Abstract
Background: Tight glycemic control (TGC) is rapidly becoming a standard of care for all hospitalized patients. However, fear of hypoglycemia has proven a potent barrier to adoption of such initiatives by physicians and medical staff. Henry Ford Hospital has pursued aggressive glycemic control for all hospital patients. Because the initial standard TGC protocol (TGCP) was insufficient to improve glycemic control in our bariatric surgery patients, we hypothesized that a more intensive protocol would be necessary to improve glycemic control for this group.
Methods: As part of an institutional quality control project involving TGC, we reviewed medical records for the bariatric surgery patients at our hospital. We divided the populations into three subgroups: prior to TGC (A), initial hospital rollout TGC (B), and intensive bariatric TGC protocol (C). Patient populations were compared using hospital administrative databases and clinical chart review. Metrics for successful glycemic control included percent hypoglycemia (glucose <50 mg/dL), in-range percent (glucose 80–150 mg/dL), mild hyperglycemia (glucose 151–250 mg/dL), and major hyperglycemia (glucose >250 mg/dL).
Results: The percent in range for group C improved to 71% but was not statistically different from the values for groups A and B. The incidence of hyperglycemia was significantly decreased in group C as compared with groups A and B at both the minor (20% vs 31% and 27%) and major levels (1% vs 4% and 2%) (p < 0.001).There were no differences in the rates of hypoglycemia.
Conclusion: As an ongoing quality improvement process, our institution has pursued TGC for all of its patients. Glucose control in bariatric surgery patients is resistant to standard TGCPs. An initial intensive TGCP can be safely implemented in bariatric surgery patients with no increase in the number of hypoglycemic events. This work represents follow-up of several plan, do, check, act (PDCA) cycles related to improvement with a hospital-wide TGCP.
Introduction
Glycemic control in both diabetic and nondiabetic hospitalized patients is a major therapeutic focus. Reversal of hyperglycemia is now linked to better clinical outcomes in medical and surgical patients.1 Initial studies of tight glycemic control (TGC) were first reported in specialized centers. Because of improved clinical outcomes, tight glycemic control protocols (TGCPs) have been disseminated throughout the hospital setting, especially for patients having acute myocardial infarction, cardiac surgery, infections, and critical illness.1–5 There is a nationwide institutional pursuit of implementation of improved glycemic control for all inpatients. A standardized method for application of TGC in inpatients outside the previously established patient populations is still emerging.
Redefining the standard of care with TGC has added an exciting new aspect to improved quality of care. The Joint Commission on Accreditation of Healthcare Organizations has listed insulin as one of the five highest-risk medications in an inpatient setting.1 The fear of hypoglycemia is an important barrier to successful implementation of TGC. The aim of improving patient care while minimizing adverse effects is challenging in TGC. Acknowledging, accepting, and applying what we have learned over the past few years will broaden the application of TGC for specialty populations. The absence of a single standard system for TGC in variable patient populations makes it necessary to establish quality improvement measures.
Our objective was to correlate TGC in bariatric surgery patients with system-wide quality improvement initiatives. Our records revealed that the initial standard TGCP for bariatric surgery patients did not lead to an improvement in glycemic control. Glucose level in subset of patients was found to be more difficult to control than in our other postoperative patients. Therefore, we hypothesized that a more intensive TGCP would be necessary to improve glycemic control in bariatric surgery patients.
Methods
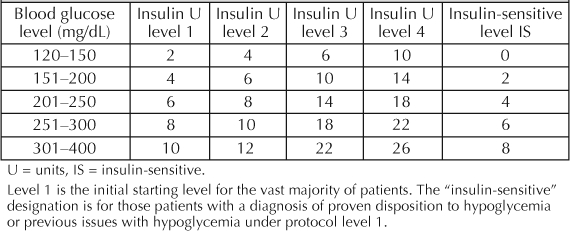
We collected data on 461 patients undergoing bariatric surgery at our institution between June 2003 and June 2005. The project was approved by our hospital institutional review board. Data from postoperative bariatric patients were collected concurrently and entered into a database. Information collected included blood glucose measurements, demographics, body mass index, surgical technique, wound infections, and length of hospital stay. The patient populations were divided into three subgroups. These included Group A: prior to TGCP, Group B: initial hospital roll out of TGCP, and Group C: intensive protocol for bariatric surgery patients. Administrative databases were used to perform chart reviews of our patient populations. Minitab version 13 software (Minitab Inc, College Station, PA) and Microsoft Office Excel (version 11, Microsoft, Seattle, WA) were used for data analysis. We defined the metrics of successful glycemic control by defining the ranges as hypoglycemia (glucose <50 mg/dL), in-range (glucose 80–150 mg/dL), mild hyperglycemia (glucose 151–250 mg/dL), and major hyperglycemia (glucose >250 mg/dL). These were the quality metrics chosen for monitoring, levels between 50 and 79 m/dL were not considered “in range” but were not counted as hypoglycemia. The sliding-scale insulin dosing was performed with Insulin Aspart (NovoLog, Novo Nordisk, Princeton, NJ), administered by subcutaneous injection. Initially all patients were started on level 1 TGCP, as seen in Table 1. Blood sugar levels were measured before meals and at bedtime or if patients were not allowed to eat or drink, their blood sugar levels were measured every six hours. The sliding-scale protocol was reassessed after two consecutive blood glucose measurements exceeding 150 mg/dL or for two consecutive blood glucose measurements less than 100 mg/dL. The TGCP was increased or decreased, respectively, by one level if glucose measurements met the above criteria. The intensive bariatric surgery protocol was initiated after it was noted to be inadequate when reviewed by the system quality improvement committee. It was noted that unlike all other surgical subgroups, bariatric surgery patients demonstrated no significant change in control (based on chosen metrics above) with the standard hospital protocol. A new approach was formulated, and consisted of the same protocol listed in Table 1. However, treatment for all postoperative bariatric surgery patients whose care followed the intensive protocol were initiated at level 2 instead of level 1. Data was summarized using Excel and Access (Microsoft, WA). Discrete variables were compared using c2 analysis with two degrees of variability. Statistical significance was assumed at p < 0.05.
Table 1.
Tight glycemic control protocol
Results
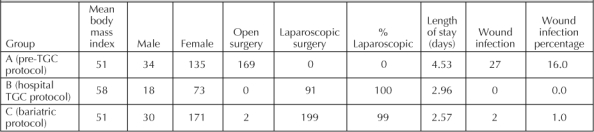
Data for a total of 461 postoperative bariatric surgery patients, divided into groups A, B, and C, were reviewed. Of these, 379 (82%) were women. The mean body mass index values for groups A, B, and C were 51, 58, and 51 kg/m2, respectively. The majority of operations performed in protocol groups B and C were laparoscopic, whereas only open operations were performed in group A. The observed increase in length of stay and incidence of wound infections in group A is likely secondary to changes in surgical technique (Table 2). Both wound infections in group C involved the only two open operations in that group.
Table 2.
Group demographics
The fear of hypoglycemia is an important barrier to successful implementation of TGC.
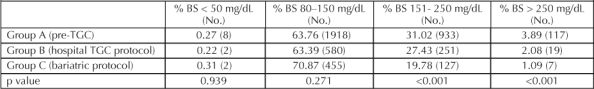
Metrics of successful TGC were measured for each group (Table 3). We found no difference in the incidence of hypoglycemia (blood sugar <50 mg/dL) in group C compared with the other two groups. The incidence of hypoglycemia in group C was observed to be 2/591 blood draws (0.31%). The incidence of in-range readings (blood sugar 80–150 mg/dL) for group C was 71%, compared with 64% and 63% for groups A and B, respectively. The incidence of mild and major hyperglycemia was significantly lower in group C than in groups A and B (Table 3). The incidence of mild hyperglycemia was 20% in group C, 31% in group A, and 27% in group B; the incidence of major hyperglycemia was 1% in group C, 4% in group A, and 2% in group B.
Table 3.
Incidence of blood sugar measurements in TGC metrics groups A, B, and C
Discussion
Improved glycemic control has become a benchmark for optimal patient care.1 This report demonstrates process improvement in glycemic control of a special group of patients. As our hospital implemented a TGCP in phases, it became clear that the bariatric surgery patient group was an outlier. Glycemic control was not adequate in this group with the initial level 1 protocol. Given our goal of achieving TGC in perioperative patients, we decided to intensify the protocol. A barrier to instituting a TGCP is the fear of hypoglycemia.1 Starting bariatric surgery patients on a level 2 TGCP required educating physicians and nurses to break down their resistance to the idea. With an initial level 2 TGCP, we were able to demonstrate significantly fewer mild and major hyperglycemic episodes, with no increase in hypoglycemic episodes.
Others have demonstrated improved clinical results with better glycemic control. Van den Berghe et al reported a reduction in mortality of the critically ill patients with a decrease in both time spent in an intensive care unit and hospital length of stay with even minor improvements in glucose control.2 Funary et al have shown a significant improvement in deep sternal wound infections with continuous insulin infusion in diabetic patients undergoing cardiac surgery.6 Although Furnary et al used continuous infusions of insulin, the endpoint was a blood sugar level <150 mg/dL, which is consistent with our in-range values. We presume that with follow-up studies in morbidly obese patients after bariatric surgery, we will see improvements also in morbidity, length of stay, infection rates, and possibly mortality from an intensive TGCP.
This report focuses on bariatric surgery patients, all of whom are morbidly obese. The failure to control glucose levels in these patients with the initial level 1 TGCP may be related to insulin resistance, which is known to be common in morbidly obese patients.7 A review of the literature did not reveal any studies of TGC in this subset of patients. The pandemic of obesity in the United States has led to an increased number of hospitalizations for morbidly obese patients. These patients may require an intensive TGCP initially in order to achieve adequate glycemic control and hopefully experience reduced morbidity and improved outcomes seen in other subset populations.2,4,6 Defining optimal TGC in hospitalized morbidly obese patients may require further modifications of a TGCP.
Improved glycemic control has become a benchmark for optimal patient care.1
Conclusion
The evidence that TGC leads to improved patient outcomes has been derived from multiple clinical studies.1–6 Performance metrics for special populations are in evolution. The work reported here represents several PDCA cycles in the optimization of perioperative glycemic control in this population. Although glucose control in bariatric surgery patients is resistant to a standard TGCP, an initial intensive TGCP can be safely initiated in these patients, with improvement in glycemic control and no increase in hypoglycemic events.
References
- ACE/ADA Task Force on Inpatient Diabetes. American College of Endocrinology and American Diabetes Association consensus statement on inpatient diabetes and glycemic control. Endocr Pract. 2006 Jul–Aug;12(4):458–68. doi: 10.4158/EP.12.4.458. [DOI] [PubMed] [Google Scholar]
- van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001 Nov 8;345(19):1359–7. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- van den Berghe G. How does blood glucose control with insulin save lives in intensive care? J Clin Invest. 2004;114:1187–95. doi: 10.1172/JCI23506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittas AG, Siegel RD, Lau J. Insulin therapy for critically ill hospitalized patients: a meta-analysis of randomized control trials. Arch Intern Med. 2004 Oct 11;164(18):2005–11. doi: 10.1001/archinte.164.18.2005. [DOI] [PubMed] [Google Scholar]
- Krinsley JS. Effect of intensive glucose management protocol on the mortality of critically ill adult patients. Mayo Clin Proc. 2004 Aug;79(8):992–1000. doi: 10.4065/79.8.992. [DOI] [PubMed] [Google Scholar]
- Furnary AP, Zerr KJ, Grunkemeier GL, Starr A. Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures. Ann Thorac Surg. 1999 Feb;67(2):352–60. doi: 10.1016/s0003-4975(99)00014-4. [DOI] [PubMed] [Google Scholar]
- Bonadonna RC, Groop L, Kraemer N, et al. Obesity and insulin resistance in humans: a dose-response study. Metabolism. 1990 May;39(5):452–9. doi: 10.1016/0026-0495(90)90002-t. [DOI] [PubMed] [Google Scholar]
- Zimmerman CR, Mlynarek ME, Jordan JA, Rajda CA, Horst HM. An insulin infusion protocol in critically ill cardiothoracic surgery patients. Ann Pharmacother. 2004 Jul–Aug;38(7–8):1123–9. doi: 10.1345/aph.1E018. [DOI] [PubMed] [Google Scholar]


