Abstract
Lipoprotein lipase (LPL) is a member of a lipase family known to hydrolyze triglyceride molecules in plasma lipoprotein particles. LPL also plays a role in the binding of lipoprotein particles to cell-surface molecules, including sulfated glycosaminoglycans (GAGs). LPL is predominantly expressed in adipose and muscle but is also highly expressed in the brain where its specific roles are unknown. It has been shown that LPL is colocalized with senile plaques in Alzheimer disease (AD) brains, and its mutations are associated with the severity of AD pathophysiological features. In this study, we identified a novel function of LPL; that is, LPL binds to amyloid β protein (Aβ) and promotes cell-surface association and uptake of Aβ in mouse primary astrocytes. The internalized Aβ was degraded within 12 h, mainly in a lysosomal pathway. We also found that sulfated GAGs were involved in the LPL-mediated cellular uptake of Aβ. Apolipoprotein E was dispensable in the LPL-mediated uptake of Aβ. Our findings indicate that LPL is a novel Aβ-binding protein promoting cellular uptake and subsequent degradation of Aβ.
Keywords: Alzheimer Disease, Amyloid, Apolipoproteins, Heparan Sulfate, Lipase, Amyloid Beta Protein, Degradation
Introduction
Lipoprotein lipase (LPL)2 catalyzes the hydrolysis of triacylglycerol and mediates the cellular uptake of lipoproteins by functioning as a “bridging molecule” between lipoproteins and sulfated glycosaminoglycans (GAGs) or lipoprotein receptors in blood vessels (1, 2). Sulfated GAGs are side chains of proteoglycans normally found in the extracellular matrix and on the cell surface in the peripheral tissues and brain. Sulfation modifications vary within the GAG chains and are crucial for interaction between GAGs and various protein ligands (3), including LPL (4, 5).
It has been shown that LPL is distributed in numerous organs and is highly expressed in the brain (6, 7). Although the catabolic activity of LPL on triacylglycerol is observed in the brain (8), the finding that apolipoprotein CII (apoCII), an essential cofactor for LPL, is not expressed in the brain (9, 10), suggests that LPL has a novel nonenzymatic function in the brain. However, little is known about LPL function in the brain. Interestingly, it has been shown that LPL is accumulated in senile plaques of Alzheimer disease (AD) brains (11). Moreover, SNPs in the coding region of the LPL gene are associated with disease incidence in clinically diagnosed AD subjects, LPL mRNA expression level, brain cholesterol level, and the severity of AD pathologies, including neurofibrillary tangles and senile plaque density (12). These results suggest that LPL may have a physiological role in the brain, whose alternation is associated with the pathogenesis of AD.
The occurrence of senile plaques in the brain is one of the pathological hallmarks of AD. They contain extracellular deposits of amyloid β protein (Aβ), and the abnormal Aβ deposition or the formation of soluble Aβ oligomers is crucial for AD pathogenesis. Aβ is a physiological peptide whose main species are 40 and 42 amino acids in length, and Aβ42 is the predominant specie in senile plaques (13). The Aβ levels are determined by the balance between its production and degradation/clearance, and an attenuated Aβ catabolism is suggested to cause Aβ accumulation in aging brains (14). Previous studies have shown that astrocytes and microglia directly take up and degrade Aβ42 (15, 16) and that Aβ degradation occurs in late endosomal-lysosomal compartments (17, 18). These lines of evidence, together with the finding that LPL mediates the cellular uptake of lipoproteins (1, 2), led us to carry out experiments to determine whether LPL interacts with Aβ to promote Aβ cellular uptake and degradation in astrocytes. Here, we provide evidence that LPL forms a complex with Aβ and facilitates Aβ cell surface binding and uptake in mouse primary astrocytes through a mechanism that is dependent on heparan sulfate and chondroitin sulfate GAG chains, leading to the lysosomal degradation of Aβ.
MATERIALS AND METHODS
Materials
Bovine LPL, heparinases, and a polyclonal anti-actin antibody were purchased from Sigma. Synthetic Aβ1–42 was purchased from the Peptide Institute (Osaka, Japan). Heparin, chondroitin, chondroitin sulfates, and chondroitinase ABC were from Seikagaku (Tokyo, Japan). Monoclonal anti-Aβ antibodies (6E10, 4G8) were purchased from Signet Laboratories (Dedham, MA), and a goat polyclonal anti-ApoE antibody and mouse control IgG were from Millipore (Bedford, MA). An anti-LPL antibody and Cy3- and FITC- conjugated secondary antibodies were purchased from Abcam, Inc. (Cambridge, MA). A monoclonal anti-Aβ antibody (2C8) was purchased from Medical and Biological Laboratories (Nagoya, Japan).
Animals
C57BL/6 mice were purchased from SLC, Inc. (Hamamatsu, Japan). ApoE-KO mice were obtained from Jackson ImmunoResearch Laboratories (Bar Harbor, ME). The National Center of Geriatrics and Gerontology Institutional Animal Care and Use Committee approved the animal studies.
Preparation of LPL
Because the sequence of LPL is highly conserved among mammalian species and the ability of LPL to interact with proteoglycans is also well conserved, we used LPL purified from bovine milk. An LPL suspension (suspended in 3.8 m ammonium sulfate, 0.02 m Tris-HCl, pH 8.0) was centrifuged (10,000 × g for 20 min at 4 °C), and the resulting pellet was dissolved in PBS. The prepared LPL was stored at 4 °C and used within 3 days.
Cell Culture
Highly astrocyte-rich cultures were prepared according to a method described previously (19). In brief, brains of postnatal day 2 C57BL/6 mice or ApoE knock-out mice were removed under anesthesia. The cerebral cortices from the mouse brains were dissected, freed from meninges, and diced into small pieces; the cortical fragments were incubated in 0.25% trypsin and 20 mg/ml DNase I in PBS at 37 °C for 20 min. The fragments were then dissociated into single cells by pipetting. The dissociated cells were seeded in 75-cm2 dishes at a density of 5 × 107 cells per flask in DMEM-containing 10% FBS. After 10 days of incubation in vitro, flasks were shaken at 37 °C overnight, and the remaining astrocytes in the monolayer were trypsinized (0.1%) and reseeded. The astrocyte-rich cultures were maintained in DMEM-containing 10% FBS until use.
Assay of Aβ Binding and Uptake in Astrocytes by Western Blotting
Assays were carried out on confluent monolayers of astrocytes grown in 12-well plates. Aβ was dissolved in dimethyl sulfoxide to a final concentration of 1 mm and stored at −40 °C. Aβ (500 nm) and LPL (1–10 μg/ml) were mixed in DMEM. Immediately, the mixture was added to the culture medium of astrocytes. Cells were incubated at 37 °C for 5 h to assess the cellular uptake of Aβ or at 4 °C for 3 h to evaluate the binding of Aβ to the cell surface of astrocytes. In these assays, cells were incubated in serum-free DMEM. After incubation, cells were washed with PBS three times, harvested using a cell scraper and lysed by sonication in radioimmune precipitation assay buffer (1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, 150 mm NaCl, 50 mm Tris-HCl (pH 8.0), 1 mm EDTA). Cell lysates were subjected to SDS-PAGE with 4–20% gradient gels (WAKO Pure Chemicals, Osaka, Japan) and transferred to polyvinylidene difluoride membranes (Millipore). Aβ was probed with 6E10 antibody followed by horseradish peroxidase-labeled anti-mouse antibody (Cell Signaling Technology, Inc., Beverly, MA) and chemiluminescent substrate ECL Plus (GE Healthcare). The protein contents of cell lysates were normalized to the expression level of actin protein. To examine the involvement of GAGs, heparin, chemically modified heparins, chondroitin, or chondroitin sulfates (3 μg/ml) were incubated with a mixture solution of Aβ and LPL. Astrocytes were pretreated with a mixture of heparinase II and heparinase III or chondroitinase ABC (0.03 units/ml) for 24 h at 37 °C to evaluate endogenously expressed glycosaminoglycans. Signals were visualized and quantified using a LAS-3000 luminescent image analyzer (Fujifilm, Tokyo, Japan) and ImageJ software (National Institutes of Health, Bethesda, MD). For analyzing protein band densities, a region of interest was drawn around a band, and protein band densities were calculated.
siRNA Interference of LPL
siRNA specific for mouse LPL (sense strand, 5′-CAGCUGAGGACACUUGUCAUCUCAUdTdT-3′; antisense strand, 5′-AUGAGAUGACAAGUGUCCUCAGCUGdTdT-3′) and control siRNA (sense strand, 5′-CAGAGGGCACAUUUGACCUUUCCAUdTdT-3′; antisense strand, 5′-AUGGAAAGGUCAAAUGUGCCCUCUG-3′) was purchased from Invitrogen. Astrocytes grown in 12-well plates for 24 h were transfected with either LPL siRNA or control siRNA with Lipofectamine RNAiMAX (Invitrogen). Forty-eight hours after transfection, cells were treated with Aβ (1 μm) and then incubated at 4 °C for 3 h, and cell-surface associated Aβ was analyzed as described above. An anti-LPL antibody (Gene Tex, Inc.) was used for the detection of LPL.
Assay of Aβ Degradation in Astrocytes
Astrocytes were incubated with Aβ (250 nm) and LPL (2 μg/ml) at 37 °C for 5 h. Subsequently, cells were washed with DMEM and incubated in DMEM for additional hours. Then, Aβ in cell lysates was analyzed by Western blotting as described above.
Immunoprecipitation
Aβ (500 nm) and LPL at various concentrations were incubated in DMEM at 37 °C for 3 h. LPL-Aβ complexes were immunoprecipitated with an anti-LPL antibody and magnetic protein G beads (Dynal, Hamburg, Germany). For detection of LPL-Aβ complexes in the mice brains, brain homogenates from 12-week-old C57BL/6 mice were used. In brief, anesthetized mice were perfused with PBS containing 35 μg/ml heparin for 15 min. The cerebrum was dissected out and homogenized by sonication in 4 volumes of PBS containing a protease inhibitor mixture (P8340; Sigma) and centrifuged at 1,000 × g for 10 min at 4 °C. The supernatants were harvested and LPL-Aβ complexes were immunoprecipitated with an anti-LPL antibody and magnetic protein G beads. The obtained precipitates were washed three times with PBS and incubated at 70 °C for 10 min in SDS sample buffer. Dissociated Aβ recovered in the supernatant was assessed by Western blotting as described above. For detection of endogenous Aβ, the supernatants were subjected to SDS-PAGE with 4–20% gradient gels and transferred to polyvinylidene difluoride membranes. The membranes were exposed to microwave irradiation for 20 s, and Aβ was probed with 4G8 antibody followed by horseradish peroxidase-labeled anti-mouse antibody and the chemiluminescent substrate ECL Plus.
Immunocytochemistry
Astrocytes grown on poly-l-lysine-coated coverslips were incubated with a mixture of Aβ (250 nm) and LPL (2 μg/ml) at 37 °C for 5 h. After treatment, the cells were fixed with 4% paraformaldehyde in PBS at room temperature for 10 min, blocked, and permeabilized with 10% normal goat serum and 0.05% saponin in PBS at room temperature for 20 min. In some experiments, cells were washed twice with DMEM followed by incubation at 37 °C for 3 h in DMEM and fixed. The cells were then incubated with primary antibodies followed by Cy3- and FITC-conjugated secondary antibodies. The stained specimens were mounted with FluorSave reagents (Calbiochem) and examined under an LSM 510 confocal laser microscope (Carl Zeiss MicroImaging GmbH, Jena, Germany).
Statistical Analysis
The collected data were analyzed by one-way analysis of variance (ANOVA) including appropriate variables followed by the Dunnett's test or unpaired Student's t test. Results were considered significant when p < 0.05.
RESULTS
LPL Binds to Aβ in Vitro
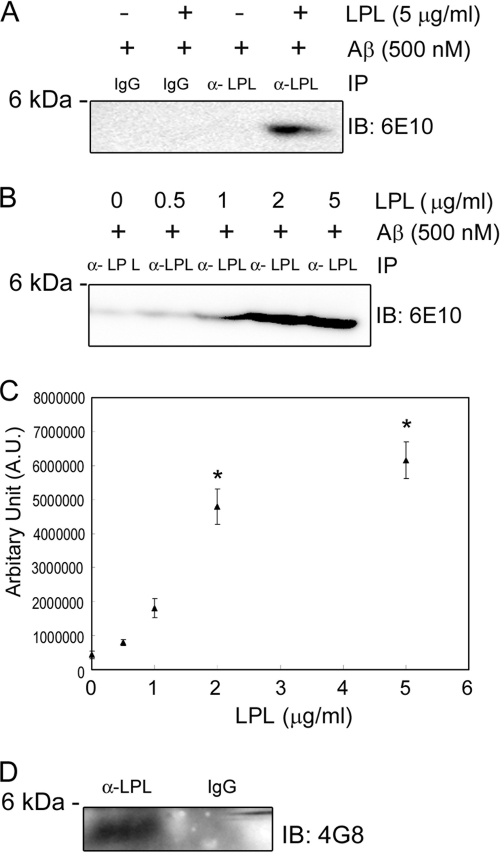
LPL was incubated with freshly prepared Aβ42 in vitro, and the complexes formed were immunoprecipitated with an anti-LPL antibody coupled with magnetic beads, followed by probing Western blots of protein complexes using an anti-Aβ antibody (Fig. 1A). Aβ42 was immunoprecipitated with an anti-LPL antibody, but not with control IgG. The levels of Aβ42 recovered in the immunoprecipitates from samples in the presence of 2–5 μg/ml LPL were significantly higher than those from samples in the presence of 0, 0.5, or 1 μg/ml of LPL (Fig. 1, B and C), suggesting that LPL directly interacts with Aβ42, and these two molecules form a complex in an LPL dose-dependent manner. Furthermore, endogenous mouse Aβ was immunoprecipitated with the anti-LPL antibody from brain homogenates prepared from C57BL/6 mice (Fig. 1D), indicating that endogenous mouse LPL directly interacts with endogenous mouse Aβ. We also determined the assembly state of Aβ that forms complex with LPL. Solutions containing Aβ oligomers were subjected to immunoprecipitation/immunoblot analysis, and Aβ42 monomers were immunoprecipitated by an anti-LPL antibody (supplemental Fig. 1).
FIGURE 1.
LPL binds to Aβ in vitro. A, LPL (5 μg/ml) and Aβ (500 nm) were incubated in DMEM at 37 °C for 3 h. Protein complexes formed were immunoprecipitated with an anti-LPL antibody (α-LPL), and the immunoprecipitates (IP) were analyzed by Western blotting using 6E10, an anti-Aβ antibody. These data are representative of three independent experiments. B, LPL at various concentrations of 0, 0.5, 1, 2, and 5 μg/ml and Aβ at 500 nm were incubated in DMEM at 37 °C for 3 h. Protein complexes formed were immunoprecipitated with an α-LPL, and the immunoprecipitates were subjected to Western blotting using 6E10. C, quantification of Aβ immunoprecipitated with α-LPL. The data presented are the means ± S.D. of three independent experiments. *, p < 0.001 versus samples without LPL treatment. D, the mouse cerebrum was homogenized by sonication in 4 volumes of PBS containing a protease inhibitor mixture and centrifuged at 1000 × g for 10 min at 4 °C. The supernatants were harvested. LPL-Aβ complexes in the supernatant were immunoprecipitated with an α-LPL, and the Aβ in the immunoprecipitates was detected by Western blotting using 4G8, an anti-Aβ antibody. IB, immunoblot.
LPL Promotes Cell Surface Binding and Cellular Uptake of Aβ in Astrocytes
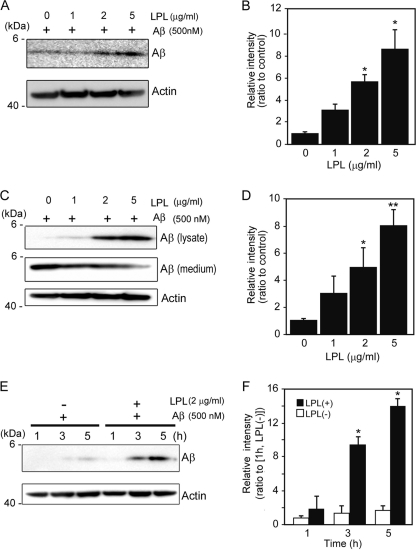
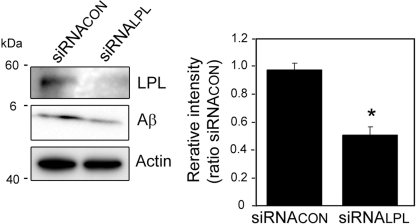
We then determined whether LPL affects the cellular binding of Aβ to astrocytes. Soluble Aβ42 and various concentrations of LPL were added to primarily cultured astrocytes prepared from WT mice and then incubated at 4 °C. LPL (2–5 μg/ml) of significantly augmented Aβ42 binding to astrocytes by 5.8- to 9-fold of that in the case without LPL (Fig. 2, A and B). To examine the effect of LPL on the cellular uptake of Aβ, we incubated primary astrocytes with soluble Aβ42 at 37 °C for 5 h. Apparently, the level of Aβ uptake by astrocytes increased in the presence of LPL at concentrations of 2 to 5 μg/ml (Fig. 2C, lysate). Consistent with the increase in the level of cellular uptake of Aβ, the level of Aβ remaining in culture medium was decreased (Fig. 2C, medium). The Aβ levels in the cell lysate quantified are shown in Fig. 2D, indicating that Aβ levels were significantly increased by 5–8-fold that in astrocytes incubated without LPL. Next, we determined the time-dependent effect of LPL-mediated Aβ uptake into astrocytes. Astrocyte cultures were incubated with Aβ (500 nm) and LPL (2 μg/ml) at 37 °C for various hours, and the Aβ level in the cell lysate was determined. The level of Aβ in the cell lysate increased in a time-dependent manner (Fig. 2E). The Aβ levels in the astrocytes incubated for 3 and 5 h were significantly higher by 9–14-fold of that in astrocytes incubated without LPL (Fig. 2F). These concentrations of LPL are comparable with the concentrations with which LPL could act as “bridging molecules” (2, 20). There were no significant differences among the values for cultures without LPL (one-way ANOVA, p = 0.1386). No change in cellular morphology or cell number in astrocyte cultures was observed during the incubation (data not shown). To examine the involvement of LPL expressed by astrocytes, we carried out experiments using the gene silencing technique for LPL. The transient knockdown of LPL expression was achieved by the transfection of siRNA specific for LPL. After transfection, cells were treated with Aβ42 (1 μm) and then incubated at 4 °C for 3 h. As shown in Fig. 3, the cellular binding of Aβ42 to astrocytes was significantly decreased by LPL protein knockdown.
FIGURE 2.
LPL augments cell-surface association and cellular uptake of Aβ in astrocytes. A, mouse primary astrocytes were incubated with LPL (0–5 μg/ml) and Aβ (500 nm) at 4 °C for 3 h. The astrocytes were washed in cold PBS three times, and the cells were harvested using a scraper. The level of Aβ on the cell surface was determined by Western blotting in a detergent extract of whole cells. B, quantification of cell-surface-associated Aβ. The data are the means ± S.D. of three independent experiments. *, p < 0.001 versus LPL at 0 μg/ml. C and D, astrocytes were incubated with Aβ (500 nm) and LPL (0, 1, 2, and 5 μg/ml) at 37 °C for 3 h. The cultured cells were then washed thoroughly in PBS for three times, and the cells were collected. The level of Aβ in the whole cell lysate (lysate), and the conditioned medium of cultured cells (medium) were determined by Western blotting using 6E10 antibody. The level of actin demonstrated by Western blotting using an anti-β-actin antibody was used as the loading control. These data are representative of at least three independent experiments. D, quantification of cellular Aβ is shown. The data presented are the means ± S.D. of three independent experiments. *, p < 0.05; **, p < 0.01 versus LPL at 0 μg/ml. E and F, astrocytes were incubated with Aβ (500 nm) and LPL (2 μg/ml) at 37 °C for 0, 3, and 5 h. The cultured cells were then washed thoroughly in PBS three times, and the cells were collected. The amount of Aβ in the whole cell lysate was determined by Western blotting using 6E10 antibody. The level of actin demonstrated by Western blotting using the anti-β-actin antibody was used as the loading control. These data are representative of at least three independent experiments. F, quantification of cellular Aβ is shown. The data are the means ± S.D. of three independent experiments. *, p < 0.001 versus LPL (+) at 1 h.
FIGURE 3.
Effect of LPL knockdown on cell-surface association of Aβ in cultured astrocytes. Astrocytes were transfected with 10 nm siRNA specific for LPL (siRNALPL) and control siRNA (siRNACON). Forty-eight hours after transfection, cells were treated with Aβ42 (1 μm) at 4 °C for 3 h. The cells were washed in cold PBS three times, and the cells were harvested using a scraper. The level of Aβ42 on the cell surface was determined by Western blotting in a detergent extract of whole cells. The graph shows the levels of cell-surface-associated Aβ. The data are the means ± S.D. of three independent experiments. *, p < 0.001 versus control siRNA by unpaired Student's t test.
Degradation of Internalized Aβ in a Lysosomal Pathway in Astrocytes
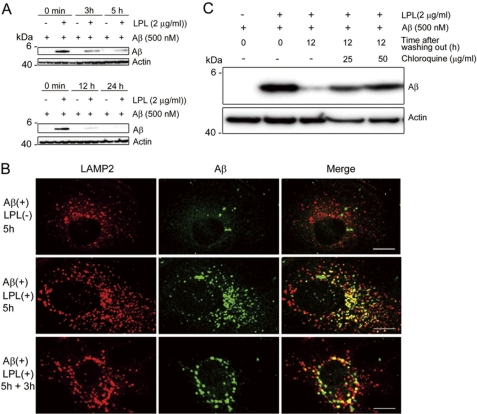
Next, we examined the degradation of internalized Aβ. Mouse primary astrocytes were incubated with soluble Aβ42 and LPL at 37 °C for 5 h, washed in DMEM three times, and cultured at 37 °C for additional time (0, 3, 5, 12, and 24 h). Cells were then harvested, and the Aβ level in the cell lysate was analyzed by Western blotting. The strong signal representing internalized Aβ during the initial incubation for 5 h was detected in the cell lysate at the point of 0 min after washing (Fig. 4A). Three to five hours after washing, the level of Aβ remaining in the cell lysate partially disappeared (Fig. 4A). Twelve and twenty-four hours after washing, the internalized Aβ completely disappeared, indicating that the internalized Aβ was degraded in astrocytes in a time-dependent manner (Fig. 4A). To gain insight into the degradation pathway of the internalized Aβ, we investigated the localization of Aβ by immunocytochemical analysis. Mouse primary astrocytes were plated on poly-l-lysine-coated coverglasses and incubated with Aβ42 (500 nm) and LPL (2 μg/ml) at 37 °C for 5 h. In some experiments, cells were washed in DMEM three times and further incubated in serum-free DMEM for 3 h. Cells were then permeabilized and stained with an anti-Aβ antibody, 6E10, and an anti-LAMP2 antibody, whose staining signal is considered as a marker of late endosomes/lysosomes (21). We found that some portions of anti-Aβ antibody-positive signals were co-localized with staining signals reactive to the anti-LAMP2 antibody, showing that the internalized Aβ was trafficked into late endosomal/lysosomal compartments (Fig. 4B). To confirm the involvement of a lysosomal pathway in the degradation of LPL-mediated internalized Aβ, we determined the effect of chloroquine on the localization of Aβ internalized in an LPL-mediated manner. Chloroquine is a weak base and is taken up by cells, which results in the neutralization of acidic organelles such as lysosomes and impairment of their functions (22, 23). Chloroquine treatment at concentrations of 25 and 50 μg/ml prevented the degradation of internalized Aβ 12 h after washing out (Fig. 4C). We also tested inhibitors of neprilysin, an insulin-degrading enzyme, and cathepsin B, all of which are known to degrade Aβ. These inhibitors failed to suppress the degradation of internalized Aβ in astrocytes (data not shown). Thus, Aβ internalized in an LPL-mediated manner was degraded in a lysosomal pathway in astrocytes.
FIGURE 4.
Aβ is trafficked to late endosomal/lysosomal compartments and degraded after the LPL-mediated uptake. A, mouse primary astrocytes were incubated with LPL (2 μg/ml) and Aβ (500 nm) at 37 °C for 5 h. Cells were washed in DMEM three times and then incubated in DMEM at 37 °C for 0, 3, 5, 12, and 24 h. The amount of Aβ remaining in the cells was determined by Western blotting using the anti-Aβ antibody, 6E10, in a detergent extract of whole cells. B, astrocytes were plated on poly-l-lysine-coated coverglasses and incubated with LPL (2 μg/ml) and Aβ (250 nm) at 37 °C for 5 h. Then, cells were permeabilized and double stained with an anti-LAMP2 antibody and 2C8. Bound antibodies were visualized with Cy3-conjugated (red) and FITC-conjugated (green) secondary antibodies for the anti-LAMP2 antibody and 6E10, respectively. Astrocytes incubated without Aβ did not show any anti-Aβ antibody-positive signals (not shown). Scale bar, 10 μm. C, astrocytes were incubated with LPL (2 μg/ml) and Aβ (500 nm) at 37 °C for 5 h. Cells were then washed in DMEM and cultured with or without chloroquine in DMEM at 37 °C for an additional 12 h. The level of Aβ in the detergent extract of whole cells was determined by Western blotting with 6E10. These are representative data of at least three independent experiments.
LPL Promotes Cellular Uptake of Aβ in a Heparan Sulfate- and Chondroitin Sulfate-dependent Manner
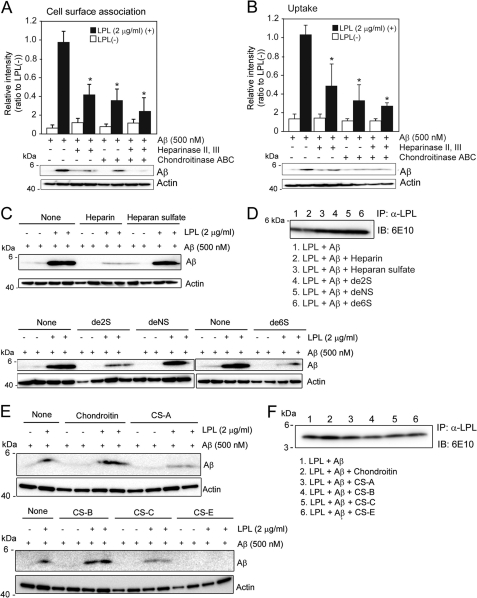
LPL has a high affinity with heparan sulfate (HS) and chondroitin sulfate (CS) (5, 24, 25). Therefore, we next investigated whether HS and CS are involved in the LPL-mediated cellular binding and cellular uptake of Aβ in astrocytes. Mouse primary astrocytes were pretreated with a mixture of heparinase II and heparinase III and/or chondroitinase ABC for 24 h at 37 °C, followed by incubation with Aβ42 and LPL at 4 °C for 3 h. There were no significant differences among the values in the absence of LPL (one-way ANOVA; p = 0.0929 for cell-surface-associated Aβ, p = 0.4350 for cellular Aβ). Pretreatment with heparinases or chondroitinase ABC partially decreased the level of LPL-mediated cellular binding of Aβ in astrocytes to 40 or 50% of that observed in the nontreated control, respectively (Fig. 5A). Interestingly, pretreatment with both heparinases and chondroitinase ABC decreased the level of LPL-mediated binding of Aβ to astrocytes to 20% of that observed in nontreated control (Fig. 5A). Next, we determined the effect of HS and/or CS on the LPL-mediated cellular uptake of Aβ. In conjunction with the effect of LPL on Aβ binding, heparinases and chondroitinase ABC decreased the level of LPL-mediated cellular uptake of Aβ in astrocytes to 30 and 50% of that observed in the nontreated control incubated with LPL, respectively (Fig. 5B). Pretreatment with both heparinases and chondroitinase ABC did not show an additive effect on the attenuation of LPL-promoted Aβ uptake (Fig. 5B). These findings indicate that HS and CS expressed in astrocytes are involved in the LPL-mediated association of Aβ with astrocytes and Aβ cellular uptake.
FIGURE 5.
LPL-mediated cellular binding and uptake of Aβ depends on heparan sulfate and chondroitin sulfate in astrocytes. A and B, astrocytes from wild-type mice were pretreated with a mixture of heparinase II (0.03 μg/ml) and heparinase III (0.03 μg/ml), and/or chondroitinase ABC (0.03 μg/ml) at 37 °C for 24 h. After washing in DMEM three times, cells were incubated with LPL (2 μg/ml) and Aβ (500 nm) at 4 °C for 3 h (for cell surface association) (A) or 37 °C for 3 h (for uptake) (B). The level of Aβ in the detergent extract of whole cells was determined by Western blotting using 6E10. The quantitative assessment of cell-surface-associated Aβ (A) and cellular Aβ (B) in the present (closed bars) or absence (open bars) of LPL are shown. The data presented are the means ± S.D. of three independent experiments. * p < 0.001 versus levels of LPL (-). (C) Mouse primary astrocytes were incubated with Aβ (500 nm) or LPL (2 μg/ml) and Aβ (500 nm) in the presence or absence of heparin or chemically modified heparins at a concentration of 3 μg/ml at 37 °C for 5 h. The level of Aβ in the detergent extract of whole cells was determined using 6E10. (D) LPL (2 μg/ml) and Aβ (500 nm) were incubated in DMEM at 37 °C for 3 h in the presence or absence of heparin, heparan sulfate, or chemically modified heparins at a concentration of 3 μg/ml. Protein complexes in DMEM were immunoprecipitated (IP) with an anti-LPL antibody (α-LPL) and the Aβ recovered in the immunoprecipitates was analyzed by Western blotting using 6E10. These data are representative of at least three independent experiments. de2S, 2-O-desulfated heparin; de6S, 6-O-desulfated heparin; deNS, N-desulfated heparin. E, astrocytes were incubated with LPL (2 μg/ml) and Aβ (500 nm) in the presence or absence of chondroitin sulfates (chondroitin, chondroitin 4-sulfate (CS-A), 2-O-, 6-O-disulfated chondroitin sulfate (CS-B), 6-O-sulfated chondroitin sulfate (CS-C), and chondroitin 4,6-disulfate (CS-E)) at a concentration of 3 μg/ml at 37 °C for 5 h. The level of Aβ in a detergent extract of whole cells was determined by Western blotting using 6E10. F, LPL (2 μg/ml) and Aβ (500 nm) were incubated in DMEM at 37 °C for 3 h in the presence or absence of chondroitin sulfates at a concentration of 3 μg/ml. Protein complexes were immunoprecipitated with the anti-LPL antibody (α-LPL), and the Aβ recovered in the immunoprecipitates was analyzed by Western blotting using 6E10. The data are representative of at least three independent experiments. IB, immunoblot.
To further confirm the involvement of HS and CS in LPL-mediated Aβ uptake, we incubated astrocytes with various glycosaminoglycans. Heparin, which is a structural analog of HS, substantially suppressed the effect of LPL on Aβ uptake at a concentration of 3 μg/ml (Fig. 5C). The suppressive effect of heparin on LPL-mediated Aβ uptake was also observed in the presence of de-N-sulfated heparin, whereas either de-2-O-sulfated heparin or de-6-O-sulfated heparin had no effect on LPL-mediated Aβ uptake (Fig. 5C). None of these heparins interfered with the interaction between LPL and Aβ (Fig. 5D). In addition, 4-O-, 6-O-disulfated chondroitin sulfate (3 μg/ml) completely suppressed the promotive effect of LPL on Aβ uptake (Fig. 5E). 4-O-Sulfated chondroitin sulfate and 6-O-sulfated chondroitin sulfate moderately attenuated the function of LPL, whereas chondroitin (a nonsulfated form of chondroitin sulfate) and 2-O-, 6-O-disulfated chondroitin sulfate (also known as dermatan sulfate) did not (Fig. 5E). None of these CS interfered with the interaction between LPL and Aβ in vitro (Fig. 5F).
ApoE Is Dispensable for LPL-mediated Cellular Uptake of Aβ in Astrocytes
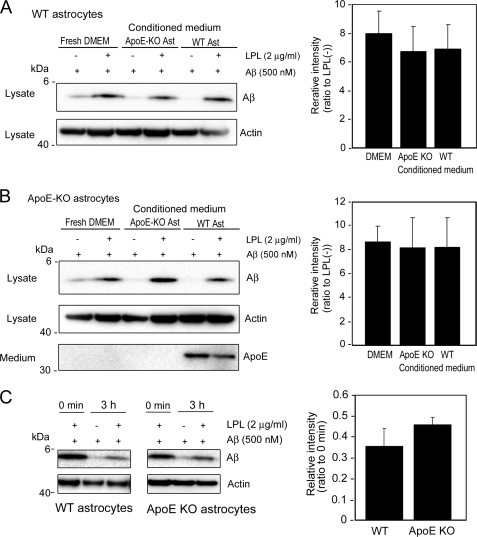
Because ApoE is reported to be involved in the metabolism of Aβ, including its aggregation and clearance (26), we analyzed the effects of ApoE on the LPL-mediated cellular uptake of Aβ in astrocytes. We collected culture media of primary astrocytes prepared from ApoE-KO mice and C57BL/6 (WT) mice. The astrocyte cultures prepared from wild-type mouse cortices were incubated in conditioned media in the presence of Aβ42 and LPL. As shown in Fig. 6A, Aβ uptake was promoted by LPL in astrocytes prepared from WT mice incubated in a fresh medium, the conditioned medium from ApoE-KO astrocytes, and the conditioned medium from WT astrocytes. There were no significant differences between these three groups (one-way ANOVA; p = 0.6419). This is also the case for ApoE-KO astrocytes (one-way ANOVA; p = 0.9467) (Fig. 6B). These findings indicate that ApoE is dispensable for the LPL-promoted cellular uptake of Aβ in astrocytes. We also examined the effects of ApoE on the degradation of internalized Aβ. Primary astrocytes from WT and ApoE-KO mice were incubated with soluble Aβ42 and LPL at 37 °C for 5 h, washed in DMEM three times, and further incubated at 37 °C for 3 h. Cells were then harvested, and the Aβ level in the cell lysate was analyzed by Western blotting. As shown in Fig. 6C, there were no significant differences between the levels of Aβ remaining in the lysate of WT astrocytes and ApoE-KO astrocytes (p = 0.1031).
FIGURE 6.
ApoE is dispensable for the LPL-mediated cellular uptake of Aβ in astrocytes. The astrocyte cultures prepared from WT or ApoE knock-out (KO) mice were incubated in fresh serum-free DMEM for 3 days at 37 °C. The conditioned media of these cultures were then collected. The astrocytes prepared from WT (A) or ApoE-KO (B) mouse brains were incubated in the conditioned medium of ApoE-KO astrocyte cultures or conditioned medium of WT astrocyte cultures, and LPL (2 μg/ml) and Aβ (500 nm) were added into each culture; the cultures were then maintained for another 5 h at 37 °C. After the incubation, the cultures were harvested, and the amount of cellular Aβ in a detergent extract of whole cells (lysate) was determined by Western blotting using 6E10. The amount of ApoE in the conditioned medium of cultured cells (medium) was determined by Western blotting using an anti-ApoE antibody, AB947. These data are representative of at least three independent experiments. The graphs show the cellular Aβ levels. The data are the means ± S.D. of three independent experiments. CM, conditioned medium; Ast, astrocytes. C, mouse primary astrocytes from WT and ApoE-KO mice were incubated with soluble Aβ42 in the presence or absence of LPL at 37 °C for 5 h, washed in DMEM three times, and further incubated at 37 °C for 3 h. Cells were then harvested, and the Aβ levels in the lysate was analyzed by Western blotting. The graph shows the cellular Aβ levels. The data are the means ± S.D. of three independent experiments.
DISCUSSION
Previous studies have shown that the mRNA expression of the LPL gene and the enzymatically active LPL are found in the brain in several mammalian species (6, 7, 27). However, considering that the main fraction of lipoproteins in the brain is HDL, which contains negligible or no triacylglycerols, and that the brain lacks an essential cofactor, apoCII, it is conceivable that LPL has a different function in the brain from that in the systemic circulation serving as an enzyme with the cofactor apoCII to catalyze the hydrolysis of triacylglycerols (28). In the present study, we found a novel function of LPL serving as an Aβ binding molecule; that is, exogenous LPL binds to Aβ and promotes cellular binding and uptake of Aβ in astrocytes. The internalized Aβ was degraded within 12 h, mainly in a lysosomal pathway. Furthermore, we have demonstrated that HS and CS glycosaminoglycans are involved in the promotion of the LPL-mediated cellular uptake of Aβ in astrocytes.
Astrocytes are a major glial cell type in the CNS and play a crucial role in neuronal development, maintenance of synapse functions, and CNS repair after injury. Additionally, astrocytes have phagocytic and proteolytic activities (29, 30) and ingest Aβ (15, 31, 32). Our results indicate that LPL strongly enhances cellular uptake of Aβ, leading to increased degradation of Aβ in astrocytes. Previous studies have shown that SNPs in the coding region of the LPL gene are associated with AD development (33) and the severity of AD pathophysiological features (12), with the molecular mechanisms underlying this association remaining unknown. It may be possible that altered function of LPL shown in this study would result in impaired Aβ clearance and subsequent accumulation of Aβ, accelerating AD development. Because the accumulation of Aβ in the extracellular space is considered to trigger Aβ aggregation and deposition, the function of LPL to enhance Aβ binding, uptake, and degradation in astrocytes may decrease Aβ levels in the brain. However, because LPL is known to regulate the uptake and transport of vitamin E to the brain, of which deficiency results in increased Aβ accumulation and presynaptic defects accompanied by impaired learning and memory function in vivo (34, 35), there may be other possibilities as well, that the altered LPL function regulating vitamin E transport may enhance Aβ accumulation and impair synaptic function.
It has been suggested that lysosomal dysfunction plays a major role in Aβ accumulation, thereby causing neuronal cell death (36, 37) and that chloroquine, which disrupts lysosomal pH balance, enhances Aβ accumulation in a microglial cell line (38). Our results show that almost all of the internalized Aβ was localized in lysosomes and degraded in a time-dependent manner, and this degradation was markedly inhibited by the treatment with chloroquine, suggesting that Aβ was degraded mainly in a lysosomal pathway. These findings suggest that lysosomal pathways play a critical role in the degradation of Aβ that is internalized via a novel pathway as LPL-Aβ complexes by astrocytes.
It has been shown that LPL associates with lipoproteins and the formed LPL-bound lipoprotein complexes bind to cell-surface HS proteoglycans and CS proteoglycans (1, 5, 39), promoting the cellular uptake of lipoproteins by acting as a bridging molecule (2, 40). HS proteoglycans and CS proteoglycans are present in astrocytes (41–43). We found that pretreatment of astrocytes with a mixture of heparinases or chondroitinase ABC partially attenuated the LPL-mediated Aβ uptake, and cotreatment with heparinases and chondroitinase ABC completely suppressed the LPL-mediated cellular uptake of Aβ (Fig. 4), indicating that the LPL-mediated cellular uptake of Aβ is mediated via HS proteoglycans and CS proteoglycans. Interestingly, heparin, a highly sulfated form of HS, and 4-O-, 6-O-disulfated chondroitin sulfate, a highly sulfated CS, selectively suppressed the promotion of Aβ uptake in astrocytes. These findings suggest that LPL could act as a bridging molecule between not only cell-surface GAGs and lipoproteins but also cell-surface GAGs and Aβ and facilitate the cellular uptake of Aβ in astrocytes and that certain domains modified by multiple sulfate groups are necessary for LPL to function in astrocytes.
ApoE is one of the major apolipoproteins in the brain and plays a key role in lipid transport in the brain. ApoE affects the aggregation of Aβ in vitro (26). PDAPP and Tg2576 transgenic mice exhibit extensive cerebral Aβ deposition. When these transgenic mice lack the murine apoE gene, a significant decrease in amyloid plaque formation was observed (44, 45). Furthermore, two in vitro studies have demonstrated that ApoE can facilitate the cellular degradation of Aβ (16, 31). These lines of evidence suggest that ApoE affects Aβ metabolism. Thus, we examined whether ApoE could be involved in the LPL-mediated cellular uptake of Aβ. LPL promoted the cellular uptake of Aβ in wild-type and ApoE-deficient astrocytes in culture. The presence or absence of ApoE in the conditioned medium of astrocytes did not alter the levels of Aβ internalized in an LPL-mediated manner. These results suggest that ApoE is not required for the LPL-mediated cellular uptake of Aβ in astrocytes.
In this study, we demonstrated a novel LPL function; that is, LPL binds to Aβ and enhances the cellular uptake of Aβ in a sulfated glycosaminoglycan-dependent manner, and the internalized Aβ is degraded in a lysosomal pathway. Although further studies will be needed to confirm the role of LPL in the clearance of Aβ in vivo, our findings provide a new insight into the molecular pathogenesis of AD and a potential strategy for AD therapy.
Supplementary Material
This work was supported by a grant-in-aid for scientific research on priority areas (Research on Pathomechanisms of Brain Disorders) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, a grant from the Program for Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation, a grant from the Ministry of Health, Labor, and Welfare of Japan (Research on Dementia, Health, and Labor Sciences Research Grant H20-007), and a grant from the Japan Health Sciences Foundation (Research on Publicly Essential Drugs and Medical Devices).

The on-line version of this article (available at http://www.jbc.org) contains supplemental “Methods” and Fig. 1.
- LPL
- lipoprotein lipase
- Aβ
- amyloid β
- ApoE
- apolipoprotein E
- CS
- chondroitin sulfate(s)
- HS
- heparan sulfate
- GAG
- glycosaminoglycan
- ANOVA
- one-way analysis of variance.
REFERENCES
- 1. Williams K. J., Fless G. M., Petrie K. A., Snyder M. L., Brocia R. W., Swenson T. L. (1992) J. Biol. Chem. 267, 13284–13292 [PubMed] [Google Scholar]
- 2. Mulder M., Lombardi P., Jansen H., van Berkel T. J., Frants R. R., Havekes L. M. (1993) J. Biol. Chem. 268, 9369–9375 [PubMed] [Google Scholar]
- 3. Kreuger J., Spillmann D., Li J. P., Lindahl U. (2006) J. Cell Biol. 174, 323–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Edwards I. J., Goldberg I. J., Parks J. S., Xu H., Wagner W. D. (1993) J. Lipid Res. 34, 1155–1163 [PubMed] [Google Scholar]
- 5. Edwards I. J., Xu H., Obunike J. C., Goldberg I. J., Wagner W. D. (1995) Arterioscler. Thromb. Vasc. Biol. 15, 400–409 [DOI] [PubMed] [Google Scholar]
- 6. Goldberg I. J., Soprano D. R., Wyatt M. L., Vanni T. M., Kirchgessner T. G., Schotz M. C. (1989) J. Lipid Res. 30, 1569–1577 [PubMed] [Google Scholar]
- 7. Yacoub L. K., Vanni T. M., Goldberg I. J. (1990) J. Lipid Res. 31, 1845–1852 [PubMed] [Google Scholar]
- 8. Eckel R. H., Robbins R. J. (1984) Proc. Natl. Acad. Sci. U.S.A. 81, 7604–7607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Havel R. J., Fielding C. J., Olivecrona T., Shore V. G., Fielding P. E., Egelrud T. (1973) Biochemistry 12, 1828–1833 [DOI] [PubMed] [Google Scholar]
- 10. Zannis V. I., Cole F. S., Jackson C. L., Kurnit D. M., Karathanasis S. K. (1985) Biochemistry 24, 4450–4455 [DOI] [PubMed] [Google Scholar]
- 11. Rebeck G. W., Harr S. D., Strickland D. K., Hyman B. T. (1995) Ann. Neurol. 37, 211–217 [DOI] [PubMed] [Google Scholar]
- 12. Blain J. F., Aumont N., Théroux L., Dea D., Poirier J. (2006) Eur. J. Neurosci. 24, 1245–1251 [DOI] [PubMed] [Google Scholar]
- 13. Iwatsubo T., Odaka A., Suzuki N., Mizusawa H., Nukina N., Ihara Y. (1994) Neuron 13, 45–53 [DOI] [PubMed] [Google Scholar]
- 14. Tanzi R. E., Moir R. D., Wagner S. L. (2004) Neuron 43, 605–608 [DOI] [PubMed] [Google Scholar]
- 15. Wyss-Coray T., Loike J. D., Brionne T. C., Lu E., Anankov R., Yan F., Silverstein S. C., Husemann J. (2003) Nat. Med. 9, 453–457 [DOI] [PubMed] [Google Scholar]
- 16. Jiang Q., Lee C. Y., Mandrekar S., Wilkinson B., Cramer P., Zelcer N., Mann K., Lamb B., Willson T. M., Collins J. L., Richardson J. C., Smith J. D., Comery T. A., Riddell D., Holtzman D. M., Tontonoz P., Landreth G. E. (2008) Neuron 58, 681–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Majumdar A., Cruz D., Asamoah N., Buxbaum A., Sohar I., Lobel P., Maxfield F. R. (2007) Mol. Biol. Cell 18, 1490–1496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mandrekar S., Jiang Q., Lee C. Y., Koenigsknecht-Talboo J., Holtzman D. M., Landreth G. E. (2009) J. Neurosci. 29, 4252–4262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Michikawa M., Gong J. S., Fan Q. W., Sawamura N., Yanagisawa K. (2001) J Neurosci. 21, 7226–7235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fernández-Borja M., Bellido D., Vilella E., Olivecrona G., Vilaró S. (1996) J. Lipid Res. 37, 464–481 [PubMed] [Google Scholar]
- 21. Fukuda M. (1991) J. Biol. Chem. 266, 21327–21330 [PubMed] [Google Scholar]
- 22. de Duve C., de Barsy T., Poole B., Trouet A., Tulkens P., Van Hoof F. (1974) Biochem. Pharmacol. 23, 2495–2531 [DOI] [PubMed] [Google Scholar]
- 23. Poole B., Ohkuma S. (1981) J. Cell Biol. 90, 665–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bengtsson G., Olivecrona T., Höök M., Riesenfeld J., Lindahl U. (1980) Biochem. J. 189, 625–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pillarisetti S., Paka L., Sasaki A., Vanni-Reyes T., Yin B., Parthasarathy N., Wagner W. D., Goldberg I. J. (1997) J. Biol. Chem. 272, 15753–15759 [DOI] [PubMed] [Google Scholar]
- 26. Kim J., Basak J. M., Holtzman D. M. (2009) Neuron 63, 287–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Brecher P., Kuan H. T. (1979) J. Lipid Res. 20, 464–471 [PubMed] [Google Scholar]
- 28. Koch S., Donarski N., Goetze K., Kreckel M., Stuerenburg H. J., Buhmann C., Beisiegel U. (2001) J. Lipid Res. 42, 1143–1151 [PubMed] [Google Scholar]
- 29. al-Ali S. Y., al-Hussain S. M. (1996) J. Anat. 188, 257–262 [PMC free article] [PubMed] [Google Scholar]
- 30. Hatten M. E., Liem R. K., Shelanski M. L., Mason C. A. (1991) Glia 4, 233–243 [DOI] [PubMed] [Google Scholar]
- 31. Koistinaho M., Lin S., Wu X., Esterman M., Koger D., Hanson J., Higgs R., Liu F., Malkani S., Bales K. R., Paul S. M. (2004) Nat. Med. 10, 719–726 [DOI] [PubMed] [Google Scholar]
- 32. Matsunaga W., Shirokawa T., Isobe K. (2003) Neurosci. Lett. 342, 129–131 [DOI] [PubMed] [Google Scholar]
- 33. Baum L., Chen L., Masliah E., Chan Y. S., Ng H. K., Pang C. P. (1999) Am. J. Med. Genet. 88, 136–139 [DOI] [PubMed] [Google Scholar]
- 34. Xian X., Liu T., Yu J., Wang Y., Miao Y., Zhang J., Yu Y., Ross C., Karasinska J. M., Hayden M. R., Liu G., Chui D. (2009) J. Neurosci. 29, 4681–4685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nishida Y., Ito S., Ohtsuki S., Yamamoto N., Takahashi T., Iwata N., Jishage K., Yamada H., Sasaguri H., Yokota S., Piao W., Tomimitsu H., Saido T. C., Yanagisawa K., Terasaki T., Mizusawa H., Yokota T. (2009) J. Biol. Chem. 284, 33400–33408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bahr B. A., Bendiske J. (2002) J Neurochem. 83, 481–489 [DOI] [PubMed] [Google Scholar]
- 37. Nixon R. A., Cataldo A. M., Mathews P. M. (2000) Neurochem. Res. 25, 1161–1172 [DOI] [PubMed] [Google Scholar]
- 38. Chu T., Tran T., Yang F., Beech W., Cole G. M., Frautschy S. A. (1998) FEBS Lett. 436, 439–444 [DOI] [PubMed] [Google Scholar]
- 39. Eisenberg S., Sehayek E., Olivecrona T., Vlodavsky I. (1992) J. Clin. Invest. 90, 2013–2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Auerbach B. J., Bisgaier C. L., Wölle J., Saxena U. (1996) J. Biol. Chem. 271, 1329–1335 [DOI] [PubMed] [Google Scholar]
- 41. Hsueh Y. P., Sheng M. (1999) J. Neurosci. 19, 7415–7425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Laabs T. L., Wang H., Katagiri Y., McCann T., Fawcett J. W., Geller H. M. (2007) J. Neurosci. 27, 14494–14501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tsuchida K., Shioi J., Yamada S., Boghosian G., Wu A., Cai H., Sugahara K., Robakis N. K. (2001) J. Biol. Chem. 276, 37155–37160 [DOI] [PubMed] [Google Scholar]
- 44. Bales K. R., Verina T., Dodel R. C., Du Y., Altstiel L., Bender M., Hyslop P., Johnstone E. M., Little S. P., Cummins D. J., Piccardo P., Ghetti B., Paul S. M. (1997) Nat. Genet. 17, 263–264 [DOI] [PubMed] [Google Scholar]
- 45. Holtzman D. M., Bales K. R., Wu S., Bhat P., Parsadanian M., Fagan A. M., Chang L. K., Sun Y., Paul S. M. (1999) J. Clin. Invest. 103, R15–R21 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.