Abstract
A reduction in extracellular K+ concentration ([K+]o) causes cardiac arrhythmias and triggers internalization of the cardiac rapidly activating delayed rectifier potassium channel (IKr) encoded by the human ether-a-go-go-related gene (hERG). We investigated the role of ubiquitin (Ub) in endocytic degradation of hERG channels stably expressed in HEK cells. Under low K+ conditions, UbKO, a lysine-less mutant Ub that only supports monoubiquitination, preferentially interacted and selectively enhanced degradation of the mature hERG channels. Overexpression of Vps24 protein, also known as charged multivesicular body protein 3, significantly accelerated degradation of mature hERG channels, whereas knockdown of Vps24 impeded this process. Moreover, the lysosomal inhibitor bafilomycin A1 inhibited degradation of the internalized mature hERG channels. Thus, monoubiquitination directs mature hERG channels to degrade through the multivesicular body/lysosome pathway. Interestingly, the protease inhibitor lactacystin inhibited the low K+-induced hERG endocytosis and concomitantly led to an accumulation of monoubiquitinated mature hERG channels, suggesting that deubiquitination is also required for the endocytic degradation. Consistently, overexpression of the endosomal deubiquitinating enzyme signal transducing adaptor molecule-binding protein significantly accelerated whereas knockdown of endogenous signal transducing adaptor molecule-binding protein impeded degradation of the mature hERG channels under low K+ conditions. Thus, monoubiquitin dynamically mediates endocytic degradation of mature hERG channels under low K+ conditions.
Keywords: Deubiquitination, Endocytosis, Ion Channels, Membrane Proteins, Potassium Channels, Protein Degradation, Ubiquitin, Ubiquitination
Introduction
The human ether-a-go-go-related gene (hERG)2 encodes the pore-forming subunits of the rapidly activating delayed rectifier K+ channels (IKr) (1, 2). IKr is important for cardiac repolarization, and its reduction causes long QT syndrome (LQTS), a disorder that predisposes individuals to life-threatening arrhythmias (3). Mutations in hERG cause type 2 inherited long QT syndrome (LQT2), and a diverse variety of medications can block hERG and cause acquired LQTS as a cardiac side effect (4). In addition, a reduction in extracellular K+ concentration ([K+]o) exacerbates LQTS (5). Using an in vivo rabbit model, we previously demonstrated that hypokalemia chronically reduces IKr and prolongs the action potential duration and the QT intervals of electrocardiograms. We further showed that extracellular K+ (K+o) is required for hERG function and membrane stability; upon depletion of K+o, hERG channels enter into a non-conducting state within minutes and are subsequently internalized and degraded within hours (6, 7). We have also demonstrated an involvement of caveolin in low K+-induced internalization of cell surface hERG channels (8). However, the cellular machinery for hERG endocytic degradation is largely unknown. In particular, although ubiquitin (Ub) is found to be involved in the internalization of cell surface hERG channels (6, 7), the nature of Ub-hERG interactions is not known.
Ub is a protein with a highly conserved sequence of 76 amino acids found in every eukaryotic cell. The covalent binding of Ub to target proteins is known as ubiquitination, a process well known for labeling proteins for degradation (9, 10). Appendage of a single Ub moiety leads to monoubiquitination, whereas attachment of a chain of Ub leads to polyubiquitination. Although polyubiquitination targets proteins for proteasomal degradation (11), monoubiquitination targets membrane proteins for internalization and lysosomal degradation. As an internalization signal of membrane proteins, monoubiquitination is well described in yeast (12–14). In mammals, evidence of this connection is also accumulating (9, 15–22).
In the present study, using biochemical and electrophysiological approaches, we demonstrated, for the first time, that under low K+ conditions monoubiquitination of cell surface hERG channels directs the channel internalization and degradation through the multivesicular body (MVB) pathway, and Ub is released from the internalized channel prior to the channel entry into lysosomes for further degradation.
EXPERIMENTAL PROCEDURES
Molecular Biology
A hERG-expressing human embryonic kidney (HEK) 293 stable cell line (hERG-HEK cells) was obtained from Dr. Craig January (University of Wisconsin-Madison). hERG cDNA was obtained from Dr. Gail A. Robertson (University of Wisconsin-Madison). The S624T mutation was generated using the overlap extension PCR technique (23). The human HA-tagged wild type (WT) Ub (plasmid number 17608), lysine-less mutant Ub (UbKO; plasmid number 17603), and FLAG/HA-tagged signal transducing adaptor molecule-binding protein (STAMBP; also known as AMSH (associated molecule with the Src homology 3 domain of STAM); plasmid number 22560) cDNAs were obtained from Addgene (Cambridge, MA). For UbKO, all lysine residues within Ub are replaced by arginine (24). Thus, UbKO can only support monoubiquitin modification of target proteins (25). Vps24 human cDNA open reading frame (ORF) clone with Myc tag in pCMV6-entry vector (hVps24; plasmid number RC220006) was obtained from OriGene Technologies (Rockville, MD). hVps24 siRNA and STAMBP siRNA were purchased from Sigma. A custom made 0 mm K+ minimum essential medium (MEM) that lacks potassium in any form was purchased from Invitrogen. Primary antibodies against hERG (anti-Kv11.1), Ub, HA, STAMBP, and actin; the proteasomal inhibitor lactacystin; and the lysosomal inhibitor bafilomycin A1 were purchased from Sigma. Control siRNA, protein A/G plus-agarose, primary antibodies against Vps24, and secondary antibodies against rabbit, goat, or mouse were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA).
Patch Clamp Electrophysiological Recordings
The whole cell patch clamp method was used for IhERG recordings. The pipette solution contained 135 mm KCl, 5 mm EGTA, 1 mm MgCl2, and 10 mm HEPES (pH 7.2). The bath solution contained 135 mm NaCl, 5 mm KCl, 2 mm CaCl2, 1 mm MgCl2, 10 mm glucose, and 10 mm HEPES (pH 7.4). For selection of transiently transfected cells with Ub, UbKO, hVps24, or STAMBP plasmid, a plasmid encoding GFP (pIRES2-EGFP; Clontech) was co-transfected, and only GFP-positive cells were used for IhERG recordings. For recording families of hERG currents at various voltages shown in Figs. 2A, 3C, 5, B and D, 6C, and 8, B and D, the currents were evoked by depolarizing steps to voltages between −70 and +70 mV in 10-mV increments. The holding potential was −80 mV. The hERG tail currents were recorded upon a repolarizing step to −50 mV for 5 s. The tail current amplitude upon −50-mV repolarization after +50-mV depolarization was used for analyzing IhERG amplitude. Patch clamp experiments were performed at room temperature (22 ± 1 °C).
FIGURE 2.
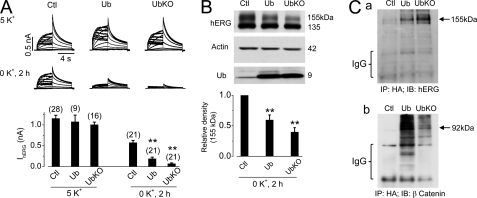
Monoubiquitination accelerates degradation of mature hERG channels in 0 mm K+ MEM. A, IhERG in control (Ctl), Ub-, or UbKO-transfected hERG-HEK cells cultured in 5 or 0 mm K+ MEM for 2 h. Thirty-six hours after transfection, the cell culture media were changed to 5 or 0 mm K+ MEM for 2 h before patch clamp experiments. The averaged tail currents are shown in the lower panel. The numbers in parentheses shown above the histograms indicate the number of cells tested. Error bars represent standard error of the mean. ** indicates p < 0.01 compared with the control (Ctl). B, hERG expression levels in control (Ctl), Ub-, or UbKO-transfected hERG-HEK cells cultured in 0 mm K+ MEM for 2 h (n = 5). C, co-IP analysis of Ub-hERG and Ub-β-catenin interactions. Panel a, immunoblotting (IB) analysis of hERG in HA-immunoprecipitated proteins from Ub- or UbKO-transfected hERG-HEK cells cultured in 0 mm K+ MEM with lactacystin (20 μm) for 1 h. Panel b, immunoblotting analysis of β-catenin in HA-immunoprecipitated proteins from Ub- or UbKO-transfected hERG-HEK cells cultured in normal MEM.
FIGURE 3.
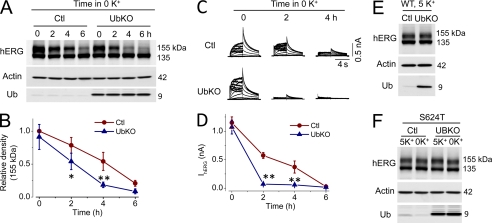
Effects of UbKO expression on WT or S624T mutant hERG expression levels in stable cell lines cultured in 5 or 0 mm K+ MEM. A, hERG expression levels in control (Ctl) or UbKO-transfected hERG-HEK cells cultured in 0 mm K+ MEM for various periods. B, relative density of the 155-kDa hERG band in cells at various periods of culture in 0 mm K+ MEM. The intensity of the 155-kDa band at each time point was normalized to the initial value and plotted against time (n = 5). C, families of IhERG in control (Ctl) or UbKO-transfected hERG-HEK cells cultured in 0 mm K+ MEM for 0, 2, or 4 h. D, the time courses of 0 mm K+ exposure-induced reduction of IhERG in control (Ctl) or UbKO-transfected hERG-HEK cells. E, the hERG expression level in control (Ctl) or UbKO-transfected hERG-HEK cells cultured in 5 mm K+ MEM for 48 h. F, the S624T hERG expression levels in a stable cell line without or with UbKO transfection cultured in 5 or 0 mm K+ MEM for 6 h. In B and D, Error bars represent standard error of the mean. * denotes p < 0.05, and ** denotes p < 0.01 compared with the respective controls (Ctl).
FIGURE 5.
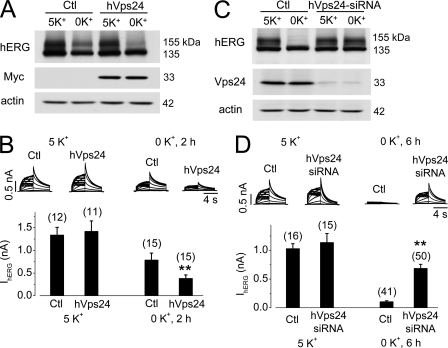
Role of hVps24 in hERG degradation. A, hERG expression levels in control (Ctl) or hVps24-transfected hERG-HEK cells cultured in 5 or 0 mm K+ MEM for 4 h (n = 5). Thirty-six hours after transfection, cell culture medium was changed to 5 or 0 mm K+ MEM for 4 h, and analysis was performed. Expression of hVps24 was detected with an anti-Myc antibody. B, IhERG in control (Ctl) or hVps24-transfected hERG-HEK cells cultured in 5 or 0 mm K+ MEM for 2 h. The averaged tail currents are shown in the lower panel. C, hERG expression levels in hERG-HEK cells transfected with control siRNA (Ctl) or hVps24 siRNA after 6-h culture in 5 or 0 mm K+ MEM (n = 3). Forty-eight hours after transfection, the culture medium was changed to 5 or 0 mm K+ MEM, and cells were cultured for 6 h and then collected for analysis. D, IhERG in hERG-HEK cells transfected with control siRNA (Ctl) or hVps24 siRNA after 6-h culture in 5 or 0 mm K+ MEM. The averaged tail currents are shown in the lower panel. The numbers in parentheses shown above the histograms in B and D indicate the number of cells tested. In B and D, Error bars represent standard error of the mean. ** denotes p < 0.01 compared with the respective controls.
FIGURE 6.
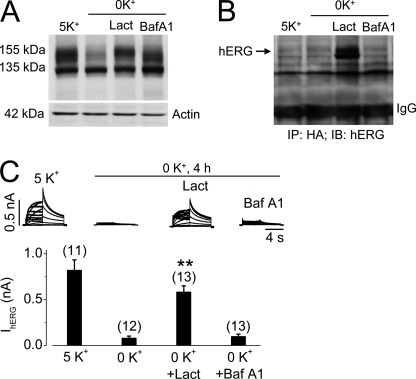
Effects of proteasomal or lysosomal inhibition on 0 mm K+-induced hERG internalization in UbKO (HA-tagged)-transfected hERG-HEK cells. A, Western blots showing hERG expression levels in UbKO-transfected hERG-HEK cells cultured for 4 h in 5 mm K+ MEM, 0 mm K+ MEM, 0 mm K+ MEM with 20 μm lactacystin (Lact), or 1 μm bafilomycin A1 (Baf A1). B, co-IP analysis between expressed UbKO and hERG channels. Proteins extracted from UbKO-transfected hERG-HEK cells cultured for 4 h in 5 mm K+ MEM, 0 mm K+ MEM, 0 mm K+ MEM with 20 μm lactacystin (Lact), or 1 μm bafilomycin A1 (Baf A1) were precipitated using an anti-HA antibody. The precipitated proteins were immunoblotted (IB) using an anti-hERG antibody. C, effects of lactacystin or bafilomycin A1 on 0 mm K+-induced reduction of IhERG. Families of IhERG were recorded in hERG-HEK cells cultured in 5 mm K+ MEM, 0 mm K+ MEM, 0 mm K+ MEM with 20 μm lactacystin (Lact), or 1 μm bafilomycin A1 (Baf A1) for 4 h. Cells were collected in drug-free 5 mm K+ MEM, and IhERG was recorded using the whole cell clamp method in 5 mm K+ bath solution. Tail current at −50 mV after a 50-mV depolarization was used for analysis. Currents from cells cultured in 0 mm K+ MEM with lactacystin (Lact) or bafilomycin A1 (Baf A1) were compared with currents from cells in 0 mm K+ MEM. The numbers in parentheses denote the number of cells tested. Error bars represent standard error of the mean. **, p < 0.01 compared with IhERG in 0 mm K+.
FIGURE 8.
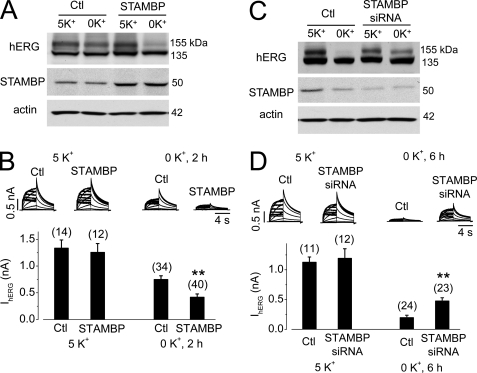
Overexpression of STAMBP accelerates and knockdown of STAMBP impedes degradation of mature hERG channels in 0 mm K+ MEM. A, hERG expression levels in control (Ctl) or STAMBP-transfected hERG-HEK cells cultured in 5 or 0 mm K+ MEM for 4 h. B, IhERG in control (Ctl) or STAMBP-transfected hERG-HEK cells cultured in 5 or 0 mm K+ MEM for 2 h. The averaged tail current amplitudes are shown below the current traces. C, hERG expression levels in hERG-HEK cells transfected with control (Ctl) or STAMBP siRNA after 6 h of culture in 5 or 0 mm K+ MEM. D, IhERG in hERG-HEK cells transfected with control (Ctl) or STAMBP siRNA after 6 h of culture in 5 or 0 mm K+ MEM. The averaged tail current amplitudes are shown below the current traces. The numbers in parentheses in B and D denote the number of cells tested. Error bars represent standard error of the mean. **, p < 0.01 compared with the respective controls.
Western Blot Analysis and Co-immunoprecipitation (Co-IP)
Total cell protein was obtained by treating cells with a lysis buffer in the presence of protease inhibitor mixture, PMSF, and N-ethylmaleimide (Sigma). Sample proteins at 10 μg/lane were separated on an 8, 12, or 15% SDS-polyacrylamide electrophoresis gel, transferred onto PVDF membranes, and blocked for 1 h with 5% nonfat milk. The blots were probed with appropriate primary and secondary antibodies and visualized with Eastman Kodak Co. film using enhanced chemiluminescence (GE Healthcare).
For immunoprecipitation, 0.5 mg of total protein was incubated with anti-Ub or anti-HA overnight at 4 °C then precipitated with protein A/G plus-agarose beads for 4 h at 4 °C. The beads were washed three times with ice-cold lysis buffer, resuspended in 2× Laemmli sample buffer, and boiled for 5 min. The samples were centrifuged at 15,000 rpm for 5 min, and the supernatants were analyzed using Western blotting.
Immunofluorescence Microscopy
Live hERG-HEK cells at 48 h after transfection with Myc-tagged hVps24 or HA-tagged UbKO were treated with a rabbit anti-hERG antibody (anti-Kv11.1; Sigma) for 30 min at room temperature. This hERG antibody targets the external S1-S2 region of the channel and thus serves to label the cell surface hERG channels. The unbound antibody was washed away with MEM. The cells were cultured in 0 or 5 mm K+ MEM for 3 or 4 h, then fixed, and permeabilized (6, 7). Antibody-labeled hERG channels were stained with Alexa Fluor 488-conjugated anti-rabbit secondary antibody. The cells were also incubated with mouse anti-Myc or anti-HA primary antibody and Alexa Fluor 594-conjugated anti-mouse secondary antibody to detect overexpressed Myc-tagged hVps24 or HA-tagged UbKO, respectively. Cells were shown by taking differential interference contrast images. Nuclei were stained using Hoechst 33342 (0.2 μg/ml; Sigma). Images were acquired using a Leica TCS SP2 Multi Photon confocal microscope.
siRNA and Plasmid Transfection
Basal Vps24 or STAMBP in hERG-HEK cells was knocked down using the respective siRNA (Sigma). Scrambled siRNA was used as the control. Cells were grown in 60-mm dishes at 60–70% confluence, and 80 pmol of duplex siRNA was transfected into cells using Lipofectamine 2000 (Invitrogen). Twenty-four to 48 h after transfection of siRNA or plasmid of interest, cells were subsequently cultured in 5 or 0 mm K+ MEM for various periods and were harvested for further experiments.
All data are expressed as the mean ± S.E. A one-way analysis of variance was used to test for statistical significance between the control and test groups. A p value of 0.05 or less was considered significant.
RESULTS
Monoubiquitination Is Involved in Low K+-induced Internalization and Degradation of WT hERG Channels but Not S624T Mutant hERG Channels
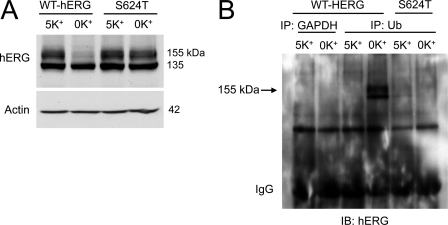
As shown in Fig. 1A, the hERG proteins extracted from hERG-HEK cells cultured in normal MEM (5 mm K+) displayed two bands with molecular masses of 155 and 135 kDa, representing the mature fully glycosylated form in the plasma membrane (155 kDa) and the immature core-glycosylated form residing in the endoplasmic reticulum (135 kDa) (26, 27). Culturing cells in 0 mm K+ MEM for 6 h eliminated the mature 155-kDa form of WT hERG channels but not the mutant hERG channel S624T (7). To investigate the role of Ub in hERG degradation in low K+ culture conditions, we analyzed Ub-hERG interactions using co-IP. We pretreated cells with the proteasomal inhibitor lactacystin (20 μm) for 1 h and cultured the cells in 5 or 0 mm K+ MEM for another hour (1 h) in the continued presence of lactacystin to prevent hERG degradation (6). Whole cell lysate was extracted and immunoprecipitated with an anti-Ub antibody. The precipitated proteins were electrophoresed and detected with an anti-hERG antibody. As shown in Fig. 1B, an increase in ubiquitinated hERG was detected in cells expressing WT hERG in 0 mm K+ culture (Fig. 1B). Thus, there is a correlation between hERG degradation and Ub modification. Interestingly, a hERG band observed in the Ub-precipitated protein is close to 155 kDa (Fig. 1B), suggesting that monoubiquitination is involved in hERG internalization.
FIGURE 1.
Ubiquitination is involved in degradation of mature hERG channels in 0 mm K+ conditions. A, WT and S624T hERG expression levels in cells cultured in 5 or 0 mm K+ MEM for 6 h. B, immunoblotting (IB) analysis of hERG channels in proteins precipitated by an anti-GAPDH antibody (control) or an anti-Ub antibody from cell lysates of WT or S624T hERG stable cell lines cultured in 5 or 0 mm K+ MEM in the presence of lactacystin (20 μm) for 1 h.
To directly examine the role of monoubiquitination in hERG degradation, hERG-HEK cells were transfected with a plasmid of either an HA-tagged WT Ub that supports both polyubiquitination and monoubiquitination or an HA-tagged mutant Ub, UbKO, in which all lysine residues are mutated to arginine, and thus it can only support monoubiquitination (24, 25). The effects of Ub or UbKO overexpression on the function of hERG channels were investigated using the whole cell patch clamp method 36 h after transfection. Under 5 mm K+ culture conditions, overexpression of either Ub or UbKO did not significantly affect the hERG current (IhERG). However, overexpression of either Ub or UbKO significantly accelerated the reduction of IhERG in cells cultured in 0 mm K+ MEM for 2 h (Fig. 2A).
The hERG expression levels were examined using Western blot analysis in hERG-HEK cells transfected with an empty vector (control), Ub, or UbKO. Thirty-six hours after transfection, the cells were exposed to 0 mm K+ MEM for 2 h, and whole cell lysate was extracted for analysis. Both Ub and UbKO overexpression accelerated the degradation of the 155-kDa form of hERG without affecting the expression of the 135-kDa form of hERG (Fig. 2B). Because UbKO can only support monoubiquitination and prevents the formation of polyubiquitin chains (24, 25), these data indicate that monoubiquitination plays a role in hERG degradation in 0 mm K+ MEM.
To evaluate the effectiveness of Ub and UbKO in ubiquitination of cellular proteins, we performed co-IP analysis of the Ub-hERG interaction in hERG-HEK cells overexpressing HA-tagged Ub or UbKO. Cells were cultured in 0 mm K+ for 1 h to facilitate the potential Ub-hERG interactions. Because Ub modification may lead to degradation of hERG proteins, lactacystin (20 μm) was used to pretreat cells for 1 h before experiments and included during 1-h culture in 0 mm K+. The cell lysates were immunoprecipitated with an anti-HA antibody and then immunoblotted with an anti-hERG antibody. As shown in Fig. 2C, panel a, ubiquitinated hERG signals were present in cells transfected with Ub or UbKO. Furthermore, the hERG signal in UbKO-transfected cells was stronger than that in Ub-transfected cells. Notably, the ubiquitinated hERG bands were around 155 kDa, indicating that overexpression of Ub or UbKO resulted in monoubiquitin conjugation to the mature hERG channels. To evaluate the effectiveness of Ub and UbKO in polyubiquitination of cellular proteins, we assessed ubiquitination of β-catenin, a well characterized substrate of polyubiquitination (28). As shown in Fig. 2C, panel b, when HA-precipitated proteins from cells transfected with Ub or UbKO were immunoblotted with an anti-β-catenin antibody, the typical ladder smear of polyubiquitinated β-catenin was observed in Ub- but not UbKO-transfected cells. These results confirm that Ub, but not UbKO, supports polyubiquitination of cellular proteins, and monoubiquitination mediates the degradation of mature hERG channels in 0 mm K+ conditions.
We investigated the effects of UbKO overexpression on the time-dependent reduction of the mature hERG channels under 0 mm K+ conditions. UbKO overexpression significantly accelerated the reduction of the 155-kDa hERG expression level and of IhERG in 0 mm K+ MEM (Fig. 3, A–D). On the other hand, overexpression of UbKO did not affect the WT hERG expression levels in 5 mm K+ culture, nor did it affect the expression levels of S624T mutant channels in either 5 or 0 mm K+ MEM (Fig. 3, E and F).
Vps24 Is Involved in Mature hERG Protein Degradation in 0 mm K+
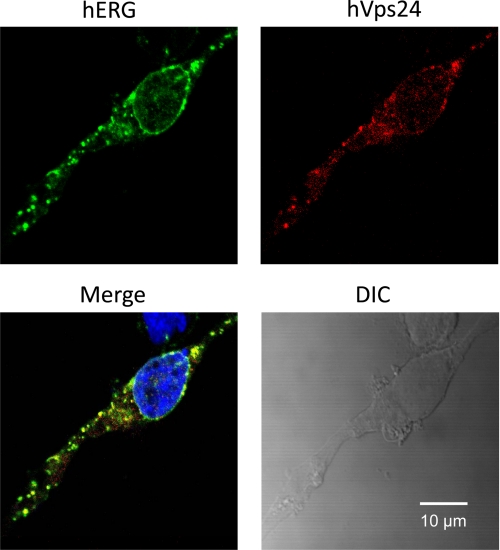
Monoubiquitination targets various proteins for lysosomal degradation through MVBs (10). Endosomal sorting complexes required for transport (ESCRTs) sort ubiquitinated cargo into the luminal vesicles of MVBs, which fuse with lysosomes, the terminal compartment of the endocytic pathway (29, 30). Vps24, also known as charged multivesicular body protein 3, is a subunit of ESCRT-III in mammals. Vps24 is important for the invagination and cleavage of intraluminal vesicles in MVBs (31) and for the fusion of MVBs with lysosomes (32). To assess the interactions between hERG and Vps24, we examined the colocalization between hERG and Myc-tagged hVps24 protein in hERG-HEK cells transfected with hVps24 plasmid. Cell surface hERG channels were labeled by incubating live hERG-HEK cells with an anti-hERG antibody that targets an extracellular region of the channel. The cells were then cultured in 0 mm K+ for 3 h, fixed, and permeabilized. Alexa Fluor 488-conjugated secondary antibody targeting the primary anti-hERG antibody was used to detect hERG, and appropriate primary and Alexa Fluor 594-conjugated secondary antibodies for Myc were used to detect the Myc-tagged hVps24 protein. Exposure of hERG-HEK cells to 0 mm K+ MEM for 3 h resulted in internalization of the labeled cell surface hERG channels, which displayed strong colocalization with Myc-tagged hVps24 proteins (Fig. 4).
FIGURE 4.
Colocalization between hERG and hVps24 in hERG-HEK cells during endocytosis. Live hERG-HEK cells transfected with Myc-tagged hVps24 were incubated with a rabbit anti-hERG primary antibody targeting an extracellularly located region of hERG for 30 min. The cells were exposed to 0 mm K+ MEM for 3 h, fixed, and permeabilized. The cells were treated with Alexa Fluor 488-conjugated secondary anti-rabbit antibody to detect hERG (green) and with mouse anti-Myc primary and Alexa Fluor 594-conjugated anti-mouse secondary antibodies to detect Myc-tagged hVps24 (red). Nuclei were stained using Hoechst 33342. DIC, differential interference contrast.
We examined the effects of Vps24 overexpression on hERG degradation. Thirty-six hours after transfection of hERG-HEK cells with the hVps24 plasmid, cells were cultured in either 5 or 0 mm K+ MEM for 4 h, and hERG expression levels were then examined. As shown in Fig. 5A, overexpression of hVps24 resulted in greater reduction in the expression levels of the 155-kDa hERG in cells cultured in 0 mm K+ MEM for 4 h. Overexpression of hVps24 also facilitated 0 mm K+ culture-induced reduction in IhERG. After the cells were cultured in 0 mm K+ MEM for 2 h, IhERG in hVps24-tranfected cells was significantly smaller than IhERG in control cells (Fig. 5B).
We also knocked down endogenous Vps24 protein expression by transfecting hERG-HEK cells with hVps24 siRNA and examined its effect on hERG expression levels. As shown in Fig. 5C, hVps24 siRNA significantly reduced the endogenous Vps24 expression level and impeded the reduction of 155-kDa hERG expression induced by 0 mm K+ culture for 6 h. The effects of hVps24 siRNA transfection on the hERG activity were also examined by analyzing IhERG. After the cells were cultured in 0 mm K+ MEM for 6 h, IhERG in cells transfected with scrambled siRNA (control) was essentially eliminated. However, significant IhERG remained in hERG-HEK cells transfected with hVps24 siRNA (Fig. 5D).
Deubiquitination Occurs during hERG Protein Endocytic Degradation in 0 mm K+ Conditions
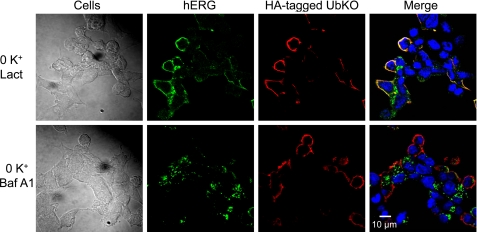
To investigate the cellular machinery for hERG endocytic degradation in low K+ conditions, we examined the effects of the proteasomal inhibitor lactacystin and the lysosomal inhibitor bafilomycin A1 on 0 mm K+-induced hERG degradation in UbKO-transfected hERG-HEK cells. Both lactacystin and bafilomycin A1 impeded the reduction of 155-kDa hERG expression induced by 0 mm K+ exposure for 4 h (Fig. 6A). Interestingly, lactacystin, but not bafilomycin A1, caused a slight upward shift of the 155-kDa band of hERG (Fig. 6A). To examine whether this shift reflects monoubiquitination of the 155-kDa hERG channels, we performed co-IP analysis of proteins extracted from cells cultured in various conditions. The extracted proteins were precipitated using an anti-HA antibody to isolate UbKO (HA-tagged)-bound proteins, and the precipitated proteins were immunoblotted using an anti-hERG antibody. As shown in Fig. 6B, strong hERG signal was only present in proteins from cells treated with 0 mm K+ plus lactacystin but not from cells treated with 0 mm K+ plus bafilomycin A1, indicating that lactacystin-preserved hERG channels are ubiquitinated. To determine the functionality of the preserved 155-kDa hERG in 0 mm K+, we recorded IhERG in cells under each condition. Although the 155-kDa hERG band is present in Western blot analysis of both lactacystin- and bafilomycin A1-treated cells exposed to 0 mm K+ for 4 h (Fig. 6A), only lactacystin-treated cells displayed IhERG (Fig. 6C). We have previously shown that the absence of IhERG in cells cultured in 0 mm K+ with bafilomycin A1 was not caused by the blocking effect of bafilomycin A1 because cells treated with bafilomycin A1 in 5 mm K+ displayed robust hERG currents (6). Instead, bafilomycin A1-preserved mature hERG channels were intracellularly localized (6). We used confocal microscopy to examine the localization of hERG and UbKO in hERG-HEK cells transfected with HA-tagged UbKO plasmid. Cell surface hERG channels were labeled using an anti-hERG antibody in live cells. After labeling, the cells were exposed to 0 mm K+ MEM in the presence of either lactacystin (20 μm) or bafilomycin A1 (1 μm) for 4 h. As shown in Fig. 7, upper row, lactacystin effectively blocked the 0 mm K+-induced internalization of membrane hERG channels that showed colocalization with UbKO. However, bafilomycin A1 failed to prevent hERG internalization. In the presence of bafilomycin A1 (1 μm), internalized hERG channels accumulated inside the cell. Interestingly, internalized hERG channels did not colocalize with UbKO (Fig. 7, lower row). Because bafilomycin A1 inhibits lysosomal degradation (33), the internalized hERG signal likely reflects the non-degraded channels accumulated in the lysosomes. The non-ubiquitinated nature of the bafilomycin A1-preserved hERG band (Fig. 6B) and lack of colocalization between internalized hERG and UbKO (Fig. 7, lower row) strongly suggest that monoubiquitinated hERG channels undergo deubiquitination before entry into lysosomes.
FIGURE 7.
Localization of hERG or UbKO in UbKO-transfected hERG-HEK cells cultured in 0 mm K+ MEM in presence of 20 μm lactacystin (Lact; upper row) or 1 μm bafilomycin A1 (Baf A1; lower row) for 4 h. hERG-HEK cells were transfected with HA-tagged UbKO. Thirty-six hours after transfection, the cell surface hERG channels were labeled by incubating the live cells with a rabbit anti-hERG primary antibody targeting the extracellular region of hERG. The cells were fixed and permeabilized. The cells were treated with Alexa Fluor 488-conjugated secondary antibody against rabbit to detect hERG (green) and with a mouse anti-HA primary antibody and an Alexa Fluor 594-conjugated secondary antibody against mouse to detect HA-UbKO (red). Nuclei were stained using Hoechst 33342.
To further examine the role of deubiquitination in hERG degradation, we enhanced or inhibited the activity of a deubiquitinating protease, STAMBP, and examined the consequences on hERG degradation. STAMBP is a metalloprotease that deubiquitinates ubiquitinated cargo (34, 35) and plays an important role in postendocytic trafficking of the epidermal growth factor receptor (29). Overexpression of STAMBP significantly enhanced the 0 mm K+-induced reduction in 155-kDa hERG expression and IhERG (Fig. 8, A and B). On the other hand, depletion of endogenous STAMBP using siRNA transfection significantly impeded 0 mm K+-induced reduction of 155-kDa hERG expression and IhERG (Fig. 8, C and D).
DISCUSSION
IKr is important for cardiac action potential repolarization, and its reduction causes LQTS (1, 2, 4, 36). A reduction in serum K+ concentration (hypokalemia) precipitates LQTS (5). We recently showed that hypokalemia enhances cell surface hERG internalization and degradation and thus identified a novel mechanism through which hypokalemia induces cardiac arrhythmias (6). Although our work showed that Ub is involved in endocytic degradation of cell surface hERG channels under low K+ conditions (6, 7), the nature of Ub-hERG interactions is unknown. The present study demonstrated that monoubiquitination mediates degradation of the voltage-gated K+ channel hERG under low K+ conditions. This conclusion is supported by the observations that overexpression of UbKO enhanced the degradation of the 155-kDa hERG channels (Figs. 2 and 3) and that UbKO preferentially associated with hERG channels under low K+ conditions in the presence of lactacystin (Fig. 6). Ub can polymerize by binding to other Ub molecules at lysine residues (37); UbKO has all lysine residues mutated to arginine residues and can only support monoubiquitination. Hence, UbKO is a useful tool in evaluating monoubiquitin modification of cargo proteins (24, 25).
Numerous cellular proteins are post-translationally modified by the addition of the small modifier protein Ub for maintaining cellular health. Whereas polyubiquitination usually leads to proteasomal degradation of various cellular proteins, monoubiquitination regulates endocytic degradation of membrane receptors in lysosomes (9, 10). The monoubiquitination-controlled attenuation of the receptor-mediated signaling pathways plays a central role in maintaining signaling homeostasis. For example, epidermal growth factor receptor, the epithelial sodium channel, the inward rectifier potassium channel ROMK1, and receptor tyrosine kinases are all monoubiquitinated, and monoubiquitin acts as a signal for internalization of targeted proteins through sorting in MVBs for lysosomal degradation (9, 28). MVBs are formed when segments of the endosomal membrane endocytose and become vesicles within a larger endosomal body. ESCRT-I first binds both Ub and phosphatidylinositol 3-phosphate and initiates the sorting mechanism (37). ESCRT-II then brings ESCRT-III to the desired site on the endosomal membrane where they sequester the MVB cargo (37). The morphological changes in the intraluminal vesicles reflect the sorting of ubiquitinated cargo toward them (31). Vps24 is an important member of ESCRT-III required for cargo protein sorting. As shown in Fig. 5, hVps24 is involved in the degradation of mature hERG channels in low K+ conditions. Overexpression of hVps24 enhanced the 155-kDa hERG degradation in low K+, whereas knockdown of hVps24 impeded this process. The hVps24 protein displays predominantly plasma membrane and vesicular localization consistent with its role in the formation of MVBs (32). Our data also showed that internalized hERG channels colocalized with the hVps24 protein, a marker protein of MVBs (Fig. 4). Because hVps24 is involved in the MVB sorting of internalized cargo proteins, depletion of hVps24 obstructs hERG sorting in MVBs, leading to an accumulation of internalized hERG channels in endosomes. Such accumulation may impede hERG endocytosis through either an increased endosome recycling to the plasma membrane or an unknown feedback mechanism.
Because UbKO is a chain elongation-defective Ub mutant, it causes premature termination of polyubiquitin chains (Fig. 2C) and only supports monoubiquitination of target proteins (24, 25). Our data showed that UbKO expression significantly accelerated low K+-induced endocytic degradation of the mature hERG channels (Figs. 2 and 3). Thus, polyubiquitination may not play a decisive role in low K+-induced hERG internalization. On the other hand, low K+-induced hERG internalization can be impeded by the proteasomal inhibitor lactacystin (Figs. 6 and 7) as well as MG132 (data not shown). The exact mechanisms for proteasomal activity in the MVB/lysosomal degradation of hERG channels under low K+ conditions warrant further investigation and may be related to the fact that proteasomal activity is required for the sorting of ubiquitinated cargo proteins in MVBs (38). Interestingly, our data demonstrated that lactacystin not only prevented 155-kDa hERG degradation but also shifted the 155-kDa hERG band toward a slightly higher molecular mass (Fig. 6A), suggesting that mature hERG channels are monoubiquitinated in the presence of lactacystin. Indeed, our co-IP data revealed that lactacystin-preserved hERG channels are monoubiquitinated (Figs. 2C and 6B). We propose that monoubiquitination triggers internalization of hERG channels, and deubiquitination is subsequently required for hERG sorting and degradation in MVBs/lysosomes (Fig. 9). The fact that lactacystin impedes degradation of mature hERG channels that are ubiquitinated raised a possibility that, as a proteasomal inhibitor, lactacystin inhibits hERG degradation in the MVB/lysosomal pathway by preventing deubiquitination of hERG channels. Previously, proteasomal activity has also been shown to be required for degradation of a number of membrane proteins that are internalized via the ubiquitination/MVB/lysosome pathway. These membrane proteins include glutamate receptors (16), tropomyosin-regulated kinase A receptor (39), growth hormone receptor (40), chloride channel cystic fibrosis transmembrane conductance regulator (41), and gap junction channel connexin 43 (42). It will be interesting to investigate the role of deubiquitination in the degradation of these membrane proteins.
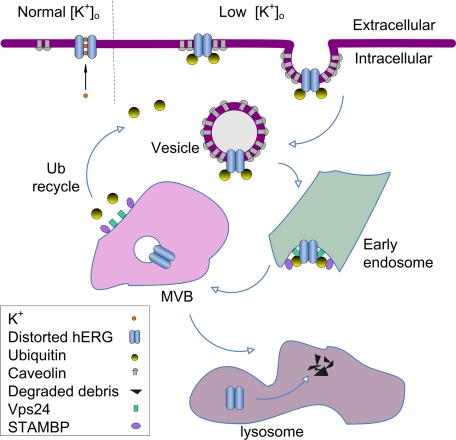
FIGURE 9.
Scheme of role of Ub in endocytic degradation of hERG channels. Under low K+ conditions, mature hERG channels adopt a non-conducting conformation, leading to monoubiquitination, which triggers hERG endocytosis through a caveolin-dependent pathway. Endocytosed hERG channels transit through early endosomes into MVBs. While Vps24 is critical for the formation and sorting of monoubiquitinated hERG channels into MVBs, STAMBP deubiquitinates the monoubiquitinated hERG to facilitate hERG sorting in the MVBs. The sorted hERG is deposited in the lysosomes for degradation. Lactacystin prevents deubiquitination, leading to accumulation of monoubiquitinated hERG channels which are primarily localized in the plasma membrane. Bafilomycin A1 prevents hERG degradation in lysosomes, leading to accumulation of deubiquitinated hERG in lysosomes.
Our data obtained using Western blot (Fig. 6) and confocal analysis (Fig. 7) further demonstrated that internalized hERG was not ubiquitinated when it reached lysosomes in the presence of lysosomal inhibitor bafilomycin A1. These data strongly suggested that deubiquitination occurs downstream of hERG sorting and degradation. Our data on the deubiquitination enzyme STAMBP directly support this notion (Fig. 8) and suggest that the monoubiquitinated and internalized 155-kDa hERG is subsequently deubiquitinated between early endosomes and lysosomes, i.e. during MVB sorting. Thus, Ub molecules are released from mature hERG channels prior to their delivery to lysosomes for degradation. Because STAMBP-mediated deubiquitination of mature hERG is likely to occur at a late stage of endocytosis during cargo sorting in MVBs where STAMBP is recruited by the ubiquitinated cargo, STAMBP does not counteract the early step of monoubiquitination of cell surface hERG channels induced by low K+ exposure. Deubiquitination plays a similar role in endocytosis of protease-activated receptor 2 (43). In short, our data showed that monoubiquitination at the earlier stages of the pathway directs the mature hERG channel for MVB sorting, and the internalized mature hERG channel is ultimately in a deubiquitinated state when it reaches lysosomes.
Endocytosis represents one of the most essential processes for cell function. We previously showed that extracellular K+ is a prerequisite for hERG function and membrane stability; under low K+ conditions, cell surface hERG channels enter into a non-conducting state, reflecting a conformational change of the channel. This conformational change may cause the channel to be tagged by Ub, which triggers the channel internalization through a caveolin-dependent endocytic pathway (8). Our present study indicates that attachment of a single Ub moiety is sufficient to trigger internalization of cell surface hERG channels; the internalized mature hERG channels are sorted by the ESCRT in MVBs and undergo deubiquitination prior to entry into lysosomes for degradation.
In summary, we have demonstrated that the cell surface voltage-gated potassium channel hERG is internalized and degraded through a monoubiquitination/MVB sorting pathway in a dynamic manner under low K+ conditions. This finding extends our understanding of ion channel regulation and endocytic degradation of plasma membrane proteins.
This work was supported in part by Canadian Institutes of Health Research Grant MOP 72911 and Heart and Stroke Foundation of Ontario Grant T 6612 (to S. Z.).
- hERG
- the human ether-a-go-go-related gene
- IKr
- the rapidly activating delayed rectifier potassium channel
- LQTS
- long QT syndrome
- Ub
- ubiquitin
- UbKO
- lysine-less Ub mutant
- MVB
- multivesicular body
- hVps24
- Homo sapiens vacuolar protein sorting 24 homolog (Saccharomyces cerevisiae)
- STAMBP
- signal transducing adaptor molecule-binding protein
- MEM
- minimum essential medium
- IP
- immunoprecipitation
- ESCRT
- endosomal sorting complex required for transport.
REFERENCES
- 1. Sanguinetti M. C., Jiang C., Curran M. E., Keating M. T. (1995) Cell 81, 299–307 [DOI] [PubMed] [Google Scholar]
- 2. Trudeau M. C., Warmke J. W., Ganetzky B., Robertson G. A. (1995) Science 269, 92–95 [DOI] [PubMed] [Google Scholar]
- 3. Sanguinetti M. C., Tristani-Firouzi M. (2006) Nature 440, 463–469 [DOI] [PubMed] [Google Scholar]
- 4. Keating M. T., Sanguinetti M. C. (2001) Cell 104, 569–580 [DOI] [PubMed] [Google Scholar]
- 5. Roden D. M., Woosley R. L., Primm R. K. (1986) Am. Heart J. 111, 1088–1093 [DOI] [PubMed] [Google Scholar]
- 6. Guo J., Massaeli H., Xu J., Jia Z., Wigle J. T., Mesaeli N., Zhang S. (2009) J. Clin. Investig. 119, 2745–2757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Massaeli H., Guo J., Xu J., Zhang S. (2010) Circ. Res. 106, 1072–1082 [DOI] [PubMed] [Google Scholar]
- 8. Massaeli H., Sun T., Li X., Shallow H., Wu J., Xu J., Li W., Hanson C., Guo J., Zhang S. (2010) J. Biol. Chem. 285, 27259–27264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Haglund K., Di Fiore P. P., Dikic I. (2003) Trends Biochem. Sci. 28, 598–603 [DOI] [PubMed] [Google Scholar]
- 10. Piper R. C., Luzio J. P. (2007) Curr. Opin. Cell Biol. 19, 459–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pickart C. M. (2000) Trends Biochem. Sci. 25, 544–548 [DOI] [PubMed] [Google Scholar]
- 12. Galan J. M., Haguenauer-Tsapis R. (1997) EMBO J. 16, 5847–5854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hicke L. (1999) Trends Cell Biol. 9, 107–112 [DOI] [PubMed] [Google Scholar]
- 14. Lucero P., Peñalver E., Vela L., Lagunas R. (2000) J. Bacteriol. 182, 241–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Haglund K., Sigismund S., Polo S., Szymkiewicz I., Di Fiore P. P., Dikic I. (2003) Nat. Cell Biol. 5, 461–466 [DOI] [PubMed] [Google Scholar]
- 16. Patrick G. N., Bingol B., Weld H. A., Schuman E. M. (2003) Curr. Biol. 13, 2073–2081 [DOI] [PubMed] [Google Scholar]
- 17. Gupta-Rossi N., Six E., LeBail O., Logeat F., Chastagner P., Olry A., Israël A., Brou C. (2004) J. Cell Biol. 166, 73–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. d'Azzo A., Bongiovanni A., Nastasi T. (2005) Traffic 6, 429–441 [DOI] [PubMed] [Google Scholar]
- 19. Sigismund S., Woelk T., Puri C., Maspero E., Tacchetti C., Transidico P., Di Fiore P. P., Polo S. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 2760–2765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen H., De Camilli P. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 2766–2771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Leithe E., Kjenseth A., Sirnes S., Stenmark H., Brech A., Rivedal E. (2009) J. Cell Sci. 122, 3883–3893 [DOI] [PubMed] [Google Scholar]
- 22. Lin D. H., Yue P., Pan C. Y., Sun P., Zhang X., Han Z., Roos M., Caplan M., Giebisch G., Wang W. H. (2009) J. Biol. Chem. 284, 29614–29624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gang H., Zhang S. (2006) J. Gen. Physiol. 128, 55–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lim K. L., Chew K. C., Tan J. M., Wang C., Chung K. K., Zhang Y., Tanaka Y., Smith W., Engelender S., Ross C. A., Dawson V. L., Dawson T. M. (2005) J. Neurosci. 25, 2002–2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhou R., Patel S. V., Snyder P. M. (2007) J. Biol. Chem. 282, 20207–20212 [DOI] [PubMed] [Google Scholar]
- 26. Zhou Z., Gong Q., Ye B., Fan Z., Makielski J. C., Robertson G. A., January C. T. (1998) Biophys. J. 74, 230–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Guo J., Massaeli H., Li W., Xu J., Luo T., Shaw J., Kirshenbaum L. A., Zhang S. (2007) J. Pharmacol. Exp. Ther. 321, 911–920 [DOI] [PubMed] [Google Scholar]
- 28. Mosesson Y., Shtiegman K., Katz M., Zwang Y., Vereb G., Szollosi J., Yarden Y. (2003) J. Biol. Chem. 278, 21323–21326 [DOI] [PubMed] [Google Scholar]
- 29. Clague M. J., Urbé S. (2006) Trends Cell Biol. 16, 551–559 [DOI] [PubMed] [Google Scholar]
- 30. Nickerson D. P., Russell M. R., Odorizzi G. (2007) EMBO Rep. 8, 644–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wollert T., Wunder C., Lippincott-Schwartz J., Hurley J. H. (2009) Nature 458, 172–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Muzioł T., Pineda-Molina E., Ravelli R. B., Zamborlini A., Usami Y., Göttlinger H., Weissenhorn W. (2006) Dev. Cell 10, 821–830 [DOI] [PubMed] [Google Scholar]
- 33. Yoshimori T., Yamamoto A., Moriyama Y., Futai M., Tashiro Y. (1991) J. Biol. Chem. 266, 17707–17712 [PubMed] [Google Scholar]
- 34. McCullough J., Clague M. J., Urbé S. (2004) J. Cell Biol. 166, 487–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McCullough J., Row P. E., Lorenzo O., Doherty M., Beynon R., Clague M. J., Urbé S. (2006) Curr. Biol. 16, 160–165 [DOI] [PubMed] [Google Scholar]
- 36. Sanguinetti M. C., Jurkiewicz N. K. (1990) J. Gen. Physiol. 96, 195–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Katzmann D. J., Odorizzi G., Emr S. D. (2002) Nat. Rev. Mol. Cell Biol. 3, 893–905 [DOI] [PubMed] [Google Scholar]
- 38. Hurley J. H. (2008) Curr. Opin. Cell Biol. 20, 4–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Geetha T., Wooten M. W. (2008) Traffic 9, 1146–1156 [DOI] [PubMed] [Google Scholar]
- 40. van Kerkhof P., Govers R., Alves dos Santos C. M., Strous G. J. (2000) J. Biol. Chem. 275, 1575–1580 [DOI] [PubMed] [Google Scholar]
- 41. Gentzsch M., Chang X. B., Cui L., Wu Y., Ozols V. V., Choudhury A., Pagano R. E., Riordan J. R. (2004) Mol. Biol. Cell 15, 2684–2696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Laing J. G., Tadros P. N., Westphale E. M., Beyer E. C. (1997) Exp. Cell Res. 236, 482–492 [DOI] [PubMed] [Google Scholar]
- 43. Hasdemir B., Murphy J. E., Cottrell G. S., Bunnett N. W. (2009) J. Biol. Chem. 284, 28453–28466 [DOI] [PMC free article] [PubMed] [Google Scholar]