Abstract
Objectives: Little is known about why people continue to smoke after learning that they have diseases and conditions that contraindicate smoking. Using data from the Adverse Childhood Experiences (ACE) Study, we examined the relation between ACEs and smoking behavior when smoking-related illnesses or conditions are present, both with and without depression as a mediator.
Methods: Participants were more than 17,000 adult HMO members who retrospectively reported on eight categories of ACEs (emotional, physical, and sexual abuse; witnessing interparental violence; parental divorce; and growing up with a substance-abusing, mentally ill, or incarcerated household member). The number of maltreatment categories was summed to form an ordinal variable called the ACE Score. We measured current smoking, conditions that contraindicate smoking (heart disease, chronic lung disease, and diabetes), and symptoms of these illnesses (chronic bronchitis, chronic cough, and shortness of breath). Logistic regression models compared the ACE Score of smokers with smoking-related illnesses to participants who reported these illnesses but were not current smokers (n = 7483).
Results: Significant dose—response relations between the ACE Score and smoking persistence were found (odds ratio = 1.69; confidence interval = 1.34–2.13 for participants with ≥4 ACEs). Depression was a significant independent predictor of smoking persistence as well as a mediator. Depression only slightly attenuated the relation between the ACE Score and persistent smoking, however.
Conclusion: Medical practitioners should consider the maltreatment history and depression status of their patients when a smoking-related diagnosis fails to elicit smoking cessation. Programs should be developed that better address the underlying motivations for continuing to smoke in the face of health problems that contraindicate smoking.
Introduction
Although nicotine addiction is a prima facie reason for continued smoking, being diagnosed with a smoking-related illness or experiencing smoking-related symptoms are strong motivations for smoking cessation.1–3 Even so, practitioners encounter patients who continue to use tobacco despite having conditions that contraindicate smoking. Quit rates among those with cardiovascular disease do not exceed quit rates for the general population.4 Similarly, about one-third of patients with cancer continue to smoke after diagnosis.5,6 Understanding why some patients seem to have greater difficulty quitting may improve the practice of medicine.
Understanding why some patients seem to have greater difficulty quitting may improve the practice of medicine.
Some researchers refer to persistent smokers as “hard-core” smokers who may never attempt to quit, regardless of their health status.7 They have been found to be younger,8,9 less well educated,10,11 and from less-advantaged socioeconomic groups.12,13 In addition, having other smokers in the household,14 attributing one's symptoms to aging rather than to smoking,15 and having weaker self-efficacy beliefs about one's ability to quit1,16 are related to lower rates of smoking cessation. Thus, it appears that health beliefs and demographics, as well as the social environment, may interfere with stop-smoking messages.
Maltreatment in childhood may lead adults to adopt risky behaviors in a variety of domains …
Mental illness is another potential barrier to smoking cessation. A recent study found that adults with psychiatric disorders are almost twice as likely as those without such disorders to be smokers.17 The interference of depression with quitting attempts has been well documented.18–21 Depressed smokers are more likely than nondepressed smokers to relapse.22,23 In addition, depression has been found to maximize withdrawal-related symptoms and discomfort.24
Maltreatment in childhood may lead adults to adopt risky behaviors in a variety of domains, including smoking,25 alcohol abuse,26,27 illicit drug use,28,29 and sexual behavior.30,31 Although child abuse has been shown to lead to higher rates of medical care use,32,33 survivors of abuse use preventive medical services such as Pap tests and other examinations less frequently.34–36 In addition, abuse survivors are less likely than adults who were not abused to follow medical regimens appropriately.37,38 Because a history of trauma is often antecedent to depression39–41 and other forms of psychological impairment,42–44 we investigated whether continuing to smoke cigarettes despite the presence of illness or symptoms often caused by smoking or conditions that are exacerbated by smoking is associated with retrospective reports of childhood trauma.
Using data from the Adverse Childhood Experiences (ACE) Study, we hypothesized that people with smoking-related illness or symptoms who persist in smoking would be substantially more likely to report childhood trauma than people with the same illnesses who are not current smokers. Furthermore, we hypothesized that depression is both directly related to smoking persistence and that it reduces the strength of association between ACEs and smoking persistence. Current thought in psychology links violence and other traumatic experiences in childhood to poorer socioemotional functioning and indeed to neurobiologic changes.45,46 The ACE Study was undertaken to assess how these experiences lead to the development of risk factors that in turn affect disease, disability, and early mortality. The conceptual framework of the study is depicted in Figure 1.
Figure 1.
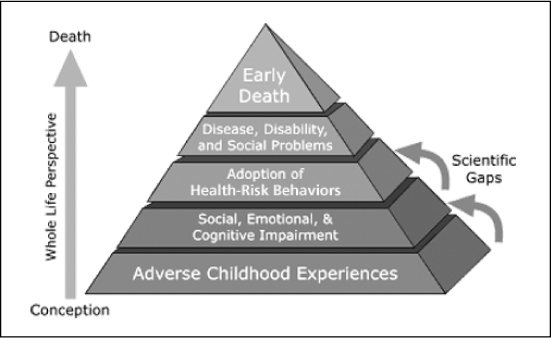
Adverse Childhood Experiences Study conceptual framework.
Reprinted from The Adverse Childhood Experiences Study. Centers for Disease Control 2006 Apr 27. Available from: www.cdc.gov/nccdphp/ace/pyramid.htm.
Methods
ACE Study participants were members of a large, metropolitan health maintenance organization (HMO) who were undergoing a comprehensive physical examination. A more detailed description of the ACE Study design and methods can be found elsewhere.47 The ACE Study was approved by the institutional review boards of the Southern California Permanente Medical Group, Emory University, and the National Institutes of Health Office of Protection from Research Risks.
We mailed a questionnaire to HMO members asking for sensitive information on childhood abuse and family dysfunction, as well as current health behaviors related to smoking, alcohol, and exercise (the Family Health History) after they had undergone their clinical examination. Information on their present health status, including their experience of a wide range of chronic diseases and disease-related symptoms, was obtained from a separate self-report previously completed by each patient (the Health Appraisal Questionnaire). During two survey periods (August to March 1996 and June to October 1997), 18,175 of 26,824 patients returned a Family Health History, for a composite response rate of 68%. Bias due to nonresponse was explored in the first survey wave48 and did not negatively impact the study's validity. We eliminated data for 280 respondents who did not provide complete demographic information and purged the second response data set for an additional 658 study subjects who participated in both survey waves. The analysis sample was a subset from this pool of 17,337 unique observations with complete demographic information and consisted of only those respondents who reported at least one of the ten smoking-related diseases or symptoms described in more detail below (n = 7483).
Definition of Persistent Smoking
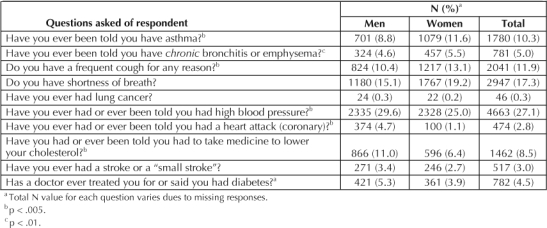
We obtained current smoking status by asking, “Do you smoke cigarettes now?” (with the response being yes or no), and we determined the number of cigarettes smoked per day by asking, “If ‘yes,’ on average, about how many cigarettes a day do you smoke?” Persistent smokers were defined as those participants who indicated that they were current smokers and who self-identified as having at least one of ten diseases or symptoms that are exacerbated by smoking. Seven of the ten are directly related to or often caused by smoking: chronic bronchitis or emphysema, asthma or wheezing, chronic cough, shortness of breath, heart attack, lung cancer, or stroke. The remaining three conditions are contraindications to smoking rather than illnesses that are a result of smoking per se: diabetes mellitus, high blood pressure, and currently taking cholesterol-lowering medication. The exact questions used to elicit the presence or absence of these diseases and conditions are listed in Table 1.
Table 1.
Prevalence of smoking-related diseases and symptoms by sex
Adverse Childhood Experiences
The Family Health History asked respondents about a variety of experiences in two broad domains: child abuse and family dysfunction. Specifically, we asked participants whether they had experienced physical, sexual, or emotional abuse; if they had witnessed interparental violence; whether their parents had separated or divorced; whether they had grown up with drug- or alcohol-abusing family members; had a family member go to prison; or had a family member who was mentally ill. In Table 2, we list the questions used to measure each of the eight domains and the response necessary to meet criterion for that category. Respondents who did not report an ACE were considered not to have had that experience. This most likely biases our results toward the null, by potentially misclassifying those who may have been exposed to an ACE as unexposed.
Table 2.
ACE questions and response categories
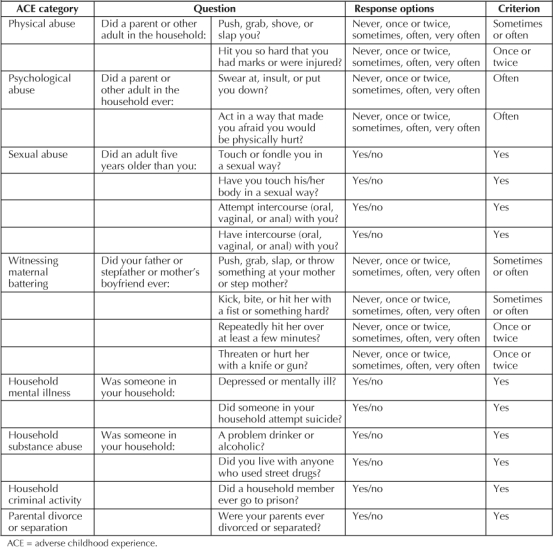
Because these experiences tend to cluster rather than to occur independently,49,50 we summed the number of ACEs that each person reported (range, 0 to 8) to form an ordinal variable referred to here as the ACE Score. We combined the upper end of the distribution to ensure adequate group size so that the ACE Score contained five levels (0, 1, 2, 3, and ≥4).
Depression
Because of the known association between depression and smoking, we investigated whether depression would mediate the relation between ACEs and smoking persistence. Respondents were considered depressed if they answered affirmatively to the question “Have you ever had or do you now have depression or feel ‘down in the dumps’?”
Persistent smokers were defined as those participants who indicated that they were current smokers and who self-identified as having at least one of ten diseases or symptoms that are exacerbated by smoking.
Statistical Analysis
We used the Statistical Package for the Social Sciences (SPSS; Chicago, IL) for all analyses. Logistic regression was used to compute adjusted odds ratios (ORs) and 95% confidence intervals (CIs) to assess the association between ACEs and smoking among people with a smoking-related illness or condition. We tested for trend in the ORs by using the summed ACE Score as an ordinal variable with five levels (0, 1, 2, 3, and ≥4 ACEs). We also modeled the relation of depression to persistent smoking, and then constructed a model including both the ACE Score and presence or history of depression to test for mediation. An analysis of variance was calculated using the ACE Score as a predictor, and the number of reported smoking-related symptoms or illnesses as the dependent measure.
Results
The ACE Study sample was composed of 9367 women (54%) and 7970 men (46%) with a mean age of 54.8 years (standard deviation [SD] = 15.7) among women and 57.5 years (SD = 14.6) among men. More than three-quarters of the participants (78.5% of the women and 81.6% of the men) described themselves as white; 34.5% of women and 45% of men were college graduates; another 37.5% of the women and 34% of the men had some college education.
The current smoking prevalence was 8.8%; men were slightly but not significantly more likely to be current smokers than were women (9.1% vs 8.6%, χ2 [1, 17,160] = 1.47, not significant). On average, smokers smoked 15 cigarettes per day (SD = 10.3). Of the 1518 current smokers in the sample, 51.2% (776) reported one or more of the tobacco-related illnesses or symptoms.
Table 1 lists the prevalence of each of the selected disease conditions and symptoms by sex, of which one or more were reported by 43.6% (7554) of the sample. The most frequently reported condition was high blood pressure, reported by >27% of the sample. Lung cancer was the rarest condition reported, having an overall prevalence of 0.3%.
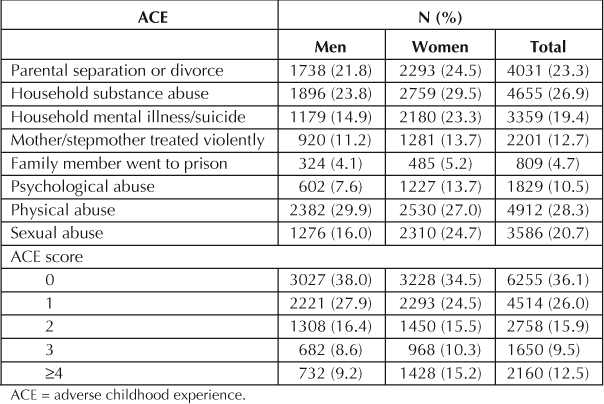
ACEs were common among participants (Table 3). Only 36.1% of individuals reported no ACEs. In contrast, 37.9% of all respondents reported ≥2 ACEs. For all ACEs except physical abuse, female respondents reported significantly higher prevalences than did men.
Table 3.
Prevalence of individual ACEs and ACE score by sex
To examine the relation between ACEs and smoking persistence, we performed a series of analyses among those participants who reported any of the diseases or symptoms listed in Table 1 and whose smoking status we knew (n = 7483). Within this group, the smoking prevalence was somewhat higher (n = 776; 10.4%) than in the total ACE sample. In addition, respondents in this group were significantly more likely to report physical, sexual, or emotional abuse (29.2% vs 27.0%, χ2 [1, 14,643] = 3.97; 21.7% vs 19.6%, χ2 [1, 14,643] = 9.72; and 11.3% vs 9.8%, χ2 [1, 14,643] = 9.38, respectively) at the .05 level. However, they did not report statistically higher prevalences of the other ACEs.
We first constructed a logistic model with the ACE Score as an ordinal predictor, adjusting for sex and age (Table 4) and current smoking as the dependent variable. The ORs and 95% CI for smoking are displayed in Table 3, under the “Separate Model” heading. The overall test for trend was significant (p < .001). Strong, graded relations were found between the ACE Score and the likelihood of continuing to smoke despite having health problems that contraindicated smoking. The adjusted likelihood of being a current, persistent smoker rose from 1.08 in individuals reporting one ACE to 1.69 in individuals reporting ≥4 ACEs. The prevalence of persistent smoking rose in a dose–response fashion as the number of reported ACEs increased, rising from 7.8% in participants with no ACEs to 16.6% in those reporting ≥4 ACEs.
Table 4.
ACEs and the prevalence and risk a (adjusted OR) of smoking among adults with smoking-related diseases or symptoms
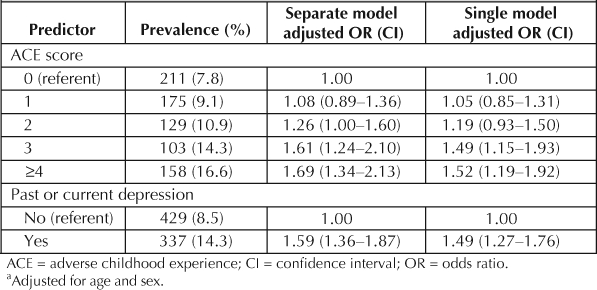
Next, we tested the relation between past or current depression and persistent smoking among those with smoking-related diseases and conditions. The adjusted odds ratio (OR) is also shown in Table 4 in the column labeled “Separate Model.” Those who reported past or current depressed affect were 1.59 times more likely to be persistent smokers than those who did not affirm past or current depression (p < .001).
Finally, we constructed a model with both the ACE Score and past or current depression as predictors. These results are shown in Table 4 under the column labeled “Single Model.” The χ-square value for the difference in the log likelihood ratios between the two models was significant (χ2 = 86.71, degrees of freedom = 1; p < .001); however, the addition of this variable only slightly attenuated the relation between the ACE Score and the odds of persistent smoking.
We also examined the ACE Score as a predictor of the number of smoking-related diseases and symptoms reported among smokers. We performed an analysis of variance with the number of smoking-related symptoms and diseases as the dependent variable (range, 0 to 10), controlling for age and sex. The ACE Score was statistically significant (F [1, 4] = 4.79; p < .001). A similar dose–response pattern of results was noted, wherein the average number of smoking-related symptoms and diseases increased as the number of reported ACEs rose (Figure 2).
Figure 2.
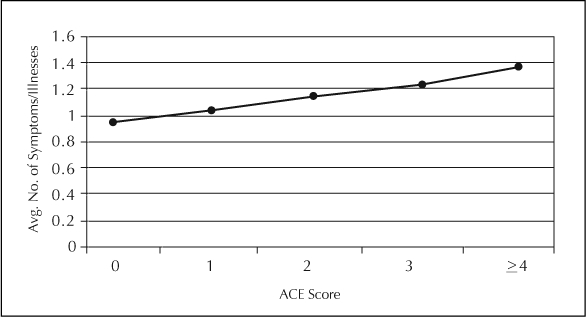
Average number of diseases/symptoms by adverse childhood experiences score (ACE), adjusted for age and sex.
Strong, graded relations were found between the ACE Score and the likelihood of continuing to smoke …
Discussion
Earlier research has demonstrated that childhood abuse is related to smoking initiation.25,51–52 Our research suggests that ACEs may play a role in the maintenance of smoking behavior in the presence of illness and poor health. These results extend our understanding of the impact of child maltreatment on adult health behavior. Furthermore, the association of ACEs with smoking persistence was sustained even after accounting for the presence of past or current depression, a condition that other researchers have related to continued smoking among patients with diabetes53,54 and patients with heart disease.55,56 Although we cannot rule out genetic influences on smoking persistence, earlier research with the ACE Study sample indicated that parental smoking had no impact on the association between ACEs and smoking initiation in the overall ACE sample.25
Despite the cross-sectional nature of these findings, it is evident that ACEs play a role in the development of health risk behaviors. The psychoactive properties of nicotine and other addictive substances may temporarily ameliorate negative affective states and thereby serve an adaptive function in coping with the aftermath of childhood trauma, regardless of the health risk involved.57 Certain psychological outcomes associated with childhood maltreatment, such as poor self-esteem,58,59 low self-efficacy,60,61 and an external locus of control,62,63 may be mechanisms by which these early negative experiences result in maladaptive patterns of health behavior later in life.
Programs that address the underlying emotional problems caused by childhood maltreatment may prove more useful than traditional cessation strategies …
The smoking prevalence of 8.8% in this sample was far below the current US prevalence of 22.5% in 2002.64 Possible explanations for this include the average age, educational attainment, and geographic location of the sample. Therefore, even though the number of persistent smokers in this sample was small, if we extrapolate these findings to the overall US population, the implications are substantial and indeed disturbing. More than 50% of current smokers in our study reported one or more conditions or symptoms that contraindicated smoking, a result that clinicians as well as smokers should keep in mind. Smoking cessation is the best treatment for reducing mortality among patients with heart disease65 and is associated with improved health among patients with other chronic conditions. Our study indicates that many current smokers are damaging their health by continuing to smoke, and for many, the current smoking-cessation strategies available are no doubt insufficient.
Limitations on these findings must be pointed out. We could not verify that the ACEs reported by participants actually occurred, because they were retrospectively assessed. However, the prevalence of each of the individual ACEs obtained here is similar to that obtained in other large samples with nonclinical populations.66,67 In addition, we relied on self-reports for smoking status as well as symptoms and disease conditions; however, the accuracy of this type of data has been established.68–70 Because our measure of smoking did not allow us to compute pack-years (= 20 cigarettes per day, per year), we could not determine smoking history, only current smoking levels. Furthermore, we were unable to determine when an individual quit smoking and whether the timing of their smoking cessation was in some way linked to a smoking-related diagnosis. Finally, our depression measure consisted of a single dichotomous question and cannot be considered a clinical diagnosis. However, sensitivity analyses performed by Dube et al71 indicated that this item achieved acceptable levels of sensitivity, specificity, and positive predictive value (83%, 60%, and 87%, respectively) when compared with a screening tool developed by the Rand Corporation72 to test for lifetime prevalence of major depression or dysthymia.
Current thinking on further reducing smoking rates includes acknowledgement of the need to better tailor smoking-cessation programs to the needs of the remaining smokers who have not yet been reached by traditional stop-smoking messages.22,73 Our findings suggest that practitioners may need to consider the abuse history and presence of depression in patients who persist in smoking despite having conditions that contraindicate smoking, when traditional smoking-cessation programs prove ineffective. Programs that address the underlying emotional problems caused by childhood maltreatment may prove more useful than traditional cessation strategies in reaching this difficult-to-treat population. To this end, we believe that universal screening for a history of ACEs as well as for depression should be part of a comprehensive medical record. Available evidence indicates that patients are comfortable with screening for childhood abuse74,75 and believe that physicians can assist them in dealing with issues arising from early maltreatment. Addressing the underlying motivations for continued smoking in the face of adverse health consequences may lead to further reductions in smoking-related illness.
The findings and conclusion in this article are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention or of the Kaiser Permanente Medical Care Program.
Acknowledgments
This research was funded by cooperative agreement TS-44-10/11 between the Centers for Disease Control and Prevention and the Association of Teachers of Preventive Medicine.
References
- Duncan CL, Cummings SR, Hudes ES, Zahnd E, Coates TJ. Quitting smoking: reasons for quitting and predictors of cessation among medical patients. J Gen Intern Med. 1992 Jul–Aug;7(4):398–404. doi: 10.1007/BF02599155. [DOI] [PubMed] [Google Scholar]
- Greenwood DC, Muir KR, Packham CJ, Madeley RJ. Stress, social support, and stopping smoking after myocardial infarction in England. J Epidemiol Community Health. 1995 Dec;49(6):583–7. doi: 10.1136/jech.49.6.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohren CL, Croghan IT, Hurt RD, et al. Predicting smoking cessation outcome in a medical center from stage of readiness: contemplation versus action. Prev Med. 1994 May;23(3):335–44. doi: 10.1006/pmed.1994.1047. [DOI] [PubMed] [Google Scholar]
- Joseph AM, Fu SS. Smoking cessation for patients with cardiovascular disease: what is the best approach? Am J Cardiovasc Drugs. 2003;3(5):339–49. doi: 10.2165/00129784-200303050-00005. [DOI] [PubMed] [Google Scholar]
- Gritz ER, Kristeller JL, Burns DM. Treating nicotine addiction in high-risk groups and patients with medical comorbidity. In: Orleans CT, Slade JD, editors. Nicotine addiction: principles and management. New York: Oxford University Press; 1993. pp. 279–309. p. [Google Scholar]
- Ostroff JS, Jacobsen PB, Moadel AB, et al. Prevalence and predictors of continued tobacco use after treatment of patients with head and neck cancer. Cancer. 1995 Jan 15;75(2):569–76. doi: 10.1002/1097-0142(19950115)75:2<569::aid-cncr2820750221>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Emery S, Gilpin EA, Ake C, Farkas AJ, Pierce JP. Characterizing and identifying “hard-core” smokers: implications for further reducing smoking prevalence. Am J Public Health. 2000 Mar;90(3):387–94. doi: 10.2105/ajph.90.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derby CA, Lasater TM, Vass K, Gonzalez S, Carleton RA. Characteristics of smokers who attempt to quit and of those who recently succeeded. Am J Prev Med. 1994 Nov–Dec;10(6):327–34. [PubMed] [Google Scholar]
- Venters MH, Kottke TE, Solberg LI, Brekke ML, Rooney B. Dependency, social factors, and the smoking cessation process: the doctors helping smokers study. Am J Prev Med. 1990 Jul–Aug;6(4):185–93. [PubMed] [Google Scholar]
- Nides MA, Rakos RF, Gonzales D, et al. Predictors of initial smoking cessation and relapse through the first 2 years of the Lung Health Study. J Consult Clin Psychol. 1995 Feb;63(1):60–9. doi: 10.1037//0022-006x.63.1.60. [DOI] [PubMed] [Google Scholar]
- Wray LA, Herzog AR, Willis RJ, Wallace RB. The impact of education and heart attack on smoking cessation among middle-aged adults. J Health Soc Behav. 1998 Dec;39(4):271–94. [PubMed] [Google Scholar]
- Hughes JR, Burns DM. The case for hardening of the target. Those who continue to smoke. Smoking and Tobacco Control Monograph No. 15. Bethesda, MD: US Department of Health and Human Services; 2003. [Google Scholar]
- Osler M, Prescott E. Psychosocial, behavioral, and health determinants of successful smoking cessation: a longitudinal study of Danish adults. Tob Control. 1998 Autumn;7(3):262–7. doi: 10.1136/tc.7.3.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hymowitz N, Cummings KM, Hyland A, et al. Predictors of smoking cessation in a cohort of adult smokers followed for five years. Tob Control. 1997;6(Suppl 2):S57–62. doi: 10.1136/tc.6.suppl_2.s57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark MA, Hogan JW, Kviz FJ, Prohaska TR. Age and the role of symptomatology in readiness to quit smoking. Addict Behav. 1999 Jan–Feb;24(1):1–16. doi: 10.1016/s0306-4603(98)00030-6. [DOI] [PubMed] [Google Scholar]
- Bolman C, deVries H. Psycho-social determinants and motivational phases in smoking behavior of cardiac inpatients. Prev Med. 1998 Sep–Oct;27(5 Part 1):738–47. doi: 10.1006/pmed.1998.0352. [DOI] [PubMed] [Google Scholar]
- Lasser K, Boyd JW, Woolhandler S, et al. Smoking and mental illness: a population-based prevalence study. JAMA. 2000 Nov 22–29;284(20):2606–10. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- Anda RF, Williamson DF, Escobedo LG, et al. Depression and the dynamics of smoking: a national perspective. JAMA. 1990 Sep 26;264(12):1541–5. [PubMed] [Google Scholar]
- Breslau N, Kilbey MM, Andreski P. Nicotine dependence and major depression. New evidence from a prospective investigation. Arch Gen Psychiatry. 1993 Jan;50(1):31–5. doi: 10.1001/archpsyc.1993.01820130033006. [DOI] [PubMed] [Google Scholar]
- Covey LS. Tobacco cessation among patients with depression. Prim Care. 1999 Sep;26(3):691–706. doi: 10.1016/s0095-4543(05)70124-x. [DOI] [PubMed] [Google Scholar]
- Glassman AH, Helzer JE, Covey LS, et al. Smoking, smoking cessation, and major depression. JAMA. 1990 Sep 26;264(12):1546–9. [PubMed] [Google Scholar]
- Covey LS, Glassman AH, Stetner F. Depression and depressive symptoms in smoking cessation. Compr Psychiatry. 1990 Jul–Aug;31(4):350–4. doi: 10.1016/0010-440x(90)90042-q. [DOI] [PubMed] [Google Scholar]
- West RJ, Hajek P, Belcher M. Severity of withdrawal symptoms as a predictor of outcome of an attempt to quit smoking. Psychol Med. 1989 Nov;19(4):981–5. doi: 10.1017/s0033291700005705. [DOI] [PubMed] [Google Scholar]
- Wetter DW, Carmack CL, Anderson CB, et al. Tobacco withdrawal signs and symptoms among women with and without a history of depression. Exp Clin Psychopharmacol. 2000 Feb;8(1):88–96. doi: 10.1037//1064-1297.8.1.88. [DOI] [PubMed] [Google Scholar]
- Anda RF, Croft JB, Felitti VJ, et al. Adverse childhood experiences and smoking during adolescence and adulthood. JAMA. 1999 Nov 3;282(17):1652–8. doi: 10.1001/jama.282.17.1652. [DOI] [PubMed] [Google Scholar]
- Dube SR, Anda RF, Felitti VJ, Edwards VJ, Croft JB. Adverse childhood experiences and personal alcohol abuse as an adult. Addict Behav. 2002 Sep–Oct;27(5):713–25. doi: 10.1016/s0306-4603(01)00204-0. [DOI] [PubMed] [Google Scholar]
- Walker EA, Gelfand A, Katon WJ, et al. Adult health status of women with histories of childhood abuse and neglect. Am J Med. 1999 Oct;107(4):332–9. doi: 10.1016/s0002-9343(99)00235-1. [DOI] [PubMed] [Google Scholar]
- Dembo R, Dertke M, Borders S. The relationship between physical and sexual abuse and tobacco, alcohol, and illicit drug use among youths in a juvenile detention center. Int J Addict. 1988 Apr;23(4):351–78. doi: 10.3109/10826088809039203. [DOI] [PubMed] [Google Scholar]
- Thompson NJ, Potter JS, Sanderson CA, Maibach EW. The relationship of sexual abuse and HIV risk behaviors among heterosexual adult female STD patients. Child Abuse Negl. 1997 Feb;21(2):149–56. doi: 10.1016/s0145-2134(96)00140-8. [DOI] [PubMed] [Google Scholar]
- Cunningham RM, Stiffman AR, Dore P, Earls F. The association of physical and sexual abuse with HIV risk behaviors in adolescence and young adulthood: implications for public health. Child Abuse Negl. 1994 Mar;18(3):233–45. doi: 10.1016/0145-2134(94)90108-2. [DOI] [PubMed] [Google Scholar]
- Rodgers CS, Lang AJ, Laffaye C, et al. The impact of individual forms of childhood maltreatment on health behavior. Child Abuse Negl. 2004 May;28(5):575–86. doi: 10.1016/j.chiabu.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Finestone HM, Stenn P, Davies F, et al. Chronic pain and health care utilization in women with a history of childhood sexual abuse. Child Abuse Negl. 2000 Apr;24(4):547–56. doi: 10.1016/s0145-2134(00)00112-5. [DOI] [PubMed] [Google Scholar]
- Newman MG, Clayton L, Zuellig A, et al. The relationship of childhood sexual abuse and depression with somatic symptoms and medical utilization. Psychol Med. 2000 Sep;30(5):1063–77. doi: 10.1017/s003329179900272x. [DOI] [PubMed] [Google Scholar]
- Borum ML. Childhood sexual trauma as a potential factor for noncompliance with endoscopic procedures. Gen Hosp Psychiatry. 1998 Nov;20(6):381–2. doi: 10.1016/s0163-8343(98)00050-4. [DOI] [PubMed] [Google Scholar]
- Farley M, Golding JM, Minkoff JR. Is a history of trauma associated with a reduced likelihood of cervical cancer screening? J Fam Pract. 2002 Oct;51(10):827–31. [PubMed] [Google Scholar]
- Springs FE, Friedrich WN. Health risk behaviors and medical sequelae of childhood sexual abuse. Mayo Clin Proc. 1992 Jun;67(6):527–32. doi: 10.1016/s0025-6196(12)60458-3. [DOI] [PubMed] [Google Scholar]
- Cohen MA, Alfonso CA, Hoffman RG, Milau V, Carrera G. The impact of PTSD on treatment adherence in persons with HIV infection. Gen Hosp Psychiatry. 2001 Sep–Oct;23(5):294–6. doi: 10.1016/s0163-8343(01)00152-9. [DOI] [PubMed] [Google Scholar]
- King TK, Clark MM, Pera V. History of sexual abuse and obesity treatment outcome. Addict Behav. 1996 May–Jun;21(3):283–90. doi: 10.1016/0306-4603(95)00058-5. [DOI] [PubMed] [Google Scholar]
- Briere J, Woo R, McRae B, Foltz J, Sitzman R. Lifetime victimization history, demographics, and clinical status in female psychiatric emergency room patients. J Nerv Ment Dis. 1997 Feb;185(2):95–101. doi: 10.1097/00005053-199702000-00005. [DOI] [PubMed] [Google Scholar]
- Chapman DP, Whitfield CL, Felitti VJ, et al. Adverse childhood experiences and the risk of depressive disorders in adulthood. J Affect Disord. 2004 Oct 15;82(2):217–25. doi: 10.1016/j.jad.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Magee WJ. Childhood adversities and adult depression: basic patterns of association in a US national survey. Psychol Med. 1993 Aug;23(3):679–90. doi: 10.1017/s0033291700025460. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Cohen P, Skodol A, Bezirganian S, Brook JS. Childhood antecedents of adolescent personality disorders. Am J Psychiatry. 1996 Jul;153(7):907–13. doi: 10.1176/ajp.153.7.907. [DOI] [PubMed] [Google Scholar]
- Flisher AJ, Kramer RA, Hoven CW, et al. Psychosocial characteristics of physically abused children and adolescents. J Am Acad Child Adolesc Psychiatry. 1997 Jan;36(1):123–31. doi: 10.1097/00004583-199701000-00026. [DOI] [PubMed] [Google Scholar]
- Gibb BE, Butler AC, Beck JS. Childhood abuse, depression, and anxiety in adult psychiatric outpatients. Depress Anxiety. 2003;17:226–8. doi: 10.1002/da.10111. [DOI] [PubMed] [Google Scholar]
- Finkelhor D, Kendall-Tackett K. A developmental perspective on the childhood impact of crime, abuse, and violent victimization. In: Cicchetti D, Toth SL, editors. Developmental perspectives on trauma: theory, research, and intervention. Rochester (NY): University of Rochester Press; 1997. pp. 1–32. p. [Google Scholar]
- De Bellis MD, Chrousos GP, Dorn LD, et al. Hypothalamic-pituitary-adrenal axis dysregulation in sexually abused girls. J Clin Endocrinol Metab. 1994 Feb;78:249–55. doi: 10.1210/jcem.78.2.8106608. [DOI] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998 May;14(4):245–58. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Edwards VJ, Anda RF, Nordenberg DF, et al. Bias assessment for child abuse survey: factors affecting probability of response to a survey about childhood abuse. Child Abuse Negl. 2001 Feb;25(2):307–12. doi: 10.1016/s0145-2134(00)00238-6. [DOI] [PubMed] [Google Scholar]
- Dong M, Anda RF, Felitti VJ, et al. The interrelatedness of multiple forms of childhood abuse, neglect, and household dysfunction. Child Abuse Negl. 2004 Jul;28:771–84. doi: 10.1016/j.chiabu.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Moeller TP, Bachmann GA, Moeller JR. The combined effects of physical, sexual, and emotional abuse during childhood: long-term health consequences for women. Child Abuse Negl. 1993 Sep–Oct;17(5):623–40. doi: 10.1016/0145-2134(93)90084-i. [DOI] [PubMed] [Google Scholar]
- Csoboth CT, Birkas E, Purebl G. Physical and sexual abuse: risk factors for substance use among young Hungarian women. Behav Med. 2003 Winter;28(4):165–71. doi: 10.1080/08964280309596055. [DOI] [PubMed] [Google Scholar]
- Simantov E, Schoen C, Klein JD. Health-compromising behaviors: why do adolescents smoke or drink? Identifying underlying risk and protective factors. Arch Pediatr Adolesc Med. 2000;154(10):1025–33. doi: 10.1001/archpedi.154.10.1025. [DOI] [PubMed] [Google Scholar]
- Solberg LI, Desai JR, O'Connor PJ, Bishop DB, Devlin HM. Diabetic patients who smoke: are they different? Ann Fam Med. 2004 Jan–Feb;2(1):26–32. doi: 10.1370/afm.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangler JG, Summerso JH, Bell RA, Konen JC. Smoking status and psychosocial variables in type 1 diabetes mellitus. Addict Behav. 2001 Jan–Feb;26(1):21–9. doi: 10.1016/s0306-4603(00)00077-0. [DOI] [PubMed] [Google Scholar]
- Havik OE, Maeland JG. Changes in smoking behavior after a myocardial infarction. Health Psychol. 1988;7(5):403–20. doi: 10.1037//0278-6133.7.5.403. [DOI] [PubMed] [Google Scholar]
- Huijbrechts IP, Duivenvoorden HJ, Deckers JW, et al. Modification of smoking habits five months after myocardial infarction: relationship with personality characteristics. J Psychosom Res. 1996;40:369–78. doi: 10.1016/0022-3999(95)00609-5. [DOI] [PubMed] [Google Scholar]
- Carmody TP. Affect regulation, nicotine addiction, and smoking cessation. J Psychoactive Drugs. 1989 Apr–Jun;24(2):111–22. doi: 10.1080/02791072.1992.10471632. [DOI] [PubMed] [Google Scholar]
- Higgins DJ, McCabe MP. Relationships between different types of maltreatment during childhood and adjustment in adulthood. Child Maltreat. 2000 Aug;5(3):261–72. doi: 10.1177/1077559500005003006. [DOI] [PubMed] [Google Scholar]
- McCauley J, Kern DE, Kolodner K, et al. Clinical characteristics of women with a history of childhood abuse: unhealed wounds. JAMA. 1997 May 7;277(17):1362–8. [PubMed] [Google Scholar]
- Johnsen LW, Harlow LL. Childhood sexual abuse linked with adult substance use, victimization, and AIDS-risk. AIDS Educ Prev. 1996 Feb;8(1):44–57. [PubMed] [Google Scholar]
- Thompson MP, Arias I, Basile KC, Desai S. The association between childhood physical and sexual victimization and health problems in adulthood in a nationally representative sample of women. J Interpers Violence. 2002;17(10):1115–29. [Google Scholar]
- Banyard VL. Childhood maltreatment and the mental health of low-income women. Am J Orthopsychiatry. 1999 Apr;69(2):161–71. doi: 10.1037/h0080418. [DOI] [PubMed] [Google Scholar]
- Toomey TC, Seville JL, Mann JD, Abashian SW, Grant JR. Relationship of sexual and physical abuse to pain description, coping, psychological distress, and health-care utilization in a chronic pain sample. Clin J Pain. 1995 Dec;11(4):307–15. doi: 10.1097/00002508-199512000-00008. [DOI] [PubMed] [Google Scholar]
- Porter S, Jackson K, Trosclair A, Pederson LL. Prevalence of current cigarette smoking among adults and changes in prevalence of current and some day smoking—United States, 1996–2001. MMWR Morb Mortal Wkly Rep. 2003 Apr 11;52(14):303–4. 306–7. [PubMed] [Google Scholar]
- Deckers JW, Agema WRP, Huijibrechts IPAM, Erdman RAM, Boersma H, Roelandt JRTC. Quitting of smoking in patients with recently established coronary artery disease reduces mortality by over 40%: results of a meta-analysis. Eur Heart J. 1994;15 (abstract suppl): 171. Abstract. [Google Scholar]
- Finkelhor D. Current information on the scope and nature of child sexual abuse. Future Child. 1994 Summer–Fall;4(2):31–53. [PubMed] [Google Scholar]
- MacMillan HL, Fleming JE, Trocme N, et al. Prevalence of child physical and sexual abuse in the community. Results from the Ontario Health Supplement. JAMA. 1997 Jul 9;278(2):131–5. [PubMed] [Google Scholar]
- Ferraro KF, Su YP. Physician-evaluated and self-reported morbidity for predicting disability. Am J Public Health. 2000 Jan;90(1):103–8. doi: 10.2105/ajph.90.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegsman DM, Penninx BW, van Eijk JT, Boeke AJ, Deeg DJ. Self-reports and general practitioner information on the presence of chronic diseases in community dwelling elderly. A study on the accuracy of patients' self-reports and on determinants of inaccuracy. J Clin Epidemiol. 1996 Dec;49(12):1407–17. doi: 10.1016/s0895-4356(96)00274-0. [DOI] [PubMed] [Google Scholar]
- Patrick DL, Cheadle A, Thompson DC, et al. The validity of self-reported smoking: a review and meta-analysis. Am J Public Health. 1994 Jul;84(7):1086–93. doi: 10.2105/ajph.84.7.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube SR, Anda RF, Felitti VJ, et al. Childhood abuse, household dysfunction and the risk of attempted suicide throughout the life span: Findings from Adverse Childhood Experiences Study. JAMA. 2001 Dec 26;286(24):3089–96. doi: 10.1001/jama.286.24.3089. [DOI] [PubMed] [Google Scholar]
- Burnam MA, Wells KB, Leake B, Landsverk J. Development of a brief screening instrument for detecting depression. Med Care. 1988 Aug;26(8):775–89. doi: 10.1097/00005650-198808000-00004. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Why does smoking so often produce dependence? A somewhat different view. Tob Control. 2001 Mar;10(1):62–4. doi: 10.1136/tc.10.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman LS, Samet JH, Roberts MS, Hudlin M, Hans P. Inquiry about victimization experiences: A survey of patient preferences and physician practices. Arch Intern Med. 1992 Jun;152(6):1186–90. doi: 10.1001/archinte.152.6.1186. [DOI] [PubMed] [Google Scholar]
- Newman E, Walker EA, Gelfand A. Assessing the ethical costs and benefits of trauma-focused research. Gen Hosp Psychiatry. 1999 May;21(3):187–96. doi: 10.1016/s0163-8343(99)00011-0. [DOI] [PubMed] [Google Scholar]


