Abstract
Smoking during pregnancy is a persistent public health problem that has been linked to later adverse outcomes. The neonatal period, the first month of life, carries substantial developmental change in regulatory skills, and is the period when tobacco metabolites are cleared physiologically. Studies to date mostly have used cross-sectional designs that limit characterizing potential impacts of prenatal tobacco exposure on the development of key self-regulatory processes and cannot disentangle short-term withdrawal effects from residual exposure-related impacts. In this study, pregnant participants (N = 304) were recruited prospectively during pregnancy and smoking was measured at multiple time points, using both self report and biochemical measures. Neonatal attention, irritable reactivity, and stress dysregulation were examined longitudinally at three time points during the first month of life, and physical growth indices were measured at birth. Tobacco-exposed infants showed significantly poorer attention skills after birth, and the magnitude of the difference between exposed and non-exposed groups attenuated across the neonatal period. In contrast, exposure-related differences in irritable reactivity were not evident and stable across the first month of life, but differed only marginally at 4-weeks of age. Third trimester smoking was associated with pervasive, deleterious, dose-response impacts on physical growth measured at birth, whereas nearly all smoking indicators throughout pregnancy predicted level and growth rates of early attention. The observed neonatal pattern is consistent with the neurobiology of tobacco on the developing nervous system and fits with developmental vulnerabilities observed later in life.
Keywords: Prenatal Tobacco Exposure, Self-Regulation, Longitudinal Modeling
Approximately 20% of women acknowledge smoking during pregnancy in the US (Office of Applied Studies National Survey on Drug Use and Health, 2005), which results in at least 500,000 prenatally tobacco infants annually. Smoking during pregnancy is substantially more prevalent than prenatal use of alcohol or illicit drugs. For most women, smoking is a daily habit that when pregnant, results in a regular dosing pattern to their fetus. As such, prenatal tobacco exposure carries broad risk for harm and potential morbidity (Koren, 1993; Slotkin, 1998b).
Tobacco contains a number of chemically active compounds. Nicotine appears to be the predominant contributor on the growth and behavior of children exposed during pregnancy. Nicotine is a powerful vasoconstrictor that reduces the flow of available nutrients and oxygen to the developing fetus. Indeed, exposure-related reductions in birth weight have been reported in the literature for several decades. Besides birth weight, prenatal tobacco exposure also is associated with dose dependent reductions in body length and head size (e.g., Hardy & Mellits, 1972; Rantakallio, 1983; Roza et al., 2007; Vik, Jacobsen, Vatten, & Bakketeig, 1996). These exposure-related physical growth differences at birth usually resolve by the infant’s first birthday (Day et al. 1992; Hardy & Mellits, 1972; Conter, Cortinovis, Rogari, & Riva 1995). The physical growth deficits and the associated tobacco exposure-related increase in perinatal complications both contribute to, but do not completely account for, a greater risk for Attention Deficit/Hyperactivity Disorder (Nigg & Breslau, 2007; Szatmari, Saigal, Rosenbaum, Campbell, & King, 1990; Willoughby, Greenberg, Blair, Stifter, & Family Life Investigative Group, 2007).
Although largely ignored for decades, nicotine is also a psychoactive compound that acts directly on the brain. Nicotine activates nicotinic acetylcholine receptors are situated on dopamine neurons in the striatum and noradrenergic neurons in the locus coeruleus (Lichtensteiger et al., 1982) and are present as early as eight weeks gestation (Hagino & Lee, 1985). In elegant pre-clinical work in non-human animals, prenatal tobacco exposure has been found to disrupt the timing of cholinergic synaptic activity during key developmental periods, alter receptor-mediated processes controlling cell replication and differentiation (Slotkin, 1998a), and result in abnormal neuronal reactivity (Seidler, Levin, Lappi, & Slotkin, 1992; Navarro, Seidler, Whitmore, & Slotkin, 1988; Slotkin, Lappi, & Seidler, 1995; Landmesser, 1994) including the disruption of developing dopaminergic circuits (Azam, Chen, & Leslie, 2007). When administered prenatally, nicotine reduces postnatal dopaminergic activity in the ventral tegmental area, nucleus accumbens, and striatum (Chen, Parker, Matta, & Sharp, 2005; Muneoka et al., 1997; Slotkin, 1998b), with a corresponding reduction in D2 dopamine receptors (Richardson & Tizabi, 1994). Serotonergic systems are affected similarly, as prenatal tobacco exposure disrupts paroxetine binding to the 5-HT transporter (Levin & Slotkin, 1998). These disruptions persist well after nicotine exposure has ceased (McFarland, Seidler, & Slotkin, 1991), suggesting that prenatal nicotine exposure alters cell development programs in an irreversible manner (Slotkin, 1998b) that is not attributable solely to the hypoxic effects of nicotine on the central nervous system (Slotkin, Greer, Faust, Cho, & Seidler, 1986).
Given the strong link between alterations of the dopaminergic and serotonergic brain systems and developmental psychopathogy, it may not be surprising that many studies have linked prenatal tobacco exposure to externalizing behaviors in childhood (e.g., Day, Richardson, Goldschmidt, & Cornelius, 2000; Wakschlag, Leventhal, Pine, Pickett, & Carter, 2006) and to the clinical diagnoses of Attention Deficit/Hyperactivity Disorder, and Oppositional Defiant Disorder (Huizink & Mulder, 2006; Wakschlag, Pickett, Cook, Benowitz, & Leventhal, 2002; Kotimaa et al., 2003; Orbelke, Knol, & Verhulst, 1999). Furthermore, self-reported prenatal smoking also has been associated with inattention, overactivity, and an impulsive response style at preschool and early school age (Day et al. 2000; Fried, Watkinson, & Gray, 1992; Johnson, Vicary, Heist, & Corneal, 2001; Leech, Richardson, Goldschmidt, & Day, 1999), working memory and inhibition deficits in adolescents (Fried & Watkinson, 2001; Jacobsen, Slotkin, Westerveld, Mencl, & Pugh, 2006; Bennett et al., 2009), and negative emotionality in infancy and young children (Fried & Makin, 1987; Wakschlag & Hans, 2002; Schuetze & Eiden, 2007; Kelmanson, Erman, & Litvina, 2002; Brook, Brook, & Whiteman, 2000; Willoughby, Greenberg, Blair, Stifter, & Family Life Investigative Group, 2007). Dose-response relations between prenatal tobacco exposure and these externalizing behaviors have been reported (e.g., Linnet et al., 2003; Williams et al., 1998, Day et al., 2000). Generally, the effect of exposure on these outcomes is robust, but may be reduced in magnitude when adjusted for confounding environmental and genetic covariates (Linnett et al., 2003; Rodriguez & Bohlin, 2005; Thapar et al., 2003; Maughan, Taylor, Caspi, & Moffitt, 2004) or is eliminated in epidemiological within-family, sibling designs (e.g., D’Onofrio et al., 2007).
Results of studies conducted on newborns in the 1970s and 80s using self-reported smoking suggest exposure-related vulnerabilities in self-regulation. Saxton (1978) examined infant behavior shortly after birth in a small sample of infants born to women who smoked 15 or more cigarettes per day. Tobacco-exposed neonates showed reduced sensitivity to auditory stimuli, evidenced by greater auditory habituation and poorer orientation to auditory inanimate and animate stimuli. Other researchers (Fried, Watkinson, Dillon, & Dulberg, 1987; Piccone, Allen, Olsen, & Ferris, 1982) confirmed these findings and noted reductions in visual attention skills (S. Jacobson, 1984; Richardson, Day, & Taylor, 1989; Streissguth, Sampson, Barr, Bookstein, & Carmichael, 1994). Alterations in state behavior (i.e., increased irritability, Jacobson, 1984; Fried & Makin 1987; Stroud et al., 2009b), disrupted cry (Nugent, Lester, Greene, & Wieczorek-Deering, 1996) and autonomic regulation (Piccone et al., 1982) also have been associated with prenatal tobacco exposure, although not in all studies (Richardson et al., 1989). More recently, a handful of studies that included bioassay-validation of exposure confirmed differences in withdrawal behaviors (heightened irritability, physiologic signs of stress) in the first few days of life (Law et al., 2003; Godding et al., 2004; Mansi et al., 2007) and hint at persistent differences later in the neonatal period in reactivity to handling (Stroud et al., 2009).
Despite its relative temporal brevity, there is substantial skill development in the neonatal period. Shortly after birth, the newborn works to independently achieve physiological stability and homeostasis, including regulation of arousal (Kopp, 1982; Riese, 1987). After homeostasis is achieved, the neonate regulates responsiveness to external stimuli through state modulation and directed orientation of attention (Emde & Buchsbaum, 1989; Bard, Coles, Platzman, & Lynch, 2000). Investigations that have focused on behavior shortly after birth likely do not fully capture the impacts of prenatal exposure on skill development across the period. From the perspective of exposure, the neonatal period begins with physiological clearing of nicotine and other tobacco compounds from maternal smoking late in pregnancy, where both exposure and withdrawal effects are evident shortly after birth. Later in the neonatal period, the persistent, residual impacts of exposure on neurobehavior can be observed without the confounding of short-term withdrawal behaviors. Furthermore, because behavioral manifestations of brain alterations may not be evident until the age at which the compromised area is called into action for skill execution (Goldman, 1974), in some cases long after the damage occurred, new insights can be gained by examining exposure-related outcome with repeated measurements. For the neonatal period, longitudinal designs permit characterization of how prenatal tobacco alters the developmental trajectory of regulatory skills and can help to disentangle short-term withdrawal from the more persistent, residual effects of exposure.
Indeed, results from a handful of studies hint at persistent differences later in the neonatal period. Fried and Makin (1987), for example, found greater impairment in TE infants in motor response at 30 days of age than at 9 days of age. More recently, Stroud and colleagues (2009a) examined the impact of prenatal tobacco exposure on the regulatory behavior of 56 neonates at 10 to 27 days. Exposed neonates did not differ from their SES- and alcohol-exposure-matched peers in stress responses or muscle tone, but exposed infants exhibited a greater need for handling and scored lower on self-regulation items. In a large sample of White and Black infants, the amount of exposure indexed by maternal serum cotinine was related to differences in arousal and regulation at 5-weeks of age (Yolton et al., 2009). To date, no studies have leveraged longitudinal data to examine exposure-related differences across early development.
The purpose of the present study, then, was to delineate the impact of prenatal tobacco exposure on the early development of emergent regulatory processes across the first month of life, the neonatal period, using a prospective, cohort design with self-reported and bioassay indices of exposure collected repeatedly during pregnancy. Using hierarchical growth modeling, the effect of prenatal tobacco exposure can be parsed into those that affect the level of self-regulatory behavior at any given age and those that affect the rate of behavior change or development, to better characterize the impact of exposure on the underlying developmental process. This modeling approach already has been demonstrated to be useful. For example, Espy, Riese, and Francis (1997) observed that prenatal cocaine exposure differentially affected the developmental level and rate parameters. Building on extant findings, we hypothesized that tobacco-exposed neonates would show reduced self-regulatory skills after birth, manifested by poorer attention and orientation, increased irritability and greater stress dysregulation, as well as persistent exposure-related differences at 4-weeks of age. Because our study was motivated by a neural systems perspective to better characterize specific tobacco exposure effects, we were particularly interested in isolating the impact of tobacco exposure as much as possible, and thus strict subject selection procedures were used to minimize other exposures and influences. With these selection methods, we postulated that differences in self-regulatory behaviors would be related in a dose response manner to the number of cigarettes smoked by the mother during pregnancy, indexed by self report and bioassays at each trimester. We also examined exposure-related differences in physical growth at birth, as these indices have been shown to be important mediators in other behavioral teratologic studies (e.g., head circumference and prenatal cocaine exposure; Eyler, Behnke, Conlon, Woods, & Wobie, 1998). Finally, exposure-related differences in the development of rudimentary self-regulatory processes were explored by evaluating differences in the rate of skill growth across the entire neonatal period.
Method
Participants
The sampling strategy was designed to compare two neonatal groups, tobacco exposed (TE) and non-exposed (NE) groups, and to minimize the influences of other exposures and sociodemographic differences. Consistent with this objective, study flyers were distributed over a 4.5 year period to all obstetric clinics in two sites in the Midwest: rural multi-county area in southern Illinois (surrounding the town of Carbondale) and a small-sized city (Lincoln, Nebraska). Interested pregnant women (N = 915) called the laboratory and were screened for study eligibility with questions regarding due date, educational attainment, maternal race, smoking history and status, alcohol and illegal drug use, and Medicaid status (a less-intrusive proxy for income). Women who reported at screening 1) illegal drug use or 2) alcohol use of four or more drinks on a single occasion (criterion for binge drinking; Centers for Disease Control, 2008) were eliminated as ineligible at screening and were not considered further for potential recruitment. Among screened women who reported no binge drinking and no illegal drug use, all women who reported smoking in the month around their last menstrual period (LMP) or current active smoking on the screening then were recruited, enrolled, and preliminarily classified as tobacco-exposed. LMP smoking was chosen as the criterion to capture women who underdisclose smoking during very early pregnancy, when in fact, they quit smoking upon learning they were pregnant (which is well into the pregnancy period) and would therefore, have been classified erroneously as non-exposed (England et al., 2007). Among screened women who reported no binge drinking, no illegal drug use, and no-smoking at screening, those with lower educational attainment (<14 years), majority race/ethnicity and Medicaid status were over-selected for subsequent recruitment to render the groups more comparable demographically given the known higher frequency of smoking in these groups (N = 387 before data exclusions described below). Most participants (65%) were enrolled prior to the 16th prenatal week, and all women were enrolled prior to the 28th prenatal week. Women’s self-reported prenatal smoking behavior then was obtained prospectively at 16-weeks, 28-weeks, and delivery (hereafter referred to as 40-weeks), using a modified timeline-follow back method (Sobell & Sobell, 1992) where dates were used to cue recall and smoking was queried month-by-month.
Next, self-reported smoking behavior was examined for consistency with the initial group assignment. Where smoking status was consistent across the interviews and agreed with the last smoking date (if applicable), the exposure group assignment remained. For those who did not meet either of these criteria, the reported last smoking dates across the interviews were examined with regards to LMP proximity. If a participant was classified initially as non-exposed but reported last smoking dates falling in the window of pregnancy, then that participant was reclassified as TE. Ten smokers reported no cigarette use during pregnancy that was inconsistent with their LMP and reported last smoking dates. For these women, the missing average smoking amounts for the applicable trimesters were imputed with regression modeling (Little & Rubin, 2002). Then, the results of the biospecimen sampling were examined to confirm smoking group assignment. Plots were created of the cotinine levels, the nicotine by-product that was assayed by US Drug Laboratories from samples collected from maternal urine at 16-, 28-, and 40-weeks and infant meconium shortly after birth (see Procedures for further details). Any non-smoking woman with at least one urine cotinine value of 30 ng/ml or greater or whose infant had a meconium cotinine level of at least 30 ng/g was scrutinized further. Two mothers were reclassified as TE who had at least one urine or infant meconium cotinine level greater than 100, the cut-off value recommended by US Drug Laboratories.
Because the purpose of this paper was to examine the impact of prenatal tobacco exposure while minimizing confounding influences and other exposures, data from women/neonates who met one of the four following criteria were excluded from analysis. First, although women who reported illegal drug use during screening were not recruited or enrolled, 53 women denied use at screening and then subsequently reported use of marijuana at either a prenatal interview (n = 38) or their infant’s meconium tested positive for marijuana at birth (n = 19). Second, one woman reported prescription anti-psychotic medication use during pregnancy, which has known negative impacts on neonatal behavior. Because women who smoke are also more likely to drink, and to drink more in one sitting, than non-smokers, alcohol use around the LMP and during each month of pregnancy was measured carefully using the same structured, timeline follow-back methods as for smoking, querying about quantity, frequency, and variability. In the present sample, 83% of the women in the TE group reported drinking before pregnancy and prior to their LMP compared to 61% percent of women in the NE group, χ2 (1, N = 304) = 18.51, p < 0.0001. Furthermore, the average alcohol drinks of consumed per day during the first trimester of pregnancy differed between TE and NE groups (shown in Table 1), as well as when comparing groups for drinkers only (TEdrinkers only: M = 0.18; SD = 0.23; NEdrinkers only: M = 0.04, SD = 0.06; t = −5.72, p < 0.0001). The data also were excluded from eight women who reported on the first interview drinking one or more drinks per day on average (criteria for heavy drinking, Centers for Disease Control, 2008) for the first trimester. Only those with heavy drinking in the first trimester were excluded because after removing the data from these eight women, 85% of participants reported no alcohol use after the end of the first trimester. Furthermore, the amount and frequency of alcohol use in those who reported use after the first trimester was no more than one drink on one specifically identified occasion (e.g., a holiday or birthday) for all but six women (Trimester 2 M = .028, SD = .028; Trimester 3 M = .076, SD = .082). Mean alcohol drinks consumed per day reported for both the second and third trimesters for each exposure group (in Table 1) were very low, as well as when comparing groups for drinkers only (TEdrinkers only: Trimester 2 M = .004, SD = .009; Trimester 3 M = .002, SD = .009; NEdrinkers only: Trimester 2 M = .007, SD = .026; Trimester 3 M = .002, SD = .010). Although our focus was on prenatal tobacco exposure while minimizing other exposures, we elected not to exclude the data from women who consumed any alcohol (even through in relatively low amounts in this sample) to conserve sample size and preserve generalizability because of the common comorbidity of smoking and alcohol use, particularly prior to pregnancy detection. Therefore, prenatal alcohol use in first trimester was included as a potential covariate (see Procedures below for further details). Finally, to minimize the well-known influence of gestational age on self-regulatory behavior (Riese, Wilson, & Matheny, 1985; Korner, Brown, Dimiceli, & Forrest, 1989) data from ten infants born preterm (before 36 weeks) also were removed. Of the ten removed eight were TE.
Table 1.
Maternal Variables by Tobacco Exposure Group
| Maternal Demographic, Health, and Perinatal Variables | Tobacco-exposed | Non-exposed | ||
|---|---|---|---|---|
| M | SD | M | SD | |
| Maternal age at delivery (years)* | 25.3 | 5.0 | 26.5 | 5.0 |
| Maternal education (years)*** | 13.01 | 1.61 | 13.89 | 1.75 |
| Median monthly family income ($) | 1742 | -- | 1820 | -- |
| Gravida | 1.77 | 2.21 | 1.50 | 1.41 |
| Parity | 1.04 | 1.33 | 1.09 | 1.05 |
| Weight gain (1st prenatal visit to delivery) | 27.7 | 15.5 | 24.7 | 14.5 |
| % | -- | % | -- | |
| Anemia | 14 | -- | 16 | -- |
| Medicaid | 84 | -- | 83 | -- |
| Married** | 36 | -- | 54 | -- |
| Placental Abruption | 0.3 | -- | 0.0 | -- |
| Delivery | ||||
| Spontaneous vaginal | 41 | -- | 54 | -- |
| Induced vaginal | 27 | -- | 28 | -- |
| Caesarean & other extraction | 32 | -- | 18 | -- |
| Asthma medication | 4 | -- | 6 | -- |
| Pain medication | 22 | -- | 17 | -- |
| Anti-depressant medication | 12 | -- | 9 | -- |
| Diabetes | 6 | -- | 7 | -- |
| Hypertension/pre-eclampsia | 11 | -- | 14 | -- |
| Infection | 11 | -- | 11 | -- |
| Heart disease | 4 | -- | 3 | -- |
| Thyroid disease | 1 | -- | 4 | -- |
| WJ-III BIA Overall IQ Estimate** | 95.52 | 10.77 | 99.23 | 12.29 |
| BSI General Severity Index T-score | 57.04 | 8.39 | 55.34 | 8.39 |
| CAARS Attention Deficit/Hyperactivity Disorder T-Score | 46.74 | 8.26 | 45.96 | 7.87 |
| Maternal Prenatal Drinking (drinks/day) | ||||
| Trimester 1 average*** | 0.127 | 0.206 | 0.015 | 0.038 |
| Trimester 2 average* | 0.003 | 0.008 | 0.001 | 0.006 |
| Trimester 3 average* | 0.004 | 0.022 | 0.001 | 0.006 |
Note. WJ-III BIA = Woodcock-Johnson III Brief Intellectual Ability Assessment (Woodcock, Johnson, & Mathers, 2001); BSI = Brief Symptom Inventory (Derogatis, 1975); CAARS = Connors Adult ADHD; Rating Scale; Short (Connors, Erhardt, & Sparrow, 1998). -- =NA;
p < 0.05;
p < 0.01;
p < 0.001.
A total of 304 women and their infants met the criteria for inclusion, with 143 infants classified as TE and 161 as NE (138 women/infants from the rural IL site and 166 women/infants from the urban Nebraska site, where exposure groups by site were comparable1). The sample included 235 White, non-Hispanic women (77.3%); 15 White, Hispanic women (4.9%); 40 African-American women (13.2%); and 9 Native American women (3.0%). On average, mothers completed 13.5 years of education (SD = 1.7). Although language spoken in the home was not collected, all women were proficient English speakers. Women in the TE and NE groups were comparable in the percent receiving Medicaid assistance, but differed in the proportion married at enrollment, age at delivery, and educational attainment. There were no differences between exposure groups in the proportion of female infants, infant gestational age at birth, or infants who were of White, non-Hispanic race/ethnicity. Tables 1 and 3 depict the respective descriptive statistics for women and neonate participants by exposure group.
Table 3.
Infant Variables and Physical Growth Parameter Outcomes by Tobacco Exposure Group
| Neonate Variables | Tobacco-exposed | Non-exposed | ||
|---|---|---|---|---|
| M | SD | M | SD | |
| Length of hospitalization (days) | 2.3 | 1.1 | 2.2 | 1.2 |
| Gestational age (weeks) | 39.04 | 1.20 | 39.13 | 1.17 |
| 5-minute APGAR | 8.78 | 0.73 | 8.83 | 0.57 |
| Birth weight (g) | 3428 | 438 | 3420 | 448 |
| Head circumference (cm) | 34.2 | 1.5 | 34.3 | 2.1 |
| Length (cm) | 50.7 | 2.2 | 50.7 | 2.6 |
| % | -- | % | -- | |
| Sex (Female) | 49 | -- | 50 | -- |
| Race/Ethnicity (White, Caucasian) | 63 | -- | 58 | -- |
| Resuscitated with oxygen | 47 | -- | 43 | -- |
Note.
p < 0.05;
p < 0.01;
p < 0.001.
Procedures
Tobacco Exposure
Women were interviewed using the structured, timeline follow-back method in a private room by trained researchers (the research technicians who conducted interviews were not the same as those who conducted neonatal evaluations to ensure blinding) at 16-, 28- and 40-weeks (just after delivery) to gain information on prenatal tobacco and alcohol use. The comprehensive interview included background and other health related questions, as well as queries about quantity, frequency, and variability information regarding tobacco and alcohol use by month. These month-by-month values were averaged into trimester indices. At each session, women provided a urine sample in a sterile cup, where 100% of women provided samples at the 16-week interview, 99% at 28-weeks, and 96% at delivery, as occasionally women had difficulty providing a sufficiently clean sample for cotinine assay after delivery. After the neonate was born, nurses collected meconium samples until a total volume of 25 grams was obtained. However, some neonates voided meconium in utero or during delivery, preventing collection of an adequate volume of meconium for later assay. A total of 255 neonate meconium cotinine results were available for analysis.
Once nicotine is absorbed by the mother during smoking, it is metabolized into cotinine and is detectable in the urine up to several days after the termination of smoking. To measure cotinine level in participants’ urine samples, the DRI® Cotinine Assay was used from US Drug Laboratories. The DRI Cotinine Assay is a liquid, ready-to-use homogeneous enzyme immunoassay, based on competition between cotinine labeled with glucose-6-phosphate dehydrogenase enzyme and free cotinine in the sample for a fixed amount of cotinine-specific antibody binding sites. The glucose-6-phosphate dehydrogenase enzyme activity is determined pectrophotometrically at 340 nm by measuring its ability to convert nicotinamide adenine dinucleotide (NAD) to NAD-Hydrogenase. This assay utilizes DRI Cotinine calibrators and controls, which are prepared by spiking negative human urine with a known quantity of cotinine. The cotinine concentration is obtained by running a standard curve with the appropriate calibrators and by quantitating samples off the standard curve.
The tobacco exposure information is provided in Table 2. Forty-three percent of the TE group reported smoking ten or more cigarettes/day on average before pregnancy and prior to the LMP. The average number of cigarettes smoked during each trimester and at the 16-, 28-, and 40-week interviews was substantially less, between three and six cigarettes/day. Although 39% reported they no longer smoked by the end of the first trimester and 50% reported no longer smoking by the end of the second trimester, the average maternal urinary cotinine values for the TE group at 28-weeks did not differ from those collected at 16-weeks (16-weeks: M = 331 ng/mL, SD = 537; 28-weeks: M = 353 ng/mL, SD = 564; t[87] = −0.72, p > 0.45). The lowest average cotinine value for the TE group was at delivery. The average cotinine values for the NE group were less than 15 ng/ml across all occasions. As expected, the mean cotinine values in maternal urine and neonate meconium differed between the TE and NE groups at all time points (all ps < .01). Table 4 provides intercorrelations of the self-reported smoking behavior variables and the cotinine assay results at all timepoints.
Table 2.
Maternal Smoking and Infant Exposure Variables by Tobacco Exposure Group
| Tobacco-related Variables | Tobacco-exposed | Non-exposed | ||
|---|---|---|---|---|
| M | SD | M | SD | |
| Maternal self-reported prenatal smoking (number of cigarettes/day) | ||||
| Trimester 1 | 5.32 | 5.74 | -- | -- |
| At 16-week interview | 3.62 | 6.21 | -- | -- |
| Trimester 2 | 3.84 | 6.17 | -- | -- |
| At 28-week interview | 3.80 | 6.31 | -- | -- |
| Trimester 3 | 3.44 | 6.18 | -- | -- |
| At 40-week interview | 3.04 | 6.07 | -- | -- |
| Cotinine level | ||||
| 16-week maternal urine (ng/ml)*** | 330.90 | 536.60 | 5.64 | 13.78 |
| 28-week maternal urine (ng/ml)*** | 352.89 | 563.65 | 10.40 | 18.22 |
| At delivery maternal urine (ng/ml)*** | 83.85 | 198.27 | 12.06 | 19.12 |
| At delivery neonate meconium (ng/g)*** | 192.74 | 856.51 | 0.39 | 3.43 |
| 2-week infant urine cotinine (ng/ml) | 21.67 | 32.14 | 16.32 | 23.27 |
| 4-week infant urine cotinine (ng/ml) | 39.80 | 156.40 | 19.39 | 23.96 |
| Exposure-Cessation Group | ||||
| %/n QUIT | 23.7% (72/143) | -- | -- | -- |
| %/n PERSIST | 23.4 % (71/143) | -- | -- | -- |
Note. -- = Not Applicable; QUIT: Quit smoking in trimester 1 or 2 and remained smoke-free through delivery, PERSIST: Smoked throughout pregnancy in all three trimesters;
p < 0.05;
p < 0.01;
p < 0.001.
Table 4.
Intercorrelations of Self-reported Smoking and Maternal Urine/infant Meconium Cotinine Value
| 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.84** | 0.79** | 0.83** | 0.78** | 0.76** | 0.69** | 0.62** | 0.51** | 0.34** | 0.16* | 0.04 |
| 2 | 1.00 | 0.96** | 0.89** | 0.96** | 0.93** | 0.65** | 0.66** | 0.59** | 0.36** | 0.27** | 0.03 |
| 3 | 1.00 | 0.87** | 0.94** | 0.96** | 0.68** | 0.65** | 0.60** | 0.34** | 0.29** | 0.03 | |
| 4 | 1.00 | 0.84** | 0.83** | 0.65** | 0.91** | 0.63** | 0.48** | 0.34** | 0.19* | ||
| 5 | 1.00 | 0.91** | 0.68** | 0.63** | 0.58** | 0.30** | 0.23** | 0.02 | |||
| 6 | 1.00 | 0.58** | 0.60** | 0.62** | 0.32** | 0.36** | 0.03 | ||||
| 7 | 1.00 | 0.83** | 0.77** | 0.46** | 0.05 | 0.17** | |||||
| 8 | 1.00 | 0.59** | 0.30** | 0.12 | 0.00 | ||||||
| 9 | 1.00 | 0.38** | 0.25** | 0.05 | |||||||
| 10 | 1.00 | 0.03 | 0.02 | ||||||||
| 11 | 1.00 | 0.17* | |||||||||
| 12 | 1.00 |
Note. 1= Number of cigarettes/day during first trimester; 2 = Number of cigarettes/day during second trimester; 3 = Number of cigarettes/day during third trimester; 4 = Number of cigarettes/day in week prior to 16-week interview; 5 = Number of cigarettes/day in the week prior to 28-week interview; 6 = Number of cigarettes/day in the week prior to the delivery interview. 7 = Urine cotinine (ng/ml) at 16-week interview; 8 = Urine cotinine (ng/ml) at 28-week interview; 9 = Urine cotinine (ng/ml) at delivery; 10 = Infant meconium cotinine level (ng/g); 11= infant urine cotinine at 2-week assessment; 12 = infant urine cotinine at 4-week assessment;
p<.05;
p<.001;
Neonate urine samples were collected from soft cloths inserted into the diaper at the 2-and 4-week session to assess environmental tobacco smoke (ETS) exposure. US Drug Laboratories conducted the DRIR Cotinine Assay on these postnatal urine samples. TE and NE group mean 2- and 4-week neonate urinary cotinine levels did not differ and are shown in Table 2.
Neonatal Assessment
Although the state ratings, auditory and visual stimuli, and reflex maneuvers are similar among most neonatal instruments due to the limited behavioral repertoire of the young neonate, the NTA was chosen to measure emergent regulatory skills because of its unique modules that include graded stressors designed to probe the regulatory system and known psychometric properties. Psychometric properties of the NTA have been reported as good (Riese, 1982), where inter-rater reliability and internal consistency range from 0.85 to 0.97 and 0.72 to 0.86, respectively (Riese, 1983). We calculated inter-rater reliabilities from co-scoring 4% of all assessments administered with obtained reliabilities ranging from 0.89 to 0.99. Regarding predictive validity, Riese and colleagues (Riese, Wilson, & Matheny, 1985; Matheny, Riese, & Wilson, 1985; Riese, 1995) have shown that neonatal temperament is related to later maternal reports and direct laboratory behavioral observations of infant temperament at 6 months, 9 months, and 2 years. In these studies, those who were more irritable perinatally were rated as more upset, more variably active, less attentive to stimuli and less responsive (Riese, 1987).
Research technicians administered a standardized neonatal temperament assessment (NTA) that was designed to evaluate individual differences in early regulatory behaviors three times in the neonatal period, at 0.2 weeks (about two days) after birth in the hospital, at 2-weeks in the university laboratory, and at 4-weeks of age in the participant’s home. Following the work of Riese (1982, 1986), research technicians were taught initially in handling and working with neonates and then were trained how to administer the NTA items. Before completion of training each research technician achieved an initial reliability greater than 90% on at least ten neonates (determined by double coding of assessments). Random cases (10%) were selected for double coding throughout the study to ensure ongoing reliability in administration remained above 90%. The NTA author conducted the extended, initial training session for study staff at the beginning of the study, and one additional follow-up session during the study. To maintain, blindness to tobacco exposure group membership, examiners who conducted the interviews with the mothers were different than those who conducted the NTA assessments with the neonates. However, it was impossible to blind the examiners to tobacco use in the home at the 4-week assessment that was conducted in the home, although tobacco use in the home is not an indicator of prenatal exposure group membership per se. Neonates averaged 0.20 weeks of age (SD = 0.14) at the birth assessment, 2.24 weeks (SD = 0.40) at the 2-week assessment, and 4.22 weeks (SD = 0.47) at the 4-week assessments. Gestational ages at birth were corrected, such that the timing of the 2- and 4-week assessments were scheduled to equate conceptional age. The age range window was ± 1 week at the 2-week assessment, and ± 1.5 weeks for 4-week assessment. There were no age differences at any assessment between TE and NE groups (all ps > 0.05).
The NTA is designed to be conducted from the initiation of feeding during the interval prior to the next feeding (approximately three to four hours, depending on feeding schedule), thereby utilizing the neonate’s natural sleep, wake, alertness, and irritability patterns. More details concerning administration procedures are provided in Riese (1982, 1986, 1987). Briefly, neonatal temperament and behavior in response to feeding, routine handling, auditory and visual stimulation, stressors (a cold disc applied to the neonate’s thigh, aversive stimuli that elicit reflexes), and interventions (pacifier, examiner talking, swaddling, picking-up) are recorded, including the degree of motor activity, and the level and quality of alertness and orientation. The states in which to present different stimuli to the neonates are specified in Riese (1987) and were followed accordingly, using traditional management methods to facilitate acceptable state for each item and module. Assessments were conducted approximately 45 minutes after last feeding in a quiet, dimly lit area in the room. The examiner first recorded the neonate’s length and weight, and rated the neonate’s state on a six-point scale (1 = quiet sleep; 2 = active sleep; 3 = drowsy; 4 = alert inactivity; 5 = waking activity; 6 = crying). Then, four modules of the NTA were administered: Attention/Orientation, Cold Disc Stressor, Pacifier Withdrawal, and Soothing Maneuvers.
The Attention/Orientation module was administered when the neonate was awake and not irritable. During administration, the neonate’s response to auditory and visual stimuli and to reflex maneuvers were scored and summary ratings of responsiveness and neonate’s reinforcement value recorded. Auditory stimuli, such as a bell, rattle, or the examiner’s voice, were presented on the right and left sides of the neonate three times each for each stimulus, for a total of 18 trials. Each auditory trial was scored on a four-point scale (1 = no orienting response; 4 = a strong orienting response with eyes searching and head turning toward the sound). For visual stimuli (e.g. bulls-eye, examiner’s face), the stimulus was positioned first at the center of the visual field, then moved around the neonate’s head to the right or left at a 90° angle, back to the center, around the other side at a 90° angle, and then back to the center. Each visual item first moved to the right or to the left twice each, for a total of four trials with each item. Visual items were scored on a four-point scale (1 = no following response; 4 = sustained fixation and following with eyes and head). Ocular reflexes, optic and acoustic blinks, and rotation were administered, as well as elicitation of rooting, sucking, withdrawal to toothpick prick, and Moro reflexes. Although these items were scored on a three- or four-point scale, such reflex testing typically results in little variability in healthy full-term neonates. Therefore in response to these maneuvers, the latency to cry was recorded and the degree of irritability was rated on a five-point scale (1 = not irritable; 5 = highly irritable). Finally, the examiner made summary ratings of the neonate’s general appearance and alertness (1= poor; 5=excellent), cuddliness (1= resists and/or thrashes and/or stiffens; 5 = always molds, relaxes and clings), responsiveness to the different stimuli (1= not irritable; 5=highly irritable), consolability (1= never irritable; 5= unconsolable), reinforcement value of the neonate to the examiner (1 = glad to be finished; 5 = fun to have at home), and predominant state throughout the module.
The Cold Disc Stressor module was administered when the neonate was quiescent and not irritable. This module consisted of five trials where a metal disc cooled by immersion in ice water was held against the quiescent neonate’s thigh for five seconds. The neonate’s latency to irritability (seconds), rated irritability during and post-stimulus, duration of soothing if required (seconds), and total latency to soothe (seconds) were recorded. Trials were presented at least 60 seconds apart, and the inter-trial interval was longer if necessary to soothe the neonate. If the neonate was not soothed after three minutes, subsequent trials were discontinued. An overall summary rating (1= not irritable to cold disc; 6 = unconsolable) also was scored at the conclusion of the module.
The Pacifier Withdrawal module was administered when the neonate reached a moderate level of irritability, with fussiness and intermittent cry vocalizations. After the examiner noted the degree of irritability, then a pacifier was given to the crying neonate. The latencies to suck and to console (in seconds) were recorded. After 30 seconds, the examiner removed the pacifier while the neonate was still sucking, and again recorded the latency to cry, behavioral state, and post-trial irritability level (1= not irritable, or no soothing needed; 5 = could not console with pacifier in 3 minutes). Up to five pacifier withdrawal trials were administered. Trials were terminated if the neonate did not become irritable after three minutes. An overall summary rating (1 = not irritable, or no soothing needed to pacifier withdrawal; 5 = could not console with pacifier in 3 minutes on any trial) were made at the conclusion of the module.
The Soothing Maneuvers module also was administered when the neonate was at a moderate level of irritability. Trials consisted of graded items designed to foster soothing, and were presented in the following order: soothingly talking to the neonate, soothing talking plus patting the neonate’s stomach, putting the neonate in the prone position, lifting the neonate to the shoulder, swaddling the neonate, and cradling the swaddled neonate horizontally. Before each trial, the examiner rated the degree of irritability, whereas after each trial, the examiner rated degree of soothability. At the end of the module, the examiner made an overall summary rating (1 = no soothing needed, 5 = not soothed by any technique). When the NTA was concluded, the examiner scored the neonate’s final behavior state.
Other potential influences on neonatal self-regulation
Smoking during pregnancy is related to maternal and neonatal risk factors, such as lower maternal education, depression, psychopathology symptoms (e.g., Schuetze & Eiden, 2006; Baghurst, Tong, Woodward, & McMichael, 1992; Wakschlag, Pickett, Cook, Benowitz, & Leventhal, 2002), maternal health and perinatal complications, which are associated with adverse developmental outcomes independent of exposure (e.g., Eyler & Behnke, 1999; Schuetze & Eiden, 2007; Schuetze, Eiden, & Dombkowski, 2006). During all study sessions, women completed questionnaires and study instruments, including a brief psychiatric symptom screening (Brief Symptom Inventory [BSI], Derogatis, 1975), the Connors Adult ADHD Rating Scale: Short (CAARS:S; Connors, Erhardt, & Sparrow, 1998), and the Woodcock-Johnson Brief Intellectual Ability assessment (BIA; Woodcock, McGrew & Mather, 2001). Standardized scores derived from instrument normative tables were used in the analyses.
Analysis
Creating Factor Scores
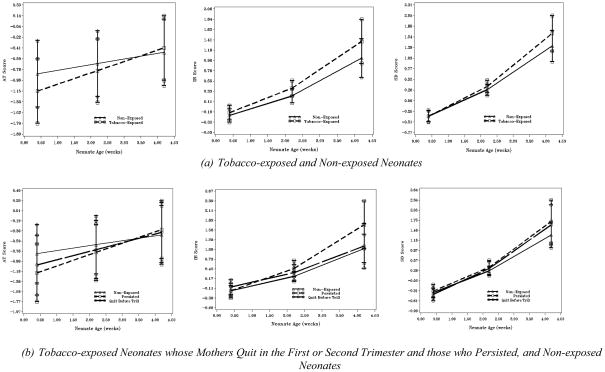
Unlike in previous studies using the NTA, we elected to pool and reduce the dependent variables into meaningful constructs to enhance reliability using principal axis factor analysis with oblique (promax) rotation. Those items (largely the reflex items) with communalities less than 0.35 were eliminated because of unreliability (Gorsuch, 1983). As recommended by Gorsuch (1983), eigenvalues (> 1), scree plots, and the percent of variance explained (>10%) were examined to select the number of factors to retain. Results indicated a three-factor solution best fit the data. The factor pattern matrix is shown in Table 5. Factor 1 was labeled Irritable Reactivity (IR), as it was composed largely of the neonate’s irritable reaction to the auditory and visual stimuli and to routine handling as a part of reflex elicitation and maneuvers. Factor 2, composed of the neonate’s orientation and attention to auditory and visual stimuli, was labeled Attention (AT). Finally, the items that involved reactions to the cold disc, pacifier, and soothing maneuvers, and behavior prior to the next feeding loaded on the third factor, labeled Stressor Dysregulation (SD). The average factor inter-correlations were: IR:AT r = −0.20, IR:SD r = 0.46, and AT:SD r = 0.11. The three computed factor scores for each subject at each time point were used as the dependent variables, and the means and standard errors of each factor by exposure group are plotted in Figure 1(a).
Table 5.
Neonatal Temperament Assessment Factor Structure and Observed Inter-factor Correlations
| Neonatal Temperament Assessment Item | Factor Loadings | ||
|---|---|---|---|
| Irritable Reactivity | Attention | Stress Dysregulation | |
| Irritability before feeding | 0.40 | 0.06 | 0.81 |
| Irritability to visual stimuli | 0.88 | −0.12 | 0.43 |
| Irritability to auditory stimuli | 0.80 | −0.19 | 0.37 |
| Irritability to handling | 0.89 | −0.11 | 0.44 |
| Irritability to reflex elicitation | 0.89 | −0.06 | 0.41 |
| Latency to soothe after Moro reflex | 0.70 | −0.02 | 0.40 |
| Soothability after reflex elicitation | 0.85 | −0.11 | 0.40 |
| Mean visual following – bullseye | −0.18 | 0.67 | 0.05 |
| Mean auditory orienting – rattle | −0.07 | 0.87 | 0.10 |
| Mean auditory orienting – bell | −0.04 | 0.87 | 0.10 |
| Mean auditory orienting – voice | −0.14 | 0.90 | 0.12 |
| Mean visual following - face and voice | −0.09 | 0.61 | 0.10 |
| Overall alertness summary | −0.19 | 0.87 | 0.08 |
| Cold Disc Stressor summary | 0.47 | 0.09 | 0.65 |
| Pacifier withdrawal summary | 0.41 | 0.13 | 0.81 |
| Soothing Maneuvers summary | 0.41 | 0.07 | 0.89 |
| Rated reinforcement value | −0.67 | 0.34 | −0.32 |
Note. Factor loadings above 0.60 or below −0.60 are presented in bold.
Figure 1.
Growth in Attention, Irritable Reactivity and Stress Dysregulation Factor Scores in Neonates.
Developing the Baseline Growth Model
To evaluate the impact of prenatal tobacco exposure on the development of neonatal regulation, hierarchical linear models were used to model change across the three time points, with a separate model for each regulatory factor. Hierarchical linear modeling takes advantage of the increased reliability of change assessments when data are collected at more than two time points to characterize developmental processes and deviations. The first analytic step was to determine the ‘baseline’ growth model derived from the unconditional model that included only a person-level variance term (i.e. a random intercept). Before any modifications were made to the structure of either age or the variance components in the model, gestational age (GA) was introduced as a covariate in each model because of its known impact on neonatal neurobehavior (e.g., Riese, Wilson, & Matheny, 1985; Korner, Brown, Dimiceli, & Forrest, 1989). GA was a significant predictor of each of the dependent variables in the linear growth models of age (AT: t [300] = 3.61, p < 0.001; IR: t [304] = 2.72, p < 0.01, SD, t [304] = 2.26, p < 0.03), and therefore was retained as a covariate in all models. Recruitment site was included in all models as a covariate to control for any spurious site-specific variance.
Visual inspection of spaghetti plots of individual factor scores across the three observations suggested that modeling age as a quadratic process might be most appropriate to describe growth in the IR and SD constructs. Although AT growth appeared linear, fit of the quadratic term was evaluated for consistency. In these analyses, age was centered at 0.2 weeks and each of the three factor scores then were fitted as functions of linear and quadratic (centered) age. The coefficient for the quadratic term did not differ from zero for AT, t (676) = −0.17, p > 0.86, but differed from zero for both IR, t (675) = −6.49, p < 0.0001, and SD, t (683) = −7.64, p < 0.0001.
Next, deviations in modeling the person-level covariance structure were considered, where the coefficient of the age term was allowed to vary in each of the models. For the linear model of AT, model fit comparisons indicated that the random-intercept-only model was preferred (AIC of 2423.1 vs. 2424.4 for the random intercept-and-slope model, and BIC of 2430.5 vs. 2439.3). For IR, the model allowing for both random intercept and slope terms improved model fit when both AIC values (2404.8 vs. 2435.2 for the random-intercept-only model) and BIC (2412.2 vs. 2442.6) are considered. A fairly wide range of quadratic trends were also exhibited in the individual plots for IR. Thus, models were fitted where the (centered) age and quadratic age terms were allowed to vary across neonates in separate models. Like SD, the growth model that included random intercept and slope terms significantly improved model fit over the random-intercept-only model when AIC values alone were considered (2260.7 vs. 2267.7 for the random-intercept-only), but not when considering BIC (2275.6 vs. 2275.2). The final baseline model for AT, then, was linear in age with only the intercept as a random coefficient, whereas for IR and SD, linear and quadratic change in age were modeled, with only the intercepts allowed to vary randomly among neonates.
Modeling the impact of exposure
Exposure-related predictors were considered in separate models, and included exposure group (TE vs. NE); exposure-cessation group, QUIT (stopped smoking during pregnancy during the first or second trimester and remained quit throughout the third trimester) vs. PERSIST (smoked throughout pregnancy); average self-reported smoking for each trimester (cigarettes/day); average self-reported smoking at the 16-, 28-, and 40-week interviews (cigarettes/day); maternal urinary cotinine level at the 16-, 28-, or 40-week interviews (scaled in units of 100 ng/mL); and neonate meconium cotinine level (scaled in units of 100 ng/g). To characterize the impact of exposure on physical growth, t-tests were run where the exposure variable was categorical, and regressions were used for continuously distributed exposure variables. For the hierarchical growth models, conditional models were developed to test the hypotheses including the effect of the exposure-related predictor on both the intercept and growth parameters. The analyses were conducted centering at .2-, 2- and 4-weeks respectively, in order to characterize the relation to neonatal behavior at each time point. Each exposure independent variable was entered as a predictor of the pattern of growth (intercept, linear change, quadratic acceleration), and then a backwards trimming procedure was used to select the best fitting conditional model, deleting those when not significant for the higher growth term and then working progressively backwards through the growth terms. The continuous predictors were scaled so that the estimated parameter represented the incremental change in the dependent variable that was associated with each additional cigarette smoked or each additional 100 cotinine units at that interview.
Selection of Covariates
A range of covariates were considered for inclusion: marital status, maternal education, family income, mother’s age at delivery, average alcohol drinks per day during the first trimester, Medicaid status, neonate sex, neonate and mother’s race/ethnicity (white vs. non-white), neonate environmental tobacco exposure (as measured by cotinine in neonate urine collected at the 2- and the 4-week assessment), maternal prescription medication use (anti-depressant, pain, or asthma medication, each coded as 0 = absent, 1 = present), gravida, parity, weight gain, mother health and delivery variables (diabetes, heart disease, placental abruption, thyroid, anemia, hypertension/pre-eclampsia, infection, delivery type [vaginal vs. cesarean/other] with the same 0, 1 coding), BSI summary index, CAARS:S Attention Deficit/Hyperactivity Disorder index, and the BIA overall IQ estimate. Covariates were analyzed separately and selected using the methods of J.L. Jacobson and S.W. Jacobson (1996), to control the influence of variables that co-occur with prenatal smoking without inappropriately reducing exposure-related variance. If the correlation of the covariate with both exposure status (NE/TE) and the NTA factor score differed from zero at the p < .10 level, the covariate was included in the models.
For AT, mother’s age at delivery, maternal education, and the maternal IQ estimate were retained for consideration as covariates through these methods. All three candidate covariates were added to the baseline model and a backwards stepwise procedure was utilized to determine which among them was to be retained in the final model. Mother’s age was removed first from the model (t [292] = 0.12, p > 0.90), followed by education (t [291] = 0.58; p > 0.55). The remaining covariate, mothers’ IQ estimate (t [291] = 3.33, p = 0.001) was retained. None of the aforementioned covariates met the criteria for model inclusion for either IR or SD.
Results
Physical growth indices at birth as a function exposure group status are shown in Table 3. Tobacco-exposed (TE) and non-exposed (NE) groups did not differ in birth weight, t (301) = −0.15, p > 0.88, head circumference, t (299) = 0.70, p > 0.48, or length, t (299) = 0.16, p > 0.87. Within the exposed neonates, there were no differences in these physical growth indices in those born to women who quit during the first or second trimester (QUIT) and those whose smoking persisted throughout pregnancy (PERSIST, all ps > 0.47). Table 6 contains the results of regression models with the self-reported smoking and biospecimen predictors. Despite the lack of overall exposure group differences in birth weight, a dose-response relation was evident. Each additional cigarette smoked in the third trimester as reported by the mother was associated with an −11.55g decrement in birth weight. A similar trend was observed for the number of cigarettes reported for the second trimester. The average number of cigarettes smoked per day in the week prior to both the 28-and 40-week interviews was associated with a respective birth weight decrement of −11.56 and −14.55 grams, respectively. Furthermore, cotinine in maternal urine at both the 16- and 40- week interviews predicted birth weight, with a respective difference of −17.24 and −46.78 per 100 ng/ml cotinine. For body length, a similar dose response pattern was evident, where the maternal urine cotinine levels at 16- and 40-weeks predicted decrements in body length at birth. Marginal trends were observed for the maternal urinary cotinine values at the 16-, 28- and 40-week interviews. The magnitude of these effects was similar. Neither self-reported smoking nor biospecimen results predicted head circumference.
Table 6.
Exposure-related Predictors and Impact on the Neonatal Physical Growth Measured at Birth
| Exposure Predictor | Physical Growth Measured at Birth |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Birth Weight | Head Circumference | Body Length | |||||||
| b | SE | β | b | SE | β | b | SE | β | |
| Maternal self-reported average smoking (number of cigarettes/day) | |||||||||
| Trimester 1 | −2.49 | 5.37 | −0.03 | −0.01 | 0.02 | −0.03 | −0.02 | 0.03 | −0.05 |
| 16-week interview | −6.70 | 6.77 | −0.07 | −0.01 | 0.03 | −0.02 | −0.05 | 0.04 | −0.10 |
| Trimester 2 | −10.41t | 5.46 | −0.11t | −0.03 | 0.02 | −0.06 | −0.04 | 0.03 | −0.08 |
| 28-week interview | −11.56* | 5.47 | −0.12* | −0.03 | 0.02 | −0.06 | −0.05 | 0.03 | −0.09 |
| Trimester 3 | −11.55* | 5.54 | −0.12* | −0.03 | 0.02 | −0.07 | −0.05 | 0.03 | −0.10 |
| 40-week interview | −14.55* | 5.69 | −0.15* | −0.04t | 0.02 | −0.98t | −0.05 | 0.03 | −0.09 |
| Cotinine level (100 ng/ml, ng/g) | |||||||||
| 16-week maternal urine**** | −17.24* | 7.85 | −0.16* | −0.04 | 0.04 | −0.09 | −0.09* | 0.04 | −0.15* |
| 28-week maternal urine**** | −9.88 | 6.06 | −0.09 | −0.03 | 0.03 | −0.07 | −0.01t | 0.03 | −0.10t |
| At delivery maternal urine**** | −46.78 | 18.32 | −0.15* | −0.07 | 0.08 | −0.05 | −0.30** | 0.10 | −0.18** |
| At delivery infant meconium** | −0.05 | 4.91 | −0.0006 | −0.01 | 0.02 | −0.02 | 0.02 | 0.03 | 0.04 |
Note. b = unstandardized b-weight; SE = Standard Error; β = standardized Beta-weight;
p <.10;
p<.05;
p <.01.
The estimated intercept and linear growth (if applicable) parameter values associated with the exposure predictors for the hierarchical growth models for the Irritable Reactivity (IR), Attention/Orientation (AT), and Stressor Dysregulation (SD) factor scores are presented in Table 7. TE and NE neonates differed in AT factor score obtained shortly after birth and in the rate of growth. The pattern of AT growth is shown in Figure 1(a). TE neonates had, on average, lower AT scores by 0.318 at two days after birth than NE neonates (t [765] = −3.232, p < 0.01). The significantly higher rate of growth (γ = 0.10) evidenced among TE neonates (t [609] =2.876, p < 0.01) resulted in a diminishing difference in AT score means between the two groups at the 2-week time point of 0.141 (t [299] = −2.014, p < 0.05), and comparable AT scores between the groups at the 4-week assessment (t [692] = 0.598, p > 0.55).
Table 7.
Exposure-related Predictors and Impact on Neonatal Self-regulation Attention/Orientation, Irritable Reactivity, and Stressor Dysregulation Growth Parameters
| Quadratic | 0.2 Weeks | SE | 2 Weeks | 4 Weeks | ||||
|---|---|---|---|---|---|---|---|---|
| Slope | Intercept | Intercept | SE | Intercept | SE | |||
| Attention/Orientation | ||||||||
| TE/NE group status | -- | 0.098** | −0.318** | 0.010 | −0.141* | 0.070 | 0.055 | 0.092 |
| Exposure-Cessation Group | ||||||||
| PERSIST | -- | 0.126** | −0.402** | 0.123 | −0.175* | 0.088 | 0.078 | 0.116 |
| QUIT | -- | 0.072t | −0.240* | 0.121 | −0.110 | 0.086 | 0.034 | 0.113 |
| Maternal self-reported average smoking (number of cigarettes/day) | ||||||||
| Trimester 1 | -- | 0.012** | −0.034** | 0.011 | −0.014t | 0.008 | 0.010 | 0.010 |
| 16-week interview | -- | 0.005 | −0.012 | 0.014 | −0.002 | 0.010 | 0.008 | 0.014 |
| Trimester 2 | -- | 0.014*** | −0.047**** | 0.012 | −0.023** | 0.008 | 0.004 | 0.011 |
| 28-week interview | -- | 0.011** | −0.042*** | 0.011 | −0.023** | 0.008 | −0.002 | 0.010 |
| Trimester 3 | -- | 0.012** | −0.045*** | 0.012 | −0.023** | 0.008 | 0.001 | 0.011 |
| 40-week interview | -- | 0.011* | −0.044*** | 0.013 | −0.025** | 0.009 | −0.003 | 0.012 |
| 16-week maternal urine | -- | 0.004 | −0.015 | 0.015 | −0.009 | 0.011 | −0.001 | 0.014 |
| 28-week maternal urine | -- | 0.007 | −0.023* | 0.012 | −0.011 | 0.008 | 0.002 | 0.011 |
| At delivery maternal urine | -- | 0.021t | −0.087* | 0.036 | −0.050t | 0.026 | −0.009 | 0.033 |
| At delivery infant meconium | -- | 0.003 | −0.016t | 0.009 | −0.010 | 0.007 | −0.004 | 0.008 |
| Irritable Reactivity | ||||||||
| TE/NE group status | −0.016 | 0.094 | 0.039 | 0.107 | 0.155 | 0.102 | 0.158t | 0.094 |
| Exposure-Cessation Group | ||||||||
| PERSIST | −0.047 | 0.229 | −0.045 | 0.135 | 0.214t | 0.128 | 0.143 | 0.119 |
| QUIT | 0.008 | −0.018 | 0.113 | 0.132 | 0.106 | 0.126 | 0.158 | 0.116 |
| Maternal self-reported average smoking (number of cigarettes/day) | ||||||||
| Trimester 1 | −0.003 | 0.016 | 0.000 | 0.012 | 0.017 | 0.011 | 0.009 | 0.010 |
| 16-week interview | 0.003 | −0.004 | −0.011 | 0.015 | −0.009 | 0.014 | 0.013 | 0.014 |
| Trimester 2 | −0.003 | 0.012 | 0.000 | 0.012 | 0.013 | 0.011 | 0.007 | 0.010 |
| 28-week interview | −0.001 | 0.009 | −0.001 | 0.012 | 0.011 | 0.011 | 0.015 | 0.010 |
| Trimester 3 | −0.002 | 0.008 | 0.003 | 0.012 | 0.012 | 0.011 | 0.010 | 0.011 |
| 40-week interview | −0.002 | 0.009 | 0.003 | 0.013 | 0.014 | 0.011 | 0.011 | 0.011 |
| Cotinine level (100 ng/ml, ng/g) | ||||||||
| 16-week maternal urine | −0.002 | 0.021 | −0.018 | 0.017 | 0.014 | 0.016 | 0.037 | 0.015 |
| 28-week maternal urine | −0.005 | 0.023 | −0.011 | 0.013 | 0.015 | 0.012 | 0.009 | 0.012 |
| At delivery maternal urine | −0.005 | 0.030 | −0.012 | 0.039 | 0.026 | 0.038 | 0.032 | 0.034 |
| At delivery infant meconium | −0.002 | 0.006 | 0.016 | 0.011 | 0.021 | 0.010 | 0.010 | 0.009 |
| Stressor Dysregulation | ||||||||
| Exposure-Cessation Group | ||||||||
| PERSIST | −0.050 | 0.144 | 0.016 | 0.123 | 0.112 | 0.117 | −0.162 | 0.109 |
| QUIT | −0.021 | 0.113 | −0.073 | 0.121 | 0.062 | 0.115 | 0.052 | 0.106 |
| Maternal self-reported average smoking (number of cigarettes/day) | ||||||||
| Trimester 1 | −0.005t | 0.017 | −0.003 | 0.011 | 0.012 | 0.010 | −0.008 | 0.009 |
| 16-week interview | 0.003 | −0.016 | 0.007 | 0.014 | −0.014 | 0.013 | −0.016 | 0.013 |
| Trimester 2 | −0.004 | 0.011 | −0.002 | 0.011 | 0.006 | 0.010 | −0.014 | 0.010 |
| 28-week interview | −0.003 | 0.007 | 0.004 | 0.011 | 0.006 | 0.010 | 0.013 | 0.012 |
| Trimester 3 | −0.003 | 0.007 | 0.001 | 0.011 | 0.006 | 0.010 | −0.008 | 0.010 |
| At 40 week interview | −0.003 | 0.010 | −0.000 | 0.012 | 0.007 | 0.010 | −0.008 | 0.010 |
| Cotinine level (100ng/ml; ng/g) | ||||||||
| 16-week maternal urine | −0.003 | 0.009 | 0.017 | 0.015 | 0.022 | 0.015 | 0.004 | 0.014 |
| 28-week maternal urine | −0.007 | 0.026 | −0.007 | 0.012 | 0.018 | 0.011 | −0.004 | 0.011 |
| At delivery maternal urine | −0.004 | −0.003 | 0.038 | 0.036 | 0.020 | 0.034 | −0.027 | 0.031 |
| At delivery infant meconium | −0.001 | 0.003 | 0.005 | 0.010 | 0.005 | 0.009 | −0.005 | 0.008 |
Note. -- = Not applicable. Covariates for the Attention/Orientation model are gestational age, site, and the estimated maternal overall intelligence score, and for the IR and SD models are gestational age and site.
p < 0.10;
p < 0.05;
p < 0.01;
p < 0.001;
Within TE neonates, the PERSIST and QUIT groups demonstrated different AT factor scores shortly after birth and different rates of growth of these scores. The PERSIST group had, on average, lower AT scores by 0.402 at two days after birth as compared to NE neonates (t [762] = −3.266, p < 0.01), and the difference of 0.240 between the QUIT and NE group was also significant (t [766] = −1.975, p < 0.05). The significantly higher rate of growth (γ = 0.126) evidenced among PERSIST neonates (t [605] = 2.941, p < 0.01) resulted in a diminishing difference in AT score means between the PERSIST and NE groups at age 2-weeks of 0.175 (t [298] = −1.984, p < 0.05), and comparable AT scores between the groups at the 4-week evaluation (t [699] = 0.674, p > 0.50). The QUIT group demonstrated a higher, but non-significant, rate of growth (γ = 0.072, t [609] = 1.708, p > 0.08) compared to the NE group. Growth trajectories are shown in Figure 1(b).
Self-reported average number of cigarettes smoked per day during the first trimester was related to the pattern of AT growth in a dose dependent fashion, with each cigarette smoked associated with a 0.034 reduction in AT score at .2-weeks (t [775] = −3.223, p < 0.01) and with a faster rate of change of 0.012 in AT score (t [606] = 3.149, p < 0.01) across the neonatal period. A similar pattern was observed for self-reported smoking during the second trimester and the average number of cigarettes smoked per day reported in the week prior to the 28-week interview, with each cigarette smoked associated with a 0.047 and 0.042 reduction, respectively in AT score at .2-weeks (t [779] = −4.026, p < 0.0001; t [784] = −3.720, p < 0.001) and with a faster rate of AT score change of 0.014 and 0.011 (t [607] = 3.361, p < 0.0001; t [602] = 2.689, p < 0.006) across the neonatal period. Average self reported smoking during the third trimester and in the week prior to the 40-week interview also was related negatively to the AT factor score, where each reported cigarette smoked per day was related to a 0.045 and 0.044 AT score reduction at .2-weeks, respectively (t [782] = −3.738, p < 0.001; t [771] = −3.425, p < 0.001), and also associated with 0.012 and 0.011 higher rate of change in AT score (t [608] = 2.938, p < 0.01; t [600] = 2.447, p < 0.02). Cotinine levels in maternal urine collected at 28-weeks and delivery were related to the pattern of growth in AT scores, such that greater assayed cotinine levels were associated a reduction in AT score of 0.023 and 0.087 at .2-weeks, respectively, (t [771] = −1.995, p < 0.05; t [737] = −2.345, p < 0.02) and with a marginally faster rate of change of 0.021 in AT scores (γ = 0.021, t [579] = 1.685, p < 0.10). At 2-weeks of age, neonatal AT scores were related negative to the average self-reported smoking during the second (t [305] = −2.744, p < 0.01) and third trimesters (t [306] = −2.714, p < 0.01), smoking in the week prior to 28- (t [305] = −2.930, p < 0.01) and 40-week interviews (t [304] = −2.711, p < 0.01), and associated marginally with self-reported smoking in the first trimester (t [307] = −1.783, p < 0.10) and with maternal urine cotinine level at delivery (t [291] = −1.886, p < 0.10). All self-reported smoking and cotinine levels were not related to AT score level at neonates’ 4-weeks of age (ps>0.30).
The group-related pattern of IR growth is shown in Figure 1(a). Unlike AT, growth patterns did not differ significantly by exposure group classification at .2- (t [831] = 0.363, p > 0.72) and 2-weeks (t [752] = 1.517, p > 0.12). The TE group was consistently more irritable (difference in intercepts between TE and NE groups of 0.039) across the neonatal period, and reached marginal significance at 4-weeks of age (γ= 0.158, t [684] = 1.674, p <0.10). Nor were differences noted between the NE group and either of the exposure cessation groups, QUIT t (827) = 0.859, p > 0.39 and PERSIST t (832) = −0.331, p > 0.74 at .2-weeks, respectively. There were marginally significant IR score differences between PERSIST and NE at 2-weeks (γ= 0.214, t [769] = 1.668, p < 0.10). Average self-reported smoking in each trimester or at each interview were not related to any IR growth parameters, nor were maternal urine or meconium cotinine levels at any time point.
Like IR, SD scores did not differ by exposure group classification (t [841] = −0.261, p > 0.79), nor were there any differences between the NE group and either the QUIT or PERSIST groups (t [836] = −0.606, p > 0.54 and t [841] = 0.127, p > 0.89, respectively). For SD, self-reported smoking at each interview, cotinine levels in maternal urine or neonatal meconium were unrelated to growth in SD scores, or at SD scores at .2-, 2-, or 4-weeks of age. Self-reported smoking during the first trimester was related marginally to quadratic growth, (γ = −0.005, t [665] = −1.794, p < 0.08), but not to SD scores at any age, and average self reported smoking in second and third trimesters were not related to any SD growth parameters over the course of neonates’ first month of life.
Discussion
The impact of prenatal tobacco exposure on the early development of emergent self-regulatory processes and on physical growth at birth was assessed using a prospective design, indexing exposure via self-report and bioassays collected at several time points during pregnancy. Neonatal self-regulatory behaviors for modulating attention, irritability, and response to stressors were assessed three times in the first month of life, and empirically parsed into meaningful constructs, enabling characterization of the dynamic impact of prenatal tobacco exposure on the trajectories of change in light of substantial growth and instability in this very early period (Korner, Kraemer, Reade, Forrest, & Dimiceli, 1987). This fine-grained analytic strategy enabled a number of new insights into the effects of exposure on the very early development of self-regulatory behaviors in several domains.
Although the prevalence, amount, and persistence of smoking in pregnant women today differs from previous decades, the oft-reported continuous dose-response relation between pregnancy smoking and birth weight was evident here. Heavier and more persistent smoking across pregnancy impacted birth weight and body length deleteriously. Self-reported second and third trimester smoking, self-reported smoking at the 28- and 40-week interview, and cotinine in maternal urine at 16- and 40-weeks, predicted birth weight decrements. The magnitude of the exposure effect on birth weight effect was largest for these third trimester smoking predictors, compared to those measured earlier in pregnancy. Maternal urinary cotinine at delivery also was associated with significant body length decrements. Marginal trends were observed for maternal urine cotinine levels at the 16-, 28- and 40-week interviews. In contrast to these dose-response relations, the lack of exposure group differences in these indices of physical growth at birth is likely a consequence of lower amount of tobacco exposure overall compared to cohorts ascertained in the 1970s and 80s, the sample selection methods to minimize other influences and exposures, and the greater sensitivity of continuous measures of exposure in comparison to gross grouping. Neither exposure group level nor dose-response differences in head circumference were evident, suggesting that the protective, brain growth sparing mechanism was not affected by prenatal tobacco exposure, unlike what has been observed in prenatally cocaine exposed neonates (Eyler, Behnke, Conlon, Woods, & Wobie,, 1998).
Importantly, the pattern of development of attention skills differed among tobacco-exposed (TE) and non-exposed (NE) neonates across the first month of life. Consistent with the stated hypotheses, TE neonates showed less orientation and attentive tracking behaviors to auditory and visual stimuli. Interestingly, exposure group differences were not constant over the first month of life. Differences were most evident on the Attention/Orientation (AT) factor scores from the assessment conducted shortly after birth, and remained apparent at 2-weeks of age. By 4-weeks of age though, AT scores were comparable between the two groups, contrary to prediction. This average trajectory is consistent with a general pattern of developmental “catchup,” where the initial reduced level of performance in TE neonates was accompanied by a more rapid rate of development to result in comparable skills at the end of the observation period. Furthermore, attention skills scores were lower shortly after birth in TE neonates whose mothers smoked more and more persistently across pregnancy, compared to those who quit smoking during or before the second trimester. Of note, too, is the relatively modest change in AT skills in NE neonates relative to the fairly steep rate of change for TE neonates, which again was greater in those who smoked more and more persistently in pregnancy.
Clearly, early in the neonatal period, attention skills differed in TE neonates relative to their NE peers. What is unclear is whether this difference reflects an effect of withdrawal or is a unique exposure-related developmental vulnerability in this period of rapid change. The differences in attention observed shortly after birth are consistent with withdrawal effects, reflecting the residual rebound impact of cessation of nicotine exposure that occurs as a consequence of birth. This interpretation is consistent with earlier findings where exposure also was measured with self-report (e.g., S.W. Jacobson, 1984; Richardson, Day, & Taylor, 1989; Streissguth, Sampson, Barr, Bookstein, & Carmichael, 1994; Fried, Watkinson, Dillon, & Dulberg, 1987; Saxton, 1978). These findings are also consistent with those from a more recent study that included bioassays of exposure and was specifically designed to examine withdrawal in the first days of life (Godding et al., 2004). The dose-response relation observed in the present between self-reported maternal smoking in the third trimester and at the delivery interview, as well as cotinine levels in maternal urine at delivery and in neonatal meconium, and the level of attention behavior observed shortly after birth support the withdrawal interpretation.
Withdrawal effects from progressive nicotine clearing also might contribute to the substantive differences observed in AT scores that persisted at 2-weeks of age. The difference in AT scores between TE and NE neonates was substantially smaller (about half in magnitude) at 2-weeks of age compared to shortly after birth. Self-reported smoking in the third trimester and at the 40-week interview, as well as cotinine levels in maternal urine at delivery, predicted the differences of AT scores between TE and NE at 2-weeks of age. However, cotinine levels in neonatal meconium were not related to attention behaviors at 2-weeks of age, which would be expected if the exposure group differences were due to receding withdrawal effects.
In the alternative, 2-weeks of age is typically considered beyond the window when acute withdrawal effects are observed (Stroud et al., 2009a). Unlike Godding et al. (2004), women in the present sample reported smoking substantially fewer cigarettes per day. The decreased number of cigarettes per day across pregnancy results in a much lower dose of nicotine to be cleared after birth and thereby might decrease the likelihood and severity of withdrawal effects shortly after birth, and certainly 2-weeks later. In the present study, neonates whose mothers quit smoking during pregnancy showed poorer attention and orientation skills shortly after birth compared to those who were non-exposed, which also is not consistent withdrawal effects. Furthermore, the amount of smoking reported in the first and second trimesters, as well as in the week prior to the 28-week interview, predicted attention skills at 2-weeks of age, earlier in pregnancy than would be a consequence of nicotine clearing. However, the general consistency is smoking across smoking and the resultant substantive correlations between smoking indicators measured at different points during pregnancy, makes it difficult to attribute independent effects on neurobehavior at each age. The observed differences in attention early in the neonatal period, though, also are not likely to be due to secondhand tobacco exposure, as secondhand smoke exposure was low in the neonatal period, confirmed by the low cotinine levels in neonate urine and the much smaller relation to prenatal smoking amounts of our present study.
Although exposure-group related differences in attention were expected at 4-weeks of age, the average trajectories did not differ between exposure groups at the end of the neonatal period. Neither self-reported smoking nor cotinine levels measured in biospecimens were related to exposure group differences in AT scores at 4-weeks of age, consistent with results obtained by Yolton et al. (2009). The pattern of skill growth for TE neonates was consistent with initial deficits followed by subsequent “recovery,” where at 4-weeks of age, all neonates showed comparable attention skills. In this period of rapid developmental growth in attention skills for all neonates, TE neonates show early deficits, as well as faster growth rates, both of which were related to the degree of exposure. The longitudinal design used here disentangled the age-specific exposure effects in the context of the developmental trajectory of skill development.
Also contrary to hypothesis, exposure group level (TE vs. NE) differences in the Irritable Reactivity (IR) factor scores shortly after birth were not significant, neither were there exposure group effects on linear or quadratic growth rates. These findings contrast those from other studies that used both self-report (Piccone, Allen, Olsen & Ferris, 1982; S.W. Jacobson, 1984; Fried, 1987; Nugent, Lester, Greene, & Wieczorek-Deering, 1996; Schuetze & Eiden, 2007) and bioassays (Law et al., 2003; Mansi, et al., 2007; Godding et al., 2004, Stroud et al., 2009b) to index prenatal tobacco exposure. What is of interest here is the impact on the developmental pattern, where the consistent exposure-related differences in IR scores between groups were visually evident at each age and persistent across the neonatal period. The magnitude of the estimated difference in irritable reactivity between TE and NE neonates was progressively greater across time points, where at 4-weeks, exposure groups differed marginally. A second look of the individual trajectories of IR scores across age suggests a high degree of between-subject variability in exposure-related impacts on IR scores that is superimposed upon a general neonatal increase in irritable reactivity across the neonatal period. Person-centered methods (e.g., Espy, Fang, Charak, Minich & Taylor, 2009) might be applied fruitfully to identify specific groups of neonate with discrepant neonatal developmental patterns related to exposure. Birth is a stressful, energy demanding event from which newborns recover via initial high levels of sleep and low irritability (Korner, 1996; Korner, Brown, Reade, & Stevenson, 1988). Because the IR factor is composed of items that score irritable reactivity to “daily living” activities – to handling, physical maneuvers, auditory and visual stimulation, exposure-related irritability would be observed routinely and persistently, at least for a subset who are most vulnerable. Given the importance of irritability to solicit care giving, these early, subtle differences perhaps set the stage for the ensuing deviations in maternal-infant behavior that have been observed (Schuetze & Eiden, 2006, 2007), and may be an early precursor to later deviations in emotional dysregulatory behavior (Wakschlag, Leventhal, Pine, Pickett, & Carter, 2006; Brook, Brook, & Whiteman, 2000).
Although the self-reported average first trimester smoking was related marginally to a decrease in the rate of deceleration in Stressor Dysregulation (SD) factor scores, none of the other exposure variables, self-report or biospecimen data, predicted differences in the rates of change in, or in the age-specific level of, the dysregulation response to mid-level stressors. Dysregulation behaviors in response to a relatively acute, substantial stressor might be more resilient to the deleterious impact of prenatal tobacco exposure, given their deeply rooted, evolution-selected, adaptive role in signaling immediate discomfort and distress. It is important to note, however, that the IR and SD factor scores were related substantively, and thus, teasing apart the isolated impact of exposure on these two dimensions is not a simple endeavor. Because TE neonates showed more irritability in response to routine handling as shown by the IR factor score difference, the introduction of a stressful stimulus (e.g., cold disc) might not have provoked as much of an increase in negative emotionality due to natural constraints in the neonatal behavioral repertoire, somewhat akin to a “ceiling effect.” Including stress biomarkers, such as cortisol or heart rate measurements, might reveal latent physiologic differences that could help to disentangle these two dimensions, as these methods have been successful in revealed exposure-related differences (e.g., Schuetze & Eiden, 2006; Franco, Chabanski, Szilwowski, Dramaix, & Kahn, 2000).
Sampling methods of this study deserve particular comment, particularly in light of the decadal changes in smoking behavior, as a contributor to the obtained pattern of findings. Women were recruited prospectively in the first trimester, and thus it was impossible to balance selection on persistence of smoking throughout pregnancy. Secondly, our goal was to minimize extraneous influences other than smoking, thus non-smoking women were selected specifically to be more comparable demographically to those who smoked, and certainly resulted in exposure groups that were more similar (although not completely so) than is typically found in community-based samples. Consistent with minimizing extraneous influences and in our interests in the neurobiologic effects of nicotine on the nervous system, our goal was to minimize the impact of other exposures, and thus women who reported illegal drug use at screening, at interview, or tested positive in biospecimens, as well as those with known heavy alcohol use were not included in the report here. The benefit of this sampling design is the highlighting of the prenatal tobacco exposure effects among the background of risks. The downside, though, is that there were fewer heavier and more persistent smokers in the present study, as higher alcohol and other drug use is substantially more common in women who smoke heavily during pregnancy. These sampling differences must be evaluated carefully in interpreting the pattern of findings across studies.
One strength of this effort is the longitudinal design that permitted characterization of the impact of prenatal tobacco exposure on the development of regulatory skills across first month of life. These findings serve to link those from other cross-sectional studies that have focused on withdrawal effects, regulatory behavior shortly after birth, and the “longer term,” residual impacts of exposure later at the end of neonatal period. The longitudinal measurement and growth modeling strategy takes advantage of the increased reliability of change to describe developmental processes and deviations (Rogosa & Willett, 1985). The average trajectory was consistent with a “catch-up” pattern for attention skills, consistent with “self-righting” resilience in development, at least in this sample with relatively low amounts of smoking and less confounding by other exposures. The observed trajectory, though, is simply a mathematical average, and is superimposed on substantial individual variation. Of course, the effects observed here in the neonatal period are only the first step in establishing the dynamic impact of prenatal tobacco exposure on the developing nervous system that supports regulatory processes within the broader context of parenting and the social environment that also interactively shapes development as it unfolds. Whether the initial developmental patterns observed in the neonatal period are related to disturbances in later attention and emotion regulation behaviors is an important future question, as these more basic neonatal skills are integrated into the increasingly complex behavior repertoire of the developing infant and child that are expressed dynamically in varying social contexts.
Acknowledgments
This research was supported in part by National Institutes of Health grants R01 DA014661, DA023653, DA015223, MH065668, and HD050309. The authors gratefully acknowledge the participating families, hospital staff, and project personnel who made this work possible.
Footnotes
Recruitment was balanced across sites, as the interaction of site by exposure group was not significant for nearly all demographic, maternal and perinatal variables. The only exception was there were more non-smoking women enrolled at the IL site who had private insurance (χ2 [1, N = 304] = 7.97, p < 0.01; 23 NE-Illinois, 4 NE-Nebraska, 11 TE-Illinois, and 12 TE-Nebraska participants had private insurance).
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/dev
References
- Azam L, Chen Y, Leslie FM. Developmental regulation of nicotinic acetylcholine receptors within midbrain dopamine neurons. Neuroscience. 2007;144:1347–1360. doi: 10.1016/j.neuroscience.2006.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baghurst PA, Tong SL, Woodward A, McMichael AJ. Effects of maternal smoking upon neuropsychological development in early childhood: importance of taking account of social and environmental factors. Paediatric and Perinatal Epidemiology. 1992;6:403–415. doi: 10.1111/j.1365-3016.1992.tb00784.x. [DOI] [PubMed] [Google Scholar]
- Bard KA, Coles CD, Platzman KA, Lynch ME. The effects of prenatal drug exposure, term status, and caregiving on arousal and arousal modulation in 8-week-old infants. Developmental Psychobiology. 2000;36:194–212. [PubMed] [Google Scholar]
- Bennett DS, Mohamed FB, Carmody DP, Bendersky M, Patel S, Khorrami M, et al. Response inhibition among early adolescents prenatally exposed to tobacco: An fMRI study. Neurotoxicology and teratology. 2009;31(5):283–290. doi: 10.1016/j.ntt.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook J, Brook D, Whiteman M. The influence of maternal smoking during pregnancy on the toddler’s negativity. Archives of Pediatric and Adolescent Medicine. 2000;154:381–385. doi: 10.1001/archpedi.154.4.381. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control. Alcohol Terms. 2008 August; Retrieved from http://www.cdc.gov/alcohol/terms.htm#excessive.
- Chen H, Parker SL, Matta SG, Sharp BM. Gestational nicotine exposure reduces nicotinic cholinergic receptor (nAChR) expression in dopaminergic brain regions of adolescent rats. European Journal of Neuroscience. 2005;22:380–388. doi: 10.1111/j.1460-9568.2005.04229.x. [DOI] [PubMed] [Google Scholar]
- Connors CK, Erhardt D, Sparrow EP. CAARS – Self-Report: Short Version (CAARS – S:S) North Tonawanda, NY: Multi-Health Systems Inc; 1998. [Google Scholar]
- Conter V, Cortinovis L, Rogari P, Riva L. Weight growth in infants born to mothers who smoked during pregnancy. British Medical Journal. 1995;310:768–776. doi: 10.1136/bmj.310.6982.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day N, Cornelius M, Goldschmidt L, Richardson G, Robles N, Taylor P. The effects of prenatal tobacco and marijuana use on offspring growth from birth through 3 years of age. Neurotoxicology and Teratology. 1992;14:407–414. doi: 10.1016/0892-0362(92)90051-b. [DOI] [PubMed] [Google Scholar]
- Day NL, Richardson GA, Goldschmidt L, Cornelius MD. Effects of prenatal tobacco exposure on preschoolers’ behavior. Journal of Developmental and Behavioral Pediatrics. 2000;21:180–188. [PubMed] [Google Scholar]
- Derogatis LR. Brief Symptom Inventory. Baltimore, MD: Clinical Psychometric Research; 1975. [Google Scholar]
- D’Onofrio B, Van Hulle C, Waldman I, Rodgers J, Rathouz P, Lahey B. Causal inferences regarding prenatal alcohol exposure and childhood externalizing problems. Archives of General Psychiatry. 2007;64:1296–1304. doi: 10.1001/archpsyc.64.11.1296. [DOI] [PubMed] [Google Scholar]
- Emde RN, Buchsbaum HK. Toward a psychoanalytic theory of affect: II. Emotional development and signaling in infancy. 1989:193–227. [Google Scholar]
- England LJ, Grauman A, Qian C, Wilkins DG, Schisterman EF, Yu KF, et al. Misclassification of maternal smoking status and its effects on an epidemiologic study of pregnancy outcomes. Nicotine Tobacco Research. 2007;9:1005–1013. doi: 10.1080/14622200701491255. [DOI] [PubMed] [Google Scholar]
- Espy KA, Fang H, Charak D, Minich N, Taylor HG. Growth mixture modeling of academic achievement in children of varying birth weight risk. Neuropsychology. 2009;23(4):460–474. doi: 10.1037/a0015676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espy KA, Riese ML, Francis DJ. Neurobehavior in preterm neonates exposed to cocaine, alcohol, and tobacco. Infant Behavior & Development. 1997;20:297–309. [Google Scholar]
- Eyler FD, Behnke M. Early development of infants exposed to drugs prenatally. Clinical Perinatology. 1999;26:107–150. [PubMed] [Google Scholar]
- Eyler FD, Behnke M, Conlon M, Woods NS, Wobie K. Birth outcome from a prospective, matched study of prenatal crack/cocaine use: I. Interactive and dose effects on health and growth. Pediatrics. 1998;101:229–237. doi: 10.1542/peds.101.2.229. [DOI] [PubMed] [Google Scholar]
- Franco P, Chabanski S, Szilwowski H, Dramaix M, Kahn A. Influence of maternal smoking on autonomic nervous system in healthy infants. Pediatric Research. 2000;47:215–220. doi: 10.1203/00006450-200002000-00011. [DOI] [PubMed] [Google Scholar]
- Fried PA, Makin JE. Neonatal behavioural correlates of prenatal exposure to marijuana, cigarettes and alcohol in a low risk population. Journal of Neurotoxicology and Teratology. 1987;9:1–7. doi: 10.1016/0892-0362(87)90062-6. [DOI] [PubMed] [Google Scholar]
- Fried PA, Watkinson B. Differential effects on facets of attention in adolescents prenatally exposed to cigarettes and marihuana. Journal of Neurotoxicology and Teratology. 2001;23:421–430. doi: 10.1016/s0892-0362(01)00160-x. [DOI] [PubMed] [Google Scholar]
- Fried PA, Watkinson B, Dillon RF, Dulberg CS. Neonatal neurological status in a low-risk population after prenatal exposure to cigarettes, marijuana, and alcohol. Journal of Developmental & Behavioral Pediatrics. 1987;8:318–326. [PubMed] [Google Scholar]
- Fried PA, Watkinson B, Gray R. A follow-up study of attentional behavior in 6-year-old children exposed prenatally to marijuana, cigarettes, and alcohol. Journal of Neurotoxicology and Teratology. 1992;14:299. doi: 10.1016/0892-0362(92)90036-a. [DOI] [PubMed] [Google Scholar]
- Godding V, Bonnier C, Fiasse L, Michel M, Longueville E, Lebecque P, et al. Does in utero exposure to heavy maternal smoking induce nicotine withdrawal symptoms in neonates? Pediatric Research. 2004;55:645–651. doi: 10.1203/01.PDR.0000112099.88740.4E. [DOI] [PubMed] [Google Scholar]
- Goldman PS. Plasticity of function in the CNS. In: Stein DG, Rosen JJ, Butters N, editors. Plasticity and recovery of function in the central nervous system. London: Academic Press; 1974. pp. 149–174. [Google Scholar]
- Gorsuch RL. Factor Analysis. 2. Hillsdale, NJ: Lawrence Erlbaum; 1983. [Google Scholar]
- Hagino N, Lee J. Effect of maternal nicotine on the development of sites for [3H]nicotine receptor binding in the fetal brain. International Journal of Developmental Neuroscience. 1985;3:567–571. doi: 10.1016/0736-5748(85)90046-2. [DOI] [PubMed] [Google Scholar]
- Hardy J, Mellits E. Does maternal smoking during pregnancy have a long term effect on the child? Lancet. 1972;2:1332–1336. doi: 10.1016/s0140-6736(72)92777-8. [DOI] [PubMed] [Google Scholar]
- Huizink AC, Mulder EJ. Maternal smoking, drinking or cannabis use during pregnancy and neurobehavioral and cognitive functioning in human offspring. Neuroscience and Biobehavioral Reviews. 2006;30:24–41. doi: 10.1016/j.neubiorev.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Jacobsen L, Slotkin T, Westerveld M, Mencl W, Pugh KR. Visuospatial memory deficits emerging during nicotine withdrawal in adolescents with prenatal exposure to active maternal smoking. Neuropsychopharmacology. 2006;31:1550–1561. doi: 10.1038/sj.npp.1300981. [DOI] [PubMed] [Google Scholar]
- Jacobson JL, Jacobson SW. Methodological considerations in behavioral toxicology in infants and children. Developmental Psychology. 1996;32:390–403. [Google Scholar]
- Jacobson SW. Neonatal correlates of prenatal exposure to smoking, caffeine, and alcohol. Infant Behavior & Development. 1984;7:253–265. [Google Scholar]
- Johnson CH, Vicary JR, Heist CL, Corneal DA. Moderate alcohol and tobacco use during pregnancy and child behavior outcomes. Journal of Primary Prevention. 2001;21:367–379. [Google Scholar]
- Kelmanson IA, Erman LV, Litvina SV. Maternal smoking during pregnancy and behavioural characteristics in 2 - 4-month-old infants. Klinische Pädiatrie. 2002;214:359–364. doi: 10.1055/s-2002-35369. [DOI] [PubMed] [Google Scholar]
- Kopp CB. Antecedents of self-regulation: A developmental perspective. Developmental Psychology. 1982;18:199–214. [Google Scholar]
- Koren G. Cocaine and the human fetus: the concept of teratophilia. Journal of Neurotoxicology and Teratology. 1993;15(5):301–304. doi: 10.1016/0892-0362(93)90029-n. discussion 311–302. [DOI] [PubMed] [Google Scholar]
- Korner AF. Reliable individual differences in preterm infants’ excitation management. Child Development. 1996;67:1793–1805. [PubMed] [Google Scholar]
- Korner AF, Brown BW, Dimiceli S, Forrest T. Stable individual differences in developmentally changing preterm infants: A replicated study. Child Development. 1989;60:502–513. [PubMed] [Google Scholar]
- Korner AF, Brown BW, Reade EP, Stevenson DK. State behavior of preterm infants as a function of development, individual and sex differences. Infant Behavior and Development. 1988;11:111–124. [Google Scholar]
- Korner AF, Kraemer HC, Reade EP, Forrest T, Dimiceli S. A methodological approach to developing an assessment procedure for testing the neurobehavioral maturity of preterm infants. Child Development. 1987;58:1478–1487. [PubMed] [Google Scholar]
- Kotimaa AJ, Moilanen I, Taanila A, Ebeling H, Smalley SL, McGough JJ, et al. Maternal smoking and hyperactivity in 8-year-old children. Journal of the American Academy of Child and Adolescent Psychiatry. 2003;42:826–833. doi: 10.1097/01.CHI.0000046866.56865.A2. [DOI] [PubMed] [Google Scholar]
- Landmesser L. Axonal outgrowth and pathfinding. Progress in Brain Research. 1994;103:67–73. doi: 10.1016/s0079-6123(08)61127-9. [DOI] [PubMed] [Google Scholar]
- Law K, Stroud L, LaGasse L, Niaura R, Liu J, Lester B. Smoking during pregnancy and newborn neurobehavior. Pediatrics. 2003;111:1318–1323. doi: 10.1542/peds.111.6.1318. [DOI] [PubMed] [Google Scholar]
- Leech SL, Richardson GA, Goldschmidt L, Day NL. Prenatal substance exposure: Effects on attention and impulsivity of 6-year-olds. Neurotoxicology and Teratology. 1999;21:109–118. doi: 10.1016/s0892-0362(98)00042-7. [DOI] [PubMed] [Google Scholar]
- Levin ED, Slotkin TA. Developmental neurotoxicity of nicotine. San Diego: Academic; 1998. [Google Scholar]
- Lichtensteiger W, Hefti F, Felix D, Huwyler T, Melamed E, Schlumpf M. Stimulation of nigrostriatal dopamine neurones by nicotine. Neuropharmacology. 1982;21:963–968. doi: 10.1016/0028-3908(82)90107-1. [DOI] [PubMed] [Google Scholar]
- Linnet KM, Dalsgaard S, Obel C, Wisborg K, Henriksen TB, Rodriguez A, et al. Maternal lifestyle factors in pregnancy risk of attention deficit hyperactivity disorder and associated behaviors: Review of the current evidence. American Journal of Psychiatry. 2003;160:1028–1040. doi: 10.1176/appi.ajp.160.6.1028. [DOI] [PubMed] [Google Scholar]
- Little RJA, Rubin DB. Statistical Analysis with Missing Data. New York: John Wiley; 2002. [Google Scholar]
- Mansi G, Raimondi F, Pichini S, Capasso L, Sarno M, Zuccaro P, et al. Neonatal urinary cotinine correlates with behavioral alterations in newborns prenatally exposed to tobacco smoke. Pediatric Research. 2007;61:257–261. doi: 10.1203/pdr.0b013e31802d89eb. [DOI] [PubMed] [Google Scholar]
- Matheny AP, Riese ML, Wilson RS. Rudiments of infant temperament: Newborn to 9 months. Developmental Psychology. 1985;21:486–494. [Google Scholar]
- Maughan B, Taylor A, Caspi A, Moffitt TE. Prenatal Smoking and Early Childhood Conduct Problems: Testing Genetic and Environmental Explanations of the Association. Archives of General Psychiatry. 2004;61:836–843. doi: 10.1001/archpsyc.61.8.836. [DOI] [PubMed] [Google Scholar]
- McFarland BJ, Seidler FJ, Slotkin TA. Inhibition of DNA synthesis in neonatal rat brain regions caused by acute nicotine administration. Developmental Brain Research. 1991;58:223–229. doi: 10.1016/0165-3806(91)90008-7. [DOI] [PubMed] [Google Scholar]
- Muneoka K, Ogawa T, Kamei K, Muraoka S, Tomiyoshi R, Mimura Y, et al. Prenatal nicotine exposure affects the development of the central serotonergic system as well as the dopaminergic system in rat offspring: involvement of route of drug administrations. Developmental Brain Research. 1997;102:117–126. doi: 10.1016/s0165-3806(97)00092-8. [DOI] [PubMed] [Google Scholar]
- Navarro HA, Seidler FJ, Whitmore WL, Slotkin TA. Prenatal exposure to nicotine via maternal infusions: effects on development of catecholamine systems. Journal of Pharmacology and Exp Therapeutics. 1988;244:940–944. [PubMed] [Google Scholar]
- Nigg JT, Breslau N. Prenatal smoking exposure, low birth weight, and disruptive behavior disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46:362–369. doi: 10.1097/01.chi.0000246054.76167.44. [DOI] [PubMed] [Google Scholar]
- Nugent JK, Lester BM, Greene SM, Wieczorek-Deering D. The effects of maternal alcohol consumption and cigarette smoking during pregnancy on acoustic cry analysis. Child Development. 1996;67:1806–1815. [PubMed] [Google Scholar]
- Office of Applied Studies. DHHS Publication No. SMA 05-4062, NSDUH Series H-28. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2005. Results from the 2004 National Survey on Drug Use and Health: National findings. [Google Scholar]
- Orlebeke JF, Knol DL, Verhulst FC. Child behavior problems increased by maternal smoking during pregnancy. Archives of Environmental Health. 1999;54:15–19. doi: 10.1080/00039899909602231. [DOI] [PubMed] [Google Scholar]
- Picone TA, Allen LH, Olsen PN, Ferris ME. Pregnancy outcome in North American women. II. Effects of diet, cigarette smoking, stress, and weight gain on placentas, and on neonatal physical and behavioral characteristics. American Journal of Clinical Nutrition. 1982;36:1214–1224. doi: 10.1093/ajcn/36.6.1214. [DOI] [PubMed] [Google Scholar]
- Rantakallio P. A follow-up study to the age of 14 of children whose mothers smoked during pregnancy. Acta Paediatrica Scandanavia. 1983;72:747–753. doi: 10.1111/j.1651-2227.1983.tb09805.x. [DOI] [PubMed] [Google Scholar]
- Richardson GA, Day NL, Taylor PM. The effect of prenatal alcohol, marijuana, and tobacco exposure on neonatal behavior. Infant Behavior & Development. 1989;12:199–209. [Google Scholar]
- Richardson SA, Tizabi Y. Hyperactivity in the offspring of nicotine-treated rats: role of the mesolimbic and nigrostriatal dopaminergic pathways. Pharmacology, Biochemistry and Behavior. 1994;47:331–337. doi: 10.1016/0091-3057(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Riese ML. Procedures and norms for assessing behavioral patterns in full-term and stable pre-term neonates. JSAS Catalog of Selected Documents in Psychology. 1982;12 (MS. No. 2415) [Google Scholar]
- Riese ML. Assessment of behavioral patterns in neonates. Infant Behavior and Development. 1983;6:241–246. [Google Scholar]
- Riese ML. Implications of sex differences in neonatal temperament for early risk and developmental/environmental interactions. Journal of Genetic Psychology. 1986;147:507–513. doi: 10.1080/00221325.1986.9914526. [DOI] [PubMed] [Google Scholar]
- Riese ML. Temperament stability between the neonatal period and 24 months. Developmental Psychology. 1987;23:216–222. [Google Scholar]
- Riese ML. Mothers’ ratings of infant temperament: Relation to neonatal latency to soothe by pacifier. Journal of Genetic Psychology. 1995;156:23–31. doi: 10.1080/00221325.1995.9914803. [DOI] [PubMed] [Google Scholar]
- Riese ML, Wilson RS, Matheny AP., Jr Multimethod assessment of temperament in twins: birth to six months. Acta Geneticae Medicae et Gemellologiae (Roma) 1985;34(1–2):15–31. doi: 10.1017/s0001566000004888. [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Bohlin G. Are maternal smoking and stress during pregnancy related to ADHD symptoms in children? Journal of Child Psychology and Psychiatry. 2005;46:246–254. doi: 10.1111/j.1469-7610.2004.00359.x. [DOI] [PubMed] [Google Scholar]
- Rogosa D, Willett J. Understanding the correlates of change by modeling individual differences in growth. Psychometrika. 1985;50:203–228. [Google Scholar]
- Roza SJ, Verburg BO, Jaddoe VW, Hofman A, Mackenbach JP, Steegers EA, et al. Effects of maternal smoking in pregnancy on prenatal brain development. The Generation R Study. European Journal of Neuroscience. 2007;25:611–617. doi: 10.1111/j.1460-9568.2007.05393.x. [DOI] [PubMed] [Google Scholar]
- Saxton DW. The behaviour of infants whose mothers smoke in pregnancy. Early Human Development. 1978;2:363–369. doi: 10.1016/0378-3782(78)90063-4. [DOI] [PubMed] [Google Scholar]
- Schuetze P, Eiden R. The association between maternal smoking and secondhand exposure and autonomic functioning at 2–4 weeks of age. Infant Behavior and Development. 2006;29:32–43. doi: 10.1016/j.infbeh.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Schuetze P, Eiden R. The association between prenatal exposure to cigarettes and infant and maternal negative affect. Infant Behavior and Development. 2007;30:387–398. doi: 10.1016/j.infbeh.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuetze P, Eiden R, Dombkowski L. The association between cigarette smoking during pregnancy and maternal behavior during the neonatal period. Infancy. 2006;10:267–288. [PMC free article] [PubMed] [Google Scholar]
- Seidler FJ, Levin ED, Lappi SE, Slotkin TA. Fetal nicotine exposure ablates the ability of postnatal nicotine challenge to release norepinephrine from rat brain regions. Developmental Brain Research. 1992;69:288–291. doi: 10.1016/0165-3806(92)90170-2. [DOI] [PubMed] [Google Scholar]
- Slotkin TA. Consensus on postnatal deficits: comparability of human and animal findings. Annals of the New York Academy of Sciences. 1998a;846:153–157. [PubMed] [Google Scholar]
- Slotkin TA. Fetal nicotine or cocaine exposure: which one is worse? Journal of Pharmacology and Experimental Therapeutics. 1998b;285:931–945. [PubMed] [Google Scholar]
- Slotkin TA, Greer N, Faust J, Cho H, Seidler FJ. Effects of maternal nicotine injections on brain development in the rat: ornithine decarboxylase activity, nucleic acids and proteins in discrete brain regions. Brain Research Bulletin. 1986;17:41–50. doi: 10.1016/0361-9230(86)90159-0. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Lappi SE, Seidler FJ. Beta-adrenergic control of c-fos expression in fetal and neonatal rat tissues: relationship to cell differentiation and teratogenesis. Toxicology and Applied Pharmacology. 1995;133:188–195. doi: 10.1006/taap.1995.1141. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: A technique for assessing self-reported alcohol consumption. In: Littenand RZ, Allen JP, editors. Measuring alcohol consumption: Psychosocial and biological methods. Totowa, NJ: Humana Press; 1992. pp. 41–72. [Google Scholar]
- Streissguth AP, Sampson PD, Barr HM, Bookstein FL, Carmichael Olson HC. The effects of prenatal exposure to alcohol and tobacco: Contributions of the Seattle Longitudinal Prospective Study and implications for public policy. In: Needleman HL, Bellinger D, editors. Prenatal exposure to toxicants: Developmental consequences. Baltimore: John Hopkins University Press; 1994. pp. b148–183. [Google Scholar]
- Stroud L, Paster R, Papandonatos G, Naura R, Salisbury A, Battle C, et al. Maternal smoking during pregnancy and newborn neurobehavior: Effects at 10 to 27 days. Journal of Pediatrics. 2009a;154(1):10–16. doi: 10.1016/j.jpeds.2008.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud L, Paster R, Goodwin M, Shenassa E, Buka S, Niaura, et al. Maternal smoking during pregnancy and neonatal behavior: A large-scale community study. Pediatrics. 2009b;123(5):842–848. doi: 10.1542/peds.2008-2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szatmari P, Saigal S, Rosenbaum P, Campbell D, King S. Psychiatric disorders at five years among children with birthweights <1000g: A regional perspective. Developmental Medicine & Child Neurology. 1990;32:954–962. [PubMed] [Google Scholar]
- Thapar A, Fowler T, Rice F, Scourfield J, van den Bree M, Thomas H, et al. Maternal Smoking During Pregnancy and Attention Deficit Hyperactivity Disorder Symptoms in Offspring. American Journal of Psychiatry. 2003;160:1985–1989. doi: 10.1176/appi.ajp.160.11.1985. [DOI] [PubMed] [Google Scholar]
- Vik T, Jacobsen G, Vatten L, Bakketeig L. Pre- and post-natal growth in children whose mothers smoked during pregnancy. Early Human Development. 1996;45:245–255. doi: 10.1016/0378-3782(96)01735-5. [DOI] [PubMed] [Google Scholar]
- Wakschlag LS, Hans SL. Maternal smoking during pregnancy and conduct problems in high-risk youth: a developmental framework. Developmental Psychopathology. 2002;14:351–369. doi: 10.1017/s0954579402002092. [DOI] [PubMed] [Google Scholar]
- Wakschlag LS, Leventhal BL, Pine DS, Pickett KE, Carter AS. Elucidating early mechanisms of developmental psychopathology: the case of prenatal smoking and disruptive behavior. Child Development. 2006;77:893–906. doi: 10.1111/j.1467-8624.2006.00909.x. [DOI] [PubMed] [Google Scholar]
- Wakschlag LS, Pickett KE, Cook E, Jr, Benowitz NL, Leventhal BL. Maternal smoking during pregnancy and severe antisocial behavior in offspring: a review. American Journal of Public Health. 2002;92:966–974. doi: 10.2105/ajph.92.6.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GM, O’Callaghan M, Najman JM, Bor W, Andersen MJ, Richards D, et al. Maternal cigarette smoking and child psychiatric morbidity: A longitudinal study. Pediatrics. 1998;102:e11. doi: 10.1542/peds.102.1.e11. [DOI] [PubMed] [Google Scholar]
- Willoughby M, Greenberg M, Blair C, Stifter C Family Life Investigative Group. Neurobehavioral consequences of prenatal exposure to smoking at 6 to 8 months of age. Infancy. 2007;12:273–301. [Google Scholar]
- Woodcock RW, McGrew KS, Mather N. Woodcock-Johnson III Tests of Cognitive Abilities: Brief Intellectual Ability. Itasca, IL: Riverside Publishing; 2001. [Google Scholar]
- Yolton K, Khoury J, Xu Y, Succop P, Lanphear B, Bernert JT, Lester B. Low-level prenatal exposure to nicotine and infant neurobehavior. Neurotoxicology & Teratology. 2009;31(6):356–363. doi: 10.1016/j.ntt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]