Abstract
Thyroid-stimulating hormone (TSH)-induced reduction in ligand binding affinity (negative cooperativity) requires TSH receptor (TSHR) homodimerization, the latter involving primarily the transmembrane domain (TMD) but with the extracellular domain (ECD) also contributing to this association. To test the role of the TMD in negative cooperativity, we studied the TSHR ECD tethered to the cell surface by a glycosylphosphatidylinositol (GPI) anchor that multimerizes despite the absence of the TMD. Using the infinite ligand dilution approach, we confirmed that TSH increased the rate of dissociation (koff) of prebound 125I-TSH from CHO cells expressing the TSH holoreceptor. Such negative cooperativity did not occur with TSHR ECD-GPI-expressing cells. However, even in the absence of added TSH, 125I-TSH dissociated much more rapidly from the TSHR ECD-GPI than from the TSH holoreceptor. This phenomenon, suggesting a lower TSH affinity for the former, was surprising because both the TSHR ECD and TSH holoreceptor contain the entire TSH-binding site, and the TSH binding affinities for both receptor forms should, theoretically, be identical. In ligand competition studies, we observed that the TSH binding affinity for the TSHR ECD-GPI was significantly lower than that for the TSH holoreceptor. Further evidence for a difference in ligand binding kinetics for the TSH holoreceptor and TSHR ECD-GPI was obtained upon comparison of the TSH Kd values for these two receptor forms at 4 °C versus room temperature. Our data provide the first evidence that the wild-type TSHR TMD influences ligand binding affinity for the ECD, possibly by altering the conformation of the closely associated hinge region that contributes to the TSH-binding site.
Keywords: G Protein-coupled Receptors (GPCRs), Glycoprotein Hormones, Receptor Structure-Function, Receptors, Thyroid, Glycoprotein Hormone Receptor, TSH Receptor
Introduction
The term “negative cooperativity” was coined in 1973 by de Meyts et al. (1) to interpret curvilinear Scatchard plots (2) observed with insulin and other receptors, i.e. occupancy of a receptor by a ligand at one site leads to an allosteric effect at another binding site, causing a reduction in binding affinity. Early studies prior to the molecular cloning of the thyroid-stimulating hormone (TSH)2 receptor (TSHR) cDNA suggested that ligand-induced negative cooperativity did not occur with this receptor (3, 4). Nearly 2 decades later, it was observed that expressing increasing numbers of recombinant TSHR on the surface of transfected cells was associated with a reduction in TSH binding affinity (5). This phenomenon suggested that enhanced interactions at higher receptor densities led to a ligand-independent form of negative cooperativity. In recent years, TSHRs were found to form homodimers or multimers on the cell surface (6, 7), like other members of the glycoprotein hormone receptor family (reviewed in Refs. 8 and 9). Using chimeric receptor molecules combined with the classical infinite ligand dilution approach of de Meyts et al., Urizar et al. (7) provided convincing evidence for ligand-induced negative cooperativity in which TSH binding to one protomer increased the rate of dissociation (koff) of prebound TSH from a second protomer.
Although glycoprotein hormone receptor dimerization is clearly established and is of functional importance in vivo (10), the contact points between protomers are unclear. Both the extracellular (ECD) and transmembrane (TMD) domains are involved in receptor dimerization. Turning first to the ECD, the leucine-rich repeat domain (LRD) of the FSH receptor ECD crystallizes as a dimer, with Tyr-110 being an important contributor to LRD-LRD interaction (11). Furthermore, the entire ECD (the LRD plus the hinge region) of the TSHR (12) and FSH receptor (9) expressed on the cell surface with a glycosylphosphatidyl-inositol (GPI) anchor multimerize even though they lack the TMD. On the other hand, there is strong evidence that the TSHR TMD plays an important role in TSH holoreceptor multimerization (7, 13). Indeed, although both the ECD and TMD are involved in dimerization, the present concept is that TMD interactions contribute to a greater extent (7).
Given that TSHR homodimerization is associated with reduced TSH binding affinity (negative cooperativity) and given the relative importance of the TMD in TSHR homodimerization, in this study, we hypothesized that the TSHR TMD is required for TSH-induced negative cooperativity. To test this hypothesis, we examined whether TSH-induced negative cooperativity occurs with TSHR ectodomains tethered to the plasma membrane by a GPI anchor that, as mentioned above, also multimerizes despite the absence of the TMD (12).
EXPERIMENTAL PROCEDURES
TSHR-expressing Cell Lines
We used a CHO cell line stably expressing the wild-type TSHR generated with the expression vector pcDNA5/FRT and the Flp-In system (Invitrogen). This system, involving selection with hygromycin, introduces only a single transgene copy per cell. Construction of this plasmid was described previously (14). We also used a CHO cell line stably expressing the cDNA for the TSHR ECD with a GPI anchor. The GPI anchor in the TSHR ECD plasmid (kindly provided by Dr. Alan Johnstone) (15) was amplified by PCR using oligonucleotide primers introducing 5′-SpeI and 3′-XbaI restriction sites. This fragment was then inserted into the same sites in pSV2neo-ECE-TSHR (16) with an SpeI site introduced in the TSHR cDNA following the codon for residue 418 (17), with the 5′- and 3′-untranslated ends deleted, and with the His-601 polymorphism corrected to Tyr-601. The cDNA for the TSHR ECD-GPI amplified by PCR introducing an XhoI site at the 3′-end was blunted, restricted with XhoI, and ligated into the AflII (blunted)-XhoI sites in pcDNA5/FRT. A stably transfected CHO cell line was obtained by selection with hygromycin. Both TSH holoreceptor and TSHR ECD-GPI cell lines were cultured in Ham's F-12 medium supplemented with 10% fetal calf serum, 100 units/ml penicillin, 50 μg/ml gentamycin, and 2.5 μg/ml Fungizone.
Flow Cytometry
Cells in 6-well plates were resuspended using 1 mm EDTA and 1 mm EGTA in PBS. After two washes with PBS containing 10 mm HEPES (pH 7.4), 2% fetal bovine serum, and 0.05% NaN3, the cells were incubated for 30 min at room temperature in 100 μl of the same buffer containing 1 μg of murine mAb CS-17 (18). The cells were then rinsed and incubated for 45 min with 100 μl of fluorescein isothiocyanate-conjugated goat anti-mouse IgG (1:100; Caltag, Burlingame, CA), washed, and analyzed using a FACScan flow cytofluorometer (BD Biosciences). Cells stained with propidium iodide (1 μg/ml final concentration) were excluded from analysis.
TSH Binding
CHO cells expressing the TSHR were cultured in 24-well plates. After aspiration of the culture medium, the cells were rinsed once with 0.5 ml of binding buffer (Hanks' buffer with 250 mm sucrose substituting for NaCl to maintain isotonicity and 0.25% bovine serum albumin). Cells were then incubated for 4 h (room temperature) or 5–8 h (4 °C) in 0.25 ml of binding buffer supplemented with 125I-TSH (B·R·A·H·M·S GmbH, Hennigsdorf, Germany), typically ∼30,000 cpm, and the indicated concentrations of unlabeled bovine TSH (Sigma). After three rapid rinses with ice-cold binding buffer, the cells were solubilized with 0.2 ml of 1 n NaOH, and radioactivity was measured in a γ-counter. Nonspecific binding was determined using untransfected cells. TSH binding affinities (Kd) were calculated by Scatchard analysis using GraphPad Prism (GraphPad Software, La Jolla, CA). (Nonlinear regression analysis provided clearly inaccurate information.)
In experiments to determine the dissociation time course of bound 125I-TSH, binding to cells in 24-well plates was performed as described above for 4 h at room temperature. Following buffer aspiration and three rinses with ice-cold binding buffer, fresh binding buffer with or without unlabeled bovine TSH (100 milliunits/ml) was added, and incubations were continued for the indicated times (0–125 min) at either room temperature or 4 °C. The medium was then aspirated, and residual radioactivity was counted on solubilized cells as described above.
RESULTS
Dissociation of Prebound TSH from the Isolated TSHR Ectodomain Versus the Holoreceptor
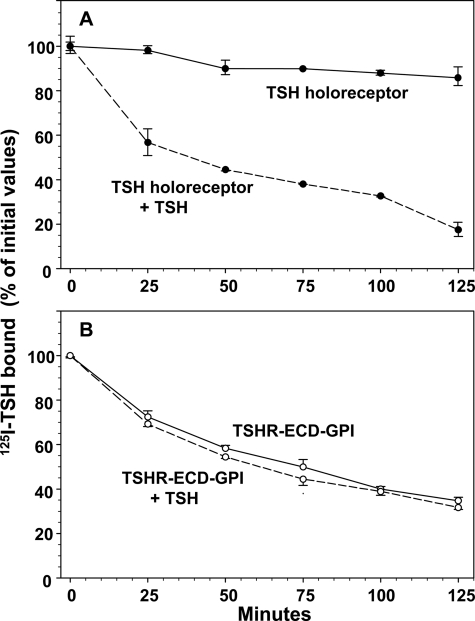
After allowing 125I-TSH to bind to monolayers of CHO cells expressing the wild-type TSHR, followed by rinsing to remove free ligand, dissociation of bound 125I-TSH from the cells was very slow, only ∼15% over 2 h (Fig. 1A). The addition of a high concentration of unlabeled TSH (100 milliunits/ml) greatly accelerated the release of 125I-TSH, 80% during the same time period (Fig. 1A). These data confirm the phenomenon of ligand-induced negative cooperativity as reported by Urizar et al. (7), i.e. TSH binding to one TSHR protomer reduces ligand binding affinity (increased koff rate) at an orthosteric site on a second protomer in the dimerized receptor.
FIGURE 1.
Dissociation of prebound 125I-TSH from cells stably expressing the TSH holoreceptor (A) and TSHR ECD-GPI (B). Confluent holoreceptor- and TSHR ECD-GPI-expressing cells in 24-well plates were preincubated for 4 h at room temperature in binding buffer containing 125I-TSH. After rinsing to remove unbound 125I-TSH, the cells were incubated at room temperature for the indicated times in binding buffer lacking 125I-TSH (see “Experimental Procedures”). Where indicated (+ TSH), the buffer was supplemented with 100 milliunits/ml bovine TSH. After buffer removal, the cells were solubilized, and residual radioactivity was measured. Each point represents the mean ± range of values from duplicate wells of cells. Similar data were obtained in replicate experiments with the TSH holoreceptor (n = 7) and TSHR ECD-GPI (n = 2).
The isolated TSHR ectodomain tethered to the cell surface by a GPI anchor (TSHR ECD-GPI) contains the entire TSH-binding site and is reported to bind TSH with an affinity comparable with the TSH holoreceptor (15, 19). For this reason, on examining the rate of dissociation of prebound 125I-TSH from cells expressing the TSHR ECD-GPI on their surface, we were surprised to observe that this rate was clearly more rapid than with the TSH holoreceptor (∼60% versus 15% after 2 h) (Fig. 1, B and A, respectively). Moreover, unlike the holoreceptor, the addition of unlabeled TSH had no effect on the rate of 125I-TSH dissociation from the TSHR ECD-GPI (Fig. 1B), i.e. despite the fact that the TSHR ECD-GPI dimerizes (12), this receptor, which lacks the membrane-spanning domain, did not exhibit TSH-induced negative cooperativity.
TSH Binding Affinity for the TSHR ECD-GPI
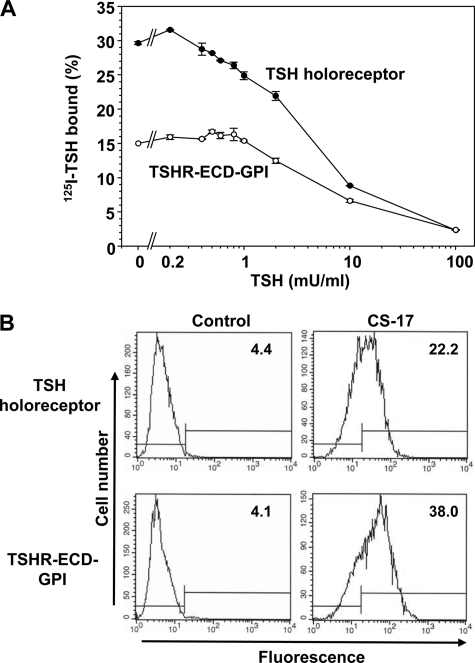
Because of the faster koff rate for the TSHR ECD-GPI versus the TSH holoreceptor, we re-examined their relative TSH binding properties. Maximum 125I-TSH binding to CHO cells stably expressing the TSHR ECD-GPI was lower than to cells expressing the wild-type TSHR (representative experiment shown in Fig. 2A), yet as determined by flow cytometry using mAb CS-17 (immunogen TSHR residues 22–289) (18), the level of the TSHR ECD-GPI expressed on the cell surface was nearly double that of the wild-type holoreceptor, with geometric means of 38.0 versus 22.2 fluorescence units, respectively (Fig. 2B). This finding was confirmed with two additional mAbs with epitopes in downstream regions of the ectodomain (data not shown).
FIGURE 2.
A, competition by unlabeled TSH for 125I-TSH binding to TSH holoreceptor- and TSHR ECD-GPI-expressing cells. Confluent cells in 24-well plates were incubated in binding buffer containing 125I-TSH and the indicated concentrations of unlabeled bovine TSH (see “Experimental Procedures”). Each point represents the mean ± range of values from duplicate wells of cells. The data shown are representative of four experiments with the TSH holoreceptor and TSHR ECD-GPI studied in parallel. Scatchard analysis revealed Kd values for the TSHR ECD-GPI and TSH holoreceptor of 2.6 ± 0.29 and 1.4 ± 0.14 milliunits/ml TSH, respectively (p = 0.02, t test). B, flow cytometric quantitation of cell-surface expression of the TSH holoreceptor and TSHR ECD-GPI. Geometric mean fluorescence values for normal mouse IgG (control) and mAb CS-17 are shown in each panel. Relatively greater TSHR expression in TSHR ECD-GPI- than in TSH holoreceptor-expressing cells was observed with two additional mAbs with epitopes in downstream regions of the ectodomain (data not shown).
Lower maximum 125I-TSH binding to the TSHR ECD-GPI than to the TSH holoreceptor despite a higher level of cell-surface expression for the former suggested that TSH binds with lower affinity to the TSHR ECD-GPI. Indeed, in all four experiments with both receptor types studied in parallel (representative experiment shown in Fig. 2A), competition by unlabeled TSH for 125I-TSH binding revealed higher Kd values (i.e. lower affinity) for the TSHR ECD-GPI than for the TSH holoreceptor (2.6 ± 0.29 versus 1.4 ± 0.14 milliunits/ml TSH; p = 0.02, t test). A nearly 2-fold reduction in TSH binding affinity for the TSHR ECD-GPI could account, at least in part, for the faster rate of dissociation (koff) of prebound 125I-TSH from the TSHR ECD-GPI versus the wild-type TSHR.
Effect of Temperature on TSH Binding Kinetics for the TSH Holoreceptor and TSHR ECD-GPI
The TSH binding affinity for the TSH holoreceptor decreases with a reduction in temperature (20). Furthermore, the TSH koff rate slows markedly at 4 °C (4). The Kd represents the ratio of the koff/kon rates. A slower koff rate would therefore increase TSH binding affinity unless the kon rate (which cannot be measured directly) also decreases. Because of the high rate of 125I-TSH dissociation from the TSHR ECD-GPI compared with the TSH holoreceptor, we examined the relative time courses of 125I-TSH binding to these two receptor types at room temperature and at 4 °C.
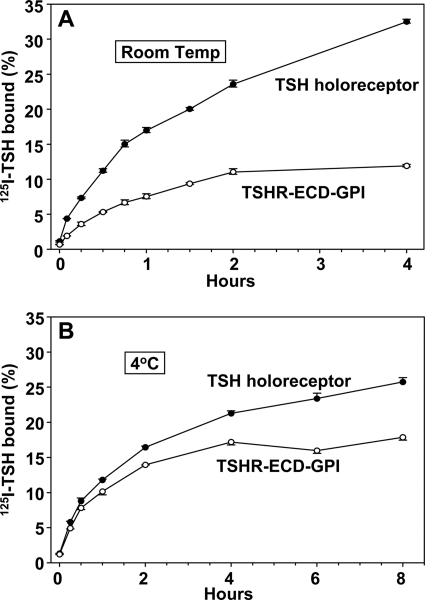
Over 4 h at room temperature, maximum 125I-TSH binding to TSHR ECD-GPI-expressing cells was lower than to cells expressing the TSH holoreceptor (Fig. 3A). These data are consistent with the foregoing TSH binding data (Fig. 2A), indicating a lower TSH binding affinity (higher Kd) for the TSHR ECD-GPI relative to the TSH holoreceptor. Over 8 h at 4 °C, the differential between 125I-TSH binding to the two receptor types was markedly narrowed, with maximum binding being reduced for the TSH holoreceptor and increased for the TSHR ECD-GPI in all three experiments performed (representative experiment shown in Fig. 3B).
FIGURE 3.
Time course of 125I-TSH binding to TSH holoreceptor- and TSHR ECD-GPI-expressing cells at room temperature (21 °C) (A) and at 4 °C (B). Confluent cells in 24-well plates were incubated for the indicated times in binding buffer containing 125I-TSH (see “Experimental Procedures”). At each time point, the buffer was removed, the cells were rinsed rapidly in ice-cold binding buffer and then solubilized, and radioactivity was measured. Each point represents the mean ± range of values from duplicate wells of cells. Similar data were obtained in three experiments.
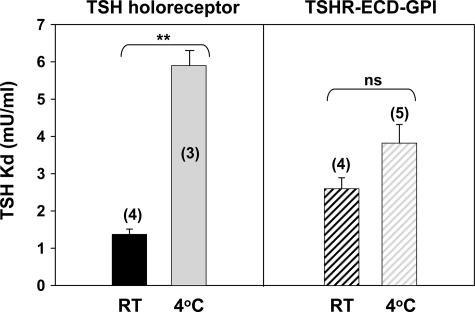
The narrowing in the maximum TSH binding attained at 4 °C could be explained by a reduction in TSH binding affinity for the TSH holoreceptor, an increase in TSH binding affinity for the TSHR ECD-GPI, or a combination of both phenomena. We therefore assessed the TSH binding affinity for these two receptor types at 4 °C (Fig. 4). In confirmation of the data of Saltiel et al. (20), the Kd for TSH binding to the TSH holoreceptor was significantly higher at 4 °C than at room temperature: 5.9 ± 0.4 (n = 3) versus 1.4 ± 0.14 (n = 4) milliunits/ml TSH (p = 0.00007, t test) (Fig. 4). In contrast, with the TSHR ECD-GPI, the Kd was not increased significantly at 4 °C compared with room temperature: 3.8 ± 0.50 (n = 5) versus 2.6 ± 0.29 (n = 4) milliunits/ml.
FIGURE 4.
Decreased TSH binding affinity at 4 °C for the TSH holoreceptor but not for the TSHR ECD-GPI. The bars represent the mean ± S.E. of Kd values (milliunits/ml) for the TSH holoreceptor and TSHR ECD-GPI at room temperature (RT) and 4 °C. The number of experiments is indicated in parentheses. ns, not significant. **, p = 0.00007 (Student's t test).
DISCUSSION
Our study was based on two phenomena reported for the glycoprotein hormone receptors. First, classical receptor negative cooperativity for the TSHR involves receptor dimerization or multimerization in which ligand binding to one protomer reduces the ligand binding affinity for a second protomer (7). Second, although the ECD contributes to or influences receptor dimerization (7, 9, 11, 12), the TMD is the major component involved in this process (7). Therefore, we hypothesized that the TSHR ECD lacking the TMD and tethered to the cell surface with a GPI anchor would not display negative cooperativity even though this truncated receptor multimerizes (12), as does the similar FSH receptor ECD-GPI construct (9). Indeed, the addition of excess unlabeled TSH did not accelerate dissociation of 125I-TSH from the TSHR ECD-GPI. However, the basis for the phenomenon was totally unexpected. Even in the absence of added unlabeled TSH, the rate of 125I-TSH dissociation from cells expressing the TSHR ECD-GPI was higher than that from the TSH holoreceptor and comparable with that from the holoreceptor in the presence of added TSH.
A faster intrinsic rate of TSH dissociation from the TSHR ECD-GPI than from the TSH holoreceptor suggested that TSH bound with lower affinity to this construct than to the latter. This finding was surprising for two reasons. First, it is intuitive and expected that the TSH binding affinities for the TSHR ECD and TSH holoreceptor should be identical because both receptor forms contain the same ECD, which contains the entire ligand-binding domain (Fig. 5) (17, 21–23). Second, previous studies of the TSH binding affinity (Kd) for the TSHR ECD-GPI and TSH holoreceptor concluded that these values were comparable (15, 19). Despite this conclusion (perhaps based on expectation), Costagliola and co-workers in the latter study reported an affinity for the TSHR ECD-GPI nearly 2-fold lower than for the holoreceptor. (No statistics were applied, and there is no indication as to whether the experiment was repeated.) Our study confirmed such a difference and determined that it is of statistical significance.
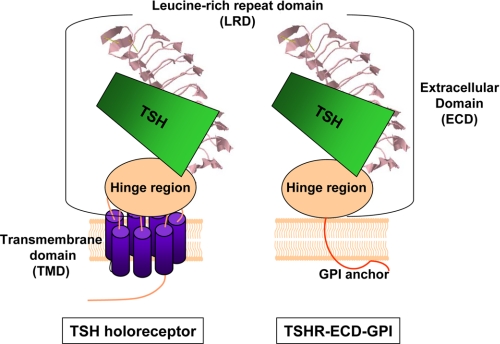
FIGURE 5.
Schematic representation of the TSH holoreceptor and TSHR ECD tethered to the cell surface by a GPI anchor. The TSHR ECD comprises two domains, the LRD and hinge region. The structure of the TSHR LRD (amino acid residues 22–260; Protein Data Bank code 3G04) (43) is known, whereas the hinge region structure has not been solved. The TSH-binding site components are entirely within the ECD, with contact points primarily within the LRD but also in the hinge region. The LRD is a rigid structure. Therefore, the difference in the TSH binding kinetics for the two receptors is likely to be a conformational change in the hinge component of the binding site. The hinge region is adjacent to the TMD, the latter being absent in the TSHR ECD-GPI.
The faster TSH dissociation rate (koff) and lower affinity (higher Kd) for the TSHR ECD-GPI compared with the TSH holoreceptor led us to explore the effect of a reduction in temperature on TSH binding kinetics for these two TSHR forms. The time course of 125I-TSH binding at room temperature to the holoreceptor and TSHR ECD-GPI confirmed the lower maximum binding attained with the latter despite its higher level of cell-surface expression as determined by flow cytometry. At 4 °C, the differential between the maximum TSH binding attained over time was narrowed (Fig. 3, A versus B). This reduced differential at 4 °C could be explained by a decrease in the TSH binding affinity for the TSH holoreceptor, an increase in the TSH binding affinity for the TSHR ECD-GPI, or a combination of both parameters. Analysis of TSH binding to the TSH holoreceptor revealed a decrease in affinity (increased Kd) at 4 °C versus room temperature, consistent with a previous report (20). In contrast, with the TSHR ECD-GPI, we observed no statistically significant difference between TSH Kd values determined at room temperature and 4 °C. These findings explain, at least in part, the differential effect of temperature on TSH binding to the two forms of the TSHR. The ratio of the dissociation and association rates (koff/kon) that determines the Kd is altered to a lesser degree with the TSHR ECD-GPI than with the TSH holoreceptor.
Our findings raise the issue of the relationship between the G protein-coupled receptor (GPCR) ECD and TMD on ligand binding affinity. The largest GPCR family (∼700 class A members) is rhodopsin-like; the great majority of the members have very small ECD components, and small ligands make direct contact with the TMD α-helices. The glycoprotein hormone receptor subfamily of the class A GPCR family (of which the TSHR is a member) is atypical in having very large ECDs (∼350–400 amino acid residues). Glycoprotein hormones do not directly interact with the TMD. Rather, the present concept is that ligand-binding sites are entirely within the ECD (11, 22), although there are reports that a component of the ligand-binding site exists on the TMD of the rat luteinizing hormone receptor (24, 25). In the case of the TSHR, the receptor ECD is converted from an inverse agonist to an agonist (44). Most glycoprotein hormone contact residues in the receptor ECD are in the LRD (reviewed in Refs. 23 and 26). However, at least in the case of the TSHR, components in the hinge region (amino acid residues 261–412) situated between the LRD and TMD contribute significantly to the TSH-binding site (depicted schematically in Fig. 5) (for example, see Refs. 14, 17, and 27–31). Although the structure of the TSHR hinge region is unsolved, functional evidence indicates that it mediates signal transduction by interacting closely with the TMD extracellular loops (reviewed in Ref. 23). The small (15-member) class B GPCR family also has relatively large ECDs (∼100–160 amino acid residues). However, the mechanism by which ligands bind and activate these receptors is quite different from the glycoprotein hormone receptor subgroup of the class A GPCR family. Ligands binding to class B GPCR family members undergo conversion to α-helices, and the ligand N-terminal cap directly engages the TMD, leading to receptor activation (32–34).
Our observation with the TSHR (class A GPCR family) indicates that even though TSH does not directly bind to the TMD, the presence of the TMD in the holoreceptor alters the TSH binding kinetics for the ECD. Such a process must involve a TMD-induced conformational change in the TSHR ECD. Because the hinge component of the ECD lies between the TMD and LRD (Fig. 5) and because the LRD is a rigid structure whose conformation does not change upon ligand binding (11), it is likely that the TMD alters the conformation of the ECD hinge region. The alteration could occur during intracellular folding of the TSHR, or the receptor TMD could induce an allosteric change in the hinge region on the cell surface. Mutations in the glycoprotein hormone receptor TMDs have been reported to alter ligand binding affinities for the ECD. For example, the rat luteinizing hormone receptor substitution D383N reduces ligand binding affinity by 280-fold (35) or ∼2-fold (36). However, the findings in this study are novel in that they relate to the wild-type TSHR, not to a mutant receptor. In addition, the marked effect of the wild-type TSHR TMD on the ligand dissociation rate has not, to our knowledge, been reported for a glycoprotein hormone receptor.
Other possible explanations are less likely in our view. First, unlike the holoreceptor, the TSHR ECD-GPI may not dimerize. However, both forms of receptor are reported to multimerize (6, 7). Second, the TSH holoreceptor and TSHR ECD-GPI could segregate in different domains in the plasma membrane and be influenced by other receptor-associated proteins. Against this possibility is the evidence that GPI-anchored proteins in general (reviewed in Ref. 37) and multimeric forms of the TSHR in particular (38, 39) both segregate in lipid rafts in the plasma membrane. Third, the type of structure anchoring the ECD to the plasma membrane may influence ligand binding affinity. For example, an intervening 31-amino acid residue component of the thrombin receptor containing the thrombin cleavage site linking the follicle-stimulating hormone and luteinizing hormone receptor ECDs to the plasma membrane increases the affinity of the former by 3-fold and reduces the affinity of the latter by 2-fold (only the former was considered to be significant) (40). In the case of the TSHR ECD-GPI, there is no intervening polypeptide sequence, and as mentioned above, previous reports for this construct have not described significant alterations in ligand binding affinity compared with the wild-type TSHR (15, 19). Finally, intramolecular cleavage of the ECD at the cell surface into disulfide-linked A- and B-subunits could vary quantitatively between the TSH holoreceptor and TSHR ECD-GPI (41). However, TSHR intramolecular cleavage into subunits has not been found to influence TSH binding affinity or function (42).
In summary, the TSHR ECD tethered to the plasma membrane by a GPI anchor does not display ligand-induced negative cooperativity despite its ability to multimerize. Moreover, TSH binds with lower affinity to the TSHR ECD-GPI than to the TSH holoreceptor. These data provide the first evidence that the TSHR TMD influences ligand binding affinity for the ECD, possibly by altering the conformation of the closely associated hinge region that contributes to the TSH-binding site.
Acknowledgments
We thank B·R·A·H·M·S GmbH for generously providing radiolabeled TSH. We are also grateful for contributions by Dr. Boris Catz.
This work was supported, in whole or in part, by National Institutes of Health Grant DK19289 (to B. R.).
- TSH
- thyroid-stimulating hormone
- TSHR
- TSH receptor
- ECD
- extracellular domain
- TMD
- transmembrane domain
- LRD
- leucine-rich repeat domain
- GPI
- glycosylphosphatidylinositol
- GPCR
- G protein-coupled receptor.
REFERENCES
- 1. de Meyts P., Roth J., Neville D. M., Jr., Gavin J. R., 3rd, Lesniak M. A. (1973) Biochem. Biophys. Res. Commun. 55, 154–161 [DOI] [PubMed] [Google Scholar]
- 2. Scatchard G. (1949) Ann. N.Y. Acad. Sci. 51, 660–672 [Google Scholar]
- 3. Powell-Jones C. H., Thomas C. G., Jr., Nayfeh S. N. (1979) Proc. Natl. Acad. Sci. U.S.A. 76, 705–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Powell-Jones C. H., Thomas C. G., Jr., Nayfeh S. N. (1980) J. Biol. Chem. 255, 4001–4010 [PubMed] [Google Scholar]
- 5. Chazenbalk G. D., Kakinuma A., Jaume J. C., McLachlan S. M., Rapoport B. (1996) Endocrinology 137, 4586–4591 [DOI] [PubMed] [Google Scholar]
- 6. Latif R., Graves P., Davies T. F. (2001) J. Biol. Chem. 276, 45217–45224 [DOI] [PubMed] [Google Scholar]
- 7. Urizar E., Montanelli L., Loy T., Bonomi M., Swillens S., Gales C., Bouvier M., Smits G., Vassart G., Costagliola S. (2005) EMBO J. 24, 1954–1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guan R., Feng X., Wu X., Zhang M., Zhang X., Hébert T. E., Segaloff D. L. (2009) J. Biol. Chem. 284, 7483–7494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guan R., Wu X., Feng X., Zhang M., Hébert T. E., Segaloff D. L. (2010) Cell. Signal. 22, 247–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rivero-Müller A., Chou Y. Y., Ji I., Lajic S., Hanyaloglu A. C., Jonas K., Rahman N., Ji T. H., Huhtaniemi I. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 2319–2324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fan Q. R., Hendrickson W. A. (2005) Nature 433, 269–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Latif R., Michalek K., Morshed S. A., Davies T. F. (2010) PLoS ONE 5, e9449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Graves P. N., Vlase H., Bobovnikova Y., Davies T. F. (1996) Endocrinology 137, 3915–3920 [DOI] [PubMed] [Google Scholar]
- 14. Mizutori Y., Chen C. R., McLachlan S. M., Rapoport B. (2008) Mol. Endocrinol. 22, 1171–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Da Costa C. R., Johnstone A. P. (1998) J. Biol. Chem. 273, 11874–11880 [DOI] [PubMed] [Google Scholar]
- 16. Nagayama Y., Kaufman K. D., Seto P., Rapoport B. (1989) Biochem. Biophys. Res. Comm. 165, 1184–1190 [DOI] [PubMed] [Google Scholar]
- 17. Nagayama Y., Wadsworth H. L., Chazenbalk G. D., Russo D., Seto P., Rapoport B. (1991) Proc. Natl. Acad. Sci. U.S.A. 88, 902–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen C. R., McLachlan S. M., Rapoport B. (2007) Endocrinology 148, 2375–2382 [DOI] [PubMed] [Google Scholar]
- 19. Costagliola S., Khoo D., Vassart G. (1998) FEBS Lett. 436, 427–433 [DOI] [PubMed] [Google Scholar]
- 20. Saltiel A. R., Thomas C. G., Jr., Nayfeh S. N. (1982) Mol. Cell. Endocrinol. 28, 299–312 [DOI] [PubMed] [Google Scholar]
- 21. Smits G., Campillo M., Govaerts C., Janssens V., Richter C., Vassart G., Pardo L., Costagliola S. (2003) EMBO J. 22, 2692–2703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Núñez Miguel R., Sanders J., Jeffreys J., Depraetere H., Evans M., Richards T., Blundell T. L., Rees Smith B., Furmaniak J. (2004) Thyroid 14, 991–1011 [DOI] [PubMed] [Google Scholar]
- 23. Kleinau G., Krause G. (2009) Endocr. Rev. 30, 133–151 [DOI] [PubMed] [Google Scholar]
- 24. Ji I., Ji T. H. (1991) Endocrinology 128, 2648–2650 [DOI] [PubMed] [Google Scholar]
- 25. Ji I. H., Ji T. H. (1991) J. Biol. Chem. 266, 13076–13079 [PubMed] [Google Scholar]
- 26. Vassart G., Pardo L., Costagliola S. (2004) Trends Biochem. Sci. 29, 119–126 [DOI] [PubMed] [Google Scholar]
- 27. Nagayama Y., Rapoport B. (1992) Endocrinology 131, 548–552 [DOI] [PubMed] [Google Scholar]
- 28. Kosugi S., Ban T., Akamizu T., Kohn L. D. (1991) J. Biol. Chem. 266, 19413–19418 [PubMed] [Google Scholar]
- 29. Costagliola S., Panneels V., Bonomi M., Koch J., Many M. C., Smits G., Vassart G. (2002) EMBO J. 21, 504–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kleinau G., Jäschke H., Neumann S., Lättig J., Paschke R., Krause G. (2004) J. Biol. Chem. 279, 51590–51600 [DOI] [PubMed] [Google Scholar]
- 31. Mueller S., Kleinau G., Jaeschke H., Paschke R., Krause G. (2008) J. Biol. Chem. 283, 18048–18055 [DOI] [PubMed] [Google Scholar]
- 32. Parthier C., Reedtz-Runge S., Rudolph R., Stubbs M. T. (2009) Trends Biochem. Sci. 34, 303–310 [DOI] [PubMed] [Google Scholar]
- 33. Siu F. Y., Stevens R. C. (2010) Structure 18, 1067–1068 [DOI] [PubMed] [Google Scholar]
- 34. Grace C. R., Perrin M. H., Gulyas J., Rivier J. E., Vale W. W., Riek R. (2010) J. Biol. Chem. 285, 38580–38589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ji I., Ji T. H. (1991) J. Biol. Chem. 266, 14953–14957 [PubMed] [Google Scholar]
- 36. Quintana J., Wang H., Ascoli M. (1993) Mol. Endocrinol. 7, 767–775 [DOI] [PubMed] [Google Scholar]
- 37. Levental I., Grzybek M., Simons K. (2010) Biochemistry 49, 6305–6316 [DOI] [PubMed] [Google Scholar]
- 38. Latif R., Ando T., Daniel S., Davies T. F. (2003) Endocrinology 144, 4725–4728 [DOI] [PubMed] [Google Scholar]
- 39. Latif R., Ando T., Davies T. F. (2007) Endocrinology 148, 3164–3175 [DOI] [PubMed] [Google Scholar]
- 40. Osuga Y., Kudo M., Kaipia A., Kobilka B., Hsueh A. J. W. (1997) Mol. Endocrinol. 11, 1659–1668 [DOI] [PubMed] [Google Scholar]
- 41. Latrofa F., Chazenbalk G. D., McLachlan S. M., Rapoport B. (2004) Thyroid 14, 801–805 [DOI] [PubMed] [Google Scholar]
- 42. Chazenbalk G. D., Tanaka K., McLachlan S. M., Rapoport B. (1999) Endocrinology 140, 4516–4520 [DOI] [PubMed] [Google Scholar]
- 43. Sanders J., Chirgadze D. Y., Sanders P., Baker S., Sullivan A., Bhardwaja A., Bolton J., Reeve M., Nakatake N., Evans M., Richards T., Powell M., Miguel R. N., Blundell T. L., Furmaniak J., Smith B. R. (2007) Thyroid 17, 395–410 [DOI] [PubMed] [Google Scholar]
- 44. Vlaeminck-Guillem V., Ho S. C., Rodien P., Vassart G., Costagliola S. (2002) Mol. Endocrinol. 16, 736–746 [DOI] [PubMed] [Google Scholar]