Abstract
Multiple transporters mediate osmoregulatory solute accumulation in Escherichia coli K-12. The larger genomes of naturally occurring strains such as pyelonephritis isolates CFT073 and HU734 may encode additional osmoregulatory systems. CFT073 is more osmotolerant than HU734 in the absence of organic osmoprotectants, yet both strains grew in high osmolality medium at low K+ (micromolar concentrations) and retained locus trkH, which encodes an osmoregulatory K+ transporter. Both lacked the trkH homologue trkG. Transporters ProP and ProU account for all glycine-betaine uptake activity in E. coli K-12 and CFT073, but not in HU734, yet elimination of ProP and ProU impairs the growth of HU734, but not CFT073, in high osmolality human urine. No known osmoprotectant stimulated the growth of CFT073 in high osmolality minimal medium, but putative transporters YhjE, YiaMNO, and YehWXYZ may mediate uptake of additional osmoprotectants. Gene betU was isolated from HU734 by functional complementation and shown to encode a betaine uptake system that belongs to the betaine-choline-carnitine transporter family. The incidence of trkG and betU within the ECOR collection, representatives of the E. coli pathotypes (PATH), and additional strains associated with urinary tract infection (UTI) were determined. Gene trkG was present in 66% of the ECOR collection but only in 16% of the PATH and UTI collections. Gene betU was more frequently detected in ECOR groups B2 and D (50% of isolates) than in groups A, B1, and E (20%), but it was similar in overall incidence in the ECOR collection and in the combined UTI and PATH collections (32 and 34%, respectively). Genes trkG and betU may have been acquired by lateral gene transfer, since trkG is part of the rac prophage and betU is flanked by putative insertion sequences. Thus, BetU and TrkG contribute, with other systems, to the osmoregulatory capacity of the species E. coli, but they are not characteristic of a particular phylogenetic group or pathotype.
Bacteria that cause food- and waterborne diseases face diverse and changing environments during processing and storage of feed, food, and water (1), within human or animal hosts (76), and outside those hosts on plants, in soil, or in water (39). Stresses faced by these bacteria may include nutrient deprivation, low pH, high organic acid levels, oxygen deprivation or exposure to reactive oxygen species, thermal fluctuations, osmotic stress (variations and extremes of salinity and/or osmolality), desiccation, or denaturant stress. Bacterial stress tolerance mechanisms are believed to increase the incidence and severity of food- and waterborne disease by increasing the frequency with which humans and animals are exposed to contaminated food or water and by enhancing bacterial virulence. Bacteria may also sense their own movement into and out of host tissues by detecting environmental changes.
Cytoplasmic accumulation of particular organic solutes (often designated compatible solutes) is a widely recognized bacterial stress response (89). Growing evidence indicates that compatible solutes confer thermal, denaturant, and/or oxidative stress tolerance in addition to being key players in osmoregulation. Trehalose accumulates in Escherichia coli in stationary phase and in response to thermal and osmotic stress, protecting the bacteria from osmotic stress (41), freezing and desiccation (53), cold stress (46), lethal heat stress (42), and nonlethal high temperature (14). Trehalose (6) and dimethylsulphoniopropionate (79) also alleviate oxidative stress. Glycine-betaine, a widely used osmoprotectant (89), promotes chill tolerance in Listeria monocytogenes (77), yet it reduces the ability of other organisms to tolerate high temperatures (31, 32, 74). The membrane-permeant solute urea is present in the urine of humans and animals at levels that can inhibit bacterial growth (up to 0.5 M for human urine) (5). Glycine-betaine confers urea tolerance on E. coli (66), as well as on renal cells (13), by counteracting its effects as a cytoplasmic denaturant (83).
Multiplicity and redundancy of homeostatic mechanisms are hallmarks of bacterial stress response. They complicate efforts to elucidate relationships among stress tolerance mechanisms, bacterial virulence and the incidence of human or animal disease. The redundancy of bacterial osmoregulatory mechanisms was first defined via studies of E. coli K-12 and Salmonella enterica serovar Typhimurium (17), and even greater redundancy has since been shown for the gram-positive bacteria Bacillus subtilis (48), Corynebacterium glutamicum (57), and L. monocytogenes (44). E. coli K-12 can achieve osmotolerance through the accumulation and release of K+ or compatible solutes (88). Osmoregulatory K+ uptake can be mediated by KdpFABC, a high-affinity K+-transporting ATPase, or by Trk, a low-affinity system present in E. coli K-12 as two variants, TrkG and TrkH. Compatible solutes stimulate bacterial growth in high-osmolality media more effectively than does K+, and compatible solute accumulation suppresses K+ accumulation in response to osmotic stress (24). Unlike compatible solute accumulation, K+ accumulation has not been reported to provide collateral thermo-, urea, or oxidative stress tolerance. Organic osmoprotectants are compounds that stimulate bacterial growth in high-osmolality media because osmoregulatory transporters, listed in Table 1, mediate their accumulation as compatible solutes. Osmoprotectants may also be converted to compatible solutes after uptake (e.g., choline uptake via BetT and conversion to glycine-betaine by BetBA) or be synthesized from central metabolic precursors (e.g., trehalose synthesis from cytoplasmic glucose mediated by OtsBA). To rigorously test the hypothesis that osmoregulatory mechanisms assist E. coli to cause human or animal disease, all systems that contribute to osmoprotection must be identified.
TABLE 1.
Known and predicted bacterial osmoprotectant transportersa
| Transporter familyb | Energy supplyc | Osmoprotectant transporter(s) of:
|
||
|---|---|---|---|---|
| E. colid | C. glutamicum | B. subtilis | ||
| MFS | ΔμH+ | ProP, [YhjE] | ProP | Nil |
| TRAP | ΔμH+ | [YiaMNO] | Nil | Nil |
| SSSS | ΔμNa+ | (PutP) | (PutP) | OpuE |
| BCCT | ΔμNa+ | BetT, BetU | EctP, BetP, LcoP | OpuD |
| ABC | ATP | ProU, [YehWXYZ] | Nil | OpuA, OpuB, OpuC |
The table lists known and predicted osmoprotectant transporters of E. coli, B. subtilis, and C. glutamicum (this article, (48, 57, 89)). Names specifying known osmoprotectant transporters are shown without parentheses or brackets. Predicted osmoprotectant transporters (E. coli only) are shown in square brackets, and transporters that are similar in sequence to known osmoprotectant transporters but are not osmoregulatory are shown in parentheses.
The transporter families are MFS (major facilitator superfamily), TRAP, SSSS (sodium-solute symporter superfamily), BCCT, and ABC (ATP-binding cassette family) (27, 70).
Each transporter class is associated with a particular energy supply (27), but the energy supplies utilized by the listed transporters have not been determined experimentally in every case.
Listed transporters are encoded by all sequenced E. coli genomes (MG1655, EDL933, RIMD0509952, and CFT073) (Table 2), with the following exceptions. YiaMNO is encoded by the genomes of E. coli MG1655 and CFT073 but not by EDL933 or RIMD0509952. Gene betU is not present in any sequenced E. coli genome, but it is present in one-third of the E. coli isolates included in this study (Tables 4 and 5).
High urea levels and fluctuating osmolality distinguish the urinary tract from other mammalian tissues. The osmolality of urine from healthy humans with a normal diet and fluid intake varies in the range of 0.5 to 0.8 mol/kg, but urinary osmolality may vary from approximately 0.04 to 1.4 mol/kg (3, 50, 69). Urea, the primary contributor to the osmolality of human urine and of renal extracellular fluid, approaches a concentration of 0.5 M. Despite its high urea content, fluctuating osmolality, and low pH, urine supports rapid and extensive growth of E. coli (18, 34). Upon demonstrating that glycine-betaine and proline-betaine conferred osmoprotective activity on human urine, Chambers and Kunin (15) inferred that osmoregulatory betaine uptake may promote growth in urine and colonization of the human urinary tract by E. coli (16). We therefore chose to probe the relationship between osmoregulatory compatible solute accumulation and bacterial virulence by focusing our attention on uropathogenic E. coli strains. Our approach is to conduct detailed studies of two pyelonephritis isolates (HU734 and CFT073) and survey commensal and virulent E. coli strains to determine the prevalence and distribution of identified osmoregulatory mechanisms (19, 23, 51).
Earlier work showed that osmoprotectant transporters ProP and ProU are present and expressed in diverse E. coli strains, both commensal and pathogenic (19). E. coli strain CFT073 is more osmotolerant than strain HU734 in the absence of organic osmoprotectants, and a defect in stationary-phase sigma factor RpoS impairs the relative ability of HU734 to grow in media of very high (over 0.8 mol/kg) but not of moderate salinity by impairing trehalose accumulation (22). In addition, HU734 harbors an osmoregulatory betaine uptake activity (BetU) not evident in CFT073, but defects eliminating ubiquitous osmoprotectant transporters ProP and ProU impair the growth of HU734 and not CFT073 in high-osmolality human urine (18, 21). The latter data suggested that osmoregulatory betaine uptake is critical for osmoregulation (and growth in urine) by HU734 but not CFT073 and that CFT073 may harbor one or more additional glycine-betaine-independent osmoregulatory systems that contribute to bacterial growth in urine and are not present in HU734.
This paper reports that both pyelonephritis isolates retain osmoregulatory K+ transporter TrkH but lack its homologue, TrkG. Both are able to grow on low-K+, high-osmolality media in the absence of organic osmoprotectants. No known osmoprotectant stimulated the growth of CFT073 in high-osmolality medium, but analysis of the CFT073 genome revealed putative osmoregulatory transporters that may mediate accumulation of urinary osmoprotectants, which are as yet unidentified. We report the isolation of betU from HU734 and evidence that BetU is a member of the betaine-carnitine-choline transporter (BCCT) family. Phylogenetic and genomic sequence analyses are revealing striking genetic diversity among E. coli isolates and elucidating the evolution of virulence (8, 40, 62, 67, 86). Osmoregulatory loci proP and proU are ubiquitous (19, 22; this work) and likely part of the core E. coli genome. We have now examined the distributions of trkG and betU within the ECOR collection and collections of pathogenic E. coli isolates to further assess their evolutionary origins and relationships to bacterial virulence.
MATERIALS AND METHODS
Bacterial strain.
The E. coli K-12 derivatives used during this study included DH5α [F− φ80dlacZΔM15 Δ(lacZYA-argF)U169 recA1 endA1 hsdR17(rK− mK+) supE44 λ− thi-1 gyrA relA1] (38), Frag-1 (F− thi rha lacZx82 gal) (29), MKH13 [F− araD139 Δ(argF-lac)U169 rpsL150 relA1 flb-5301 deoC1 ptsF25 rbsR Δ(putPA)101 Δ(proP)2 Δ(proU)608] (36), and TK2420 [Frag-1 nagA trkD1 Δ(trkA) Δ(kdpABC)5 kup] (28). Pyelonephritis isolates HU734 and CFT073 and their derivatives deficient in transporters putP, proP, and/or proU were previously described (18, 22). HU734 is a lacZ derivative of acute pyelonephritis isolate GR12 with the following properties: streptomycin and spectinomycin resistance, cysteine auxotrophy, serotype O75:K5, possession of type 1 and P pili (the latter encoded by a single pap operon), carriage of a ColV plasmid, resistance to killing by human and mouse serum, and failure to produce hemolysin. CFT073 was isolated from the blood of a patient with acute pyelonephritis. It has no antibiotic resistance or auxotrophy, is O nontypeable and nonmotile, expresses type 1, S, and P pili (the latter encoded by two pap operons), produces hemolysin, and is cytotoxic for cultured human renal epithelial cells. The derivatives of these strains used for this study included WG695 [HU734 Δ(putPA)566 Δ(proP)218 Δ(proV-proX)567], WG696 [CFT073 Δ(proP)218 Δ(proV-proX)567], WG745 [CFT073 Δ(rpoS)2062], and WG746 [CFT073 Δ(proP)218 Δ(proV-proX)567 Δ(rpoS)2062]. Two collections of E. coli strains representing diverse pathotypes were used. A collection of urinary tract and intestinal E. coli isolates was described previously (19, 23). The urinary tract infection (UTI) collection included the 30 urinary tract isolates from that collection (7 catheter-associated, 1 bacteriuria, 12 cystitis, 6 pyelonephritis [including strain HU734], and 4 unspecified UTI) plus strain CFT073. The E. coli pathotype (PATH) collection, including 21 strains with a broader array of pathotypes, is described in Table 2. Pyelonephritis isolates HU734 and CFT073 were common to both the UTI and the PATH Collections. Genomic DNA samples derived from the 72 E. coli reference (ECOR) collection strains (59) were a generous gift from C. Whitfield (University of Guelph), and R. Y. C. Lo (University of Guelph) provided plasmid pBR322 (9, 85).
TABLE 2.
E. coli strains of clinical origin used during this study (the PATH collection)
| Pathotypea | Name | Sourceb | Serotypec | Reference(s) |
|---|---|---|---|---|
| UPEC | HU734d | Urine, pyelonephritis | O75:K5 | 37, 80 |
| UPEC | CFT073 | Blood, pyelonephritis | O6:K2:H1 | 56 |
| UPEC | J96 | Urine, pyelonephritis | O4:K6:H5 | 45, 80 |
| UPEC | 536 | Pyelonephritis | O6:K15:H31 | 25 |
| EHEC | EDL933 | Ground beef | O157:H7 | 49, 62, 68 |
| EHEC | RIMD0509952 | Bloody diarrhea | O157:H7 | 40, 84 |
| EHEC | 93-001 | Bloody diarrhea | O157:H7 | 49 |
| VTEC | FRIK920 | Bovine feces | O157:H7 | 49 |
| EPEC | E2348/69 | Infantile diarrhea | O127:H6 | 52, 67 |
| EPEC | B170 | Infantile diarrhea | O111:NM | 61, 67 |
| EPEC | DEC12a | Infantile diarrhea | O111:H2 | 60, 67 |
| ETEC | H10407 | Liquid stool | O78:H11 | 30, 81 |
| ETEC | 339 | Jejunal fluid | O15:H11 | 33 |
| NEMEC | S3 | Neonatal meningitis | NA | 7, 10, 11 |
| NEMEC | S4 | Neonatal meningitis | NA | 7, 10, 11 |
| NEMEC | C5 (S25) | Cerebrospinal fluid | O18:K1:H7 | 7, 10, 11 |
| NEMEC | S39 | Neonatal meningitis | NA | 7, 10, 11 |
| NEMEC | S41 | Neonatal meningitis | NA | 7, 10, 11 |
| NEMEC | S51 | Neonatal meningitis | NA | 7, 10, 11 |
| NEMEC | S76 | Neonatal meningitis | NA | 7, 10, 11 |
| NEMEC | S82 | Neonatal meningitis | Ont:K1 | 7, 10, 11 |
UPEC, uropathogenic E. coli; EHEC, enterohemorrhagic E. coli; VTEC, verotoxigenic E. coli; EPEC, enteropathogenic E. coli; ETEC, enterotoxigenic E. coli; NEMEC, E. coli isolates from neonatal meningitis.
Bacteria were isolated from humans unless otherwise noted, and all strains except FRIK920 were associated with human disease.
NA, not available.
Media and growth measurements.
Culture media included Luria-Bertani (LB) (55), morpholinepropanesulfonic acid-based minimal medium (MOPS) (58), and K5 (24). MOPS minimal medium was supplemented with d-glucose (0.2% wt/vol) as the carbon source and NH4Cl (9.5 mM) as the nitrogen source. Antibiotics were used at the following concentrations: ampicillin (AMP), 100 μg/ml; tetracycline, 25 μg/ml. The abilities of organic compounds to provide osmoprotection to E. coli were assessed, as described previously (54), by measuring bacterial plating efficiencies on MOPS medium supplemented with NaCl (0.6 M) and/or osmoprotectant (1 mM).
Isolation of gene betU.
Molecular biological manipulations were performed as described by Sambrook et al. (71) unless otherwise stated. Plasmid DNA was prepared by using the QIAprep Spin Miniprep kit or Plasmid Midi kit (QIAGEN, Mississauga, Ontario, Canada). Electroporation was performed with the Micropulser Electroporater [Bio-Rad (Canada) Inc., Mississauga, Ontario, Canada] according to the manufacturer's instructions, and chemical transformation was performed as described by Hanahan (38).
To construct a DNA library, chromosomal DNA isolated from E. coli WG695 was partially digested with Sau3A to yield DNA fragments 3 to 10 kb in length. Vector pGEM-7z (Promega Corp., Madison, Wis.) was digested with BamHI, dephosphorylated with shrimp alkaline phosphatase (USB Corp., Cleveland, Ohio), and treated with T4 DNA ligase [Boehringer Ingelheim (Canada) Ltd., Burlington, Ontario, Canada]. The resultant recombinant plasmids were introduced to E. coli DH5α via electroporation, and transformants were selected on LB plates containing AMP and X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) (40 μg/ml). Plasmid DNA was isolated from the pooled transformants (approximately one-third of which were Lac−) and stored.
To isolate gene betU, the DNA bank was introduced to E. coli MKH13 via electroporation and transformants were selected on MOPS supplemented with AMP, NaCl (0.6 M), and glycine-betaine (1 mM). Three clones that appeared after 48 h of incubation at 37°C were streak purified, and plasmid DNA was isolated and retransformed into MKH13 to confirm complementation. One of these clones, containing a plasmid with a 7.4-kb insert, was designated pAL1. The entire insert was sequenced by primer walking (Laboratory Services, Guelph, Ontario, Canada; MOBIX, Hamilton, Ontario, Canada). For sequences not represented in the genome of E. coli K-12, the reverse strand was also sequenced. DNA sequences were assembled and analyzed by using Sequencher (Gene Codes Corporation, Ann Arbor, Mich.).
To subclone betU, plasmid DNA (both pAL1 and vector pBR322) was digested with ScaI and PstI restriction endonucleases, mixed, ligated with T4 DNA ligase, and transformed into DH5α. Colonies were selected on LB plates containing tetracycline. Plasmid DNA was isolated from 16 randomly selected clones and subjected to restriction analysis. Plasmids with the expected fragment sizes were further transformed into MKH13 to confirm complementation, and one clone, containing a plasmid designated pAL3, was retained as E. coli WG855.
Analysis of the occurrence of genetic loci by PCR.
Genomic template DNAs were prepared, duplex PCRs were performed, and amplicons were analyzed as described previously (23), except that the annealing temperature was 52°C and the extension time was 1 min for amplification of putP and betU. The primers are listed in Table 3. Amplification of proV was used as an internal positive control for the detection of trkA, trkG, trkH, and sapF, whereas putP was used as the positive control during detection of sapD and betU.
TABLE 3.
PCR primers
| Primer | Sequence (5′ → 3′) | Amplicon
|
|
|---|---|---|---|
| Origin | Size (bp) | ||
| putP1 | GGT TGC GTG TGC ATA CCG A | bp 287-488 of putP | 202 |
| putP2 | GCC GTT TCG TAG CTC ATG C | ||
| proV1 | GCA TCC ACA GCG AGC GTT CA | bp 48-301 of proV | 254 |
| proV2 | GGA GTT CGG CGT CGG ATA T | ||
| betU1 | ACC GTC ATC CAC ATT AGC | bp 496-1118 of betU | 623 |
| betU2 | GCG TTC TTC AGT TAC CG | ||
| trkA1 | CTG GTT GGC GAG AAC AAC | bp 55-263 of trkA | 209 |
| trkA2 | GAG TAG GCT ACC TGG CAG | ||
| trkG1 | GCC CAC GCA CAC GAT AGT G | bp 992-1354 of trkG | 362 |
| trkG2 | GCA CTC CGA ATG ACG ATG C | ||
| trkH1 | GCG ATC CGG AAT TTC GCA TG | bp 809-983 of trkH | 175 |
| trkH2 | CGG GCA ATG CTG TCA GTT G | ||
| sapD1 | GAC ACG ACT GCG GTT CCT G | bp 53-304 of sapD | 252 |
| sapD2 | GTG GGT TAA AGC CGT CGA C | ||
| sapF1 | GGC AAT CAG CCG TTT GGT G | bp 543-756 of sapF | 214 |
| sapF2 | GGA TAT GTC GAT GCG TTC GC | ||
Analytical procedures.
Initial rates of choline and glycine-betaine uptake were measured as described by Culham et al. (20) with [methyl-14C]choline or [1-14C]glycine-betaine (American Radiolabeled Chemicals, Inc., St. Louis, Mo.) at final concentrations and specific radioactivities of 8 μM and 10 Ci/mol for choline and 200 μM and 5 Ci/mol for glycine-betaine. The protein content of cell suspensions was determined with the bicinchoninic acid assay (78), with bovine serum albumin as the standard. Osmolalities of growth and assay media were measured with a vapor pressure osmometer (Wescor).
Nucleotide sequence accession number.
The betU sequence was deposited in GenBank with accession number AF532988.
RESULTS
E. coli isolates HU734 and CFT073 lack TrkG but retain osmoregulatory K+ uptake.
Pyelonephritis isolate CFT073 was similar in osmotolerance to E. coli K-12 when both were cultivated in NaCl-supplemented MOPS minimal medium (without osmoprotectants), but pyelonephritis isolate HU734 was less osmotolerant (18, 22). Failure to synthesize trehalose due to an RpoS defect accounted for the poor osmotolerance of HU734 at very high osmolality (more than 0.8 mol/kg) but not in the lower osmolality range characteristic of normal human urine (0.5 to 0.8 mol/kg) (22). In addition, elimination of ProP and ProU impaired the growth of HU734, but not CFT073, in high-osmolality urine (which contains osmoprotectants).
The difference in osmotolerance between E. coli HU734 and isolates K-12 and CFT073 could result from a difference in osmoregulatory K+ uptake capacity. In E. coli K-12, transporters Kdp, Trk, and Kup contribute to K+ uptake while Kdp and Trk also contribute to osmotolerance (26). Kdp is an optional, high-affinity K+ uptake system that allows bacteria lacking Trk and Kup to grow on media that are very low in K+ (micromolar concentrations) and low or high in osmolality (2). The abilities of strains HU734, CFT073, and WG745 (CFT073 ΔrpoS) to use K+ were tested by comparing the growth of each strain on NaCl-supplemented K5 medium (24) with that of E. coli K-12 strain Frag-1 (wild type for K+ uptake) and its K+ uptake-null derivative TK2420 (28). As reported previously, strain TK2420 was unable to grow in K5 medium supplemented with less than 25 mM K+. In contrast, strains HU734, CFT073, WG745, and Frag-1 grew well on K5 medium supplemented with up to 0.4 M NaCl even if no K+ salt was added (typically, the level of K+ contaminating such media is micromolar). Thus, no K+ uptake deficiency was evident in E. coli HU734.
Since the low-affinity, high-capacity Trk systems are most likely to mediate osmoregulatory K+ uptake in the relatively high-K+ environments of the urinary tract or MOPS, we further tested the incidence of those systems in the pyelonephritis isolates. Trk refers to a pair of low-affinity, high-capacity K+ transporters which are expressed constitutively in E. coli K-12 (17). Multiple components contribute to each Trk system, including TrkA and TrkG or TrkH (73). Mutations in trkE impair K+ uptake via the TrkH system, and TrkE has been redefined as an ABC transporter (SapABCDF) that is probably not part of Trk itself (88). Primers targeting sequences internal to trkA, trkG, trkH, sapD, and sapF were used in PCR to compare the incidence of the trk loci in strains HU734, CFT073, Frag-1, and TK2420 with that of loci putP and proV, which are ubiquitous (23). PCR products representative of putP and proV were detected with template DNA from all four strains, whereas PCR products representative of trkA were obtained with template DNA from strains HU734, CFT073, and Frag-1 but not TK2420 (which is known to be ΔtrkA). Products representative of trkH, sapD, and sapF were detected with template DNA from all four strains, whereas only Frag-1 and TK2420 DNAs served as templates for amplification of trkG. These observations are consistent with the fact that the trkG locus of E. coli K-12 is part of Rac (72), a lambdoid prophage that is absent from the sequenced genome of E. coli CFT073 (86). Since trkG is absent from both HU734 and CFT073, this trk defect does not account for the fact that HU734 is intrinsically less osmotolerant than CFT073 (22). Further studies will be required to determine the basis for that difference.
Osmoprotectant specificities of pyelonephritis isolates HU734 and CFT073.
Transporters ProP and ProU accounted for all glycine-betaine uptake activity in strain CFT073 but not in strain HU734. The residual glycine-betaine uptake activity in HU734 was named BetU (22). Paradoxically, elimination of ProP and ProU impaired the growth of HU734, but not CFT073, in high-osmolality human urine (22). HU734 harbors an RpoS defect that blocked osmoregulatory trehalose accumulation, but deletion of rpoS did not impair growth of CFT073 in high-osmolality human urine (22). Analysis of extracts from CFT073 cells cultivated at high osmolality in the absence of osmoprotectants failed to reveal compatible solutes of biosynthetic origin other than trehalose (22). Choline is an osmoprotectant for E. coli K-12 because BetT mediates choline uptake while BetB and BetA mediate choline oxidation to glycine-betaine (88). Choline provided osmoprotection to HU734 and CFT073, both retain locus betT (data not shown), and the choline uptake activity of strain HU734 exceeded that of strain CFT073. For bacteria cultivated in NaCl-supplemented MOPS (0.94 mol/kg), the choline uptake activities of HU734 and CFT073 were 31 ± 0.4 and 7 ± 0.1 nmol/min/mg of cell protein, respectively, and they were not affected by deletion of loci proP and proU. Differences in BetT activity are thus unlikely to accelerate the growth of CFT073 over that of HU734 in high-osmolality human urine. HU734 is known to harbor a betaine uptake activity (BetU) that is not expressed by E. coli K-12 or CFT073. Perhaps CFT073 harbors an osmoprotectant uptake system that is not present in E. coli K-12 or HU734.
The transporters listed in Table 1 mediate accumulation of diverse osmoprotectants (some of which are illustrated in Fig. 1). We screened diverse compounds to identify osmoprotectant activity for derivatives of HU734 and CFT073 lacking transporters ProP and ProU (see Materials and Methods). Both glycine-betaine and proline-betaine increased the plating efficiency of strain WG695 (HU734 ΔputPA ΔproP ΔproU) on MOPS supplemented with 0.6 M NaCl. None of the protein amino acids provided osmoprotection to this strain, and none of them reduced the osmoprotective activity of glycine-betaine, indicating that BetU is not a broad-specificity amino acid transporter. Proline, ectoine, pipecolate, dimethyl glycine, sarcosine, and carboxymethyl pyridinium also failed to provide osmoprotection. BetU was thereby tentatively defined as a betaine-specific transporter. In contrast, neither the compounds listed above nor d-carnitine, l-carnitine, taurine, betonicin, butyrobetaine, thiaproline, or trigonelline conferred osmoprotection on E. coli WG696 (CFT073 ΔproP ΔproU). Further efforts will be required to identify urinary compounds, other than those listed, which are osmoprotective for E. coli CFT073. Additional, putative osmoprotectant transporters have been identified via analysis of the CFT073 genome (see Discussion).
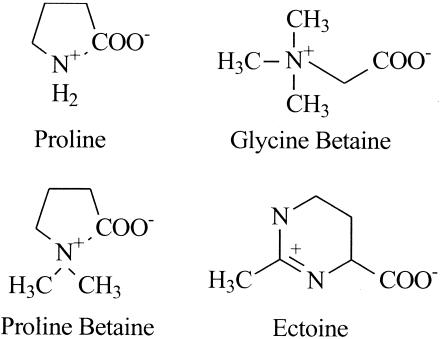
FIG. 1.
Compatible solutes. The structures of compatible solutes commonly used by E. coli are illustrated. Glycine-betaine and proline-betaine (but not proline or ectoine) are substrates for BetU.
Identification of the betU locus of HU734.
E. coli MKH13, derived from E. coli K-12 derivative MC4100, lacks the BetT, ProP, and ProU transport systems and therefore cannot grow on high-osmolality minimal media containing glycine-betaine (36). A gene library prepared from E. coli WG695 (HU734 ΔproP ΔproU) was transformed into MKH13, and transformants were selected on MOPS containing NaCl and glycine-betaine as described in Materials and Methods. Plasmid pAL1, which contained a 7.4-kb insert, enabled E. coli MKH13 to grow on MOPS containing either glycine-betaine or proline-betaine but not choline or ectoine (1 mM). Therefore, the cloned insert encoded a system with the expected substrate specificity.
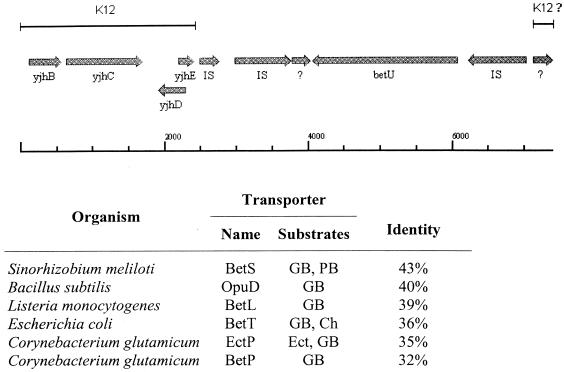
The entire 7.4-kb insert was sequenced, revealing a region identical with residues 4501566 to 4504677 of the E. coli K-12 genome, including genes yjhB, yjhC, yjhD, and yjhE (functions unknown) (Fig. 2, top). An open reading frame (ORF) flanked by putative insertion sequences was found upstream from the yjh genes and was not present in the E. coli K-12 genome, as expected given the absence of BetU activity from E. coli K-12 (22). The insertion sequences up- and downstream from betU (Fig. 2, top) (flanking betU to the left and right, respectively) encoded putative transposases identical to InsB and Hp1, present in IS911 and IS600 of Shigella flexneri, respectively. These insertion sequences and their close homologues (E values less than e−10) are present in many copies in each of the sequenced E. coli and S. flexneri genomes, occurring least frequently in E. coli K-12 (4 copies each), at intermediate frequencies in the pathogenic E. coli isolates CFT073, EDL933, and RIMD0509952 (8 to 15 copies), and most frequently in S. flexneri (42 to 56 copies).
FIG. 2.
Isolation of betU. (Top) A 7,383-bp DNA fragment including betU was isolated from E. coli WG695 (HU734 ΔputP ΔproP ΔproU), inserted in vector pGEM-7z, and recovered by functional complementation of transporter defects in E. coli MKH13 (ΔputP ΔbetTIBA ΔproP ΔproU) as described in Materials and Methods. Sequencing of this fragment revealed that betU is inserted adjacent to yjhE in the backbone sequence shared by E. coli K-12 and E. coli O157:H7 and that it is flanked by insertion sequences, as would be expected if it had appeared by lateral gene transfer. (Bottom) BetU is similar to known osmoregulatory transporters in diverse organisms (Sinorhizobium meliloti [12], B. subtilis [47], L. monocytogenes [75], E. coli [4], and C. glutamicum [63, 64]).These systems mediate accumulation of quaternary ammonium compounds including carnitine (Car), choline (Cho), ectoine (Ect), glycine-betaine (GB), and proline-betaine (PB).
The ORF flanked by the insertion sequences was subcloned into vector pBR322, creating plasmid pAL3 that was transformed into E. coli MKH13. (To do this, the ScaI-PstI fragment of pBR322 was replaced with the 3,061-bp ScaI-PstI fragment from pAL1, which extended from 423 bp upstream to 635 bp downstream of the ORF.) Like pAL1, pAL3 restored growth of E. coli MKH13 on MOPS containing NaCl and glycine-betaine. These results indicated that the isolated ORF was betU and that plasmid pAL3 included the betU promoter. The betU sequence was deposited in GenBank with accession number AF532988. The putative insertion sequences flanking betU imply its arrival by lateral gene transfer. However, gene betU is not differentiated from the E. coli genome by its base composition (50.4% G+C).
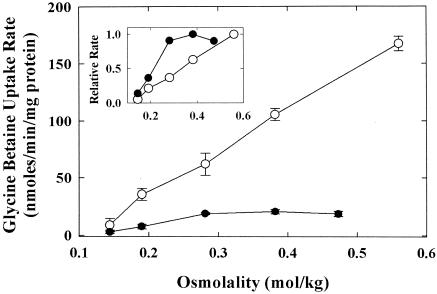
E. coli MKH13 is devoid of glycine-betaine uptake activity (47). Initial rates of glycine-betaine uptake by E. coli strains WG695 [HU734 Δ(putPA)566 Δ(proP)218 Δ(proV-proX)567] and WG855 (MKH13 pAL3) were determined as a function of medium osmolality (Fig. 3). The glycine-betaine uptake activity of BetU in its native host (WG695) was half maximal at approximately 0.2 mol/kg (Fig. 3, inset) and reached a maximum of approximately 21 nmol/min/mg of cell protein. In contrast, the glycine-betaine uptake activity of E. coli WG855 was much higher (fivefold higher at an osmolality of 0.2 mol/kg) and it did not reach a limiting value within the osmolality range tested (Fig. 3, inset). These differences were unlikely to result solely from copy number effects.
FIG. 3.
betU encodes a glycine-betaine transporter. Initial rates of glycine-betaine uptake by E. coli strains WG695 [HU734 Δ(putPA)566 Δ(proP)218 Δ(proV-proX)567] (closed circles) and WG855 (MKH13 pAL3) (open circles) were determined as a function of assay medium osmolality as described in Materials and Methods. Relative rates of glycine-betaine uptake, calculated by setting the maximum rate to a value of 1 for each strain, are shown in the inset.
The betU gene encodes a 667-residue protein. A BLAST search showed that BetU is similar to members of the BCCT family (70) that are known to catalyze osmoregulatory accumulation of quaternary ammonium compounds such as glycine-betaine (Fig. 2, bottom, and Table 1). Transporters with strong sequence similarity to BetU are also predicted to occur in a number of other organisms, many of which are pathogens. They include the following (by organism and percent sequence identity): Proteus vulgaris, 66%; Pseudomonas aeruginosa, 58, 42, and 40%; Xanthomonas campestris, 41%; Vibrio cholerae, 41%; Staphylococcus aureus, 39%; Mycobacterium tuberculosis, 38%; Yersinia pestis, 37%; Erwinia amylovora, 37%; Bacillus anthracis, 36%; Neisseria meningitidis, 33%. No insertion sequences could be found flanking the genes encoding BetT, EctP, BetP, LcoP, and OpuD. Hydropathy analysis (e.g., TopPred) (82) predicts BetU to be a membrane protein with 12 membrane spanning α-helices and cytoplasmic termini.
Distributions of trkG and betU among E. coli isolates.
The DNA sequences flanking trkG and betU suggest that both were or are components of mobile genetic elements. The distributions of these loci were determined to further assess their evolutionary origins and relationships to E. coli virulence. Locus trkG was present in 66% of the 72 ECOR collection isolates without strong bias among the ECOR groups (Tables 4 and 5). However, trkG was detected in only 16% of a group of isolates associated with UTI (the UTI collection) and 14% of a group of strains representing diverse E. coli pathotypes (the PATH collection) (Tables 2 and 5). The 22.9-kb rac prophage interrupts locus c1819 (function unknown) of E. coli K-12. A different, 1.7-kb DNA sequence occupies the corresponding position in E. coli CFT073 (86). That 1.7-kb insert encodes a homologue of sitD. Iron uptake locus sitABCD is present elsewhere in the genome of CFT073 and in the centisome 63 pathogenicity island of S. enterica serovar Typhimurium (91).
TABLE 4.
Incidence of trkG and betU in the ECOR collection strainsa
| EGb | Strain | Incidence ofc:
|
EGb | Strain | Incidence ofc
|
|||
|---|---|---|---|---|---|---|---|---|
| betU | trkG | betU | trkG | |||||
| A | ECOR-01 | − | + | |||||
| A | ECOR-02 | − | + | |||||
| A | ECOR-03 | − | + | |||||
| A | ECOR-04 | − | + | |||||
| A | ECOR-05 | − | − | |||||
| A | ECOR-06 | − | + | |||||
| A | ECOR-07 | − | − | |||||
| A | ECOR-08 | − | − | |||||
| A | ECOR-09 | − | − | |||||
| A | ECOR-10 | − | − | |||||
| A | ECOR-11 | + | + | |||||
| A | ECOR-12 | + | − | |||||
| A | ECOR-13 | + | − | |||||
| A | ECOR-14 | + | + | |||||
| A | ECOR-15 | − | + | |||||
| A | ECOR-16 | + | + | |||||
| A | ECOR-17 | − | + | |||||
| A | ECOR-18 | − | + | |||||
| A | ECOR-19 | + | + | |||||
| A | ECOR-20 | − | + | |||||
| A | ECOR-21 | − | − | |||||
| A | ECOR-22 | − | + | |||||
| A | ECOR-23 | − | + | |||||
| A | ECOR-24 | + | + | |||||
| A | ECOR-25 | − | + | |||||
| B1 | ECOR-26 | − | + | |||||
| B1 | ECOR-27 | − | + | |||||
| B1 | ECOR-28 | − | − | |||||
| B1 | ECOR-29 | − | + | |||||
| B1 | ECOR-30 | − | + | |||||
| B1 | ECOR-32 | − | + | |||||
| B1 | ECOR-33 | − | − | |||||
| B1 | ECOR-34 | − | − | |||||
| B1 | ECOR-46 | − | + | |||||
| B1 | ECOR-59 | + | − | |||||
| B1 | ECOR-67 | − | − | |||||
| B1 | ECOR-68 | − | − | |||||
| B1 | ECOR-69 | + | − | |||||
| B1 | ECOR-70 | − | + | |||||
| B1 | ECOR-71 | − | + | |||||
| B1 | ECOR-72 | − | + | |||||
| B1 | ECOR-42 | − | + | |||||
| B2 | ECOR-52 | − | + | |||||
| B2 | ECOR-53 | + | − | |||||
| B2 | ECOR-54 | + | + | |||||
| B2 | ECOR-55 | + | + | |||||
| B2 | ECOR-56 | − | − | |||||
| B2 | ECOR-57 | − | + | |||||
| B2 | ECOR-58 | − | + | |||||
| B2 | ECOR-60 | − | + | |||||
| B2 | ECOR-61 | + | + | |||||
| B2 | ECOR-62 | + | + | |||||
| B2 | ECOR-63 | − | + | |||||
| B2 | ECOR-64 | + | + | |||||
| B2 | ECOR-65 | − | − | |||||
| B2 | ECOR-66 | − | − | |||||
| D | ECOR-35 | + | − | |||||
| D | ECOR-36 | + | − | |||||
| D | ECOR-38 | + | + | |||||
| D | ECOR-39 | + | + | |||||
| D | ECOR-40 | + | + | |||||
| D | ECOR-41 | + | − | |||||
| D | ECOR-45 | − | + | |||||
| D | ECOR-47 | − | − | |||||
| D | ECOR-48 | − | + | |||||
| D | ECOR-49 | − | + | |||||
| D | ECOR-50 | + | + | |||||
| D | ECOR-51 | − | + | |||||
| E | ECOR-31 | − | + | |||||
| E | ECOR-37 | − | − | |||||
| E | ECOR-43 | + | − | |||||
| E | ECOR-44 | − | + | |||||
The incidence of each locus was determined by duplex PCR as described in Materials and Methods. To detect betU, PCR was performed with primers putP1, putP2, betU1, and betU2 (Table 3). A 0.2-kb putP amplicon was detected in every reaction mixture. To detect trkG, PCR was performed with primers proV1, proV2, trkG1, and trkG2 (Table 3). A 0.3-kb proV amplicon was detected in every reaction mixture. +, an additional amplicon of the expected size was observed (0.6 kb for betU and 0.4 kb for trkG); −, no additional amplicon was observed.
EG, ECOR group. The ECOR groups represent distinct evolutionary clones of E. coli detected via multilocus enzyme electrophoresis and DNA sequence analysis of housekeeping loci (43, 87).
Percent incidences of betU are as follows: group A, 28%; group B1, 6%; group B2, 50% group D, 58%; group E, 25%. Percent incidences of trkG are as follows: group A, 68%; group B1, 63%; group B2, 71%; group D, 67%; Group E, 50%.
TABLE 5.
Incidence of osmoregulatory loci in representative clinical isolates (the UTI and PATH collections)
| Group or strain namea | Incidence of locusb
|
|
|---|---|---|
| betU | trkG | |
| UTI collection | 29 | 16 |
| HU734 | + | − |
| CFT073 | − | − |
| J96 | + | + |
| 536 | − | − |
| EDL933 | − | − |
| RIMD0509952 | − | − |
| 93-001 | − | − |
| FRIK920 | − | − |
| E2348/69 | − | − |
| B170 | + | − |
| DEC12a | + | − |
| H10407 | + | + |
| 339 | + | + |
| S3 | − | − |
| S4 | − | − |
| C5 (S25) | − | − |
| S39 | + | − |
| S41 | − | − |
| S51 | − | − |
| S76 | + | − |
| S82 | + | − |
| PATH collection | 43 | 14 |
The UTI collection includes 30 urinary tract isolates from a previously described collection (19, 23) plus E. coli CFT073. The PATH collection strains are listed in Table 2.
The incidence of each genetic locus, determined by duplex PCR as described in Materials and Methods and footnote a to Table 4, is reported as percent occurrence for the collections and presence (+) or absence (−) for individual isolates. Loci putP and proV were detected in all strains.
Locus betU was present in one-third of the ECOR collection strains, its incidence being highest (50% or more) in ECOR groups B2 and D (Tables 4 and 5). It has been suggested that genomic sequences common to group B2 organisms diverge deeply from those of commensal E. coli strains in ECOR groups A and B1 and have provided an essential context for the evolution of extraintestinal virulence (7, 65). Since betU is present in only one-half of the group B2 strains, either it is not part of that essential context or it has been selectively lost. betU was present in less than one-half of the isolates in the UTI and PATH collections (29 and 43%, respectively) (Table 5). Given that betU of HU734 is flanked by putative insertion sequences, it may be a nonessential gene that is present in a subset of pathogenic and nonpathogenic E. coli strains due to lateral gene transfer.
DISCUSSION
Our goal is to test the hypothesis that osmoregulatory betaine accumulation contributes to growth in urine and urinary tract colonization by uropathogenic E. coli (16). Before this hypothesis can be rigorously tested, all the osmoregulatory systems that contribute to osmoprotection must be identified. We are therefore conducting detailed studies of pyelonephritis isolates HU734 and CFT073 (18, 22) and surveying commensal and virulent E. coli strains to determine the prevalence and distribution of the identified osmoregulatory mechanisms (this study) (19, 23, 51). Earlier work (i) revealed BetU, an osmoregulatory system present in HU734 but not E. coli K-12 or CFT073, (ii) suggested that osmoregulatory betaine uptake is critical for osmoregulation (and growth in urine) by HU734 but not CFT073, and (iii) implied that CFT073 may harbor yet another glycine-betaine-independent osmoregulatory system that contributes to bacterial growth in urine and is not present in HU734.
In this paper, we report the isolation and characterization of the betU locus from E. coli HU734. Gene betU encodes a 667-residue protein that is a member of the BCCT family and predicted to have 12 transmembrane helices (Fig. 2). The BCCT family continues to grow (Fig. 2), and it appears to dominate osmoregulation in some organisms (e.g., C. glutamicum), whereas osmoregulatory ABC transporters appear to be dominant in others (e.g., B. subtilis) (Table 1). Gene betU could have coevolved with paralogue betT after a gene duplication event in E. coli. Alternatively, since betU is flanked by insertion sequences, it could have arrived by lateral transfer. In contrast, the genes encoding BCCTs BetT, BetP, EctP, and OpuD are not flanked by insertion sequences. Much higher glycine-betaine uptake activity could be attributed to BetU when betU was expressed from its own promoter in E. coli K-12 than when betU was expressed in its native genetic background (Fig. 3). This may indicate that elements required to regulate betU expression are absent from E. coli K-12.
One-third of the E. coli strains included in this study harbored locus betU (Tables 4 and 5). The incidence of betU among pathogenic E. coli strains included in this study (34% overall) was similar to that in the ECOR collection (32%) but lower than that in ECOR groups B2 and D (Tables 4 and 5). Clearly the presence of betU was not selected during the evolution of urovirulence. In contrast, locus trkG, which encodes an osmoregulatory K+ transporter similar in structure and function to TrkH, occurred much less frequently among pathogenic E. coli isolates (overall incidence, 16%) than among the (predominantly) commensal isolates of the ECOR collection (overall incidence, 66%) (Tables 4 and 5). Indeed, the sequenced genomes of two E. coli O157:H7 isolates harbor lambdoid prophages at the Rac insertion site, but both lack trkG. Thus, TrkG is not essential for virulence and incorporation of trkG in the Rac prophage, loss of the genetic material that is replaced by Rac or the presence of genetic material within Rac may impair virulence. The presence of insertion sequences flanking betU (at least in the genome of E. coli HU734) as well as the fact that trkG is encoded by (and may be a latecomer to) the Rac prophage (at least in E. coli K-12) imply distribution of these genes by lateral transfer. The UTI and PATH collections are small (31 and 21 isolates, respectively). More extensive analyses conducted with larger numbers of isolates from each pathotype, each characterized by phylogenetic group, may reveal additional evolutionary relationships.
Differences in osmoregulatory trehalose synthesis, K+, or choline uptake did not account for the greater osmotolerance of CFT073 relative to HU734, and no known osmoprotectant stimulated the growth of CFT073 derivatives lacking loci proP, proU, and/or rpoS in high-osmolality medium (see Results). The four sequenced E. coli genomes were analyzed to determine whether CFT073 might contain additional osmoprotectant transporters with new substrate specificities (Table 1). BLAST searches were conducted with known osmoregulatory transporters as query sequences. The significance of each identified relationship between a known osmoregulatory transporter and a protein of unknown function was assessed by comparing the percent identity and extent of sequence alignment with those parameters for pairs of known osmoregulatory transporters.
PutP, BetT, ProP, and ProU (the latter comprised of ProV, ProW, and ProX) are encoded by all four E. coli genomes, since according to BLAST analysis, these genomes share loci which show 99 to 100% sequence identity over alignments covering 100% of the query sequence length. Proline accumulation via PutP is not osmoregulatory for E. coli K-12 (88). PutP could be an osmoregulator in other E. coli strains, since its homologues in B. subtilis and S. aureus have that activity (OpuE and PutP, respectively). However, this seems unlikely, since proline is not an osmoprotectant for a derivative of strain CFT073 which lacks ProP and ProU but not PutP (22). Each secondary transporter (PutP, BetT, or ProP) is comprised of a single integral membrane protein subunit. Homologues of those proteins were considered putative paralogues if there was more than 30% sequence identity over more than 80% of the query sequence length. On that basis, a putative paralogue was found for E. coli ProP but not for PutP or BetT. YhjE, encoded by all four E. coli genomes, shares 33% sequence identity with ProP over an alignment that covers 84% of the ProP sequence. (By comparison, C. glutamicum ProP, a known osmoregulatory transporter, is 39% identical and E. coli ShiA, a shikimate transporter that is not an osmoregulator, is 31% identical to E. coli ProP.)
ABC transporters (e.g., ProU) are comprised of an ATP binding cassette (ABC subunit, e.g., ProV), an integral membrane protein subunit (e.g., ProW), and a periplasmic substrate binding protein (PBP subunit, e.g., ProX). The components of E. coli YehXYWZ were similar to the corresponding components of E. coli ProU but even more closely related to those of OpuC from B. subtilis. Taking into account the presence of yehXYWZ in all four sequenced E. coli genomes, the encoded proteins showed more than 40% sequence identity over more than 70% of the query sequence length (ABC subunit OpuCA), more than 40% sequence identity over more than 80% of the query sequence length (integral membrane protein subunits OpuCB and OpuCD) or more than 25% sequence identity over 97% of the alignment length (PBP subunit OpuCC). Similar relationships were seen to OpuA and OpuB of B. subtilis, with the exception of the PBP subunits. Thus, YehXYWZ is likely paralogous to ProU and may be orthologous to OpuC, a glycine-betaine transporter, but differ in substrate specificity.
No osmoregulatory tripartite ATP-independent periplasmic (TRAP) transporter has been identified in an organism with a sequenced genome, but Gramman et al. have shown that TRAP transporter TeaABC is an osmoregulatory ectoine transporter in Halomonas elongata (35). YiaOMN of E. coli may be orthologous with TeaABC, since the subunits show 24, 24, and 33% identity over alignments that cover 95, 62, and 87% of the query (Tea) sequence length, respectively. However, the YiaOMN subunits are also similar in sequence to those of transporters not implicated in osmoregulation, and yiaOMN is part of a gene cluster implicated in carbon metabolism by E. coli (reference 90 and references cited therein). Interestingly, YiaOMN is encoded by the genomes of E. coli MG1655 and CFT073 but not by the sequenced genomes of E. coli O157:H7 isolates.
Further work will be required to determine whether YhjE, YehXYWZ, and YiaOMN are transporters, whether they transport osmoprotectants, what their substrate specificities are, and how they are distributed among E. coli isolates. Past failure to detect contributions of these systems to osmoregulation in E. coli K-12 or CFT073 could result from failure to offer the appropriate osmoprotectant or failure of these systems to be expressed under past experimental conditions. For example, the latter problem has to date prevented study of C. glutamicum ProP in its native context (64). These data suggest that E. coli and other organisms share a pool of genes encoding osmoregulatory transporters, some of which can be readily transferred among organisms. No particular complement of osmoregulatory systems is common to all E. coli strains.
Acknowledgments
We are grateful to the following individuals and to the American Type Culture Collection for providing clinical E. coli isolates: S. Bonacorsi (Hôpital Robert Debré, Paris, France), B. B. Finlay (University of British Columbia), C. L. Gyles (University of Guelph), J. Hacker (Universität Würzburg), R. P. Johnson (Health Canada, Guelph, Canada), H. L. Mobley (University of Maryland), G. Reid (University of Western Ontario), and T. S. Whittam (Michigan State University).
This research was supported by Operating Grant MT-15113, awarded to J.M.W. by the Canadian Institutes for Health Research.
REFERENCES
- 1.Abee, T., and J. A. Wouters. 1999. Microbial stress response in minimal processing. Int. J. Food Microbiol. 50:65-91. [DOI] [PubMed] [Google Scholar]
- 2.Altendorf, K., and W. Epstein. 1993. Kdp-ATPase of Escherichia coli. Cell. Physiol. Biochem. 4:160-168. [Google Scholar]
- 3.Altman, P. L. 1961. Physical properties and chemical composition of urine: mammals. Part 1: man, p. 363-369. In D. S. Dittmer (ed.), Blood and other bodily fluids. Federation of American Societies for Experimental Biology, Washington, D.C.
- 4.Andresen, P. A., I. Kaasen, O. B. Styrvold, G. Boulnois, and A. R. Strøm. 1988. Molecular cloning, physical mapping and expression of bet genes governing the osmoregulatory choline-glycine betaine pathway of Escherichia coli. J. Gen. Microbiol. 134:1737-1746. [DOI] [PubMed] [Google Scholar]
- 5.Bankir, L. 1996. Urea and the kidney, p. 571-606. In B. M. Brenner (ed.), The kidney. Saunders, Philadelphia, Pa.
- 6.Benaroudj, N., D. H. Lee, and A. L. Goldberg. 2001. Trehalose accumulation during cellular stress protects cells and cellular proteins from damage by oxygen radicals. J. Biol. Chem. 276:24261-24267. [DOI] [PubMed] [Google Scholar]
- 7.Bingen, E., B. Picard, N. Brahimi, S. Mathy, P. Desjardins, J. Elion, and E. Denamur. 1998. Phylogenetic analysis of Escherichia coli strains causing neonatal meningitis suggests horizontal gene transfer from a predominant pool of highly virulent B2 group strains. J. Infect. Dis. 177:642-650. [DOI] [PubMed] [Google Scholar]
- 8.Blattner, F. R., G. Plunkett III, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, J. Collado-Vides, J. D. Glasner, C. K. Rode, G. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrick, M. A. Goeden, D. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453-1462. [DOI] [PubMed] [Google Scholar]
- 9.Bolivar, F., R. L. Rodriguez, P. J. Greene, M. C. Betlach, H. L. Heyneker, H. W. Boyer, J. H. Crosa, and S. Falko. 1977. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene 2:95-113. [PubMed] [Google Scholar]
- 10.Bonacorsi, S., O. Clermont, V. Houdouin, C. Cordevant, N. Brahimi, A. Marecat, C. Tinsley, X. Nassif, M. Lange, and E. Bingen. 2003. Molecular analysis and experimental virulence of French and North American Escherichia coli neonatal meningitis isolates: identification of a new virulent clone. J. Infect. Dis. 187:1895-1906. [DOI] [PubMed] [Google Scholar]
- 11.Bonacorsi, S. P. P., O. Clermont, C. Tinsley, I. LeGall, J.-C. Beaudoin, J. Elion, X. Nassif, and E. Bingen. 2000. Identification of regions of the Escherichia coli chromosome specific for neonatal meningitis-associated strains. Infect. Immun. 68:2096-2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boscari, A., K. Mandon, L. Dupont, M. C. Poggi, and D. Le Rudulier. 2002. BetS is a major glycine betaine/proline betaine transporter required for early osmotic adjustment in Sinorhizobium meliloti. J. Bacteriol. 184:2654-2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burg, M. B. 1995. Molecular basis of osmotic regulation. Am. J. Physiol. 268:F983-F996. [DOI] [PubMed] [Google Scholar]
- 14.Canovas, D., S. Fletcher, M. Hayashi, and L. N. Csonka. 2001. Role of trehalose in growth at high temperature of Salmonella enterica serovar Typhimurium. J. Bacteriol. 183:3365-3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chambers, S. T., and C. M. Kunin. 1987. Isolation of glycine betaine and proline betaine from human urine. Assessment of their role as osmoprotective agents for bacteria and the kidney. J. Clin. Investig. 79:731-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chambers, S. T., and M. Lever. 1996. Betaines and urinary tract infections. Nephron 74:1-10. [DOI] [PubMed] [Google Scholar]
- 17.Csonka, L. N., and W. Epstein. 1996. Osmoregulation, p. 1210-1223. In F. C. Neidhardt (ed.), Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington, D.C.
- 18.Culham, D. E., C. Dalgado, C. L. Gyles, D. Mamelak, S. MacLellan, and J. M. Wood. 1998. Osmoregulatory transporter ProP influences colonization of the urinary tract by Escherichia coli. Microbiology 144:91-102. [DOI] [PubMed] [Google Scholar]
- 19.Culham, D. E., K. S. Emmerson, B. Lasby, D. Mamelak, B. A. Steer, C. L. Gyles, M. Villarejo, and J. M. Wood. 1994. Genes encoding osmoregulatory proline/glycine betaine transporters and the proline catabolic system are present and expressed in diverse clinical Escherichia coli isolates. Can. J. Microbiol. 40:397-402. [DOI] [PubMed] [Google Scholar]
- 20.Culham, D. E., J. Henderson, R. A. Crane, and J. M. Wood. 2003. Osmosensor ProP of Escherichia coli responds to the concentration, chemistry and molecular size of osmolytes in the proteoliposome lumen. Biochemistry 42:410-420. [DOI] [PubMed] [Google Scholar]
- 21.Culham, D. E., B. Lasby, A. G. Marangoni, J. L. Milner, B. A. Steer, R. W. van Nues, and J. M. Wood. 1993. Isolation and sequencing of Escherichia coli gene proP reveals unusual structural features of the osmoregulatory proline/betaine transporter, ProP. J. Mol. Biol. 229:268-276. [DOI] [PubMed] [Google Scholar]
- 22.Culham, D. E., A. Lu, M. Jishage, K. A. Krogfelt, A. Ishihama, and J. M. Wood. 2001. The osmotic stress response and virulence in pyelonephritis isolates of Escherichia coli: contributions of rpoS, proP, proU and other systems. Microbiology 147:1657-1670. [DOI] [PubMed] [Google Scholar]
- 23.Culham, D. E., and J. M. Wood. 2000. An ECOR group B2- and uropathogen-associated polymorphism in the rpoS-mutS region of the Escherichia coli chromosome. J. Bacteriol. 182:6272-6276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dinnbier, U., E. Limpinsel, R. Schmid, and E. P. Bakker. 1988. Transient accumulation of potassium glutamate and its replacement by trehalose during adaptation of growing cells of Escherichia coli K-12 to elevated sodium chloride concentrations. Arch. Microbiol. 150:348-357. [DOI] [PubMed] [Google Scholar]
- 25.Dobrindt, U., B. Janke, K. Piechaczek, G. Nagy, W. Ziebuhr, G. Fischer, A. Schierhorn, M. Hecker, G. Blum-Oehler, and J. Hacker. 2000. Toxin genes on pathogenicity islands: impact for microbial evolution. Int. J. Med. Microbiol. 290:307-311. [DOI] [PubMed] [Google Scholar]
- 26.Dosch, D. C., G. L. Helmer, S. H. Sutton, F. F. Salvacion, and W. Epstein. 1991. Genetic analysis of potassium transport loci in Escherichia coli: evidence for three constitutive systems mediating uptake of potassium. J. Bacteriol. 173:687-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Driessen, A. J. M., B. P. Rosen, and W. N. Konings. 2000. Diversity of transport mechanisms: common structural principles. Trends Biochem. Sci. 25:397-401. [DOI] [PubMed] [Google Scholar]
- 28.Epstein, W., E. T. Buurman, D. McLaggan, and J. Naprstek. 1993. Multiple mechanisms, roles and controls of K+ transport in Escherichia coli. Biochem. Soc. Trans. 21:1006-1010. [DOI] [PubMed] [Google Scholar]
- 29.Epstein, W., and M. Davies. 1970. Potassium-dependent mutants of Escherichia coli K-12. J. Bacteriol. 101:836-843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Evans, D. G., D. J. Evans, Jr., and N. F. Pierce. 1973. Differences in the response of rabbit small intestine to heat-labile and heat-stable enterotoxins of Escherichia coli. Infect. Immun. 7:873-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fletcher, S., D. Rhodes, and L. N. Csonka. 2001. Analysis of the effects of osmoprotectants on the high osmolality-dependent induction of increased thermotolerance in Salmonella typhimurium. Food Microbiol. 18:345-354. [Google Scholar]
- 32.Fletcher, S. A., and L. N. Csonka. 1998. Characterization of the induction of increased thermotolerance by high osmolality in Salmonella typhimurium. Food Microbiol. 15:307-317. [Google Scholar]
- 33.Gorbach, S. L., J. G. Banwell, B. D. Chatterjee, B. Jacobs, and R. B. Sack. 1971. Acute undifferentiated human diarrhea in the tropics. I. Alterations in intestinal micrflora. J. Clin. Investig. 50:881-889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gordon, D. M., and M. A. Riley. 1992. A theoretical and experimental analysis of bacterial growth in the bladder. Mol. Microbiol. 6:555-562. [DOI] [PubMed] [Google Scholar]
- 35.Gramman, K., A. Volke, and H. J. Kunte. 2002. New type of osmoregulated solute transporter identified in halophilic members of the Bacteria domain: TRAP transporter TeaABC mediates uptake of ectoine and hydroxyectoine in Halomonas elongata DSM 2581T. J. Bacteriol. 184:3078-3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haardt, M., B. Kempf, E. Faatz, and E. Bremer. 1995. The osmoprotectant proline betaine is a major substrate for the binding-protein-dependent transport system ProU of Escherichia coli K-12. Mol. Gen. Genet. 246:783-786. [DOI] [PubMed] [Google Scholar]
- 37.Hagberg, L., I. Engberg, R. Freter, J. Lam, S. Olling, and C. Svanborg Eden. 1983. Ascending, unobstructed urinary tract infection in mice caused by pyelonephritogenic Escherichia coli of human origin. Infect. Immun. 40:273-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557-569. [DOI] [PubMed] [Google Scholar]
- 39.Hancock, D., T. Besser, J. Lejeune, M. Davis, and D. Rice. 2001. The control of VTEC in the animal reservoir. Int. J. Food Microbiol. 66:71-78. [DOI] [PubMed] [Google Scholar]
- 40.Hayashi, T., K. Makino, M. Ohnishi, K. Kurokawa, K. Yokoyama, C.-G. Han, E. Ohtsubo, K. Nakayama, T. Murata, M. Tanaka, T. Tobe, T. Iida, H. Takami, T. Honda, C. Sasakawa, N. Ogasawara, T. Yasunaga, S. Kuhara, T. Shiba, M. Hattori, and H. Shinagawa. 2001. Complete genome sequence of enterohemorrhagic Escherichia coli O157:H7 and genomic comparison with a laboratory strain K-12. DNA Res. 8:11-22. [DOI] [PubMed] [Google Scholar]
- 41.Hengge-Aronis, R. 1996. Back to log phase: sigma S as a global regulator in the osmotic control of gene expression in Escherichia coli. Mol. Microbiol. 21:887-893. [DOI] [PubMed] [Google Scholar]
- 42.Hengge-Aronis, R., R. Lange, N. Henneberg, and D. Fischer. 1993. Osmotic regulation of rpoS-dependent genes in Escherichia coli. J. Bacteriol. 175:259-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Herzer, P. J., S. Inouye, M. Inouye, and T. S. Whittam. 1990. Phylogenetic distribution of branched RNA-linked multicopy single-stranded DNA among natural isolates of Escherichia coli. J. Bacteriol. 172:6175-6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hill, C., P. D. Cotter, R. D. Sleator, and C. G. M. Gahan. 2002. Bacterial stress response in Listeria monocytogenes: jumping the hurdles imposed by minimal processing. Int. Dairy J. 12:273-283. [Google Scholar]
- 45.Hull, R. A., R. E. Gill, P. Hsu, B. H. Minshew, and S. Falkow. 1981. Construction and expression of recombinant plasmids encoding type 1 or d-mannose-resistant pili from a urinary tract infection Escherichia coli isolate. Infect. Immun. 33:933-938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kandror, O., A. DeLeon, and A. L. Goldberg. 2002. Trehalose synthesis is induced upon exposure of Escherichia coli to cold and is essential for viability at low temperatures. Proc. Natl. Acad. Sci. USA 99:9727-9732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kappes, R. M., B. Kempf, and E. Bremer. 1996. Three transport systems for the osmoprotectant glycine betaine operate in Bacillus subtilis: characterization of OpuD. J. Bacteriol. 178:5071-5079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kempf, B., and E. Bremer. 1998. Uptake and synthesis of compatible solutes as microbial stress responses to high osmolality environments. Arch. Microbiol. 170:319-330. [DOI] [PubMed] [Google Scholar]
- 49.Kim, J., J. Nietfeldt, J. Ju, J. Wise, N. Fegan, P. Desmarchelier, and A. K. Benson. 2001. Ancestral divergence, genome diversification, and phylogeographic variation in subpopulations of sorbitol-negative, β-glucuronidase-negative enterohemorrhagic Escherichia coli O157. J. Bacteriol. 183:6885-6897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kunin, C. M. 1987. Detection, prevention and management of urinary tract infections. Lea & Febiger, Philadephia, Pa.
- 51.Kunin, C. M., T. H. Hua, L. Van Arsdale White, and M. Villarejo. 1992. Growth of Escherichia coli in human urine: role of salt tolerance and accumulation of glycine betaine. J. Infect. Dis. 166:1311-1315. [DOI] [PubMed] [Google Scholar]
- 52.Levine, M. M., E. J. Bergquist, D. R. Nalin, D. H. Waterman, R. B. Hornick, C. R. Young, and S. Sotman. 1978. Escherichia coli strains that cause diarrhea but do not produce heat-labile or heat-stable enterotoxins and are non-invasive. Lancet i:1119-1122. [DOI] [PubMed] [Google Scholar]
- 53.Louis, P., H. G. Trüper, and E. A. Galinski. 1994. Survival of Escherichia coli during drying and storage in the presence of compatible solutes. Appl. Microbiol. Biotechnol. 41:684-688. [Google Scholar]
- 54.MacMillan, S. V., D. A. Alexander, D. E. Culham, H. J. Kunte, E. V. Marshall, D. Rochon, and J. M. Wood. 1999. The ion coupling and organic substrate specificities of osmoregulatory transporter ProP in Escherichia coli. Biochim. Biophys. Acta 1420:30-44. [DOI] [PubMed] [Google Scholar]
- 55.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 56.Mobley, H. L., K. G. Jarvis, J. P. Elwood, D. I. Whittle, C. V. Lockatell, R. G. Russell, D. E. Johnson, M. S. Donnenberg, and J. W. Warren. 1993. Isogenic P-fimbrial deletion mutants of pyelonephritogenic Escherichia coli: the role of alpha Gal(1-4) beta Gal binding in virulence of a wild-type strain. Mol. Microbiol. 10:143-155. [DOI] [PubMed] [Google Scholar]
- 57.Morbach, S., and R. Krämer. 2002. Body shaping under water stress: osmosensing and osmoregulation of solute transport in bacteria. Chembiochem 3:384-397. [DOI] [PubMed] [Google Scholar]
- 58.Neidhardt, F. C., P. L. Bloch, and D. F. Smith. 1974. Culture medium for enterobacteria. J. Bacteriol. 119:736-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ochman, H., and R. K. Selander. 1984. Standard reference strains of Escherichia coli from natural populations. J. Bacteriol. 157:690-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Orskov, F., T. S. Whittam, A. Cravioto, and I. Orskov. 1990. Clonal relationships among classic enteropathogenic Escherichia coli (EPEC) belong to different O groups. J. Infect. Dis. 162:76-81. [DOI] [PubMed] [Google Scholar]
- 61.Paulozzi, L. J., K. E. Johnson, L. M. Kamahele, C. R. Clausen, L. W. Riley, and S. D. Helgerson. 1986. Diarrhea associated with adherent enteropathogenic Escherichia coli in an infant and toddler center, Seattle, Washington. Pediatrics 77:296-300. [PubMed] [Google Scholar]
- 62.Perna, N. T., G. Plunkett III, V. Burland, B. Mau, J. D. Glasner, D. J. Rose, G. F. Mayhew, P. S. Evans, J. Gregor, H. A. Kirkpatrick, G. Posfai, J. Hackett, S. Klinck, A. Boutin, Y. Shao, L. Miller, E. J. Grotbeck, N. W. Davis, A. Lim, E. T. Dimalanta, K. D. Potamousis, J. Apodaca, T. S. Anantharaman, J. Lin, G. Yen, D. C. Schwartz, R. A. Welch, and F. R. Blattner. 2001. Genome sequence of enterohaemorrhagic Escherichia coli O157:H7. Nature 409:529-533. [DOI] [PubMed] [Google Scholar]
- 63.Peter, H., A. Burkovski, and R. Krämer. 1996. Isolation, characterization, and expression of the Corynebacterium glutamicum betP gene, encoding the transport system for the compatible solute glycine betaine. J. Bacteriol. 178:5229-5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Peter, H., B. Weil, A. Burkovski, R. Krämer, and S. Morbach. 1998. Corynebacterium glutamicum is equipped with four secondary carriers for compatible solutes: identification, sequencing, and characterisation of the proline/ectoine uptake system ProP and the ectoine/proline/glycine betaine carrier EctP. J. Bacteriol. 180:6005-6012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Picard, B., J. S. Garcia, S. Gouriou, P. Duriez, N. Brahimi, E. Bingen, J. Elion, and E. Denamur. 1999. The link between phylogeny and virulence in Escherichia coli extraintestinal infection. Infect. Immun. 67:546-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Randall, K., M. Lever, B. A. Peddie, and S. T. Chambers. 1996. Natural and synthetic betaines counter the effects of high NaCl and urea concentrations. Biochim. Biophys. Acta 1291:189-194. [DOI] [PubMed] [Google Scholar]
- 67.Reid, S. D., C. J. Hrebelin, A. C. Bumbaugh, R. K. Selander, and T. S. Whittam. 2000. Parallel evolution of virulence in pathogenic Escherichia coli. Nature 406:64-67. [DOI] [PubMed] [Google Scholar]
- 68.Riley, L. W., R. S. Remis, S. D. Helgerson, H. B. McGee, J. G. Wells, B. R. Davis, R. J. Hebert, E. S. Olcott, L. M. Johnson, N. T. Hargrett, P. A. Blake, and M. L. Cohen. 1983. Hemorrhagic colitis associated with a rare Escherichia coli serotype. N. Engl. J. Med. 308:681-685. [DOI] [PubMed] [Google Scholar]
- 69.Ross, D. L., and A. E. Neely. 1983. Textbook of urinalysis and bodily fluids. Appleton Century Crofts, Norwalk, Va.
- 70.Saier, M. H., Jr. 2000. Families of transmembrane transporters selective for amino acids and their derivatives. Microbiology 146:1775-1795. [DOI] [PubMed] [Google Scholar]
- 71.Sambrook, J., E. F. Frisch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 72.Schlösser, A., S. Kluttig, A. Hamann, and E. P. Bakker. 1991. Subcloning, nucleotide sequence, and expression of trkG, a gene that encodes an integral membrane protein involved in potassium uptake via the Trk system of Escherichia coli. J. Bacteriol. 173:3170-3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schlösser, A., M. Meldorf, S. Stumpe, E. P. Bakker, and W. Epstein. 1995. TrkH and its homolog, TrkG, determine the specificity and kinetics of cation transport by the Trk system of Escherichia coli. J. Bacteriol. 177:1908-1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shebuski, J. R., O. Vilhelmsson, and K. J. Miller. 2000. Effects of growth at low water activity on the thermal tolerance of Staphylococcus aureus. J. Food Prot. 63:1277-1281. [DOI] [PubMed] [Google Scholar]
- 75.Sleator, R. D., C. G. M. Gahan, T. Abee, and C. Hill. 1999. Identification and disruption of BetL, a secondary glycine betaine transport system linked to the salt tolerance of Listeria monocytogenes LO28. Appl. Environ. Microbiol. 65:2078-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sleator, R. D., and C. Hill. 2001. Bacterial osmoadaptation: the role of osmolytes in bacteria stress and virulence. FEMS Microbiol. Rev. 731:1-23. [DOI] [PubMed] [Google Scholar]
- 77.Smith, L. T. 1996. Role of osmolytes in adaptation of osmotically stressed and chill-stressed Listeria monocytogenes grown in liquid media and on processed meat surfaces. Appl. Environ. Microbiol. 62:3088-3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Smith, P. K., R. I. Krohn, G. T. Hermanson, A. K. Mallia, F. H. Gartner, M. D. Provenzano, E. K. Fujimoto, N. M. Goeke, B. J. Olson, and D. C. Klenk. 1985. Measurement of protein using bicinchoninic acid. Anal. Biochem. 150:76-85. [DOI] [PubMed] [Google Scholar]
- 79.Sunda, W., D. J. Kieber, R. P. Kiene, and S. Huntsman. 2002. An antioxidant function for DMSP and DMS in marine algae. Nature 418:317-320. [DOI] [PubMed] [Google Scholar]
- 80.Svanborg Eden, C., R. Hull, S. Falkow, and H. Leffler. 1983. Target cell specificity of wild-type E. coli and mutants and clones with genetically defined adhesins. Prog. Food Nutr. Sci. 7:75-89. [PubMed] [Google Scholar]
- 81.Tauschek, M., R. J. Gorrell, R. A. Strugnell, and R. M. Robins-Browne. 2002. Identification of a protein secretory pathway for the secretion of heat-labile enterotoxin by an enterotoxigenic strain of Escherichia coli. Proc. Natl. Acad. Sci. USA 99:7066-7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.von Heinje, G. 1992. Membrane protein structure prediction, hydrophobicity analysis and the positive-inside rule. J. Mol. Biol. 225:487-494. [DOI] [PubMed] [Google Scholar]
- 83.Wang, A. J., and D. W. Bolen. 1997. A naturally occurring protective system in urea-rich cells: mechanism of osmolyte protection of proteins against urea denaturation. Biochemistry 36:9101-9108. [DOI] [PubMed] [Google Scholar]
- 84.Watanabe, H., A. Wada, Y. Inagaki, K. Itoh, and K. Tamura. 1996. Outbreaks of enterohaemorrhagic Escherichia coli O157:H7 infection by two different genotype strains in Japan. Lancet 348:831-832. [DOI] [PubMed] [Google Scholar]
- 85.Watson, N. 1988. A new revision of the sequence of plasmid pBR322. Gene 70:399-403. [DOI] [PubMed] [Google Scholar]
- 86.Welch, R. A., V. Burland, G. Plunkett III, P. Redford, P. Roesch, D. Rasko, E. L. Buckles, S. R. Liou, A. Boutin, J. Hackett, D. Stroud, G. F. Mayhew, D. J. Rose, S. Zhou, D. C. Schwartz, N. T. Perna, H. L. Mobley, M. S. Donnenberg, and F. R. Blattner. 2002. Extensive mosaic structure revealed by the complete genome sequence of uropathogenic Escherichia coli. Proc. Natl. Acad. Sci. USA 99:17020-17024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Whittam, T. S. 1996. Genetic variation and evolutionary processes in natural populations of Escherichia coli, p. 2708-2722. In F. C. Neidhardt (ed.), Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington, D.C.
- 88.Wood, J. M. 1999. Osmosensing by bacteria: signals and membrane-based sensors. Microbiol. Mol. Biol. Rev. 63:230-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wood, J. M., E. Bremer, L. N. Csonka, R. Krämer, B. Poolman, T. van der Heide, and L. T. Smith. 2001. Osmosensing and osmoregulatory compatible solute accumulation by bacteria. Comp. Biochem. Physiol. 130:437-460. [DOI] [PubMed] [Google Scholar]
- 90.Yew, W. S., and J. A. Gerlt. 2002. Utilization of l-ascorbate by Escherichia coli K-12: assignments of functions to products of the yjf-sga and yia-sgb operons. J. Bacteriol. 184:302-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhou, D., W. D. Hardt, and J. E. Galan. 1999. Salmonella typhimurium encodes a putative iron transport system within the centisome 63 pathogenicity island. Infect. Immun. 67:1974-1981. [DOI] [PMC free article] [PubMed] [Google Scholar]