Abstract
Phosphoprotein phosphatase 2A (PP2A) is a major phosphoserine/threonine protein phosphatase in all eukaryotes. It has been isolated as a heterotrimeric holoenzyme composed of a 65 kDa A subunit, which serves as a scaffold for the association of the 36 kDa catalytic C subunit, and a variety of B subunits that control phosphatase specificity. The C subunit is reversibly methyl esterified by specific methyltransferase and methylesterase enzymes at a completely conserved C-terminal leucine residue. Here we show that methylation plays an essential role in promoting PP2A holoenzyme assembly and that demethylation has an opposing effect. Changes in methylation indirectly regulate PP2A phosphatase activity by controlling the binding of regulatory B subunits to AC dimers.
Keywords: phosphoprotein phosphatase 2A regulation/protein methylesterase/protein methyltransferase/signal transduction
Introduction
Phosphorylation on serine and threonine residues is catalyzed by hundreds of different protein kinases (Hunter, 1995). In principle, protein phosphatases play an equally important role in the regulation of protein phosphorylation, but there are relatively few different protein phosphatase enzymes. In mammalian cells, two closely related phosphoprotein phosphatases, PP1 and PP2A, account for >90% of the total phosphatase activity (DePaoli-Roach et al., 1994; Oliver and Shenolikar, 1998). In contrast to protein kinase activation, which tends to be associated with cell growth and proliferation, PP2A phosphatase activity is associated with growth inhibition and cell cycle arrest (Schonthal, 1998).
PP2A is a multimeric protein that has been highly conserved during the evolution of eukaryotes. The 36 kDa catalytic C subunit is found tightly associated with a 65 kDa A subunit (Groves et al., 1999) that serves as a scaffold for the association of one of a variety of regulatory B subunits. B subunits are thought to target PP2A to different spectra of phosphoprotein substrates (Hubbard and Cohen, 1993; Molloy et al., 1998). There appear to be distinct tissue and subcellular localizations for different B subunits (McCright et al., 1996; Tehrani et al., 1996), and B subunits have been shown to alter substrate specificity (Imaoka et al., 1983; Mumby et al., 1987; Cegielska et al., 1994; Kamibayashi et al., 1994; Agostinis et al., 1996; Zhao et al., 1997; Turowski et al., 1999). Three different, apparently unrelated, families of B subunits have been identified: B (DePaoli-Roach et al., 1994; Kamibayashi et al., 1994; Strack et al., 1999), B′ (McCright et al., 1996) and B′′ (Hendricks et al., 1993; Voorhoeve et al., 1999). In addition, several types of viral proteins, such as the small and middle tumor antigens of polyoma virus and small tumor antigen of simian virus 40 (SV40), can also bind to PP2A heterodimers (AC). These viral proteins inhibit PP2A activity, presumably as part of the viral infection strategy (Pallas et al., 1990; Mumby and Walter, 1991; Ulug et al., 1992). It should be noted, however, that B subunit targeting and inhibitory functions are not necessarily exclusive. B subunits generally increase the relative activity of PP2A towards some phosphoprotein targets while at the same time inhibiting activity toward others (Zolnierowicz et al., 1996). Although biochemical and genetic studies strongly suggest that B subunits play an important role in the control of PP2A activity, little is known about the mechanisms that regulate complex formation.
The PP2A catalytic subunit has a highly conserved C-terminal extension that seems to play an important role in phosphatase regulation. The last six residues, TPDYFL, are identical in all known PP2As, ranging from human to yeast. The tyrosine in this sequence, Y307, is subject to phosphorylation by receptor-associated tyrosine kinase activities (Chen et al., 1992, 1994. This has been shown to cause a dramatic decrease in PP2A phosphatase activity. The C-terminal leucine, L309, is subject to α-carboxyl methyl esterification (Lee and Stock, 1993; Xie and Clarke, 1994). This modification appears to be completely specific to PP2A and a closely related phosphatase, PP4/PPX (Kloeker et al., 1997). The methylation reaction is catalyzed by a specific 38 kDa AdoMet-dependent PP2A-methyltransferase, PPMT (Lee and Stock, 1993; De Baere et al., 1999), and the methyl group is removed by a specific 46 kDa PP2A-methylesterase, PPME (Lee et al., 1996; Ogris et al., 1999).
We have investigated the relationship between methylation and PP2A subunit composition. Using a monoclonal antibody that specifically detects methylated PP2A, we show that essentially all of the methyl-esterified C subunits in bovine brain extracts are associated with ABC heterotrimers. This is explained by the fact that methylation is essential for B subunit binding, and B subunit binding precludes demethylation by the methylesterase. Previously reported effects of methylation on PP2A activities in cell extracts (Favre et al., 1994) can be readily understood in terms of indirect effects related to altered B subunit associations. We see no direct effect of methylation on the phosphatase activity of purified PP2A dimers in the absence of B subunits. Our results strongly support the hypothesis that methylation regulates PP2A by modulating holoenzyme assembly.
Results
Relationship between PP2A subunit composition and methylation in extracts from bovine brain
The various forms of PP2A in bovine brain cytosol were separated by hydrophobic interaction chromatography (Figure 1A). Fractions were analyzed for phosphatase activity, methyl-accepting activity, subunit composition and endogenous levels of C subunit methylation. Immunoblots using polyclonal antibodies directed against A, C, B′ and Bα (a member of the B family) indicated that the A and C subunits eluted between fractions 30 and 60, B′ eluted between fractions 34 and 42, and Bα eluted between fractions 44 and 50. Thus, at least three major forms of PP2A were apparent in the elution profile: AB′C, ABαC and AC. Two peaks of p-nitrophenylphosphate (pNPP) phosphatase activity were detected: a minor peak corresponding to AB′C, and a major peak centered at fraction 50 that overlaps ABαC and AC. Although PP2A heterotrimers have been shown to enhance activities toward some phosphoprotein substrates, results presented below indicate that AC dimers have a much greater activity towards the artificial small molecule substrate, pNPP.
Fig. 1. Resolution of different forms of PP2A from bovine brain. (A) Hydrophobic interaction chromatography. Bovine brain extracts were subjected to hydrophobic interaction chromatography and 20 ml fractions were analyzed for PP2A [3H]methyl-accepting (open circles) and pNPP phosphatase (filled circles) activities as described in Materials and methods. Aliquots of indicated column fractions were analyzed by immunoblotting with anti-A, -B′, -Bα and -C antibodies. The positions of subunits A, Bα, B′ and C are shown on the right. (B) Purification of PP2A dimers. The major peak of methyl-accepting activity (fractions 44–56) was pooled, dialyzed and subjected to DEAE–Toyopearl column chromatography. Fractions with methyl-accepting activity were pooled (13 mg protein), concentrated and chromatographed on a Superose 6 HR column. Peak fractions were pooled, concentrated, desalted and subjected to Mono Q HR column chromatography. A Coomassie Blue-stained SDS–polyacrylamide gel of the purified PP2A dimer obtained from this procedure is indicated. Subunit identities were confirmed by western blotting with polyclonal antibodies as well as by microsequence analysis.
PP2A methyl-accepting activity eluted with ABαC. One might suppose from this result that ABαC is the preferred PPMT substrate, but further purification showed that the methyl-accepting activity in these fractions was due to the presence of AC dimers (Figure 1B). Because there are substantial levels of PPME demethylating activity in all fractions (data not shown), methylation could only be detected in the presence of B subunits that bind to methylated AC dimers and protect against demethylation (see below).
Purified AC dimers form tight complexes with PPMT, which are dissociated either by AC methylation or by incubation with okadaic acid (Figure 2). The latter observation explains why okadaic acid inhibits PP2A methylation (Floer and Stock, 1994; Li and Damuni, 1994).
Fig. 2. Methyltransferase binding to PP2A AC dimers. Protein samples were prepared in 200 µl of column buffer (50 mM MOPS pH 7.2, 0.2 M NaCl, 1 mM EDTA, 1 mM DTT, 5% glycerol) containing the carrier proteins β-amylase, alcohol dehydrogenase and ovalbumin (100 µg each). The samples were chromatographed at a flow rate of 0.40 ml/min on a Superose 12 column and fractions were collected every 30 s. Methyltransferase activity was measured by adding [3H]AdoMet plus excess AC to aliquots of each fraction (filled circles); AC–PPMT complexes were detected by measuring [3H]methyl-labeled AC after incubation with [3H]AdoMet (open circles). (A) A mixture of purified AC (100 pmol, 10 µg) and PPMT (10 pmol, 0.4 µg) was incubated for 5 min at 24°C. PPMT activity and AC methyl-accepting activity co-eluted at an apparent molecular weight corresponding to AC–PPMT trimers (∼150 kDa). (B) After incubation for 15 min in the presence of unlabeled 50 µM AdoMet, the AC–PPMT complex dissociated into free PPMT plus methylated AC. The latter is not subject to further methylation by [3H]AdoMet and therefore has no methyl-accepting activity. Unmethylated AC remained associated with PPMT. (C) Addition of 1.0 µM okadaic acid caused the dissociation of PPMT from AC. No AC–PPMT complex was detected in this experiment.
Identification of major methylated PP2A proteins with ABC and AB′C heterotrimers
We have generated a series of monoclonal antibodies against a peptide corresponding to the C-terminus of the C subunit of bovine PP2A. Antibody 4D9, which specifically recognizes only the methylated C-terminus of PP2A (Figure 3A), was used to determine the state of methylation of the various forms of PP2A that had been resolved by hydrophobic interaction chromatography. Antibody 6A3, which does not differentiate between the methylated and unmethylated proteins, was used as a control. The results indicated that whereas AB′C trimers in bovine cell extracts were highly methylated, there was relatively little methylation in fractions that contained ABαC and AC (data not shown). The same results were obtained with brain extracts that were incubated with [3H]AdoMet prior to hydrophobic interaction chromatography (Figure 3B). Western blot analysis with the non-specific monoclonal antibody 6A3 indicated that the overall distribution of C subunits was not significantly affected by incubation with [3H]AdoMet. Moreover, the distribution of [3H]methyl-labeled PP2A was essentially the same as the total distribution of methyl groups detected with the methylation-specific antibody 4D9. Although the majority of the methylated C subunits always appeared in a peak corresponding to AB′C (peak I), a small, but significant, portion of the methylated PP2A eluted in later fractions that contained ABαC and AC (peak II).
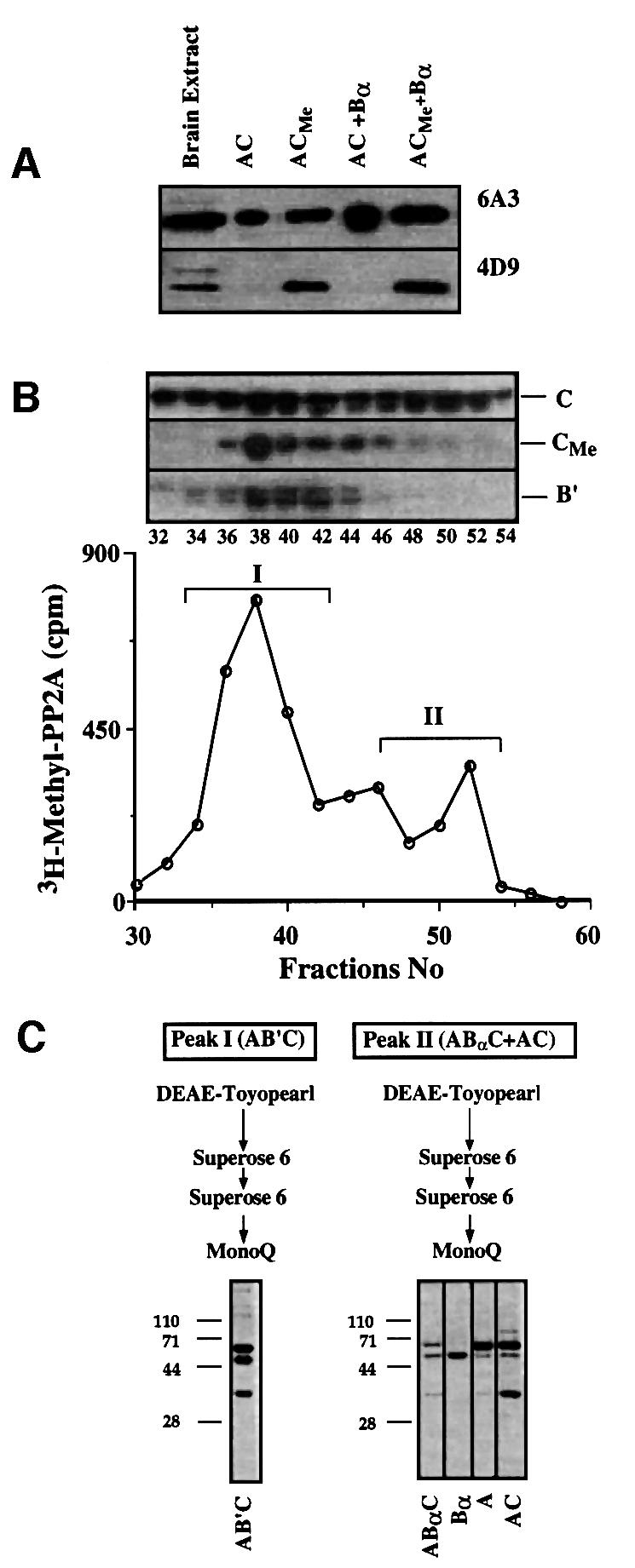
Fig. 3. Methylated forms of PP2A. (A) A monoclonal antibody that specifically recognizes methylated PP2A. The indicated forms of PP2A were subjected to western blot analysis using the non-specific monoclonal antibody 6A3 and the methylation-specific antibody 4D9. (B) Separation of [3H]methyl-labeled PP2A in bovine brain extract by hydrophobic interaction chromatography. Fractions were analyzed by the methanol diffusion assay to detect [3H]methyl-labeled C subunits of PP2A (open circles) and by immunoblotting with non-specific anti-C monoclonal antibody 6A3, methylation-specific anti-C monoclonal antibody 4D9 and anti-B′ polyclonal antibody. (C) Purification of PP2A from peaks I and II. Coomassie Blue-stained SDS–polyacrylamide gels of the purified PP2A components obtained from each peak are indicated. Subunit identities were confirmed by western blotting with polyclonal antibodies as well as by microsequence analysis.
The [3H]methyl-labeled forms of PP2A in peaks I and II were purified by ion exchange and size exclusion chromatography (Figure 3C). Peak I yielded a heterotrimeric form of PP2A that contained 65, 55 and 36 kDa polypeptides in approximately a 1:1:1 molar ratio with a >75% yield of 3H-labeled protein. Based on the specific radioactivity of [3H]AdoMet used for labeling, there appeared to be approximately one [3H]methyl group per purified C subunit. The 65 and 36 kDa polypeptides were identified by immunoblotting and partial amino acid sequence analysis as the Aα and Cα subunits of mammalian PP2A. The 55 kDa subunit was identified immunologically as a member of the B′ family, and micro-sequencing of peptides generated by endoproteinase LysC digestion gave sequences corresponding to B′ε (McCright et al., 1996). Purification of the [3H]methyl-labeled PP2A from peak II yielded an ABαC heterotrimeric form of PP2A (Figure 3C). In contrast to the results with peak I, however, a large fraction of the [3H]methyl esters in peak II were hydrolyzed during the purification procedure (yield <15%). This loss of [3H]methyl groups correlated with the dissociation of [3H]methyl-labeled ABαC into free Bα plus AC dimers. The latter could be stoichiometrically methylated after addition of PPMT and [3H]AdoMet. These results indicate that the methylated PP2As in brain extracts correspond to the two major PP2A holoenzymes in brain, i.e. heterotrimers with either B′ or B regulatory subunits bound to A and C.
B subunit binding to methylated AC prevents PPME-mediated demethylation
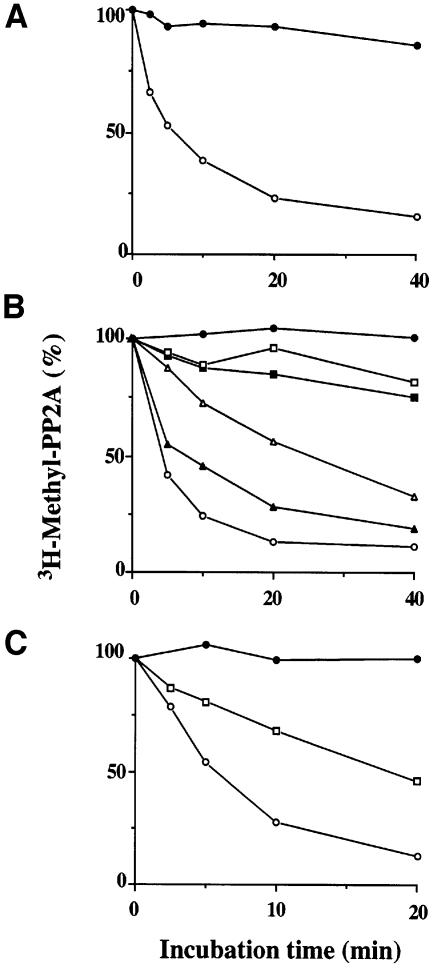
Although AC dimers are the preferred PPMT substrate, the predominant forms of methylated PP2A that can be detected in crude cell extracts are heterotrimeric. This can be accounted for by the fact that B subunit binding protects PP2A from demethylation by PPME. Purified [3H]methyl-labeled AB′C was resistant to demethylation by PPME (Figure 4A) under conditions where methylated dimers were readily demethylated, and addition of purified B′ or Bα inhibited the demethylation of [3H]methyl-labeled AC dimers (Figure 4B and C). These results indicate that PPME and B subunits may bind to overlapping sites on the A subunit scaffold so that binding of one precludes binding of the other. Based on the relative concentrations of Bα and B′ needed to protect [3H]methyl-labeled AC, it appears that B′ has at least five times greater affinity than Bα for methylated AC.
Fig. 4. Methylated PP2A trimers are not demethylated by PPME. Demethylation assays were performed as described previously (Lee et al., 1996). (A) Aliquots of 25 nM [3H]methyl-labeled AC dimers (open circles) or [3H]methyl-labeled AB′εC trimers (filled circles) were incubated with 10 nM PPME for the indicated times. (B) Aliquots of 1.0 nM [3H]methyl-labeled AC dimers were incubated in buffer alone (filled circles), in the presence of 1.0 nM PPME (open circles) or in the presence of 1.0 nM PPME plus 2.5 nM B′ε (filled triangles), 5.0 nM B′ε (open triangles), 10 nM B′ε (filled squares) or 20 nM B′ε (open squares). (C) [3H]methyl-labeled AC dimer, 10 nM in buffer alone (filled circles), was incubated in the presence of 5.0 nM PPME (open circles) or in the presence of 5.0 nM PPME plus 250 nM Bα (open squares).
Methylation promotes the stability of ABC and AB′C PP2A holoenzymes
In the presence of a 2-fold molar excess of methylated AC, Bα was stoichiometrically incorporated into stable methylated ABαC holoenzyme (Figure 5A). Under the same conditions with unmethylated AC, a significant fraction of Bα remained free, and the unmethylated ABαC trimers that formed tended to dissociate during molecular sieve chromatography (Figure 5B). Thus, AC methylation causes a substantial increase in the stability of the ABαC holoenzyme.

Fig. 5. Methylation increases the affinity of AC dimers for Bα. Size exclusion HPLC and western blot analysis of complex formation between Bα and methylated (A) or unmethylated (B) AC dimers. AC dimers (120 µM) were incubated for 30 min at 32°C in the presence (A) or absence (B) of 6.25 µM PPMT plus 50 µM AdoMet. An equal volume of 120 µM Bα was then added and the resulting mixtures were separated by size exclusion chromatography using a Superose 12 HR column. Samples to be analyzed were prepared in 200 µl of column buffer (50 mM MOPS pH 7.2, 0.20 M NaCl, 1.0 mM EDTA, 1.0 mM DTT, 5% glycerol) containing the carrier proteins β-amylase, alcohol dehydrogenase and ovalbumin (100 µg each). The samples were chromatographed at a flow rate of 0.40 ml/min and the indicated 0.40 ml fractions were collected and analyzed by western blotting using a mixture of anti-A, -Bα and -C antibodies. Control experiments in which AC dimer and Bα were incubated with either AdoMet or PPMT alone (not shown) gave the same results as those obtained with unmethylated AC. The elution profiles of free Bα, AC dimer, ABαC trimer and molecular weight standards are indicated.
No unmethylated AB′C could be detected in brain cell extracts. Nevertheless, incubation of these extracts with [3H]AdoMet resulted in efficient labeling of AB′C, which was subsequently purified to obtain a good yield of pure [3H]methyl-labeled AB′C trimer with a specific activity corresponding to that of the [3H]AdoMet used as methyl donor (Figure 3). It is apparent that the generation of [3H]methyl-labeled AB′C trimers resulted from an exchange of B′ from unlabeled methylated AC dimers to [3H]methyl-labeled dimers. Essentially all of the B′ in the brain extracts co-eluted with AB′C complexes, and incubation with AdoMet did not result in a significant increase in the total level of methylated AB′C. Moreover, incubation of unlabeled AB′C with purified [3H]methyl-labeled AC resulted in incorporation of ∼30% of the labeled subunits into AB′C heterotrimers, but when unlabeled AB′C trimers were incubated with PPMT in the presence of [3H]AdoMet no incorporation of label could be detected. Thus, it is apparent that B′ binds very tightly, yet reversibly, to methylated AC dimers, and has a relatively low affinity for unmethylated dimers.
Methylation regulates PP2A phosphatase activity by promoting holoenzyme assembly
We have observed that the specific activity of purified PP2A heterotrimers toward pNPP is much less than that of AC dimers. Since methylation increases heterotrimer formation, one would expect that methylation of AC in the presence of B would inhibit pNPP phosphatase activity. To test this prediction, AC and equimolar amounts of AC plus Bα were methylated using purified PPMT and [3H]AdoMet. Under the conditions used, methylation reached a plateau after 20 min with a >95% yield of methylated C subunits (Figure 6A). The presence of Bα did not significantly affect the rate of methylation. Phosphatase activities were measured for equivalent molar amounts of unmethylated AC, methylated AC, unmethylated AC + Bα, and methylated AC + Bα (Figure 6B). In the absence of Bα, the activity of AC was unaffected by methylation, but in the presence of Bα, methylation caused a substantial reduction in phosphatase activity. The hypothesis that this effect was due to increased formation of ABαC is supported by the observation that a similar inhibitory effect was obtained in the absence of methylation by increasing the concentration of Bα in the mixture. Thus, the effects of PP2A methylation on pNPP phosphatase activity appear to derive from the effects of methylation on PP2A holoenzyme formation.
Fig. 6. Effects of methylation on PP2A phosphatase activity depend on methylation-induced changes in the association of B subunits with AC dimers. (A) Unmethylated AC dimers (120 nM) in the presence (filled circles) or absence (open circles) of equimolar Bα were incubated with 12 nM PPMT plus 10 µM [3H]AdoMet (5.5 Ci/mmol). Aliquots were removed at the indicated times and assayed for incorporation of [3H]methyl esters. The results are presented as the percentage of total possible methyl ester incorporation, assuming a maximum of 1 mol of methyl esters per mol PP2A. (B) AC alone, AC plus one equivalent of Bα or AC plus two equivalents of Bα was incubated for 20 min at 32°C. In a parallel experiment, AC alone or AC plus Bα were incubated in the presence of PPMT plus AdoMet for 20 min at 32°C. Aliquots of each protein mixture were then assayed for pNPP phosphatase activity. The data presented, expressed as change in A410/min/nmol PP2A, are the averages of triplicate determinations (standard deviation <10%).
Discussion
Our results indicate that methyl esterification of the C-terminus of the PP2A catalytic subunit controls the formation of heterotrimeric PP2A holoenzymes by increasing the binding of regulatory B subunits. PP2A heterotrimers appear to be assembled in a multi-step process involving methylation of AC dimers, followed by tight, but reversible, binding of regulatory B subunits (Figure 7). Assembly seems to begin with the formation of AC heterodimers, which are then subject to methylation and demethylation by the PP2A-specific methyltransferase and methylesterase enzymes. Methylation of AC dramatically enhances the affinity for B or B′ subunits, which leads to the formation of ABC or AB′C holoenzymes. PPME cannot demethylate AC in the presence of a bound B or B′ subunit, so holoenzyme formation essentially locks in the methyl group for the lifetime of the complex. Given the general role of PP2A in the dephosphorylation of numerous different phosphoproteins, together with the role of B/B′ subunits in regulating phosphoprotein target specificities, it seems likely that changes in rates of PP2A methylation and demethylation could have wide ranging effects on the global regulation of cellular activities. This is not to say that the subunit composition or activity of PP2A can be inferred from measurements of levels of methylation, however. Our results do not imply that heterodimeric PP2A is unmethylated in vivo. In fact, we show that it is the dimeric AC form of PP2A that is the substrate for both PPMT and PPME. Presumably, other factors being equal, the balance of these activities determines the level of AC dimer methylation, which acts in turn to control the rate of B subunit association. For instance, we find that most of the PP2A in NIH 3T3 cells is methyl esterified (our unpublished results), but from this information alone little can be said concerning the subunit composition or activity of the phosphatase. For a long time, PP2A has been thought to exist in the cell predominantly as a trimer, but several recent studies have indicated that C subunits alone, AC dimers and/or C subunits associated with other cellular regulatory proteins may play significant roles in vivo (Kremmer et al., 1997; Murata et al., 1997; Ruediger et al., 1997; Chung et al., 1999).
Fig. 7. PP2A methylation functions in the assembly of PP2A ABC holoenzymes. The PP2A methyltransferase (PPMT) and methylesterase (PPME) bind to unmethylated and methylated AC dimers, respectively, in place of the regulatory B subunits. Methylation dramatically enhances the binding of B and B′ regulatory subunits to facilitate the assembly of PP2A holoenzyme trimers.
Studies of PP2A mutants are consistent with the conclusion that methylation at the C-terminus of the PP2A C subunit is essential for the binding of B subunits. Deletion of nine C-terminal amino acids completely abolishes binding of the Bα subunit (Ogris et al., 1997) and mutation of the C-terminal leucine, L309→A (Bryant et al., 1999) or L309→Q (Chung et al., 1999), exhibited similar, but less severe defects. Results with these mutations, as well as recent results with similar PP2A mutations in yeast (Evans et al., 1999), do not indicate that lack of holoenzyme formation leads to dramatic changes in cell growth, however. This has been most clearly shown in Saccharomyces cerevisiae, whose genome encodes an A subunit, Tpd3 (van Zyl et al., 1992), and two regulatory subunits: a B subunit, Cdc55 (Healy et al., 1991), and a B′ subunit, Rts1 (Zhao et al., 1997). Strains deficient in the A subunit and/or one or both of the B/B′ subunits appear to be viable (van Zyl et al., 1992; Shu et al., 1997). Nevertheless, these cells exhibit defects in growth regulation under a variety of different stress-related situations.
In addition to regulating the partitioning of the PP2A catalytic subunit between the trimeric holoenzymes and other forms, changes in methylation might be expected to influence the partitioning between different types of holoenzyme. Although binding of both B′ and Bα to AC dimers is substantially enhanced by methylation, B′ clearly binds much more tightly to methylated AC than does Bα. Moreover, at least in the case of Bα, it is clear that the effect of methylation on binding is not absolute, so that substantial levels of ABαC can form in the absence of methylation. From these considerations, one would expect that the effects of methylation in vivo would be highly dependent on the relative levels of A, B, B′ and C that are present at a given location within a particular cell type. A relatively simple example where these considerations come into play is provided by studies of the interactions of viral proteins with PP2A. SV40 small tumor antigen binds to AC heterodimers, thereby inhibiting PP2A activity. The SV40 small t antigen can displace Bα but not B′, presumably because the affinity of small t for methylated AC falls between that of Bα and B′ (Yang et al., 1991; Sontag et al., 1993). The effect of methylation on small t antigen binding has not been assessed, but, in the case of polyomavirus middle T antigen, which also inhibits PP2A by binding to AC, methylation is probably not required since middle T binding is unaffected by C-terminal deletions (Ogris et al., 1997). Thus, one would expect that inhibition of PP2A by viral proteins in vivo would be antagonized by PP2A methylation due to increased competitive binding by B subunits. From these considerations, it seems likely that methylation is inhibited during viral infections.
The methylating and demethylating enzymes (PPMT and PPME, respectively) appear to function as B subunits insofar as they bind to AC dimers. In fact, PPME has recently been purified as part of a trimeric complex with AC (Ogris et al., 1999). The reported sequence of the purified human enzyme is virtually identical to the sequence of the PPME enzyme we have purified from bovine brain (Lee et al., 1996). Our characterization of the binding of PPMT to AC dimers also shows the formation of stable heterotrimers, which are disrupted by methylation. In this sense, PPMT provides an example of a B-like subunit that binds preferentially to unmethylated AC. It seems likely that other proteins will be identified that have a similar specificity for binding to unmethylated as opposed to methylated AC, in which case changes in methylation would cause a switch between different heterotrimeric forms.
We find that although methylation has no effect on the activity of isolated AC dimers towards pNPP, methylation causes a substantial decrease in activity in the presence of Bα. This inhibitory effect was clearly due to the formation of relatively inactive ABαC heterotrimers. It seems likely that previously reported increases in PP2A activity towards phosphorylase a and other phosphoproteins are attributable to the formation of heterotrimers between AC dimers and B subunits that were present in the relatively crude enzyme preparations used in these studies (Favre et al., 1994; Kowluru et al., 1996). This notion is supported by the finding that methylation has no effect on the activity of purified PP2A dimers towards phosphorylase a (De Baere et al., 1999). Thus, the effects of methylation on PP2A catalytic activity appear to be secondary consequences of the effects of methylation on the stability of B subunit binding to the AC dimer.
What regulates the level of PP2A methylation in vivo? Preliminary results indicate that the level of PP2A methylation may be controlled, at least in part, by PPMT phosphorylation. PP2A methylation in frog egg cytoplasm has been shown to increase in response to addition of cAMP (Floer and Stock, 1994), and we have observed cAMP-independent phosphorylation of PPMT in brain extracts (our unpublished results). Given the complexity of PP2A regulation and the plethora of potential phosphoprotein targets, it seems likely that the regulation of PP2A methylation involves several overlapping mechanisms that depend on both kinase and phosphatase activities.
Since PP2A has been implicated in the regulation of cell proliferation, it seems likely that changes in methylation function in cell cycle regulation. Results with mammalian fibroblasts in tissue culture indicate that methylation levels may depend on the cell cycle, with different patterns of regulation in the nucleus and cytoplasm (Turowski et al., 1995). In these studies, cytoplasmic PP2A appeared to become demethylated at the G0/G1 boundary and remethylated as cells entered S phase, at which point nuclear PP2A was demethylated. In another study, methylation of PP2A was shown to occur in S phase during granulocytic differentiation of leukemia cells (Zhu et al., 1997). The method used to assess PP2A methylation in these experiments was not definitive, however. Polyclonal antibodies directed against the unmethylated C-terminus of the C subunit give slightly stronger signals on western blots with unmethylated (i.e. alkali treated) as opposed to methylated C subunits. Inferences concerning changes in methylation were based on quantitative differences in immunoreactivity in fixed cells or cell extracts before and after alkali treatment. In this context, it should be noted that in one of the first general screens for protein carboxyl methylation, metabolic labeling of mammalian cells with [3H]methyl-methionine was shown to generate a soluble 36 kDa [3H]methyl esterified species that almost certainly corresponded to PP2A (Chelsky et al., 1985). Methylation of this protein appeared to be confined to the cytoplasm; however, no PP2A methylation was observed in the nucleus, and there was no indication that methylation levels varied with the cell cycle (Chelsky et al., 1987). More rigorous results concerning levels of methylation in vivo will undoubtedly be forthcoming with the recent development of better immunological tools. The methylation-specific monoclonal antibody reported here complements the recently reported monoclonal antibody that specifically recognizes unmethylated PP2A (Ogris et al., 1999).
There are two homologs of mammalian PPMT in S.cerevisiae (De Baere et al., 1999). A mutant strain lacking one of these genes exhibits the same pattern of phenotypes exhibited by cdc55 or rts1 mutants, which lack the B or B′ subunits, respectively (Wu et al., 2000). Moreover, direct assays confirm that the yeast PPMT-deficient strain lacks the ability to form stable ABC heterotrimers. From these results, it is apparent that the role of methylation in PP2A holoenzyme assembly is a highly conserved feature of eukaryotic cell regulation. The results with yeast indicate that although PP2A methylation per se is not essential for growth, it does play an important role in the regulation of growth under conditions of stress. It is as if the unmethylated form of PP2A signals unrestricted growth and PP2A methylation counteracts this effect. The simplest hypothesis to explain these results is that B subunit binding is required to target PP2A to phosphoprotein substrates that are phosphorylated by growth-promoting kinases, most notably the kinases themselves. There is already considerable evidence that PP2A holoenzymes serve this general role (for a recent review see Millward et al., 1999). It is apparent that the PP2A methylation system serves to modulate this growth restraining aspect of PP2A activity.
Materials and methods
Materials
DEAE– and phenyl–Toyopearl TSK 650M were from Supelco (Bellefonte, PA). Superose 6 HR10/30, Superose 12 HR10/30 and Mono Q HR 5/5 columns were from Pharmacia-LKB Biotechnology Inc. DEAE–cellulose DE-52 was from Whatman. AdoMet, aprotinin, leupeptin and pepstatin were from Boehringer Mannheim. [3H]AdoMet (65–80 Ci/mmol) was from New England Nuclear. Prestained molecular weight electrophoresis markers were from Life Technologies, Inc. PVDF membranes were from Bio-Rad. SuperSignal Ultra Substrate, goat anti-mouse IgG-coupled peroxidase, goat anti-mouse IgG-coupled alkaline phosphatase and Imject Activated Immunogen Conjugation Kit were from Pierce. Fast Blue RR salt, α-naphthyl phosphate, size-exclusion molecular weight markers [β-amylase, alcohol dehydrogenase, bovine serum albumin (BSA) and ovalbumin] and pNPP were from Sigma. Centricon and Centriprep ultrafiltration devices were purchased from Amicon. Microdialysis filter membranes (diameter 2.5 cm, pore size 0.025 mm) were from Millipore.
Hydrophobic interaction chromatography
Frozen cow brains were prepared as described previously (Lee and Stock, 1993). The 0.10 and 0.30 M NaCl eluates from the DEAE step, which contained PPMT and PP2A, were combined and stored in aliquots at –20°C. Aliquots of 1.0 g of protein were used for each hydrophobic interaction chromatography experiment. Ammonium sulfate was added to a final concentration of 0.80 M, and after centrifugation the supernatant was applied at 60 ml/h on to a phenyl–Toyopearl column (3.2 × 12.5 cm) equilibrated in buffer A [50 mM MOPS pH 7.2, 1.0 mM EDTA, 1.0 mM dithiothreitol (DTT), 0.50 µg/ml each of aprotinin, leupeptin and pepstatin] containing 0.80 M ammonium sulfate. The column was eluted with 10 column volumes of this buffer followed by a 1.0 l linear gradient to 20% (v/v) ethylene glycol in buffer A.
[3H]methyl labeling of bovine brain extracts
An aliquot of bovine brain extract (see above) was mixed with [3H]AdoMet (13 nM; 5.0 µCi/nmol), incubated at 32°C. After 15 min, 50 µM unlabeled AdoMet was added. After 15 min, protein was precipitated with 70% ammonium sulfate, dissolved in 200 ml of buffer A and subjected to hydrophobic interaction chromatography as described above. The fractions were analyzed for [3H]methyl esters by the [3H]methanol diffusion assay (Lee and Stock, 1993) and for A, B, B′ and C subunits by western blotting. Fractions eluting between 0.3 and 0.2 M ammonium sulfate (peak I; ∼60 mg of total protein) and between 0.1 and 0.0 M ammonium sulfate (peak II; ∼80 mg of total protein) were combined and dialysed against buffer A for further purification.
Purification of [3H]methyl-labeled AB′C
Peak I. Dialysed material of peak I was loaded on to a DEAE–Toyopearl TSK 650M column (1.6 × 12 cm) and eluted with a linear gradient of 0–300 mM NaCl in 200 ml of buffer A; 4.0 ml fractions were collected and analyzed. Fractions (∼8 mg protein) eluting between 190 and 240 mM NaCl were pooled, concentrated to 0.90 ml using Centriprep 30 and applied to a Superose 6 HR column in buffer B (50 mM MOPS pH 7.2, 1.0 mM EDTA, 1.0 mM DTT, 5% glycerol) containing 0.2 M NaCl; 0.3 ml fractions were collected and analyzed. Fractions with an apparent Mr of ∼200 kDa (90% of [3H]PP2A; 2.4 mg protein) were combined, concentrated and applied to a Superose 6 HR column as described above. The radioactive material (1.5 mg protein) was concentrated and desalted using Centricon 30 and applied to a Mono Q HR column (5 × 50 mm) equilibrated in buffer B containing 50 mM NaCl. The proteins were eluted with consecutive linear gradients of 50–200 mM NaCl in 10 ml of buffer B at 1.0 ml/min, 200–300 mM NaCl in 20 ml at 0.50 ml/min and 300–500 mM NaCl in 5.0 ml at 1.0 ml/min. [3H]AB′C eluted as a single major peak at 260 mM NaCl. This material was stored at –20°C in 40% glycerol. The yield of [3H]methyl-labeled AB′C was ∼1.0 nmol based on the final specific radioactivity of [3H]methyl-labeled protein (0.50 µCi/nmol). Similar values were obtained estimating the total level of PP2A subunits by Coomassie Blue staining.
Peak II. Dialysed material of peak II was subjected to DEAE–Toyopearl column chromatography as described above, except that fractions eluting between 180 and 200 mM NaCl were collected (∼11 mg of protein), concentrated by Centriprep-30 to a final volume of 1.2 ml and chromatographed on a Superose 6 HR column. The radioactive material eluting in fractions corresponding to ∼200 kDa was collected, concentrated and subjected to Superose 6 HR column chromatography. The resulting material (0.80 mg protein) was concentrated, desalted by Centricon-30 and subjected to Mono Q HR column chromatography as described above. During this procedure most of the methylated ABαC dissociated to free Bα (∼50 µg, eluting at 215 mM NaCl) plus unmethylated AC dimer (∼100 µg, eluting at 270 mM NaCl). [3H]ABαC eluted at 240 mM NaCl. The yield was 120 pmol, based on the amount of label incorporated and the original specific radioactivity of [3H]AdoMet. The same value was obtained by estimating the total level of C subunit by Coomassie Blue staining.
Purification of ABα C, AB′C, Bα and B′ subunits, PPMT and PPME
Unlabeled ABαC was obtained by the same procedure as [3H]ABαC except that the Mono Q step was replaced by chromatography on a DEAE–Toyopearl TSK column (9 × 50 mm) to minimize the dissociation of Bα. AB′C and Bα were isolated as described above. To obtain B′, purified AB′C (50 µl, 150 µg) was diluted to 1.0 ml with buffer B containing 7.0 M guanine hydrochloride (GuHCl), concentrated by Centricon 10 to 0.10 ml and chromatographed on a Superose 12 HR column equilibrated in buffer B containing 7.0 M GuHCl. Peak fractions of B′ (25 µg) were pooled, concentrated to a final volume of 50 µl and dialysed against buffer B containing 0.10 M NaCl plus 10% glycerol for 3 h at 4°C using a microdialysis membrane filter (0.025 mm). PPMT and PPME were purified from crude bovine brain extract as described previously (Lee and Stock, 1993; Lee et al., 1996).
Assay for methyl-accepting activity
Methyl-accepting activity of purified PP2A proteins was assayed by incubating them with the indicated concentrations of pure PPMT at 32°C for 15 min in 50 mM MOPS pH 7.2, 1.0 mM EDTA, 5.0 mM DTT, 1.0 mg/ml BSA and 1–10 µM [3H]AdoMet (5.5–55 µCi/nmol). Aliquots were spotted on to Whatman filter paper and washed three times with 10% trichloroacetic acid (TCA) and once with methanol. After drying under vacuum, radioactivity was assayed by liquid scintillation spectrometry. To assay crude extracts, reactions were stopped by addition of 10% TCA and subjected to SDS–PAGE; the C subunit band was then excised and analyzed for incorporation of [3H]methyl esters by the methanol diffusion assay (Stock et al., 1984).
Phosphatase assay
Phosphatase activity was assayed by adding a source of enzyme to 10 mM pNPP in 50 mM Tris–HCl pH 8.5, 1.0 mM DTT, 10 mM MgCl2 and 0.10 mg/ml BSA in a final volume of 500 µl. Unless otherwise indicated, the reaction was stopped after 15 min of incubation at 32°C by addition of 500 µl of 1.0 M Na2CO3, and adsorbance was measured at 410 nm.
Protein analyses
Protein concentrations were determined by the method of Bradford (1976) and 12.5% SDS–PAGE was performed according to the method of Laemmli (1970). Lys-C protease digestion of A, B′ and C subunits of [3H]methyl-labeled PP2A, separation of the digestion products by reversed phase HPLC and N-terminal sequencing of peptides were carried out at the Princeton University Synthesizing/Sequencing Facility.
Immunoblotting
Proteins were transferred to PVDF membranes, which were then blocked with 5% skimmed milk in Tris-buffered saline with 0.05% Tween-20 and incubated with primary antibody (1:1000). Goat anti-mouse IgG-coupled peroxidase (1:100 000) or goat anti-mouse IgG-coupled alkaline phosphatase (1:5000) was used as the secondary antiserum. Peroxidase activity was detected using enhanced chemiluminescent SuperSignal Ultra Substrate. Alkaline phosphatase activity was detected with Fast Blue RR Salt and α-naphthyl phosphate as a substrate. The following primary antibodies were used: polyclonal antibodies against Aα, Bα and B′ subunits of PP2A from Mark Mumby (Mumby et al., 1987; Kamibayashi et al., 1994), and polyclonal anti-PP2A C subunit antibody (Upstate Biotechnology). Monoclonal anti-PP2A C subunit antibodies 6A3 and 4D9 were prepared as described below.
Production of monoclonal antibodies specific for methylated PP2A
To generate methylation-specific monoclonal antibodies, we used a synthetic peptide corresponding to amino acid residues 299–309 of the catalytic subunit of PP2A, HVTRRTPDYFL, and the amidated derivative, HVTRRTPDYFL-amide. Each peptide was synthesized with an N-terminal cysteine residue for coupling to maleimide-activated keyhole limpet haemocyanin using an Imject Activated Immunogen Conjugation Kit. Monoclonal antibodies were generated at Princeton University Monoclonal Antibody Facility by immunizing mice using standard procedures (Harlow and Lane, 1988). Briefly, two mice were injected three times at intervals of 2 weeks with each peptide–keyhole limpet hemocyanin conjugate, serum titers were determined by ELISA, hybridoma cell lines were generated in myeloma–lymphocyte cell fusion and the culture supernatants were screened by ELISA using plates coated with 1.0 µg/well synthetic peptides. Cells from positive wells were cloned and rescreened. No signals specific to unmodified peptide were detected. Most of the clones from positive wells recognized both forms of the peptide. Three of these clones were chosen for production of monoclonal antibodies that bind the C subunit C-terminus irrespective of its state of methylation. One clone, 4D9, which recognized only the amidated peptide, was chosen for production of the methylation-specific monoclonal antibody. The monoclonal antibodies produced by these clones were then tested for their ability to detect different forms of PP2A on western blots. Monoclonal antibody 4D9 binds only the methylated form of the C subunit. The other monoclonal antibodies recognize both methylated and unmethylated C subunits. Antibody 6A3 was chosen for our studies.
Acknowledgments
Acknowledgements
We thank Mark Mumby for providing PP2A antibodies, Sergei Borukhov and Oskar Rokhlin for assistance, and James Broach, Sandra Da Re and Jeannie Wu for critical reading of the manuscript. S.V. was supported by a fellowship from the Beckman Scholars Program. This work was supported by a grant from the NIH (GM61284 to J.B.S.).
References
- Agostinis P., Donella-Deana,A., Van Hoof,C., Cesaro,L., Brunati,A.M., Ruzzene,M., Merlevede,W., Pinna,L.A. and Goris,J. (1996) A comparative study of the phosphotyrosyl phosphatase specificity of protein phosphatase type 2A and phosphotyrosyl phosphatase type 1B using phosphopeptides and the phosphoproteins p50/HS1, c-Fgr and Lyn. Eur. J. Biochem., 236, 548–557. [DOI] [PubMed] [Google Scholar]
- Bradford M.M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem., 72, 248–254. [DOI] [PubMed] [Google Scholar]
- Bryant J.C., Westphal,R.S. and Wadzinski,B.E. (1999) Methylated C-terminal leucine residue of PP2A catalytic subunit is important for binding of regulatory Bα subunit. Biochem. J., 339, 241–246. [PMC free article] [PubMed] [Google Scholar]
- Cegielska A., Shaffer,S., Derua,R., Goris,J. and Virshup,D.M. (1994) Different oligomeric forms of protein phosphatase 2A activate and inhibit simian virus 40 DNA replication. Mol. Cell. Biol., 14, 4616–4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelsky D., Ruskin,B. and Koshland,D.E.,Jr (1985) Methyl-esterified proteins in a mammalian cell line. Biochemistry, 24, 6651–6658. [DOI] [PubMed] [Google Scholar]
- Chelsky D., Olson,J.F. and Koshland,D.E.,Jr (1987) Cell cycle-dependent methyl esterification of lamin B. J. Biol. Chem., 262, 4303–4309. [PubMed] [Google Scholar]
- Chen J., Martin,B.L. and Brautigan,D.L. (1992) Regulation of protein serine–threonine phosphatase type-2A by tyrosine phosphorylation. Science, 257, 1261–1264. [DOI] [PubMed] [Google Scholar]
- Chen J., Parsons,S. and Brautigan,D.L. (1994) Tyrosine phosphorylation of protein phosphatase 2A in response to growth stimulation and v-src transformation of fibroblasts. J. Biol. Chem., 269, 7957–7962. [PubMed] [Google Scholar]
- Chung H., Nairn,A.C., Murata,K. and Brautigan,D.L. (1999) Mutation of Tyr307 and Leu309 in the protein phosphatase 2A catalytic subunit favors association with the α4 subunit which promotes dephosphorylation of elongation factor-2. Biochemistry, 38, 10371–10376. [DOI] [PubMed] [Google Scholar]
- De Baere I., Derua,R., Janssens,V., Van Hoof,C., Waelkens,E., Merlevede,W. and Goris,J. (1999) Purification of porcine brain protein phosphatase 2A leucine carboxyl methyltransferase and cloning of the human homologue. Biochemistry, 38, 16539–16547. [DOI] [PubMed] [Google Scholar]
- DePaoli-Roach A.A. et al. (1994) Serine/threonine protein phosphatases in the control of cell function. Adv. Enzyme Regul., 34, 199–224. [DOI] [PubMed] [Google Scholar]
- Evans D.R., Myles,T., Hofsteenge,J. and Hemmings,B.A. (1999) Functional expression of human PP2Ac in yeast permits the identification of novel C-terminal and dominant-negative mutant forms. J. Biol. Chem., 274, 24038–24046. [DOI] [PubMed] [Google Scholar]
- Favre B., Zolnierowicz,S., Turowski,P. and Hemmings,B.A. (1994) The catalytic subunit of protein phosphatase 2A is carboxyl-methylated in vivo. J. Biol. Chem., 269, 16311–16317. [PubMed] [Google Scholar]
- Floer M. and Stock,J. (1994) Carboxyl methylation of protein phosphatase 2A from Xenopus eggs is stimulated by cAMP and inhibited by okadaic acid. Biochem. Biophys. Res. Commun., 198, 372–379. [DOI] [PubMed] [Google Scholar]
- Groves M.R., Hanlon,N., Turowski,P., Hemmings,B.A. and Barford,D. (1999) The structure of the protein phosphatase 2A PR65/A subunit reveals the conformation of its 15 tandemly repeated HEAT motifs. Cell, 96, 99–110. [DOI] [PubMed] [Google Scholar]
- Harlow E. and Lane,D. (1988) Antibodies: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Healy A.M., Zolnierowicz,S., Stapleton,A.E., Goebl,M., DePaoli-Roach,A.A. and Pringle,J.R. (1991) CDC55, a Saccharomyces cerevisiae gene involved in cellular morphogenesis: identification, characterization and homology to the B subunit of mammalian type 2A protein phosphatase. Mol. Cell. Biol., 11, 5767–5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks D.D., Ono,E., Seyer,J.M. and Gupta,K.C. (1993) Phosphorylation of the Sendai virus C proteins. Virology, 197, 471–474. [DOI] [PubMed] [Google Scholar]
- Hubbard M.J. and Cohen,P. (1993) On target with a new mechanism for the regulation of protein phosphorylation. Trends Biochem. Sci., 18, 172–177. [DOI] [PubMed] [Google Scholar]
- Hunter T. (1995) Protein kinases and phosphatases: the yin and yang of protein phosphorylation and signaling. Cell, 80, 225–236. [DOI] [PubMed] [Google Scholar]
- Imaoka T., Imazu,M., Usui,H., Kinohara,N. and Takeda,M. (1983) Resolution and reassociation of three distinct components from pig heart phosphoprotein phosphatase. J. Biol. Chem., 258, 1526–1535. [PubMed] [Google Scholar]
- Kamibayashi C., Estes,R., Lickteig,R.L., Yang,S.I., Craft,C. and Mumby,M.C. (1994) Comparison of heterotrimeric protein phosphatase 2A containing different B subunits. J. Biol. Chem., 269, 20139–20148. [PubMed] [Google Scholar]
- Kloeker S., Bryant,J.C., Strack,S., Colbran,R.J. and Wadzinski,B.E. (1997) Carboxymethylation of nuclear protein serine/threonine phosphatase X. Biochem. J., 327, 481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowluru A., Seavey,S.E., Rabaglia,M.E., Nesher,R. and Metz,S.A. (1996) Carboxylmethylation of the catalytic subunit of protein phosphatase 2A in insulin-secreting cells: evidence for functional consequences on enzyme activity and insulin secretion. Endocrinology, 137, 2315–2323. [DOI] [PubMed] [Google Scholar]
- Kremmer E., Ohst,K., Kiefer,J., Brewis,N. and Walter,G. (1997) Separation of PP2A core enzyme and holoenzyme with monoclonal antibodies against the regulatory A subunit: abundant expression of both forms in cells. Mol. Cell. Biol., 17, 1692–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U.K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227, 680–685. [DOI] [PubMed] [Google Scholar]
- Lee J. and Stock,J. (1993) Protein phosphatase 2A catalytic subunit is methyl-esterified at its carboxyl terminus by a novel methyltransferase. J. Biol. Chem., 268, 19192–19195. [PubMed] [Google Scholar]
- Lee J., Chen,Y., Tolstykh,T. and Stock,J. (1996) A specific protein carboxyl methylesterase that demethylates phosphoprotein phosphatase 2A in bovine brain. Proc. Natl Acad. Sci. USA, 93, 6043–6047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M. and Damuni,Z. (1994) Okadaic acid and microcystin-LR directly inhibit the methylation of protein phosphatase 2A by its specific methyltransferase. Biochem. Biophys. Res. Commun., 202, 1023–1030. [DOI] [PubMed] [Google Scholar]
- McCright B., Rivers,A.M., Audlin,S. and Virshup,D.M. (1996) The B56 family of protein phosphatase 2A (PP2A) regulatory subunits encodes differentiation-induced phosphoproteins that target PP2A to both nucleus and cytoplasm. J. Biol. Chem., 271, 22081–22089. [DOI] [PubMed] [Google Scholar]
- Millward T.A., Zolnierowicz,S. and Hemmings,B.A. (1999) Regulation of protein kinase cascades by protein phosphatase 2A. Trends Biochem. Sci., 24, 186–191. [DOI] [PubMed] [Google Scholar]
- Molloy S.S., Thomas,L., Kamibayashi,C., Mumby,M.C. and Thomas,G. (1998) Regulation of endosome sorting by a specific PP2A isoform. J. Cell Biol., 142, 1399–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby M.C. and Walter,G. (1991) Protein phosphatases and DNA tumor viruses: transformation through the back door? Cell Regul., 2, 589–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby M.C., Russell,K.L., Garrard,L.J. and Green,D.D. (1987) Cardiac contractile protein phosphatases. Purification of two enzyme forms and their characterization with subunit-specific antibodies. J. Biol. Chem., 262, 6257–6265. [PubMed] [Google Scholar]
- Murata K., Wu,J. and Brautigan,D.L. (1997) B cell receptor-associated protein α4 displays rapamycin-sensitive binding directly to the catalytic subunit of protein phosphatase 2A. Proc. Natl Acad. Sci. USA, 94, 10624–10629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogris E., Gibson,D.M. and Pallas,D.C. (1997) Protein phosphatase 2A subunit assembly: the catalytic subunit carboxy terminus is important for binding cellular B subunit but not polyomavirus middle tumor antigen. Oncogene, 15, 911–917. [DOI] [PubMed] [Google Scholar]
- Ogris E., Du,X., Nelson,K.C., Mak,E.K., Yu,X.X., Lane,W.S. and Pallas,D.C. (1999) A protein phosphatase methylesterase (PME-1) is one of several novel proteins stably associating with two inactive mutants of protein phosphatase 2A. J. Biol. Chem., 274, 14382–14391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver C.J. and Shenolikar,S. (1998) Physiologic importance of protein phosphatase inhibitors. Front. Biosci., 3, D961–972. [DOI] [PubMed] [Google Scholar]
- Pallas D.C., Shahrik,L.K., Martin,B.L., Jaspers,S., Miller,T.B., Brautigan,D.L. and Roberts,T.M. (1990) Polyoma small and middle T antigens and SV40 small t antigen form stable complexes with protein phosphatase 2A. Cell, 60, 167–176. [DOI] [PubMed] [Google Scholar]
- Ruediger R., Brewis,N., Ohst,K. and Walter,G. (1997) Increasing the ratio of PP2A core enzyme to holoenzyme inhibits Tat-stimulated HIV-1 transcription and virus production. Virology, 238, 432–443. [DOI] [PubMed] [Google Scholar]
- Schonthal A. (1998) Role of PP2A in intracellular signal transduction pathways. Front. BioSci., 3, D1262–D1273. [DOI] [PubMed] [Google Scholar]
- Shu Y., Yang,H., Hallberg,E. and Hallberg,R. (1997) Molecular genetic analysis of Rts1p, a B′ regulatory subunit of Saccharomyces cerevisiae protein phosphatase 2A. Mol. Cell. Biol., 17, 3242–3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sontag E., Fedorov,S., Kamibayashi,C., Robbins,D., Cobb,M. and Mumby,M. (1993) The interaction of SV40 small tumor antigen with protein phosphatase 2A stimulates the map kinase pathway and induces cell proliferation. Cell, 75, 887–897. [DOI] [PubMed] [Google Scholar]
- Stock J.B., Clarke,S. and Koshland,D.E.,Jr (1984) The protein carboxylmethyltransferase involved in Escherichia coli and Salmonella typhimurium chemotaxis. Methods Enzymol., 106, 310–321. [DOI] [PubMed] [Google Scholar]
- Strack S., Chang,D., Zaucha,J.A., Colbran,R.J. and Wadzinski,B.E. (1999) Cloning and characterization of Bδ, a novel regulatory subunit of protein phosphatase 2A. FEBS Lett., 460, 462–466. [DOI] [PubMed] [Google Scholar]
- Tehrani M.A., Mumby,M.C. and Kamibayashi,C. (1996) Identification of a novel protein phosphatase 2A regulatory subunit highly expressed in muscle. J. Biol. Chem., 271, 5164–5170. [DOI] [PubMed] [Google Scholar]
- Turowski P., Fernandez,A., Favre,B., Lamb,N.J. and Hemmings,B.A. (1995) Differential methylation and altered conformation of cytoplasmic and nuclear forms of protein phosphatase 2A during cell cycle progression. J. Cell Biol., 129, 397–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turowski P., Myles,T., Hemmings,B.A., Fernandez,A. and Lamb,N.J. (1999) Vimentin dephosphorylation by protein phosphatase 2A is modulated by the targeting subunit B55. Mol. Biol. Cell, 10, 1997–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulug E.T., Cartwright,A.J. and Courtneidge,S.A. (1992) Characterization of the interaction of polyomavirus middle T antigen with type 2A protein phosphatase. J. Virol., 66, 1458–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zyl W., Huang,W., Sneddon,A.A., Stark,M., Camier,S., Werner,M., Marck,C., Sentenac,A. and Broach,J.R. (1992) Inactivation of the protein phosphatase 2A regulatory subunit A results in morphological and transcriptional defects in Saccharomyces cerevisiae. Mol. Cell. Biol., 12, 4946–4959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorhoeve P.M., Hijmans,E.M. and Bernards,R. (1999) Functional interaction between a novel protein phosphatase 2A regulatory subunit, PR59 and the retinoblastoma-related p107 protein. Oncogene, 18, 515–524. [DOI] [PubMed] [Google Scholar]
- Wu J., Tolstykh,T., Lee,J., Boyd,K., Stock,J. and Broach,J.R. (2000) Carboxyl methylation of the phosphoprotein phosphatase 2A catalytic subunit promotes its functional association with regulatory subunits in vivo. EMBO J., 19, 5672–5681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H. and Clarke,S. (1994) Protein phosphatase 2A is reversibly modified by methyl esterification at its C-terminal leucine residue in bovine brain. J. Biol. Chem., 269, 1981–1984. [PubMed] [Google Scholar]
- Yang S.I., Lickteig,R.L., Estes,R., Rundell,K., Walter,G. and Mumby,M.C. (1991) Control of protein phosphatase 2A by simian virus 40 small-t antigen. Mol. Cell. Biol., 11, 1988–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Boguslawski,G., Zitomer,R.S. and DePaoli-Roach,A.A. (1997) Saccharomyces cerevisiae homologs of mammalian B and B′ subunits of protein phosphatase 2A direct the enzyme to distinct cellular functions. J. Biol. Chem., 272, 8256–8262. [DOI] [PubMed] [Google Scholar]
- Zhu T., Matsuzawa,S., Mizuno,Y., Kamibayashi,C., Mumby,M.C., Andjelkovic,N., Hemmings,B.A., Onoe,K. and Kikuchi,K. (1997) The interconversion of protein phosphatase 2A between PP2A1 and PP2A0 during retinoic acid-induced granulocytic differentiation and a modification on the catalytic subunit in S phase of HL-60 cells. Arch. Biochem. Biophys., 339, 210–217. [DOI] [PubMed] [Google Scholar]
- Zolnierowicz S., Van Hoof,C., Andjelkovic,N., Cron,P., Stevens,I., Merlevede,W., Goris,J. and Hemmings,B.A. (1996) The variable subunit associated with protein phosphatase 2A0 defines a novel multimember family of regulatory subunits. Biochem. J., 317, 187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]