Abstract
C-reactive protein (CRP), a phylogenetically highly conserved plasma protein, is the classical acute phase reactant in humans. Upon infection, inflammation, or tissue damage, its plasma level can rise within hours >1000-fold, providing an early, nonspecific disease indicator of prime clinical importance. In recent years, another aspect of CRP expression has attracted much scientific and public attention. Apart from transient, acute phase-associated spikes in plasma concentration, highly sensitive measurements have revealed stable interindividual differences of baseline CRP values in healthy persons. Strikingly, even modest elevations in stable baseline CRP plasma levels have been found to correlate with a significantly increased risk of future cardiovascular disease. These observations have triggered intense controversies about potential atherosclerosis-promoting properties of CRP. To directly assess potential effects of CRP on atherogenesis, we have generated CRP-deficient mice via gene targeting and introduced the inactivated allele into atherosclerosis-susceptible ApoE−/− and LDLR−/− mice, two well established mouse models of atherogenesis. Morphometric analyses of atherosclerotic plaques in CRP-deficient animals revealed equivalent or increased atherosclerotic lesions compared with controls, an experimental result, which does not support a proatherogenic role of CRP. In fact, our data suggest that mouse CRP may even mediate atheroprotective effects, adding a cautionary note to the idea of targeting CRP as therapeutic intervention against progressive cardiovascular disease.
Keywords: Apolipoproteins, Atherosclerosis, Gene Knock-out, Inflammation, Mouse Genetics, C-reactive Protein, Acute Phase Proteins
Introduction
Atherosclerosis is a complex, multifactorial disease initiated by focal lipid deposition in the arterial wall and formation of characteristic lesions known as atherosclerotic plaques. Such plaques can narrow the lumen and eventually rupture, causing thrombotic arterial occlusion responsible for heart attacks and strokes, the cardiovascular disease events that are the leading cause of death worldwide. Research during the last two decades has firmly established chronic arterial inflammation as key participant in atherogenesis at all stages of pathology, from its initiation through to its life-threatening clinical manifestations (1, 2). There is thus intense interest in systemic markers of inflammation in relation to both risk assessment for cardiovascular disease and the possible role of such markers in pathogenesis of atherosclerosis and atherothrombosis. C-reactive protein (CRP),2 the classical acute phase plasma protein, which is an exquisitely sensitive, nonspecific marker of inflammation and tissue damage (3, 4), has become a particular focus of attention and controversy. Despite the dynamic behavior of CRP in response to injury and disease, baseline values in apparently healthy general populations are surprisingly stable, comparable with repeat measurements of plasma cholesterol. Importantly, modestly higher CRP concentrations have been found to correlate significantly with increased risk of cardiovascular disease (5–7), an observation that has fueled much debate about a potential causative role of CRP in cardiovascular pathogenesis (6, 8–12).
Hypercholesterolemic apolipoprotein E (ApoE−/−) and LDL receptor knock-out (LDLR−/−) mice are established animal models of atherosclerosis, widely used to unveil environmental and genetic effects on atherogenesis (13–17). In several independent studies, transgenes overexpressing rabbit (18) or human CRP (19–24) have been introduced into these and other atherosclerosis-prone mouse strains. Although the authors of one study interpreted their findings as evidence for a proatherogenic role of CRP (23), other investigators could not find such indications. However, as some authors rightly caution (18–20), expression of human or rabbit CRP in a xenogeneic murine environment is associated with limitations, which may presage a negative result. To avoid such limitations, we have chosen a complementary approach and generated mice with a targeted deletion of the CRP gene on B6.ApoE−/− as well as B6.LDLR−/− genetic backgrounds. Based on quantitative analysis of atherosclerotic lesions in such animals, we here provide decisive evidence against a proatherogenic role of CRP in the two most widely used mouse models of atherosclerosis.
EXPERIMENTAL PROCEDURES
Generation of CRP-deficient Mice
Mice with inactivated CRP alleles were generated with C57BL/6-derived BRUCE4 ES cells (25) by classical gene targeting. The targeting vector consisted of a short arm of homology, a loxP-flanked neomycin resistance cassette, a long arm of homology, and a herpes simplex thymidine kinase-encoding cassette for selection against random integration. Relevant DNA fragments homologous to endogenous CRP sequences were obtained by PCR with genomic DNA from BRUCE-4 ES cells as template using an Expand Long Template PCR system (Roche Diagnostics) and 5′-CAATGTACCAACTCTGGGTCAGGC-3′/5′-ATGATCAGAAGGCACCAGAGTAGC-3′ and 5′-TGGTCCTCAGCACTAGAGCAC-3′/5′-CAGTTAACAGTCCCTGCTGATTC-3′ as specific primer combinations, respectively. The complete nucleotide sequence of the final targeting construct pCR-XH are available upon request. Gene targeting experiments were performed with C57BL/6-derived BRUCE4 ES cells (25) (kind gift of Ralf Kühn) as described (26). In two separate experiments, three independent ES clones (25, 87, and 147) with a correctly targeted CRP allele were identified after screening a total of 541 colonies by PCR with the Expand Long Template PCR system (Roche Diagnostics) and 5′-GAGAAATGAGCAGGAAATACTGGC-3′/5′-TTCTGAGGGGATCGGCAATA-3′ as primers. Correct homologous recombination events in candidate clones were confirmed by Southern blotting with a probe located outside of the targeting construct. Considering the adenine in the ATG start codon of the CRP gene as nucleotide position +1, this probe covers positions −5627 to −4998 (5′-CATGGATACC…609 nt…ACTCTGGGTC-3′). Two of the three independently generated CRP+/− ES clones (25 and 147) were injected into BALB/c blastocysts. Coat color chimeras derived from both clones were mated with C57BL/6 partners, and offspring carrying the inactivated CRP allele was used to establish the two separate CRP knock-out mouse lines 25 and 147, both on inbred C57BL/6 background.
Atherosclerosis Studies
For atherosclerosis studies, CRP−/− mice were crossed onto the atherosclerosis sensitizing ApoE−/− and LDLR−/− backgrounds. ApoE−/− and LDLR−/− mice, originally generated in the laboratories of N. Maeda (27, 28) and J. Herz (29), were purchased from The Jackson Laboratory, both at the >10th generation onto C57BL/6 (strain C57BL/6J-Apoetm1Unc, stock no. 002052 and strain B6.129S7-Ldlrtm1Her/J, stock no. 002207, respectively). To introduce inactivated CRP alleles onto the ApoE−/− background, heterozygous CRP+/− mice of line 147 were backcrossed to ApoE−/− animals to generate CRP+/−ApoE−/− “master breeders.” Pups of CRP+/−ApoE−/− parents were weaned at the age of 4 weeks onto a lowfat, semisynthetic diet, genotyped, and used to assess artherosclerotic lesion size at indicated time points. Inactivated CRP alleles were introduced onto the LDLR−/− background following an analogous breeding strategy. The CRP genotype of all experimental animals was determined initially by PCR and subsequently confirmed by Southern blotting. Homozygous ApoE−/− and LDLR−/− breeders were identified by PCR; predicted ApoE and LDLR genotypes of respective offspring were routinely confirmed in the first litter by PCR and in all sacrificed animals by analysis of serum lipoprotein levels at the time of death. All animals used in this study were housed under specific pathogen free (SPF) conditions in individually ventilated cages in the same room with access to food ad libidum. The lowfat, semisynthetic diet containing 0.02% cholesterol (30) was purchased from SNIFF Spezialdiäten GmbH, Soest, Germany (modified AIN76; article no. S8619-E010). For composition/ingredients, see supplemental Table S1. Mice were exsanguinated by a left ventricular puncture, and blood was collected into syringes containing EDTA. The circulatory system was flushed with PBS (20 ml), and the heart and brachiocephalic artery (BCA) were removed and snap-frozen in Tissue-Tek optimum cutting temperature compound (Sakura Finetek).
Morphometric Determination of Atherosclerotic Lesion Size
To quantify atherosclerosis at the aortic root, optimum cutting temperature-embedded hearts were processed as described previously (31). In brief, atherosclerotic lesion size at the aortic root was determined as the mean of five Oil Red O-stained sections through the aortic valve area, with each section 50 μm apart from each other. Atherosclerotic lesions in the BCA were quantified luminal to the internal elastic lamina in three equidistant Oil Red O-stained sections 200, 400, and 600 μm from the branching point of the BCA into the carotid and subclavian arteries. For en face analysis, the aorta was dissected to the iliac bifurcation, opened longitudinally, and fixed between glass slides.
Immunohistochemistry
Frozen sections were fixed in ice-cold acetone/methanol (mixture, 1:1 for 15 min) and washed with PBS. Peroxidases were quenched with 3% H2O2 in PBS, 10% methanol (4 min). Sections were blocked with 2.5% normal serum (goat serum for CD68 and complement C3, horse serum for α-actin) for 30 min. Primary antibodies were incubated at 4 °C overnight at dilutions shown in supplemental Table S2. Sections were washed and incubated with HRP-conjugated secondary antibodies as indicated in supplemental Table S2 for 30 min at room temperature. After washing, peroxidase was visualized by incubation with ImmPACT Nova Red (Vector Laboratories), and sections were counterstained with hematoxylin. Sections were dehydrated and permanently mounted with Vecta Mount mounting medium (Vector Laboratories). CD68-stained area of aortic root sections was assessed by planimetry.
Blood Analysis
In immunoblots, mouse CRP was detected with a biotinylated goat anti-mouse CRP antibody from R&D Systems (catalog no. BAF1829), followed by a horseradish peroxidase-labeled donkey anti-goat secondary antibody from Santa Cruz Biotechnology (Santa Cruz, CA; catalog no. SC-3851). α1-Antitrypsin as loading control was detected with a rabbit anti-human α1-antitrypsin serum from Sigma-Aldrich (catalog no. A-0409), which cross-reacts with the mouse ortholog, followed by a horseradish peroxidase-labeled goat anti-rabbit secondary antibody from Santa Cruz Biotechnology (catalog no. SC-2768). The concentration of CRP in serum was measured with an anti-mouse CRP ELISA (catalog no. 2210-01) purchased from Life Diagnostics (West Chester, PA). Lipoproteins were isolated by sequential ultracentrifugation from 60 μl plasma at d < 1.006 g/ml (very low density lipoprotein), 1.006 ≤ d ≤ 1.063 g/ml (intermediate density lipoprotein, low density lipoprotein), and d > 1.063 g/ml (HDL) in a TL-100 ultracentrifuge (Beckman Coulter) as described (31). Cholesterol was determined enzymatically using a colorimetric method (Roche Applied Science).
Statistical Analysis
Distributions were tested for normality, and statistical analysis was done by analysis of variance for normally distributed data and Kruskal-Wallis test for non-normally distributed data using the Prism software (version 4.0). Differences were considered statistically significant at p < 0.05.
RESULTS
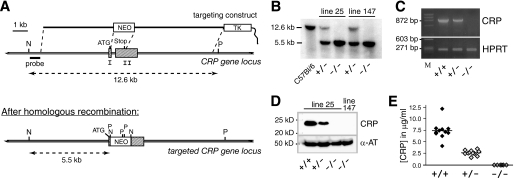
CRP knock-out mice were generated via classical gene targeting, following the strategy depicted in Fig. 1 (A and B). As genetic background significantly influences atherosclerosis susceptibility (32, 33), we generated and maintained CRP-deficient mouse mutants on a pure, inbred C57BL/6 background. Homozygous CRP-deficient animals were obtained from heterozygous parents with the expected Mendelian frequency. Complete absence of CRP message in liver and CRP protein in serum of CRP−/− mice was demonstrated by RT-PCR (Fig. 1C) and immunoblotting (Fig. 1D), respectively. In heterozygous mutant animals, circulating CRP levels were reduced by ∼50%, demonstrating a potent gene-dosage effect (Fig. 1E). Neither heterozygous nor homozygous CRP-deficient mice exhibited any gross abnormalities and proved healthy and fertile when maintained under specific pathogen free conditions.
FIGURE 1.
Generation of CRP-deficient mice on an inbred C57BL/6 background. A, targeting strategy. The two exons of the CRP gene are represented by shaded rectangles. NEO, neomycin resistance gene; TK, thymidine kinase gene for selection against random integration; P, PstI; N, NheI. The targeting vector was designed to delete all coding sequences of the CRP gene except the first 11 triplets, which code for the N-terminal part of the signal peptide. Targeting experiments were performed in BRUCE-4 ES cells derived from the inbred, atherosclerosis-susceptible strain C57BL/6. Mutant mice were maintained on a pure C57BL/6 background. B, Southern blot analysis of genomic DNA from tail biopsies of a wild-type C57BL/6 control animal and heterozygous as well as homozygous CRP-deficient mice of both independently generated knock-out lines 25 and 147. The DNA was double digested with NheI/PstI, blotted, and hybridized with a probe located outside of the targeting construct, as indicated in A. C, RT-PCR analysis of CRP expression in the liver of mice with the indicated genotypes. Specific oligonucleotide primers (5′-ATGGAGAAGCTACTCTGGTGCCTTC-3′/5′-CTAGTGGGATGCTTAT GCTGGAAGC-3′) were designed to anneal within CRP exons 1 and 2 just upstream and downstream of the deleted region. HPRT, hypoxanthine-guanine-phosphoribosyl transferase. D, immunoblot demonstrating complete absence of CRP protein in serum of homozygous knock-out mice (−/−) and reduced levels in a representative heterozygous animal (+/−). After hybridization with a goat anti-mouse CRP antibody, the filter was stripped and rehybridized with an antibody against mouse α-antitrypsin (α-AT) as loading control. E, CRP serum levels in wild-type (+/+; n = 11), heterozygous (+/−; n = 11), and homozygous (−/−; n = 6) CRP knock-out mice as determined by ELISA. CRP+/+ and CRP+/− mice represent four complete litters from two separate matings between heterozygous males and C57BL/6 females. CRP−/− mice were offspring of homozygous CRP−/− parents.
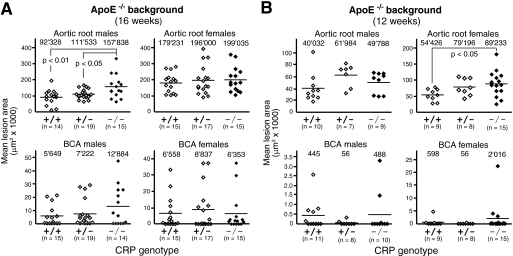
To assess potential effects of CRP deficiency on atherosclerosis susceptibility, we first bred the CRP null mutation onto the B6.ApoE-deficient background. Littermates with all three CRP genotypes were obtained from CRP+/−ApoE−/− parents and weaned to a lowfat, semisynthetic diet containing 0.02% cholesterol (supplemental Table S1), which has been shown previously to promote hypercholesterolemia and atherosclerosis while avoiding obesity with its attendant metabolic complications (31). When atherosclerotic lesion size was determined in BCAs and aortic roots of a first cohort of 16-week-old mice, no protective effect neither of heterozygous nor homozygous CRP deficiency was noticeable. In fact, lesions were increased with statistical significance by 71% (157,838 ± 71,398 μm2 versus 92,328 ± 47,889 μm2; p < 0.01) in aortic roots of CRP−/− males (Fig. 2A), whereas no significant differences were observed in females. Lesions were increased in male CRP−/− mice in the absence of significant changes in plasma lipids and lipoproteins (supplemental Table S3). As lesion development is known to be slower in males, detection of CRP effects in ApoE−/− females might require an earlier time point of analysis. We thus analyzed a second group of animals sacrificed 4 weeks earlier, i.e. at 12 weeks of age. There was again no indication that CRP deficiency would delay or inhibit atherogenesis in any of the experimental groups. Instead, we observed once more a statistically significant 64% increase of lesion size (89,233 ± 39,598 μm2 versus 54,426 ± 18,662 μm2; p < 0.05) in aortic roots of CRP−/− animals, this time in females (Fig. 2B). Plasma total cholesterol was not significantly different between groups (supplemental Table S3). Representative micrographs of atherosclerotic tissue sections from BCAs and aortic roots of CRP+/+ and CRP−/− animals on the ApoE−/− background are shown in Fig. 3. To test whether differences in lesion formation might be seen at other parts of the aorta, we performed en face evaluations of the whole aorta at 36 weeks of age but detected no significant differences (Fig. 4).
FIGURE 2.
No beneficial effect of CRP deficiency on atherosclerotic lesion size in ApoE−/− mice. Each data point represents the mean lesion area in the aortic root and BCA of an individual ApoE-deficient mouse with the indicated CRP genotype at the age of 16 (A) and 12 (B) weeks. The mean lesion area was determined morphometrically for each mouse in a total of five and three equidistant Oil Red O-stained cross-sections at the aortic root and BCA, respectively. Numbers on top of each cohort give means of individual data points, as indicated by lines. OCT-embedded hearts and BCA were processed as described (31). All morphometric measurements were done by investigators blinded for CRP genotypes.
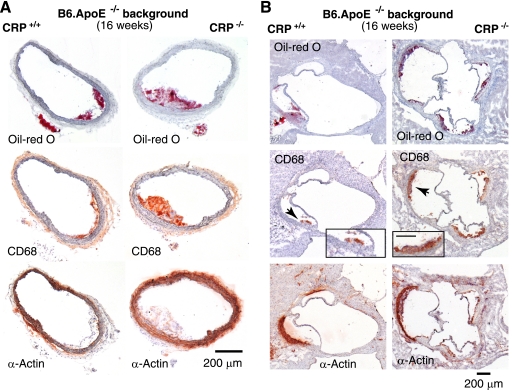
FIGURE 3.
Representative photomicrographs of atherosclerosis histology from 16-week-old CRP+/+ApoE−/− and CRP−/−ApoE−/− mice. Serial sections from BCAs (A) and aortic roots (B) were stained with Oil Red O to visualize lipid depositions (top panels) or immunostained with an antibody against CD68 to highlight macrophage infiltrates typical for atherosclerotic lesions (middle panel). Black arrowheads point to areas depicted enlarged in the insets, with the scale bar representing 100 μm. The lower panels show immunostaining for smooth muscle actin (α-actin).
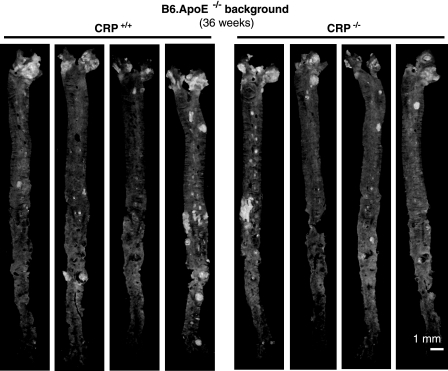
FIGURE 4.
Representative images of en face aorta preparations from four CRP+/+ and four CRP−/− mice on the ApoE−/− background. When 4 weeks old, mice were weaned and fed a standardized semisynthetic diet until sacrifice at 36 weeks of age. No significant differences between CRP-proficient and -deficient mice were observed.
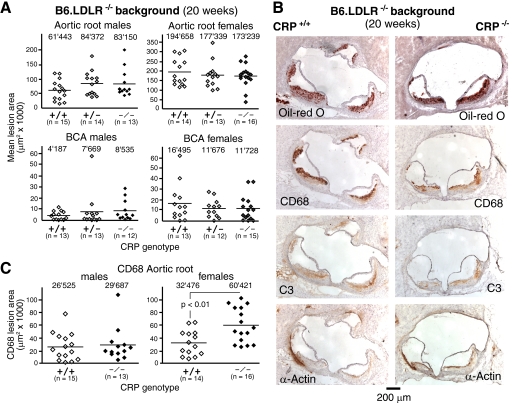
We also bred the CRP null mutation onto the LDL receptor-deficient (LDLR−/−) background, an alternative mouse model of atherogenesis, to test whether the mode of atherosclerosis induction might influence the result (13, 14). Offspring of CRP+/−LDLR−/− breeders were sacrificed when 20 weeks old. Importantly, morphometric analysis of plaques revealed similar levels of atherosclerosis within male and female cohorts irrespective of CRP genotype (Fig. 5A), confirming data from the ApoE−/− model that CRP does not promote atherosclerosis. Because male mice on the LDLR−/− background showed differences in lipid levels (supplemental Table S3), these analyses were repeated after adjusting for these parameters, and the lack of effect of CRP deficiency on atherosclerosis was confirmed (supplemental Table S4). In addition, a more detailed analysis of lesion composition of these animals showed no general differences in lesion composition between CRP+/+ and CRP−/− animals (Fig. 5B). However, female LDLR−/− mice had a significantly larger content of macrophages in lesions in CRP−/− mice compared with CRP+/+ animals (Fig. 5C). Thus, even though neither male nor female CRP-deficient mice exhibited any statistically significant increase in lesion size on LDLR−/− background, the more macrophage-rich plaque phenotypes in female CRP−/− mice might provide additional evidence for an atheroprotective effect of CRP.
FIGURE 5.
Equivalent atherosclerotic lesion size in LDLR−/− mice irrespective of CRP genotype. A, each data point represents the mean lesion area in the aortic root and BCA of an individual 20-week-old LDLR-deficient mouse with the indicated CRP genotype. The mean lesion area was obtained morphometrically for each mouse from a total of five and three equidistant Oil Red O-stained cross-sections at the aortic root and BCA, respectively. Numbers on top of each cohort give means of individual data points, as indicated by lines. OCT-embedded hearts and BCAs were processed as described (31). B, representative photomicrographs of atherosclerotic lesions in the aortic root from 20-week-old male CRP+/+LDLR−/− and CRP−/−LDLR−/− mice. Serial sections were stained with Oil Red O to visualize lipid depositions, CD68+ macrophages, complement C3 deposition, and smooth muscle actin (α-actin) as indicated. The scale bar represents 200 μm. C, the CD68 stained area of sections was assessed by planimetry and represents the macrophage content of lesions at a defined site (section 2) in the aortic root. Interestingly, lesions in female mice lacking CRP showed significantly larger areas of CD68 staining than CRP+/+ control lesions reflecting a greater percentage of macrophage content (16% in CRP+/+ versus 40% in CRP−/− mice). All morphometric measurements were done by investigators blinded for CRP genotypes.
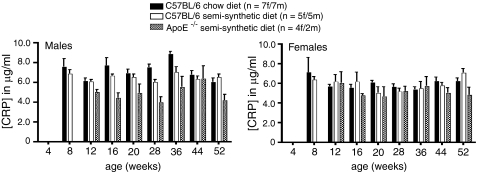
To test for potentially confounding effects of diet or atherosclerotic load on CRP serum levels in experimental animals, we collected blood over a period of 1 year at regular intervals from three informative cohorts of mice. Essentially stable CRP serum levels in all animals analyzed (Fig. 6) exclude indirect effects of the experimental set-up on CRP concentration as complicating factor.
FIGURE 6.
CRP serum levels in different cohorts of mice. Neither progressive atherogenesis in ApoE-deficient mice nor type of diet or age exhibited a significant influence on CRP serum concentration. Blood samples were taken from the same animals at the indicated intervals over a period of 1 year. CRP concentrations were determined by ELISA (Life Diagnostics, Inc.). f, female; m, male.
DISCUSSION
A possible role of CRP in atherogenesis would be of considerable clinical and potential therapeutic importance. Here, we demonstrate that neither a reduction of CRP serum concentration nor its complete absence result in a measurable reduction of atherosclerotic lesions in two distinct mouse models of atherogenesis. Our data are based on the morphometric analysis of brachiocephalic arteries and aortic roots from a total of >240 animals, providing powerful evidence against a proatherogenic role of CRP in mice. In fact, in two CRP−/− subgroups on the ApoE−/− background, we observed a suspicious increase in aortic root lesions, more compatible with an overall beneficial, antiatherogenic influence of CRP. This was corroborated by a more unstable, macrophage-rich plaque phenotype in female CRP−/− mice on the LDLR−/− background, even though lesion size was comparable with wild-type animals on that background. However, we did not find independent evidence for such an atheroprotective activity in the LDLR−/− mouse model. Currently, we thus cannot exclude that the apparent atheroprotective effect of CRP with respect to lesion size and lesion composition may be linked to physiologic peculiarities associated with any of the atherosclerosis-prone backgrounds used in the current study. For example, ApoE itself has been reported to have immunomodulatory effects and to be essential for some innate immune functions (34). On the other hand, mild atheroprotective effects may manifest phenotypically only within a limited range of specific atherogenic conditions. Modest effects of the CRP deletion might be obscured when the course of atherogenesis is too aggressive. A conclusive evaluation of potential athero-protective functions of CRP thus requires additional, comprehensive studies, for instance in mice with more indolent atherosclerosis-sensitizing backgrounds, like the ApoE*-Leiden or the ApoB100/100LDLR−/− strains (14). Actually, a mild beneficial influence of CRP has been observed previously in mice of the ApoB100/100LDLR−/− strain, when overexpressing human CRP (22). Although a conclusive demonstration of potential atheroprotective functions of CRP needs further investigation, our data clearly refute a proatherogenic role of CRP in mice. Our findings along with the latter report (22) therefore question the effectiveness of proposed strategies to slow atherosclerosis by pharmaceutical reduction of CRP serum levels or CRP activity and caution that such attempts could actually bring about the adverse effect.
Atherosclerosis is an intricate, systemic disease with complex contributions from the immune system, the vascular system, and from genetic and environmental determinants. Experimental investigation of individual effects of these determinants on the development and progression of atherosclerosis thus necessitates in vivo studies. So far, all published investigations designed to assess the in vivo role of CRP in atherogenesis have been based on experimental elevation of CRP serum levels, either by injecting purified mouse CRP into animals (35) or by overexpressing rabbit (18) or human CRP (19–24) in transgenic mice. Notably, none of the transgenic studies were able to detect any pro-atherogenic effect of CRP (or any other adverse effects, for that matter), with one exception (23). The authors of this latter report interpreted their findings as evidence that transgenic expression of human CRP accelerates the progression of atherosclerosis in ApoE-deficient mice. This interpretation has been criticized vehemently (18, 19), as differences in lesion size were observed only in males, only at one time point at the end of the study, as the reported difference “was of marginal statistical significance that could be abolished by elimination of a single outlier” (19) and for relying on the poorly controlled use of turpentine to periodically boost circulating CRP levels, which itself might induce active inflammatory pathology in the animals studied. However, failure to detect effects of elevated CRP levels in other studies could be due to the inability of human or rabbit CRP to interact with critical mouse effector molecules, such as complement, cellular receptors, the extracellular matrix, lipoproteins etc., in a xenogeneic environment, as prudently pointed out in most of these previous reports. By avoiding xenogeneic complications, our approach eliminates this pitfall entirely. Moreover, our in vivo study is the first, which investigates atherosclerosis under conditions of CRP deficiency rather than CRP overload.
No currently available animal model of atherosclerosis can fully reflect the human situation, which also holds true for our study. In mice, the major acute phase protein is the homologous pentraxin serum amyloid protein, whereas CRP plasma levels rise only moderately following appropriate stimulation ((3) and our own data, data not shown). However, the reported association between CRP and cardiovascular disease in humans strictly refers to stable baseline serum levels of CRP and not to highly transient acute-phase associated peaks. In fact, to obtain meaningful correlations, data from patients or controls with an ongoing acute phase response are rigorously excluded in such studies. Pertinent publications frequently reiterate that serum concentrations of CRP in the mouse are distinctly low compared with human. Surprisingly, it is quite difficult to find convincing experimental evidence for such statements in the available literature. If references are given at all, they either refer to review articles devoid of experimental data or to a single publication from the late 1970s, which reports results of an electroimmunoassay using sheep anti-human CRP antibodies cross-reacting with mouse CRP, which failed to detect any mouse CRP in sera before acute phase induction (36). Reliable reagents specific for mouse CRP have become available just recently. Using such commercial reagents along with serum from our CRP knock-out mice as stringent negative control, our own measurements appear to indicate robust expression of mouse CRP even in the noninduced state (Fig. 1, D and E), which suggests that serum levels might be vastly underestimated and comparable with humans. Until currently available assays have been rigorously calibrated, the genuine CRP concentration in normal mouse serum should simply be considered as not yet established. Importantly, the CRP knock-out mice described here provide a critical negative control for assessing the specificity of anti-CRP reagents, which has not been available so far. In any case, the phylogenetic conservation of mouse CRP, its conserved structure and phosphocholin ligand binding specificity imply that mouse CRP serum levels are obviously high enough to provide a biologically significant activity and a distinct survival advantage. This not withstanding, there are certainly marked inter-species differences, for instance in secondary effects of ligand binding, such as precipitation and complement activation. Extrapolation from mouse to man therefore requires caution. Irrespective of these limitations, our findings are entirely consistent with the most recent, largescale human observational (37) and genetic epidemiology studies (38), which also provide no support for a proatherogenic role of human CRP.
Supplementary Material
Acknowledgments
We thank Dr. Ralf Kühn for BRUCE4 ES cells and advice; Franziska Beckel, Franziska Jeromin, and Claudia Weise for excellent technical assistance; Carmen Blum for blastocyst injections; Dr. Markus Scholz (LIFE Center for LIFE –Leipzig Research Center for Civilization Diseases, Universität Leipzig) for statistical support; the Tierforschungszentrum Ulm for expert care of our mouse colony; and Hans-Reimer Rodewald for discussions and many helpful suggestions.
This work was supported by SFB451-Teilprojekt B12 and by the Leipzig Interdisciplinary Research Cluster of Genetic Factors, Clinical Phenotypes, and Environment at the Universität Leipzig. LIFE is funded by the European Union, the European Regional Development Fund, and the Free State of Saxony within the framework of the excellence initiative.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Tables S1–S4.
- CRP
- C-reactive protein
- BCA
- brachiocephalic artery
- LDLR
- LDL receptor.
REFERENCES
- 1. Hansson G. K., Libby P. (2006) Nat. Rev. Immunol. 6, 508–519 [DOI] [PubMed] [Google Scholar]
- 2. Hansson G. K. (2005) N. Engl. J. Med. 352, 1685–1695 [DOI] [PubMed] [Google Scholar]
- 3. Pepys M. B., Hirschfield G. M. (2003) J. Clin. Invest. 111, 1805–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Black S., Kushner I., Samols D. (2004) J. Biol. Chem. 279, 48487–48490 [DOI] [PubMed] [Google Scholar]
- 5. Liuzzo G., Biasucci L. M., Gallimore J. R., Grillo R. L., Rebuzzi A. G., Pepys M. B., Maseri A. (1994) N. Engl. J. Med. 331, 417–424 [DOI] [PubMed] [Google Scholar]
- 6. Casas J. P., Shah T., Hingorani A. D., Danesh J., Pepys M. B. (2008) J. Intern. Med. 264, 295–314 [DOI] [PubMed] [Google Scholar]
- 7. Verma S., Szmitko P. E., Ridker P. M. (2005) Nat. Clin. Pract. Cardiovasc. Med. 2, 29–36 [DOI] [PubMed] [Google Scholar]
- 8. Nilsson J. (2005) Arterioscler. Thromb. Vasc. Biol. 25, 1527–1528 [DOI] [PubMed] [Google Scholar]
- 9. Pepys M. B. (2008) Nat. Clin. Pract. Nephrol. 4, 234–235 [DOI] [PubMed] [Google Scholar]
- 10. Paffen E., DeMaat M. P. (2006) Cardiovasc. Res. 71, 30–39 [DOI] [PubMed] [Google Scholar]
- 11. Scirica B. M., Morrow D. A. (2006) Circulation 113, 2128–2134 [DOI] [PubMed] [Google Scholar]
- 12. Verma S., Devaraj S., Jialal I. (2006) Circulation 113, 2135–2150 [PubMed] [Google Scholar]
- 13. Knowles J. W., Maeda N. (2000) Arterioscler. Thromb. Vasc. Biol. 20, 2336–2345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zadelaar S., Kleemann R., Verschuren L., de Vries-Van der Weij J., van der Hoorn J., Princen H. M., Kooistra T. (2007) Arterioscler. Thromb. Vasc. Biol. 27, 1706–1721 [DOI] [PubMed] [Google Scholar]
- 15. Breslow J. L. (1996) Science 272, 685–688 [DOI] [PubMed] [Google Scholar]
- 16. Smith J. D., Topol E. J. (2006) Expert Rev. Cardiovasc. Ther. 4, 703–709 [DOI] [PubMed] [Google Scholar]
- 17. Wouters K., Shiri-Sverdlov R., van Gorp P. J., van Bilsen M., Hofker M. H. (2005) Clin. Chem. Lab. Med. 43, 470–479 [DOI] [PubMed] [Google Scholar]
- 18. Reifenberg K., Lehr H. A., Baskal D., Wiese E., Schaefer S. C., Black S., Samols D., Torzewski M., Lackner K. J., Husmann M., Blettner M., Bhakdi S. (2005) Arterioscler. Thromb. Vasc. Biol. 25, 1641–1646 [DOI] [PubMed] [Google Scholar]
- 19. Hirschfield G. M., Gallimore J. R., Kahan M. C., Hutchinson W. L., Sabin C. A., Benson G. M., Dhillon A. P., Tennent G. A., Pepys M. B. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 8309–8314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tennent G. A., Hutchinson W. L., Kahan M. C., Hirschfield G. M., Gallimore J. R., Lewin J., Sabin C. A., Dhillon A. P., Pepys M. B. (2008) Atherosclerosis 196, 248–255 [DOI] [PubMed] [Google Scholar]
- 21. Trion A., de Maat M. P., Jukema J. W., van der Laarse A., Maas M. C., Offerman E. H., Havekes L. M., Szalai A. J., Princen H. M., Emeis J. J. (2005) Arterioscler. Thromb. Vasc. Biol. 25, 1635–1640 [DOI] [PubMed] [Google Scholar]
- 22. Kovacs A., Tornvall P., Nilsson R., Tegnér J., Hamsten A., Björkegren J. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 13768–13773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Paul A., Ko K. W., Li L., Yechoor V., McCrory M. A., Szalai A. J., Chan L. (2004) Circulation 109, 647–655 [DOI] [PubMed] [Google Scholar]
- 24. Torzewski M., Reifenberg K., Cheng F., Wiese E., Küpper I., Crain J., Lackner K. J., Bhakdi S. (2008) Thromb. Haemost. 99, 196–201 [DOI] [PubMed] [Google Scholar]
- 25. Köntgen F., Süss G., Stewart C., Steinmetz M., Bluethmann H. (1993) Int. Immunol. 5, 957–964 [DOI] [PubMed] [Google Scholar]
- 26. Luche H., Weber O., Nageswara Rao T., Blum C., Fehling H. J. (2007) Eur. J. Immunol. 37, 43–53 [DOI] [PubMed] [Google Scholar]
- 27. Piedrahita J. A., Zhang S. H., Hagaman J. R., Oliver P. M., Maeda N. (1992) Proc. Natl. Acad. Sci. U.S.A. 89, 4471–4475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang S. H., Reddick R. L., Piedrahita J. A., Maeda N. (1992) Science 258, 468–471 [DOI] [PubMed] [Google Scholar]
- 29. Ishibashi S., Brown M. S., Goldstein J. L., Gerard R. D., Hammer R. E., Herz J. (1993) J. Clin. Invest. 92, 883–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Teupser D., Pavlides S., Tan M., Gutierrez-Ramos J. C., Kolbeck R., Breslow J. L. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 17795–17800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Teupser D., Persky A. D., Breslow J. L. (2003) Arterioscler. Thromb. Vasc. Biol. 23, 1907–1913 [DOI] [PubMed] [Google Scholar]
- 32. Dansky H. M., Charlton S. A., Sikes J. L., Heath S. C., Simantov R., Levin L. F., Shu P., Moore K. J., Breslow J. L., Smith J. D. (1999) Arterioscler. Thromb. Vasc. Biol. 19, 1960–1968 [DOI] [PubMed] [Google Scholar]
- 33. Su Z., Li Y., James J. C., McDuffie M., Matsumoto A. H., Helm G. A., Weber J. L., Lusis A. J., Shi W. (2006) Genetics 172, 1799–1807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Davignon J. (2005) Arterioscler. Thromb. Vasc. Biol. 25, 267–269 [DOI] [PubMed] [Google Scholar]
- 35. Schwedler S. B., Amann K., Wernicke K., Krebs A., Nauck M., Wanner C., Potempa L. A., Galle J. (2005) Circulation 112, 1016–1023 [DOI] [PubMed] [Google Scholar]
- 36. Pepys M. B. (1979) Immunology 37, 637–641 [PMC free article] [PubMed] [Google Scholar]
- 37. Kaptoge S., Di Angelantonio E., Lowe G., Pepys M. B., Thompson S. G., Collins R., Danesh J. (2010) Lancet 375, 132–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Elliott P., Chambers J. C., Zhang W., Clarke R., Hopewell J. C., Peden J. F., Erdmann J., Braund P., Engert J. C., Bennett D., Coin L., Ashby D., Tzoulaki I., Brown I. J., Mt-Isa S., McCarthy M. I., Peltonen L., Freimer N. B., Farrall M., Ruokonen A., Hamsten A., Lim N., Froguel P., Waterworth D. M., Vollenweider P., Waeber G., Jarvelin M. R., Mooser V., Scott J., Hall A. S., Schunkert H., Anand S. S., Collins R., Samani N. J., Watkins H., Kooner J. S. (2009) JAMA 302, 37–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.