Abstract
BACKGROUND
Late implantation and the pattern of early rise in hCG have been associated with early pregnancy loss. We explored factors that might be predictive of these markers of poor embryonic health in spontaneously conceived pregnancies.
METHODS
Participants in the North Carolina Early Pregnancy Study collected daily first-morning urine specimens while attempting to conceive. Samples were assayed for estrogen and progesterone metabolites (to identify day of ovulation) and hCG (to detect conception). Data were available for 190 pregnancies, 48 of which ended in early loss (within 6 weeks of the last menstrual period). We used logistic regression to identify characteristics associated with late implantation (≥10 days post-ovulation). For pregnancies surviving at least 6 weeks (n= 142), we used linear mixed models to identify factors associated with variations in hCG rise in the first 7 days from detection.
RESULTS
Later implantation was associated with current maternal smoking [odds ratio (OR): 5.7; 95% confidence interval (CI): 1.1–30] and with oocytes that were likely to have been fertilized late in their post-ovulatory lifespan (OR: 5.1; CI: 1.9–16). Older women had a faster rise in hCG (P= 0.01), as did women who had relatively late menarche (P for trend = 0.02). Women exposed in utero to diethylstilbestrol showed an unusual pattern of slow initial hCG rise followed by a fast increase, a pattern significantly different from that of unexposed women (P= 0.002).
CONCLUSIONS
Although limited by small numbers and infrequent exposures, our analyses suggest that a woman's exposures both early in life and at the time of pregnancy may influence early development of the conceptus.
Keywords: smoking, DES, age at menarche, oocyte quality, early pregnancy loss
Introduction
hCG is secreted by trophoblast cells of the early conceptus and the developing placenta, and performs vital functions in early pregnancy. These include maintenance of the corpus luteum (Zeleznik and Pohl, 2006) and formation of the placental syncytium (Yang et al., 2003). The appearance of hCG in maternal urine can be used as a marker of embryonic implantation (Wilcox et al., 1999). Delayed implantation has been associated with early pregnancy loss (Wilcox et al., 1999). Lower hCG levels and slower rates of hCG rise early in pregnancy are seen in ectopic pregnancies (Kadar et al., 1981; Check et al., 1992; Seeber et al., 2006) and have been associated with pregnancy loss (Check et al., 1992; Bjercke et al., 1999; Urbancsek et al., 2002; Baird et al., 2003; Alahakoon et al., 2004; Stone et al., 2006; Shamonki et al., 2009) (reviewed in Chung and Allen, 2008). Most of these studies have been based on clinical populations undergoing fertility treatment, which may not be representative of spontaneously conceived pregnancies. Given that timing of implantation and early pregnancy hCG rise may be markers of pregnancy health, we explored maternal and pregnancy characteristics that may influence these markers. We studied a cohort of women with spontaneously conceived pregnancies under observation from before the time of conception.
Materials and Methods
Study population and design
The North Carolina Early Pregnancy Study (NCEPS) (1982–1986) enrolled 221 women at the time they discontinued birth control in order to conceive a pregnancy. The original purpose of the study was to determine the incidence of very early pregnancy loss (Wilcox et al., 1988). Women were eligible to participate if they had no known chronic health or fertility problems and were not using hormone medications. Participants collected daily first-morning urine specimens and kept daily diaries that included information on menstrual bleeding and sexual intercourse. All participants provided informed consent. The research was approved by the Institutional Review Board of the National Institute of Environmental Health Sciences.
Hormonal assays
To detect day of ovulation, first-morning urine specimens from a 17-day mid-cycle window were assayed for estrone 3-glucuronide (E1G) and pregnanediol 3-glucuronide (PdG). E1G and PdG were measured by direct radioimmunoassay (Wright et al., 1978; Samarajeewa et al., 1979). Specimens were analyzed in duplicate or triplicate, and the geometric means of the steroid hormone concentrations were divided by the corresponding creatinine concentration to adjust for variations in dilution. The day of ovulation for each cycle was defined based on the rapid drop in the estrogen-to-progesterone ratio (Baird et al., 1991), a measure validated by LH measurements (Baird et al., 1995) and by ultrasound (Ecochard et al., 2001).
We employed a highly sensitive immunoradiometric assay to quantify hCG in urine samples (Wilcox et al., 1985). Variations in urinary creatinine were very small compared with the exponential rise in hCG (McChesney et al., 2005), making the adjustment of hCG for creatinine unnecessary.
Potential correlates
All potential correlates except two (season of implantation and oocyte-waiting time) were based on information reported by the participant at enrollment. ‘Recent' oral contraceptive use was defined as use within 90 days of enrollment. Recent use of alcohol, caffeine, marijuana and vitamins was defined as any use in the 3 months prior to enrollment. Other characteristics of interest included age, reproductive history, menstrual cycle characteristics and BMI.
We considered season because in a prior analysis, risk of early pregnancy loss had been elevated in the last quarter of the year (Weinberg et al., 1994). We assessed whether late implantation was associated with season of implantation by assigning each day of the year to an angle in radians (subtracting 1 January from the date of implantation, dividing by 365 and then multiplying by 2π). By including the sine and cosine of these angles as exposures in our models using trigonometric regression, we allow a sinusoidal pattern for the risk of later implantation, with amplitude and day of greatest risk (phase) determined by the data.
Oocyte-waiting time is a measure of the time between ovulation and fertilization (presumably a matter of hours). We can measure oocyte-waiting time only indirectly, using the days of sexual intercourse (recorded in the woman's daily diary), relative to her measured day of ovulation (Wilcox et al., 1998). All conceptions in this study occurred with intercourse during a 6-day window ending on the day of ovulation. No pregnancies were observed with intercourse only on the day after ovulation (Wilcox et al., 1995). From this we can infer that the oocyte is short-lived and a long oocyte-waiting time would be most likely in pregnancies for which there was intercourse on the day of ovulation but not on the previous 5 days. That is, without capacitated sperm present when the oocyte arrives in the oviduct, the oocyte may need to wait many hours before capacitated sperm are present and fertilization can occur. The optimum analysis to address oocyte-waiting time would be limited to conceptions with intercourse only on the day of ovulation and not during the preceding 5 days. However, very few conceptions met that criterion. At the cost of some reduced specificity but with the benefit of added cycles, we defined a long oocyte-waiting time as intercourse on the day of ovulation but not during the 2 days before ovulation. [Intercourse before those 2 days being much less likely to result in conception (Wilcox et al., 1995).]
All of the potential correlates were examined with each of the reproductive end-points; however, we only present results from those variables that were statistically significant or suggestive of an association.
Reproductive end-points
A pregnancy was identified based on elevated hCG (≥0.025 ng/ml) for three consecutive days (Wilcox et al., 1988). Once a pregnancy was identified, the day of implantation was assigned as the first day of a sustained rise in hCG in which each subsequent day exceeded 0.015 ng/ml. We dichotomized days to implantation as 9 or less and 10 or more. A pregnancy was categorized as an early loss if the initial rise in hCG was followed by a decline with menstrual-like bleeding within 6 weeks (42 days) of the start of the previous menstrual period. Pregnancies lasting longer than 42 days were considered clinical pregnancies.
Statistical analysis
We used logistic regression to identify factors correlated with late implantation (10 or more days after ovulation). To identify factors related to the pattern of hCG rise, we used a linear mixed model and generated P-values. The model was of the following general form for woman i on Day j:
where β0i and β1i are zero-mean woman-specific random effects, j is the number of days starting with implantation and U (=1 or 0) indicates a dichotomous maternal or pregnancy factor that may be associated with the rise in hCG. This general model was expanded to accommodate multiple categories of a variable (U1, U2, U3, etc.). To determine whether a variable was associated with the rate of hCG rise, the importance of β4 and β5 was assessed using a likelihood ratio test, based on a two-degree-of-freedom χ2 distribution under the null hypothesis that U has no effect on the pattern of rise.
Each characteristic was assessed in a separate model; thus, all analyses are unadjusted for other factors except where specified in the text.
For graphical display, the patterns of rise in hCG over the 7 days beginning with implantation were quantified using an average relative increase in hCG for each day. This was calculated for Day j as
where ‘ln(hCG)1' indicates the natural log of the hCG concentration on Day 1, which is the day of implantation, ‘ln(hCG)j' indicates the natural log of the hCG concentration on Day j, which ranges from 2 to 7, and the ‘average' is taken across pregnancies.
The absolute hCG curves from this population have been previously published (Nepomnaschy et al., 2008). In addition, that paper examined the association of hCG curves with twin pregnancies, pregnancy loss after 42 days gestation and infant sex. None of these factors were found to be important and thus all pregnancies are included here unless otherwise noted.
Study sample
There were 199 pregnancies detected in the Early Pregnancy Study. Of these, 151 were clinical pregnancies and 48 were early pregnancy losses. There were 10 clinical pregnancies which were missing a day of ovulation or a day of implantation, leaving 189 pregnancies in the sample used to analyze late implantation. The patterns of hCG rise were assessed only among the clinical pregnancies. This is because early losses often do not last 7 days and do not necessarily have a clear pattern of rise (Baird et al., 2003). One additional clinical pregnancy could be used in this analysis because it had a measured day of implantation (day of ovulation was missing), thus providing 142 clinical pregnancies for the rate of rise analysis. Clinical pregnancies were mostly singleton live births (n = 121); other outcomes were 6 twin births, 13 miscarriages, 1 ectopic pregnancy and 1 molar pregnancy.
Some women contributed more than one pregnancy to the study (3 had more than one early loss, 21 had both an early loss and a clinical pregnancy and 1 had two early losses and a clinical pregnancy). We evaluated the effect of adjustment for this statistical dependency. By design, no woman had more than one clinical pregnancy in the study. Thus, the number of pregnancies is related to risk of loss: if a woman had more than one pregnancy, we know that at least one of those pregnancies was an early pregnancy loss. When (as in this case) cluster size is ‘informative', the standard methods for adjusting for dependent observations (such as generalized estimating equations) are biased. Thus we used within-cluster resampling (Hoffman et al., 2001) to account for the multiple observations per woman. Other than a slight loss of precision, results were unchanged (data not shown).
Results
The median age was 29, and about two-thirds of the women had previously been pregnant (Table 1). About half of pregnancies in the study had implantation on Day 10 or later. Only 11 pregnancies (6%) were to women who were self-reported smokers. Seven pregnancies (5%) were to women who reported prenatal diethylstilbestrol (DES) exposure, and 47 (28%) pregnancies were to women who reported that their mother had smoked while pregnant with them.
Table I.
Distribution of maternal and pregnancy characteristics within the two study samples, all conceptions (n= 189) and clinical pregnancies only (n= 142).
| Number of conceptionsa (%) | Number of clinical pregnanciesb (%) | |
|---|---|---|
| Time from ovulation to implantation | ||
| ≥10 days | 87 (46) | 52 (37) |
| ≤9 days | 102 (54) | 89 (63) |
| Age | ||
| ≥29 | 97 (51) | 72 (51) |
| <29 | 92 (49) | 70 (49) |
| Prior pregnancy | ||
| Yes | 128 (68) | 94 (66) |
| No | 60 (32) | 48 (34) |
| History of miscarriage | ||
| Yes | 27 (14) | 18 (13) |
| No | 101 (54) | 76 (53) |
| Never pregnant | 60 (32) | 48 (34) |
| Age at menarche | ||
| <12 | 24 (13) | 20 (14) |
| 12, 13 | 114 (60) | 83 (58) |
| >13 | 51 (27) | 39 (28) |
| Cycle length | ||
| <29 | 86 (48) | 66 (49) |
| ≥29 | 95 (52) | 69 (51) |
| Regular cycles | ||
| Yes | 162 (86) | 120 (84) |
| No | 27 (14) | 22 (16) |
| Recent oral contraceptive use | ||
| Yes | 17 (9) | 12 (9) |
| No | 172 (91) | 130 (91) |
| Oocyte-waiting time | ||
| Long | 23 (12) | 11 (8) |
| Short or average | 166 (88) | 130 (92) |
| BMI | ||
| <18 | 18 (10) | 14 (10) |
| 18 to <25 | 155 (82) | 115 (82) |
| ≥25 | 16 (8) | 13 (9) |
| Prenatal DES exposure | ||
| Yes | 7 (5) | 4 (4) |
| No | 135 (95) | 100 (96) |
| Prenatal exposure to maternal smoking | ||
| Yes | 47 (28) | 36 (29) |
| No | 118 (72) | 88 (71) |
| Number of smokers in the home when participant was <10 years old | ||
| 0 | 64 (35) | 44 (31) |
| 1 | 64 (35) | 49 (36) |
| ≥2 | 56 (30) | 46 (33) |
| Husband's current smoking | ||
| Yes | 15 (8) | 12 (9) |
| No | 174 (92) | 130 (91) |
| Smoking status at enrollment | ||
| Current | 11 (6) | 8 (6) |
| Former | 52 (27) | 40 (28) |
| Never | 126 (67) | 94 (66) |
| Alcohol intake | ||
| Any | 150 (79) | 114 (80) |
| None | 39 (21) | 28 (20) |
| Caffeine intake | ||
| Any | 179 (95) | 133 (94) |
| None | 10 (5) | 9 (6) |
| Marijuana use | ||
| Any | 26 (14) | 20 (14) |
| None | 163 (86) | 122 (86) |
| Vitamin use | ||
| Yes | 99 (53) | 79 (55) |
| No | 89 (47) | 63 (45) |
aThis sample includes early pregnancy losses and clinical pregnancies, women could have experienced either or both leading to more than one observation per woman.
bThis sample includes pregnancies that survived to clinical recognition. Women only contributed one clinical pregnancy leading to only one observation per woman.
Time from ovulation to implantation
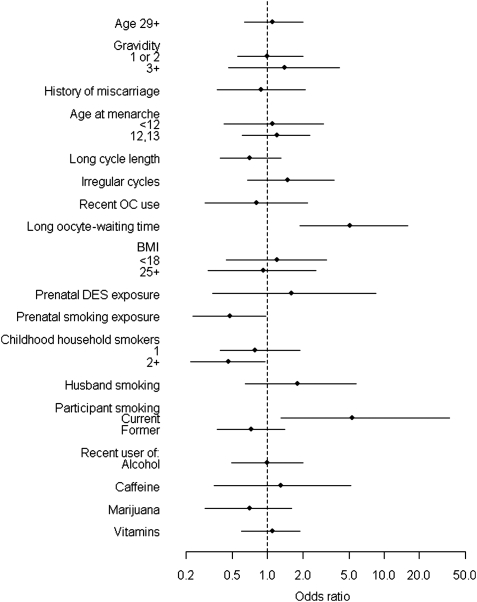
Women who were current smokers had five times the odds of late implantation as never or former smokers [odds ratio (OR) (95% confidence interval, CI): 5.3 (1.3, 36), P= 0.03] (Fig. 1). There were not enough current smokers to evaluate a dose–response relationship. The association between husband's smoking and late implantation was also positive but weaker [OR (CI): 1.8 (0.64, 5.7)].
Figure 1.
ORs and 95% CIs for late implantation (≥10 days post-ovulation).
Implantation was also later among conceptions that resulted from a long oocyte-waiting time [OR (CI): 5.1 (1.9, 16), P= 0.0008] (Fig. 1). Late implantation was more common in exposed early losses, but not exclusive to them. When we restricted analysis to clinical pregnancies, the association between oocyte-waiting time and late implantation was still evident, but attenuated [OR (CI): 2.2 (0.63, 8.0)].
Given our previous observation that a long oocyte-waiting time led to early loss (Wilcox et al., 1998), we explored whether that association might be mediated by late implantation. We included both oocyte-waiting time and late implantation as predictors in a logistic regression of early loss. While the effect of oocyte-waiting time was attenuated when adjusted for late implantation (from an OR of 3.9 to 2.7), it was still significantly associated with early loss (P= 0.04).
Women who reported that their mother had smoked while pregnant with them had less late implantation [OR (CI): 0.48 (0.23, 0.96), P= 0.04]. The odds of late implantation also tended to be lower for women who had lived with one or more household smokers (mother, father or other) during their childhood (P= 0.11) (Fig. 1).
We explored the robustness of these associations with current smoking and prenatal exposure to maternal smoking by limiting the analysis to clinical pregnancies. The association between current smoking and late implantation was similar [5.5 (1.2, 39)] and the protective association of prenatal exposure to maternal smoking and late implantation was slightly stronger, 0.35 (0.14, 0.83). After further restricting to only live births (i.e. excluding the 15 clinical miscarriages), the associations again became slightly stronger [current smoking, 6.1 (1.3, 44) and prenatal maternal smoking exposure 0.31 (0.11, 0.77)].
Season of the year [previously associated with early loss in these data (Weinberg et al., 1994)] was not associated with the timing of implantation (P= 0.97, data not shown).
Rate of hCG rise
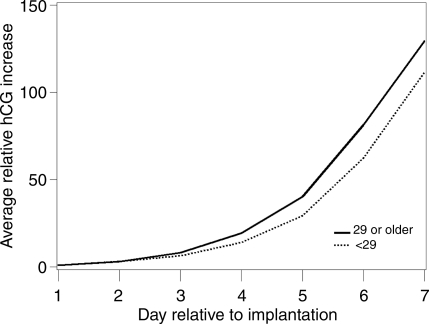
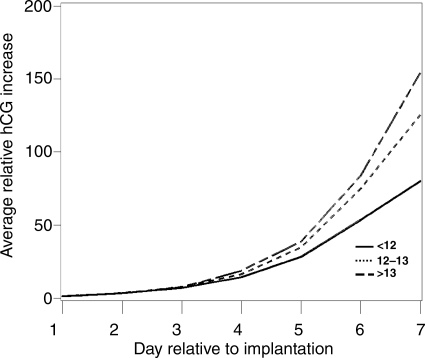
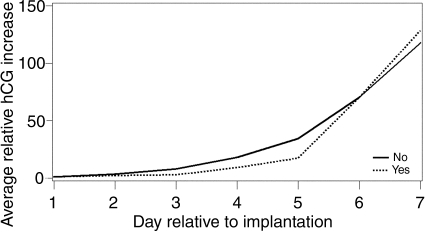
Three factors were associated with the steepness of the initial hCG rise: age, age of menarche and DES. Older women had a faster rise (P= 0.01) (Fig. 2). This could not be attributed to their increased gravidity which was not associated with rate of rise (P= 0.25). When pregnancy losses were removed, the P-value increased to 0.08. The P-value for age when age at menarche was included in the model was unchanged (0.01). Excluding the DES-exposed women led to a stronger P-value (P= 0.006). Age at menarche was weakly associated with hCG rise, with women who were younger at menarche having a slower hCG rise (P= 0.09) (Fig. 3). A linear trend test of the age at menarche categories was significant, P= 0.02. This P-value was not affected by adjustment for BMI (P= 0.02) or removing the pregnancy losses (P= 0.03); however removing the DES-exposed participants increased the P-value (0.06). Exposure to DES in utero was associated with a distinct pattern of hCG increase (P= 0.002) (Fig. 4). Exposed women had a slower hCG rise up to Day 4 and then a faster rise thereafter. Interpretation of this finding is limited by the fact that only four pregnancies were to DES-exposed mothers. Removing the pregnancy losses did not alter the P-value (P= 0.002).
Figure 2.
Average relative increase in hCG over the first 7 days of pregnancy beginning with the day of implantation (Day 1), stratified by participant age at intake, P= 0.01. Solid line represents women 29 or older; the dotted line represents women under 29.
Figure 3.
Average relative increase in hCG over the first 7 days of pregnancy beginning with the day of implantation (Day 1), stratified by age at menarche, P= 0.01. The solid line represents women <12 at menarche, the small dashed line represents women aged 12–13 at menarche and the long dashes represent women >13 at menarche.
Figure 4.
Average relative increase in hCG over the first 7 days of pregnancy beginning with the day of implantation (Day 1), stratified by in utero exposure to DES, P= 0.002. The solid line represents women not exposed to DES in utero; the dotted line represents women who did report exposure to DES in utero.
Discussion
We found a number of maternal factors associated with time to implantation and with the pattern of initial hCG rise. Current smoking was associated with late implantation, while prenatal exposure to maternal smoking was associated with earlier implantation. Conceptions resulting from a long oocyte-waiting time were also more likely to implant later, but this pathway does not appear to explain their increased risk of early loss that we previously reported. Among those conceiving a clinical pregnancy, older women and women with a later age at menarche experienced a faster initial rise of hCG. Conceptions to the few women who were DES daughters showed a distinct pattern of rise, slower over the first 4 days and faster thereafter.
These data are unique in describing peri-conceptional events in a group of naturally conceived pregnancies. Nonetheless, the analysis has notable limitations. One is small numbers. For example, few women smoked or had been prenatally exposed to DES. While sample size limits the conclusiveness of our analysis, these preliminary data may serve to generate hypotheses for future research. Given that we explored a total of ∼20 variables and 2 outcomes, a few statistically significant results would be expected by chance. We have therefore focused our interpretations on biological plausibility and coherence rather than statistical significance. Our data set represents a relatively healthy population of embryos; in order to be observed, embryos had to be able to implant and secrete hCG. Embryos that failed sooner or were unable to produce enough hCG to meet our cutoffs are unobservable; as in any pregnancy study, their unobservability may have influenced the observed associations. Another possible limitation is that our measure of hCG was in urine. Some of the observed differences could be due to exposure effects on maternal disposition of hCG rather than to the actual amount of hCG secreted by the conceptus, although urinary and serum concentrations are in general highly correlated (Wehmann and Nisula, 1981; Norman et al., 1987; McChesney et al., 2005).
Time to implantation
Experimental data suggest that when oocytes wait many hours before fertilization they produce less viable embryos (Lanman, 1968; Butcher, 1976; Longo and So, 1982; Juetten and Bavister, 1983; Perreault, 1992). Our earlier finding of oocyte-waiting time being associated with early pregnancy loss is consistent with those experimental data, and suggests that aging of the oocyte prior to its fertilization (even by a few hours) may be detrimental to embryonic development (Wilcox et al., 1998). Such effects on development may contribute to delays in implantation. In the present study, we find that long oocyte-waiting time is associated with late implantations both in conceptions destined to be early losses and (albeit more weakly) in surviving conceptions.
After restricting to clinical pregnancies only, a long oocyte-waiting time still tended to be followed by later implantation. This could reflect a less competent conceptus. It is also possible that the association is a natural consequence of later fertilization. Oocytes are thought to remain viable for ∼24 h. Oocytes that are fertilized quickly will produce embryos that are as much as a day ahead of embryos that result from late fertilization. Thus, the late fertilizations may be up to 1 day behind in their implantation time. Consistent with this hypothesis, the implantation delay we observed in the clinical pregnancies was primarily a shift from post-ovulation Day 9 to Day 10. Such a delay may or may not have negative consequences for the embryo.
The delay in implantation among smokers, if not a chance finding, could be due to disruption of ovum retrieval by the oviduct or of tubal transport (Neri and Marcus, 1972; Mitchell and Hammer, 1985; Knoll et al., 1995; Knoll and Talbot, 1998) (reviewed in Lyons et al., 2006). The IVF literature suggests that maternal smoking can also have adverse effects on a range of other reproductive functions. Smoking has been associated with decreased ova retrieval (Van Voorhis et al., 1996; Joesbury et al., 1998; Fuentes et al., 2010), lower fertilization rates (Elenbogen et al., 1991; Rosevear et al., 1992), lower implantation rates (Van Voorhis et al., 1996) and increased miscarriage risk (Maximovich and Beyler, 1995; Van Voorhis et al., 1996). A meta-analysis reported lower IVF pregnancy rates for smokers compared with non-smokers (Augood et al., 1998).
We previously reported that women with prenatal exposure to maternal smoking had reduced fertility (Weinberg et al., 1989). It is possible that among these women only the most robust of the embryos succeed in implanting. This selection could explain both a fertility effect and the observed association between prenatal exposure to maternal smoking and earlier implantation if the slower-developing embryos often fail to implant.
hCG rise
The slower initial hCG rise among women with earlier age at menarche was unexpected. Early menarche has been associated with endometrial pathology [endometrial cancer (Fujita et al., 2008; Dossus et al., 2010), adenomyosis (Templeman et al., 2008) and endometriosis (Treloar et al., 2010)]. Early menarche may also be associated with endometrial factors that retard early development of an implanted conceptus (and thus slow the production or release of hCG). Polymorphisms in chemokine receptor 3 (CCR3) have been associated with age at menarche and CCR3 is associated with endometrial inflammation (Yang et al., 2007). Since embryonic implantation is an inflammatory process, it is possible that this gene indirectly influences the early rise of hCG.
DES was associated with a distinct pattern of hCG rise in these data. Women who were DES daughters have been found to have numerous uterine and cervical abnormalities (Kaufman et al., 1984) that may contribute to their higher risk for infertility (Herbst et al., 1980; Kaufman et al., 1984) and pre-eclampsia (Troisi et al., 2007). DES exposure in utero may also influence immune function (Ford et al., 1983; Ways et al., 1987). Structural abnormalities of the uterus and immune impairments may influence the ability of an embryo to invade the endometrium, which might have resulted in the unusual pattern of hCG rise.
Conclusion
In summary, these data from a group of naturally conceiving women who participated in the NCEPS provide an opportunity to examine factors affecting the timing of implantation and the patterns of early pregnancy hCG. These data suggest that early life factors, as well as current exposures, may affect early pregnancy.
Authors' roles
A.M.Z.J. performed all analyses and wrote the manuscript. C.R.W. provided statistical expertise. C.R.W., D.D.B. and A.J.W. designed and implemented the original study, provided input on these analyses and edited the manuscript.
Funding
This research was supported by the intramural research program of the NIH, National Institute of Environmental Health Sciences.
Acknowledgements
We appreciate D. Robert McConnaughey's contributions to data organization and management.
References
- Alahakoon TI, Crittenden J, Illingworth P. Value of single and paired serum human chorionic gonadotropin measurements in predicting outcome of in vitro fertilisation pregnancy. Aust N Z J Obstet Gynaecol. 2004;44:57–61. doi: 10.1111/j.1479-828X.2004.00172.x. [DOI] [PubMed] [Google Scholar]
- Augood C, Duckitt K, Templeton AA. Smoking and female infertility: a systematic review and meta-analysis. Hum Reprod. 1998;13:1532–1539. doi: 10.1093/humrep/13.6.1532. [DOI] [PubMed] [Google Scholar]
- Baird DD, Weinberg CR, Wilcox AJ, McConnaughey DR, Musey PI. Using the ratio of urinary oestrogen and progesterone metabolites to estimate day of ovulation. Stat Med. 1991;10:255–266. doi: 10.1002/sim.4780100209. [DOI] [PubMed] [Google Scholar]
- Baird DD, McConnaughey DR, Weinberg CR, Musey PI, Collins DC, Kesner JS, Knecht EA, Wilcox AJ. Application of a method for estimating day of ovulation using urinary estrogen and progesterone metabolites. Epidemiology. 1995;6:547–550. doi: 10.1097/00001648-199509000-00015. [DOI] [PubMed] [Google Scholar]
- Baird DD, Weinberg CR, McConnaughey DR, Wilcox AJ. Rescue of the corpus luteum in human pregnancy. Biol Reprod. 2003;68:448–456. doi: 10.1095/biolreprod.102.008425. [DOI] [PubMed] [Google Scholar]
- Bjercke S, Tanbo T, Dale PO, Morkrid L, Abyholm T. Human chorionic gonadotrophin concentrations in early pregnancy after in-vitro fertilization. Hum Reprod. 1999;14:1642–1646. doi: 10.1093/humrep/14.6.1642. [DOI] [PubMed] [Google Scholar]
- Butcher RL. Pre-ovulatory and post-ovulatory overripeness. Int J Gynaecol Obstet. 1976;14:105–110. doi: 10.1002/j.1879-3479.1976.tb00577.x. [DOI] [PubMed] [Google Scholar]
- Check JH, Weiss RM, Lurie D. Analysis of serum human chorionic gonadotrophin levels in normal singleton, multiple and abnormal pregnancies. Hum Reprod. 1992;7:1176–1180. doi: 10.1093/oxfordjournals.humrep.a137817. [DOI] [PubMed] [Google Scholar]
- Chung K, Allen R. The use of serial human chorionic gonadotropin levels to establish a viable or a nonviable pregnancy. Semin Reprod Med. 2008;26:383–390. doi: 10.1055/s-0028-1087104. [DOI] [PubMed] [Google Scholar]
- Dossus L, Allen N, Kaaks R, Bakken K, Lund E, Tjonneland A, Olsen A, Overvad K, Clavel-Chapelon F, Fournier A, et al. Reproductive risk factors and endometrial cancer: the European prospective investigation into cancer and nutrition. Int J Cancer. 2010;127:442–451. doi: 10.1002/ijc.25050. [DOI] [PubMed] [Google Scholar]
- Ecochard R, Boehringer H, Rabilloud M, Marret H. Chronological aspects of ultrasonic, hormonal, and other indirect indices of ovulation. BJOG. 2001;108:822–829. doi: 10.1111/j.1471-0528.2001.00194.x. [DOI] [PubMed] [Google Scholar]
- Elenbogen A, Lipitz S, Mashiach S, Dor J, Levran D, Ben-Rafael Z. The effect of smoking on the outcome of in-vitro fertilization—embryo transfer. Hum Reprod. 1991;6:242–244. doi: 10.1093/oxfordjournals.humrep.a137314. [DOI] [PubMed] [Google Scholar]
- Ford CD, Johnson GH, Smith WG. Natural killer cells in in utero diethylstilbesterol-exposed patients. Gynecol Oncol. 1983;16:400–404. doi: 10.1016/0090-8258(83)90168-3. [DOI] [PubMed] [Google Scholar]
- Fuentes A, Munoz A, Barnhart K, Arguello B, Diaz M, Pommer R. Recent cigarette smoking and assisted reproductive technologies outcome. Fertil Steril. 2010;93:89–95. doi: 10.1016/j.fertnstert.2008.09.073. [DOI] [PubMed] [Google Scholar]
- Fujita M, Tase T, Kakugawa Y, Hoshi S, Nishino Y, Nagase S, Ito K, Niikura H, Yaegashi N, Minami Y. Smoking, earlier menarche and low parity as independent risk factors for gynecologic cancers in Japanese: a case–control study. Tohoku J Exp Med. 2008;216:297–307. doi: 10.1620/tjem.216.297. [DOI] [PubMed] [Google Scholar]
- Herbst AL, Hubby MM, Blough RR, Azizi F. A comparison of pregnancy experience in DES-exposed and DES-unexposed daughters. J Reprod Med. 1980;24:62–69. [PubMed] [Google Scholar]
- Hoffman E, Sen P, Weinberg C. Within-cluster resampling. Biometrika. 2001;88:1121–1134. [Google Scholar]
- Joesbury KA, Edirisinghe WR, Phillips MR, Yovich JL. Evidence that male smoking affects the likelihood of a pregnancy following IVF treatment: application of the modified cumulative embryo score. Hum Reprod. 1998;13:1506–1513. doi: 10.1093/humrep/13.6.1506. [DOI] [PubMed] [Google Scholar]
- Juetten J, Bavister B. Effects of egg aging on in vitro fertilization and first cleavage division in the hamster. Gamete Res. 1983;8:219–230. [Google Scholar]
- Kadar N, Caldwell BV, Romero R. A method of screening for ectopic pregnancy and its indications. Obstet Gynecol. 1981;58:162–166. [PubMed] [Google Scholar]
- Kaufman RH, Noller K, Adam E, Irwin J, Gray M, Jefferies JA, Hilton J. Upper genital tract abnormalities and pregnancy outcome in diethylstilbestrol-exposed progeny. Am J Obstet Gynecol. 1984;148:973–984. doi: 10.1016/0002-9378(84)90540-4. [DOI] [PubMed] [Google Scholar]
- Knoll M, Talbot P. Cigarette smoke inhibits oocyte cumulus complex pick-up by the oviduct in vitro independent of ciliary beat frequency. Reprod Toxicol. 1998;12:57–68. doi: 10.1016/s0890-6238(97)00100-7. [DOI] [PubMed] [Google Scholar]
- Knoll M, Shaoulian R, Magers T, Talbot P. Ciliary beat frequency of hamster oviducts is decreased in vitro by exposure to solutions of mainstream and sidestream cigarette smoke. Biol Reprod. 1995;53:29–37. doi: 10.1095/biolreprod53.1.29. [DOI] [PubMed] [Google Scholar]
- Lanman JT. Delays during reproduction and their effects on the embryo and fetus. 2. Aging of eggs. N Engl J Med. 1968;278:1047–1054. doi: 10.1056/NEJM196805092781906. [DOI] [PubMed] [Google Scholar]
- Longo F, So F. Transformations of sperm nuclei incorporated into aged and unaged hamster eggs. J Androl. 1982;3:420–428. [Google Scholar]
- Lyons RA, Saridogan E, Djahanbakhch O. The reproductive significance of human Fallopian tube cilia. Hum Reprod Update. 2006;12:363–372. doi: 10.1093/humupd/dml012. [DOI] [PubMed] [Google Scholar]
- Maximovich A, Beyler SA. Cigarette smoking at time of in vitro fertilization cycle initiation has negative effect on in vitro fertilization-embryo transfer success rate. J Assist Reprod Genet. 1995;12:75–77. doi: 10.1007/BF02211373. [DOI] [PubMed] [Google Scholar]
- McChesney R, Wilcox AJ, O'Connor JF, Weinberg CR, Baird DD, Schlatterer JP, McConnaughey DR, Birken S, Canfield RE. Intact HCG, free HCG beta subunit and HCG beta core fragment: longitudinal patterns in urine during early pregnancy. Hum Reprod. 2005;20:928–935. doi: 10.1093/humrep/deh702. [DOI] [PubMed] [Google Scholar]
- Mitchell JA, Hammer RE. Effects of nicotine on oviductal blood flow and embryo development in the rat. J Reprod Fertil. 1985;74:71–76. doi: 10.1530/jrf.0.0740071. [DOI] [PubMed] [Google Scholar]
- Nepomnaschy PA, Weinberg CR, Wilcox AJ, Baird DD. Urinary hCG patterns during the week following implantation. Hum Reprod. 2008;23:271–277. doi: 10.1093/humrep/dem397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neri A, Marcus SL. Effect of nicotine on the motility of the oviducts in the rhesus monkey: a preliminary report. J Reprod Fertil. 1972;31:91–97. doi: 10.1530/jrf.0.0310091. [DOI] [PubMed] [Google Scholar]
- Norman RJ, Menabawey M, Lowings C, Buck RH, Chard T. Relationship between blood and urine concentrations of intact human chorionic gonadotropin and its free subunits in early pregnancy. Obstet Gynecol. 1987;69:590–593. [PubMed] [Google Scholar]
- Perreault SD. Chromatin remodeling in mammalian zygotes. Mutat Res. 1992;296:43–55. doi: 10.1016/0165-1110(92)90031-4. [DOI] [PubMed] [Google Scholar]
- Rosevear SK, Holt DW, Lee TD, Ford WC, Wardle PG, Hull MG. Smoking and decreased fertilisation rates in vitro. Lancet. 1992;340:1195–1196. doi: 10.1016/0140-6736(92)92895-m. [DOI] [PubMed] [Google Scholar]
- Samarajeewa P, Cooley G, Kellie AE. The radioimmunoassay of pregnanediol-3 alpha-glucuronide. J Steroid Biochem. 1979;11:1165–1171. doi: 10.1016/0022-4731(79)90169-9. [DOI] [PubMed] [Google Scholar]
- Seeber BE, Sammel MD, Guo W, Zhou L, Hummel A, Barnhart KT. Application of redefined human chorionic gonadotropin curves for the diagnosis of women at risk for ectopic pregnancy. Fertil Steril. 2006;86:454–459. doi: 10.1016/j.fertnstert.2005.12.056. [DOI] [PubMed] [Google Scholar]
- Shamonki MI, Frattarelli JL, Bergh PA, Scott RT. Logarithmic curves depicting initial level and rise of serum beta human chorionic gonadotropin and live delivery outcomes with in vitro fertilization: an analysis of 6021 pregnancies. Fertil Steril. 2009;91:1760–1764. doi: 10.1016/j.fertnstert.2008.02.171. [DOI] [PubMed] [Google Scholar]
- Stone BA, Vargyas JM, Ringler GE, March CM, Marrs RP. The rate at which serum total beta-subunit human chorionic gonadotropin increases after embryo transfer is a predictor of the viability of pregnancy and an identifier of determinants of pregnancy. Fertil Steril. 2006;86:1626–1633. doi: 10.1016/j.fertnstert.2006.04.048. [DOI] [PubMed] [Google Scholar]
- Templeman C, Marshall SF, Ursin G, Horn-Ross PL, Clarke CA, Allen M, Deapen D, Ziogas A, Reynolds P, Cress R, et al. Adenomyosis and endometriosis in the California Teachers Study. Fertil Steril. 2008;90:415–424. doi: 10.1016/j.fertnstert.2007.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treloar SA, Bell TA, Nagle CM, Purdie DM, Green AC. Early menstrual characteristics associated with subsequent diagnosis of endometriosis. Am J Obstet Gynecol. 2010;202:534.e1–6. doi: 10.1016/j.ajog.2009.10.857. [DOI] [PubMed] [Google Scholar]
- Troisi R, Titus-Ernstoff L, Hyer M, Hatch EE, Robboy SJ, Strohsnitter W, Palmer JR, Oglaend B, Adam E, Kaufman R, et al. Preeclampsia risk in women exposed in utero to diethylstilbestrol. Obstet Gynecol. 2007;110:113–120. doi: 10.1097/01.AOG.0000268796.75591.02. [DOI] [PubMed] [Google Scholar]
- Urbancsek J, Hauzman E, Fedorcsak P, Halmos A, Devenyi N, Papp Z. Serum human chorionic gonadotropin measurements may predict pregnancy outcome and multiple gestation after in vitro fertilization. Fertil Steril. 2002;78:540–542. doi: 10.1016/s0015-0282(02)03278-8. [DOI] [PubMed] [Google Scholar]
- Van Voorhis BJ, Dawson JD, Stovall DW, Sparks AE, Syrop CH. The effects of smoking on ovarian function and fertility during assisted reproduction cycles. Obstet Gynecol. 1996;88:785–791. doi: 10.1016/0029-7844(96)00286-4. [DOI] [PubMed] [Google Scholar]
- Ways SC, Mortola JF, Zvaifler NJ, Weiss RJ, Yen SS. Alterations in immune responsiveness in women exposed to diethylstilbestrol in utero. Fertil Steril. 1987;48:193–197. doi: 10.1016/s0015-0282(16)59341-8. [DOI] [PubMed] [Google Scholar]
- Wehmann RE, Nisula BC. Metabolic and renal clearance rates of purified human chorionic gonadotropin. J Clin Invest. 1981;68:184–194. doi: 10.1172/JCI110234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg CR, Wilcox AJ, Baird DD. Reduced fecundability in women with prenatal exposure to cigarette smoking. Am J Epidemiol. 1989;129:1072–1078. doi: 10.1093/oxfordjournals.aje.a115211. [DOI] [PubMed] [Google Scholar]
- Weinberg CR, Moledor E, Baird DD, Wilcox AJ. Is there a seasonal pattern in risk of early pregnancy loss? Epidemiology. 1994;5:484–489. [PubMed] [Google Scholar]
- Wilcox AJ, Weinberg CR, Wehmann RE, Armstrong EG, Canfield RE, Nisula BC. Measuring early pregnancy loss: laboratory and field methods. Fertil Steril. 1985;44:366–374. [PubMed] [Google Scholar]
- Wilcox AJ, Weinberg CR, O'Connor JF, Baird DD, Schlatterer JP, Canfield RE, Armstrong EG, Nisula BC. Incidence of early loss of pregnancy. N Engl J Med. 1988;319:189–194. doi: 10.1056/NEJM198807283190401. [DOI] [PubMed] [Google Scholar]
- Wilcox AJ, Weinberg CR, Baird DD. Timing of sexual intercourse in relation to ovulation. Effects on the probability of conception, survival of the pregnancy, and sex of the baby. N Engl J Med. 1995;333:1517–1521. doi: 10.1056/NEJM199512073332301. [DOI] [PubMed] [Google Scholar]
- Wilcox AJ, Weinberg CR, Baird DD. Post-ovulatory ageing of the human oocyte and embryo failure. Hum Reprod. 1998;13:394–397. doi: 10.1093/humrep/13.2.394. [DOI] [PubMed] [Google Scholar]
- Wilcox AJ, Baird DD, Weinberg CR. Time of implantation of the conceptus and loss of pregnancy. N Engl J Med. 1999;340:1796–1799. doi: 10.1056/NEJM199906103402304. [DOI] [PubMed] [Google Scholar]
- Wright K, Collins DC, Musey PI, Preedy JR. Direct radioimmunoassay of specific urinary estrogen glucosiduronates in normal men and nonpregnant women. Steroids. 1978;31:407–426. doi: 10.1016/0039-128x(78)90053-3. [DOI] [PubMed] [Google Scholar]
- Yang M, Lei ZM, Rao Ch V. The central role of human chorionic gonadotropin in the formation of human placental syncytium. Endocrinology. 2003;144:1108–1120. doi: 10.1210/en.2002-220922. [DOI] [PubMed] [Google Scholar]
- Yang F, Xiong DH, Guo Y, Shen H, Xiao P, Zhang F, Jiang H, Recker RR, Deng HW. The chemokine (C-C-motif) receptor 3 (CCR3) gene is linked and associated with age at menarche in Caucasian females. Hum Genet. 2007;121:35–42. doi: 10.1007/s00439-006-0295-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeleznik A, Pohl R. Control of follicul1ar development, corpus luteum function, the maternal recognition of pregnancy, and the neuroendocrine regulation of the menstrual cycle in higher primates. In: Neill JD, editor. Knobil and Neill's Physiology of Reproduction. San Diego: Elsevier, Academic Press; 2006. pp. 2470–2475. [Google Scholar]