Abstract
Scientists and health officials are concerned that an H5N1 influenza pandemic could be both imminent and catastrophic. Managing it will be difficult. Supplies of antiviral agents will be limited and expensive. Clinical development of adjuvant-combined, antigen-sparing, inactivated vaccines has been slow; the vaccines will take several months to produce and the global capacity to produce them will remain limited for several years. People who live in countries without vaccine companies — more than 85% of humankind — will have little prospect for being immunized. Thus, new approaches are needed to confront an imminent pandemic. The interventions must be scientifically promising and already licensed or near licensure. Moreover, the global industrial capacity to produce them must be large and already in place. Three interventions meet these criteria. Within a few months, several billion doses of live-attenuated H5N1 vaccines could be produced in existing egg-based or cell culture production facilities and several billion doses of an H5 recombinant hemagglutinin (rHA) vaccine could be produced in existing pharmaceutical bioreactors. In addition, generic medications such as statins might be able to moderate the aberrant innate immune response that characterizes human cases of H5N1 influenza. Statins would be affordable and available worldwide on the first day of the pandemic. Given the limitations of current efforts to develop and produce antivirals and conventional vaccines, urgent attention must be given these promising new approaches to pandemic control.
The February 2007 decision of the Indonesian minister of health to suspend shipments of H5N1 influenza virus isolates to World Health Organization Collaborating Centers indicated that international cooperation is likely to be inadequate in any upcoming influenza pandemic. Access to pandemic-relevant influenza vaccines will be difficult for people in all countries, not just those in developing nations. Three affordable alternatives are proposed here that could be made available to people worldwide. The potential role of statins, in spite of important epidemiologic and experimental support, has not yet been discussed in the general medical literature, much less in the public press. The authors believe that all three approaches must be considered if we are to think and act seriously about responding to the possibility of an imminent pandemic.
— Vincent J Felitti, MD, Senior Editor
Introduction
Health officials throughout the world are concerned that the H5N1 avian influenza virus could be the cause of the next human influenza pandemic. It is estimated that if the 1918 pandemic were to recur today, it would kill between 51 million and 82 million people worldwide.1–3 However, the case fatality rate for H5N1 influenza is approximately 60%, and thus a pandemic caused by this virus could kill hundreds of millions and conceivably lead to a partial global population die-off. No one can estimate the probability that a pandemic of this magnitude will occur, but it is certainly possible.
Many influenza scientists have said that the next pandemic could be imminent and the molecular evolution of the H5N1 virus looks increasingly threatening. Yet scientists are also concerned that “pandemic fatigue” will lead governments to reduce their commitments to develop and produce the vaccines, antivirals, and other agents that will be needed. What can be done to focus government attention on practical approaches to confronting an imminent pandemic?
Yet scientists are also concerned that “pandemic fatigue” will lead governments to reduce their commitments to develop and produce the vaccines, antivirals, and other agents that will be needed.
The Inadequacy of Current Antivirals and Inactivated Vaccines for Pandemic Control
For control of the next influenza pandemic, only limited supplies of antiviral agents will be produced. In addition, they will be expensive and available only in countries that can afford to stockpile them. Moreover, experience with the neuraminidase inhibitors (Tamiflu, etc) used to treat patients with H5N1 infection indicates that at current dose levels, they fail to improve survival rates.4 Studies of larger doses, longer treatment periods and newer agents are planned, yet even if these studies are successful, the difficult problems of inadequate supply and high cost will remain.
Inactivated (killed-virus) vaccines are the mainstay for controlling seasonal influenza and are regarded as the primary intervention for controlling the next pandemic. A pandemic vaccine, however, will take many months to produce, and producing an H5N1 vaccine will be especially difficult. The reverse genetics-engineered seed strain used for producing an H5N1 vaccine gives poor yields in vaccine production facilities (less than one third of those normally expected).1,2 Moreover, the vaccine by itself is poorly immunogenic and requires an adjuvant to improve immunogenicity. Consequently, if confronted today with an imminent H5N1 pandemic, the world's vaccine companies could produce in six months enough doses of the most promising vaccine formulation (an adjuvanted vaccine containing 3.75 μg hemagglutinin [HA]) to immunize with two doses approximately 700 million people.2 This is fewer than the number of people who live in the nine major vaccine-producing countries: Australia, Canada, France, Germany, Italy, Japan, the Netherlands, the United Kingdom, and the United States. In the US, the sole domestic vaccine producer could produce in six months enough doses to immunize only 130 million people. Recently, the United States licensed a nonadjuvanted H5N1 vaccine containing 90 μg HA per dose, but this vaccine will never be used. In Europe, several adjuvanted H5N1 vaccines containing 3.75 to 30 μg HA per dose have been registered, yet none of these developments improve the prospects for global vaccine supply. More people might be immunized if vaccines considered to be potentially cross-protective were stockpiled in advance, but this approach will be adopted in a limited way by very few countries.
Inactivated (killed-virus) vaccines are the mainstay for controlling seasonal influenza and are regarded as the primary intervention for controlling the next pandemic.
Currently, almost all doses of inactivated influenza vaccine are produced in embryonated eggs. To increase production capacity, the US government has invested $1 billion to accelerate the construction of cell culture vaccine production facilities. In other countries, vaccine companies are making similar, though smaller, investments, usually without any government assistance. Despite these initiatives, four to five years are required to construct and obtain regulatory approval for new production facilities. Consequently, there is little chance that the potential global supply of conventional pandemic vaccines will increase substantially within this period.
Given these facts and the possibility that the next pandemic could be imminent, it is clear that antiviral agents will be available to only some of the people who live in a few developed countries. Moreover, people who live in countries that do not have vaccine companies (>85% of humankind) will have virtually no chance of receiving pandemic vaccines. For those who do live in countries with vaccine companies, vaccination will still be difficult.
Three New Approaches to Confronting an Imminent Pandemic
The fundamental reason why most of the world's people will remain vulnerable to an imminent pandemic is the lack of global industrial capacity that will allow us to quickly produce adequate supplies of affordable inactivated vaccines and antiviral agents. None of the current efforts to develop vaccines and antivirals addresses this fundamental need. Thus, it is not surprising that initiatives for pandemic preparedness concentrate on community mitigation and non-pharmaceutical interventions.5
Three new approaches could help us better confront an imminent pandemic. Two involve vaccines, one that is already licensed for seasonal use and another that could be licensed within one year. The third approach involves a generic medication that is widely available and inexpensive. It is not clear whether time will show these three approaches to be the best ways to confront a pandemic; other promising interventions in early stages of development may eventually prove to be better. Nonetheless, all three of these interventions share one unique and fundamental advantage: the industrial capacity already exists to produce them quickly in sufficient supply to meet global demand. What is lacking is the social imagination and political will to demonstrate that they are efficacious and ensure they can be produced.
A Live-Attenuated Pandemic Vaccine
A safe and effective live-attenuated, cold-adapted, intranasal, trivalent influenza vaccine has been available for seasonal use in the US for several years, and it is now licensed in a refrigerator-stable formulation. Investigators are working to develop a similar vaccine for pandemic use. One such vaccine has been shown to protect against challenge infection with various H5N1 viruses in experimental animals.6
A live-attenuated H5N1 pandemic vaccine could have several practical advantages—one-dose, needle-free, intranasal administration; enhanced local immunity in the respiratory tract; and perhaps reliable cross-protection against different clades of H5N1 virus. A small clinical trial has shown that a live-attenuated vaccine incorporating the HA of a current human H5N1 isolate is poorly immunogenic,7 but there are good reasons to believe that this would not be seen with a vaccine incorporating the HA from a highly transmissible pandemic virus. If an acceptably immunogenic, live-attenuated H5N1 vaccine could be developed, it could not be used for prepandemic immunization because it might reassort with a circulating seasonal influenza virus and thereby gain increased transmissibility. However, it could be stockpiled for use once a new pandemic virus had emerged.
The company that produces the seasonal live-attenuated influenza vaccine currently has a limited capacity to produce a pandemic vaccine (45 million doses per month), although production capacity will increase significantly by 2011.8 However, its production technology is highly efficient and this is an enormous advantage; compared with trivalent vaccine production in cell culture or eggs, the number of doses of monovalent pandemic vaccine that could be produced could increase by either 100- or 180-fold, respectively.8 The vaccine virus itself could be produced in any compatible facility: for example, the licensed facility of a company that would ordinarily produce virus for an inactivated H5N1 vaccine. Once the bulk vaccine virus had been produced, the other steps in the vaccine production process could, if necessary, be transferred to other facilities. It is also possible that animal influenza vaccine production facilities could be used if they meet regulatory specifications. This would not disrupt production of conventional pandemic vaccines in human vaccine production facilities and would take advantage of the very large existing global capacity for producing animal influenza vaccines, 80% of which is located in Asia.9 Given focused attention to clinical, regulatory, and industrial development, it is conceivable that several billion doses of a live-attenuated, pandemic vaccine could be produced within a few months of the emergence of a new pandemic virus.
A live-attenuated H5N1 pandemic vaccine could have several practical advantages— one-dose, needle-free, intranasal administration; enhanced local immunity in the respiratory tract; and perhaps reliable cross-protection against different clades of H5N1 virus.
A Recombinant Hemagglutinin Pandemic Vaccine
Recently, a randomized controlled trial of a trivalent, seasonal, recombinant HA (rHA) vaccine showed it to be safe, immunogenic and probably clinically efficacious.10,11 and an application for registration will be filed in the US in 2007. Given the similar immunogenicity of nonadjuvanted rHA H512 and split-virus H5N113 vaccines and the demonstrated antigen-sparing effects of adjuvanted H5N1 vaccines, an efficacious low-dose adjuvanted rHA H5 pandemic vaccine is a very real possibility.
Producing a pandemic vaccine involving rHA would first require cloning the gene for the pandemic virus HA in a baculovirus vector.11 This vector would be used to transfect insect cells that are then grown in a pharmaceutical bioreactor. A similar system is already being used to produce a new human papillomavirus vaccine. Thus, rHA antigen for pandemic vaccines could be manufactured in any existing pharmaceutical bioreactor facility.
Pilot studies show that a 10,000 L bioreactor could produce one million doses of a 135 μg rHA vaccine every five days.11 This information has been used to estimate the number of doses of pandemic rHA vaccine that might be produced worldwide (Dunnill P, Fedson DS, unpublished observations). Our estimate assumes that
Five 5-day production cycles could be accommodated each month
An effective adjuvanted rHA would need to contain only 10 μg or 3.75 μg rHA per dose
Twenty-five percent of the existing global pharmaceutical bioreactor capacity of two million L (approximately 60% of which is located in the US) could be diverted for several months to produce rHA antigen
Large-scale production yields in existing bioreactor facilities might be only 25% of those projected from pilot study experience.
Given these assumptions, the estimate of the number of doses of rHA vaccine that could be produced can be used to compare hypothetically the number of people who could be immunized worldwide with conventional egg-based inactivated H5N1 and rHA H5 vaccines (Table 1). Three months’ global production of the conventional vaccine (3.75 μg HA, adjuvanted) would be enough to immunize with two doses 350 million people. In contrast, three months’ production of an adjuvanted 3.75 μg rHA pandemic vaccine would be enough to immunize almost 3.4 billion people. This number is probably greater than the number of people who could be effectively immunized by all of the world's health care systems.
Table 1.
Number of people who could be immunized against an H5N1 pandemic with conventional egg-based or rHA vaccines a (hypothetical)
Statins might help control the aberrant innate immune response (cytokine storm) that characterizes human H5N1 infection,4 …
The contrasting scenarios shown in Table 1 are hypothetical. Nonetheless, they give a good indication of the potential scale and speed of rHA H5 vaccine production when compared with that of conventional egg-based H5N1 vaccines; the increase per month might be ten-fold, if not greater. Producing an rHA vaccine would improve dramatically the global prospects for pandemic vaccination.
Statins: A Possible Alternative for Treatment and Prophylaxis of Pandemic Influenza
Because of the inadequacies of the current antiviral and vaccine approach to control an influenza pandemic, an effective alternative is needed. One possibility is to use medications that are already produced as generics and are available and affordable worldwide. Statins, the drugs used to treat high cholesterol levels and prevent heart disease, are one such group that should be considered.14
The scientific rationale for the potential role of statins in pandemic influenza prophylaxis and treatment is based on their known anti-inflammatory and immunomodulatory effects.15 Statins might help control the aberrant innate immune response (cytokine storm) that characterizes human H5N1 infection,4 a response that could accompany infection with a virulent pandemic virus.14,16,17
Several observational studies have shown that recent prescriptions for statins are associated with 30% to 50% reductions in hospitalizations for chronic obstructive pulmonary disease and pneumonia and 40% to 60% reductions in pneumonia and all-cause mortality18–21 (Table 2). One observational study22 showed that statins had no effect on outcomes in patients with pneumonia, although there were several problems with its methods, including the definition of statin users and the way propensity scores were used in risk adjustment (Table 2). None of these studies was able to address directly the question of whether it is necessary to continue or to initiate statin treatment after hospital admission. However, early inhospital statin treatment benefits patients with other conditions associated with cytokine dysregulation, such as acute myocardial infarction,23 major noncardiac surgery,24 and bacteremia.25 Moreover, withdrawing statin treatment after hospital admission can be harmful.23,26 These findings strongly suggest that in patients with pneumonia, the full benefits of statins will require continued treatment after the onset of illness.
Table 2.
Improved outcomes from recent treatment with statins in patients with COPD and pneumonia
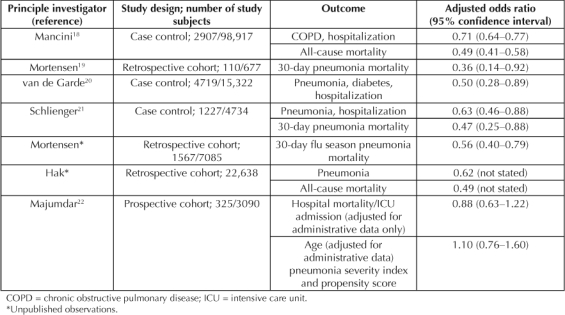
The observational studies of statins and pneumonia have provided an “epidemiological signal of protection” and clearly indicate the urgent need for additional research. Studies in animal models (especially ferrets and nonhuman primates) of acute H5N1 and 1918 influenza16,17 could help determine whether statin treatment, with or without concomitant antiviral treatment, could help control the aberrant immune response that accompanies these infections. In addition, observational studies of inpatient statin treatment of pneumonia patients in and out of influenza seasons would provide a strong indication of whether statins could benefit patients with seasonal as well as pandemic influenza.
The scientific argument for pandemic treatment and prophylaxis with statins is persuasive, but it must be confirmed in experimental and clinical studies.14 If, however, statins are shown to be of benefit, the public health argument for their use in a pandemic would be hugely compelling. Currently, a five-day course of treatment with a neuraminidase inhibitor costs $60 to $90 in the US, and the global availability of these agents is limited. By contrast, generic statins are available worldwide and are inexpensive. In the US, a five-day course of treatment would cost approximately $1.75, whereas in a developing country such as India, it would probably cost less than $1.00.14 Moreover, unlike vaccines and antivirals, statins would be available in almost all countries on the first day of the pandemic.
Requirements for Developing and Implementing These New Approaches
Any effort to quickly produce very large numbers of doses of live-attenuated and/or rHA pandemic vaccines would first require demonstrating their immunogenicity and safety, a process that, if tightly organized, should take no more than a few months. Far more important and more difficult would be the planning and development needed to identify production facilities, validate scale-up and bioprocessing procedures, sort out intellectual property and liability issues, ascertain overall demand for the vaccines, and arrange guaranteed financing for vaccine production and distribution. This could best be done by a top-down management system. Ideally, it would be coordinated by an international agency such as the World Health Organization. If this were not politically feasible, it would have to be undertaken by the governments of one or two countries. Government management could be especially important for brokering and funding the collaborative arrangements between companies that otherwise might not have commercial reasons to work together. Although this enterprise would be unprecedented in scale and complexity, the governments of vaccine-producing countries must be mindful of the economic and political consequences of not undertaking it.
A strong argument can be made for simultaneously pursuing a bottom-up approach that seeks to demonstrate the rationale for treatment and prophylaxis using existing generic medications that modify the host response to serious influenza virus infections. Whether agents such as statins would be more effective when used alone or with concomitant administration of an antiviral agent will have to be determined. If they were found to be effective, their global availability and affordability could be critically important, especially for developing countries.
If, however, statins are shown to be of benefit, the public health argument for their use in a pandemic would be hugely compelling.
Conclusion
Influenza scientists have repeatedly warned that the next pandemic could be imminent and might be catastrophic. Yet the collective efforts of governments, companies, and international organizations such as the World Health Organization have failed to match the magnitude of the pandemic threat. In an analysis of another disaster that scientists had foreseen—the Challenger launch explosion—an American sociologist concluded27 that the failure to prepare “… was a mistake embedded in the banality of organizational life and facilitated by an environment of scarcity and competition, elite bargaining, uncertain technology, incrementalism, patterns of information … (and) … organizational structures … that normalized signals of potential danger and re-aligned action with organizational goals.” Stripped of its academic jargon, this statement is a stinging indictment of “business as usual.” In an analysis of cultural challenges to envisioning worst case scenarios,28 another author concluded, “A less than perfect trajectory cannot deter us. … We can do significantly better in fighting calamity and catastrophe than current efforts allow. Given the stakes, … we would be truly remiss if we simply failed to try.”
The continuing occurrence of human H5N1 infections represents a very real and possibly imminent pandemic threat, one that in a worst case scenario could have unimaginable consequences for humankind. Current evidence indicates that we cannot count on having adequate supplies of antivirals and inactivated vaccines to respond to this threat. Failure to understand our current predicament could be costly to people everywhere.
Failure to understand our current predicament could be costly to people everywhere.
Live-attenuated and rHA vaccines offer realistic near-term possibilities for global immunization. Statins represent another potentially promising approach to pandemic treatment and prophylaxis. Developing the scientific basis for the clinical use of these three interventions will require the efforts of investigators from many disciplines. At the same time, governments must begin to harness the resources and facilities that will be needed for their production and distribution to all countries that will want to use them. The costs of not doing so could be incalculable.
Acknowledgments
Katharine O'Moore-Klopf of KOK Edit provided editorial assistance.
References
- Fedson DS. Preparing for pandemic vaccination: an international policy agenda for vaccine development. J Public Health Policy. 2005 Apr;26(1):4–29. doi: 10.1057/palgrave.jphp.3200008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedson DS. Vaccine development for an imminent pandemic: why we should worry, what we must do. Hum Vaccin. 2006 Jan–Feb;2(1):38–42. doi: 10.4161/hv.2.1.2554. [DOI] [PubMed] [Google Scholar]
- Murray CJ, Lopez AD, Chin B, Feehan D, Hill KH. Estimation of potential global pandemic influenza mortality on the basis of vital registry data from the 1918–20 pandemic: a quantitative analysis. Lancet. 2006 Dec 23;368(9554):2211–8. doi: 10.1016/S0140-6736(06)69895-4. [DOI] [PubMed] [Google Scholar]
- de Jong MD, Simmons CP, Thanh TT, et al. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat Med. 2006 Oct;12(10):1203–7. doi: 10.1038/nm1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control & Prevention. Interim pre-pandemic planning guidance: community strategy for pandemic influenza mitigation in the United States—early, targeted, layered use of nonpharmaceutical interventions [monograph on the Internet] Washington (DC): Centers for Disease and Control and Prevention, US Department of Health and Human Services; 2007 Feb. [cited 2007 Apr 19]. Available from: www.pandemicflu.gov/plan/community/community_mitigation.pdf. [Google Scholar]
- Suguitan AL, Jr, McAuliffe J, Mills KL, et al. Live, attenuated influenza A H5N1 candidate vaccines provide broad cross-protection in mice and ferrets [serial on the Internet] PLoS Med. 2006;3(9):e360. doi: 10.1371/journal.pmed.0030360. [cited 2007 Apr 29] [about 15 pages]. Available from http://medicine.plosjournals.org/perlserv/?request=get-document&doi=10.1371/journal.pmed.0030360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karron R, Callahan K, Luke C, et al. Phase I evaluation of live attenuated H9N2 and H5N1 ca reassortant vaccines in health adults [PowerPoint presentation on the Internet] Presented at the Third World Health Organization meeting on evaluation of pandemic influenza prototype vaccines in clinical trials, Geneva, Switzerland, 15–16 February 2007 [cited 2007 May 8]. Available from: www.who.int/vaccine_research/diseases/influenza/meeting_150207/en/print.html (Click on: Selected Presentations: NIH, MedImmune, CDC, JHU in USA [pdf 65kb])
- MedImmune presentation. 2006 analyst and investor day, Gaithersburg MD, December 6, 2006.
- Kieny MP, Costa A, Hombach J, et al. A global pandemic influenza vaccine action plan. Vaccine. 2006 Sep 29;24(40–41):6367–70. doi: 10.1016/j.vaccine.2006.07.021. [DOI] [PubMed] [Google Scholar]
- Treanor JJ, Schiff GM, Couch RB, et al. Dose-related safety and immunogenicity of a trivalent baculovirus-expressed influenza-virus hemagglutinin vaccine in elderly adults. J Infect Dis. 2006 May 1;193(9):1223–8. doi: 10.1086/503050. [DOI] [PubMed] [Google Scholar]
- Treanor JJ, Schiff GM, Hayden FG, et al. Safety and immunogenicity of a baculovirus-expressed hemagglutinin influenza vaccine: a randomized controlled trial. JAMA. 2007 Apr 11;297(14):1577–82. doi: 10.1001/jama.297.14.1577. [DOI] [PubMed] [Google Scholar]
- Treanor JJ, Wilkinson BE, Masseoud F, et al. Safety and immunogenicity of a recombinant hemagglutinin vaccine for H5 influenza in humans. Vaccine. 2001 Feb 8;19(13–14):1732–7. doi: 10.1016/s0264-410x(00)00395-9. [DOI] [PubMed] [Google Scholar]
- Treanor JJ, Campbell JD, Zangwill KM, Rowe T, Wolff M. Safety and immunogenicity of an inactivated subvirion influenza A (H5N1) vaccine. N Engl J Med. 2006 Mar 30;354(13):1343–51. doi: 10.1056/NEJMoa055778. [DOI] [PubMed] [Google Scholar]
- Fedson DS. Pandemic influenza: a potential role for statins in treatment and prophylaxis. Clin Infect Dis. 2006 Jul 15;43(2):199–205. doi: 10.1086/505116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao JK, Laufs U. Pleiotropic effect of statins. Annu Rev Pharmacol Toxicol. 2005;45:89–118. doi: 10.1146/annurev.pharmtox.45.120403.095748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kash JC, Tumpey TM, Proll SC, et al. Genomic analysis of increased host immune and cell death responses induced by 1918 influenza virus. Nature. 2006 Oct 5;443(7111):578–81. doi: 10.1038/nature05181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobasa D, Jones SM, Shinya K, et al. Aberrant innate immune response in lethal infection of macaques with the 1918 influenza virus. Nature. 2007 Jan 18;445(7125):319–23. doi: 10.1038/nature05495. [DOI] [PubMed] [Google Scholar]
- Mancini GB, Etminan M, Zhang B, et al. Reduction of morbidity and mortality by statins, angiotensin-converting enzyme inhibitors, and angiotensin receptor blockers in patients with chronic obstructive pulmonary disease. J Am Coll Cardiol. 2006 Jun 20;47(12):2554–60. doi: 10.1016/j.jacc.2006.04.039. [DOI] [PubMed] [Google Scholar]
- Mortensen EM, Restrepo MI, Anzueto A, Pugh J. The effect of prior statin use on 30-day mortality for patients hospitalized with community-acquired pneumonia. Respir Res. 2005 Jul 25;6:82. doi: 10.1186/1465-9921-6-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Garde EM, Hak E, Souverein PC, et al. Statin therapy and reduced risk of pneumonia in patients with diabetes. Thorax. 2006 Nov;61(11):957–61. doi: 10.1136/thx.2006.062885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlienger RG, Fedson DS, Jick SS, Jick H, Meier CR. Statin and risk of pneumonia: a population-based, nested case-control study. Pharmacotherapy. 2007 Mar;27(3):325–32. doi: 10.1592/phco.27.3.325. [DOI] [PubMed] [Google Scholar]
- Majumdar SR, McAlister FA, Eurich DT, Padwal RS, Marrie TJ. Statins and outcomes in patients admitted to hospital with community acquired pneumonia: population based prospective cohort study. BMJ. 2006 Nov 11;333(7576):999–1004. doi: 10.1136/bmj.38992.565972.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonarow GC, Wright RS, Spencer FA, et al. National Registry of Myocardial Infarction 4 Investigators Effect of statin use within the first 24 hours of admission for acute myocardial infarction on early morbidity and mortality. Am J Cardiol. 2005 Sep 1;96(5):611–6. doi: 10.1016/j.amjcard.2005.04.029. [DOI] [PubMed] [Google Scholar]
- Lindenauer PK, Pekow P, Wang K, Gutierrez B, Benjamin EM. Lipid-lowering therapy and in-hospital mortality following major non-cardiac surgery. JAMA. 2004 May 4;291(17):2092–9. doi: 10.1001/jama.291.17.2092. [DOI] [PubMed] [Google Scholar]
- Kruger P, Fitzsimmons K, Cook D, Jones M, Nimmo G. Statin therapy is associated with fewer deaths in patients with bacteraemia. Intensive Care Med. 2006 Jan;32(1):75–9. doi: 10.1007/s00134-005-2859-y. [DOI] [PubMed] [Google Scholar]
- Cubeddu LX, Seamon MJ. Statin withdrawal: clinical implications and molecular mechanisms. Pharmacotherapy. 2006 Sep;26(9):1288–96. doi: 10.1592/phco.26.9.1288. [DOI] [PubMed] [Google Scholar]
- Vaughan D. The Challenger launch decision: risky technology, culture, and deviance at NASA. Chicago: The University of Chicago Press; 1996. p. xii.p. xiv. p. [Google Scholar]
- Cerulo KA. Never saw it coming: cultural challenges to envisioning the worst. Chicago: The University of Chicago Press; 2006. p. 244. p. [Google Scholar]


