Abstract
Wistar Kyoto (WKY) rats, an animal model of anxiety vulnerability, acquire lever-press avoidance faster than outbred Sprague Dawley (SD) rats. Faster avoidance acquisition may reflect an inherent ability to acquire cue-outcome associations, response-outcome associations or both. To evaluate cue-outcome learning, acquisition of classically conditioned eyeblink response was compared in SD and WKY rats using a delay-type paradigm (500-ms conditioned stimulus (CS) coterminating with a 10-ms unconditional stimulus (US)). WKY rats demonstrated enhanced classical conditioning, with both faster acquisition and greater asymptotic performance in delay-type training than SD rats. To evaluate response-outcome learning, separate SD and WKY rats were given control over US delivery through imposition of an omission contingency into delay-type training (emitting a conditioned response (CR) prevented delivery of the US). The schedule of US delivery derived by these rats became the training regimen for a separate group of SD and WKY rats, yoked within strain. In SD rats, no differences in acquisition were detected between those given control over US delivery and those trained with the same partial reinforcement schedule. Acquisition rates of those WKY rats with control exceeded those trained with a yoked-schedule of US presentation. Collectively, WKY rats exhibit enhanced classical conditioning and sensitivity to schedules of reinforcement compared to outbred SD rats. Anxiety vulnerability, in particular inhibited temperament, may be traced to active processes in the prediction and control of aversive events.
Keywords: anxiety, anxiety vulnerability, temperament, classical conditioning, instrumental sensitivity, negative reinforcement, avoidance
Introduction
Individuals with anxiety disorders demonstrate greater sensitivity to avoidance, that is, they exhibit avoidance to a larger degree and in situations where normal individuals do not [31]. Avoidance is a process; growth or acquisition of avoidance may be an etiological factor in the development of anxiety disorders. This is most evident in post traumatic stress disorder (PTSD), in which the onset of the disorder is traceable to overt events or experiences. For PTSD, avoidance becomes more pervasive over a period of time subsequent to trauma [14,25,34] and the degree of avoidance expression differentiates between those who develop PTSD and those who recover [14,35]. Vulnerability to development anxiety disorders may be traced to enhanced sensitivity to express avoidance in the face of adverse events. Young children and adolescents with trait behavioral inhibition (BI), a risk factor for anxiety disorders typified by withdrawal from social and novel-nonsocial challenges [15], exhibit greater avoidance sensitivity [11].
A similar sensitivity to acquire or express avoidance is observed in Wistar-Kyoto (WKY) rats, an animal model of BI. WKY rats exhibit decreased activity in the open-field and reduced social interactions [39,29,12]. These rats also demonstrate increased physiological [4,38,37,44] and behavioral [44,39] reactivity to stressors. Further, WKY rats demonstrate faster acquisition, higher asymptotic performance, and resistance to extinction of lever-press avoidance compared to outbred Sprague Dawley (SD) rats [50]. These features place WKY rats in sharp contrast to strains bred for either avoidance performance (avoidance performance is inversely related to inhibited temperament [58,6]) or reactive temperament [5,36]. Although WKY rats acquire avoidance faster, the nature of the facilitated learning is not known. Facilitated avoidance acquisition may reflect enhanced cue-outcome learning (classical conditioning), response-outcome learning (sensitivity to negative reinforcement), or both [10,33,42,62].
One potential means for independently assessing cue-outcome and response-outcome learning in a single protocol is eyeblink conditioning, a form of defensive new motor learning. Cue-outcome associations may be evaluated with a delay type paradigm (the conditioned stimulus [CS] and the unconditional stimulus [US] coterminate). Delay-type acquisition of the classically conditioned eyeblink response measures a general ability to form stimulus-stimulus associations. A recent study found an overall tendency for WKY rats to acquire an eyeblink conditioned response (CR) faster than SD rats [43]. The learning differences, however, primarily reflected a reduction in proactive interference. Acquisition was similar with a direct comparison of the acquisition of the two strains in response to delay-type conditioning. Yet, an extended acclimation period preceding training (necessary to match the period needed for stimulus pre-exposures) may have differentially affected the strains. Thus, the question of associability with regards to classical conditioning remains open.
Although not an instrumental task per se, classic work suggests that eyeblink conditioning could illustrate sensitivity to negative reinforcement through the imposition an omission contingency, that is, performance of a CR prevents the delivery of the US. Under these conditions, avoidance may be acquired. Avoidance is assessed in comparison to those given the same pattern of CS and US exposures but without control over US delivery (yoked comparisons). There is evidence avoidance responses may be acquired in both humans (without the complication of special instructions) [32,18,19,30,45,28,13,30,22,21] and rabbits [40,17,9]. In general, avoidance is apparent as greater responding in comparison to yoked subjects, however arguments may be made to the stability and suitability of yoked schedules [8,26]. As rats and humans have demonstrated similar sensitivity to other manipulations in eyeblink conditioning, such as the inter-stimulus interval [49,64], and proactive interference [1,43,60], acquisition of eyeblink avoidance in rats may resemble the human data. By implication, the enhanced acquisition of avoidance in WKY rats may be similarly expressed during eyeblink conditioning.
Therefore we assessed simple classical conditioning and sensitivity to negative reinforcement in SD and WKY rats utilizing eyeblink conditioning. Classical conditioning was assessed via a delay-type paradigm, while instrumental sensitivity was assessed through negative reinforcement of a CR in comparison to yoked schedules of reinforcement. We hypothesized that WKY and SD rats would equivalently acquire delay eyeblink conditioning. We also expected SD rats to exhibit eyeblink avoidance and WKY rats to exhibit facilitated avoidance acquisition. These data would support a generalized sensitivity to negative reinforcement in WKY rats.
Methods
Subjects
Male SD and WKY rats were obtained from Harlan Sprague Dawley (Indianapolis, IN) and individually housed in shoebox cages with ad libitum access to food and water upon arrival and throughout the experiment. Shoebox cages were kept in open air animal housing rooms with controlled temperature and humidity. Rats were kept on a 12:12 light:dark cycle with lights on at 0600 hr. All experiments were conducted during the light phase, between 0800 and 1500 hrs. Rats were 3–4 months of age when the experiment was conducted.
Surgery
Electrodes were surgically implanted into the upper eyelid of the rats as previously described [46]. Briefly, rats were anesthetized with a ketamine (80 mg/kg)/xylazine (10 mg/kg) mixture. Rats were then fitted with a headstage with four metal wire electrodes, two each for US administration and electromyography (EMG) recording. These electrodes were then threaded subcutaneously and emerged through the eyelid. Animals were allowed at least 72 hrs to recover before eyelid conditioning.
Apparatus
Eyelid conditioning was conducted within a 27 cm × 29 cm × 43 cm sound-attenuating test chamber (Med Associates, St. Albans, VT). The EMG electrodes were connected to a differential AC amplifier equipped with a 300-to 500- Hz bandpass filter (A-M Systems Model 1700, Everett, WA) and amplified by 10,000. A computer with an A/D board (National Instruments, Austin, TX) collected the EMG signal at 1000 Hz and a Labview (National Instruments) program was used to control stimulus presentations and EMG recording. The US, a 10-ms, 10-V square-wave stimulus, was produced by a Bioelectric Stimulus Isolator (Coulbourn Instruments, Whitehall, PA). The CS was a 500-ms white noise pulse of 82 dB with a rise/fall of 10 ms. During avoidance training, if the filtered EMG during the CS period reached a threshold value of 0.3 V and was 2.5 times baseline on that trial, a cutoff signal was sent to the stimulator and the US was aborted for that trial. The last 10 ms before the initiation of the US were not utilized for calculating whether a CR occurred because this time window is too close to initiate the cutoff signal.
Procedure
After surgery and recovery as described above, rats were acclimated to the experimental apparatus for 30 min during which their EMG signals were evaluated to determine signal quality. The following day training began. To assess simple delay-type acquisition (CS and US coterminate), SD (n = 10) and WKY (n= 10) were trained in three daily sessions, each consisting of 100 paired trials per session. For avoidance training, separate groups of SD (n = 8) and WKY (n = 10) were trained with delay-type training with the added imposition of an omission contingency for US delivery, that is, detection of a CR prevented US delivery for that trial. Lastly, a separate group of rats were trained on a pattern of CS and US presentations exactly matching their avoidance counterpart. The yoked group consisted of SD (n = 8) and WKY (n = 10) rats. For all training procedures the intertrial interval varied between 20-30 s with an average of 25 s.
Data processing and statistics
EMG data were analyzed using S-Plus, version 7 (Insightful Corporation, Seattle, WA). The data were filtered using a locally weighted low-pass filter with a time constraint of 0.01 and a smoothing interval of 3. The initial 250 ms of a trial were treated as a baseline for comparison for the rest of the trial. An eyeblink was recorded when the filtered EMG activity 30 ms after the onset of the CS, but before the onset of the US, exceeded the baseline activity level [46]. Threshold activity was established as the mean plus one standard deviation (to account for general noise level in the EMG signal) plus the maximum amplitude (to account for spontaneous blinks that may signal false CRs) of the EMG activity during the baseline period. Responses during the first 30 ms of the CS duration were treated as orienting responses and not considered for analysis (orienting responses rarely occur in the preparation given the gradual rise\fall of the acoustic stimulus).
The efficiency of the processing to prevent US delivery was evaluated according to signal detection theory for each rat for hits (a CR emitted, US omitted), miss (CR emitted, US delivered), correct rejections (no CR, US delivered) and false alarms (no CR, US omitted) (see Table 1). As can be seen, errors were predominantly misses which occurred for the most part when eyeblink occurred too close to the US delivery to allow adequate time for the cutoff signal. For two SD rats, eyelid activity while significant was sufficient to trigger the online detector resulting in excessive (>30%) misses; these two pairs (avoidance and associated yoke) were eliminated from further analysis.
Table 1.
Signal detection breakdown of performance of the US-cutoff protocol. Efficiency reached 84% for both strains, while the majority of errors consisted of misses, that is, non-reinforced eyeblinks (US was not prevented).
| Hits | Misses | Correct Reject | False Alarm | |
|---|---|---|---|---|
| SD | 44 ± 8% | 13 ± 4% | 40 ± 9% | 3 ± 1% |
| WKY | 52 ± 6% | 11 ± 3% | 32 ± 7% | 5 ± 1% |
Efficiency reached 84% for both strains, while the majority of errors consisted of misses, that is, non-reinforced eyeblinks (US was not prevented).
For evaluation of CR percentage, each session was grouped into 5 blocks, with 20 trials per block. Mixed analyses of variance (ANOVA) for repeated measures were then used to analyze CR percentage. F-tests for simple effects were used for post-hoc analysis [41]. Statistical significance was established at an α level of p < .05. All data are expressed as mean ± standard error of the mean. Dunn’s test (tD) was used for post-hoc analyses.
Results
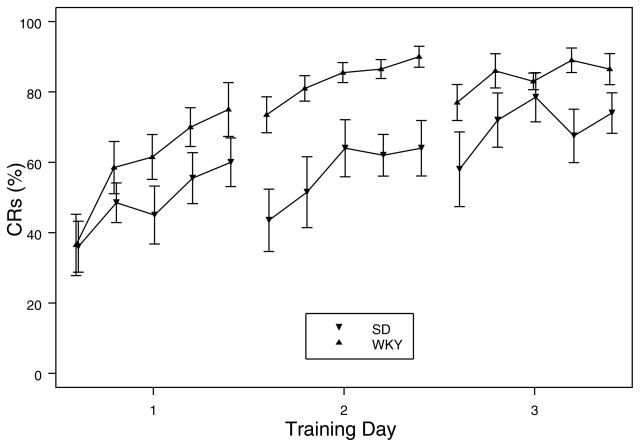
To evaluate simple acquisition of the eyeblink CR, the SD and WKY rats that received delay-type training were directly compared. WKY rats acquired the response faster and to a greater degree than SD rats (see Fig. 1). This difference emerged on the first day and persisted throughout the remainder of training. These impressions were confirmed via a 2 × 5 × 3 (Strain x Block x Day) mixed ANOVA analysis, which yielded a main effect of strain, F(1, 18) = 8.5, p < .01. In addition, main effects of both Block, F(4, 72) = 19.0, and Day, F(2, 36) = 14.1, which were subordinate to a Block by Day interaction, F(8,144) = 2.4, all ps < .05, reached significance. For both SD and WKY rats, less within session differences in acquisition were apparent as training progressed.
Figure 1.
Acquisition of eyeblink CR in paired training in SD and WKY rats. Each day of training consisted of 100 trials, divided into 5 blocks. Strain designations are in the figure legend. WKY rats acquire the eyeblink response faster and reach a high asymptotic performance than SD rats.
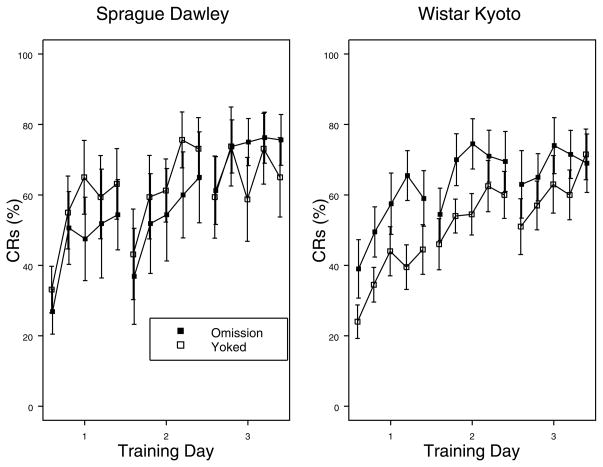
To evaluate avoidance learning of the two strains, rats trained with an omission contingency and their respective yoked counterparts were directly compared. For SD rats, acquisition did not differ between those with control over US delivery and those that did not (see Fig. 2). For WKY rats, those with control over US delivery acquired an eyeblink CR faster than those trained with a yoked schedule for reinforcement (see Fig. 2). This difference was most apparent early in training. These observations were confirmed with a 2 × 2 × 5 × 3 (Strain x Training x Block x Day) mixed ANOVA, which yielded a main effect of Block, F(4, 128) = 39.9, p < .001, and a main effect of Day, F(2, 64) = 39.3, which was subordinate to a Strain x Training x Day interaction, F(2, 64) = 4.4, ps < .05. Post-hoc analysis revealed that avoidance trained and yoked WKY rats only differed on the first day of training, tD = 2.84, p < .05.
Figure 2.
Acquisition of the eyeblink CR in avoidance and yoked training in SD and WKY rats. Each day of training consisted of 100 trials, divided into 5 blocks. Group designations are in the figure legend. In SD rats, performance in by those with avoidance training did not differ from their yoked counterparts. In WKY rats, performance by those with avoidance training exceeded their yoked counterparts.
Discussion
This study marks the first investigation of sensitivity to negative reinforcement in eyeblink conditioning in rats. For outbred SD rats, acquisition was similar under all three types of training: a simple delay-type protocol, avoidance training, and their yoked counterparts. These latter two groups did not differ from the former even though the partial reinforcement schedule of avoidance and yoked trained rats resulted in CS-US pairing on only 54% of trials. Training under similar schedules of reinforcement typically results in a step-wise pattern of acquisition curves: delay-type training with the highest asymptotic performance, avoidance with intermediate rates, and the partial reinforcement of yoked counterparts having significantly lower asymptotic performance [32,18,45,19,23]. These data support an interpretation that humans and rabbits acquire an eyeblink avoidance response. In contrast, outbred rats failed to display this pattern. Moreover, SD rats did not appear to be sensitive to partial reinforcement which would be expected to degrade the acquisition curve. However, partial reinforcement was not expressly manipulated; the patterns of CS-alone trials and paired CS-US trials were vastly different for individual yoked rats. A more focused examination controlling patterns of CS-alone and CS-US trials would be needed to determine whether SD rats indeed exhibit reduced sensitivity to partial reinforcement. Nonetheless, there was no evidence SD rats acquired an eyeblink avoidance response.
As to the strain comparisons, WKY rats acquired the eyeblink response faster and to a greater degree than SD rats. While greater cue-outcome learning is consistent with previous investigation into eyeblink conditioning in WKY rats [43], prior differences were mostly mediated through reduced proactive interference in WKY rats. In that prior study, acquisition with a similar delay protocol did not differ between the strains. One difference between the present study and the earlier study is the degree of exposure to the experimental context prior to training. In the former study of proactive interference, rats undergoing delay training had an extended period of exposure to the experimental context prior to the initiation of training. This exposure corresponded to the time needed to deliver CS and US pre-exposures. The pre-exposure period could have served to reduce emotionality in WKY rats, resulting in equivalent learning. Nevertheless, the two studies when taken together do indicate greater potential for the acquisition of cue-outcome associations in WKY rats.
In contrast to SD rats, WKY rats demonstrated the step-wise progression of curves described above. Thus it appears that WKY rats are more sensitive to negative reinforcement than SD rats. Although it is tempting to interpret the performance of WKY rats as facilitated avoidance acquisition, there are two factors casting doubt. Firstly, if the WKY rats acquired an eyeblink avoidance response, one may expect the rate of avoidance to exceed that of simple delay-type training and that of the SD rats trained under similar conditions. Eyeblink avoidance of WKY rats was not superior to SD rats. Leverpress avoidance performance of WKY rats nears unity with extensive training [50], leaving room for the possibility that avoidance performance could be further improved with more extensive training than was examined here. Secondly, the lack of decrease by yoked-trained SD rats suggests that WKY rats demonstrated greater sensitivity to partial reinforcement. Greater sensitivity to partial reinforcement must be tempered by the strain differences in the number of CS-US pairings for the respective yoked groups reflecting the slightly better overall performance of avoidance trained WKY rats. Yoked WKY rats as a group received 44% of CS-US pairings, an average of 10% fewer paired trials during yoking training than SD rats. The lower overall rate of US delivery for WKY yoked rats compared to SD rats would produce patterns of exposure more conducive to degraded performance. The enhanced partial reinforcement may also provide an alternative explanation for the larger strain differences in delay acquisition between the present study and our earlier work. In the earlier examination of proactive interference, the delay-type protocol included CS-alone and US-alone trials. Thus, greater sensitivity to partial reinforcement by WKY rats in the earlier study may account for the similar acquisition curves in that study. A more specific analysis of partial reinforcement effects in eyeblink conditioning would need to be conducted to ascertain whether WKY rats are indeed more sensitive to partial reinforcement.
Faster acquisition of the classically conditioned eyeblink response in stress-sensitive WKY rats is consistent with a body of research detailing the impact of stress on acquisition in male rats [51,49,47,48,63,53,27,3]. Strain differences in eyeblink acquisition also reminiscent of the work done in humans. These studies demonstrated faster eyeblink conditioning in a variety of studies in subjects who scored at the extremes of a distribution of an anxiety scale [57,55,54,56]. Thus WKY rats are similar to humans in that innate differences in emotionality correlate with persistent facilitation of eyeblink conditioning. Yet, despite the concordance in the current study and research in non-pathological anxiety states in humans, no clear consensus has been determined for the acquisition of eyeblink conditioning in pathological anxiety [2,20,16].
Greater cue-outcome and response-outcome learning might suggest a common underlying neurobiology. One potential brain region is the cerebellum. The cerebellum is intrinsic to acquisition of an eyeblink conditioned response, being studied extensively in cue-outcome associations [7]. Thus heightened cerebellar function could potentiate cue-outcome learning in WKY rats. It may also be involved in response-outcome learning. Cerebellar lesions have been found to specifically reduced escape and avoidance responses, but not food-reinforced bar pressing [59]. While no direct testing of cerebellar enhancement has been conducted, it is not unreasonable to suggest that heightened activity in the cerebellum may potentiate aversively motivated response-outcome learning as well. The modulatory structures may also play a role in greater associative learning. Among the modulatory structures for eyeblink conditioning, the amygdala is also a likely mediator. The basolateral amygdala has been shown to mediate the stress-induced facilitation of eyeblink conditioning [61,3]. The amygdala has been implicated in measuring valence of reward in instrumental tasks, affecting dopamine release in the dorsal striatum [24,3]. WKY rats demonstrate greater basal levels of corticotrophin releasing hormone (CRH) mRNA in the central amygdala [52]. Greater CRH could indicate hyperresponsivity of the amygdala in this strain thereby enhancing both cue-and response-outcome learning in this strain. Further specific testing would be needed to pinpoint neurobiological systems underlying greater associative learning in WKY rats.
As WKY rats are an animal model of BI, a vulnerability factor for anxiety in humans, features of this strain may shed light on the manner by which vulnerability translates to psychopathology. WKY rats acquire avoidance faster, and demonstrate enhanced associative learning in eyeblink conditioning compared to outbred strains. Enhanced associative learning, especially in fear conditioning, has been proposed as a central component of anxiety disorders. These concepts may be complementary as greater associative learning in WKY rats suggests that enhanced defensive learning capabilities are a predisposing factor for anxiety disorders. Either enhanced cue-outcome or response-outcome learning would bias towards the acquisition of avoidant behavior, a core feature of anxiety disorders. An interesting implication of the present work is the prediction that behaviorally inhibited individuals would demonstrate a similar pattern of enhanced cue-outcome learning during eyeblink conditioning.
In summary, inbred WKY rats demonstrated superior associative learning compared to SD rats. In delay-type eyeblink conditioning, WKY rats acquired the eyeblink response faster and to a greater degree than SD rats, indicating enhanced cue-outcome learning. During avoidance training WKY rats performed better than yoked rats indicating greater sensitivity to negative reinforcement or inhibition from partial reinforcement. By implication, faster lever-press avoidance acquisition may reflect enhanced associative learning related to cues of warning and safety as well as negative reinforcement from avoidance of shock. These learning enhancements were discussed as they relate to anxiety vulnerability.
Acknowledgments
Support for this study was provided by Department of Veterans Affairs Medical Research Funds, the Stress & Motivated Behavior Institute of the New Jersey Medical School and NIH grant NS044373. The authors wish to thank Toni Marie Dispenziere, Tracey Longo, Swamini Sinha, and Eric Zaccone for their technical assistance.
References
- 1.Allen MT, Chelius L, Masand V, Gluck MA, Myers CE, Schnirman G. A comparison of latent inhibition and learned irrelevance pre-exposure effects in rabbit and human eyeblink conditioning. Integr Physiol Behav Sci. 2002;37:188–214. doi: 10.1007/BF02734181. [DOI] [PubMed] [Google Scholar]
- 2.Ayers ED, White J, Powell DA. Pavlovian eyeblink conditioning in combat veterans with and without post-traumatic stress disorder. Integr Physiol Behav Sci. 2003;38:230–247. doi: 10.1007/BF02688856. [DOI] [PubMed] [Google Scholar]
- 3.Bangasser DA, Santollo J, Shors TJ. The bed nucleus of the stria terminalis is critically involved in enhancing associative learning after stressful experience. Behav Neurosci. 2005;119:1459–1466. doi: 10.1037/0735-7044.119.6.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beck KD, Zhu G, Beldowicz D, Brennan FX, Ottenweller JE, Moldow RL, Servatius RJ. Central nervous system effects from a peripherally acting cholinesterase inhibiting agent: interaction with stress or genetics. Ann N Y Acad Sci. 2001;933:310–314. doi: 10.1111/j.1749-6632.2001.tb05833.x. [DOI] [PubMed] [Google Scholar]
- 5.Blizard DA, Adams N. The Maudsley Reactive and Nonreactive strains: a new perspective. Behav Genet. 2002;32:277–299. doi: 10.1023/a:1020206120248. [DOI] [PubMed] [Google Scholar]
- 6.Brush FR. Selection for differences in avoidance learning: the Syracuse strains differ in anxiety, not learning ability. Behav Genet. 2003;33:677–696. doi: 10.1023/a:1026135231594. [DOI] [PubMed] [Google Scholar]
- 7.Christian KM, Thompson RF. Neural substrates of eyeblink conditioning: acquisition and retention. Learn Mem. 2003;10:427–455. doi: 10.1101/lm.59603. [DOI] [PubMed] [Google Scholar]
- 8.Church RM. Systematic effect of random error in the yoked control design. Psychological Bulletin. 1964;62:122–131. doi: 10.1037/h0042733. [DOI] [PubMed] [Google Scholar]
- 9.Coleman SR. Consequences of response-contingent change in unconditioned stimulus intensity upon the rabbit (Oryctolagus cuniculus) nictitating membrane response. J Comp Physiol Psychol. 1975;88:591–595. doi: 10.1037/h0076413. [DOI] [PubMed] [Google Scholar]
- 10.Dinsmoor JA. Stimuli inevitably generated by behavior that avoids electric shock are inherently reinforcing. J Exp Anal Behav. 2001;75:311–333. doi: 10.1901/jeab.2001.75-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dubi K, Rapee RM, Emerton JL, Schniering CA. Maternal modeling and the acquisition of fear and avoidance in toddlers: influence of stimulus preparedness and child temperament. J Abnorm Child Psychol. 2008;36:499–512. doi: 10.1007/s10802-007-9195-3. [DOI] [PubMed] [Google Scholar]
- 12.Ferguson SA, Cada AM. Spatial learning/memory and social and nonsocial behaviors in the spontaneously hypertensive, Wistar-Kyoto and Sprague-Dawley rat strains. Pharmacol Biochem Behav. 2004;77:583–594. doi: 10.1016/j.pbb.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 13.Fleming RA, Grant DA. A comparison of rate and contingency of classical and instrumental reinforcement upon the acquisition and extinction of the human eyelid CR. J Exp Psychol. 1966;72:488–491. doi: 10.1037/h0023771. [DOI] [PubMed] [Google Scholar]
- 14.Foa EB, Stein DJ, McFarlane AC. Symptomatology and psychopathology of mental health problems after disaster. J Clin Psychiatry. 2006;67(Suppl 2):15–25. [PubMed] [Google Scholar]
- 15.Fox NA, Henderson HA, Marshall PJ, Nichols KE, Ghera MM. Behavioral inhibition: linking biology and behavior within a developmental framework. Annu Rev Psychol. 2005;56:235–262. doi: 10.1146/annurev.psych.55.090902.141532. [DOI] [PubMed] [Google Scholar]
- 16.Ginsberg JP, Ayers E, Burriss L, Powell DA. Discriminative delay Pavlovian eyeblink conditioning in veterans with and without posttraumatic stress disorder. J Anxiety Disord. 2008;22:809–823. doi: 10.1016/j.janxdis.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 17.Gormezano I, Coleman SR. The law of effect and CR contingent modification of the UCS. Cond Reflex. 1973;8:41–56. doi: 10.1007/BF03000282. [DOI] [PubMed] [Google Scholar]
- 18.Gormezano I, Moore JW, DEAUX E. Supplementary report: yoked comparisons of classical and avoidance eyelid conditioning under 3 UCS intensities. J Exp Psychol. 1962;64:551–552. doi: 10.1037/h0047193. [DOI] [PubMed] [Google Scholar]
- 19.Gormezano I, MOORE JW. Yoked comparisons of contingent and noncontingent US presentations in human eyelid conditioning. Psychonomic Science. 1964;1:231–232. [Google Scholar]
- 20.Grillon C, Lissek S, McDowell D, Levenson J, Pine DS. Reduction of trace but not delay eyeblink conditioning in panic disorder. Am J Psychiatry. 2007;164:283–289. doi: 10.1176/ajp.2007.164.2.283. [DOI] [PubMed] [Google Scholar]
- 21.Hellige JB, Grant DA. Eyelid conditioning performance when the mode of reinforcement is changed from classical to instrumental avoidance and vice versa. J Exp Psychol. 1974;102:710–719. doi: 10.1037/h0036341. [DOI] [PubMed] [Google Scholar]
- 22.Hellige JB, Grant DA. Response rate and development of response topography in eyelid conditioning under different conditions of reinforcement. J Exp Psychol. 1974;103:574–582. doi: 10.1037/h0037169. [DOI] [PubMed] [Google Scholar]
- 23.Hupka RB, Massaro DW, Moore JW. Yoked Comparisons of instrumental avoidance and classical conditioning of the rabbit nictitating membrane response as a function of interstimulus interval and number of trials per day. Psychonomic Science. 1968;12:93–94. [Google Scholar]
- 24.Jones JL, Day JJ, Aragona BJ, Wheeler RA, Wightman RM, Carelli RM. Basolateral amygdala modulates terminal dopamine release in the nucleus accumbens and conditioned responding. Biol Psychiatry. 2010;67:737–744. doi: 10.1016/j.biopsych.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karamustafalioglu OK, Zohar J, Guveli M, Gal G, Bakim B, Fostick L, Karamustafalioglu N, Sasson Y. Natural Course of Posttraumatic Stress Disorder: A 20-Month Prospective Study of Turkish Earthquake Survivors. J Clin Psychiatry. 2006;67:882–889. doi: 10.4088/jcp.v67n0604. [DOI] [PubMed] [Google Scholar]
- 26.Kimmel HD, Terrant FR. Bias due to individual differences in yoked control designs. Behavior Research Methods & Instrumentation. 1968;1:11–14. [Google Scholar]
- 27.Leuner B, Mendolia-Loffredo S, Shors TJ. Males and females respond differently to controllability and antidepressant treatment. Biol Psychiatry. 2004;56:964–970. doi: 10.1016/j.biopsych.2004.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Logan FA. A comparison of avoidance and nonavoidance eyelid conditionings. Journal of Experimental Psychology. 1951;42:390–393. doi: 10.1037/h0054332. [DOI] [PubMed] [Google Scholar]
- 29.Malkesman O, Braw Y, Zagoory-Sharon O, Golan O, Lavi-Avnon Y, Schroeder M, Overstreet DH, Yadid G, Weller A. Reward and anxiety in genetic animal models of childhood depression. Behav Brain Res. 2005;164:1–10. doi: 10.1016/j.bbr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 30.Massaro DW, Moore JW. Differential classical and avoidance eyelid conditioning. J Exp Psychol. 1967;75:151–157. doi: 10.1037/h0024973. [DOI] [PubMed] [Google Scholar]
- 31.Mineka S, Zinbarg R. A contemporary learning theory perspective on the etiology of anxiety disorders: it’s not what you thought it was. Am Psychol. 2006;61:10–26. doi: 10.1037/0003-066X.61.1.10. [DOI] [PubMed] [Google Scholar]
- 32.Moore JW, Gormezano I. Yoked comparisons of instrumental and classical eyelid conditioning. J Exp Psychol. 1961;62:552–559. doi: 10.1037/h0044551. [DOI] [PubMed] [Google Scholar]
- 33.Mowrer OH. Two-factor learning theory reconsidered, with special reference to secondary reinforcement and the concept of habit. Psychol Rev. 1956;63:114–128. doi: 10.1037/h0040613. [DOI] [PubMed] [Google Scholar]
- 34.North CS, Pfefferbaum B, Tivis L, Kawasaki A, Reddy C, Spitznagel EL. The course of posttraumatic stress disorder in a follow-up study of survivors of the Oklahoma City bombing. Ann Clin Psychiatry. 2004;16:209–215. doi: 10.1080/10401230490522034. [DOI] [PubMed] [Google Scholar]
- 35.O’donnell ML, Elliott P, Lau W, Creamer M. PTSD symptom trajectories: From early to chronic response. Behav Res Ther. 2006 doi: 10.1016/j.brat.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 36.Overstreet DH, Rezvani AH, Janowsky DS. Maudsley reactive and nonreactive rats differ only in some tasks reflecting emotionality. Physiology & Behavior. 1992;52:149–152. doi: 10.1016/0031-9384(92)90444-7. [DOI] [PubMed] [Google Scholar]
- 37.Pardon MC, Gould GG, Garcia A, Phillips L, Cook MC, Miller SA, Mason PA, Morilak DA. Stress reactivity of the brain noradrenergic system in three rat strains differing in their neuroendocrine and behavioral responses to stress: implications for susceptibility to stress-related neuropsychiatric disorders. Neuroscience. 2002;115:229–242. doi: 10.1016/s0306-4522(02)00364-0. [DOI] [PubMed] [Google Scholar]
- 38.Pare WP. Stress ulcer susceptibility and depression in Wistar Kyoto (WKY) rats. Physiol Behav. 1989;46:993–998. doi: 10.1016/0031-9384(89)90203-5. [DOI] [PubMed] [Google Scholar]
- 39.Pare WP. Open field, learned helplessness, conditioned defensive burying, and forced-swim tests in WKY rats. Physiol Behav. 1994;55:433–439. doi: 10.1016/0031-9384(94)90097-3. [DOI] [PubMed] [Google Scholar]
- 40.Polenchar BE, Patterson MM, Lavond DG, Thompson RF. Cerebellar lesions abolish an avoidance response in rabbit. Behav Neural Biol. 1985;44:221–227. doi: 10.1016/s0163-1047(85)90226-2. [DOI] [PubMed] [Google Scholar]
- 41.Kirk RE. Experimental Design: Procedures fo the Behavioral Sciences. 2 1982. [Google Scholar]
- 42.Rescorla RA, Solomon RL. Two-process learning theory: Relationships between Pavlovian conditioning and instrumental learning. Psychol Rev. 1967;74:151–182. doi: 10.1037/h0024475. [DOI] [PubMed] [Google Scholar]
- 43.Ricart TM, De Niear MA, Jiao X, Pang KC, Beck KD, Servatius RJ. Deficient proactive interference of eyeblink conditioning in Wistar-Kyoto rats. Behav Brain Res. 2010 doi: 10.1016/j.bbr.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rittenhouse PA, Lopez-Rubalcava C, Stanwood GD, Lucki I. Amplified behavioral and endocrine responses to forced swim stress in the Wistar-Kyoto rat. Psychoneuroendocrinology. 2002;27:303–318. doi: 10.1016/s0306-4530(01)00052-x. [DOI] [PubMed] [Google Scholar]
- 45.Runquist WN, Sidowski J, Gormezano I. Yoked comparisons of classical and avoidance conditioning in differential conditioning of the eyelid response. Psychological Reports. 1962;11:43–50. [Google Scholar]
- 46.Servatius RJ. Eyeblink conditioning in the freely moving rat: square-wave stimulation as the unconditioned stimulus. J Neurosci Methods. 2000;102:35–42. doi: 10.1016/s0165-0270(00)00276-4. [DOI] [PubMed] [Google Scholar]
- 47.Servatius RJ, Beck KD. Facilitated acquisition of the classically conditioned eyeblink response in male rats after systemic IL-1beta. Integr Physiol Behav Sci. 2003;38:169–178. doi: 10.1007/BF02688851. [DOI] [PubMed] [Google Scholar]
- 48.Servatius RJ, Beck KD, Moldow RL, Salameh G, Tumminello TP, Short KR. A stress-induced anxious state in male rats: corticotropin-releasing hormone induces persistent changes in associative learning and startle reactivity. Biol Psychiatry. 2005;57:865–872. doi: 10.1016/j.biopsych.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 49.Servatius RJ, Brennan FX, Beck KD, Beldowicz D, Coyle-DiNorcia K. Stress facilitates acquisition of the classically conditioned eyeblink response at both long and short interstimulus intervals. Learning and Motivation. 2001;32:178–192. [Google Scholar]
- 50.Servatius RJ, Jiao X, Beck KD, Pang KC, Minor TR. Rapid avoidance acquisition in Wistar-Kyoto rats. Behav Brain Res. 2008;192:191–197. doi: 10.1016/j.bbr.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 51.Servatius RJ, Shors TJ. Exposure to Inescapable Stress Persistently Facilitates Associative and Nonassociative Learning in Rats. Behavioral Neuroscience. 1994;108:1101–1106. doi: 10.1037//0735-7044.108.6.1101. [DOI] [PubMed] [Google Scholar]
- 52.Shepard JD, Myers DA. Strain differences in anxiety-like behavior: Association with corticotropin-releasing factor. Behavioural Brain Research. 2008;186:239–245. doi: 10.1016/j.bbr.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 53.Shors TJ. Acute Stress Rapidly and Persistently Enhances Memory Formation in the Male Rat. Neurobiology of Learning and Memory. 2001;75:10–29. doi: 10.1006/nlme.1999.3956. [DOI] [PubMed] [Google Scholar]
- 54.Spence KW, BEECROFT RS. Differential conditioning and level of anxiety. J Exp Psychol. 1954;48:399–403. doi: 10.1037/h0057825. [DOI] [PubMed] [Google Scholar]
- 55.Spence KW, FARBER IE. Conditioning and extinction as a function of anxiety. J Exp Psychol. 1953;45:116–119. doi: 10.1037/h0055234. [DOI] [PubMed] [Google Scholar]
- 56.Spence KW, Spence JT. Sex and anxiety differences in eyelid conditioning. Psychol Bull. 1966;65:137–142. doi: 10.1037/h0022982. [DOI] [PubMed] [Google Scholar]
- 57.Spence KW, TAYLOR J. Anxiety and strength of the UCS as determiners of the amount of eyelid conditioning. J Exp Psychol. 1951;42:183–188. doi: 10.1037/h0061580. [DOI] [PubMed] [Google Scholar]
- 58.Steimer T, Driscoll P. Divergent stress responses and coping styles in psychogenetically selected Roman high-(RHA) and low-(RLA) avoidance rats: behavioural, neuroendocrine and developmental aspects. Stress. 2003;6:87–100. doi: 10.1080/1025389031000111320. [DOI] [PubMed] [Google Scholar]
- 59.Steinmetz JE, Logue SF, Miller DP. Using signaled barpressing tasks to study the neural substrates of appetitive and aversive learning in rats: behavioral manipulations and cerebellar lesions. Behav Neurosci. 1993;107:941–954. doi: 10.1037//0735-7044.107.6.941. [DOI] [PubMed] [Google Scholar]
- 60.Taylor JA. Level of conditioning and intensity of the adaptation stimulus. Journal of Experimental Psychology. 1956;51:127–130. doi: 10.1037/h0042941. [DOI] [PubMed] [Google Scholar]
- 61.Waddell J, Bangasser DA, Shors TJ. The basolateral nucleus of the amygdala is necessary to induce the opposing effects of stressful experience on learning in males and females. J Neurosci. 2008;28:5290–5294. doi: 10.1523/JNEUROSCI.1129-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Williams BA. Two-factor theory has strong empirical evidence of validity. J Exp Anal Behav. 2001;75:362–365. doi: 10.1901/jeab.2001.75-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wood GE, Shors TJ. Stress facilitates classical conditioning in males, but impairs classical conditioning in females through activational effects of ovarian hormones. Proc Natl Acad Sci U S A. 1998;95:4066–4071. doi: 10.1073/pnas.95.7.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Woodruff-Pak DS, Thompson RF. Classical conditioning of the eyeblink response in the delay paradigm in adults aged 18-83 years. Psychol Aging. 1988;3:219–229. doi: 10.1037//0882-7974.3.3.219. [DOI] [PubMed] [Google Scholar]