Abstract
Breast cancer remains a major cause of death in the United States as well as the rest of the world. In view of the limited treatment options for patients with advanced breast cancer, preventive and novel therapeutic approaches play an important role in combating this disease. The plant-derived triterpenoids, commonly used for medicinal purposes in many Asian countries, posses various pharmacological properties. A large number of triterpenoids are known to exhibit cytotoxicity against a variety of tumor cells as well as anticancer efficacy in preclinical animal models. Numerous triterpenoids have been synthesized by structural modification of natural compounds. Some of these analogs are considered to be the most potent antiinflammatory and anticarcinogenic triterpenoids known. This review examines the potential role of natural triterpenoids and their derivatives in the chemoprevention and treatment of mammary tumors. Both in vitro and in vivo effects of these agents and related molecular mechanisms are presented. Potential challenges and future directions involved in the advancement of these promising compounds in the prevention and therapy of human breast cancer are also identified.
Keywords: Apoptosis, Breast Cancer, Chemoprevention, Review, Treatment, Triterpenoids, Tumor Cells
2. INTRODUCTION
Breast cancer is the leading worldwide cause of death among women between the ages of 40 and 55 (1) with an estimated 1 million women diagnosed annually (2). According to the American Cancer Society, nearly 193,000 new cases and more than 40,000 deaths are estimated to occur in 2009 in women in the United States alone (3). In the United States, a woman will die from breast cancer every 13 min and over 1 million women have died from this disease since 1970. Surprisingly, the incidence of male breast cancer is also on the rise (4). In 2009, nearly 2,000 cases of breast cancer and about 450 deaths are expected to occur among men in the United States (3).
Breast cancer is known to be influenced by several hormones, specifically estrogen and progesterone which are known to be capable of increasing breast cancer risk. Approximately two-thirds of breast cancers are positive for estrogen and/or progesterone receptors. In premenopausal women, the primary source of estrogen is the ovaries and in postmenopausal women, estrogen is produced in adipose tissue and the adrenal glands. The enzyme aromatase plays a critical role in the production of estrogen in postmenopausal women. At present, tamoxifen is widely used for estrogen receptor (ER)-positive breast cancers (5). Trustazumab is used in HER2/neu-positive breast cancers and have proven benefits (6). Nevertheless, these drugs carry significant adverse effects along with their known benefits. Tamoxifen shows a positive effect on bone decreasing the osteoporosis and on the other hand increases the risk of endometrial cancer and venous thromboembolism (5). Trustazumab, a monoclonal antibody, has potential concern of severe cardiac dysfunction (7).
Unfortunately, the significant morbidity of breast cancer has been only somewhat impacted by current treatment modalities including surgery, radiotherapy, and adjuvant chemotherapy and/or hormone therapies (8, 9). There is still no effective cure for the vast majority of patients with advanced stages of the disease (10). There is a critical and urgent need for developing agents that will be effective in decreasing the incidence of breast cancer in high-risk women. These necessitate the search for novel preventive and therapeutic approaches for this disease. One such approach to consider is chemoprevention, an approach by which the occurrence of the disease can be prevented, slowed, or reversed by the administration of one or more naturally occurring and/or synthetic compounds (11–13). Due to generally being an estrogen-dependent cancer, breast carcinomas are ideal candidates for hormonal and other types of chemoprevention (14). During the last decade several bioactive agents have been identified in plants and in human diets and are being developed as chemopreventive and therapeutic agents for various cancers including breast cancer (14–20). One such group of phytochemicals, the terpenoids, is found widely in nature. Terpenoids, also referred to as terpenes or isoprenoids, are the largest group of natural compounds found in plants and are synthesized from two five-carbon building blocks. Based on the number of building blocks, terpenoids are classified as monoterpenes, sequiterpenes, diterpenes, sesterterpenes, triterpenes, tetraterpenes, and polyterpenes (21) with almost 40,000 different terpenoids having been isolated from plants, animals and microbial species (22–23). Among the terpenoids, triterpenoids have recently emerged as a unique group of phytochemicals with multifunctional anticancer activities as demonstrated by promising results in preclinical studies.
The use of triterpenoids and related compounds for either chemoprevention or therapy of mammary carcinoma has not been extensively discussed previously although several excellent articles provide an overview of the antitumor potential of these agents against various cancers (24–27). Accordingly, this review critically examines the current knowledge about naturally occurring and semi-synthetic triterpenoids and their mechanisms of breast cancer chemopreventive and therapeutic activity both in vitro and in vivo with a goal of finding realistic use of these compounds for the prevention and treatment of breast cancer.
3. TRITERPENOIDS
Triterpenoids are metabolites of isopentenyl pyrophosphate oligomers and represent the largest group of phytochemicals. It has been estimated that more than 20,000 triterpenoids exist in nature (25). They predominantly are found in various plants including sea-weeds as well as in wax-like coatings of various fruits and medicinal herbs, including apples, cranberries, figs, olives, mistletoe, lavender, oregano, rosemary and thyme (21, 26, 28–30). Triterpenoids are biosynthesized in plants by the cyclization of squalene, a triterpene hydrocarbon and precursor of all steroids (31). They can further be subclassified into diverse groups including cucurbitanes, cycloartanes, dammaranes, euphanes, friedelanes, holostanes, hopanes, isomalabaricanes, lanostanes, limonoids, lupanes, oleananes, protostanes, sqalenes, tirucallanes, ursanes and miscellaneous compounds (24, 27, 32). Although triterpenoids were considered to be biologically inactive for a long period of time, accumulating evidence on their broad spectrum pharmacological activities coupled with a low toxicity profile has sparked renewed interest with regard to human health and disease. Triterpenoids are used for medicinal purposes in many Asian countries for antiinflammatory, analgesic, antipyretic, hepatoprotective, cardiotonic, sedative and tonic effects (28, 33, 34). Recent studies have not only confirmed some of the aforementioned pharmacological properties of several triterpenoids, but also identified a variety of additional biological activities including antioxidant, antimicrobial, antiviral, antiallergic, antipruritic, antiangiogenic and spasmolytic activity (35, 36). An increasing number of triterpenoids have been reported to exhibit cytotoxicity against a variety of cancer cells without manifesting any toxicity in normal cells (24, 26, 27). They also demonstrate antitumor efficacy in preclinical animal models of cancer (26, 27). A large number of triterpenoids have been synthesized by structural modification of natural compounds for optimization of bioactivity, and some of these semi-synthetic analogs are considered to be the most potent antiinflammatory and anticarcinogenic triterpenoids known to mankind (25). The antitumor efficacy of several triterpenoids are currently being evaluated in phase I clinical trials (27).
4. TRITERPENOIDS AND BREAST CANCER
The following sections of this review present in vitro and in vivo studies carried out to explore chemotherapeutic as well as chemopreventive potential of triterpenoids of plant origin and their semi-synthetic analogs in breast cancer. Saponins as well as triterpenoids derived from animal and microbial species are not included.
4.1. In vitro studies
There are studies being done in vitro that demonstrate the inhibitory effects of various triterpenoids against proliferation, growth and invasion of a large variety of breast cancer cell lines (Table 1).
Table 1.
In vitro effects of natural and semi-synthetic triterpenoids on various breast cancer cells
| Triterpenoids | Compounds | Cellular effects | Mechanisms | IC50 | References |
|---|---|---|---|---|---|
| Cucurbitanes | Cucurbitacin I | Suppressed the proliferation of MDA-MB-468 cells | ↑Apoptosis; ↓phospho-STAT3 | Blaskovich et al. (40) | |
| Cucurbitacin Q | Induced apoptosis in MDA-MB-435 and MDA-MB-453 cells | Sun et al. (41) | |||
| 2-Deoxycucurbitacin D, 25-Acetylcucurbitacin F, Cucurbitacin D | Demonstrated cytotoxicity against MCF-7 cells | 0.02–0.11 μg/ml | Rodriguez et al. (42) | ||
| Cucurbitacin B, Cucurbitacin D, Cucurbitacin E, Cucurbitacin I | Displayed inhibitory effects on the growth of MCF-7 cells | ↓COX-2 | Jayaprakasam et al. (43) | ||
| 23,24-Dihydro-cucurbitacin F, 23,24-Dihydro-25-acetylcucurbitacin F | Showed antitumor activity against MCF-7 cells | 1.9–>10 μg/ml | Olmedo et al. (44) | ||
| 23,24-Dihydro-cucurbitacin B | Possessed antiproliferative activities against Bcap37 and MCF-7 cells | ⊥G2-M; ↑apoptosis; cyt. c release; PARP cleavage; caspase activation | Yang et al. (45) | ||
| Cucurbitacin B | Inhibited the proliferation of BT474, MCF-7, T47D, ZR-75-1, MDA-MB-231 and MDA-MB-453 cells | Disruption of microtubules and F-actin | 3.03 × 10−2–4.18 × 10−1 μM | Wakimoto et al. (46) | |
| Cucurbitacin B | Exhibited cytotoxicity in MCF-7, T47D, MDA-MB-435 and SKBR3 cells | 25.8–73.3 μg/ml | Kongtum et al. (47) | ||
| Balsaminapentanol, Balsaminol A, Balsaminol B, Cucurbalsaminol B | Suppressed the growth of MCF-7 cells | 30.7–55.3 μM | Ramalhete et al. (48) | ||
| Dammaranes | Cabraleadiol, Cabraleahydroxylactone, Cabralealactone, Eichlerialactone | Exhibited week cytotoxic effects in breast cancer cell line | 12.5–18.0 μg/ml | Phongmaykin et al. (49) | |
| Ergostanes | Methyl antcinate B, Zhankuic acid A, Zhankuic acid C | Inhibited the proliferation of MCF-7 and MDA-MB-231 cells | 25.1–57.8 μM | Yeh et al. (51) | |
| Friedelanes | Netzahualcoyonol Tigenone | Showed cytotoxic effect in MDA-MB-231 cells | 1.2–1.5 μM | Setzer et al. (52) | |
| Celastrol, Pristimerin | Exhibited cytotoxic effect in MCF-7 cells | 0.14–0.21 μg/ml | Chang et al. (53) | ||
| Pristimerin | Inhibited cell viability of MCF-7 and MDA-MB-231 cells | ↑Apoptosis; cyt. c release; caspase activation | 0.42 μM | Wu et al. (54) | |
| Celastrol | Inhibited the proliferation and migration of W256 cells | ↑Apoptosis; ↓IL-1β; ↓NF-κ B; IKKα/β; ↓MMP-9; ↓uPA | 0.43 μM | Idris et al. (55) | |
| Celastrol | Potentiated cytotoxic effect of TRAIL in T47D and MDA-MB-231 | ↑Apoptosis; ↓cFLIP; ↓cIAP-1; ↓Bcl-2; ↓Bcl-xL; ↓survivin; ↓XIAP; ↑Bax | Sung et al. (56) | ||
| Fridelin, Fridelin-1-3-dione | Suppressed the proliferation of MDA-MB-231 cells | 39.6–>40.0 μg/ml | Ee et al. (57) | ||
| Lanostanes | 15α-Acetyl-dehydrosulphurenic acid, Sulphurenic acid | Inhibited the proliferation of MCF-7 and MDA-MB-231 cells | 55.9– >500μM | Yeh et al. (51) | |
| Limonoids | Meliavolkenin | Showed cytotoxicity in MCF-7 cells | 4.3μg/ml | Zeng et al. (58) | |
| Melianin B, Melianin C, Meliavolkinin | Exhibited cytotoxicity in MCF-7 cells | 3.3–27.8 μg/ml | Rogers et al. (59) | ||
| Lupanes | Betulinic acid | Displayed cytotoxic activity in MDA-MB-231 cells | 9.6 μM | Setzer et al. (61) | |
| Betulinic acid | Displayed antiproliferative activity in MCF-7 cells | 0.27 μM | Amico et al. (62) | ||
| Betulinic acid | Suppressed the proliferation of T47D cells | ↓Bcl-2; ↓cyclin D1; ↑Bax | 2.4 μM | Rzeski et al. (63) | |
| Betulinic acid | Demonstrated lethal effects in SKBR3 cells | ↑Apoptosis; caspase activation | Basu et al. (64) | ||
| Betulinic acid | Exhibited cytotoxicity in SKBR3, MDA-MB-231, MDL13E, BT438, BT474, BT549 and T47D cells | 5.5–16.2 μg/ml | Kessler et al. (65) | ||
| Betulin | Inhibited the proliferation of T47D cells | 5.2 μM | Rzeski et al. (66) | ||
| Lupeol | Suppressed the proliferation of MDA-MB-231 cells | ↑ERα protein and mRNA | Lambertini et al. (67) | ||
| Oleananes | Remangilones A Remangilones C |
Elicited cytotoxic effects in MDA-MB-231 and MDA-MB-435 cells | ↑Apoptosis | 1.6–8.5μM | Deng et al. (68) |
| 3β,23,28-Trihydroxy-12-oleanene 23-caffeate, 3β,23,28-Trihydroxy-12-oleanene 3β-caffeate, | Exhibited cytotoxicity in MCF-7 cells | ↓Lipid peroxidation | 1.8–2.2 μg/ml | Yun et al. (69) | |
| Oleanolic acid | Displayed negligible cytotoxicity in MCF-7 and MDA-MB-231 cells | Hsu et al. (70) | |||
| CDDO | Inhibited the proliferation of MCF-7, MDA-MB-231, 21-MT-1, 21-MT-2, 21-NT and 21-PT cells | 3 × 10−2–1 μM | Suh et al. (71) | ||
| CDDO | Exerted the growth inhibition of MCF-7, MDA-MB-231 and MDA-MB-435 cells | ↑Apoptosis; ⊥G1-S; ⊥G2-M | Lapillonne et al. (74) | ||
| CDDO | Inhibited the growth of MCF-7 and MDA-MB-435 cells | ↓HER2 phospho-rylation; ↓HER2 kinase; ↓caveolin-1 | 3.5–7.5 μM | Konopleva et al. (75) | |
| CDDO, CDDO-Im | Displayed the growth suppression of MCF-7 cells | 10–30 nM | Place et al. (76) | ||
| CDDO, CDDO-Im | Sensitized T47D and MDA-MB-468 cells to TRAIL | ↑Apoptosis; ↑DR4; ↑DR5; ↓FLIPL | Hyer et al. (77) | ||
| CDDO, CDDO-Me | Suppressed the proliferation of MCF-7 cells | 0.06–0.16 μM | Honda et al. (78) | ||
| CDDO-Me | Suppressed the proliferation and invasion of 4T1 cells | ⊥G2-M; ↓STAT3; ↓Src; ↓Akt; ↓c-Myc | Ling et al. (79) | ||
| CDDO-Me | Inhibited IL-6-induced and constitutive JAK1 activity in MDA-MB-231 cells | ↓JAK1; ↓STAT3 | Ahmad et al. (78) | ||
| AMR | Displayed the growth inhibitory effect in MCF-7 cells | 2.5 μg/ml | Rabi et al. (84) | ||
| AMR | Exhibited cytotoxicity to MCF-7 and MCF-7/TH cells | ↑Apoptosis; ⊥G2-M; ↑total caspase; ↑caspase-8 | 3.8–6.8 μg/ml | Rabi et al. (85) | |
| AMR | Inhibited the survival of MCF-7 and MDA-MB-468 cells | ↑Apoptosis; ⊥G2-M; ↑caspase-3/7; ↑p53; ↑Bax; ↓Bcl2 | 1.8–7.0 μM | Rabi et al. (86) | |
| AMR-Me | Showed cytotoxic effects against MCF-7 cells | 1.8 μg/ml | Rabi et al. (84) | ||
| AMR-Me | Suppressed the proliferation of MCF-7 and MCF-7/p53siRNA cells | ↑Apoptosis; ⊥G2-M; ↑caspase-3/7; ↑Bax; ↓Bcl2; ↓cyclin A; ↓cyclin B1; ↑JNK; ↑MAPK | 0.5–0.6 μM | Rabi and Banerjee (87) | |
| Tirucallanes | Masticadienonic acid, Masticadienolic acid, 3-α-Hydroxy-masticadienolic acid, 24,25S-Dihydro-masticadienoic acid | Possessed cytotoxic effects against MCF-7 cells | 18.4–31.5 μg/ml | Chávez et al. (88) | |
| Ursanes | Ursolic acid | Exhibited cytostatic and cytotoxic effects in MCF-7 cells | ⊥G1 | Es-Saadyet al. (89) | |
| Ursolic acid, Promolic acid, 2-Oxopromolic acid, 3-O-acetyl promolic acid | Elicited cytotoxicity in MCF-7 cells | 13.7–184 μg/ml | Neto et al. (90) | ||
| Ursolic acid | Inhibited PMA-mediated induction of COX-2 and PGE2 in 184B4/HER cells | ↓PKC; ↓ERK; ↓JNK; ↓p38 MAPK; ↓AP-1 | Subbaramaiah et al. (91) | ||
| Ursolic acid, α-Amyrine | Displayed cytotoxicity in MCF-7 cells | 11.2–22.5 μM | Martín-Cordero et al. (92) | ||
| Ursolic acid | Displayed the growth inhibitory effects in MCaIV and BT-20 cells | ↓O2 consumption | 14–48 μM | Lee et al. (93) | |
| Ursolic acid | Elicited the antiproliferative activity in MCF-7 cells | 11.7 μM | He and Liu (94) | ||
| cis- and trans-3-O-p- Hydroxycinnamoyl ursolic acid | Suppressed the growth of MCF-7 cells | 18.8–100 μM | Murphy et al. (95) | ||
| Ursolic acid | Exhibited cytotoxicity against MCF-7 cells | ↑NO | 17.5 μg/ml | Chávez et al. (88) | |
| Ursolic acid | Showed cytotoxicity against MDA-MB-231 cells | 1.5 μg/ml | Chen et al. (96) | ||
| Ursolic acid, 2α-Hydroxyursolic acid, 3β-trans-p-Coumaroyloxy-2α-hydroxyolean-12-en-28-oic acid | Prevented the proliferation of MCF-7 cells | 4.7–20.9 μM | He and Liu (97) | ||
| 2α-Hydroxyursolic acid | Suppressed the proliferation of MCF-7 cells | ↓NF-κ B; ↓proteosomal activity | 37.1 μM | Yoon and Liu (98) | |
| Ursolic acid | Displayed cytotoxicity in MCF-7 cells | ↑Apoptosis; ↓Bcl-2; PARP cleavage; GR modulation | 53 μM | Kassi et al. (99) | |
| Ursolic acid | Exerted inhibitory effects on growth, migration and invasiveness of MDA-MB-231 cells | ↓MMP-2; ↓uPA; ↓VEGF; ↓NF-κ B; ↓c-Jun; ↓c-Fos; ↓JNK; ↓Akt; ↓mTOR | Yeh et al. (100) | ||
| Uncarinic acid C, Uncarinic acid D, Uncarinic acid E | Inhibited the proliferation of MCF-7 cells | 0.6–5.9 μg/ml | Lee et al. (101) | ||
| Asiatic acid | Exhibited growth inhibition in MCF-7 and MDA-MB-231 cells | ↑Apoptosis; ⊥S-G2/M; ↑p21/WAF1; ↓cyclin B1, ↓cyclin A, ↓Cdc2, ↓Cdc25C; ↓Bcl-2; ↑Bax; ↑ERK 1/2; ↑p38 | 5.9–8.2 μM | Hsu et al. (102) | |
| Miscellaneous | 9,19-Cycloart-23-ene-3β,25-diol, 9,19-Cycloart-25-ene-3β,24-diol | Displayed inactivating effects in MCF-7 cells | 7.5–15 μM | Smith-Kielland et al. (103) | |
| Bryonolic acid | Exhibited cytotoxicity in MCF-7, T47D, MDA-MB-435 and SKBR3 cells | 90.5–131.9 μg/ml | Kongtum et al. (47) | ||
| AECHL-1 | Suppressed the proliferation of MCF-7 and MDA-MB-231 cells | ↑Apoptosis; ⊥S-G2/M; ↓microtubules | Lavhale et al. (104) |
Abbrevaitions: AECHL-1, Ailanthus excelsa chloroform extract-1; AMR, amooranin; AMR-Me, methyl ester of amooranin; AP-1, activator protein-1; CDDO, 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid; CDDO-Im, 1-(2-cyano-3,12-dioxooleana-1,9-dien-28-oyl) imidazole; CDDO-Me, methyl ester of 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid; COX-2, cyclooxygenase-2; DR4, death receptor 4; DR5, death receptor 5; cyt. c, cytochrome c; GR, glucocorticoid receptor; IC50, inhibitory concentration 50%; ERα, estrogen receptor α; ERK, extracellular signal-regulated kinase; IKKα/β, Iκ B kinase α/β; IL-1β, interleukin 1β; IL-6, interlukin-6; JAK1, Janus-activated kinase 1; JNK, c-Jun N-terminal kinase; MAPK, mitogen-activated protein kinase; MMP-2, matrix metalloproteinase-2; MMP-9, matrix metalloproteinase-9; mTOR, mammalian target of rapamycin; NO, nitric oxide; PARP, polypeptide poly(ADP-ribose) polymerase; PGE2, prostaglandin E2; PKC, protein kinase C; PMA, phorbol 12-myristate; NF-κ B, nuclear factor-kappa B; STAT3, signal transducer and activator of transcription 3; TRAIL, tumor necrosis factor-related apoptosis-inducing ligand; uPA, urinary plasmogen activator; VEGF, vascular endothelial growth factor.
4.1.1. Cucurbitanes
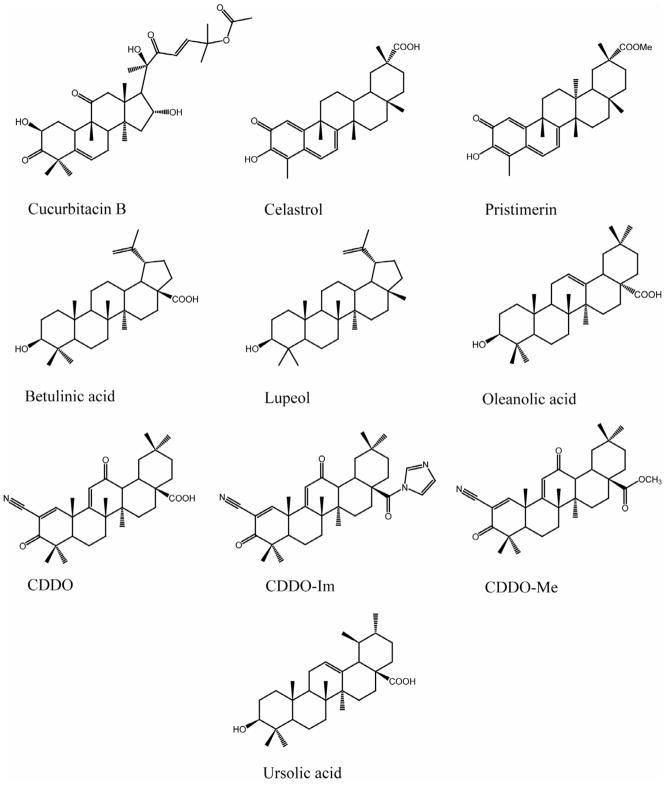
Cucurbitacins, originally isolated from several plants of the Cucurbitaceae family which possess medicinal properties, represent a group of diverse triterpenoids containing a cucurbitane skeleton (37). Cucurbitaceae plants have been used as folk medicine for centuries in India and China. Accumulating evidence indicates that constitutively activated, tyrosine-phosphorylated signal transducer and activators of transcription 3 (STAT3) plays a vital role in several human malignancies including breast cancer (38, 39). It has also been found that aberrant STAT3 activation is required for growth and survival of these neoplastic cells. In an attempt to discover disruptors of aberrant STAT3 signaling as novel anticancer agents, Blaskovich et al. (40) have identified cucurbitacin I from the National Cancer Institute Diversity Set of nearly 2,000 compounds. Cucurbitacin I has been found to inhibit the proliferation of MDA-MB-468 human breast cancer cells that express constitutively activated STAT3 (40). Cucurbitacin I also reduced the levels of phosphotyrosine of constitutively activated STAT3 and induced apoptosis in the same tumor model. The structure-activity relationship studies carried out by Sun and colleagues (41) with five cucurbitacin analogs, cucurbitacin A, B, E, I and Q, led to the discovery of cucurbitacin Q, which has also been shown to inhibit the STAT3. The same study has documented that cucurbitacin Q induces apoptosis more potently in MDA-MB-435 human breast cancer cells that contain constitutively activated STAT3 compared to MDA-MB-453 cells that do not. A new cucurbitacin D analog, 2-deoxycucurbitacin D as well as cucurbitacin D and 25-acetylcucurbitacin F have been isolated from the leaves of Sloanea zuliaensis. All these compounds have exhibited potent cytotoxic activity against MCF-7 human breast cancer cells (42). Bioassay-guided purification of the fruit extract of Cucurbita andreana yielded cucurbitacin B, D, E and I, which were evaluated for their inhibitory effects on the growth of MCF-7 cells. All these compounds displayed antitumor effects with specific cyclooxygenase-2 (COX-2) enzyme inhibition; however, cucurbitacin B (Figure 1) has been found to be the most potent compound (43). 23,24-Dihydrocucurbitacin F and 23,24-dihydro-25-acetylcucurbitacin F have been isolated from the ethanolic extract of Coutarea hexandra fruits. Both compounds have shown moderate cytotoxic activity against MCF-7 cells (44). Another cucurbitacin-derived compound, 23,24-dihyderocucurbitacin B, isolated from the roots of Trichosanthes kirilowli, possesses anticancer activity in a dose- and time-dependent manner against human breast cancer cell lines including Bcap37 and MCF-7. Mechanistic studies have shown that this triterpenoid exerts cell cycle arrest at G2/M phase and induces apoptosis in Bcap37 cells through mitochondria-dependent pathway (45). In another study (46), cucurbitacin B, the most abundant member, has been tested for antiproliferative effects against six human breast cancer cell lines that represent a diverse mix of breast cancer subtypes varying in the expression of ER, HER2/neu, and p53 mutation. The results indicate that MDA-MB-231 cells are the most sensitive to cucurbitacin B and no relationship has been observed between the sensitivity and ER, HER2/neu, and p53 mutation status of cells under investigation. Another interesting finding of this study is cucurbitacin B-induced rapid morphological changes within 20 min of exposure which was associated with disruption of microtubules and F-actin as observed by confocal microscopy. Thus, cucurbitacin B may represent the third member of antimicrotubule drugs (after taxol and vincristine) with antitumor activity in breast cancer. Cucurbitacin B, isolated from the fruit juice of Trichosanthes curcumerina, has been tested for cytotoxicity against four breast cancer cell lines, namely SKBR3, MCF-7, T47D and MDA-MB-435. Though cucurbitacin B exhibited antiproliferative effects against all the cell lines tested, MDA-MB-435 was found to be the most sensitive (47). Phytochemical investigation of the aerial parts of Momordica balsamina, commonly known as African pumpkin, has led to the isolation of several new cucurbitane-type triterpenoids, including balsaminapentanol, balsaminol A, balsaminol B and cucurbalsaminol B. All these compounds have suppressed the growth of MCF-7 cells with varying potency (48).
Figure 1.
Chemical structures of selected natural and semi-synthetic triterpenoids studied to explore the breast cancer chemopreventive and therapeutic potential.
4.1.2. Dammaranes
Six dammarane triterpenoids have been isolated from the leaves and wood of the tropical plant Chisocheton penduliflorus and four of them namely, cabraleadiol, carbraleahydroxylactone, carbralealactone and eichlerialactone possess weak anti-breast cancer activity in vitro (49).
4.1.3. Ergostanes
Antrodia camphorata, a parasistic fungus, has been widely used in China for treatment of various ailments including cancer (50). Three ergostane-type triterpenoids (methyl antcinate B, zhankuic acid A and zhankuic acid C), isolated from the fruiting bodies of A. camphorate, displayed potent cytotoxic effects against MCF-7 as well as MDA-MB-231 cells (51).
4.1.4. Friedelanes
Several members of the genus Salacia find use as traditional herbal medicine (33). Bioactivity-directed isolation, purification and elucidation of biological activities of the active components from the bark extract of Salacia petenensis led to the discovery of tingenone and netzahualcoyonol, which exhibited cytotoxicity against MDA-MB-231 cells (52). Chang et al. (53) have found that two major triterpenoids, celastrol and its methyl ester pristimerin, are significantly active against the proliferation of MCF-7 cell lines. These compounds have been derived from the plant Reissantia buchananii. Subsequent studies by the same group have shown that pristimerin inhibited the viability of MCF-7 and MDA-MB-231 cells. Mechanistic studies using MDA-MB-231 cell culture model have revealed that pristimerin induces rapid apoptosis characterized by caspase activation, decrease of mitochondrial membrane potential and a rapid release of cytochrome c. Pristimerin, however, has no effect on the protein expression of Bcl-2 family members and also on the generation of reactive oxygen species (54). Recently, celastrol has been shown to inhibit the growth and induce apoptosis of W256 cells as evidenced by caspase-3 activation and nuclear morphology. Celastrol also abolished interlukin-1β- and tumor necrosis factor-α (TNF-α)-induced IκB phosphorylation, IκB kinase activation and prevented nuclear translocation of nuclear factor-kappa B (NF-κB) and DNA binding, reduced the expression of matrix metalloproteinase-9 (MMP-9) and urinary plasminogen activator (uPA) leading to inhibition of migration of W256 cells (55). Very recently, celastrol has been reported to potentiate tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced cytotoxicity and apoptosis of T47D and MDA-MB-231 cells. This triterpenoid also downregulated the expression of cell survival proteins, namely cFILP, IAP-1, Bcl-2, Bcl-xL, survivin and XIAP and upregulated Bax expression. The cell surface expression of both the TRAIL receptors–death receptor 4 (DR4) and DR5–have also been found to be induced by celastrol treatment (56). Ee et al. (57) have isolated several phytochemicals, including two triterpenoids namely friedelin and friedelan-1,3-dione, from the stem bark extract of Mesua daphnifolia. Both compounds have exhibited growth inhibitory activities against MBA-MD-231 cells.
4.1.5. Lanostanes
Yeh and coworkers (51) have recently isolated five lanostane-type triterpenes from the fruits of A. camphorate and investigated for their potential as anticancer agents against MCF-7 and MDA-MB-231 breast cancer models. Two compounds, namely sulphurenic acid and its analog 15α-acetyl-dehydrosulphurenic acid have also shown antitumor efficacy with both compounds being more potent against MDA-MB-231 cells as compared to MCF-7 cells.
4.1.6. Limonoids
pMeliavolkenin, a new limonoid triterpene, has been isolated from the root bark of Melia volkensii and found to be cytotoxic against MCF-7 cells (58). The same plant has been used to isolate other limonoids, namely melianin B, melianin C and meliavolkinin–all of which are active in killing MCF-7 cells (59).
4.1.7. Lupanes
Betulinic acid, a pentacyclic triterpene found in many plant species especially the white-barked birch tree such as Betula alba, has been discovered through a drug screening program of the National Cancer Institute and is known to possess diverse pharmacological properties (60). Subsequently, betulinic acid has been isolated from the bark extract of Syncarpia glomulifera and almond hulls of Prunus delcis and has shown antiproliferative activities toward MCF-7 as well as MDA-MB-231 cells (61, 62). Betulinic acid also exhibited a remarkable cytotoxic effect against T47D cells with a simultaneous induction of apoptosis, decrease of Bcl-2 and cyclin D1 and increase of Bax gene expression (63). Basu and colleagues (64) have demonstrated lethal effects of betulinic acid in another breast cancer cell line–SKBR3. Additionally, betulinic acid has been shown to induce early apoptotic events accompanied by flopping of phosphatidylserine on the outer lamella of the plasma membrane as evidenced by elegant fluorescent microscopy. The mitochondrial signaling pathway has been implicated in the observed apoptosis-inducing property of betulinic acid. Using nine breast cancer cell lines, Kessler et al. (65) have confirmed the antitumor effects of betulinic acid against mammary carcinoma with various degree of sensitivity. Unlike betulinic acid, betulin, a pentacyclic triterpene present in white birch bark, has not been subjected to extensive studies for its anticancer efficacy in breast malignancy. In a recent study, betulin has been found to inhibit the proliferation of T47D breast carcinoma cells (66). Lupeol, another natural pentacyclic triterpenoid present in many fruits and vegetables such as mango, olive and fig, possesses a wide variety of biological activities including antiinflammatory and antioxidant properties (27). Gas chromatographic/mass sprectromeric analysis has enabled researchers to identify lupeol as the major bioactive component of Aegle marmelos plant extract. Lupeol has caused significant inhibition of growth of MDA-MB-231 cells in a dose-responsive manner. Interestingly, it has also been able to induce ERα expression in this ERα-negative breast cancer cell line (67).
4.1.8. Oleananes
In 1999, three new 24,28-dinorolean-3-one derivatives, namely remangilones A, B and C, were isolated from the dried leaves of Physena madagascariensis. Remangilones A and C have been found to be cytotoxic against MBA-MD-231 and MDA-MB-435 cells (68). In the same year, two new triterpene caffeates (3β,23,28-trihydroxy-12-oleanene 23-caffeate and 3β,23,28-trihydroxy-12-oleanene 3β-caffeate) have been derived from the root bark of Hibiscus syriacus. Both compounds have exhibited antiproliferative response against MCF-7 cells possibly due to antioxidative properties (69). Oleanolic acid, isolated from Glossogyne tenuifolia, has been reported to possess negligible antioxidant activity and also displayed weak cytotoxicity against MCF-7 and MDA-MB-231 cells (70). Since the biological activities of naturally occurring oleanolic acid are relatively weak, efforts have been undertaken to synthesize new analogs with the objective of enhancing their potency. Suh et al. (71) has been successful in developing one such synthetic oleanane triterpenoid, 2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid (CDDO), which has been found to be 100–500 fold more potent than any previous triterpenoid in suppressing inducible nitric oxide synthase and COX-2–two inflammatory enzymes having important roles in the development of malignancy (72, 73). Consequently, CDDO inhibits the proliferation of an array of premalignant and malignant cells including ER-positive and ER-negative breast cancer cells (71). In another study, CDDO (at 1 μM) completely abrogated ER-negative, p53-mutated and HER2-expressing breast cancer cells. In all of these in vitro mammary cancer models, CDDO transactivated peroxisome prolifeartor-activated receptor γ, induced cell cycle arrest in G1-S and G2-M as well as apoptosis. It has been confirmed that CDDO regulated the expression of cyclin D1, p21Waf1/CIP1, and Bcl-2 in the aforementioned tumor models (74). The effect of HER2 overexpression on the sensitivity of breast cancer cells to the growth-inhibitory properties of CDDO has been investigated. While both tumor cell growth and colony formation were preferentially suppressed in HER2-overexpressing cell lines at low concentrations, the growth-inhibitory effects at high concentrations did not correlate with the expression level of HER2. CDDO also inhibited phosphorylation of HER2 in HER2-overexpressing cells, diminished HER2 kinase activity and induced breast tumor suppressor gene caveolin-1 (75). In order to increase the potency of CDDO, various C-28 derivatives have been synthesized. One such synthetic triterpenoid, 1[2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oyl]imidazole (CDDO-Im), has been found to be more potent than CDDO in inhibiting the proliferation of MCF-7 cells as measured by thymidine incorporation (76). In a separate study, CDDO and CDDO-Im sensitized TRAIL-resistant T47D and MDA-MB-468 breast cancer cells to TRAIL-mediated apoptosis. Sensitization of these tumor cells to TRAIL by both compounds have been associated with upregulation of cell surface DR4 and DR5 and downregulation of the antiapoptotic protein FLIPL (77). Another synthetic triterpenoid, C-28 methyl ester of CDDO (CDDO-Me), inhibited the proliferation of MCF-7 cells (78). To investigate the effect of CDDO-Me on constitutively activated STAT3 signaling, Ling and coworkers (79) have utilized chemotherapy-resistant murine 4T1 breast cancer cells. These investigators have found that CDDO-Me diminished the invasive growth of 4T1 cells with a parallel accumulation of cells in G2-M phase, inactivation of STAT3, Src and Akt as well as inhibition of c-Myc. CDDO-Me has also been shown to block the janus-activated kinase-1 (JAK1)→STAT3 pathway by directly inhibiting both JAK1 and STAT3 leading to suppression of growth and survival genes (80, 81).
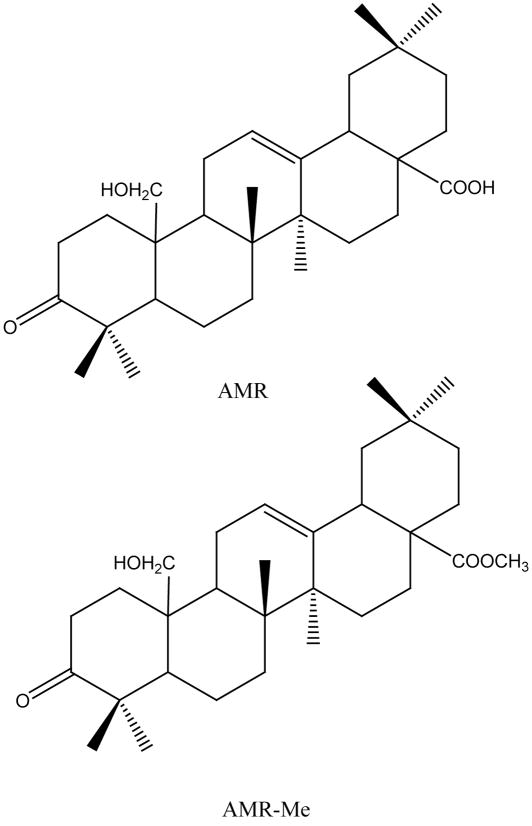
Amoora rohituka, a tropical tree that grows wild in India, has been traditionally used for the treatment of human malignancies (82, 83). Amooranin (AMR), a triterpene acid (25-hydroxy-3-oxoolean-12-en-28-oic acid) with a novel structure (Figure 2) isolated from the stem bark of A. rohituka, has been shown to inhibit the growth of MCF-7 cells (84). Subsequent studies by Rabi and coworkers (85, 86) have investigated underlying mechanisms of action of AMR using breast cancer cell lines of diverse origin. In one study, AMR has been found to induce oligonucleosome-sized DNA ladder formation (characteristic of apoptosis) in breast carcinoma cells accompanied by the elevation of total caspase and caspase-8 activities (85). In another study, AMR has displayed a strong inhibitory effect on survival of MCF-7 and MDA-MB-468 breast carcinoma cells compared to MCF-10A normal breast epithelial cells. Ancillary studies suggest that AMR induces apoptosis in human breast carcinoma cells via caspase activation pathway which could be independent of p53 involvement (86). Because the antineoplastic activity of the plant-derived compound AMR is relatively weak, new analogues of this molecule have been prepared by chemical transformations in an attempt to identify more potent agents. One of these analogues, methyl ester of AMR (AMR-Me, Figure 2), has been found to inhibit proliferation of MCF-7 cancer cells with greater potency than the parent compound AMR (84). Additional studies have confirmed the astonishing potency of AMR-Me (at nanomolar range) against MCF-7 breast cancer cells. Killing of MCF-7 cells proceeded more effectively (IC50 = 0.5 μM) than killing of normal breast epithelial cells, which required a 25-fold increase in the concentration of AMR-Me (IC50 = 12.5 μM). Moreover, AMR-Me has been shown to induce apoptosis in MCF-7 cells through a mitochondrial apoptotic pathway associated with DNA fragmentation and poly(ADP-ribose) polymerase (PARP) degradation. Such degradation is preceded by altering the Bax:Bcl-2 ratios, cytochrome c release, and subsequent induction of caspases. AMR-Me also stimulated two different mitogen-activated protein kinase (MAPK) signaling pathways of p38 MAPK and c-jun N-terminal kinase (JNK) for amplifying the apoptosis cascade (87).
Figure 2.
Chemical structures of novel oleanane triterpenoids: AMR, amooranin (25-hydroxy-3-oxoolean-12-en-28-oic acid); AMR-Me, methyl ester of amooranin (methyl-25-hydroxy-3-oxoolean-12-en-28-oate).
4.1.9. Tirucallanes
Several tirucallane triterpenoids have been extracted from the bark of Amphipterygium adstringens and all these agents exhibited cytotoxicity against MCF-7 cells with varying sensitivity (88).
4.1.10. Ursanes
The first study on the anti-mammary cancer effects of ursolic acid was published by Es-Saady and coworkers (89) who obtained this agent from several sources including Calluna vulgaris. According to this study, ursolic acid acted as a potent inhibitor of MCF-7 cell proliferation, exerting an early cytostatic response at G1 phase of cell cycle followed by cell death. Neto et al. (90) have isolated ursolic acid, promolic acid and related compounds from the bark and stem extract of Polylepis racemosa and all these natural products elicited cytotoxicity in MCF-7 cells. Ursolic acid suppressed phorbol 12-myristate 13-acetate-mediated induction of COX-2, prostaglandin E2, protein kinase C, extracellular signal-regulated kinase 1/2, JNK, p38 MAPK and activator protein in 184B4/HER cells (91). Ursolic acid and α-amyrine, both isolated from the aerial parts of Erica andevalensis, have been reported to display tumor cell killing effects in MCF-7 tumor model (92). Ursolic acid has been shown to exhibit tumoricidal effects with induction of apoptosis and inhibition of oxygen consumption in MCaIV murine mammary adenocarcinoma and BT-20 human breast carcinoma cells (93). Bioactivity-guided fractionation of the cranberry fruit (Vaccinium macrocarpon) has led to identification of ursolic acid and novel ursolic acid esters with antitumor activities against MCF-7 cells (94, 95). Ursolic acid has also been isolated from A. adstringens and Hydrotis biflora and found to inhibit the growth of various human breast cancer cells (88, 96). He and Liu (97) have isolated ursolic acid along with its natural analogs from the apple (Malus pumila) peel that show antiproliferative activities against MCF-7 cells. In another study (98), 2α-hydroxyursolic acid was isolated from apple peel and its antitumor mechanisms were examined using MCF-7 cells. The results have shown that this natural compound inhibited tumor cell proliferation, suppressed TNF-α-induced NF-κB activation and diminished proteasomal activity (a critical step in the NF-κB activation pathway). These studies suggest that apple peel should not be considered as waste since it contains important triterpenoids with the potential to prevent and treat breast cancer. Ursolic acid has been found to induce apoptosis, PARP cleavage and also decrease in Bcl-2 protein in MCF-7 cells, thus supporting the hypothesis that the compound induces apoptosis through the intrinsic mitochondrial pathway. Additionally, ursolic acid has been shown to bind to glucocorticoid receptor (GR) and translocate GR into nucleus suggesting its potential as an anticancer agent in breast cancer through GR modulation (99). Very recently, it has been observed that ursolic acid exerted dose- and time-dependent suppressive effects on the migration and invasion of highly metastatic MDA-MB-231 cells at non-cytotoxic concentrations. This effect has been associated with inhibition of JNK, Akt, and mammalian target of rapamycin phosphorylation and reduction of NF-κB p65 in the nucleus leading to downmodulation of MMP-2 and uPA expression (100). All these results underscore the potential of its potential both as a preventive and therapeutic agent for metastatic breast cancer. Phytochemical investigation of the hooks of Uncaria rhynchophylla resulted in the isolation of three uncarinic acids that inhibited the proliferation of MCF-7 cells (101). Another triterpenoid, asiatic acid, has been reported to exhibit breast cancer cell growth inhibition by inducing cancer cells to undergo S-G2/M phase arrest and apoptosis. The cell cycle arrest has been associated with the elevation of p21/WAF1 and reduction in cyclinB1, cyclinA, Cdc2, and Cdc25C in a p53-dependent manner. Additional studies have indicated that asiatic acid triggered the mitochondrial apoptotic pathway as evidenced by a shift in Bax/Bcl-2 ratio, caspase-9 and caspase-9 activation and cytochrome c release (102).
4.1.11. Miscellaneous
Two stereoisomeric cyclic triterpenoids have been isolated from the leaves of Euphorbia pulcherrima and investigated for anticancer efficacy in MCF-7 cells with positive results (103). The root extract of T. cucumerina has been found to contain bryonolic acid which shows antitumor activities against four different breast cancer cell lines, namely MCF-7, T47D, MDA-MB-435 and SKBR3 with a maximum antitumor effect on MDA-MB-435 cells (47). Ailanthus excelsa chloroform extract-1 (AECHL-1), a new triterpenoid isolated from the root bark of A. excelsa (Tree of Heaven), has been evaluated for anti-cancer activity using breast cancer cells of different lineage. AECHL-1-mediated growth suppression has been linked to S-G2/M arrest, apoptosis induction and microtubule disruption (104).
4.2. In vivo studies
As a large number of in vitro studies (discussed above) have successfully shown that triterpenoids possess potent cytotoxic effects against several breast cancer cell lines, in vivo studies by different laboratories have investigated whether some of these triterpenoids and their semi-synthetic analogs lead to positive outcomes in preclinical animal models of breast cancer (Table 2). Most of these studies have utilized tumor growth in immunocompromised mouse model whereas a few investigators have used the chemically-induced mammary tumor development protocol.
Table 2.
In vivo effects of natural and semi-synthetic triterpenoids on development and growth of breast cancer
| Triterpenoids | Compounds | Effects | Mechanisms | Dose/duration | Route | References |
|---|---|---|---|---|---|---|
| Cucurbitanes | Cucurbitacin I | Suppressed the growth of implanted MDA-MB-468 cells in nude mice | 1 mg/kg/day; Up to 60 days | i.p. | Blaskovich et al. (40) | |
| Cucurbitacin B | Reduced tumor growth in nude mice implanted with the MDA-MB-231 cells | ↓Blood lymphocyte; ↓platelet; ↑uric acid; ↓SGPT; ↓glucose | 1 mg/kg/day; three times a week, 6 weeks | i.p. | Wakimoto et al. (46) | |
| Friedelanes | Celastrol | Inhibited osteolytic bone metastasis in Wistar rats injected with W256 cells | ↓Tumor size and number, ↓bone loss | 1 mg/kg/day; 10 days | i.p. | Idris et al. (55) |
| Oleananes | CDDO | Reduced tumor growth in nude mice inoculated with MDA-MB-435 cells | 40 mg/kg/day; twice a week, 3 weeks | i.v. | Lapillonne et al. (74) | |
| CDDO | Arrested the rate of tumor growth and tumor size in nude mice transplanted with MCF/Neo or MCF7/HER2 cells | ↓Proliferation; ↓cyclin D1; ↑apoptosis; | 20 mg/kg/day; three times a week, 3 weeks | i.v. | Konopleva et al. (75) | |
| CDDO-Im | In combination with TRAIL inhibited the growth of MDA-MB-468 tumor cells in BALB/c nu/nu mice | ↓Proliferation | 5 mg/kg/day; 14 days | i.p. | Hyer et al. (77) | |
| CDDO-Me | Inhibited growth and metastases of 4T1 breast cancer cells transplanted in BALB/c mice | ↑Mature spleen dendritic cells | 200 μg/mouse; 5 times at 2-day intervals | i.v. | Ling et al. (79) | |
| CDDO-Me | Alone or in combination with rexinoid delayed the development or reduced the volume of mammary tumors in MMTV-neu mice | ↓Proliferation; ↑apoptosis; | 60–100 mg/kg diet; 4–45 weeks | diet | Liby et al. (107) | |
| AMR | Suppressed the growth of implanted adenocarcinoma induced by NMU in rats | 10, 20 mg/kg/day; 3 weeks | i.p. | Rabi (108) | ||
| AMR-Me | Did not exhibit antineoplastic activity against Ehrlich ascites tumor in Swiss mice | 50, 100 mg/kg/day; 7 days | i.p. | Rabi et al. (84) | ||
| Ursanes | Ursolic acid | Failed to influence DMBA-induced mammary tumorigenesis in Sprague-Dawley rats | 200 mg/kg/day; 5 days | i.p. | Singletary et al. (109) | |
| Ursolic acid | Did not suppress the tumor growth in C3H mice transplanted with MCaIV cells | 100 mg/kg; once | i.p. | Lee et al. (93) | ||
| Miscellaneous | AECHL-1 | Regressed tumor volume in nude mice xenografted with MCF-7 cells | 5 or 10 μg/day; 10 days | s.c. | Lavhale et al. (104) |
Abbrevaitions: AECHL-1, Ailanthus excelsa chloroform extract-1; AMR, amooranin; AMR-Me, methyl ester of amooranin; CDDO, 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid; CDDO-Im, 1-(2-cyano-3,12-dioxooleana-1,9-dien-28-oyl) imidazole; CDDO-Me, methyl ester of 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid; DMBA, 7,12-dimethylbenz(a)anthracene; i.p., intraperitoneal; i.v., intravenous; NMU, N-nitrosomethyl urea; s.c., subcutaneous; SGPT, serum glutamic pyruvic transaminase; TRAIL, tumor necrosis factor-related apoptosis-inducing ligand.
4.2.1. Cucurbitanes
In order to evaluate the efficacy of cucurbitacin I in inhibiting breast tumor growth, nude mice have been injected subcutaneously (s.c.) MDA-MB-468 human breast carcinoma cells and subsequently dosed with cucurbitacin I (1 mg/kg/day) when tumor volume reached around 150 mm3. This treatment regimen has been found to significantly inhibit tumor growth by 76% with a prolongation of survival time compared to control animals (40). Wakimoto et al. (46) have examined the antitumor activity of cucurbitacin B against MDA-MB-231 human breast cancer cells orthotopically grown in the mammary glands of female nude mice. Intraperitoneal (i.p.) administration of cucurbitacin B that was initiated one day following tumor cell implantation and continued for 6 weeks significantly reduced the tumor volume. Additionally, cucurbitacin B-treated animals exhibited lack of ascitic fluid accumulation, elevated platelet counts, increased serum uric acid and reduced serum glutamic pyruvic transaminase and glucose levels compared to control mice.
4.2.2. Friedelanes
Administration of celastrol has been found to suppress trabecular bone loss and lessened the number and size of osteolytic bone lesions following intracardiac injection of W256 mammary carcinosarcoma tumor cells in male Wistar rats (55). Accompanying histomorphometric analysis has revealed that celastrol decreased osteoclast number and inhibited bone resorption indicating its potential in the prevention and treatment of bone metastases associated with breast cancer.
4.2.3. Oleananes
Lapillonne and coworkers (74) have investigated the effects of CDDO on the growth of breast tumors in female nude immunodeficient mice inoculated (s.c.) with MDA-MB-435 cells. Following the establishment of tumors (10 days after tumor cell inoculation), mice were injected intravenously with CDDO for three weeks. The results demonstrated a significant reduction in tumor growth in CDDO-treated animals compared to their control counterparts. Konopleva et al. (75) have confirmed the antitumor activity of CDDO using a xenograft model of breast cancer. According to this study, parenteral administration of liposomally encapsulated CDDO abrogated the rate of tumor growth and the percentage of change in the mean tumor size in female nude mice injected with highly tumorigenic MCF-7/HER2 cells. Mechanistic studies showed that CDDO significantly decreased HER2 phosphorylation and nuclear cyclin D1 expression in tumors as well as induced tumor cell apoptosis and thereby supporting in vitro results of the same group (75). These findings suggest that CDDO could be beneficial for therapy of patients with HER2-overexpressing breast cancer, an aggressive form of disease characterized by significantly reduced survival times (105). CDDO-Im, an analog of CDDO, has also been investigated for in vivo efficacy against a xenograft model of mammary cancer. Although no inhibition of MDA-MB-468 xenograft growth has been observed in female BALB/C nu/nu mice treated with CDDO-Im, a combination of CDDO-Im and TRAIL has been shown to be effective in reducing the tumor burden with simultaneous inhibition of tumor cell proliferation (77). Accompanying toxicity studies suggest that the combination is well tolerated by the tumor-bearing animals. This study also underscores an apparent synergy of CDDO-Im and TRAIL in achieving tumor growth suppression in this breast cancer model. In another study conducted by Ling et al. (79), CDDO-Me completely eliminated breast cancer growth and lung metastases induced by 4T1 mouse breast cancer cells when the treatment started one day following tumor implantation, and significantly arrested tumor growth when initiated five days post tumor cell inoculation. Additional experiments have indicated that CDDO-Me maintained the mature spleen dendritic cell population which possibly contributed to the potent antitumor and antimetastatic effect of this promising agent (79). Unlike the incidence of ER-positive breast cancer, the incidence of ER-negative breast cancer remains virtually unaltered during the last 30 years (106) and novel preventive and therapeutic approaches are urgently needed for this type of breast cancer. According to a recent study executed by Liby et al. (107), dietary CDDO-Me (60 mg/kg diet) significantly delayed the development of ER-negative mammary tumors in female MMTV-neu mice. Interestingly, a combination of CDDO-Me (60 mg/kg diet) and the rexinoid LG100268 (20 mg/kg diet) has been found to be more potent than the individual agents for the prevention of mammary tumorigenesis. Dietary CDDO-Me (100 mg/kg diet) has also been effective in regressing established tumors or arresting the growth of tumors in this model. The combination of CDDO-Me (100 mg/kg diet) and LG100268 (60 mg/kg diet) has been found to be more effective in reducing the tumor volume than CDDO-Me alone. A combined treatment has also been shown to suppress cell proliferation and induce apoptosis in tumors as evidenced by immunohistochemical analyses.
The earliest in vivo antitumor activity of natural oleanane-type triterpenoid AMR involved its inhibitory effects on N-nitrosomethyl urea (NMU)-induced mammary adenocarcinomas in female Sprague-Dawley rats as reported by Rabi (108). In this study, NMU-induced primary breast carcinomas were transplanted (s.c.) beneath the lower thoracic mammary fat pads of the animals. Fifty days following tumor transplantation (tumor volume 50–150 mm3), the animals were treated with AMR (i.p.) once every day for three weeks. It has been observed that AMR at a dose of 10 or 20 mg/kg/day prolonged the mean survival time of tumor-bearing animals and significantly reduced tumor size. When tested in the Ehrlich ascites tumor model in Swiss mice, parenteral administration (i.p.) of AMR-Me at 50 or 100 mg/kg/day for seven days was found to be inactive. As AMR-Me has exhibited substantial cytotoxic effects against human breast adenocarcinoma cells (84, 87), this compound should be tested against other in vivo models of experimental mammary carcinomas. Accordingly, we have initiated a series of experiments to evaluate mechanism-based chemopreventive and therapeutic effects of AMR-Me in a well established, chemically-induced animal model of breast cancer which closely mimics the human disease.
4.2.4. Ursanes
Another triterpenoid, ursolic acid, has been investigated for its potential as chemopreventive as well as therapeutic agent against breast cancer. Administration of ursolic acid prior to 7,12-dimethylbenz[a]anthracene (DMBA) initiation had no effect on rat mammary DMBA-DNA adduct formation as well as DMBA-induced mammary tumors and adenocarcinomas in female Sprague-Dawley rats (109). Similarly, an i.p. administration of ursolic acid did not inhibit the growth of existing tumor established in female C3H mice by subcutaneously injecting BT-20 human breast carcinoma cells (93). These results are in contrast of significant cytotoxic effects of ursolic acid against various breast cancer cell lines and necessitate the need for additional in vivo studies to evaluate the potential of ursolic acid in breast cancer.
4.2.5. Miscellaneous
AECHL-1, a triterpenoid with antitumor effects against various breast cancer cells, has also been tested for its in vivo efficacy. In this experiment, xenografted tumor has been developed by (s.c.) injecting MCF-7 cells into female athymic nude mice. Injection of AECHL-1 to the site of tumor for once a day for 10 days exhibited a significant antitumor effect leading to regression of established tumors. Histological analysis of tumor tissues from the triterpenoid-treated animals showed a decrease in neoplastic cell density, loss of neovascularization (indication of inhibition of angiogenesis) and absence of hemorrhagic areas (104). The positive results of this study underscore the potential of AECHL-1 as a novel therapeutic agent for human breast cancer.
5. CONCLUSION AND FUTURE DIRECTIONS
Even though mortality due to breast cancer is decreasing, it is still the second most common cause of death in women. Using naturally occurring compounds, including those derived from fruits, vegetables, herbs and spices, as potential breast cancer preventive and/or therapeutic agents has become a fascinating strategy. Among these plant-based agents, triterpenoids have emerged as a promising group of phytochemicals that selectively kill breast cancer cells with a pleiotropic mode of action while sparing normal cells. From numerous studies using cell culture assays and animal models of cancer as described in this review, it is clear that triterpenoids hold great potential not only in the therapy of a wide variety of breast cancers but also in preventing these diseases. The chemopreventive potential of triterpenoids would have broader implications if we consider that these secondary plant metabolites represent the largest group of naturally occurring phytochemicals and that are also present in common foods like apples and olives. Triterpenoids exert a plethora of biological activities including suppression of inflammation, reduction of oxidative stress, regulation of cell cycle, inhibition of cell proliferation, induction of apoptosis, and interaction with tumor microenvironment through modulation of multiple signal transduction pathways. In essence, this could explain, at least in part, their antineoplastic properties in breast cancer. It is well known that tumor cells use multiple survival pathways to prevail over their normal counterparts. According to a recent landmark study, mutations in nearly 200 genes have been detected in human breast and colon cancers showing the genetic complexity of these specific neoplastic diseases (110). With this background, agents like triterpenoids that can alter multiple dysregulated cellular pathways may have a significant potential for breast cancer prevention and control.
From the numerous studies presented here, it is apparent that triterpenoids, belonging to various groups, are unique as they have characteristic mechanisms of action. However, in most of the studies, individual compounds have been tested against in vitro or in vivo breast cancer models. It is possible that there exists a better preventive or therapeutic potential in the synergistic action of these triterpenoids. Further studies are needed to explore the full potential of these multifunctional agents in combinational settings.
Accumulating evidence suggests that even in the event of lack of efficacy for a single agent at low concentrations, combinations of two or more compounds could be much more effective. Combinations of several chemotherapeutic drugs also offer the possibility of lowering their doses and consequently reducing unwanted adverse effects. Considering these advantages, triterpenoids may be used in combination with other chemotherapeutic drugs and radiation therapy to enhance their therapeutic efficacy while limiting chemo- and radio-therapy-associated unwanted side effects. However, more studies are required to validate these premises.
Scanning of pertinent literature reveals that although there are a large number of in vitro studies demonstrating the cytotoxicity of triterpenoids against various breast cancer cells, only a few compounds have yet been evaluated in preclinical animal models of breast cancer. One of the reasons for the limited number of preclinical breast cancer studies on triterpenoids, including lack of in vivo studies on agents which have already showed efficacy in cell culture systems, could be due to the fact that most of the triterpenoids are insoluble in aqueous media limiting their bioavailability in the body which is very important for in vivo efficacy. One approach to enhance the water solubility of triterpenoids could be the structural modification of naturally occurring compounds to generate more polar analogs. Several other possibilities of improving the hydrophilicity of triterpenoids include design and generation of formulations containing cyclodextrin complexes, liposomes, colloids, micelles as well as nanoparticles (111–113).
As presented in this review, an impressive body of work has unraveled many unique biological properties of triterpenoids with an objective of evaluating its clinical potential in the prevention and intervention of breast cancer. Nevertheless, a considerable amount of work needs to be done. This includes identification of novel target proteins and pathways in which they function for further drug interventions, and development of selective end-point and surrogate biomarkers for evaluating positive clinical outcome. Long-term epidemiological studies and well-designed clinical trials are also warranted. In summary, the in vitro and in vivo data examined in this review strongly suggest that triterpenoids are promising candidates in the chemopreventive and chemotherapeutic strategies to lower the burden of human breast cancer.
Acknowledgments
The research on triterpenoid and breast cancer chemoprevention at the corresponding author’s laboratory is supported by the award R03CA13614 from the National Cancer Institute. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. The authors thank Altaf S. Darvesh, Ph.D., for carefully editing the manuscript and providing valuable comments and Werner J. Geldenhuys, Ph.D., for assistance with the chemical structures.
Footnotes
Publisher's Disclaimer: This is an, un-copyedited, author manuscript that has been accepted for publication in the Frontiers in Bioscience”. Cite this article as appearing in the Journal of Frontiers in Bioscience. Full citation can be found by searching the Frontiers in Bioscience (http://bioscience.org/search/authors/htm/search.htm) following publication and at PubMed (http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?CMD=search&DB=pubmed) following indexing. This article may not be duplicated or reproduced, other than for personal use or within the rule of “Fair Use of Copyrighted Materials” (section 107, Title 17, U.S. Code) without permission of the copyright holder, the Frontiers in Bioscience. From the time of acceptance following peer review, the full final copy edited article of this manuscript will be made available at http://www.bioscience.org/. The Frontiers in Bioscience disclaims any responsibility or liability for errors or omissions in this version of the un-copyedited manuscript or in any version derived from it by the National Institutes of Health or other parties.
References
- 1.Baselga J, Mendelsohn J. The epidermal growth factor receptor as a target for therapy in breast carcinoma. Breast Cancer Res Treat. 1994;29:127–138. doi: 10.1007/BF00666188. [DOI] [PubMed] [Google Scholar]
- 2.Jordan VC. SERMs: meeting the promise of multifunctional medicines. J Natl Cancer Inst. 2007;99:350–356. doi: 10.1093/jnci/djk062. [DOI] [PubMed] [Google Scholar]
- 3.American Cancer Society. Breast Cancer Facts and Figures 2009–2010. Atlanta: American Cancer Society; 2009. [Google Scholar]
- 4.Stang A, Thomssen C. Decline in breast cancer incidence in the United States: what about male breast cancer? Breast Cancer Res Treat. 2008;112:595–596. doi: 10.1007/s10549-007-9882-3. [DOI] [PubMed] [Google Scholar]
- 5.Vogel VG. Chemoprevention strategies. Curr Treat Options Oncol. 2007;8:74–88. doi: 10.1007/s11864-007-0019-z. [DOI] [PubMed] [Google Scholar]
- 6.Sutherland S, Sutherland A, Miles D, Chan S, Wardley A, Davidson N, Bhatti R, Shehata M, Nouras H, Camburn T, Johnston SRD. Treatment of HER2-positive metastatic breast cancer with lapatinib and capecitabine in the lapatinib expanded access programme, including efficacy in brain metastases - the UK experience. Br J cancer. 2010;102:995–1002. doi: 10.1038/sj.bjc.6605586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chien J, Rugo HS. The cardiac safety of trastuzumab in the treatment of breast cancer. Expert Opin Drug Saf. 2010;2:335–346. doi: 10.1517/14740331003627441. [DOI] [PubMed] [Google Scholar]
- 8.Bange J, Zwick E, Ullrich A. Molecular targets for breast cancer therapy and prevention. Nat Med. 2001;7:548–552. doi: 10.1038/87872. [DOI] [PubMed] [Google Scholar]
- 9.Wong JS, Harris JR. Importance of local tumor control in breast cancer. Lancet Oncol. 2001;2:11–17. doi: 10.1016/S1470-2045(00)00190-X. [DOI] [PubMed] [Google Scholar]
- 10.Loncaster J, Dodwell D. Adjuvant radiotherapy in breast cancer. Are there factors that allow selection of patients who do not require adjuvant radiotherapy following breast-conserving surgery for breast cancer? Minerva Med. 2002;93:101–107. [PubMed] [Google Scholar]
- 11.Sporn MB, Suh N. Chemoprevention of cancer. Carcinogenesis. 2000;21:525–530. doi: 10.1093/carcin/21.3.525. [DOI] [PubMed] [Google Scholar]
- 12.Jordan VC. Chemoprevention of breast cancer with selective oestrogen-receptor modulators. Nature. 2007;7:46–53. doi: 10.1038/nrc2048. [DOI] [PubMed] [Google Scholar]
- 13.Castrellon AB, Glüch S. Chemoprevention of breast cancer. Expert Rev Anticancer Ther. 2008;8:443–452. doi: 10.1586/14737140.8.3.443. [DOI] [PubMed] [Google Scholar]
- 14.Zhao X, Li L, Wang Z. Chemoprevention of breast cancer: current status and future prospects. Front Biosci. 2006;11:2249–2256. doi: 10.2741/1967. [DOI] [PubMed] [Google Scholar]
- 15.Pan M-H, Ho C-T. Chemopreventive effects of natural dietary compounds on cancer development. Chem Soc Rev. 2008;37:2558–2574. doi: 10.1039/b801558a. [DOI] [PubMed] [Google Scholar]
- 16.Naithani R, Huma LC, Moriarty RM, McCormick DL, Mehta RG. Comprehensive review of cancer chemopreventive agents evaluated in experimental carcinogenesis models and clinical trials. Curr Med Chem. 2008;15:1044–1071. doi: 10.2174/092986708784221403. [DOI] [PubMed] [Google Scholar]
- 17.Bishayee A. Cancer prevention and treatment with resveratrol: from rodent studies to clinical trials. Cancer Prev Res. 2009;2:409–418. doi: 10.1158/1940-6207.CAPR-08-0160. [DOI] [PubMed] [Google Scholar]
- 18.Bishayee A, Waghray A, Patel MA, Chatterjee M. Vanadium in the detection, prevention and treatment of cancer: the in vivo evidence. Cancer Lett. 2010 doi: 10.1016/j.canlet.2010.01.030. in press. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 19.Korkina LG, De Luca C, Kostyuk VA, Pastore S. Plant polyphenols and tumors: from mechanisms to therapies, prevention, and protection against toxicity of anti-cancer treatments. Curr Med Chem. 2009;16:3943–3965. doi: 10.2174/092986709789352312. [DOI] [PubMed] [Google Scholar]
- 20.Dennis T, Fanous M, Mouse S. Natural products for chemopreventive and adjunctive therapy in oncologic disease. Nutr Cancer. 2009;61:587–597. doi: 10.1080/01635580902825530. [DOI] [PubMed] [Google Scholar]
- 21.Rabi T, Bishayee A. Terpenoids and breast cancer prevention. Breast Cancer Res Treat. 2009;115:223–239. doi: 10.1007/s10549-008-0118-y. [DOI] [PubMed] [Google Scholar]
- 22.Rohdich F, Bacher A, Eisenreich W. Isoprenoid biosynthetic pathways as anti-infective drug targets. Biochem Soc Trans. 2005;33:785–791. doi: 10.1042/BST0330785. [DOI] [PubMed] [Google Scholar]
- 23.Withers ST, Keasling JD. Biosynthesis and engineering of isoprenoid small molecules. Appl Microbiol Biotechnol. 2007;73:980–990. doi: 10.1007/s00253-006-0593-1. [DOI] [PubMed] [Google Scholar]
- 24.Setzer WN, Setzer MC. Plant-derived triterpenoids as potential antineoplastic agents. Mini Rev Med Chem. 2003;3:540–556. doi: 10.2174/1389557033487854. [DOI] [PubMed] [Google Scholar]
- 25.Liby KT, Yore MM, Sporn MB. Triterpenoids and rexinoids as multifunctional agents for the prevention and treatment of cancer. Nat Rev Cancer. 2007;7:357–369. doi: 10.1038/nrc2129. [DOI] [PubMed] [Google Scholar]
- 26.Laszczyk MN. Pentacyclic triterpenes of the lupane, oleanane and ursane group as tools in cancer therapy. Planta Med. 2009;75:1549–1560. doi: 10.1055/s-0029-1186102. [DOI] [PubMed] [Google Scholar]
- 27.Petronelli A, Pannitteri G, Testa U. Triterpenoids as new promising anticancer drugs. Anti-Cancer Drugs. 2009;20:880–892. doi: 10.1097/CAD.0b013e328330fd90. [DOI] [PubMed] [Google Scholar]
- 28.Ovensná Z, Vachalková A, Horváthová K, Táthová D. Pentacyclic triterpenoic acids: new chemoprotective compounds. Neoplasma. 2004;51:327–333. [PubMed] [Google Scholar]
- 29.Neto CC. Cranberry and its phytochemicals: a review in in vitro anticancer studies. J Nutr. 2007;137:1865–1935. doi: 10.1093/jn/137.1.186S. [DOI] [PubMed] [Google Scholar]
- 30.Gerhauser C. Cancer chemopreventive potential of apples, apple juice, and apple components. Planta Med. 2008;74:1608–1624. doi: 10.1055/s-0028-1088300. [DOI] [PubMed] [Google Scholar]
- 31.Phillips DR, Rasbery JM, Bartel B, Masuda SP. Biosynthetic diversity in plant triterpene cyclization. Curr Opin Plant Biol. 2006;9:305–314. doi: 10.1016/j.pbi.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 32.Mullauer FB, Kessler JH, Madema JP. Betulinic acid, a natural compound with potent anticancer effects. Anti-Cancer Drugs. 2010;21:215–227. doi: 10.1097/CAD.0b013e3283357c62. [DOI] [PubMed] [Google Scholar]
- 33.The Wealth of India Raw Materials. Vol. 9. New Delhi: Publications and Information Directorate, Council of Scientific and Industrial Research; 1948–1976. [Google Scholar]
- 34.Huang KC. The Pharmacology of Chinese Herbs. Tokyo: CRC Press; 1993. [Google Scholar]
- 35.Sultana N, Ata A. Oleanolic acid and related derivatives as medicinally important compounds. J Enzyme Inhibition Med Chem. 2008;23:739–756. doi: 10.1080/14756360701633187. [DOI] [PubMed] [Google Scholar]
- 36.Shah BA, Qazi GN, Taneja SC. Boswellic acids: a group of medicinally important compounds. Nat Prod Rep. 2009;26:72–89. doi: 10.1039/b809437n. [DOI] [PubMed] [Google Scholar]
- 37.Chen JC, Chiu MH, Nie RL, Cordell GA, Qin SX. Cucurbitacins and cucurbitane glycosides structures and biological activities. Nat Prod Rep. 2005;22:386–399. doi: 10.1039/b418841c. [DOI] [PubMed] [Google Scholar]
- 38.Turkson J, Jove R. STAT proteins: novel molecular targets for cancer drug discovery. Oncogene. 2000;19:6613–6626. doi: 10.1038/sj.onc.1204086. [DOI] [PubMed] [Google Scholar]
- 39.Bowman T, Garcia R, Turkson J, Jove R. STATs in oncogenesis. Oncogene. 2000;19:2474–2488. doi: 10.1038/sj.onc.1203527. [DOI] [PubMed] [Google Scholar]
- 40.Blaskovich MA, Sun J, Cantor A, Turkson J, Jove R, Sebti SM. Discovery of JSI-124 (cucurbitacin I), a selective Janus kinase/signal transducer and activator of transcription 3 signaling pathway inhibitor with potent antitumor activity against human and murine cancer cells in mice. Cancer Res. 2003;63:1270–1279. [PubMed] [Google Scholar]
- 41.Sun J, Blaskovich MA, Jove R, Livingston SK, Coppola D, Sebti SM. Cucurbitacin Q: a selective STAT3 activation inhibitor with potent antitumor activity. Oncogene. 2005;24:3236–3245. doi: 10.1038/sj.onc.1208470. [DOI] [PubMed] [Google Scholar]
- 42.Rodriguez N, Vasquez Y, Hussain AA, Coley PD, Solis PN, Gupta MP. Cytotoxic cucurbitacin constituents from Sloanea zuliaensis. J Natl Prod. 2003;66:1515–1516. doi: 10.1021/np0303106. [DOI] [PubMed] [Google Scholar]
- 43.Jayaprakasam B, Seeram NP, Nair MG. Anticancer and antiinflammatory activities of cucurbitacins from Cucurbita andreana. Cancer Lett. 2003;189:11–16. doi: 10.1016/s0304-3835(02)00497-4. [DOI] [PubMed] [Google Scholar]
- 44.Olmedo D, Rodriguez N, Vasquez Y, Solis PN, Lopez-Perez JL, San Feliciano A, Gupta MP. A new coumarin from the fruits of Coutarea hexandra. Nat Prod Res. 2007;21:625–331. doi: 10.1080/14786410701371116. [DOI] [PubMed] [Google Scholar]
- 45.Yang L, Wu S, Zhang Q, Liu F, Wu P. 23–24-Dihydrocucurbitacin B induces G2/M cell-cycle arrest and mitochondria-dependent apoptosis in human breast cancer cells (Bcap37) Cancer Lett. 2007;256:267–278. doi: 10.1016/j.canlet.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 46.Wakimoto N, Yin D, O’Kelley J, Haritunians T, Said J, Xing H, Phillip Koeffler H. Cucurbitacin B has a potent antiproliferative effect on breast cancer cells in vitro and in vivo. Cancer Sci. 2008;99:1793–1797. doi: 10.1111/j.1349-7006.2008.00899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kongtun S, Jiratchariyakul W, Kummalue T, Tan-ariya P, Kunnachak S, Wilhelm Frahm A. Cytotoxic properties of root extract and fruit juice of Trichosanthes cucurbitacinumerina. Planta Med. 2009;75:839–842. doi: 10.1055/s-0029-1185455. [DOI] [PubMed] [Google Scholar]
- 48.Ramalhete C, Mansoor TA, Mulhovo S, Molnár J, Ferreira M-JU. Cucurbitane-type triterpenoids from the African plant Momordica balsamina. J Natl Prod. 2009;72:2009–2013. doi: 10.1021/np900457u. [DOI] [PubMed] [Google Scholar]
- 49.Phongmaykin J, Kumamoto T, Ishikawa T, Suttisri R, Saifah E. A new sesquiterpene and other terpenoid constituents of Chisocheton penduliflorus. Arch Pharm Res. 2008;31:21–27. doi: 10.1007/s12272-008-1115-8. [DOI] [PubMed] [Google Scholar]
- 50.Tsai ZT, Liaw SL. The Use and the Effect of Ganoderma. Taichung: San Yun Press; 1985. [Google Scholar]
- 51.Yeh C-T, Rao YK, Yao C-J, Yeh C-F, Li C-H, Chuang S-E, Luong JHT, Lai G-M, Tzeng Y-M. Cytotoxic triterpenes from Antrodia camphorate and their mode of action in HT-29 human colon cancer cells. Cancer Lett. 2009;285:73–79. doi: 10.1016/j.canlet.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 52.Setzer WN, Holland MT, Bozeman CA, Rozmus GF, Setzer MC, Moriarity DM, Reeb S, Vogler B, Bates RB, Haber WA. Isolation and frontier molecular orbital investigation of bioactive quinine-methide triterpenoids from the bark ofSalacia petenesis. Planta Med. 2001;67:65–69. doi: 10.1055/s-2001-10879. [DOI] [PubMed] [Google Scholar]
- 53.Chang F-R, Hayashi K-I, Chen I-H, Liaw CC, Bastow KF, Nakanishi Y, Nozaki H, Cragg GM, Wu Y-C, Lee K-H. Antitumor agents 228. Five new agarofurans, reissantins A-E, and cytotoxic principles fromReissantia buchananii. J Natl Prod. 2003;66:1416–1420. doi: 10.1021/np030241v. [DOI] [PubMed] [Google Scholar]
- 54.Wu C-C, Chan M-L, Chen W-Y, Tsai C-Y, Chang F-R, Wu Y-C. Pristimerin induces caspase-dependent apoptosis in MDA-MB-231 cells via direct effects on mitochondria. Mol Cancer Ther. 2005;4:1277–1285. doi: 10.1158/1535-7163.MCT-05-0027. [DOI] [PubMed] [Google Scholar]
- 55.Idris AI, Libouban H, Nyangoga H, Landao-Bassonga E, Chappard D, Ralston SH. Pharmacologic inhibitors of Iκβ kinase suppress growth and migration of mammary carcinosarcoma cells in vitro and prevent osteolytic bone metastasisin vivo. Mol Cancer Ther. 2009;8:2339–2347. doi: 10.1158/1535-7163.MCT-09-0133. [DOI] [PubMed] [Google Scholar]
- 56.Sung B, Park B, Yadav VR, Aggarwal BB. Celastrol, a triterpene, enhances TRAIL-induced apoptosis through the downregulation of cell survival proteins and upregulation of death receptors. J Biol Chem. 2010 doi: 10.1074/jbc.M109.090209. in press. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 57.Ee GCL, Lim CK, Rahmat A, Lee HL. Cytoxic activities of chemical constituents from Mesua daphnifolia. Trop Biomed. 2005;22:99–102. [PubMed] [Google Scholar]
- 58.Zeng L, Gu Z-M, Chang C-J, Wood KV, McLaughlin JL. Meliavolkenin, a new bioactive triterpenoid from Melia volkensii. Bioorg Med Chem. 1995;3:383–390. doi: 10.1016/0968-0896(95)00034-e. [DOI] [PubMed] [Google Scholar]
- 59.Rogers LL, Zeng L, Kozlowski JF, Shimada H, Alali FQ, Johnson HA, McLaughlin JL. New bioactive triterpenoids from Melia volkensii. J Natl Prod. 1998;61:64–70. doi: 10.1021/np9704009. [DOI] [PubMed] [Google Scholar]
- 60.Pisha E, Chai H, Lee IS, Chagwedera TE, Farnsworth NR, Cordell GA, Beecher CW, Fong HH, Kinghorn AD, Brown DM, Wani MC, Hall ME, Hieken TJ, Das Gupta TK, Pezzuto JM. Discovery of betulinic acid as a selective inhibitor of human melanoma that functions by induction of apoptosis. Nat Med. 1995;1:1046–1051. doi: 10.1038/nm1095-1046. [DOI] [PubMed] [Google Scholar]
- 61.Setzer WN, Setzer MC, Bates RB, Jackes BR. Biologically active triterpenoids of Syncarpia glomulifera extract from Paluma, North Queensland, Australia. Planta Med. 2000;66:176–177. doi: 10.1055/s-2000-11129. [DOI] [PubMed] [Google Scholar]
- 62.Amico V, Barresi V, Condorelli D, Spatafora C, Tringali C. Antiproliferative terpenoids from almond hulls (Prunus dulcis): identification and structure-activity relationships. J Agric Food Chem. 2006;54:810–814. doi: 10.1021/jf052812q. [DOI] [PubMed] [Google Scholar]
- 63.Rzeski W, Stepulak A, Szymański M, Sifringer M, Kaczor J, Wejksza K, Zdzisińska B, Kandefer-Szerszen M. Betulinic acid decreases expression of bcl-2 and cyclin D1, inhibits proliferation, migration and induces apoptosis in cancer cells. Naunyn-Schmeiedeberg’s Acrh Pharmacol. 2006;374:11–20. doi: 10.1007/s00210-006-0090-1. [DOI] [PubMed] [Google Scholar]
- 64.Basu S, Ma R, Boyle PJ, Mikulla B, Bradley M, Smith B, Basu M, Banerjee S. Apoptosis of human carcinoma cells in the presence of potential anti-cancer drugs: III. Treatment of Colo-205 and SKB3 cells with: cis-platin, tamoxifen, melphalan, betulic acid, L-PDMP, L-PPMP, and GD3 ganglioside. Glycoconj J. 2004;20:563–577. doi: 10.1023/B:GLYC.0000043293.46845.07. [DOI] [PubMed] [Google Scholar]
- 65.Kessler JH, Mallauer FB, de Roo GM, Medema JP. Broad in vitro efficacy of plant-derived betulinic acid against cell lines derived from the most prevalent human cancer types. Cancer Lett. 2007;251:132–145. doi: 10.1016/j.canlet.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 66.Rzeski W, Stepulak A, Szymański M, Juszczak M, Grabarska A, Sifringer M, Kaczor J, Kandefer-Szerszeń M. Betulin elicits anti-cancer effects in tumor primary cultures and cell lines in vitro. Basic Clin Pharmacol Toxicol. 2009;105:425–432. doi: 10.1111/j.1742-7843.2009.00471.x. [DOI] [PubMed] [Google Scholar]
- 67.Lambertini E, Lampronti I, Penolazzi L, Khan MT, Ather A, Giorgi G, Gambari R, Piva R. Expression of estrogen receptor alpha gene in breast cancer cells treated with transcription factor decoy is modulated by Bangladeshi natural plant extracts. Oncol Res. 2005;14:69–79. [PubMed] [Google Scholar]
- 68.Deng Y, Jiang T-Y, Sheng S, Tianasoa-Ramamonjy M, Snyder JK. Remangilones A–C, new cytotoxic triterpenes from Physena madagascariensis. J Natl Prod. 1999;62:471–476. doi: 10.1021/np9805140. [DOI] [PubMed] [Google Scholar]
- 69.Yun B-S, Ryoo I-J, Lee I-K, Park K-H, Choung D-H, Han K-H, Yoo I-D. Two bioactive pentacyclic triterpene esters from the root bark ofHibiscus syriacus. J Natl Prod. 1999;62:764–766. doi: 10.1021/np9804637. [DOI] [PubMed] [Google Scholar]
- 70.Hsu H-F, Houng J-Y, Chang C-L, Wu C-C, Chang F-R, Wu Y-C. Antioxidant activity, and DNA information of Glossogyne tenuifolia. J Agric Food Chem. 2005;53:6117–6125. doi: 10.1021/jf050463u. [DOI] [PubMed] [Google Scholar]
- 71.Suh N, Wang Y, Honda T, Gribble GW, Dmitrovsky E, Hickey WF, Maue RA, Place AE, Porter DM, Spinella MJ, Williams CR, Wu G, Dannenberg AJ, Flanders KC, Letterio JJ, Mangelsdorf DJ, Nathan CF, Nguyen L, Porter WW, Ren RF, Roberts AB, Roche NS, Subbaramaiah K, Sporn MB. A novel synthetic oleanane triterpenoid, 2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid, with potent differentiating, antiproliferative, and anti-Inflammatory activity. Cancer Res. 1999;59:336–341. [PubMed] [Google Scholar]
- 72.Murakami A, Ohigashi H. Targeting NOX, INOS and COX-2 in inflammatory cells: chemoprevention using food phytochemicals. Int J Cancer. 2007;121:2357–2363. doi: 10.1002/ijc.23161. [DOI] [PubMed] [Google Scholar]
- 73.Kundu JK, Surh Y-J. Inflammation: gearing the journey to cancer. Mutat Res. 2008;659:15–30. doi: 10.1016/j.mrrev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 74.Lapillonne H, Konopleva M, Tsao T, Gold D, Mcqueen T, Sutherland RL, Madden T, Andreeff M. Activation of peroxisome proliferators-activated receptor gamma by a novel synthetic triterpenoid 2-cyano-3,12-dioxoleana-1,9-dien-28oic acid induces growth arrest and apoptosis in breast cancer cells. Cancer Res. 2003;63:5926–5939. [PubMed] [Google Scholar]
- 75.Konopleva M, Zhang W, Shi Y, McQueen T, Tsao T, Abdelrahim M, Munsell MF, Johansen M, Yu D, Hung M, Andreeff M. Synthetic triterpenoid 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid induces growth arrest in HER2-over expressing breast cancer cells. Mol Cancer Ther. 2006;5:317–328. doi: 10.1158/1535-7163.MCT-05-0350. [DOI] [PubMed] [Google Scholar]
- 76.Place E, Suh N, Williams CR, Risingsong R, Honda T, Honda Y, Gribble GW, Leesnitzer LM, Stimmel JB, Wilson TM, Rosen E, Sporn MB. The novel synthetic triterpenoid, CDDO-imidazolide, inhibits inflammatory response and tumor growth in vivo. Clin Cancer Res. 2003;9:2798–2806. [PubMed] [Google Scholar]
- 77.Hyer ML, Croxton R, Krajewska M, Krajewski S, Kress CL, Lu M, Suh N, Sporn MB, Cryns VL, Zapata J, Reed JC. Synthetic triterpenoids co-operate with tumor necrosis factor-related apoptosis-inducing ligand to induce apoptosis of breast cancer cells. Cancer Res. 2005;65:4799–4808. doi: 10.1158/0008-5472.CAN-04-3319. [DOI] [PubMed] [Google Scholar]
- 78.Honda T, Janosik T, Honda Y, Han J, Liby KT, Williams CR, Couch RD, Anderson AC, Sporn MB, Gribble GW. Design, synthesis and biological evaluation of biotin conjugates of 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid for the isolation of the protein targets. J Med Chem. 2004;47:4923–4932. doi: 10.1021/jm049727e. [DOI] [PubMed] [Google Scholar]
- 79.Ling X, Konopleva M, Zeng Z, Ruvolo V, Stephens LC, Schober W, Mcqueen T, Dietrich M, Madden TL, Andreeff M. The novel triterpenoid C-28 methyl ester of 2-cyano-3,12-dioxolen-1,9-dien-28-oic acid inhibits metastatic murine breast tumor growth through inactivation of STAT3 signaling. Cancer Res. 2007;67:4210–4218. doi: 10.1158/0008-5472.CAN-06-3629. [DOI] [PubMed] [Google Scholar]
- 80.Ahmad R, Raina D, Meyer C, Kufe D. Triterpenoid CDDO-methyl ester inhibits the Janus-activated kinase-1 (JAK1)→signal transducer and activator of transcription-3 (STAT3) pathway by direct inhibition of JAK1 and STAT3. Cancer Res. 2008;68:2920–2926. doi: 10.1158/0008-5472.CAN-07-3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Duan Z, Ames RY, Ryan M, Hornicek FJ, Mankin H, Seiden MV. CDDO-Me, a synthetic triterpenoid, inhibits expression of IL-6 and Stat3 phosphorylation in multi-drug resistant ovarian cancer cells. Cancer Chemother Pharmacol. 2009;63:681–689. doi: 10.1007/s00280-008-0785-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chopra RN, Chopra IC. Glossary of Indian Medicinal Plants. New Delhi: Council of Scientific and Industrial Research; 1965. [Google Scholar]
- 83.Prasad GC. Studies on cancer in Ayurveda and its management. J Res Ayurveda Siddha. 1987;8:147–167. [Google Scholar]
- 84.Rabi T, Karunagaran D, Krishnan Nair M, Bhattathiri VN. Cytotoxic activity of amooranin and its derivatives. Phytother Res. 2002;16:S84–S86. doi: 10.1002/ptr.803. [DOI] [PubMed] [Google Scholar]
- 85.Rabi T, Ramachandran C, Fonseca HB, Nair RP, Alamo A, Melnick SJ, Escalon E. Novel drug amooranin induces apoptosis through caspase activity in human breast carcinoma cell lines. Breast Cancer Res Treat. 2003;80:321–330. doi: 10.1023/A:1024911925623. [DOI] [PubMed] [Google Scholar]
- 86.Rabi T, Wang L, Banerjee S. Novel triterpenoid 25-hydroxy-3-oxoolean-12-en-28-oic acid induces growth arrest and apoptosis in breast cancer cells. Breast Cancer Res Treat. 2007;101:27–36. doi: 10.1007/s10549-006-9275-z. [DOI] [PubMed] [Google Scholar]
- 87.Rabi T, Banerjee S. Novel synthetic triterpenoid methyl 25-hydroxy-3-oxoolean-12-en-28-oate induces apoptosis through JNK and p38 MAPK pathways in human breast adenocarcinoma MCF-7 cells. Mol Carcinog. 2008;47:415–423. doi: 10.1002/mc.20399. [DOI] [PubMed] [Google Scholar]
- 88.Chávez IO, Apan TR, Martínez-Vázquez M. Cytotoxic activity and effect on nitric oxide production of tirucallane-type triterpenes. J Pharm Pharmacol. 2005;57:1087–1091. doi: 10.1211/jpp.57.9.0003. [DOI] [PubMed] [Google Scholar]
- 89.Es-Saady D, Simon A, Jayat-Vignoles C, Chulia AJ, Delage C. MCF-7 cell cycle arrested at G1 through ursolic acid, and increased reduction of tetrazolium salts. Anticancer Res. 1996;16:481–486. [PubMed] [Google Scholar]
- 90.Neto CC, Vaisberg AJ, Zhou B-N, Kingston DGI, Hammond GB. Cytotoxic triterpene acids from the Peruvian medicinal plant Polylepis racemosa. Planta Med. 2000;66:483–484. doi: 10.1055/s-2000-8583. [DOI] [PubMed] [Google Scholar]
- 91.Subbaramaiah K, Michaluart P, Sporn MB, Dannenberg AJ. Ursolic acid inhibits cyclooxygenase-2 transcription in human mammary epithelial cells. Cancer Res. 2000;60:2399–2404. [PubMed] [Google Scholar]
- 92.Martín-Cordero C, Reyes M, Jesus M, Toro MV. Cytotoxic triterpenoids from Erica andvalensis. Z Naturforsch. 2001;56c:45–48. doi: 10.1515/znc-2001-1-208. [DOI] [PubMed] [Google Scholar]
- 93.Lee I, Lee J, Lee YH, Leonard J. Ursolic acid-induced changes in tumor growth, O2 consumption, and tumor interstitial fluid pressure. Anticancer Res. 2001;21:2827–2834. [PubMed] [Google Scholar]
- 94.He X, Liu RH. Cranberry phytochemicals: isolation, structure elucidation, and their antiproliferative and antioxidant activities. J Agric Food Chem. 2006;54:7069–7074. doi: 10.1021/jf061058l. [DOI] [PubMed] [Google Scholar]
- 95.Murphy BT, MacKinnon SL, Yan X, Hammond GB, Valisburg AJ, Neto CC. Identification of triterpene hydroxycinnamates with in vitro antitumor activity from whole cranberry fruit (Vaccinium macrocarpon) J Agric Food Chem. 2003;51:3541–3545. doi: 10.1021/jf034114g. [DOI] [PubMed] [Google Scholar]
- 96.Chen Y-H, Chang F-R, Wu C-C, Yen M-H, Liaw C-C, Huang H-C, Kuo Y-H, Wu Y-C. New cytotoxic 6-oxygenated 8,9-dihydrofurocoumarins, Hedyotiscone A–C, from Hedyotis biflora. Planta Med. 2006;72:75–78. doi: 10.1055/s-2005-873178. [DOI] [PubMed] [Google Scholar]
- 97.He X, Liu RH. Triterpenoids isolated from apple peels have potent antiproliferative activity and may be partially responsible for apple’s anticancer activity. J Agric Food Chem. 2007;55:4366–4370. doi: 10.1021/jf063563o. [DOI] [PubMed] [Google Scholar]
- 98.Yoon H, Liu RH. Effect of 2α-hydroxyursolic acid on NF- κB activation induced by TNF-α in human breast cancer MCF-7 Cells. J Agric Food Chem. 2008;56:8412–8417. doi: 10.1021/jf8012844. [DOI] [PubMed] [Google Scholar]
- 99.Kassi E, Sourlingas TG, Spiliotaki M, Papouts Z, Pratsinis H, Moutsatsou P. Ursolic acid triggers apoptosis and Bcl-2 downregulation in MCF-7 breast cancer cells. Cancer Invest. 2009;27:723–733. doi: 10.1080/07357900802672712. [DOI] [PubMed] [Google Scholar]
- 100.Yeh C-T, Wu C-H, Yen G-C. Ursolic acid, a naturally occurring triterpenoid, suppresses migration and invasion of human breast cancer cells by modulating c-Jun N-terminal kinase, Akt and mammalian target of rapamycin signaling. Mol Nutr Food Res. 2010;54:1–11. doi: 10.1002/mnfr.200900414. [DOI] [PubMed] [Google Scholar]
- 101.Lee JS, Kim J, Kim BY, Lee HS, Ahn JS, Chang YS. Inhibition of phospholipase Cγ1 and cancer cell proliferation by triterpene esters from Uncaria rhynchophylla. J Natl Prod. 2000;63:753–756. doi: 10.1021/np990478k. [DOI] [PubMed] [Google Scholar]
- 102.Hsu H-L, Kuo PL, Lin LT, Lin CC. Asiatic acid, a triterpene, induces apoptosis and cell cycle arrest through activation of extracellular signal-regulated kinase and p38 mitogen-activated protein kinase pathways in human breast cancer cells. J Pharmacol Exp Ther. 2005;313:333–344. doi: 10.1124/jpet.104.078808. [DOI] [PubMed] [Google Scholar]
- 103.Smith-Kielland I, Domish JM, Malterud KE, Hvistendahl G, Rømming C, Bøckman OC, Kolsaker P, Stenstrøm Y, Nordal A. Cytotoxic triterpenoids from the leaves of Euphorbia pulcherrima. Planta Med. 1996;62:322–325. doi: 10.1055/s-2006-957893. [DOI] [PubMed] [Google Scholar]
- 104.Lavhale MS, Kumar S, Misra SH, Sitasawad SL. A novel triterpenoid isolated from the root bark of Ailanthus excelsa Roxb (tree of heaven), AECHL-1 as a potential anti-cancer agent. PLoS One. 2009;4:e5365. doi: 10.1371/journal.pone.0005365. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 105.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 106.Ravdin PM, Cronin KA, Howlader N, Berg CD, Chlebowski RT, Feuer EJ, Edwards BK, Berry DA. The decrease in breast-cancer incidence in 2003 in the United States. N Eng J Med. 2007;356:1670–1674. doi: 10.1056/NEJMsr070105. [DOI] [PubMed] [Google Scholar]
- 107.Liby K, Song RR, Royce DB, Williams CR, Yore MM, Honda T, Gribble GW, Lamph WW, Vannini N, Sogno I, Albini A, Sporn MB. Prevention and treatment of experimental estrogen receptor negative carcinogenesis by the synthetic triterpenoid CDDO-methyl ester and the rexinoid LG100268. Clin Cancer Res. 2008;14:4556–4563. doi: 10.1158/1078-0432.CCR-08-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rabi T. Antitumor activity of amooranin from Amoora rohituka stem bark. Currr Sci. 1996;70:80–81. [Google Scholar]
- 109.Singletary K, MacDonald C, Wallig M. Inhibition of rosemary and carnosol of 7,12-dimethylbenz(a)anthracene (DMBA)-induced rat mammary tumorigenesis and in vivo DMBA-DNA adduct formation. Cancer Lett. 1996;104:43–48. doi: 10.1016/0304-3835(96)04227-9. [DOI] [PubMed] [Google Scholar]
- 110.Sjöblom T, Jones S, Wood LD, Parsons DW, Lin J, Barber TD, Mandelker D, Leary RJ, Ptak J, Silliman N, Szabo S, Buckhaults P, Farrell C, Meeh P, Markowitz SD, Willis J, Dawson D, Willson JK, Gazdar AF, Hartigan J, Wu L, Liu C, Parmigiani G, Park BH, Bachman KE, Papadopoulos N, Vogelstein B, Kinzler KW, Velculescu VE. The consensus coding sequences of human breast and colorectal cancers. Science. 2006;314:268–274. doi: 10.1126/science.1133427. [DOI] [PubMed] [Google Scholar]
- 111.Guo M, Zhang S, Song F, Wang D, Liu Z, Liu S. Studies on the non-covalent complexes between oleanolic acid and cyclodextrins using electrospray ionization tandem mass spectroscopy. J Mass Spectrom. 2003;38:723–731. doi: 10.1002/jms.486. [DOI] [PubMed] [Google Scholar]
- 112.Chen Y, Liu J, Yang X, Zhao X, Xu H. Oleanolic acid nanosuspensions: preparation, in-vitro characterization and enhanced hepatoprotective effect. J Pharm Pharmacol. 2005;57:259–264. doi: 10.1211/0022357055407. [DOI] [PubMed] [Google Scholar]
- 113.Kang HS, Park JE, Lee YJ, Chang IS, Chung YI, Tae G. Preparation of liposomes containing oleanolic acid via micelle-to-vesicle transition. J Nanosci Nanotechnol. 2007;7:3944–3948. doi: 10.1166/jnn.2007.099. [DOI] [PubMed] [Google Scholar]