Abstract
In Escherichia coli, the active transport of phenylalanine is considered to be performed by two different systems, AroP and PheP. However, a low level of accumulation of phenylalanine was observed in an aromatic amino acid transporter-deficient E. coli strain (ΔaroP ΔpheP Δmtr Δtna ΔtyrP). The uptake of phenylalanine by this strain was significantly inhibited in the presence of branched-chain amino acids. Genetic analysis and transport studies revealed that the LIV-I/LS system, which is a branched-chain amino acid transporter consisting of two periplasmic binding proteins, the LIV-binding protein (LIV-I system) and LS-binding protein (LS system), and membrane components, LivHMGF, is involved in phenylalanine accumulation in E. coli cells. The Km values for phenylalanine in the LIV-I and LS systems were determined to be 19 and 30 μM, respectively. Competitive inhibition of phenylalanine uptake by isoleucine, leucine, and valine was observed for the LIV-I system and, surprisingly, also for the LS system, which has been assumed to be leucine specific on the basis of the results of binding studies with the purified LS-binding protein. We found that the LS system is capable of transporting isoleucine and valine with affinity comparable to that for leucine and that the LIV-I system is able to transport tyrosine with affinity lower than that seen with other substrates. The physiological importance of the LIV-I/LS system for phenylalanine accumulation was revealed in the growth of phenylalanine-auxotrophic E. coli strains under various conditions.
It has been reported that Escherichia coli has five distinct transport systems (AroP, Mtr, PheP, TnaB, and TyrP) for the accumulation of aromatic amino acids (36). A general amino acid permease, encoded by the aroP gene, transports three aromatic amino acids with high affinity (8, 11, 21, 36). The closely related PheP protein transports phenylalanine in preference to tyrosine but does not exhibit tryptophan uptake activity (10, 34-36). Mtr and TyrP are specific for tryptophan and tyrosine, respectively (19, 36, 42, 51, 52), and TnaB is a low-affinity, tryptophan-specific transporter encoded in the tryptophanase operon together with the tnaA gene (13, 36, 43).
In a previous study, we cloned the tyrosine transporter tutB gene of Erwinia herbicola and used E. coli cells to determine the properties of its product (23). In the course of that study, we found that the aromatic amino acid transporter-deficient E. coli strain TK1135 (ΔaroP ΔpheP mtr24 Δtna ΔtyrP) (23) has the ability to accumulate phenylalanine in an energy-dependent manner, although the initial rate of uptake, as well as the steady-state level, was quite low. This finding prompted us to examine the basis for this activity and whether this transport activity is physiologically important in E. coli. Here, we present evidence indicating that a branched-chain amino acid transporter, the LIV-I/LS system (1-3, 18, 24, 28, 29, 32, 37, 38, 45, 50), acts as the third phenylalanine transporter, plays a significant role in the accumulation of phenylalanine, and has a broader substrate specificity than previously reported.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The bacterial strains used in this study are derivatives of E. coli K-12. The strains and plasmids are listed in Table 1 with their characteristics. The aroP, pheP, tna, and tyrP genes were disrupted as described previously (23), and the mtr gene was disrupted, using mtr-1 and mtr-2 (Table 1) as primers, by the method described by Datsenko and Wanner (12). Disruption of the brnQ gene was carried out as follows. The brnQ gene was amplified by PCR using KOD polymerase (Toyobo, Japan) with the genomic DNA of MG1655 as the template and brnQ-F and brnQ-R (Table 1) as the primer pair, and the amplified DNA fragment was ligated with the 3.5-kb NsiI (blunt-ended)-NruI fragment of pACYC177 (6). The internal region of the brnQ gene was then removed by EcoRV-PvuII digestion and replaced with the Flp recognition target (FRT)-flanked kanamycin resistance gene (FRT-kan+-FRT), which was amplified by PCR using pKD13 (12) as the template and pKD13-1 and pKD13-4 (Table 1) as the primer pair. The resulting ΔbrnQ::(FRT-kan+-FRT) gene was introduced into strain MG1655 harboring pKD46 (12) by electroporation and allowed to integrate into the chromosome through a double-crossover event. Elimination of the kan gene from the integrated locus was carried out as described previously (12), with the aid of plasmid pCP20 carrying the Flp recombinase gene (7). Disruption of the livHMGF region and the livJ-yhhK-livKHMGF gene cluster was performed similarly. In these cases, primer pair livH-F and livF-R and primer pair liv-F1 and liv-R1 (Table 1) were used for amplification of the livHMGF and livJ-yhhK-livKHMGF genes, respectively. The internal 2.9-kb BglII-PvuII region in the livHMGF cluster and the 6.4-kb PvuII region within the livJ-yhhK-livKHMGF cluster were deleted and replaced with the FRT-kan+-FRT gene. Integration into the chromosome and subsequent elimination of the kan+ gene were carried out as described above.
TABLE 1.
Strains, plasmids, and oligonuclotides used in this work
| Strain, plasmid, or oligonucleotide | Characteristic(s) or sequence | Source or reference |
|---|---|---|
| Strains | ||
| MG1655 | λ−rph-1 | Laboratory stock |
| NK6024 | λ− Δ(gpt-lac)5 pheA18::Tn10 relA1 spoT1 thi-1 | B. J. Bachmann |
| TK1135 | MG1655 ΔaroP mtr24 ΔpheP Δtna ΔtyrP | 23 |
| TK1170 | MG1655 ΔaroP Δmtr ΔpheP Δtna ΔtyrP | This study |
| TK1173 | TK1170 pheA18::Tn10 | This study |
| YG74 | MG1655 ΔaroP ΔlivHMGF ΔpheP | This study |
| YG106 | MG1655 ΔaroP ΔbrnQ ΔpheP | This study |
| YG108 | MG1655 ΔaroP ΔbrnQ ΔlivHMGF | This study |
| YG109 | MG1655 ΔbrnQ ΔlivHMGF ΔpheP | This study |
| YG195 | MG1655 ΔaroP ΔbrnQ Δmtr ΔpheP Δtna ΔtyrP | This study |
| YG198 | MG1655 ΔaroP ΔbrnQ ΔlivHMGF Δmtr ΔpheP Δtna ΔtyrP | This study |
| YG201 | MG1655 ΔaroP ΔbrnQ ΔlivHMGF ΔpheP | This study |
| YG208 | MG1655 pheA18::Tn10 | This study |
| YG210 | YG106 pheA18::Tn10 | This study |
| YG211 | YG108 pheA18::Tn10 | This study |
| YG212 | YG109 pheA18::Tn10 | This study |
| YG213 | YG201 pheA18::Tn10 | This study |
| YG228 | MG1655 ΔaroP ΔbrnQ Δ(livJ-yhhK-livKHMGF) ΔpheP | This study |
| YG256 | YG228 ΔtyrP | This study |
| Plasmids | ||
| pACYC177 | p15A replicon bla+kan+ | 6 |
| pCP20 | pSC101 replicon (Ts) bla+cat+ Flp(λRp) cI857 | 7 |
| pKD13 | oriRγ bla+ FRT-kan+-FRT | 12 |
| pKD46 | oriR101 repA101(Ts) bla+araC+gam+-bet+-exo+ (araBp) | 12 |
| pMBO131 | Mini-F replicon cat+ | 30 |
| pMW118 | pSC101 replicon bla+lacZα+ | Nippon Gene |
| pYG218 | pSC101 replicon bla+livH+M+G+F+ | This study |
| pYG237 | Mini-F replicon kan+livJ+ | This study |
| pYG239 | Mini-F replicon kan+livK+ | This study |
| pYG249 | Mini-F replicon kan+; the 1.7-kb NheI-BamHI fragment containing the kan+ gene was recovered from pACYC177, blunt ended, and then ligated with the 4.8-kb ScaI-XhoI (blunt-ended) fragment of pMBO131 | This study |
| Oligonucleotides | ||
| brnQ-F | 5′-GATTAGCCATGTCTTTTTCACGGAA-3′ (for cloning the brnQ gene; upstream end) | |
| brnQ-R | 5′-ATGCTTTGATCCCGTCGAGAATAC-3′ (for cloning the brnQ gene; downstream end) | |
| livH-F | 5′-AGGTTACCTTATGTCTGAGCAG-3′ (for cloning the livHMGF genes; upstream end) | |
| livF-R | 5′-CGGTTTCATCGTTTATCTCTCTT-3′ (for cloning the livHMGF genes; downstream end) | |
| liv-F1 | 5′-CCAATCCCCACGCAGATTGTTAATAAACTG-3′ (for cloning the livJ-yhhK-livK genes and the livJ-yhhK-livKHMGF genes; upstream end) | |
| liv-R1 | 5′-GTGAGGGAAAATGGGAGATGGGGC-3′ (for cloning the livJ-yhhK-livKHMGF genes; downstream end) | |
| liv-R2 | 5′-CAATCATATAAACCTCGCCGTGGG-3′ (for cloning the livJ-yhhK-livK genes; downstream end) | |
| mtr-1 | 5′-CACCGTCGCTGCTTGGCGGCGTGGTGATTATCGGCGGCACGTGTAGGCTGGAGCTGCTTC-3′ (for disruption of the mtr gene; upstream end) | |
| mtr-2 | 5′-GCTGCCAAAGCGTTTACGCGATGCACGGGCTAACAGCGCCATTCCGGGGATCCGTCGACC-3′ (for disruption of the mtr gene; downstream end) | |
| pKD13-1 | 5′-GTGTAGGCTGGAGCTGCTGCTTC-3′ (for amplifying the kanamycin resistance gene; upstream end) | |
| pKD13-4 | 5′-ATTCCGGGGATCCGTCGACC-3′ (for amplifying the kanamycin resistance gene; downstream end) |
After confirmation of the correct recombination event by Southern hybridization analysis (41) and/or genomic PCR analysis with primers designed to anneal external regions that had been used for the homologous recombination event, the disrupted gene was transferred to other strains by P1 transduction (26).
Media and chemicals.
Luria-Bertani (LB) (26) broth was routinely used for the cultivation of E. coli strains. M63-glucose (26) was used as the minimal medium, and, when necessary, phenylalanine and pantothenate were added as growth requirements to final concentrations of 10 μM to 1 mM and 5 μg/ml, respectively. Ampicillin, tetracycline, and kanamycin were used at final concentrations of 100, 15, and 30 μg/ml for LB medium and 50, 7.5, and 15 μg/ml for the minimal medium, respectively. For the disk inhibition assay, disks were impregnated with 1 mM concentrations of various amino acids and then put onto the plates. l-(U-14C)-isoleucine (314 mCi/mmol, 0.05 mCi/ml), l-(U-14C)-leucine (306 mCi/mmol, 0.05 mCi/ml), l-(U-14C)-valine (256 mCi/mmol, 0.05 mCi/ml), and l-(U-14C)-tyrosine (434 mCi/mmol, 0.05 mCi/ml) were purchased from Amersham Pharmacia Biotech. l-(U-14C)-phenylalanine (496 mCi/mmol, 0.1 mCi/ml) and l-(side chain-3-14C)-tryptophan (58.1 mCi/mmol, 0.02 mCi/ml) were from Perkin-Elmer Life Sciences Inc. The chemicals were all obtained commercially and not purified further.
Genetic techniques.
Standard genetic techniques were used essentially as described by Sambrook and Russell (41). The method used for generalized transduction involving the P1 phage was that described by Miller (26).
Cloning of the liv gene cluster.
The chromosomal locus including the liv gene cluster consists of the livJ, yhhK, livK, livH, livM, livG, and livF genes in that order. While the livJ and livK genes encode periplasmic binding proteins, the livH, livM, livG, and livF genes specify membrane channel components (1, 45). The function of the yhhK gene has not been clarified yet. The DNA fragment containing the livJ-yhhK-livK region was amplified by high-fidelity PCR using KOD polymerase (Toyobo, Japan) with the genomic DNA of MG1655 as the template and liv-F1 and liv-R2 (Table 1) as the primer pair. To clone the livJ gene, the amplified fragment was digested with AatI to remove the yhhK and livK genes and then inserted into the SalI (blunt-ended) site of pYG249. The livK gene was recovered by BglII digestion of the amplified fragment, blunt ended, and then inserted into the SalI (blunt-ended) site of pYG249. The amplified livK and livJ genes were entirely sequenced to ensure that no misincorporation of nucleotides had occurred during the PCR amplification.
The genes for the livHMGF cluster were cloned as follows. The DNA fragment containing the livJ-yhhK-livKHMGF gene cluster was amplified by high-fidelity PCR with the genomic DNA of MG1655 as the template and liv-F1 and liv-R1 (Table 1) as the primer pair. After insertion of the fragment into the PvuII site of pMW118 (Nippon Gene, Tokyo, Japan), the 2.3-kb EcoRV fragment containing the livJ-yhhK-livK genes was removed and the remaining large fragment carrying the livHMGF genes was circularized by self-ligation. Although the livKHMGF genes constitute an operon and are usually transcribed in one unit, it has been shown that a weak internal promoter present just upstream of the livH gene can direct synthesis of the downstream genes (1). Sequence analysis of the amplified fragment revealed a two-base discordance compared to data reported by Blattner et al. (4) at a locus downstream of the stop codon of the livF gene, which would have no substantial effect on the properties of the LIV-I/LS system. The resulting plasmid, pYG218, was introduced into strain YG201 (ΔaroP ΔbrnQ ΔlivHMGF ΔpheP) and examined for the ability to complement the chromosomal livHMGF lesion with respect to phenylalanine transport.
Transport assays.
Transport assays were performed as described previously (23, 51), with slight modifications as follows. Cells grown in minimal medium were harvested at mid-exponential phase and then washed twice with M63-glucose containing 60 μg of chloramphenicol/ml to stop protein synthesis. The assay was initiated by adding the cell suspension to the reaction mixture containing various concentrations of labeled substrates in the presence or absence of cold competitive inhibitors. The rate of nonspecific diffusion was determined using energy-starved cells that had been prepared by incubating cells in the presence of 100 μM carbonylcyanide-m-chlorophenylhydrazone (CCCP) for 30 min prior to starting the assay. The uptake of substrates was expressed as picomoles per milligram of dry cells as a function of time.
RESULTS AND DISCUSSION
It has so far been considered that in E. coli, the active transport of aromatic amino acids across the inner membrane is mediated by five distinct permeases, AroP, Mtr, PheP, TnaB, and TyrP (8, 10, 11, 13, 19, 21, 34-36, 42, 43, 51, 52) and that among them, the AroP and PheP systems are responsible for the accumulation of phenylalanine in cells. However, as described above, in the course of studying tyrosine transporter TutB of E. herbicola through the use of E. coli cells (23), a low level of accumulation of phenylalanine was observed in the aromatic amino acid transporter-deficient strain TK1135 (ΔaroP ΔpheP mtr24 Δtna ΔtyrP) (data not shown). At first we speculated that this activity might be due to altered specificity of the mutant Mtr protein, i.e., Mtr24 (20), although the nature of the mtr24 allele has not been elucidated. This possibility, however, was ruled out by the observation that an E. coli strain, TK1170 (ΔaroP ΔpheP Δmtr Δtna ΔtyrP), accumulated as much phenylalanine (Fig. 1B) as strain TK1135 in an energy-dependent manner. Even though the initial rate of uptake and the steady-state level of phenylalanine in cells were not so high compared to those with known phenylalanine transport systems, AroP and PheP, reported previously (5, 49) (Fig. 2), this activity seems to be important for cells to accumulate phenylalanine because a phenylalanine-auxotrophic (Phe−) strain could be obtained by transducing TK1170 (ΔaroP ΔpheP Δmtr Δtna ΔtyrP) with a P1 phage lysate prepared from strain NK6024 (pheA18::Tn10) (PheA, chorismate mutase-prephenate dehydratase) and subsequent selection with Tn10 as a marker. These findings suggested that E. coli might have at least one additional phenylalanine transporter.
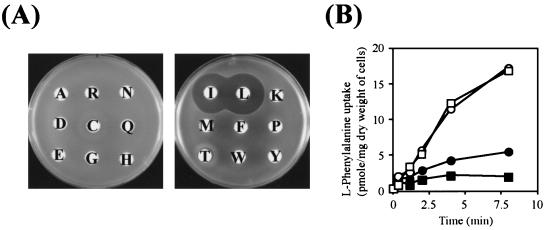
FIG. 1.
Inhibition of phenylalanine uptake by branched-chain amino acids. (A) Growth inhibition of phenylalanine-auxotrophic (Phe−) E. coli strain TK1173 (ΔaroP Δmtr ΔpheP Δtna ΔtyrP pheA18::Tn10) in the presence of branched-chain amino acids, as observed on disk assaying. Cells were grown in LB medium, washed twice with M63 minimal buffer, mixed with the top agar, and then overlaid on M63 minimal solid medium containing 100 μM phenylalanine. Disks were impregnated with 1 mM concentrations of various amino acids (indicated by a one-letter code). (B) Phenylalanine uptake activity of E. coli strain TK1170 (ΔaroP Δmtr ΔpheP Δtna ΔtyrP). l-Phenylalanine was added to cell suspensions to a final concentration of 50 μM in either the absence (□) or presence of 5 μM glutamate (○), leucine (▪), or valine (•). Samples were withdrawn at the indicated times. The experiments were repeated three times with essentially the same results; the data for a representative experiment are shown.
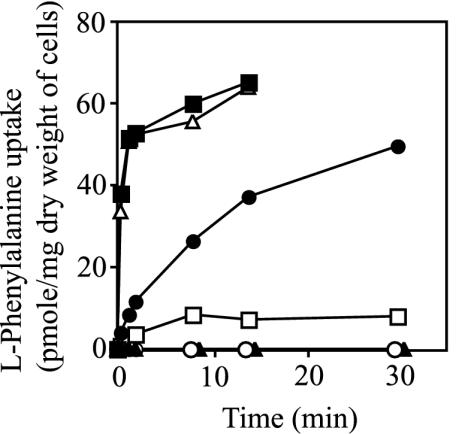
FIG. 2.
The LIV-I/LS system as the third phenylalanine transporter in E. coli. l-Phenylalanine uptake was measured in various E. coli cells, including YG74 (ΔaroP ΔlivHMGF ΔpheP) (BrnQ) (▴), YG106 (ΔaroP ΔbrnQ ΔpheP) (LIV-I/LS) (□), YG108 (ΔaroP ΔbrnQ ΔlivHMGF) (PheP) (•), YG109 (ΔbrnQ ΔlivHMGF ΔpheP) (AroP) (▵), and YG201 (ΔaroP ΔbrnQ ΔlivHMGF ΔpheP) (○) cells, and compared to that in wild-type strain MG1655 (▪). Cell suspensions were incubated in the presence of 1 μM l-(U-14C)-phenylalanine, and samples were withdrawn at the times indicated. The experiments were repeated three times with essentially the same results; the data for a representative experiment are shown.
Inhibition of phenylalanine uptake by branched-chain amino acids.
The question is what system is involved in this transport: a protein encoded by an undefined open reading frame (function unknown ORF) or a defined small molecule transporter with broad substrate specificity? We found by using a disk inhibition assay (Fig. 1A) that the growth of the aromatic transporter-negative Phe− strain TK1173 (ΔaroP ΔpheP Δmtr Δtna ΔtyrP pheA18::Tn10) in minimal medium supplemented with phenylalanine was severely inhibited in the presence of isoleucine and leucine. In this assay, valine and serine were omitted since both cause a serious growth defect by blocking the synthesis of intermediates required for the synthesis of other amino acids (17, 25, 44). While large clear zones of inhibition were observed around the disks impregnated with isoleucine and leucine, a small inhibition zone was also observed around the disk impregnated with threonine (Fig. 1A), which is discussed later. No significant inhibition zones appeared around the other 15 amino acids tested. The results in Fig. 1A suggested that phenylalanine might be accumulated in cells through a branched-chain amino acid transport system. This notion was further supported by a transport assay with l-(U-14C)-phenylalanine. Whereas the presence of glutamate (Fig. 1B) in the assay mixture did not affect phenylalanine uptake, the addition of valine and leucine decreased the phenylalanine uptake activity even with a low concentration (5 μM valine and leucine each versus 50 μM phenylalanine). These results strongly suggested that the active transport of phenylalanine into E. coli cells with the ΔaroP ΔpheP Δmtr Δtna ΔtyrP background could be dependent on a system that transports branched-chain amino acids.
Identification of the LIV-I/LS system as the third phenylalanine transporter in E. coli.
Branched-chain amino acids are transported into E. coli cells by an osmotic-shock-sensitive system designated LIV-I/LS (1-3, 18, 24, 28, 29, 32, 37, 38, 45, 50) and by an osmotic-shock-resistant system, BrnQ (15, 16, 31, 45, 53, 54), formerly called LIV-II (3, 31, 37, 38, 45, 50). Whereas transport by the BrnQ system is mediated by a single membrane protein (38, 45, 50), uptake by the LIV-I/LS system depends on two substrate-binding proteins (BP), LIV-BP and LS-BP, located in the periplasm (2, 14, 24, 33, 38, 45, 50). Previous studies involving purified BPs showed that LIV-BP, encoded by the livJ gene, binds isoleucine, leucine, and valine with Kd values of 10−6 to 10−7 M and threonine, serine, and alanine with lower affinity and that LS-BP, encoded by the livK gene, binds leucine with a Kd value of approximately 10−6 M (24). To enable the ATP-hydrolysis-coupled transport of their substrates into the cytoplasm, LIV-BP and LS-BP interact with the common inner-membrane components LivHMGF, which constitute the LIV-I and LS systems, respectively (1, 28, 29, 45, 50). These six liv genes are clustered at 77 min on the chromosome (45) and divided into two transcription units, one for livJ and the other for livKHMGF (1, 18, 45). In the region between livJ and livK there is the yhhK gene; the deletion of this region results in pantothenate auxotrophy (1).
To determine whether BrnQ or LIV-I/LS carries out the uptake of phenylalanine, a series of E. coli strains expressing individual transport systems was constructed and assayed for transport: AroP-expressing strain YG109 (ΔbrnQ ΔlivHMGF ΔpheP), BrnQ-expressing strain YG74 (ΔaroP ΔlivHMGF ΔpheP), LIV-I/LS-expressing strain YG106 (ΔaroP ΔbrnQ ΔpheP), and PheP-expressing strain YG108 (ΔaroP ΔbrnQ ΔlivHMGF). The transport activity was measured in the presence of 1 μM labeled phenylalanine and compared to that of wild-type strain MG1655 and strain YG201 lacking portions of the aroP, brnQ, livHMGF, and pheP genes.
As shown in Fig. 2, neither BrnQ-expressing strain YG74 (ΔaroP ΔlivHMGF ΔpheP) nor strain YG201 (ΔaroP ΔbrnQ ΔlivHMGF ΔpheP) could accumulate phenylalanine. A sodium gradient made by adding NaCl (final concentration, 1 mM) to the assay mixture did not have any effect on the uptake activity of these strains. On the other hand, LIV-I/LS-expressing strain YG106 (ΔaroP ΔbrnQ ΔpheP) was able to accumulate phenylalanine, demonstrating the involvement of the LIV-I/LS system, but not BrnQ, in phenylalanine transport, although the initial rate and the steady-state level were considerably lower than those in the strains expressing AroP and PheP. It seemed likely that the small inhibition halo observed around the disk impregnated with threonine shown in Fig. 1A reflected the substrate preference of LIV-BP (24). Despite the low phenylalanine transport activity, the LIV-I/LS system alone could support the growth of Phe− strain YG210 (ΔaroP ΔbrnQ ΔpheP pheA18::Tn10) in minimal medium supplemented with 10 μM phenylalanine, indicating the participation of the LIV-I/LS system in the accumulation of phenylalanine. The Km value for phenylalanine in the LIV-I/LS system was determined to be 30 μM, which is considerably higher than those for AroP (0.4 μM) and PheP (2 μM) (36).
AroP-expressing strain YG109 (ΔbrnQ ΔlivHMGF ΔpheP) exhibited the highest uptake activity, and its activity was essentially equal to that of wild-type strain MG1655, suggesting that the AroP protein ordinarily acts as the major phenylalanine transport system in wild-type cells. As for PheP-expressing strain YG108 (ΔaroP ΔbrnQ ΔlivHMGF), more than 40 pmol of phenylalanine/mg (dry weight of cells) was accumulated in the cells, which was comparable to the steady-state level in the case of the AroP system, although the initial rate of uptake was significantly lower than that for AroP.
Next, we tested which binding protein, LIV-BP or LS-BP, participates in the transport of phenylalanine. For this end, LIV-BP and LS-BP were expressed in E. coli cells individually in the presence of membrane machinery components LivHMGF. Strain YG228 [ΔaroP ΔbrnQ Δ(livJ-yhhK-livKHMGF) ΔpheP] was transformed with two compatible plasmids; one was a pSC101-derived vector carrying the genes for membrane components LivHMGF (pYG218), and the other was a Mini-F-derived plasmid carrying either the livJ gene (LIV-I) (pYG237; Fig. 3A) or the livK gene (LS) (pYG239; Fig. 3B). These strains were used for uptake assay in the presence of 10 to 300 μM phenylalanine (Fig. 3A and B). The results clearly show that both BPs are capable of effecting transport of the substrate. The amounts of phenylalanine accumulated in the cells significantly differed between them, but we cannot comment about this difference because the organization of the liv genes on plasmids was different from that on the chromosome. In the absence of BP, no accumulation was observed in the cells (Fig. 3A and B). Considering that the disruption of livHMGF, the genes encoding the membrane components, completely abolished phenylalanine transport (Fig. 2), it can be concluded that both BPs interact only with LivHMGF.
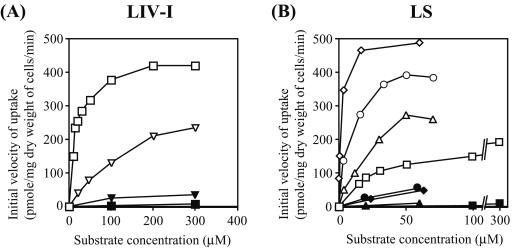
FIG. 3.
Uptake studies of various amino acids with E. coli cells expressing the LIV-I (A) and LS (B) systems. (A) Strain YG228 [ΔaroP ΔbrnQ Δ(livJ-yhhK-livKHMGF) ΔpheP] carrying pYG218 (pSC101 replicon bla+ livH+M+G+F+) (membrane components) was transformed with either pYG237 (Mini-F replicon kan+ livJ+) (LIV-I; open symbols) or pYG249 (Mini-F replicon kan+) (control; filled symbols). For the tyrosine transport assay, YG256 [ΔaroP ΔbrnQ Δ(livJ-yhhK-livKHMGF) ΔpheP ΔtyrP] was used instead of YG228. Cell suspensions were incubated in the presence of 10 to 300 μM l-phenylalanine (□, ▪) and 25 to 300 μM l-tyrosine (▿, ▾). (B) Strain YG228 carrying pYG218 was transformed with either pYG239 (Mini-F replicon kan+ livK+) (LS; open symbols) or pYG249 (control; filled symbols). Cell suspensions were incubated in the presence of 10 to 300 μM l-phenylalanine (□, ▪), 0.4 to 70 μM l-leucine (⋄, ♦), l-isoleucine (○, •), or l-valine (▵, ▴). All experiments were repeated three times with essentially the same results; the data for a representative experiment are shown.
Recently, using NMR with fluorine-labeled LS-BP, Salopek-Sondi and Luck revealed that LS-BP binds phenylalanine in addition to leucine and that the phenylalanine-binding ability is specific for LS-BP but not for LIV-BP (39). Our transport studies corroborated the ability of LS-BP to bind phenylalanine but contradicted the results obtained for LIV-BP.
Taken together, these results led us to the conclusion that in E. coli, there are three phenylalanine uptake systems, AroP, PheP, and LIV-I/LS, all of which may allow phenylalanine accumulation.
Kinetic studies of the LIV-I and LS systems.
To further characterize the LIV-I and LS systems, kinetic constants for both systems were determined by monitoring phenylalanine uptake in the absence or presence of probable competitive inhibitors, branched amino acids. The Km values for phenylalanine in the LIV-I and LS systems were determined to be 19 and 30 μM, respectively, by double-reciprocal plotting of the data in Fig. 3 (Table 2). In inhibition assays, as expected from the substrate specificity of LIV-BP, phenylalanine uptake by the LIV-I system was found to be decreased in a concentration-dependent manner upon the addition of isoleucine, leucine, and valine (data not shown), the Ki values for them having been determined to be 2.3, 1.7, and 1.5 μM, respectively (Table 2). These Ki values were comparable to the respective Km values determined by means of transport assays with LIV-I-expressing cells incubated in the presence of 0.4 to 70 μM labeled branched-chain amino acids (Table 2). The Ki values for valine and phenylalanine inhibition of leucine uptake were also determined by incubating cells under conditions of 0.4 to 20 μM labeled leucine in the presence of cold valine (0.5 to 20 μM) and phenylalanine (15 to 50 μM). The values obtained (Ki = 1.4 μM for valine and 30 μM for phenylalanine) were in good agreement with the Km values (2.4 μM for valine and 19 μM for phenylalanine) (Table 2).
TABLE 2.
Kinetic constants (Km and Ki) for various substrates in the LIV-I and LS systems as determined by in vivo uptake assays
| Systema and substrate | Kmb (μM) | Kib for inhibition of Phe uptake (μM) | Kib for inhibition of Leu uptake (μM) |
|---|---|---|---|
| LIV-I (LivJ) | |||
| Ile | 8.0 | 2.3 | NDd |
| Leu | 2.3 | 1.7 | |
| Val | 2.4 | 1.5 | 1.4 |
| Phe | 19 | 30 | |
| Tyrc | 230 | 120 | 200 |
| LS (LivK) | |||
| Ile | 5.0 | 6.6 | ND |
| Leu | 2.3 | 2.1 | |
| Val | 9.2 | 2.7 | 8.3 |
| Phe | 30 | 74 |
E. coli strain YG228 [λ− rph-1 ΔaroP ΔbrnQ Δ(livJ-yhhK-livKHMGF) ΔpheP] carrying pYG218 (pSC101 replicon bla+ livH+ M+ G+ F+) (membrane component) was transformed with either pYG237 (Mini-F replicon kan+ livJ+) (LIV-BP) or pYG239 (Mini-F replicon kan+ livK+) (LS-BP) and then used for assaying.
The apparent Km and Ki values were determined by double-reciprocal plotting of the data. For determination of Km, assays were carried out in the presence of 0.4 to 70 μM Ile (l-isoleucine), Leu (l-leucine), and Val (l-valine), 10 to 300 μM Phe (l-phenyalanine), and 25 to 300 μM Tyr (l-tyrosine). Values are the averages of three independent results. For Ki determination, the phenylalanine and leucine concentrations were varied (from 10 to 200 μM and 0.4 to 20 μM, respectively) in the presence of cold isoleucine, leucine, valine (0.5 to 20 μM each), phenylalanine (15 to 50 μM), or tyrosine (100 to 500 μM). Values are the averages of two independent experiments.
tyrP-disruptant YG228 [λ− rph-1 ΔaroP ΔbrnQ Δ(livJ-yhhK-livKHMGF) ΔpheP ΔtyrP] (YG256) transformed with pYG218 and pYG237 was used for assaying.
ND, not determined.
The finding that phenylalanine is a good substrate prompted us to examine the possibility of other aromatic amino acids being transported by the LIV-I system. We found that the uptake of phenylalanine by the LIV-I system was inhibited in the presence of tyrosine with a Ki value of 120 μM (Table 2), suggesting the ability of the system to transport tyrosine. ΔtyrP YG228 was constructed (YG256), similarly transformed with pYG218 (livHMGF) and pYG237 (livJ), and then examined for transport. Low-level accumulation of labeled tyrosine was observed (Fig. 3A) but not in the strain carrying the empty vector. The Km value for tyrosine (230 μM) in the LIV-I system was comparable to the Ki value (200 μM) determined by its inhibition of leucine uptake. Although the accumulation of tyrosine was appreciable, the presence of the LIV-I/LS system alone could not support the growth of a tyrosine-auxotrophic strain (ΔaroP ΔbrnQ Δmtr ΔpheP Δtna ΔtyrP ΔtyrA::kan+) in minimal medium even in the presence of 100 μM tyrosine, maybe due to the low affinity for tyrosine. Therefore, it seems likely that the LIV-I/LS system is not a physiologically important tyrosine transporter in E. coli. As for tryptophan, neither inhibition of leucine uptake nor accumulation in the cells by the LIV-I system was observed (10 to 300 μM) (data not shown). Alanine, serine, and threonine acted as inhibitors of phenylalanine transport by the LIV-I system (data not shown), as expected from the results obtained in binding studies with LIV-BP by Rahmanian et al. (38).
Similar experiments were performed with LS-expressing cells, and not only leucine but also isoleucine and valine were found to inhibit phenylalanine uptake. This was surprising, because it has been shown that purified LS-BP preferentially binds leucine (0.4 μM) but not isoleucine or valine (>1 mM each) (24). We carried out transport assays with labeled substrates (Fig. 3B) and found that the LS system was able to transport isoleucine and valine in addition to leucine. The DNA sequence of the livK gene on pYG239 was again analyzed, but no difference was found from the results reported by Blattner et al. (4). The Km values for isoleucine, leucine, and valine in the LS system were determined to be 5.0, 2.3, and 9.2 μM, respectively. There are apparent contradictions between the results of binding studies (14, 33, 40) and transport studies; is an auxiliary protein involved in the recognition of substrates by LS-BP or does the presence of membrane components LivHMGF alter the substrate specificity of LS-BP? In vitro uptake studies with the LS system reconstituted in liposomes are necessary to explain this discrepancy.
Of the aromatic amino acids tested (10 to 300 μM), only phenylalanine acted as a substrate for the LS system. Phenylalanine inhibited leucine uptake with a Ki value of 74 μM, which was comparable to the Km value of 30 μM. Likewise, the Ki values estimated for isoleucine (6.6 μM), leucine (2.1 μM), and valine (2.7 μM) in inhibition assays of phenylalanine uptake were in good accordance with the Km values obtained for them (5.0, 2.3, and 9.2 μM, respectively). The presence of alanine, serine, and threonine (each at 100 μM) did not affect phenylalanine uptake by the LS system at the saturating concentration.
Thus, consistent results were obtained in our transport studies, which revealed new aspects of the substrate specificity of the LIV-I and LS systems. The neutral amino acid ATP-binding cassette-type transport system (Nat) of Synechocystis sp. strain PCC 6803 has been identified by means of insertional mutagenesis, and it was shown that the strain inactivated for NatB, a periplasmic binding protein, leaked significant amounts of amino acids alanine, isoleucine, leucine, valine, and phenylalanine into the medium (27), indicating a role of the Nat system in the recapture of these amino acids. Although the LivJ (LIV-BP) and LivK (LS-BP) proteins of E. coli exhibit low levels (16%) of identity with NatB with respect to amino acid sequences, a similar substrate specificity was suggested, which may help us understand the mechanism underlying the substrate recognition by these proteins.
Functional distinction among the three phenylalanine uptake systems AroP, PheP, and LIV-I/LS.
To obtain a better understanding of the LIV-I/LS system as the phenylalanine transporter, the physiological significance of the AroP, PheP, and LIV-I/LS systems was evaluated. A strain expressing one of the three transport systems was made Phe− (pheA18::Tn10) by P1 transduction and then streaked onto an M63-glucose minimal medium plate containing phenylalanine (MMF) and onto an MMF plate including isoleucine, tryptophan, or tyrosine (MMF+I, MMF+W, or MMF+Y) (Fig. 4A). In parallel, a Phe− strain possessing either all or none of the phenylalanine transporters was constructed and streaked onto similar plates. A phenylalanine transport-deficient Phe− strain was obtained by spreading the transductants [YG201 and P1(NK6024)] on LB plates containing 1 mM phenylalanine. Since phenylalanine accumulation could not be detected in the YG74 (ΔaroP ΔlivHMGF ΔpheP) cells, even in the presence of 100 μM phenylalanine (data not shown), it seems likely that nonspecific diffusion of phenylalanine at the high concentration (1 mM) can support the growth of the strain.
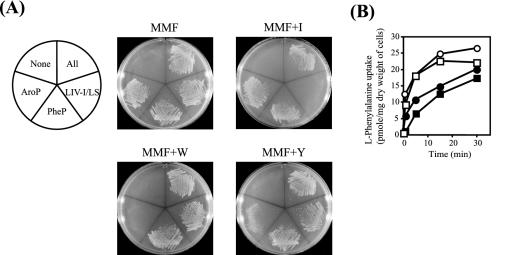
FIG. 4.
Physiological role of each phenylalanine transport system in E. coli cells grown under various conditions. (A) AroP-, BrnQ-, LIV-I/LS-, and PheP-expressing Phe− strain YG208 (All), LIV-I/LS-expressing Phe− strain YG210 (LIV-I/LS), PheP-expressing Phe− strain YG211 (PheP), AroP-expressing Phe− strain YG212 (AroP), and Phe− strain YG213 not carrying any of these transport systems (None) were streaked on M63-glucose minimal medium plates containing 100 μM phenylalanine (MMF) and on MMF supplemented with 1 mM isoleucine (MMF+I), tryptophan (MMF+W), or tyrosine (MMF+Y). (B) Accumulation of phenylalanine in E. coli cells with various phenylalanine transport systems in the presence of 1 mM tyrosine. Strains MG1655 (□), YG106 (LIV-I/LS) (○), YG108 (PheP) (•), and YG109 (AroP) (▪) were grown in MMF+Y, and after the optical density at 600 nm had reached 0.5, the cells were harvested and suspended in MMF+Y containing 60 μg of chloramphenicol/ml. Cell suspensions were incubated in the presence of 100 μM labeled phenylalanine, and samples were withdrawn at the times indicated. The experiments were repeated three times with essentially the same results; the data for a representative experiment are shown.
As shown in Fig. 4A, growth of the Phe− strain in the presence of phenylalanine was dependent on the presence of any one of the AroP, PheP, or LIV-I/LS systems and no growth was observed for the Phe− strain lacking them. The same results were obtained regardless of the presence or absence of the BrnQ system (data not shown). The growth rates of the strains carrying the respective phenylalanine transporters did not differ significantly. These results confirm the physiological importance of these three transporters in phenylalanine accumulation.
The addition of isoleucine, which is a good substrate for both the LIV-I and LS systems, to the MMF medium severely inhibited the growth of LIV-I/LS-expressing Phe− strain YG210 (ΔaroP ΔbrnQ ΔpheP pheA18::Tn10), whereas the growth of the AroP- and PheP-expressing Phe− strains was not affected. Likewise, the presence of tryptophan or tyrosine, either of which acts as a competitor for phenylalanine transport in the AroP system, caused significant retardation of the growth of AroP-expressing Phe− strain YG212 (ΔbrnQ ΔlivHMGF ΔpheP pheA18::Tn10) on the MMF+W or +Y medium. The inhibitory effect was greater for tryptophan than for tyrosine. The same results were obtained for five independently constructed strains. This was surprising, because the expression of the aroP gene is known to be strongly repressed by tyrosine but not by tryptophan (9, 22, 36, 46-48, 55) and the AroP system is known to exhibit almost equal affinity for the three aromatic amino acids (5, 36). At present, the reason for this phenomenon is unclear. PheP-expressing Phe− strain YG211 (ΔaroP ΔbrnQ ΔlivHMGF pheA18::Tn10) grew well under all conditions tested (PheP). Although the PheP and LIV-I systems are capable of transporting tyrosine, no inhibitory effect was observed in the presence of tyrosine (MMF+Y), reflecting the high Km values for tyrosine compared to those for phenylalanine in these systems.
Transport studies were carried out using these cells grown under the same conditions, and the results were consistent with the growth behavior shown in Fig. 4A. It is notable that when cells expressing the individual phenylalanine transport systems were grown in MMF+Y and then assayed for transport (Fig. 4B), the LIV-I/LS-expressing cells exhibited the highest phenylalanine uptake activity, which was almost the same as that of wild-type strain MG1655. Similar results were obtained when these cells were grown in MMF+W (data not shown). Thus, in the presence of tryptophan or tyrosine, the LIV-I/LS system plays a major role in phenylalanine accumulation in E. coli cells.
In conclusion, the LIV-I/LS system was identified as the third phenylalanine transporter in E. coli, which plays a significant role in the accumulation of phenylalanine in cells, especially when grown in the presence of tryptophan or tyrosine. The substrate specificities of the LIV-I and LS systems revealed by transport studies contradicted those found previously in binding studies; the reason for this contradiction remains to be elucidated.
Acknowledgments
This work was partly supported by a Grant-in-Aid for Scientific Research (B), no. 14360056, from the Ministry of Education, Culture, Sports, Science, and Technology, Japan, by a Grant-in-Aid for Fine Enzymatic Synthesis of Useful Compounds from Research for the Future (RFTF) of the Japan Society for the Promotion of Science, and by a Grant-in-Aid for Aromatic Amino Acid Metabolism in Bacteria from the Noda Institute for Scientific Research. T. Koyanagi is supported by the 21st Century COE Program of the Ministry of Education, Culture, Sports, Science and Technology.
REFERENCES
- 1.Adams, M. D., L. M. Wagner, T. J. Graddis, R. Landick, T. K. Antonucci, A. L. Gibson, and D. L. Oxender. 1990. Nucleotide sequence and genetic characterization reveal six essential genes for the LIV-I and LS transport systems of Escherichia coli. J. Biol. Chem. 265:11436-11443. [PubMed] [Google Scholar]
- 2.Anderson, J. J., and D. L. Oxender. 1977. Escherichia coli transport mutants lacking binding protein and other components of the branched-chain amino acid transport systems. J. Bacteriol. 130:384-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson, J. J., and D. L. Oxender. 1978. Genetic separation of high- and low-affinity transport systems for branched-chain amino acids in Escherichia coli K-12. J. Bacteriol. 136:168-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blattner, F. R., G. Plunkett III, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, J. Collado-Vides, J. D. Glasner, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrick, M. A. Goeden, D. J. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453-1474. [DOI] [PubMed] [Google Scholar]
- 5.Brown, K. D. 1970. Formation of aromatic amino acid pools in Escherichia coli K-12. J. Bacteriol. 104:177-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang, A. C. Y., and S. N. Cohen. 1978. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the p15A cryptic miniplasmid. J. Bacteriol. 134:1141-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cherepanov, P. P., and W. Wackernagel. 1995. Gene disruption in Escherichia coli: TcR and KmR cassettes with the option of Flp-catalyzed excision of the antibiotic-resistance determinant. Gene 158:9-14. [DOI] [PubMed] [Google Scholar]
- 8.Chye, M.-L., J. R. Guest, and J. Pittard. 1986. Cloning of the aroP gene and identification of its product in Escherichia coli K-12. J. Bacteriol. 167:749-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chye, M.-L., and J. Pittard. 1987. Transcription control of the aroP gene in Escherichia coli K-12: analysis of operator mutants. J. Bacteriol. 169:386-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cosgriff, A. J., G. Brasier, J. Pi, C. Dogovski, J. P. Sarsero, and A. J. Pittard. 2000. A study of AroP-PheP chimeric proteins and identification of a residue involved in tryptophan transport. J. Bacteriol. 182:2207-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cosgriff, A. J., and A. J. Pittard. 1997. A topological model for the general aromatic amino acid permease, AroP, of Escherichia coli. J. Bacteriol. 179:3317-3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deeley, M. C., and C. Yanofsky. 1981. Nucleotide sequence of the structural gene for tryptophanase of Escherichia coli K-12. J. Bacteriol. 147:787-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furlong, C. E., and J. H. Weiner. 1970. Purification of a leucine-specific binding protein from Escherichia coli. Biochem. Biophys. Res. Commun. 38:1076-1083. [DOI] [PubMed] [Google Scholar]
- 15.Guardiola, J., M. De Felice, T. Klopotowski, and M. Iaccarino. 1974. Mutations affecting the different transport systems for isoleucine, leucine, and valine in Escherichia coli K-12. J. Bacteriol. 117:393-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guardiola, J., and M. Iaccarino. 1971. Escherichia coli K-12 mutants altered in the transport of branched-chain amino acids. J. Bacteriol. 108:1034-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hama, H., Y. Sumita, Y. Kakutani, M. Tsuda, and T. Tsuchiya. 1990. Target of serine inhibition in Escherichia coli. Biochem. Biophys. Res. Commun. 168:1211-1216. [DOI] [PubMed] [Google Scholar]
- 18.Haney, S. A., J. V. Platko, D. L. Oxender, and J. M. Calvo. 1992. Lrp, a leucine-responsive protein, regulates branched-chain amino acid transport genes in Escherichia coli. J. Bacteriol. 174:108-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heatwole, V. M., and R. L. Somerville. 1991. Cloning, nucleotide sequence, and characterization of mtr, the structural gene for a tryptophan-specific permease of Escherichia coli K-12. J. Bacteriol. 173:108-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hiraga, S., K. Ito, T. Matsuyama, H. Ozaki, and T. Yura. 1968. 5-Methyltryptophan-resistance mutations linked with the arginine G marker in Escherichia coli. J. Bacteriol. 96:1880-1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Honore, N., and S. T. Cole. 1990. Nucleotide sequence of the aroP gene encoding the general aromatic amino acid transport protein of Escherichia coli K-12: homology with yeast transport proteins. Nucleic Acids Res. 18:653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katayama, T., H. Suzuki, T. Koyanagi, and H. Kumagai. 2000. Cloning and random mutagenesis of the Erwinia herbicola tyrR gene for high-level expression of tyrosine phenol-lyase. Appl. Environ. Microbiol. 66:4764-4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katayama, T., H. Suzuki, T. Koyanagi, and H. Kumagai. 2002. Functional analysis of the Erwinia herbicola tutB gene and its product. J. Bacteriol. 184:3135-3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Landick, R., and D. L. Oxender. 1985. The complete nucleotide sequences of the Escherichia coli LIV-BP and LS-BP genes. Implication for the mechanism of high-affinity branched-chain amino acid transport. J. Biol. Chem. 260:8257-8261. [PubMed] [Google Scholar]
- 25.Lawther, R. P., D. H. Calhoun, C. W. Adams, C. A. Hauser, J. Gray, and G. W. Hatfield. 1981. Molecular basis of valine resistance in Escherichia coli K-12. Proc. Natl. Acad. Sci. USA 78:922-925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller, J. H. 1992. A short course in bacterial genetics. A laboratory manual and handbook for Escherichia coli and related bacteria. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 27.Montesinos, M. L., A. Herrero, and E. Flores. 1997. Amino acid transport in taxonomically diverse cyanobacteria and identification of two genes encoding elements of a neutral amino acid permease putatively involved in recapture of leaked hydrophobic amino acids. J. Bacteriol. 179:853-862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nazos, P. M., T. K. Antonucci, R. Landick, and D. L. Oxender. 1986. Cloning and characterization of livH, the structural gene encoding a component of the leucine transport system in Escherichia coli. J. Bacteriol. 166:565-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nazos, P. M., M. M. Mayo, T. Z. Su, J. J. Anderson, and D. L. Oxender. 1985. Identification of livG, a membrane-associated component of the branched-chain amino acid transport in Escherichia coli. J. Bacteriol. 163:1196-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Connor, M., M. Peifer, and W. Bender. 1989. Construction of large DNA segments in Escherichia coli. Science 244:1307-1312. [DOI] [PubMed] [Google Scholar]
- 31.Ohnishi, K., A. Hasegawa, K. Matsubara, T. Date, T. Okada, and K. Kiritani. 1988. Cloning and nucleotide sequence of the brnQ gene, the structural gene for a membrane-associated component of the LIV-II transport system for branched-chain amino acids in Salmonella typhimurium. Jpn. J. Genet. 63:343-357. [DOI] [PubMed] [Google Scholar]
- 32.Oxender, D. L., J. J. Anderson, C. J. Daniels, R. Landick, R. P. Gunsalus, G. Zurawski, E. Selker, and C. Yanofsky. 1980. Structural and functional analysis of cloned DNA containing genes responsible for branched-chain amino acid transport in Escherichia coli. Proc. Natl. Acad. Sci. USA 77:1412-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Penrose, W. R., G. E. Nichoalds, J. R. Piperno, and D. L. Oxender. 1968. Purification and properties of a leucine-binding protein from Escherichia coli. J. Biol. Chem. 243:5921-5928. [PubMed] [Google Scholar]
- 34.Pi, J., and A. J. Pittard. 1996. Topology of the phenylalanine-specific permease of Escherichia coli. J. Bacteriol. 178:2650-2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pi, J., P. J. Wookey, and A. J. Pittard. 1991. Cloning and sequencing of the pheP gene, which encodes the phenylalanine-specific transport system of Escherichia coli. J. Bacteriol. 173:3622-3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pittard, A. J. 1996. Biosynthesis of the aromatic amino acids, p. 458-484. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. American Society for Microbiology, Washington, D.C.
- 37.Quay, S. C., T. E. Dick, and D. L. Oxender. 1977. Role of transport systems in amino acid metabolism: leucine toxicity and the branched-chain amino acid transport systems. J. Bacteriol. 129:1257-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rahmanian, M., D. R. Claus, and D. L. Oxender. 1973. Multiplicity of leucine transport systems in Escherichia coli K-12. J. Bacteriol. 116:1258-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salopek-Sondi, B., and L. A. Luck. 2002. 19F NMR study of the leucine-specific binding protein of Escherichia coli: mutagenesis and assignment of the 5-fluorotryptophan-labeled residues. Prot. Eng. 15:855-859. [DOI] [PubMed] [Google Scholar]
- 40.Salopek-Sondi, B., D. Swartz, P. S. Adams, and L. A. Luck. 2002. Exploring the role of amino acid-18 of the leucine binding proteins of E. coli. J. Biomol. Struct. Dyn. 20:381-387. [DOI] [PubMed] [Google Scholar]
- 41.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 42.Sarsero, J. P., and A. J. Pittard. 1995. Membrane topology analysis of Escherichia coli K-12 Mtr permease by alkaline phosphatase and β-galactosidase fusions. J. Bacteriol. 177:297-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sarsero, J. P., P. J. Wookey, P. Gollnick, C. Yanofsky, and A. J. Pittard. 1991. A new family of integral membrane proteins involved in transport of aromatic amino acids in Escherichia coli. J. Bacteriol. 173:3231-3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suzuki, H., W. Hashimoto, and H. Kumagai. 1993. Escherichia coli K-12 can utilize an exogenous γ-glutamyl peptide as an amino acid source, for which γ-glutamyltranspeptidase is essential. J. Bacteriol. 175:6038-6040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Umbarger, H. E. 1996. Biosynthesis of the branched-chain amino acids, p. 442-457. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed., vol. 1. American Society for Microbiology, Washington, D.C.
- 46.Wang, P., J. Yang, A. Ishihama, and A. J. Pittard. 1998. Demonstration that the TyrR protein and RNA polymerase complex formed at the divergent P3 promoter inhibits binding of RNA polymerase to the major promoter, P1, of the aroP gene of Escherichia coli. J. Bacteriol. 180:5466-5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang, P., J. Yang, B. Lawley, and A. J. Pittard. 1997. Repression of the aroP gene of Escherichia coli involves activation of a divergent promoter. J. Bacteriol. 179:4213-4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang, P., J. Yang, and A. J. Pittard. 1997. Promoters and transcripts associated with the aroP gene of Escherichia coli. J. Bacteriol. 179:4206-4212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Whipp, M. J., D. M. Halsall, and A. J. Pittard. 1980. Isolation and characterization of an Escherichia coli K-12 mutant defective in tyrosine- and phenylalanine-specific transport systems. J. Bacteriol. 143:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wood, J. M. 1975. Leucine transport in Escherichia coli. The resolution of multiple transport systems and their coupling to metabolic energy. J. Biol. Chem. 250:4477-4485. [PubMed] [Google Scholar]
- 51.Wookey, P. J., J. Pittard, S. M. Forrest, and B. E. Davidson. 1984. Cloning of the tyrP gene and further characterization of the tyrosine-specific transport system in Escherichia coli K-12. J. Bacteriol. 160:169-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wookey, P. J., and A. J. Pittard. 1988. DNA sequence of the gene (tyrP) encoding the tyrosine-specific transport system of Escherichia coli. J. Bacteriol. 170:4946-4949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamato, I., and Y. Anraku. 1980. Genetic and biochemical studies of transport systems for branched-chain amino acids in Escherichia coli K-12: isolation and properties of mutants defective in leucine-repressible transport activities. J. Bacteriol. 144:36-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yamato, I., M. Ohki, and Y. Anraku. 1979. Genetic and biochemical studies of transport systems for branched-chain amino acids in Escherichia coli. J. Bacteriol. 138:24-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang, J., P. Wang, and A. J. Pittard. 1999. Mechanism of repression of the aroP P2 promoter by the TyrR protein of Escherichia coli. J. Bacteriol. 181:6411-6418. [DOI] [PMC free article] [PubMed] [Google Scholar]