for large vessel flows will also be performed with integration of the MR perfusion data.
Organization
The organizational elements of VERiTAS Study group are listed in the appendix, and consist of the CCC, DMC, participating sites, and committees (Operations Committees, Advisory committee, Adjudication Committee, and Angiography Review Committee).
CONCLUSION
VERiTAS is the first prospective study of hemodynamics and stroke risk in the posterior circulation. The results may impact the selection criteria and likelihood for success of future clinical trials aimed at assessing the efficacy of endovascular or surgical interventions for VBD. Moreover, defining a low risk population in whom the risks of invasive interventions would be unnecessary would have an equally important impact on VBD management, both from a clinical, and cost, perspective. The data regarding the hemodynamic effects of VBD, and their temporal progression, may also enhance our understanding of the basic pathophysiology and mechanisms of stroke in this morbid disease entity.
Acknowledgments
FUNDING AND DISCLOSURES
Supported by the National Institutes of Health/National Institute of Neurological Disorders and Stroke (NINDS R01 NS 059745), and the Dr. Ralph and Marian Falk Research Trust Foundation. Other research support (no direct funding) provided by GE Healthcare (supplying gadolinium) and VasSol, Inc. (supplying NOVA technology and technical support) Dr Charbel has financial interest in VasSol, Inc.
Appendix A: VERiTAS Study Group: Organizational Structure and Participants as of January 31, 2010
Clinical Coordinating Center
University of Illinois at Chicago
PI: Sepideh Amin-Hanjani, MD
Project Manager: Linda Rose-Finnell, MPA CCRA
NIH/NINDS Program Officer: Tom P. Jacobs, MD
Data Management Center
Center for Stroke Research, University of Illinois at Chicago
Director: DeJuran Richardson, PhD
Biostatistician: Hui Xie, PhD
Database Administrator: Vic Simkus
Participating sites (sites that are open for enrollment as of January 31, 2010, in descending order of number of enrollees)
University of Illinois at Chicago
Site PI: Sepideh Amin-Hanjani, MD
Site MR Team: Keith Thulborn, MD PhD, Michael P Flannery, Hagai Ganin
Study Physicians: Rebecca Grysecwicz, MD, Aslam Khaja, MD, Laura Pedelty, MD, Fernando Testai, MD
Coordinator: Karriem Watson, MD, Nada Mlinarevich RN
Washington University, St. Louis
Site PI: Gregory J. Zipfel, MD
Site MR Team: Katie Vo, MD, Glenn Foster
Study Physicians: Andria Ford, MD
Coordinator: Abbie Bradley, RN, BSN, MSW
University of California, Los Angeles
Site PI: David S. Liebeskind, MD
Site MR Team: Jeffrey Alger, PhD, Sergio Godinez
Study Physicians/Co-Investigators: Jeffrey Saver, MD, Latisha Ali, MD, Doojin
Kim, MD, Radoslav Raychev, MD, Sarah Song, MD, Bruce Ovbiagele, MD
Coordinator: Hannah Smith, BS
Columbia University, New York
Site PI: Mitchell S.V. Elkind, MD
Site MR Team: Joy Hirsch, PhD, Stephen Dashnaw
Study Physicians: Philip Meyers, MD
Coordinator: Edwina McNeill-Simaan, BS
University of Toronto -Toronto Western Hospital, Toronto
Site PI: Frank L. Silver, MD
Site MR Team: David Mikulis, MD, Jorn Fierstra, Eugen Hlasny
Study Physicians: Leanne K. Casaubon, MD, Mervyn Vergouwen, MD, J.C.
Martin del Campo, MD, Cheryl S. Jaigobin, MD
Coordinator: Cherissa Astorga, RN
Mercy Hospital and Medical Center, Chicago
Site PI: Jeffrey Kramer, MD
Study Physician: Susan Vaughan, MD
Coordinator: Laura Owens, RN
Committees and Panels
Operations Committee
Sepideh Amin-Hanjani, MD, FACS (Chair)
Fady T. Charbel, MD, FACS
Philip B. Gorelick, MD, MPH, FACP
Dilip K. Pandey, MD, PhD
Sean D. Ruland, DO
Keith R. Thulborn, MD, PhD
Advisory Committee
Colin P. Derdeyn, MD (Chair)
Louis R. Caplan, MD
Adjudication Committee
Scott E. Kasner, MD (Chair)
Brett Kissela, MD
Tanya N. Turan, MD
Angiography Committee
Y. Pierre Gobin, MD
References
- 1.Whisnant JP, Cartlidge NE, Elveback LR. Carotid and vertebral-basilar transient ischemic attacks: effect of anticoagulants, hypertension, and cardiac disorders on survival and stroke occurrence--a population study. Ann Neurol. 1978;3(2):107–15. doi: 10.1002/ana.410030204. [DOI] [PubMed] [Google Scholar]
- 2.Caplan LR. Posterior circulation disease: clinical findings, diagnosis, and management. Cambridge, Mass: Blackwell Science; 1996. [Google Scholar]
- 3.Caplan LR. Vertebrobasilar disease. Adv Neurol. 2003;92:131–40. [PubMed] [Google Scholar]
- 4.Caplan LR, Wityk RJ, Glass TA, et al. New England Medical Center Posterior Circulation registry. Ann Neurol. 2004;56(3):389–98. doi: 10.1002/ana.20204. [DOI] [PubMed] [Google Scholar]
- 5.Voetsch B, DeWitt LD, Pessin MS, Caplan LR. Basilar artery occlusive disease in the New England Medical Center Posterior Circulation Registry. Arch Neurol. 2004;61(4):496–504. doi: 10.1001/archneur.61.4.496. [DOI] [PubMed] [Google Scholar]
- 6.Caplan LR, Hennerici M. Impaired clearance of emboli (washout) is an important link between hypoperfusion, embolism, and ischemic stroke. Arch Neurol. 1998;55(11):1475–82. doi: 10.1001/archneur.55.11.1475. [DOI] [PubMed] [Google Scholar]
- 7.Grubb RL, Jr, Derdeyn CP, Fritsch SM, et al. Importance of hemodynamic factors in the prognosis of symptomatic carotid occlusion [see comment] Jama. 1998;280(12):1055–60. doi: 10.1001/jama.280.12.1055. [DOI] [PubMed] [Google Scholar]
- 8.Labauge R, Pages M, Blard JM. Long term survival after basilar artery occlusion. 4 cases. Rev Neurol (Paris) 1989;145(11):789–94. [PubMed] [Google Scholar]
- 9.Ogasawara K, Ogawa A, Yoshimoto T. Cerebrovascular reactivity to acetazolamide and outcome in patients with symptomatic internal carotid or middle cerebral artery occlusion: a xenon-133 single-photon emission computed tomography study. Stroke. 2002;33(7):1857–62. doi: 10.1161/01.str.0000019511.81583.a8. [DOI] [PubMed] [Google Scholar]
- 10.Anonymous Prognosis of patients with symptomatic vertebral or basilar artery stenosis. The Warfarin-Aspirin Symptomatic Intracranial Disease (WASID) Study Group. Stroke. 1998;29(7):1389–92. doi: 10.1161/01.str.29.7.1389. [DOI] [PubMed] [Google Scholar]
- 11.Moufarrij NA, Little JR, Furlan AJ, Leatherman JR, Williams GW. Basilar and distal vertebral artery stenosis: long-term follow-up. Stroke. 1986;17(5):938–42. doi: 10.1161/01.str.17.5.938. [DOI] [PubMed] [Google Scholar]
- 12.Qureshi AI, Ziai WC, Yahia AM, et al. Stroke-free survival and its determinants in patients with symptomatic vertebrobasilar stenosis: a multicenter study. Neurosurgery. 2003;52(5):1033–9. discussion 9–40. [PubMed] [Google Scholar]
- 13.Chimowitz MI, Kokkinos J, Strong J, et al. The Warfarin-Aspirin Symptomatic Intracranial Disease Study. Neurology. 1995;45(8):1488–93. doi: 10.1212/wnl.45.8.1488. [DOI] [PubMed] [Google Scholar]
- 14.Barakate MS, Snook KL, Harrington TJ, Sorby W, Pik J, Morgan MK. Angioplasty and stenting in the posterior cerebral circulation. J Endovasc Ther. 2001;8(6):558–65. doi: 10.1177/152660280100800604. [DOI] [PubMed] [Google Scholar]
- 15.Gomez CR, Misra VK, Liu MW, et al. Elective stenting of symptomatic basilar artery stenosis. Stroke. 2000;31(1):95–9. doi: 10.1161/01.str.31.1.95. [DOI] [PubMed] [Google Scholar]
- 16.Nahser HC, Henkes H, Weber W, Berg-Dammer E, Yousry TA, Kuhne D. Intracranial vertebrobasilar stenosis: angioplasty and follow-up. AJNR Am J Neuroradiol. 2000;21(7):1293–301. [PMC free article] [PubMed] [Google Scholar]
- 17.Fiorella D, Chow MM, Anderson M, Woo H, Rasmussen PA, Masaryk TJ. A 7-year experience with balloon-mounted coronary stents for the treatment of symptomatic vertebrobasilar intracranial atheromatous disease. Neurosurgery. 2007;61(2):236–42. doi: 10.1227/01.NEU.0000255521.42579.31. discussion 42–3. [DOI] [PubMed] [Google Scholar]
- 18.Anonymous Stenting of Symptomatic Atherosclerotic Lesions in the Vertebral or Intracranial Arteries (SSYLVIA): study results. Stroke. 2004;35(6):1388–92. doi: 10.1161/01.STR.0000128708.86762.d6. [DOI] [PubMed] [Google Scholar]
- 19.Meier D, Maier S, Bosiger P. Quantitative flow measurements on phantoms and on blood vessels with MR. Magn Reson Med. 1988;8(1):25–34. doi: 10.1002/mrm.1910080104. [DOI] [PubMed] [Google Scholar]
- 20.Hofman MB, Visser FC, van Rossum AC, Vink QM, Sprenger M, Westerhof N. In vivo validation of magnetic resonance blood volume flow measurements with limited spatial resolution in small vessels. Magn Reson Med. 1995;33(6):778–84. doi: 10.1002/mrm.1910330606. [DOI] [PubMed] [Google Scholar]
- 21.Amin-Hanjani S, Shin JH, Zhao M, Du X, Charbel FT. Evaluation of extracranial-intracranial bypass using quantitative magnetic resonance angiography. J Neurosurg. 2007;106(2):291–8. doi: 10.3171/jns.2007.106.2.291. [DOI] [PubMed] [Google Scholar]
- 22.Amin-Hanjani S, Du X, Zhao M, Walsh K, Malisch TW, Charbel FT. Use of quantitative magnetic resonance angiography to stratify stroke risk in symptomatic vertebrobasilar disease. Stroke. 2005;36(6):1140–5. doi: 10.1161/01.STR.0000166195.63276.7c. [DOI] [PubMed] [Google Scholar]
- 23.Zhao M, Amin-Hanjani S, Ruland S, Curcio AP, Ostergren L, Charbel FT. Regional cerebral blood flow using quantitative MR angiography. AJNR Am J Neuroradiol. 2007;28(8):1470–3. doi: 10.3174/ajnr.A0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao M, Charbel FT, Alperin N, Loth F, Clark ME. Improved phase-contrast flow quantification by three-dimensional vessel localization. Magn Reson Imaging. 2000;18(6):697–706. doi: 10.1016/s0730-725x(00)00157-0. [DOI] [PubMed] [Google Scholar]
- 25.Special report from the National Institute of Neurological Disorders and Stroke. Classification of cerebrovascular diseases III. Stroke. 1990;21(4):637–76. doi: 10.1161/01.str.21.4.637. [DOI] [PubMed] [Google Scholar]
- 26.Anonymous Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–421. [PubMed] [Google Scholar]
- 27.Adams RJ, Albers G, Alberts MJ, et al. Update to the AHA/ASA recommendations for the prevention of stroke in patients with stroke and transient ischemic attack. Stroke. 2008;39(5):1647–52. doi: 10.1161/STROKEAHA.107.189063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sacco RL, Adams R, Albers G, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co-sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline. Stroke. 2006;37(2):577–617. doi: 10.1161/01.STR.0000199147.30016.74. [DOI] [PubMed] [Google Scholar]
- 29.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42(6):1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 30.Samuels OB, Joseph GJ, Lynn MJ, Smith HA, Chimowitz MI. A standardized method for measuring intracranial arterial stenosis. AJNR Am J Neuroradiol. 2000;21(4):643–6. [PMC free article] [PubMed] [Google Scholar]


