Summary
Background
Ca2+ signaling mechanisms are crucial for proper regulation of vascular smooth muscle contractility and vessel diameter. In cerebral artery myocytes, a rise in global cytosolic Ca2+ concentration ([Ca2+]i) causes contraction while an increase in local Ca2+ release events from the sarcoplasmic reticulum (Ca2+ sparks) leads to increased activity of large-conductance Ca2+-activated (BK) K+ channels, hyperpolarization and relaxation. Here, we examined the impact of SAH on Ca2+ spark activity and [Ca2+]i in cerebral artery myocytes following SAH.
Methods
A rabbit double injection SAH model was used in this study. Five days after the initial intracisternal injection of whole blood, small diameter cerebral arteries were dissected from the brain for study. For simultaneous measurement of arterial wall [Ca2+]i and diameter, vessels were cannulated and loaded with the ratiometric Ca2+ indicator fura-2. For measurement of Ca2+ sparks, individual myocytes were enzymatically isolated from cerebral arteries and loaded with the Ca2+ indicator fluo-4. Sparks were visualized using laser scanning confocal microscopy.
Results
Arterial wall [Ca2+]i was significantly elevated and greater levels of myogenic tone developed in arteries isolated from SAH animals compared with arteries isolated from healthy animals. The L-type voltage-dependent Ca2+ channel (VDCC) blocker nifedipine attenuated increases in [Ca2+]i and tone in both groups suggesting increased VDCC activity following SAH. Membrane potential measurement using intracellular microelectrodes revealed significant depolarization of vascular smooth muscle following SAH. Further, myocytes from SAH animals exhibited significantly reduced Ca2+ spark frequency (~50%).
Conclusions
Our findings suggest decreased Ca2+ spark frequency leads to reduced BK channel activity in cerebral artery myocytes following SAH. This results in membrane potential depolarization, increased VDCC activity, elevated [Ca2+]i and decreased vessel diameter. We propose this mechanism of enhanced cerebral artery myocyte contractility may contribute to decreased cerebral blood flow and development of neurological deficits in SAH patients.
Keywords: Ca2+ channels, K+ channels, Ca2+ sparks, vascular smooth muscle, vasospasm
Introduction
Intracellular Ca2+ is a ubiquitous second messenger, playing critical roles in a wide array of physiological processes including muscle contraction [2]. In the cerebral vasculature, average intracellular Ca2+ concentration or global cytosolic Ca2+ ([Ca2+]i) dictates smooth muscle contraction (and arterial diameter) via regulation of myosin light chain kinase activity [4]. Thus, an elevation in global cytosolic Ca2+ leads to enhanced vasoconstriction and potentially a decrease in cerebral blood flow [12]. Paradoxically, localized intracellular Ca2+ release events, termed Ca2+ sparks, promote a decrease in [Ca2+]i and relaxation of cerebral artery myocytes [15, 27]. Ca2+ sparks are generated by the coordinated opening of ryanodine receptors (RyRs) located on the sarcoplasmic reticulum of smooth muscle cells and activate plasmamemmal large conductance Ca2+-activated K+ (BK) channels leading to membrane potential hyperpolarization, decreased activity of voltage-dependent Ca2+ channels (VDCCs), decreased [Ca2+]i, and vasodilation. Currently, the impact of subarachnoid hemorrhage on local and/or global Ca2+ signals in myocytes from small diameter cerebral arteries is unclear [24].
Methods
SAH model
A rabbit double-injection model of SAH was used in this study. Briefly, anesthetized New Zealand white rabbits (males, 3.0-3.5 kg) received an intracisternal injection of autologous arterial blood (2.5 ml) using a previously described surgical procedure [8, 9]. Forty-eight hours after the initial injection, the procedure was repeated with animals receiving a second injection of 2.5 ml of arterial blood. Five days after the initial surgery, rabbits were euthanized and posterior cerebral and cerebellar arteries (100-200 μm diameter) were dissected for in vitro studies. All protocols were conducted in accordance with the guidelines for the care and use of laboratory animals (NIH publication 85-23, 1985) and followed protocols approved by the Institutional Animal Use and Care Committee of the University of Vermont, USA.
Simultaneous measurement of global cytosolic Ca2+ and arterial diameter
Intact cerebral arteries were cannulated on glass micropipettes mounted in a Living Systems Inc. (Burlington, VT) arteriograph chamber. Arteries were loaded, in the dark, with the ratiometric Ca2+ indicator fura-2-AM (5 μM) in a MOPS solution containing pluronic acid (0.05%) for 45 minutes at room temperature. The MOPS loading solution had the following composition (in mM): 145 NaCl, 5 KCl, 1 MgSO4, 2.5 CaCl2, 1 KH2PO4, 0.02 EDTA, 3 3-(N-morpholino)propanesulfonic acid (MOPS), 2 pyruvate, 5 glucose, 1% bovine serum albumin (pH 7.4). Arteries were then continuously superfused with aerated artificial cerebral spinal fluid (aCSF) at 37° C for the remainder of the experiment. The composition of the aCSF was (in mM): 125 NaCl, 3 KCl, 18 NaHCO3, 1.25 NaH2PO4, 1 MgCl2, 2 CaCl2, 5 glucose aerated with 5% CO2, 20% O2, 75% N2 (pH 7.35). Ratio images of the arterial wall were obtained from background corrected images of the 510 nm emission from arteries alternately excited at 340 and 380 nm using software developed by IonOptix Inc. (Milton, MA). Arterial wall [Ca2+] is calculated using the following equation [3] : [Ca2+] = Kd × β × (R-Rmin)/(Rmax-R). An apparent Kd of 282 nM of fura-2 for Ca2+ was used [12]. Arterial constriction was expressed as a percent decrease from the maximal (fully dilated) diameter obtained at the end of each experiment in Ca2+-free aCSF containing the vasodilators nifedipine (1 μM) and forskolin (1 μM). In some studies intracellular microelectrodes were used to measure smooth muscle membrane potential in intact pressurized arteries, as described previously [25].
Measurement of Ca2+ sparks in isolated cerebral artery myocytes
Individual smooth muscle cells were enzymatically isolated from posterior cerebral and cerebellar artery segments [26]. Isolated myocytes were then loaded with fluo-4-AM (10 μM) for 60 minutes (21 C) in a HEPES-buffered physiological saline solution (PSS) containing pluronic acid (0.05%). The HEPES-PSS had the following composition (in mM): 135 NaCl, 5.4 KCl, 1.8 CaCl2, 1 MgCl2, 10 HEPES, 10 glucose (pH 7.4 with NaOH). Myocyte images were acquired with a Noran Oz laser scanning confocal microscope [19]. Fluo-4 was excited using the 488 nm line of a krypton/argon laser and the light emitted by this dye (520 nm) was separated from the excitation light and collected. Images were acquired at a frequency of ≈60 Hz for a period of 20 seconds. Ca2+ sparks are detected and analyzed using custom software (written by Dr. Adrian Bonev, University of Vermont, using IDL 5.0.2; Research Systems Inc., Boulder, CO). Baseline fluorescence (Fo) was determined by averaging 10 images without Ca2+ spark activity. Fractional fluorescence increases (F/Fo) are determined in areas (2.1 μm × 2.1 μm) where Ca2+ sparks were observed. Ca2+ sparks are defined as local fractional fluorescence increases greater than 1.3. All measurements were recorded at room temperature.
Results
Elevated global cytosolic Ca2+ and enhanced myogenic tone in small diameter cerebral arteries following SAH
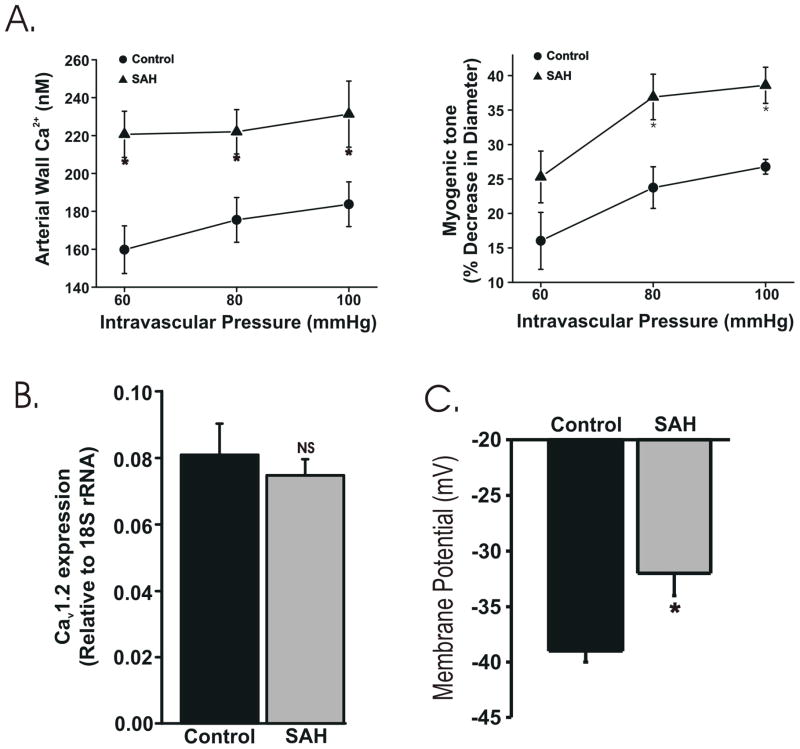
The relationship between intravascular pressure, arterial Ca2+ and myogenic tone was examined in small diameter (150-200 μm) cerebral arteries isolated from healthy control and SAH model rabbits. Arterial wall Ca2+ and constriction increased as intravascular pressure was elevated within the range (60-100 mmHg) typically experienced by these arteries in vivo. Arteries from SAH animals exhibited significant elevations in both arterial wall Ca2+ and constriction compared to similar arteries from control animals (Figure 1A). For example, at 100 mmHg, arterial wall Ca2+ was ~26% higher in arteries isolated from SAH animals (231 ± 17 nM, n = 4) compared with arteries isolated from healthy (184 ± 12 nM, n = 4). The level of constriction (myogenic tone) at 100 mmHg was ~1.5 fold higher in arteries isolated from SAH animals (39 ± 3 % decrease in diameter, n = 4) compared with arteries isolated from healthy animals (27 ± 2 % decrease in diameter, n = 4). In the presence of the L-type VDCC blocker nifedipine (1 μM), arterial Ca2+ was greatly reduced in arteries from both SAH (141 ± 15 nM) and control (118 ± 7 nM) rabbits. Nifedipine (1 μM) also reduced pressure-induced constrictions by 92 ± 1 % and 93 ± 1 % in arteries from SAH and control animals, respectively. These data suggest that increased VDCC activity underlies enhanced pressure-induced constriction observed in small diameter cerebral arteries from SAH.
Figure 1. Elevated global cytosolic Ca2+ following SAH.
A. Summary data from simultaneous measurement of arterial wall Ca2+ (using fura-2) and diameter. Arterial wall Ca2+ and tone were significantly increased at intravascular pressures between 60 and 100 mmHg in arteries isolated from SAH animals compared with controls. B. Summary data using quantitative real-time PCR. Total RNA was collected from posterior cerebral arteries. CaV1.2 expression was not altered following SAH. NS: not statistically significant. C. Summary data from membrane potential measurement using intracellular microelectrodes. Vascular smooth muscle membrane potential in arteries from SAH animals was significantly depolarized following SAH. * P<0.05.
Increased VDCC activity could reflect either increased L-type VDCC expression or enhanced VDCC activation due to smooth muscle membrane potential depolarization following SAH. Quantitative real-time PCR was used to assess L-type VDCC expression encoded by the gene CaV1.2 [17]. Using this approach, no significant difference in CaV1.2 mRNA levels was detected in cerebral artery homogenates from control and SAH animals (Figure 1B). Next, we used intracellular microelectrodes to directly measure vascular smooth muscle membrane potential from intact pressurized cerebral arteries. At 80 mmHg, smooth muscle membrane potential was significantly depolarized by approximately 8 mV in arteries isolated from SAH animals compared with arteries from control animals (Figure 1C). These findings suggest that membrane potential depolarization of vascular smooth muscle leads to increased VDCC activity, elevated global cytosolic Ca2+ and enhanced constriction of small diameter cerebral arteries following SAH.
Ca2+ spark frequency is decreased in cerebral artery myocytes from SAH animals
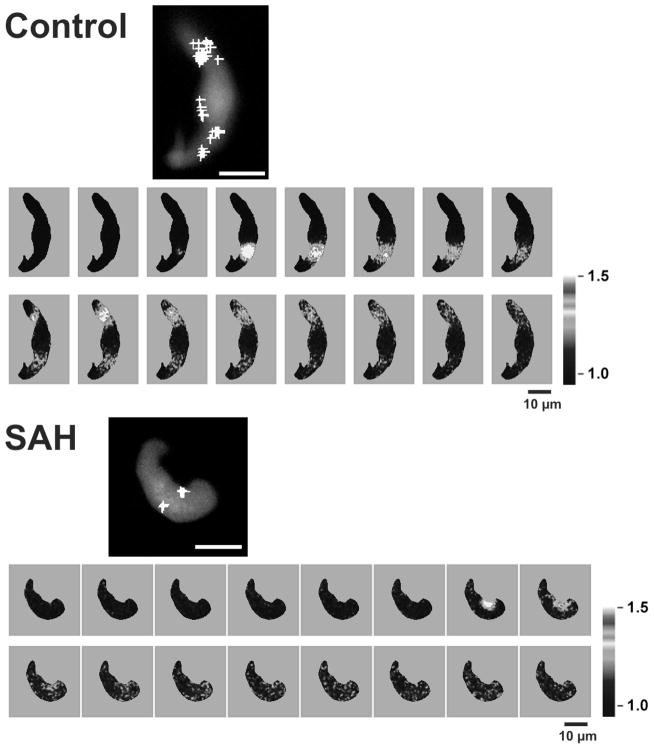
A decrease in Ca2+ spark frequency and associated BK activity promotes membrane potential depolarization, elevated global cytosolic Ca2+, and vasoconstriction [15, 27]. To explore whether decreased Ca2+ spark frequency may contribute to enhanced cerebral artery constriction, Ca2+ sparks were measured in isolated cerebral artery myocytes using laser scanning confocal microscopy and the Ca2+ indicator dye fluo-4. As illustrated in figure 2, Ca2+ sparks were observed in cerebral artery myocytes obtained from both control and SAH animals. However, Ca2+ spark frequency was markedly decreased (by approximately 50%) in myocytes isolated from SAH animals. Consistent with the observed decrease in Ca2+ spark frequency, the frequency of transient BK currents detected using patch clamp electrophysiology was also reduced by approximately 50 % in freshly isolated cerebral artery myocytes from SAH animals (Koide and Wellman, unpublished observations). These data suggest that a decrease in the frequency of Ca2+ sparks and their associated BK channel currents may contribute to enhanced constriction of small diameter cerebral arteries following SAH.
Figure 2. Decreased Ca2+ spark frequency in cerebral myocytes from SAH animals.
Imaging of Ca2+ sparks in cerebral artery myocytes loaded with the fluorescent Ca2+ indicator fluo-4. Fluorescent images were detected using laser scanning confocal microscopy with Ca2+ sparks defined as a fractional fluorescent increase of greater than 30% within 2.1 μm by 2.1 μm analysis areas. Large size images represent an average of 30 images in gray scale without Ca2+ spark activity. White crosses depict where individual Ca2+ sparks occurred during the 20 sec recordings. Scale bars represent 10 μm. Smaller images illustrate the time course of Ca2+ sparks in control and SAH myocytes. Images were obtained every 19 msec.
Discussion
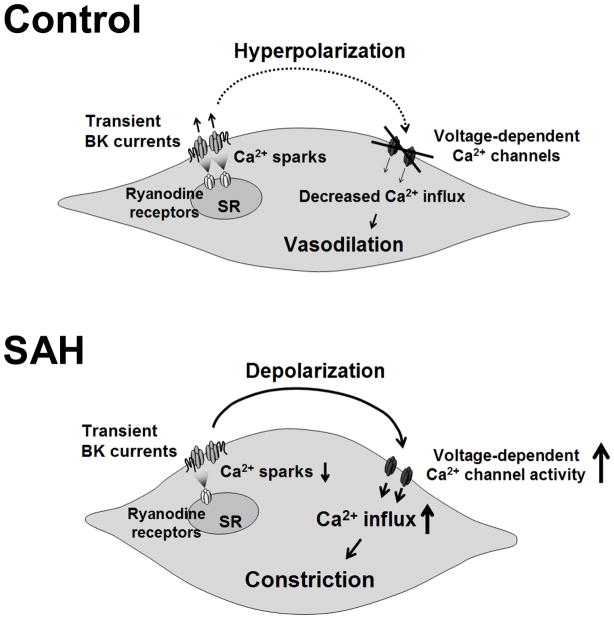
Aneurysmal SAH is associated with high rates of morbidity and mortality [1]. It has been a long-held belief that delayed and sustained large diameter (conduit) cerebral artery vasospasm (“angiographic vasospasm”) is a major contributor to SAH-induced death and disability. However, there is a growing appreciation that a host of other factors are also likely involved in the pathological consequences associated with cerebral aneurysm rupture [5, 20]. Here, we provide evidence that SAH enhances the dynamic constriction of small diameter pial arteries in response to physiological increases in intravascular pressure, an effect that could have a pronounced influence to decrease cerebral blood flow. Our findings indicate that this augmented constriction is associated with enhanced smooth muscle contraction due to membrane potential depolarization, enhanced voltage-dependent Ca2+ channel activity and elevated global cytosolic calcium. Further, our recent findings suggest a decrease in the frequency of Ca2+ sparks and associated BK channel activity may contribute to enhanced cerebral artery constriction and elevated global cytosolic Ca2+ (figure 3).
Figure 3. Summary cartoon.
In control cerebral artery myocytes, local Ca2+ release events from the sarcoplasmic reticulum (Ca2+ sparks) activate large conductance Ca2+ activated (BK) K+ channels, causing membrane potential hyperpolarization, decreased voltage-dependent Ca2+ channel (VDCC) activity and decreased global cytosolic Ca2+. Following SAH, the frequency of Ca2+ sparks and therefore BK channel activity is decreased, promoting membrane potential depolarization, increased VDCC activity and increased global cytosolic Ca2+ levels.
The cerebral circulation maintains a constant level of blood flow to the brain despite physiological fluctuations in cerebral perfusion pressure [14]. To achieve stable cerebral blood flow in the face of changes in blood pressure, cerebral arteries must constrict in response to increased intravascular pressure and dilate when intravascular pressure is reduced. In pial arteries from healthy animals, physiological increases in intravascular pressure lead to smooth muscle membrane potential depolarization, an increase in the activity of L-type CaV1.2 channels (encoded by the gene CACNA1C), and increased global cytosolic Ca2+ [12, 17]. The open-state probability of CaV1.2 is steeply voltage-dependent [16]; thus, small changes in membrane potential can have a profound impact on smooth muscle Ca2+. Our present results suggest membrane potential depolarization and enhanced L-type VDCC activity in small diameter arteries is likely to contribute to SAH-induced impairment in the autoregulation of cerebral blood flow reported by others [18, 23]. Our evidence also suggests enhanced activity of L-type VDCCs play a large part in the SAH-induced elevation in global cytosolic Ca2+. However, in the presence of the L-type VDCC blocker nifedipine, global Ca2+ remained elevated (by approximately 20%) in arteries from SAH animals. This nifedipine-resistant increase in global Ca2+ in cerebral arteries from SAH animals may reflect the emergence of R-type VDCC channels [9], or the possible upregulation of additional Ca2+ entry pathways.
Our present findings suggest decreased Ca2+ spark and associated BK channel activity contribute to SAH-induced membrane potential depolarization and enhanced VDCC activity in cerebral artery myocytes. Harder and colleagues [6] were the first to report that the membrane potential of cerebral artery myocytes is depolarized following SAH and a number of subsequent studies have provided further evidence for decreased voltage-dependent K+ (KV) channel activity in pial arteries following SAH [7, 10, 13, 21, 22, 24]. Interestingly, BK channel activity and expression have been reported to be unchanged in basilar artery myocytes obtained from a canine SAH model [11]. Our current work demonstrates that decreased BK channel activity following SAH results from a decrease in local Ca2+ signaling from the SR to the plasma membrane (i.e. decreased Ca2+ spark activity), rather than a direct effect on BK channel properties or expression.
Conclusions
In this study, we examined global and local Ca2+ signaling in cerebral artery myocytes following SAH. As for global Ca2+, we observed a significant increase in averaged cytosolic Ca2+ and constriction in cerebral arteries from SAH animals at physiological intravascular pressures. Regarding local Ca2+ signaling, we report a decrease in the frequency of Ca2+ sparks and associated transient outward BK currents, which likely contribute to membrane potential depolarization, increased VDCC activity and enhanced cerebral constriction artery following SAH. These data suggest that increased global Ca2+ and impaired local Ca2+ signaling may contribute to decreased cerebral blood flow and the development of neurological deficits frequently observed following aneurysmal SAH.
Acknowledgments
This work was supported by the Totman Medical Research Trust Fund, the Peter Martin Brain Aneurysm Endowment, the NIH (NIHLBI, R01 HL078983 and NCRR, P20 RR16435) and the American Heart Association (0725837T, 0815736D). The authors wish to thank to Ms. Sheila Russell for her assistance with this study. The authors would also like to acknowledge the University of Vermont Neuroscience COBRE molecular biology and imaging core facilities.
References
- 1.Bederson JB, Connolly ES, Jr, Batjer HH, Dacey RG, Dion JE, Diringer MN, Duldner JE, Jr, Harbaugh RE, Patel AB, Rosenwasser RH. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 2009;40:994–1025. doi: 10.1161/STROKEAHA.108.191395. [DOI] [PubMed] [Google Scholar]
- 2.Clapham DE. Calcium signaling. Cell. 1995;80:259–268. doi: 10.1016/0092-8674(95)90408-5. [DOI] [PubMed] [Google Scholar]
- 3.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 4.Hai CM, Murphy RA. Ca2+, crossbridge phosphorylation, and contraction. Annu Rev Physiol. 1989;51:285–298. doi: 10.1146/annurev.ph.51.030189.001441. [DOI] [PubMed] [Google Scholar]
- 5.Hansen-Schwartz J, Vajkoczy P, Macdonald RL, Pluta RM, Zhang JH. Cerebral vasospasm: looking beyond vasoconstriction. Trends Pharmacol Sci. 2007;28:252–256. doi: 10.1016/j.tips.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Harder DR, Dernbach P, Waters A. Possible cellular mechanism for cerebral vasospasm after experimental subarachnoid hemorrhage in the dog. J Clin Invest. 1987;80:875–880. doi: 10.1172/JCI113146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishiguro M, Morielli AD, Zvarova K, Tranmer BI, Penar PL, Wellman GC. Oxyhemoglobin-induced suppression of voltage-dependent K+ channels in cerebral arteries by enhanced tyrosine kinase activity. Circ Res. 2006;99:1252–1260. doi: 10.1161/01.RES.0000250821.32324.e1. [DOI] [PubMed] [Google Scholar]
- 8.Ishiguro M, Puryear CB, Bisson E, Saundry CM, Nathan DJ, Russell SR, Tranmer BI, Wellman GC. Enhanced myogenic tone in cerebral arteries from a rabbit model of subarachnoid hemorrhage. Am J Physiol Heart Circ Physiol. 2002;283:H2217–H2225. doi: 10.1152/ajpheart.00629.2002. [DOI] [PubMed] [Google Scholar]
- 9.Ishiguro M, Wellman TL, Honda A, Russell SR, Tranmer BI, Wellman GC. Emergence of a R-type Ca2+ channel (CaV 2.3) contributes to cerebral artery constriction after subarachnoid hemorrhage. Circ Res. 2005;96:419–426. doi: 10.1161/01.RES.0000157670.49936.da. [DOI] [PubMed] [Google Scholar]
- 10.Jahromi BS, Aihara Y, Ai J, Zhang ZD, Nikitina E, Macdonald RL. Voltage-gated K+ channel dysfunction in myocytes from a dog model of subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2008;28:797–811. doi: 10.1038/sj.jcbfm.9600577. [DOI] [PubMed] [Google Scholar]
- 11.Jahromi BS, Aihara Y, Ai J, Zhang ZD, Weyer G, Nikitina E, Yassari R, Houamed KM, Macdonald RL. Preserved BK channel function in vasospastic myocytes from a dog model of subarachnoid hemorrhage. J Vasc Res. 2008;45:402–415. doi: 10.1159/000124864. [DOI] [PubMed] [Google Scholar]
- 12.Knot HJ, Nelson MT. Regulation of arterial diameter and wall [Ca2+] in cerebral arteries of rat by membrane potential and intravascular pressure. J Physiol. 1998;508 ( Pt 1):199–209. doi: 10.1111/j.1469-7793.1998.199br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koide M, Penar PL, Tranmer BI, Wellman GC. Heparin-binding EGF-like growth factor mediates oxyhemoglobin-induced suppression of voltage-dependent potassium channels in rabbit cerebral artery myocytes. Am J Physiol Heart Circ Physiol. 2007;293:H1750–H1759. doi: 10.1152/ajpheart.00443.2007. [DOI] [PubMed] [Google Scholar]
- 14.Lee KR, Hoff JT. Intracranial Pressure. In: Youmans JR, editor. Neurological Surgery. W. B. Saunders Co; Philadelphia: 1996. pp. 491–518. [Google Scholar]
- 15.Nelson MT, Cheng H, Rubart M, Santana LF, Bonev AD, Knot HJ, Lederer WJ. Relaxation of arterial smooth muscle by calcium sparks. Science. 1995;270:633–637. doi: 10.1126/science.270.5236.633. [DOI] [PubMed] [Google Scholar]
- 16.Nelson MT, Patlak JB, Worley JF, Standen NB. Calcium channels, potassium channels, and voltage dependence of arterial smooth muscle tone. Am J Physiol. 1990;259:C3–18. doi: 10.1152/ajpcell.1990.259.1.C3. [DOI] [PubMed] [Google Scholar]
- 17.Nystoriak MA, Murakami K, Penar PL, Wellman GC. Ca(v)1.2 splice variant with exon 9* is critical for regulation of cerebral artery diameter. Am J Physiol Heart Circ Physiol. 2009;297:H1820–H1828. doi: 10.1152/ajpheart.00326.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohkuma H, Ogane K, Tanaka M, Suzuki S. Assessment of cerebral microcirculatory changes during cerebral vasospasm by analyzing cerebral circulation time on DSA images. Acta Neurochir Suppl. 2001;77:127–130. doi: 10.1007/978-3-7091-6232-3_27. [DOI] [PubMed] [Google Scholar]
- 19.Perez GJ, Bonev AD, Patlak JB, Nelson MT. Functional coupling of ryanodine receptors to KCa channels in smooth muscle cells from rat cerebral arteries. J Gen Physiol. 1999;113:229–238. doi: 10.1085/jgp.113.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pluta RM, Hansen-Schwartz J, Dreier J, Vajkoczy P, Macdonald RL, Nishizawa S, Kasuya H, Wellman G, Keller E, Zauner A, Dorsch N, Clark J, Ono S, Kiris T, Leroux P, Zhang JH. Cerebral vasospasm following subarachnoid hemorrhage: time for a new world of thought. Neurol Res. 2009;31:151–158. doi: 10.1179/174313209X393564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quan L, Sobey CG. Selective effects of subarachnoid hemorrhage on cerebral vascular responses to 4-aminopyridine in rats. Stroke. 2000;31:2460–2465. doi: 10.1161/01.str.31.10.2460. [DOI] [PubMed] [Google Scholar]
- 22.Sobey CG, Faraci FM. Subarachnoid haemorrhage: what happens to the cerebral arteries? Clin Exp Pharmacol Physiol. 1998;25:867–876. doi: 10.1111/j.1440-1681.1998.tb02337.x. [DOI] [PubMed] [Google Scholar]
- 23.Takeuchi H, Handa Y, Kobayashi H, Kawano H, Hayashi M. Impairment of cerebral autoregulation during the development of chronic cerebral vasospasm after subarachnoid hemorrhage in primates. Neurosurgery. 1991;28:41–48. doi: 10.1097/00006123-199101000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Wellman GC. Ion channels and calcium signaling in cerebral arteries following subarachnoid hemorrhage. Neurol Res. 2006;28:690–702. doi: 10.1179/016164106X151972. [DOI] [PubMed] [Google Scholar]
- 25.Wellman GC, Bonev AD, Nelson MT, Brayden JE. Gender differences in coronary artery diameter involve estrogen, nitric oxide, and Ca2+-dependent K+ channels. Circ Res. 1996;79:1024–1030. doi: 10.1161/01.res.79.5.1024. [DOI] [PubMed] [Google Scholar]
- 26.Wellman GC, Nathan DJ, Saundry CM, Perez G, Bonev AD, Penar PL, Tranmer BI, Nelson MT. Ca2+ sparks and their function in human cerebral arteries. Stroke. 2002;33:802–808. doi: 10.1161/hs0302.104089. [DOI] [PubMed] [Google Scholar]
- 27.Wellman GC, Nelson MT. Signaling between SR and Plasmalemma in Smooth Muscle: Sparks and the activation of Ca2+-senstive ion channels. Cell Calcium. 2003;34:211–229. doi: 10.1016/s0143-4160(03)00124-6. [DOI] [PubMed] [Google Scholar]