Abstract
The capacity to walk independently is a central component of independent living. Numerous large and well designed longitudinal studies have shown that gait speed, a reliable marker of mobility, tends to decline with age and as a consequence of chronic disease. This decline in performance is of utmost importance as slow walking speed is a strong, independent predictor of disability, health care utilization, nursing home admission, and mortality. Based on these robust findings, it has been postulated that the age-associated decline in walking speed is a reliable barometer of the impact of biological aging on health and functional status. Despite the extraordinary prognostic information provided by walking speed, which is often superior to traditional medical information, we have a limited understanding of the mechanisms that underlie age- and disease-related gait speed decline. Identifying the mechanisms that underlie the prognostic value of walking speed should be a central theme in the design of the next generation of longitudinal studies of aging, with appropriate measures introduced, and analytical approaches incorporated.
Here, we argue that the decline in customary walking speed with aging and disease is induced by a scarcity of available energy. Based on our work in the Baltimore Longitudinal Study of Aging, we provide examples of measures, operationalized dimensions, and analytical models that may be implemented to tackle this hypothesis. Our main premise is simple: the biochemical processes that maintain life, secure homeostatic equilibrium, and prevent the collapse of health require energy. If energy becomes deficient, adaptive behaviors develop aimed at conserving energy.
Keywords: Energy, Aging, Physical Function, Gait Speed, Fatigue
INTRODUCTION
Exceptionally slow gait speed, a hallmark of frailty, frequently accompanies reports of fatigue and reduced overall activity. Although commonly observed in daily life, the contribution of fatigue to disability in older persons is not well-understood. Research conducted across a variety of animal species, from worms to mice, demonstrates that mobility remains largely stable with age until late life and then declines rapidly and that within species, animals which remain mobile later in life tend to survive longer. Even simple movements critical for life such as pharyngeal peristalsis in C. Elegans decline with aging 1. Laboratory animals show lower activity levels 2 and reduced speed of voluntary movement 3 with age and those with higher baseline performance and greater time spent in voluntary physical activity exhibit lower rates of mortality 2, 3. These observations imply that the root cause of age-related mobility decline is not a function of cultural expectations for aged individuals, but rather core physiological changes that occur with aging.
The observation that severe mobility loss or gait-slowing occurs in the latest stages of life suggests that mobility is so central to life that energy is shifted away from walking activity only when other vital activities are threatened. If this interpretation is correct, poor mobility develops only when a critical threshold of energy scarcity has been passed, a condition that is associated with high mortality risk via collapse of homeostatic regulation 4. This is consistent with the work of Priede and coll. who found fish that spent more time swimming at maximal aerobic capacity tended to have the highest risk of death 4, because they needed to maintain a level of energetic demand close to the upper limits of their metabolic boundaries. Further, the relationship between metabolic rate and mortality is U-shaped with increased risk at the extremes and lowest risk found in animals with a moderate metabolic rate 4, 5. These findings support a direct connection between mortality and metabolic rate and underscore the importance of the ability to perform essential functions at a moderate, or submaximal, level of metabolic capacity.
HOW DOES THIS HYPOTHESIS FIT THE RESULTS OF LONGITUDINAL STUDIES?
In humans, age-related decline in physical performance and the prognostic significance of such decline is well-established 6–8. In the Established Populations for Epidemiologic Studies of the Elderly (EPESE), lower baseline lower extremity performance scores were associated with increased frequency of mobility disability and dependence in one or more activities of daily living at follow-up 6, 7. Gait speed alone was almost as strong at predicting incident disability as the full performance battery 7. In the Women’s Health and Aging Study II (WHAS II), mobility limitation defined as difficulty walking ½ mile or climbing stairs or slowed walking speed predicted incident mobility disability in older women 9. Together, these results indicate physical performance particularly walking speed reflects current and predicts future functional ability.
Although collectively these findings establish slow and declining walking speed as hallmarks of impending functional limitation, disability, and death 7, 9–11, it is unknown why gait speed declines with age or why it is strongly predictive of adverse outcomes. Two main hypotheses should be considered: 1) Slow walking speed may be a marker of reduced mobility that in itself plays a causal role in health deterioration and 2) Biological and physiological conditions that contribute to reduced speed are also primarily responsible for subsequent negative health events. Addressing this dichotomy may help develop appropriate interventions for disability prevention in older persons.
Multiple factors have been associated with gait speed and its decline, including maximal energy expenditure, body composition, and biomechanics of gait and balance 7, 12, but little is known about the role of changing energy availability with age. Maximal energy expenditure (VO2 max), the upper limit of energy availability or the capacity to perform vigorous activities, declines with age 13, 14. The ability to maintain fast walking over moderate distances, a proxy measure of VO2 max in older adults, also declines with age 15, 16. We contend that, as VO2 max approaches the energy required for usual walking, walking speed declines as an adaptation which allows individuals to remain within safe limits of their energetic boundaries.
Previous research has also demonstrated that sedentary behavior increases risk of future disability and that physical activity in the form of volitional walking preserves functional capacity 17, 18. Walking at least eight blocks per week was strongly inversely associated with severity of walking difficulty and gait speed decline in the Women’s Health and Aging Study (WHAS) 19. Similarly, in EPESE, an increased likelihood of dying without disability was found in the most physically active older adults relative to their sedentary counterparts 17. These studies suggest that preserving aerobic capacity and fitness, in general is a potential strategy to prevent mobility disability. Indeed, aerobic capacity strongly predicts longevity 20 18. In the Health, Aging, and Body Composition Study, aerobic capacity, as defined by performance on the long distance corridor walk (LDCW) a validated measure of cardiorespiratory fitness in older adults 21, 22, was inversely associated with mobility limitation, disability, and death 23.
Maximum oxygen consumption (VO2 max) declines with age 16 starting around age thirty and continuing at approximately 10% per decade, depending on health and activity level 13. Previous research suggests that VO2 max declines to approximately 12–16 ml/kg/min between the ages of 80 and 90 24. This is pivotal for understanding the pathway to mobility loss as peak VO2 of 20 ml/kg/min has been shown to predict functional limitations 25 and individuals with VO2 max of less than 18 ml/kg/min tend to report significant difficulty in performing daily tasks 26. To fully explore our proposed hypothetical model we need to face some methodological challenges. VO2 max represents an approximation of the maximal amount of energy that an individual can expend in 24 hours, which is the true determinant of what can be accomplished in daily life. Whether and to what extent VO2 max predicts the total maximal energy expendable in a one-day cycle, is unknown.
Resting metabolic rate (RMR) generally declines with age in part because of diminished lean mass. However, such a decline is often offset by increased energetic demands required to counteract the destabilizing effects on homeostasis of a host of metabolic disorders highly prevalent in older individuals. Partially confirming this hypothesis, in the Baltimore Longitudinal study of Aging, after accounting for lean mass individuals with little or no decline in RMR with aging had higher mortality than those who had the normal RMR age-associated decline 27. Noteworthy, the excess in RMR emerged as early as 10 to 15 years prior to death.
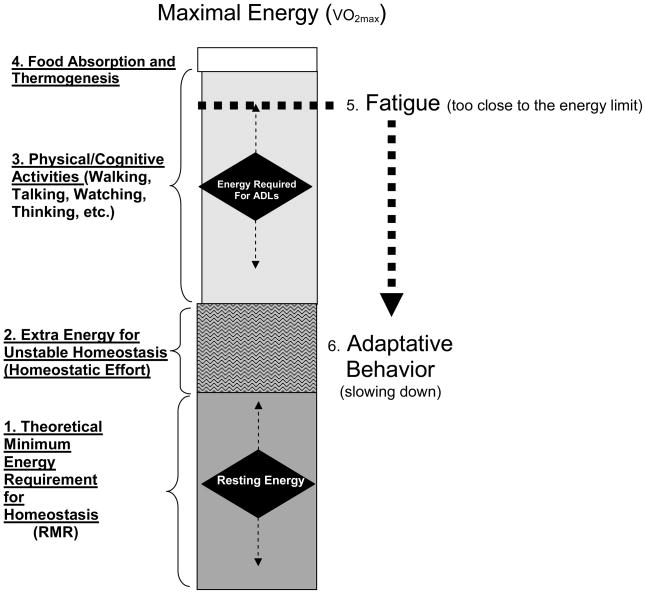
Thus, if maximum available energy declines and the cost of basic life support increases, the energy remaining for productive and essential activity will become progressively smaller Young and healthy individuals perform, most activities of daily living at a workload well below their maximum energetic capacity 28 and therefore can sustain such activity for a prolonged period 29. In late life when aerobic capacity has declined substantially even the most basic tasks challenge energetic limits and anaerobic pathways must be accessed to meet energetic demands 29 (Figure 1). It is reasonable to hypothesize that sustained anaerobic metabolism will trigger feelings of fatigue as commonly observed in endurance athletics. We believe this compression and downward shift of energetic scope may be a major contributor to age-related decline in physical activity. In sum, with aging and illness, a greater proportion of energy is required to perform daily tasks, contributing to greater fatigue, sedentary behavior, reduced endurance, and further decline in fitness 28, 30.
Figure 1. An Extended Model of Aging Energetics.
The box represents the total amount of energy available to an individual over 24 hours. The height of the box is determined by an individual’s maximal oxygen consumption (VO2 max), or the maximum amount of energy an individual can expend during physical activity. Total energy availability can be divided into sections which reflect energy utilization. Section (1) represents the theoretical minimal energy required to maintain life, or resting metabolic rate (RMR). Section (2) depicts extra energy required for unstable homeostasis. In older individuals, this may reflect the energy needed to combat multiple comorbidities as the body attempts to heal itself and/or the extra energy needed to perform physical tasks due to reduced biomechanical efficiency. Section (3) represents the energy used for daily activities, ranging from activities of daily living to volitional exercise. Section (4) represents the energy needed to break down food and maintain body temperature. In older adults, declines in maximal oxygen consumption compress the size of the box, resulting in less energy availability overall. Further, more energy is required to maintain homeostasis and perform daily tasks due to reduced metabolic and biomechanical efficiency which results in reduced energy available for “essential” tasks related to independent living and increased feelings of fatigue (5). These feelings represent a signal to the brain that energy resources are limited and that there is a need to slow down (6).
Of course, this is a simplification of a complex phenomenon. Physical fatigue, or exhaustion or weariness from labor or stress 31, can arise from changes at the level of skeletal muscle cells and/or decreased activation from the central nervous system. These changes and their association with the aging process are also not well understood, but are believed to involve biological, psychological, and physiological factors 28, which together influence decline in energy availability and hence the threshold of fatigue. . When the fatigue threshold is low, individuals tire easily and lead a sedentary existence which contributes to further declines in fitness. For example, climbing stairs and walking ¼ mile may become increasingly difficult due to deteriorations in muscle quality and development of clinical/subclinical disease. A low fatigue threshold may explain why, despite strong evidence that physical activity prevents disability and mortality in older adults, only a small percentage exercise regularly 15.
The theory presented above is highly plausible and consistent with findings from animal and limited human studies of metabolic rate and the risk of death 4 and physical performance and longevity 3. In the second part of this paper we offer some methodological clues on how the energetic hypothesis of disability could be tested in future longitudinal studies of aging. The methodological work described here was developed in the context of the BLSA. We also present preliminary data collected in the same population, for illustrative purposes.
METHODOLOGICAL DEVELOPMENT AND PRELIMINARY DATA
Energy Availability
To estimate overall energy availability, energy required to sustain life and to perform essential tasks; we measured energy expenditure in participants of the BLSA in three different states:
rest;
standardized submaximal walking (0.67 m/s);
walking at maximal speed for a prolonged period (400m);
Although treadmill-based VO2max is also assessed in the BLSA, this parameter is not addressed in this report. Rather, peak sustained VO2, as measured during the “long-distance corridor walk” (LDCW), a validated measure of cardiorespiratory fitness in older adults 21, 22, was used to allow for a measure of over-ground energy expenditure that is more consistent with the energy required for the daily living.
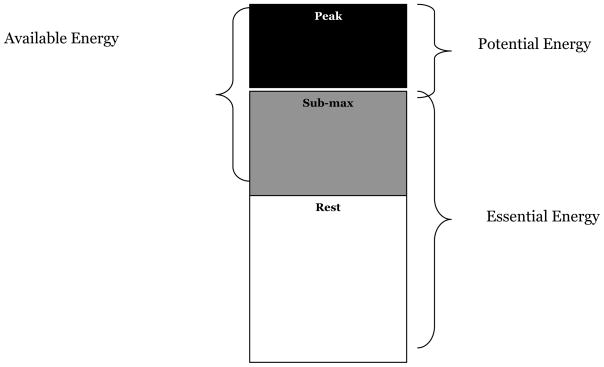
Using these measurements, we developed the following energy constructs (Figure 2):
Figure 2. Energy Constructs.
1. Essential energy (submaximal & resting EE*)
2. Potential energy (peak EE – submaximal EE*)
3. Available energy (peak EE – resting EE*)
*EE = Energy Expenditure
essential energy, the energy essential for independent living,
potential energy, the energy available above the energy essential for independent living, sometimes referred to as reserve capacity.
available energy, the total energy available to an individual above the energy required solely for living (rest).
We hypothesize that the age-related decline in physical activity and ultimately walking speed are compensatory mechanisms for decreased energy availability. Understanding factors that specifically affect these energy constructs may help explain why older persons tend to have a low fatigability threshold and identify new targets for disability prevention in older individuals.
To test this hypothesis, we studies 350 BLSA participants aged 50 years or older (48% female, mean age 70 ± 10.2 years, range: 50–96) evaluated between July 2007 and May 2009. The BLSA is a clinical research study on human aging supported by the National Institute on Aging Intramural Research Program (NIA - IRP), conducted in Baltimore since 1958. A general description of the sample and enrollment criteria of the BLSA has been previously reported 32. All protocols were approved by the Institutional Review Board of the Medstar Research Institute, and all participants provided written informed consent.
Energy Expenditure
Energy expenditure was calculated from the volume of oxygen consumed per kilogram of body weight (VO2 ml/kg/min) during rest, walking at a fixed speed, and walking at max speed for 400 meters. Breath-by-breath measurement of VO2 data (ml/kg/min) was collected and averaged into thirty second intervals to reduce variability.
Resting energy expenditure, as measured by resting metabolic rate (RMR)
RMR was assessed using indirect calorimetry in a quiet, thermo-neutral environment, in the morning prior to eating or drinking. Participants were asked to breathe into a mask attached to a Cosmed K4b2 portable metabolic analyzer (Cosmed, Rome, Italy) for approximately 20 minutes. At test completion, oxygen consumption (VO2), and respiratory quotient (RQ) were used to assess RMR.
To calculate average resting energy expenditure, readings from the first five minutes were discarded to allow time for the participant to adjust to the face mask and the testing procedure, and the remaining 10 minutes were averaged to arrive at a single measure of resting energy expenditure (ml/kg/min).
Essential energy expenditure, as represented by submaximal, slow paced walking
The submaximal walking test was performed on a treadmill at a speed of 0.67 m/s for 5 minutes, at 0% grade. This speed was chosen to allow assessment of the amount of energy expended at a level of walking that is predictive of future mobility disability 9, 10, and that is slow enough for even frail or impaired individuals to endeavor. Although it has been demonstrated that treadmill walking in older adults can alter step cadence, stance times, and energy expenditure compared to overground walking 33–35, use of a treadmill for this test was essential to ensure that participants walk at exactly the same speed, providing a standardized measure by which to gauge the amount of energy expended during a task essential for independent living.
Breath-by-breath measurement of expired air was conducted throughout the test and analyzed for oxygen and carbon dioxide content using a standard laboratory metabolic cart (Medical Graphics Corp., St. Paul, MN). To calculate average energy expended, readings from the first 1.5 minutes of testing were discarded to allow the participant time to adjust to the treadmill workload, and the remaining 3.5 minutes were averaged to arrive at a single measure of average energy expended (ml/kg/min) during submaximal standardized walking.
Peak energy expenditure
Peak energy expenditure was assessed during the 400 meter segment of the LDCW, a two-part, self-paced endurance walking test. The test was performed on a course in an uncarpeted corridor while the participant was wearing the Cosmed unit, a lightweight and non-cumbersome, portable device. After the Cosmed was placed on the participant, he/she was seated for 2 minutes, to allow acclimation to the face mask and harness. After two minutes, the participant stood, was escorted to the starting line, and instructed to walk at his/her preferred, comfortable walking speed until directed to stop. After 2.5 minutes, the participant was escorted back to the starting line, and instructed to walk “as fast as possible, at a pace you can sustain for 400 meters.” The course was 20 meters long and marked by cones at both ends. Standardized encouragement was given each lap along with the number of laps remaining. Split times for each lap and total time to walk 400m was recorded. The Cosmed remained on the participant for 2 minutes after the completion of the test to ensure adequate breath collection.
To calculate average peak energy expenditure, readings from the first 1.5 minutes of the 400m walk were discarded to allow the participant time to adjust to the workload and the remaining readings were averaged to arrive at single measure of the average energy expended (ml/kg/min) during 400 meters of peak sustained walking.
Energy Constructs
From these three basic measures of energy expenditure, we operationalized our energy constructs (Figure 2):
- Essential energy, the energy essential for independent living, that is the average energy expended during standardized submaximal walking inclusive of resting energy expenditure:
- Potential energy, the energy available above that which is essential for independent living. Potential energy is the difference between the average energy expended at peak sustained walking speed and during standardized submaximal walking:
- Available energy, the total energy available to an individual above the energy required solely for living (resting energy). Available energy is the difference between the average energy expended at peak sustained walking speed and resting energy expenditure:
Height, weight, and waist circumference were assessed using standardized methods. Body composition was assessed using most recent dual energy x-ray absorptiometry (DEXA) scan to provide information on fat and lean tissue over the total body. From this information, a ratio of fat-to-lean mass was calculated.
Statistical Analyses
The relationship between energy availability and age was explored using scatterplots and analyzed by linear regression models for each energy construct adjusted for: age, sex, height, and body composition. All models initially included interaction terms between: age and sex, age and body composition, and sex and body composition. All analyses were performed using Intercooled Stata version 10 (Statacorp., College Station, TX).
RESULTS
Table 1 shows study population characteristics. Mean resting metabolic rate was 2.6 ± 0.7 ml/kg/min, mean submaximal workload energy expenditure was 8.9 ± 1.7 ml/kg/min, and mean peak energy expenditure was 18.1 ± 4.6 ml/kg/min. These values are similar to those previously reported from studies conducted in healthy, older adult populations 21, 29, 36 All participants successfully completed the LDCW and the submaximal treadmill test without a walking aid.
Table 1.
Participant Characteristics
| Characteristics (n = 350) | Mean | s.d. |
|---|---|---|
| Age | 70.3 | (10.2) |
| BMI | 26.8 | (4.5) |
| Fat free mass (kg) | 50.7 | (9.7) |
| Fat mass (kg) | 26.4 | (9.8) |
| Resting VO2 (ml/kg/min) | 2.6 | (0.7) |
| Submaximal VO2 (ml/kg/min) | 8.9 | (1.7) |
| Peak VO2 (ml/kg/min) | 18.1 | (4.6) |
| N | (%) | |
| Age Group | ||
| 50–65 | 112 | 31.9 |
| 65–74 | 114 | 32.5 |
| 75–84 | 94 | 26.8 |
| 85 + | 31 | 8.8 |
| Sex | ||
| Men | 182 | 51.8 |
| Women | 169 | 48.2 |
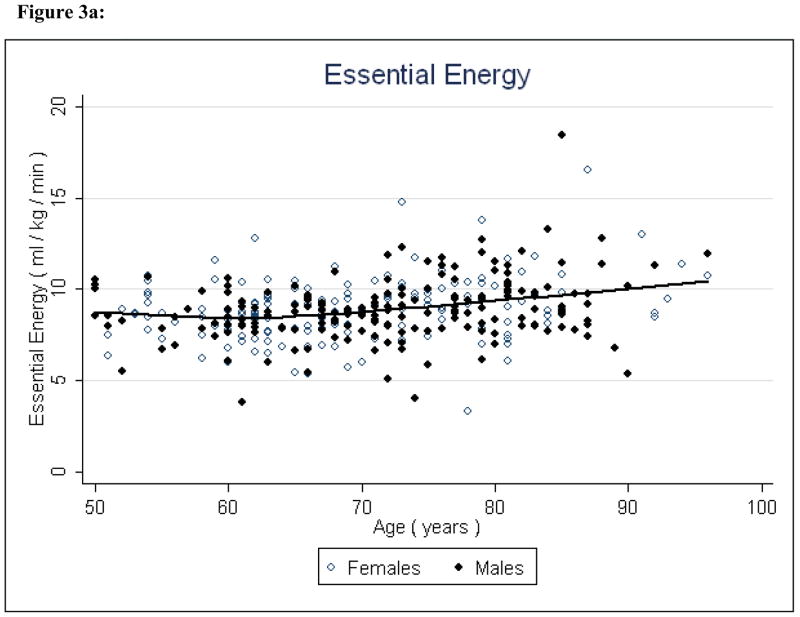
Essential energy increased significantly (P < 0.001) with age, indicating the energy needed to walk at a submaximal level increases with age, independent of sex, height, and body composition (Figure 3, Table 2). Sex and height did not contribute to the model (P = .60 and P = .10, respectively), but, the ratio of fat to lean mass was (P = 0.003) an important factor.
Figure 3.
Figure 3a: Essential Energy and Age
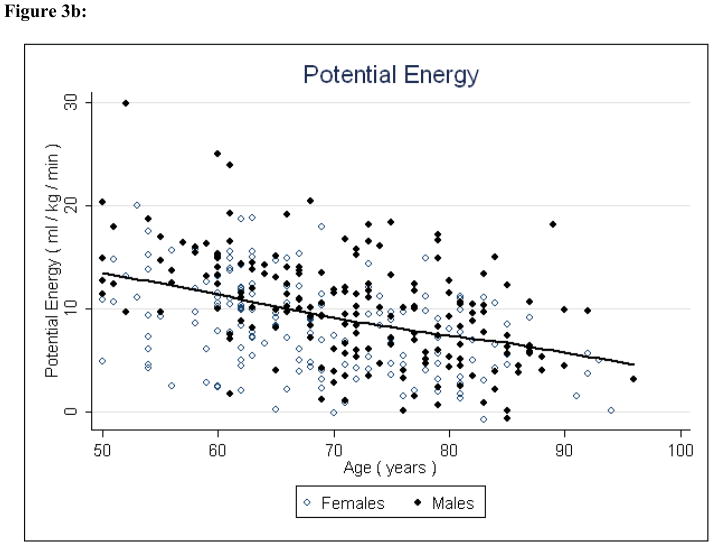
Figure 3b: Potential Energy and Age
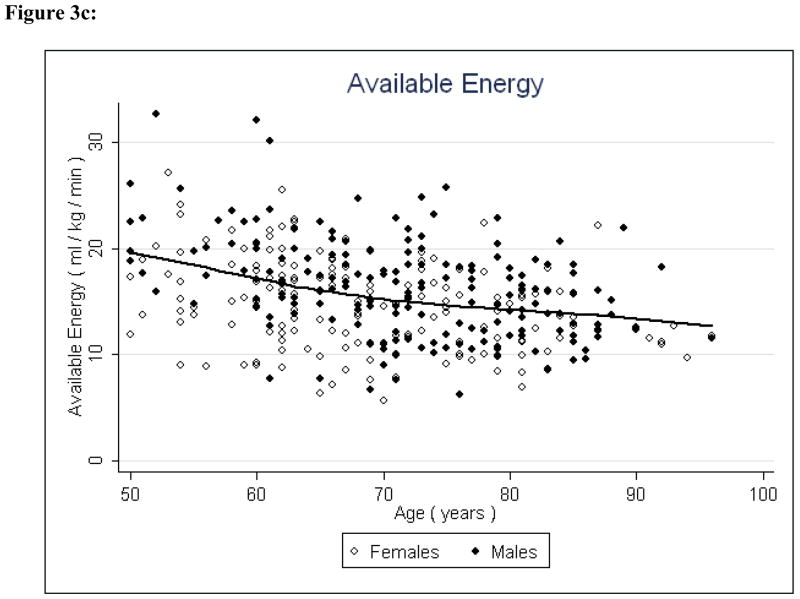
Figure 3c: Available Energy and Age
Table 2.
Parameter Estimates
| Variable | Parameter Estimate | Standard Error | p-value |
|---|---|---|---|
| Model 1: Essential Energy | |||
| Age | 0.039 | 0.009 | 0.000* |
| Male Sex | −0.145 | 0.277 | 0.601 |
| Height | −0.023 | 0.014 | 0.098 |
| Ratio | −1.537 | 0.519 | 0.003* |
| Model 2: Potential Energy | |||
| Age | −0.232 | 0.023 | 0.000* |
| Male Sex | 0.529 | 0.694 | 0.446 |
| Height | 0.029 | 0.034 | 0.405 |
| Ratio | −8.095 | 1.300 | 0.000* |
| Model 3: Available Energy | |||
| Age | −0.184 | 0.022 | 0.000* |
| Male Sex | 0.241 | 0.659 | 0.714 |
| Height | 0.011 | 0.033 | 0.746 |
| Ratio | −8.730 | 1.236 | 0.000* |
p<0.05
Ratio = ratio of fat-to-lean mass
Potential energy and available energy both decreased significantly (P < 0.001 and P < 0.001, respectively) with age, indicating that both reserve capacity and energy availability for all physical activity decline with age (Figures 4 & 5, Table 2). Fat to lean ratio was again a significant factor (P < 0.001) indicating that those with higher levels of fat mass have lower reserve capacity and lower overall energy available for physical activity.
Correlation analyses were conducted to further examine the trends between energy availability and age and the relationships between energy constructs (Table 3). The results were consistent with the regression analyses, showing a positive correlation between essential energy and age (ρ = .27, P < 0.001), and negative correlations between potential energy and age (ρ = −0.41, P < 0.0001), and available energy and age (ρ = −0.33, P < 0.001). Essential energy and potential energy were negatively correlated ((ρ = −0.32, P < 0.0001), but the relationship between essential energy and available energy was not statistically significant (ρ = 0.02, P = .69).
Table 3.
Pearson Correlation Coefficients for Energy Constructs
| Variable | Age | Essential Energy | Potential Energy | Available Energy |
|---|---|---|---|---|
| Age | 1.000 | |||
| Essential Energy | 0.273* | 1.000 | ||
| Potential Energy | −0.411* | −0.316* | 1.000 | |
| Available Energy | −0.334* | 0.021 | 0.931* | 1.000 |
p<0.001
Potential and available energy were highly positively correlated (ρ = 0.93, P < 0.001) in the overall sample population (Table 3). However, further examination revealed that the strength of the available-potential energy correlation diminished with age, from ρ = 0.95 (P < 0.001) in persons under age 75 to ρ = 0.74 (P < 0.001) in those 85 and older. Additionally, the correlation between essential and potential energy increased with age, from ρ = −0.158 (P = 0.02) under age 75 to ρ = −0.49 (P = 0.005) in those 85 and older (Table 4).
Table 4.
Pearson Correlation Coefficients for Energy Constructs by Age Group
| Age | Essential/Potential Energy | Essential/Available Energy | Potential/Available Energy |
|---|---|---|---|
| <75 | −0.158* | 0.120 | 0.951* |
| 75–84 | −0.357* | 0.027 | 0.908* |
| 85 + | −0.492* | 0.218 | 0.737* |
p<0.02
DISCUSSION AND PERSPECTIVES FOR FUTURE STUDIES
In this report we describe how the study of energy availability and consumption may better inform understanding of the aging process. We reviewed some of the animal and human literature on energetics and movement speed and used an example from the BLSA to demonstrate how the paradigm of the energetic pathway to disability could be implemented in longitudinal studies of aging. This includes new logical constructs, new measures and new ways to combine measurements to operationalize these concepts.
The preliminary findings suggest that with increasing age, energy availability declines and energy needs for independent living increase. Available energy appears to substantially diminish between middle-age and late life - approximately 55% to 60% of total energetic capacity is lost, which may explain at least in part the decline in physical activity commonly observed in older individuals. Concurrent with reduced available energy, energy requirements for independent living increased, as demonstrated by the marked increase in essential energy with age. Over time, these processes in combination serve to shift downward and compress the amount of energy available for activity beyond that necessary for maintaining homeostatic equilibrium. This supports the notion that slowed gait speed is an adaptive response to conserve task-specific energy expenditure. The primacy of slow gait as an auger of frailty and mortality further supports this premise.
The well established relationship between physical activity and mobility, indicates that these changes may be modifiable through maintaining or increasing physical activity in adulthood and late life, to preserve muscle mass and quality and reduce comorbidities 37, 38. Although maximal aerobic capacity declines with age regardless of physical activity level 39, voluntary aerobic activity could help sustain peak energy expenditure and minimize essential energy expenditure into old age through maintenance of aerobic capacity, strength, and endurance 40.
Findings from this preliminary analysis indicate that body composition may have an important role in the complex interaction between energetics, mobility and aging. This relationship is consistent with age-related changes in body composition 41, 42 such as loss of lean body mass, an important contributor to RMR 43. Most studies have ignored the basal metabolic consumption of fat, presuming it to be negligible. However, as excessive fatness becomes increasingly prevalent in older adults, equations for estimating RMR may need to re-evaluate the differential contributions of lean and fat mass. Additionally, results from several pre-clinical and clinical studies suggest that aging, obesity and selected metabolic diseases substantially impact the anatomical, metabolic and functional characteristics of muscle, regardless of changes in mass 36, 44, 45. Such changes, which can be captured using novel imaging techniques and analysis of tissue samples, likely affect metabolic rate. Lastly, the amount of energy consumed per kilogram of weight in activities as basic as walking are certainly related to body composition, but the relationship may be complex and somewhat counterintuitive. For example, more muscle may facilitate activity and help stabilize gait, but also may increase energy consumption. In support of this possibility, in the findings presented here, we found a high fat to lean ratio was associated with lower energy requirements for essential activities.
If our energetic hypothesis is correct, interventions for disability prevention should be maximally effective in individuals in late middle-to-old age just prior to the age when gait speed starts to decline at the population level. It would be particularly interesting to study energetics in long-lived, healthy and highly functional individuals and persons affected by specific chronic conditions. Several comorbidities known to affect gait, such as osteo- and/or rheumatoid arthritis, may also influence energy expenditure 46, 47. We attempted to minimize the effect of disease by using a healthy, fairly active, mobile population for the analyses, but a large amount of heterogeneity with age remains. Further untangling the energy expenditure-walking speed-disease relationship may prove complex. Future studies with longitudinal data are needed to investigate the effect that comorbidities may have on the energy/walking speed trade-off.
Considerable methodological development is also necessary. For example, there currently exists no field applicable assessment of maximum energy expenditure over 24 hours. The symptom-limited treadmill exercise and the 400m walk estimate maximum capacity, which are crude approximations of maximum expenditure. Thus, a more direct measure would be highly desirable. The role of fatigue and factors that modulate the threshold of fatigue independent of energetics should be operationalized and evaluated as well. The deficit between energy available and energy required probably generates the first signal for fatigue, but this signal may be modulated by other concurrent factors, including inflammation, depression, pain etc. In spite of these difficulties, this is an exciting new area of investigation that may shed light on the mechanisms of late life disability and identify new targets for prevention and intervention.
Acknowledgments
This paper is based on a conference on Longitudinal Studies in Aging held in January 2008, supported by a grant from the Robert Wood Johnson Foundation.
Sponsor’s Role: None.
Footnotes
Conflict of Interest: This research was supported by the Intramural Research Program of the NIH, National Institute on Aging.
Data for these analyses were obtained from the Baltimore Longitudinal Study of Aging, a study performed by the National Institute on Aging.
Author Contributions: Study concept and design: Ferrucci (BLSA “energy project”), Ferrucci, Schrack, Simonsick (energy measurements and constructs). Acquisition of subjects: Ferrucci, Simonsick. Acquisition of data: Schrack. Data analysis and interpretation: Schrack (primary); Ferrucci, Simonsick (critical review). Manuscript preparation: Schrack.
References
- 1.Chiang JT, Steciuk M, Shtonda B, et al. Evolution of pharyngeal behaviors and neuronal functions in free-living soil nematodes. J Exp Biol. 2006;209:1859–1873. doi: 10.1242/jeb.02165. [DOI] [PubMed] [Google Scholar]
- 2.Wax TM, Goodrick CL. Nearness to death and wheelrunning behavior in mice. Exp Gerontol. 1978;13:233–236. doi: 10.1016/0531-5565(78)90017-7. [DOI] [PubMed] [Google Scholar]
- 3.Carter CS, Sonntag WE, Onder G, Pahor M. Physical Performance and Longevity in Aged Rats. 2002;57:B193–197. doi: 10.1093/gerona/57.5.b193. [DOI] [PubMed] [Google Scholar]
- 4.Priede IG. Natural selection for energetic efficiency and the relationship between activity level and mortality. Nature. 1977;267:610–611. doi: 10.1038/267610a0. [DOI] [PubMed] [Google Scholar]
- 5.Priede IG. Fish Energetics: New Perspectives. Baltimore: The Johns Hopkins University Press; 1985. pp. 33–66. [Google Scholar]
- 6.Guralnik JM, Ferrucci L, Simonsick EM, et al. Lower extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: Consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Med Sci. 2000;55:M221–231. doi: 10.1093/gerona/55.4.m221. [DOI] [PubMed] [Google Scholar]
- 8.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 9.Fried LP, Bandeen-Roche K, Chaves PH, et al. Preclinical mobility disability predicts incident mobility disability in older women. J Gerontol A Biol Med Sci. 2000;55:M43–52. doi: 10.1093/gerona/55.1.m43. [DOI] [PubMed] [Google Scholar]
- 10.Cesari M, Kritchevsky SB, Penninx BWHJ, et al. Prognostic Value of usual gait speed in well-functioning older people; results from the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2005;53:1675–1680. doi: 10.1111/j.1532-5415.2005.53501.x. [DOI] [PubMed] [Google Scholar]
- 11.Shumway-Cook A, Guralnik JM, Phillips CL, et al. Age-associated declines in complex walking task performance: The walking InCHIANTI Toolkit. J Am Geriatr Soc. 2007;55:58–65. doi: 10.1111/j.1532-5415.2006.00962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrucci L, Bandinelli S, Benvenuti E, et al. Subsystems contributing to the decline in ability to walk: Bridging the gap between epidemiology and geriatric practice in the InCHIANTI Study. J Am Geriatr Soc. 2000;48:1618–1625. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 13.McArdle WD, Katch FL, Katch VL. Exercise Physiology: Energy, Nutrition, and Human Performance. 3. Philadelphia: Lea & Febiger; 1991. [Google Scholar]
- 14.Astrand I, Astrand PO, Hallback I, et al. A reduction in normal oxygen uptake with age. J Appl Physiol. 1973;35:649–654. doi: 10.1152/jappl.1973.35.5.649. [DOI] [PubMed] [Google Scholar]
- 15.Cunningham DA, Rechnitzer PA, Pearce ME, Donner AP. Determinants of self-selected walking pace across ages 19 to 66. J Gerontol. 1982;37(5):560–564. doi: 10.1093/geronj/37.5.560. [DOI] [PubMed] [Google Scholar]
- 16.Fleg JL, Morrell CH, Bos AG, et al. Accelerated Longitudinal Decline of Aerobic Capacity in Healthy Older Adults. Circulation. 2005 August 2;112(5):674–682. doi: 10.1161/CIRCULATIONAHA.105.545459. [DOI] [PubMed] [Google Scholar]
- 17.Leveille SG, Guralnik JM, Ferrucci L, et al. Aging successfully until death in old age: opportunities for increasing active life expectancy. Am J Epidemiol. 1999;149:654–664. doi: 10.1093/oxfordjournals.aje.a009866. [DOI] [PubMed] [Google Scholar]
- 18.Blair SN, Kohl HWI, Paffenbarger RSJ, et al. Physical fitness and all-cause mortality: A prospective study of healthy men and women. JAMA. 1989;262:2395–2401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- 19.Simonsick EM, Guralnik JM, Fried LP. Who walks? Factors associated with walking behavior in disabled older women with and without self-reported walking difficulty. J Am Geriatr Soc. 1999;47:672–680. doi: 10.1111/j.1532-5415.1999.tb01588.x. [DOI] [PubMed] [Google Scholar]
- 20.Myers J, Prakash M, Froelicher V, et al. Exercise capacity and mortality among men referred for exercise testing. N Engl Med J. 2002;346:793–801. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- 21.Simonsick EM, Fan E, Fleg JL. Estimating Cardiorespiratory fitness in well-functioning older adults: Treadmill Validation of the Long Distance Corridor Walk. J Am Geriatr Soc. 2006;54:127–132. doi: 10.1111/j.1532-5415.2005.00530.x. [DOI] [PubMed] [Google Scholar]
- 22.Simonsick EM, Montgomery PS, Newman AB, et al. Measuring fitness in healthy older adults: The Health ABC Long Distance Corridor Walk. J Am Geriatr Soc. 2001;49:1544–1548. doi: 10.1046/j.1532-5415.2001.4911247.x. [DOI] [PubMed] [Google Scholar]
- 23.Newman A, Simonsick EM, Naydeck BL, et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006;295:2018–2026. doi: 10.1001/jama.295.17.2018. [DOI] [PubMed] [Google Scholar]
- 24.Shephard RJ. Maximal oxygen intake and indepedence in old age. Br J Sports Med. 2009;43:342–346. doi: 10.1136/bjsm.2007.044800. [DOI] [PubMed] [Google Scholar]
- 25.Cress ME, Meyer M. Maximal voluntary and functional performance needed for independence in adults aged 65 to 97 years. Phys Ther. 2003;83:37–48. [PubMed] [Google Scholar]
- 26.Morey MC, Pieper CF, Cornoni-Huntley J. Is there a threshold between peak oxygen uptake and self-reported physical functioning in older adults? Med Sci Sports Exerc. 1998;30:1223–1229. doi: 10.1097/00005768-199808000-00007. [DOI] [PubMed] [Google Scholar]
- 27.Ruggiero C, Metter EJ, Melenovsky V, et al. High Basal metabolic rate is a risk factor for mortality: The Baltimore Longitudinal Study of Aging. J Gerontol A Biol Med Sci. 2008;63:698–706. doi: 10.1093/gerona/63.7.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Evans WJ, Lambert CP. Physiological basis of fatigue. Am J Phys Med Rehabil. 2007;86(1 Suppl):S29–46. doi: 10.1097/phm.0b013e31802ba53c. [DOI] [PubMed] [Google Scholar]
- 29.Waters RL, Lunsford BR, Perry J, et al. Energy speed relationship of walking - standard tables. J Orthop Res. 1988:215–222. doi: 10.1002/jor.1100060208. [DOI] [PubMed] [Google Scholar]
- 30.Fleg JL, Lakatta EG. Role of muscle loss in the age-associated reduction in VO2 max. J Appl Physiol. 1988;65:1147–1151. doi: 10.1152/jappl.1988.65.3.1147. [DOI] [PubMed] [Google Scholar]
- 31.Fatigue. Merriam-Webster’s Medical Dictionary; 2007. [Google Scholar]
- 32.Stone JL, Norris AH. Activities and attitudes of participants in the Baltimore Longitudinal Study. J Gerontol. 1966;21:575–580. doi: 10.1093/geronj/21.4.575. [DOI] [PubMed] [Google Scholar]
- 33.Parvataneni K, Ploeg L, Olney SJ, et al. Kinematic, kinetic and metabolic parameters of treadmill versus overground walking in healthy older adults. Clin Biomech (Bristol, Avon) 2009:95–100. doi: 10.1016/j.clinbiomech.2008.07.002. Epub 2008 Oct 30. [DOI] [PubMed] [Google Scholar]
- 34.Marsh AP, Katula JA, Pacchia CF, et al. Effect of treadmill and overground walking on function and attitudes in older adults. Med Sci Sports Exerc. 2006;38:1157–1164. doi: 10.1249/01.mss.0000222844.81638.35. [DOI] [PubMed] [Google Scholar]
- 35.Greig C, Butler F, Skelton D, et al. Treadmill walking in old age may not reproduce the real life situation. J Am Geriatr Soc. 1993;41:15–18. doi: 10.1111/j.1532-5415.1993.tb05941.x. [DOI] [PubMed] [Google Scholar]
- 36.Frisard MI, Fabre JM, Russell RD, et al. Physical activity level and physical functionality in nonagenarians compared to individuals aged 60 74 years. J Gerontol A Biol Sci Med Sci. 2007;62:783–788. doi: 10.1093/gerona/62.7.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fiatarone MA, O’Neill EF, Ryan ND, et al. Exercise Training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med. 1994;330:1769–1775. doi: 10.1056/NEJM199406233302501. [DOI] [PubMed] [Google Scholar]
- 38.Pahor M, Blair SN, Espeland MA, et al. Effects of a physical activity intervention on measures of physical performance: Results of the lifestyle interventions and independence for Elders Pilot (LIFE-P) study. J Gerontol A Biol Med Sci. 2006;61:1157–1165. doi: 10.1093/gerona/61.11.1157. [DOI] [PubMed] [Google Scholar]
- 39.Fitzgerald MD, Tanaka H, Tran ZV, et al. Age-related declines in maximal aerobic capacity in regularly exercising vs. sedentary women: A meta-analysis. J Appl Physiol. 1997;183:160–165. doi: 10.1152/jappl.1997.83.1.160. [DOI] [PubMed] [Google Scholar]
- 40.Arnett SW, Laity JH, Agrawal SK, et al. Aerobic reserve and physical functional performance in older adults. Age Ageing. 2008;37:384–389. doi: 10.1093/ageing/afn022. [DOI] [PubMed] [Google Scholar]
- 41.Thomas DR. Loss of skeletal muscle mass in aging: Examining the relationship of starvation, sarcopenia and cachexia. Clin Nutr. 2007;26:389–399. doi: 10.1016/j.clnu.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 42.Wallace JI, Schwartz RS. Epidemiology of weight loss in humans with special reference to wasting in the elderly. Int J Cardiol. 2002;85:15–21. doi: 10.1016/s0167-5273(02)00246-2. [DOI] [PubMed] [Google Scholar]
- 43.Vaughn L, Zurlo F, Ravussin E. Aging and energy expenditure. Am J Clin Nutr. 1991;53:821–825. doi: 10.1093/ajcn/53.4.821. [DOI] [PubMed] [Google Scholar]
- 44.Edwards JD, Redmond AD, Nightingale P, et al. Oxygen consumption following trauma: A reappraisal in severely injured patients requiring mechanical ventilation. Br J Surg. 1988;75:690–692. doi: 10.1002/bjs.1800750722. [DOI] [PubMed] [Google Scholar]
- 45.Kotler DP. Cachexia. Ann Intern Med. 2000;133:622–634. doi: 10.7326/0003-4819-133-8-200010170-00015. [DOI] [PubMed] [Google Scholar]
- 46.Davies MJ, Dalsky GP. Economy of mobility in older adults. J Orthop Sports Phys Ther. 1997;26:69–72. doi: 10.2519/jospt.1997.26.2.69. [DOI] [PubMed] [Google Scholar]
- 47.Waters RL, Mulroy S. The energy expenditure of normal and pahtologic gait. Gait Posture. 1999;9:207–231. doi: 10.1016/s0966-6362(99)00009-0. [DOI] [PubMed] [Google Scholar]