Abstract
The natural history of lifespan cognitive performance and its late-life determinants have been studied from an array of perspectives. Significant insights come from psychological disciplines including cognitive, developmental, and neuropsychology, as well as from medical specialties such as geriatrics, neurology, psychiatry, neuroradiology and neuropathology which contribute to the growing interdisciplinary scientific field, cognitive neuroscience of aging. Our survey of longitudinal studies of aging suggests that disease-oriented investigations commonly do not adequately consider normative cognitive changes, while developmental studies do not sufficiently measure and model non-normative cognitive aging. We argue for an integrative perspective that considers both of these influences on cognitive trajectories, and present a series of methodological issues that have not been addressed comprehensively. We conclude that interdisciplinary methods from longitudinal observational studies should be leveraged to enable translational interventions to promote brain longevity.
Keywords: cognitive aging, change point, robust norms, measurement burst
OVERVIEW
Cognitive trajectories across the lifespan are determined by a combination of normative and non-normative processes which affect brain function1. These influences have been studied in the context of the aging brain through an array of perspectives. Significant insights come from psychological disciplines including cognitive, developmental, and neuropsychology, as well as from medical specialties such as geriatrics, neurology, psychiatry, neuroradiology and neuropathology which contribute to a growing interdisciplinary scientific field: cognitive neuroscience of aging.
Lifespan developmental studies often focus on the natural history of human cognition common to all or most individuals. These changes transpire during obligate developmental and involutional stages and are often conceptualized as normative cognitive aging. Medical disciplines, such as neurology and psychiatry, with their interests in disease, often focus on changes which occur only in particular individuals or carefully specified groups. These non-normative influences may increase in intensity or prevalence with increasing age (e.g. atherosclerosis or Alzheimer’s) but they are not an inevitable part of the aging process, and are not well predicted by chronological age. Our survey of longitudinal studies of aging suggests that disease-oriented investigations commonly do not adequately consider normative cognitive changes, while developmental studies do not sufficiently measure and model non-normative cognitive decline. Herein, we argue for an integrative perspective that considers both the normative and non-normative aspects of cognitive aging.
We recognize that both normative and non-normative cognitive aging are stunningly complex phenomena influenced by a broad range of factors acting on various timescales. Normative aging is associated with improving cognitive abilities through early adulthood, followed by a period of relative stability during mid-life and late-life decline. Non-normative influences produce additional effects superimposed on the complex normative landscape. For example, Alzheimer’s disease (AD) may produce accelerated cognitive decline for a number of years before it is diagnosed2; prior to diagnosis, individuals with preclinical or prodromal AD contribute to cognitive decline that may be interpreted as normative3. In certain individuals, cerebrovascular disease, cardiopulmonary conditions, and other comorbidities alter cognitive trajectories in idiosyncratic ways. The Table outlines the most common etiologic mechanisms which contribute to non-normative cognitive change in aging individuals.
TABLE 1.
Selected non-normative influences on cognitive trajectories by mechanism
| Neurodegenerative Proteinopathies | Cerebrovascular | Systemic/Homeostatic |
|---|---|---|
| β-amyloidopathy | Stroke | Stress, pain, sleep |
| Tauopathy | White matter degeneration (leukoaraiosis) | Emotional dysregulation |
| α-synucleinopathy | Diabetes, hypertension, dyslipidemia | Diabetes, hypertension, dyslipidemia |
| TDP-43opathy | Cardiorespiratory insufficiency | CNS inflammation |
| Hippocampal sclerosis | Hippocampal sclerosis | Medications, toxins |
CNS=Central nervous system. β-amyloid, tau, α-synuclein, and TDP-43 (Transactivation response DNA binding protein) are examples of proteins which aggregate and are detectable via pathologic evaluation in neurodegenerative diseases. Note that mechanistic categories may not be entirely distinct, for example if ischemic changes lead to neurodegeneration. As such, pathobiologic conditions may pertain to more than one category.
In addition, there are important within-person processes that influence cognitive performance over a range of timescales (moment-to-moment, day-to-day, months-to-years) whose detection requires temporal sampling strategies tailored to the process under investigation. Intra-individual variability in cognitive performance is often attributed to the unreliability of cognitive tests. We will argue that the unreliable component of intra-individual variability can be minimized with optimal measurement strategies. Much of the variation that is considered unreliable may be explained by state fluctuations associated with changes in mood, pain, sleep, stress, and motivation. Additional influences on cognitive trajectories include environmental exposures, medications, acute and episodic neuropsychiatric syndromes as well as social behaviors. We acknowledge that unmeasured error can never be fully eliminated and that selective attrition from longitudinal studies presents particular challenges.
Herein we approach a series of complex issues that merit further attention: 1) How to better characterize changes in cognitive function at the group level and within individuals; 2) How to evaluate within-person cognitive change on various timescales; 3) How to disentangle normative and non-normative factors which affect cognitive trajectories of older adults.
ASSESSING COGNITIVE STATUS AND CHANGE
Cognitive status can be assessed using 1) tests of global function (mental status tests); 2) conventional neuropsychological tests designed to gauge one or more specific cognitive domains (e.g. verbal memory, attention); or 3) experimental cognitive procedures. Global measures of cognitive function, such as the Mini-Mental State Exam (MMSE)4 are widely used. The advantages of familiarity and ease of use are offset by some disadvantages for studies which model lifespan cognitive trajectories or differentiate patterns and causes of longitudinal change. These tests have scores that are not normally distributed across the lifespan and that may be difficult to normalize through transformations. They have non-linear measurement properties5, including notable ceiling and floor effects (which artificially inflate reliability). They have poor sensitivity to normative age-related changes, and for studying non-normative processes which preferentially influence specific cognitive domains (e.g. memory in AD), averaging across domains may attenuate the effects that are being measured. For the preponderance of longitudinal studies which include such instruments, these instruments provide useful summary information. Contemporary statistical approaches can make use of item-level responses to transform global cognitive outcomes into psychometrically cocalibrated measures which outperform unidimensional total scores when quantifying longitudinal change5, 6.
A single global measure intended to gauge multiple aspects of cognition should be distinguished from a general underlying factor, such as processing speed, which may prove successful in characterizing patterns of relationships between chronological age and a range of cognitive domains7. “Common factor” theories of cognitive aging have been supported by numerous cross-sectional studies demonstrating shared relationships between increasing age and a range of specific cognitive measures. Longitudinal studies are required to estimate rates of within-person change in specific cognitive domains; to the extent that domain-specific rates of change may be correlated, this may result from the cumulative effects of multiple age-associated conditions and does not necessarily support a common factor theory of cognitive aging8, 9. Multivariate modeling techniques suggest that hierarchical models made up of general and specific factors characterize normative and non-normative cognitive aging10, 11. Such methods can be applied to disentangle longitudinal changes in general and specific aspects of cognitive function.
Traditional neuropsychological domains such as memory and executive functioning are measured with one or several instruments. Factor analysis is sometimes used to identify specific domains of cognitive function summarizing across a number of tests. These approaches, though useful, may depend upon unsupported psychometric assumptions. Caution is required when summarizing, combining, or transforming conventional neuropsychological measures to develop summary scores. Nonlinear measurement properties, especially at upper and lower bounds of performance can be circumvented, at least partially, by developing psychometrically-matched composite scales12.
When measuring cognitive performance, instrument validity and reliability are crucial. Unreliable measures make it difficult to detect change in cognitive performance and attenuate power for identifying group differences. Tests of mental status and general cognition are not well suited to detecting domain-specific change over time. Conventional neuropsychological tests provide greater domain specificity and are a mainstay of longitudinal cognitive studies. Their utility is sometimes limited by modest test-retest reliability, practice effects, and the limited availability of multiple equivalent forms. Nevertheless, cross-sectional diagnosis and ascertainment of clinical samples at elevated risk for future decline is still best accomplished via neuropsychological tests evidencing selective cognitive impairment. For the purposes of improving reliability, modeling repeated-administration performance trends, and increasing precision of estimated cognitive decline, experimental assessments—typically reaction time (RT) tasks adapted from cognitive psychology paradigms—can be integrated within clinical and epidemiological studies of aging via intensive scheduled observation, as described below in the context of measurement bursts.
Assessments of cognitive status in longitudinal studies should be selected based on the nature of the sample under study. Defining groups by chronological age or even by disease status inevitably leaves substantial heterogeneity of cognitive trajectories within groups. In longitudinal studies, heterogeneity is likely to increase over time, an effect attributable to non-normative processes affecting subsets of individuals. Put another way, samples of apparently normal elders will predictably include individuals with undetected disease, leading to heterogeneous cognitive trajectories. Summary measures of cognitive status and change in mixed groups reflect both normative and non-normative aging. Equally, biological or disease-oriented studies which define groups by diagnosis, genetics, or other biomarkers must contend with the substantial influence of normative changes and other unmeasured influences on their cognitive outcomes of choice.
In the following sections, we review some of the intrinsic conceptual challenges for longitudinal studies of aging and describe methodological alternatives applicable to specific research settings and goals.
MEASUREMENT BURSTS INCREASE POWER TO DETECT CHANGE
Most longitudinal studies employ a succession of single-timepoint assessments at infrequent intervals. By contrast, measurement-burst protocols involve closely-spaced clusters of assessment sessions13. This temporal sampling strategy provides an opportunity to improve reliability, enhance sensitivity to change, to measure variability, and to identify factors that account for that variability. This procedure has been mathematically and empirically shown to increase reliability and sensitivity to change14 and offers significant advantages to longitudinal studies. The power of the measurement burst is augmented when cognitive assessment is combined with assessment of psychological state at each session, allowing investigators to directly assess the influence of short-term changes in mood, pain, sleep, and stress on cognitive performance. Physiological measures, such as serum glucose, blood pressure, or oxygen saturation may be similarly coupled to variation in cognitive performance.
Studies with measurement-burst designs can address variability and changes in human behavior that transpire over distinct temporal intervals13. For example, intra-individual variability, in the sense of moment-to-moment or day-to-day instability of task performance, is generally treated as an unmeasured source of error or attributed to test-retest effects. Using measurement bursts, one can endeavor to explain the within-burst variability which reflects a balance of resilience (the degree to which cognitive performance is affected by fluctuating psychological state) and neuroplasticity (reflecting enduring session-to-session learning). Combining fine-grained cognitive tasks and intensive temporal sampling strategies with multilevel modeling of within-person relationships allows investigators to explore brain-behavioral relationships in novel ways. Additionally, by explaining important sources of variability, one can model the “true” level of cognitive abilities over each burst, and thereby improve the precision of estimated cognitive change. Detection of intra-individual change that takes place over months to years is critically important for understanding normative cognitive aging as well as the cognitive effects of neurodegeneration and other non-normative processes. By sampling multiple times within each burst, and by taking short-term variability into account, the measurement-burst approach improves the assessment of latent cognitive abilities and the quantification of longitudinal change.
NON-NORMATIVE AGING: MODELING THE ACCELERATED COGNITIVE DECLINE OF BRAIN FAILURE
In the setting of acquired cognitive impairments which interfere with daily activities, the term dementia is used to describe the “clinically observable result of the cumulative burden of multiple pathological insults in the brain”15. Widely-applied diagnostic criteria require impairment of memory and of at least one other cognitive domain of sufficient severity to interfere with occupational and social function. This seemingly simple definition is complex to operationalize as there are many approaches to measuring each cognitive domain and controversy in the definitions of domain-specific impairment. Disabling cognitive disorders with prominent cerebrovascular contributions will not necessarily meet the criteria for memory impairment.16 Interference with occupational and social function is often the last feature to develop and is related, at least in part, to an individual’s premorbid occupational and social function. Time of dementia onset is a somewhat arbitrary temporal referent determined by social factors as well as brain biology. Because the term dementia is stigmatic and lacks specificity, some have argued for its excision from scientific and common lexicon17. We have observed diffuse and inconsistent usage of that term across disciplines and contexts; its most useful application appears to be for rating clinical severity. Indeed, in research settings among consistently-trained clinicians, the reliable staging of symptomatology can be achieved, even for mild disease18.
As operationalized, dementia is ascertained only after an extended period of non-normative cognitive decline. Evidence suggests that pathobiological changes unfold in the brain during this period, producing accelerated cognitive decline over years. To describe this trajectory, longitudinal studies typically adopt analytical approaches using one of three candidate time scales: 1) Chronological age; 2) Time since enrollment or study year; 3) Time prior to diagnosis. The first two strategies, though widely used, do not reveal the relationship between unfolding of brain disease and cognitive changes. Individuals of the same age or who enrolled during the same period are likely to be at different stages of normative brain aging and non-normative brain disorders. Thus, chronological age and time since enrollment are not well correlated with unfolding of biological processes resulting in dementia. As such, heterogeneity in rates of age-based decline and their resulting large covariances derive from the chosen alignment of individuals. Only the third option—aligning individuals at time of diagnosis—attempts to associate cognitive changes with roughly equivalent stages of a causal process8. Diagnoses need to be assigned using consistently-applied research criteria; alternatively, one can align subjects at other terminal outcomes such as a cognitive performance threshold, study attrition, or death. This procedure shifts the emphasis from a time-based to a process-based approach to understanding non-normative cognitive aging.
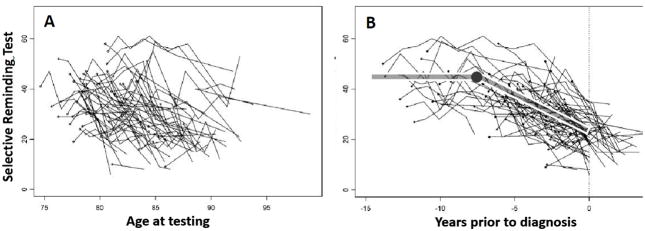
Figure 1 demonstrates that aligning individuals at time of diagnosis reveals the preclinical trajectory in a way which is obscured by using chronological age as the index variable. The resulting trajectories can be estimated using change point models, which have been employed in describing cognitive trajectories preceding dementia2, AD19, 20 and mild cognitive impairment (MCI)21. The basic conceptual model assumes that a cognitive measure declines at a constant rate prior to some unknown change point (presumed to be several years prior to diagnosis), after which the decline would be more rapid. This assumption is supported by theory and by previously reported findings. Consistent results from several longitudinal studies applying change-point models to verbal episodic memory performance observed a change point and accelerated memory decline 7–8 years prior to AD diagnosis19, 20, 22.
Figure 1. Spaghetti plots of memory scores as a function of age in 72 participants who developed dementia.
Within-individual trajectories of Buschke Selective Reminding Test (SRT) scores for the participants in a longitudinal aging study who develop dementia as a function of chronological age (Figure 1A) and time before the clinical diagnosis (Figure 1B). Superimposed on the plots in 1B is the change-point trajectory which is apparent only when cases are aligned at time of diagnosis. Model-estimated change point was 7.6 years prior to diagnosis, with 95% confidence interval of 4.6–9.0 years. Figure adapted from Hall, et al. 2000, data are reported in Hall, et al. 2003.
Change-point models are relevant to estimating trajectories in individuals who develop clinical disorders, as well as in cases with terminal events such as death or study attrition23, 24. The change-point methodology provides a parsimonious and interpretable descriptive model applicable to such study subjects, provided there is sufficient follow-up. On the other hand, many subjects will not have such terminal events, and would be omitted from many analyses. Change-point methodology has recently been extended to jointly model the cognitive trajectories of individuals who do and do not achieve the terminal event of interest 25 as well as the competing risks of developing dementia and dying without dementia26. In any case, change-point models require substantial longitudinal data and cannot address the problem of accurate classification or diagnosis of subjects before they have had sufficient follow-up required to model longitudinal cognitive trajectories or ascertain terminal events.
ROBUST NORMS: REDUCING NON-NORMATIVE INFLUENCES ON STUDIES OF NORMATIVE COGNITIVE AGING
Because cognitive function declines for many years prior to a clinical diagnosis, studies of cognitively normal elders screened for dementia may include individuals in the stage of accelerated decline. The possible inclusion of individuals with this and other forms of non-normative cognitive aging is a frequently cited limitation of normative aging studies. By failing to exclude individuals with preclinical dementia, for example, studies underestimate the mean, overestimate the variance, and overestimate the effect of age on cognitive measures3, 27. In practice, this problem makes it difficult to distinguish normative cognitive aging from disease-related change at cross-section, as individuals with preclinical conditions may still perform within normal limits. Moreover, group declines in cognitive performance may be attributed to normative aging, whereas the effects may be driven by a subset of individuals with preclinical disease. Similarly, it is possible that strictly normative cognitive trajectories may be overlooked or attributed to undetected disease.
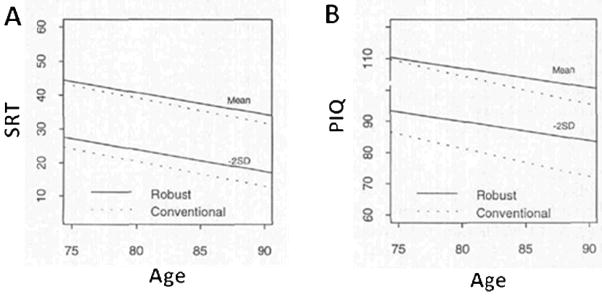
Insight into the extent of this problem, and approaches to disentangle normative and non-normative cognitive performance, come from studies which employ robust norming procedures28. The conventional method of identifying cognitive impairment involves comparing an individual’s cognitive performance with that of a reference or normative group studied at a single timepoint. Robust norms are generated using baseline cognitive data from a group constructed following exclusion of individuals who subsequently manifest dementia within a specified follow-up period3, 28. As opposed to conventional norms, robust norms more accurately estimate the common effect of age on cognition in the absence of very early disease. While conventional norms may underestimate the recognition of impairment and future cognitive decliners, robust norms can improve cross-sectional diagnosis of cognitive impairment3, 29, and better predict subsequent cognitive decline27. Figure 2 illustrates the differences between robust and conventional norms, which increase as a function of age and departure from the mean.
Figure 2. Mean and impairment cutoff by age using conventional and robust norms.
Estimates of Buschke Selective Reminding Test (SRT) scores (Figure 2A) and Wechsler Performance IQ (PIQ) scores (Figure 2B) corresponding to the mean and 2 standard deviations (SD) below the mean obtained from the conventional (dashed line) and robust (solid line) normative samples. The differences between the robust and conventional norms increase as a function of age and as scores depart from the mean. Adapted from Sliwinski, et al. 1996.
As discussed, robust norms can successfully be generated from longitudinal studies with extensive follow-up, and can be profitably applied to other settings. Robust norming may ultimately incorporate strategies which reduce longitudinal follow-up requirements. That is, instead of removing individuals who go on to develop dementia, combining robust norms at cross-section with neuroimaging and other biomarkers may provide a basis for prospectively identifying prodromal disease. Future investigations which aim to carefully characterize the process of normative cognitive aging might extend the concept of robust cross-sectional norms to specify normative rates of intra-individual change in cognitive performance. Among the advantages would be using individuals as their own controls in defining normative cognitive trajectories, as well as reducing misclassification of individuals who perform below cross-sectional cut scores due to factors such as culture or education rather than aging or disease.
SUMMARY AND SPECULATIONS
Despite the complexities of measurement, timescales, influences and perspectives, herein, we present strategies to address conceptual challenges in cognitive aging. As discussed, applying change-point models to longitudinal data approximates the cognitive trajectory as a prolonged pre-change linear decline over the mid-to-late adult lifespan followed by a post-change acceleration of cognitive decline. We expect that normative and non-normative biological determinants of brain function will have differential effects on the pre- and post-change point trajectories as well as the onset of accelerated cognitive decline.
Combining the change point models with disease-specific measures, biomarkers, and mediating factors can provide a framework for testing hypotheses and producing interpretable research findings. For example, by estimating distinct trajectories based on individual differences in lifespan experience, change-point techniques have been adapted to model the effect of cognitive reserve30. Indeed, educational attainment and participation in leisure activities has been shown to delay the onset of accelerated memory decline in preclinical stages31, 32. Further, distinct neurodegenerative proteinopathies may have dissociable effects on cognitive trajectories, both with respect to time-to-diagnosis and to cognitive domains affected. White matter changes or diabetes may have relatively strong effects on the pre-change trajectory, whereas cerebral amyloidosis may drive the post-change accelerated decline. Pain, stress, and sleep deprivation may contribute to intra-individual variability around the pre-change trajectory, propositions which can be explored using intensive measurement strategies tailored to the phenomenon and timescale under investigation. Studies will be greatly facilitated by employing robust normative approaches to improve diagnostic accuracy and minimize the effects of admixed individuals with preclinical neurodegeneration.
Alternatives to the change point model incorporate a plateau33 or a period of cognitive stability34 preceding clinical AD diagnosis. These may be regarded as variants of the generic change-point trajectory to resolve performance dynamics surrounding the onset of accelerated cognitive decline. A multiple change-point model could also be posited to account for concurrent neurodegenerative, cerebrovascular, and systemic/homeostatic mechanisms.
Identifying, disentangling, and accounting for normative and non-normative influences on lifespan cognitive trajectories will establish a shared conceptual framework for future interdisciplinary investigations into the complexity of the aging brain. Without delay, cognitive scientific methods from longitudinal observational studies can enhance and enable translational interventions to promote brain longevity.
Acknowledgments
This paper is based on a conference on Longitudinal Studies in Aging, held in January 2009 and sponsored by a grant from the Robert Wood Johnson Foundation.
Support received from NIH program project grant AG003949
Sponsor’s Role: None
Footnotes
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
Author Contributions: Steinerman: study concept and design, analysis and interpretation of data, preparation of manuscript.
Hall: acquisition of subjects and/or data, analysis and interpretation of data, and preparation of manuscript.
Sliwinski: analysis and interpretation of data, and preparation of manuscript.
Lipton: study concept and design, acquisition of subjects and/or data, analysis and interpretation of data, and preparation of manuscript
References
- 1.Sliwinski MJ, Hofer SM, Hall C, et al. Modeling memory decline in older adults: the importance of preclinical dementia, attrition, and chronological age. Psychol Aging. 2003;18:658–671. doi: 10.1037/0882-7974.18.4.658. [DOI] [PubMed] [Google Scholar]
- 2.Hall CB, Ying J, Kuo L, et al. Bayesian and profile likelihood change point methods for modeling cognitive function over time. Computational Statistics & Data Analysis. 2003;42:91–109. [Google Scholar]
- 3.Sliwinski M, Lipton RB, Buschke H, et al. The effects of preclinical dementia on estimates of normal cognitive functioning in aging. J Gerontol B Psychol Sci Soc Sci. 1996;51:217–225. doi: 10.1093/geronb/51b.4.p217. [DOI] [PubMed] [Google Scholar]
- 4.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 5.Mungas D, Reed BR. Application of item response theory for development of a global functioning measure of dementia with linear measurement properties. Stat Med. 2000;19:1631–1644. doi: 10.1002/(sici)1097-0258(20000615/30)19:11/12<1631::aid-sim451>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 6.Crane PK, Narasimhalu K, Gibbons LE, et al. Item response theory facilitated cocalibrating cognitive tests and reduced bias in estimated rates of decline. J Clin Epidemiol. 2008;61:1018–1027. e1019. doi: 10.1016/j.jclinepi.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychol Rev. 1996;103:403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- 8.Sliwinski MJ, Hofer SM, Hall C. Correlated and coupled cognitive change in older adults with and without preclinical dementia. Psychol Aging. 2003;18:672–683. doi: 10.1037/0882-7974.18.4.672. [DOI] [PubMed] [Google Scholar]
- 9.Wilson RS, Beckett LA, Barnes LL, et al. Individual differences in rates of change in cognitive abilities of older persons. Psychol Aging. 2002;17:179–193. [PubMed] [Google Scholar]
- 10.Salthouse TA. Decomposing age correlations on neuropsychological and cognitive variables. J Int Neuropsychol Soc. 2009;15:650–661. doi: 10.1017/S1355617709990385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson DK, Storandt M, Morris JC, et al. Cognitive profiles in dementia: Alzheimer disease vs healthy brain aging. Neurology. 2008;71:1783–1789. doi: 10.1212/01.wnl.0000335972.35970.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mungas D, Reed BR, Kramer JH. Psychometrically matched measures of global cognition, memory, and executive function for assessment of cognitive decline in older persons. Neuropsychology. 2003;17:380–392. doi: 10.1037/0894-4105.17.3.380. [DOI] [PubMed] [Google Scholar]
- 13.Sliwinski MJ. Measurement-burst designs for social health research. Soc Personal Psychol Compass. 2008;2:245–261. [Google Scholar]
- 14.Nesselroade JR, Salthouse TA. Methodological and theoretical implications of intraindividual variability in perceptual-motor performance. J Gerontol B Psychol Sci Soc Sci. 2004;59:49–55. doi: 10.1093/geronb/59.2.p49. [DOI] [PubMed] [Google Scholar]
- 15.Jack CR, Jr, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9:119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roman GC, Sachdev P, Royall DR, et al. Vascular cognitive disorder: A new diagnostic category updating vascular cognitive impairment and vascular dementia. J Neurol Sci. 2004;226:81–87. doi: 10.1016/j.jns.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 17.Trachtenberg DI, Trojanowski JQ. Dementia: A word to be forgotten. Arch Neurol. 2008;65:593–595. doi: 10.1001/archneur.65.5.593. [DOI] [PubMed] [Google Scholar]
- 18.Williams MM, Roe CM, Morris JC. Stability of the Clinical Dementia Rating, 1979–2007. Arch Neurol. 2009;66:773–777. doi: 10.1001/archneurol.2009.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hall CB, Lipton RB, Sliwinski M, et al. A change point model for estimating the onset of cognitive decline in preclinical Alzheimer’s disease. Stat Med. 2000;19:1555–1566. doi: 10.1002/(sici)1097-0258(20000615/30)19:11/12<1555::aid-sim445>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 20.Hall CB, Ying J, Kuo L, et al. Estimation of bivariate measurements having different change points, with application to cognitive ageing. Stat Med. 2001;20:3695–3714. doi: 10.1002/sim.1113. [DOI] [PubMed] [Google Scholar]
- 21.Howieson DB, Carlson NE, Moore MM, et al. Trajectory of mild cognitive impairment onset. J Int Neuropsychol Soc. 2008;14:192–198. doi: 10.1017/S1355617708080375. [DOI] [PubMed] [Google Scholar]
- 22.Grober E, Hall CB, Lipton RB, et al. Memory impairment, executive dysfunction, and intellectual decline in preclinical Alzheimer’s disease. J Int Neuropsychol Soc. 2008;14:266–278. doi: 10.1017/S1355617708080302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laukka EJ, MacDonald SW, Backman L. Contrasting cognitive trajectories of impending death and preclinical dementia in the very old. Neurology. 2006;66:833–838. doi: 10.1212/01.wnl.0000203112.12554.f4. [DOI] [PubMed] [Google Scholar]
- 24.Wilson RS, Beckett LA, Bienias JL, et al. Terminal decline in cognitive function. Neurology. 2003;60:1782–1787. doi: 10.1212/01.wnl.0000068019.60901.c1. [DOI] [PubMed] [Google Scholar]
- 25.Jacqmin-Gadda H, Commenges D, Dartigues JF. Random change point model for joint modeling of cognitive decline and dementia. Biometrics. 2006;62:254–260. doi: 10.1111/j.1541-0420.2005.00443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu B, Ghosh P. Joint Modeling for Cognitive Trajectory and Risk of Dementia in the Presence of Death. Biometrics. 2009 May 4; doi: 10.1111/j.1541-0420.2009.01261.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Santi S, Pirraglia E, Barr W, et al. Robust and conventional neuropsychological norms: diagnosis and prediction of age-related cognitive decline. Neuropsychology. 2008;22:469–484. doi: 10.1037/0894-4105.22.4.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holtzer R, Goldin Y, Zimmerman M, et al. Robust norms for selected neuropsychological tests in older adults. Arch Clin Neuropsychol. 2008;23:531–541. doi: 10.1016/j.acn.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manly JJ, Bell-McGinty S, Tang MX, et al. Implementing diagnostic criteria and estimating frequency of mild cognitive impairment in an urban community. Arch Neurol. 2005;62:1739–1746. doi: 10.1001/archneur.62.11.1739. [DOI] [PubMed] [Google Scholar]
- 30.Stern Y. Cognitive reserve. Neuropsychologia. 2009;47:2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hall CB, Derby C, LeValley A, et al. Education delays accelerated decline on a memory test in persons who develop dementia. Neurology. 2007;69:1657–1664. doi: 10.1212/01.wnl.0000278163.82636.30. [DOI] [PubMed] [Google Scholar]
- 32.Hall CB, Lipton RB, Sliwinski M, et al. Cognitive activities delay onset of memory decline in persons who develop dementia. Neurology. 2009;73:356–361. doi: 10.1212/WNL.0b013e3181b04ae3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith GE, Pankratz VS, Negash S, et al. A plateau in pre-Alzheimer memory decline: evidence for compensatory mechanisms? Neurology. 2007;69:133–139. doi: 10.1212/01.wnl.0000265594.23511.16. [DOI] [PubMed] [Google Scholar]
- 34.Backman L, Small BJ, Fratiglioni L. Stability of the preclinical episodic memory deficit in Alzheimer’s disease. Brain. 2001;124:96–102. doi: 10.1093/brain/124.1.96. [DOI] [PubMed] [Google Scholar]