Abstract
The human α2β1 integrin binds collagen and acts as a cellular receptor for rotaviruses and human echovirus 1. These ligands require the inserted (I) domain within the α2 subunit of α2β1 for binding. Previous studies have identified the binding sites for collagen and echovirus 1 in the α2 I domain. We used CHO cells expressing mutated α2β1 to identify amino acids involved in binding to human and animal rotaviruses. Residues where mutation affected rotavirus binding were located in several exposed loops and adjacent regions of the α2 I domain. Binding by all rotaviruses was eliminated by mutations in the activation-responsive αC-α6 and αF helices. This is a novel feature that distinguishes rotavirus from other α2β1 ligands. Mutation of residues that co-ordinate the metal ion (Ser-153, Thr-221, and Glu-256 in α2 and Asp-130 in β1) and nearby amino acids (Ser-154, Gln-215, and Asp-219) also inhibited rotavirus binding. The importance of most of these residues was greatest for binding by human rotaviruses. These mutations inhibit collagen binding to α2β1 (apart from Glu-256) but do not affect echovirus binding. Overall, residues where mutation affected both rotavirus and collagen recognition are located at one side of the metal ion-dependent adhesion site, whereas those important for collagen alone cluster nearby. Mutations eliminating rotavirus and echovirus binding are distinct, consistent with the respective preference of these viruses for activated or inactive α2β1. In contrast, rotavirus and collagen utilize activated α2β1 and show an overlap in α2β1 residues important for binding.
Keywords: Cell Surface Receptor, Double-stranded RNA Viruses, Integrin, Mutant, Protein Domains, Signal Transduction, Viral Replication, Virus Entry, Rotavirus
Introduction
Integrins are heterodimers of α and β subunits anchored in the cell membrane that interact with the extracellular matrix, the actin cytoskeleton and signaling pathways to regulate cell processes including survival, differentiation, and migration (1). Their abundant expression, moderate affinity for natural ligands and ability to connect with endocytic processes means integrins are frequently commandeered by invading viruses to act as cellular receptors (2, 3).
Rotaviruses are the major etiologic agents of infantile gastroenteritis in humans and animals worldwide, causing at least 500,000 deaths annually (4). The multiple rotavirus cell interactions leading to penetration of intestinal epithelial cells are likely to be major determinants of rotavirus tropism. Many rotaviruses, including human (e.g. Wa and K8), monkey (RRV and SA11), and bovine (NCDV) strains, utilize the α2β1 integrin as a key cellular receptor or entry cofactor in epithelial cell lines (5–9). The rotavirus non-structural protein NSP4, which is produced from viral RNA after cell entry, also binds α2β1 (10). Cellular α2β1 expression plays a role in mouse susceptibility to rotavirus-induced biliary atresia (11). The α2β1 integrin is one of four human collagen-binding integrins that comprises a distinct subgroup along with the five leukocyte integrins (including αXβ2), because of possession of an additional “inserted” (I) ligand-binding α domain (1, 12). Rotaviruses also bind terminal and subterminal sialic acids, and can recognize other integrins including αXβ2 during cell entry (6, 7, 13–16). Previous studies indicated that rotavirus usage of α2β1 requires the presence of the α2 subunit I domain (α2I),3 as cells expressing α2β1 that lacks α2I do not support monkey rotavirus binding or infection via α2β1 (17). Rotavirus binding to α2β1 is inhibited by type I collagen and several function-blocking anti-α2I monoclonal antibodies, but not by anti-α2 antibodies that map outside α2I (7, 17, 18). Human echovirus 1 (EV1) also uses α2β1 as a cellular receptor, with α2I being sufficient for EV1 binding (19, 20).
The α2I is an independently folding domain of ∼200 amino acids (aa) containing the divalent cation-binding sequence DXSXS within the metal ion-dependent adhesion site (MIDAS) (21). The MIDAS is the major site for ligand binding. Integrins exist in inactive or active states for ligand binding and signaling that are proposed to relate to conformational change between bent (closed) and extended (open) extracellular domains, respectively (1, 12). Ligand binding to α2β1 appears to alter the closed conformation to the open form (21, 22). Titers of SA11 rotavirus bound to cellular α2β1 were increased following β1 activation with 8A2 antibody, providing evidence that rotavirus preferentially binds to activated α2β1 (7). Natural α2β1 ligands also show higher binding levels to the open over the closed form of α2I (23, 24). However, EV1 preferentially recognizes the bent, inactive α2β1 form (25).
The rotavirus spike protein VP4 is a major virulence determinant, eliciting neutralizing antibodies and dictating the P serotype (26). VP4 is the major rotavirus recognition protein for host cell surface receptors (7, 27–29). Protease cleavage of VP4 facilitates rotavirus infectivity and generates the virion-associated VP5* (60 kDa) and VP8* (28 kDa) subunits. Truncated RRV VP5* binds expressed α2I protein and competes with RRV for binding to cell surface-expressed α2β1 (7, 30). Mutation of the putative α2β1 ligand sequence DGE located in VP5* at positions 308 to 310 abrogates binding of truncated VP5* to α2I and VP5* competition with RRV cell binding and infectivity (7, 30). Additionally, DGE-containing peptides specifically inhibit rotavirus-cell binding and infection mediated through α2β1 (6, 7, 30, 31). The DGE sequence is externally located on the trypsin-primed and putative post-penetration structural forms of VP5* (32, 33).
These findings demonstrate the importance of α2I in monkey rotavirus recognition of α2β1. However, the role of α2I in α2β1 recognition by rotaviruses from other species (including humans) has not been determined, and the α2I residues involved have not been identified for any rotavirus strain. Monkey rotaviruses preferentially recognize human α2β1 over human α2 combined with hamster β1 (17), suggesting a role for the β1 subunit that requires further analysis. In this study, the α2I was shown to be necessary for α2β1 binding by human, monkey and bovine rotaviruses. The effect of mutations in exposed loops and adjacent regions of α2I, and in β1, on α2β1 binding by these rotaviruses was determined. Interestingly, rotaviruses showed strain-specific differences in their dependence on particular α2β1 residues for binding. We have identified regions of α2β1 that are involved in rotavirus recognition, including sites that differentiate rotavirus binding from that of other α2β1 ligands. Notably, all rotaviruses required residues in the activation-responsive αC-α6 and αF helices of α2I, in contrast to other α2β1 ligands.
EXPERIMENTAL PROCEDURES
Cell Lines
The derivation, mutagenesis, and propagation with G418 sulfate selection (Invitrogen) of CHO cells transfected with cDNA encoding empty vector (PBJ-1); human α2 combined with hamster β1 (Hu α2); human α2 containing a single point mutation or deletion, combined with hamster β1 (n = 51); human α2 with a human-to-mouse swap at amino acids 212–216 in α2I, combined with hamster β1 (Hu-mur α2I); human α2 containing a human-to-mouse swap of α2I, combined with hamster β1 (Mur α2I); human α2 combined with human β1 (Hu α2β1); and human α2 and human β1 with a D130A mutation (Hu α2β1 D130A) were as described previously (17, 34–36). The presence of the mutations was confirmed by nucleotide sequence analysis, as before (35).
Antibodies and Rotavirus Strains
Anti-human α2 monoclonal antibodies AK7 (37, 38) and HAS4 (39), and isotype control antibodies MOPC21 and UPC10, were obtained as described previously (7, 40). Mouse anti-human α2 antibody Gi9 was provided through participation of B.S.C. in the Leukocyte Typing VI Workshop. FITC-conjugated anti-rat α2 antibody Ha1/29 and isotype control antibody Ha4/8 were donated by Dale Godfrey, Department of Microbiology and Immunology, The University of Melbourne (41). The origins and characterization of rotavirus strains RRV and SA11 (monkey), Wa and K8 (human), NCDV (bovine) and CRW-8 (porcine) have been described previously (7, 42). Rotaviruses were propagated in MA104 cells following trypsin activation of infectivity, harvested by two freeze-thawing cycles and stocks clarified by low-speed centrifugation (RRV cell culture harvest), as previously (7, 8, 17). RRV was further purified by glycerol gradient ultracentrifugation, as before (17). Rotavirus infectivity titers were determined by indirect immunofluorescent staining of MA104 cells inoculated with serial dilutions of samples followed by microscopy, as described previously (8, 17). Titers were expressed as the number of fluorescent cell-forming units (FCFU)/ml.
Flow Cytometric Analysis
Surface α2β1 integrin expression was detected on CHO cell lines by direct (Ha1/29) or indirect (AK7, Gi9, Has4) immunofluorescent staining of 8 × 105 cells followed by flow cytometry as described previously (8). The relative linear median fluorescence intensity (RLMFI) was calculated, defined as the ratio of the median fluorescence intensities obtained with the test and isotype control antibodies. A positive RLMFI was defined as ≥1.2, as before (8).
Rotavirus Cell Binding and Rotavirus Replication Assays
Assays of infectious rotavirus binding at 4 °C for 1 h to confluent CHO cell lines (8 × 105 cells) were performed as previously described (8, 17). Rotavirus doses of 4 × 106 and 8 × 106 FCFU, corresponding to 5 and 10 FCFU/cell, respectively, were bound to cells. The multiplicity of infection used, defined as the number of FCFU/cell, was 5 or 10. Rotavirus-cell binding assays were conducted at 4 °C rather than 37 °C to avoid inaccuracy due to the virus entry that begins within 5 min at 37 °C (43). Titers of bound virus were measured by inoculation and immunofluorescent staining of MA104 cell monolayers as described above (8, 17). Rotavirus replication in CHO cell lines (8 × 105 cells) was assayed at 16 h post-infection at a multiplicity of infection of 1. Rotavirus titers (expressed in FCFU/ml) were determined by titration in MA104 cells as described above.
Data Analysis
In graphs, data are given as the mean of at least three independent experiments, and the bar indicates the S.D. A probability level of 0.05 was considered significant in statistical analysis. Data were analyzed by Student's t test unless otherwise indicated.
RESULTS
Surface Expression of Mutated α2β1 Integrin on Transfected CHO Cells, and Recognition of Mutated α2β1 by AK7 Antibody
A panel of CHO cell lines was produced, each transfected to express human α2 with a single point mutation (n = 50) or a short deletion (n = 1) in α2I. As transfection produces cell surface expression of heterodimers of human α2 combined with the endogenous hamster β1, these cell lines were examined for surface α2 expression by flow cytometry. The binding of monoclonal antibodies HAS4 and AK7, directed to α2 outside the I domain and to the I domain, respectively, was determined for a subset of 14 cell lines, including parental CHO K1 cells transfected with empty vector (PBJ-1) or with cDNA encoding human α2 (Hu α2). With both antibodies, PBJ-1 cells showed a negative RLMFI of <1.2, and Hu α2 showed positive RLMFI of 13.6 (HAS4) and 10.5 (AK7; Fig. 1A). All other CHO cell lines showed positive RLMFI values, with means ± 95%CI of 5.7 ± 2.6 (HAS4) and 6.2 ± 2.1 (AK7). As shown in Fig. 1A, a highly significant correlation was observed between the α2 RLMFI values determined using these antibodies (Spearman r = 0.94; p < 0.0001). In the 13 α2-positive cell lines, the percentage of cells expressing α2 (mean ± 95%CI) was 40.9 ± 10.5 with HAS4 and 45.9 ± 12.8 with AK7. These percentages also were significantly correlated (Spearman r = 0.83; p = 0.0001). Levels of α2 expression on the full mutant CHO cell panel (n = 51) were determined using AK7. With one exception, AK7 detected α2 on all cell lines. These α2-positive cell lines showed RLMFI values and percentages of positive cells (mean ± 95%CI) of 10.5 ± 5.0 and 50.2 ± 7.3, respectively. The exception was the CHO α2 R288A cell line, which showed a negative RLMFI of 1.1 ± 0.1 with AK7, and a positive RLMFI of 5.1 ± 0.1 when tested with HAS4. This implicates Arg-288 in AK7 recognition of α2I.
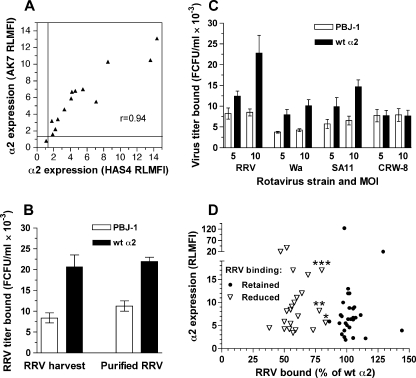
FIGURE 1.
Determining the parameters of rotavirus binding to native and mutant α2 on CHO cells. A, levels of α2 expression determined by flow cytometry using AK7 and HAS4 antibodies, expressed as RLMFI, were compared using cells expressing empty vector (PBJ-1), wt human α2 (Hu α2), wt human α2β1, and mutated human α2 (randomly selected; Y157A, Q212A, R213A, G240A, D259A, K264A, D273A, L291A, D292A, K298A, E309A). Lines indicate the positive-negative RLMFI cut-off of 1.2. PBJ-1 cells exhibited the single negative RLMFI value. B, RRV purification did not alter virus titers bound to PBJ-1 and Hu α2 CHO cells. RRV cell culture harvest (RRV harvest) and purified RRV were bound to cells at a multiplicity of infection of 10. C, dose-dependence of rotavirus binding to α2β1 on CHO cells. Binding of representative rotavirus strains RRV, Wa, SA11, and CRW-8 was evaluated at a multiplicity of infection (MOI) of 5 and 10. Data obtained with rotaviruses K8 and NCDV were similar to Wa binding levels. D, comparison of α2 RLMFI values and RRV binding levels for cells expressing mutated α2 that retained RRV binding (n = 29) or showed reduced RRV binding (n = 22, comprising 21 point mutants and the αC deletion mutant). RLMFI values for α2 were determined using AK7 except for CHO α2 R288A, which was recognized by HAS4 only. Data marked *, **, and *** represent CHO α2 E256A, N295A, and K163A, respectively. RRV and Wa bound these mutants at intermediate and low levels, respectively (Fig. 2).
Establishing Assay Parameters for Rotavirus Binding to CHO Cells Expressing Recombinant α2β1
Initially, binding to PBJ-1 or Hu α2 cells by purified RRV was compared with binding by RRV cell culture harvest (Fig. 1B). As purification did not affect infectious RRV binding to these cell lines (p > 0.05), cell culture harvests were used for all further experiments.
The effect of rotavirus dose on α2β1 binding was determined. Previously, we were unable to detect Wa binding to α2β1 on CHO cells using a multiplicity of infection of 1 (17). However, use of higher doses allowed significant levels of dose-dependent Wa binding to Hu α2 to be demonstrated (Fig. 1C; p < 0.01). The other integrin-using rotavirus strains RRV, SA11, K8, and NCDV also showed dose-dependent binding to α2β1 on Hu α2 cells (p < 0.01), and a multiplicity of infection of 10, corresponding to 8 × 106 FCFU, was optimal for all rotaviruses (Fig. 1C). Dose-independent binding to Hu α2 cells at control levels by the integrin-independent rotavirus CRW-8 demonstrated the specificity of α2β1 binding by the other rotaviruses.
To examine if the level of CHO cell α2 expression affected RRV binding, α2 RLMFI values on the 29 cell lines that bound RRV rotavirus (titer of virus bound ≥80% of the titer bound to cells expressing wt α2) were compared with those on the 22 cell lines showing RRV binding at or near the level of the PBJ-1 negative control (Fig. 1D). The RLMFI values determined with AK7 were used for all cell lines except CHO α2 R288A, for which the HAS4 RLMFI was used. Irrespective of the effect of the α2 mutation of RRV binding, there was no correlation between levels of RRV binding and cellular α2 expression (Spearman r<-0.02; p > 0.75). This shows that the level of α2 integrin expression on transfected CHO cell lines was not limiting for detection of rotavirus binding, and lack of binding above control levels was an indication that the α2 mutation inhibited virus binding.
Regions of α2I Required for Binding by Rotaviruses RRV and Wa
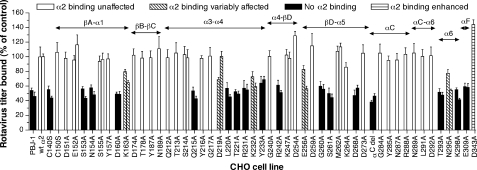
To determine the effect of α2I mutations on rotavirus recognition, the titer of RRV bound to the CHO cells expressing recombinant α2 with mutated α2I residues was assayed. As shown in Fig. 2, the 21 point mutations in α2I that abolished or reduced RRV binding were located in the N-terminal region (Cys-140), the loop between βA and α1 (Ser-153, Asn-154), the α1 helix (Asp-160, Lys-163), the loop between helices α3 and α4 (Gln-215, Asp-219, Leu-220, Thr-221), the region between α4 and βD (Arg-231, Lys-232, Tyr-233, Arg-242), the loop between βD and α5 (Glu-256, Gly-260, Ser-261), the α5 helix (Asp-268), the α6 loop (Thr-293, Asn-295, Lys-298), and the αF loop (Glu-309). Our earlier data indicated a trend for a reduction in RRV binding to CHO cells expressing α2β1 with the T221A mutation (17). Improvements in assay reproducibility led to a clear demonstration of RRV binding loss due to this mutation (Fig. 2). Deletion of the αC loop (amino acids 283–290) also abolished RRV binding, although individual alanine point mutations within and immediately adjacent to this region (Gly-284, Tyr-285, Asn-287, Arg-288, Asn-289, Leu-291, Asp-292) did not affect RRV binding (Fig. 2). This CHO cell-expressed α2I deletion was shown previously to have no effect on collagen binding affinity, demonstrating its functionality (34). A single C terminus mutation (D343A) increased RRV binding. This mutation appeared to increase collagen binding in a previous study (44).
FIGURE 2.
Effect of mutations in α2I on α2β1 binding by rotavirus strains RRV and Wa. CHO cells stably expressing empty vector (PBJ-1), wild-type human α2 (wt α2), or mutant human α2 were used to determine binding by monkey rotavirus RRV (first bar in each pair, or single bar) and human rotavirus Wa (second bar of each pair). The mutations consisted of single amino acid changes as indicated, with the exception of αC del that contained a deletion of the αC loop (amino acids 283–290). Data are presented as a percentage of the virus titer bound to wt α2 cells. These cells bound twice the level of virus bound by cells containing the empty vector. As cellular α2 expression was similar between mutant cell lines bound by rotaviruses and those that did not bind virus (Fig. 1), these data were not normalized for α2 expression levels. Secondary structural elements of α2I (α-helices and β-sheets as defined in Ref. 34) are indicated above the bars.
With one exception (D219A), all mutations that reduced RRV binding also reduced Wa binding to α2β1 (Fig. 2). However, mutations E256A and N295A reduced Wa binding to a greater extent than RRV binding. The findings indicated that Wa binding to α2β1 also involves α2I, and rotavirus binding to α2I was affected by mutations of many residues in a manner novel to α2β1 ligands. In addition, several α2I mutations affected binding by individual rotavirus strains to differing extents.
Differential Effects of α2I Mutations on α2β1 Binding by Human, Monkey, and Bovine Rotaviruses
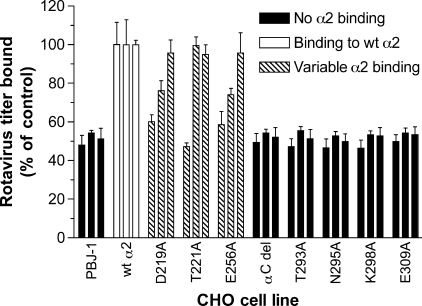
Mutations differentially affecting RRV and Wa binding to α2β1 (D219A, E256A, N295A) or involving the αC, α6, or αF loops were investigated for their effects on binding by other human (K8), monkey (SA11), and bovine (NCDV) rotaviruses (Fig. 3). As previous studies had shown SA11 binding to be unaffected by the T221A mutation, whereas RRV binding was reduced (Fig. 2), the effects of this mutation also were further analyzed. Initially, the ability of rotaviruses K8 and NCDV to bind human α2 on CHO cells was demonstrated (wt α2; Fig. 3). This is consistent with the K8 and NCDV binding to human α2β1 on K562 cells shown previously (7). Each virus strain showed a distinct pattern of reactivity with cells expressing the D219A, T221A, or E256A mutations in α2I (Fig. 3). Although K8 binding was almost eliminated by all 3 mutations, SA11 binding was reduced by D219A and E256A and unaffected by T221A, and NCDV binding was unaffected by any of the three mutations. In contrast, mutation of αC, the loop between αC and α6, α6 helix or αF region abrogated binding by K8, SA11, and NCDV (Fig. 3). These findings also show the dependence on α2I of K8 and NCDV binding to α2β1. Overall, D219A, T221A, E256A, and N295A mutations differentially affected rotavirus binding to α2β1, whereas mutations of other residues in the αC, α6, and αF regions and/or intervening loops abolished binding by all rotaviruses tested.
FIGURE 3.
Analysis of binding by rotaviruses K8 (human), SA11 (monkey), and NCDV (bovine) to α2I domain mutants that variably affected RRV and Wa binding, and α2I containing an αC loop deletion (amino acids 283–290) or single α6 loop mutations. Rotaviruses were bound to CHO cell lines selected from those described in the legend to Fig. 2. Titers bound by K8, SA11, and NCDV are represented by the first, second, and third bars in each group, respectively. Data are presented as a percentage of the virus titer bound to CHO parental cells expressing wild-type human α2 (wt α2).
Effect of Human-to-Mouse Swapping Mutations in α2I on Rotavirus Cell Binding and Infection
A region surrounding the MIDAS in α2I contains residues critically important for type I collagen binding (Asp-219, Thr-221), and shown above to be involved in rotavirus binding (Gln-215, Asp-219, Leu-220, Thr-221). Residues 217–221 are conserved among human, monkey,4 bovine and mouse α2I, whereas amino acids 212–216 vary between species, being QTSQY in humans and ETRQH in mice (19, 36, 45). To further test the importance of this region, CHO cells expressing human α2 with residues 212–216 (Hu-mur α2I) or the entire α2I (Hu-mur α2I) swapped to the mouse sequence were studied. The functional effect of these mutations was evaluated by flow cytometry using α2 antibodies (Table 1). Binding of anti-human α2I antibody Gi9 has been mapped to aa 212–216 (46). Swapping either this sequence (Hu-mur α2I) or the entire α2 I domain (Mur α2I) to the corresponding mouse residues abolished Gi9 binding as would be predicted. Conversely, the anti-mouse α2I antibody Ha1/29 bound both the human-to-mouse swapping mutants, but not human α2. This confirms the presence of functional mouse sequences in the mutants, and indicates that provision of mouse amino acids 212–216 is sufficient to confer Ha1/29 binding. Thus, Ha1/29 requires amino acids 212–216 for binding, similarly to Gi9. Non-function blocking antibody HAS4, which maps outside α2I (19, 44), detected the human α2 non-I region present in all transfected CHO cell lines, as expected. Overall, these data demonstrate the functionality of these swapping mutations in recombinant α2 expressed on CHO cells.
TABLE 1.
Functional verification of human-to mouse swapping mutations in α2I by reactivity of transfected CHO cells with monoclonal antibodies directed to human or mouse α2
CHO cells expressing wild-type human α2 (wt α2), human α2 with amino acids 212–216 swapped to the mouse sequence (Hu-mur α2I) or mouse α2I combined with human α2 (Mur α2I) were evaluated. Levels of α2 are expressed as the relative linear median fluorescence intensity (RLMFI), as defined in “Experimental Procedures.” Positive RLMFI values are given in bold. The fluorescence histograms of CHO cells expressing empty vector (PBJ-1) were indistinguishable from isotype control antibody profiles, showing RLMFI values of <1.1.
| Antibody | Specificity | CHO cell line |
|||||
|---|---|---|---|---|---|---|---|
| wt α2 |
Hu-mur α2I |
Mur α2I |
|||||
| % positive | RLMFI | % positive | RLMFI | % positive | RLMFI | ||
| Gi9 | Human α2I | 54 | 5.8 | 2 | 1.1 | 2 | 1.0 |
| Has4 | Human α2 (non-I) | 62 | 2.6 | 75 | 6.8 | 70 | 15.0 |
| Ha1/29 | Mouse α2I | 2 | 1.0 | 75 | 3.8 | 35 | 1.8 |
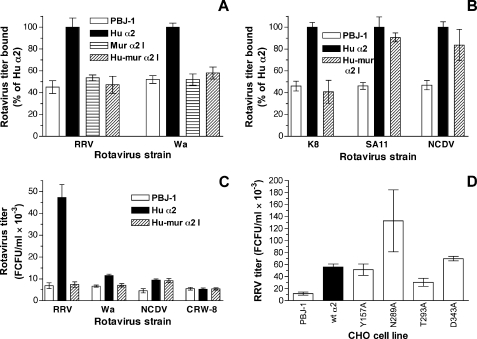
The impact of these human-to-mouse swapping mutations in α2I on α2β1 recognition by rotaviruses was measured. As shown previously for RRV and SA11 (17), rotaviruses Wa, K8, and NCDV bound to human α2β1 expressed on CHO cells (Fig. 4, A and B). Binding by rotaviruses RRV, Wa, and K8 was reduced to background levels by swapping residues 212–216 (Hu-mur α2I), whereas SA11 and NCDV binding was unaffected (Fig. 4, A and B). The effect was similar to that of the T221A mutation on binding by these rotaviruses. This finding was confirmed with Mur α2I cells, as swapping to the mouse I domain abrogated binding by RRV and Wa (Fig. 4A). The relation of the degree of virus binding to productive infection was determined (Fig. 4C). Expression of human α2 in CHO cells allows limited replication of RRV and Wa rotaviruses (5, 17). Consistent with this and the binding studies above, CHO cell yields of infectious RRV and Wa due to the presence of human α2 were reduced to background levels by the human-to-mouse swap of amino acids 212–216. Although we demonstrated α2-dependent NCDV rotavirus replication in CHO cells, this mutation did not affect the NCDV yield. This is in complete agreement with the lack of effect of this mutation on NCDV binding to α2β1. Porcine rotavirus CRW-8 does not use human or monkey α2β1 as a cellular receptor (7, 30, 31). Consistent with this, the very low level of CRW-8 replication in CHO cells was unaffected by the presence of human α2 or its mutation. Overall, the ability of rotaviruses to bind α2β1 with this swapping mutation correlated with their success in α2β1-mediated replication. Taken together with the data presented in Figs. 2 and 3, these studies indicate the importance of the entire α3-α4 region in human rotavirus and RRV usage of α2β1 as a cellular receptor, and the lesser importance of this region for α2β1 usage by SA11 and NCDV rotaviruses.
FIGURE 4.
Effect of human-to-mouse swapping and point mutations in α2I on rotavirus binding and infection through cell surface-expressed α2β1. Relative levels of binding by rotaviruses RRV and Wa (A) and K8, SA11, and NCDV (B) to CHO cells expressing empty vector (PBJ-1), human α2 (Hu α2), mouse α2I within human α2 (Mur α2I), or human α2 with amino acids 212–216 swapped to the mouse sequence (Hu-mur α2I) are illustrated. C, infectious rotavirus titers produced at 16 h after infection of PBJ-1, Hu α2, and Hu-mur α2I by RRV, Wa, NCDV, or integrin-independent porcine rotavirus CRW-8 at a multiplicity of infection of 1. D, infectious RRV titers produced at 16 h after infection of PBJ-1, Hu α2, Y157A, N289A, T293A, and D243A.
Effects of Point Mutations in α2I on Rotavirus Replication
The effect on RRV growth of representative point mutations in α2I that had shown varying effects on rotavirus binding to α2β1 was determined (Fig. 4D). The T293A mutation that reduced RRV binding to control levels also reduced RRV yield, by 54% (p < 0.0001). Similarly, the enhanced RRV binding to the D343A mutant (140% of wt α2 control; Fig. 2) translated to a 120% increase in RRV yield (p < 0.0001). Mutations that did not affect RRV binding to α2β1 either had no effect (Y157A; 92% of control, p = 0.07) or increased infectious yield (N289A; 240% of control, p < 0.0001). The CHO-N289A cells displayed an elongated morphology that was distinct from the other CHO cell lines analyzed for virus yield. This may indicate changes in cellular function that facilitated virus entry or replication independently of α2β1 expression. Taking into account the limitation provided by the varying effects of α2I mutation on cell function, these data provide further evidence that the outcome of α2I mutations for rotavirus replication is strongly influenced by their effect on virus-cell binding.
Comparison of α2β1 Recognition by Rotaviruses, Echoviruses, and Type 1 Collagen
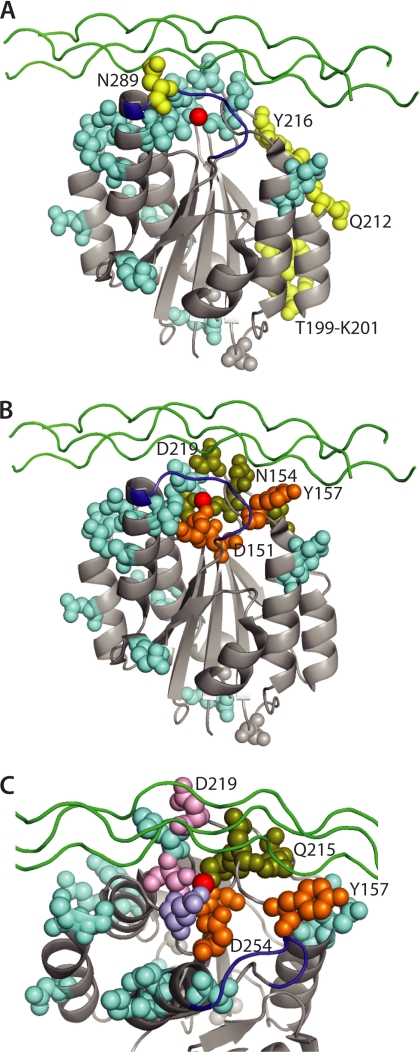
Studies described above indicated that the α2I point mutations eliminating rotavirus and EV1 binding were distinct, whereas mutations in the DXSXS region at Ser-153, Asn-154 and in the α3-α4 loop surrounding the MIDAS at Gln-215, Asp-219, and Thr-221 affected both rotavirus and collagen binding (summarized in Table 2). Native α2β1 expressed on CHO cells is activated, based on recognition by antibody 12F1 (47). When visualized in the crystal structure of activated α2I, mutations affecting rotavirus binding were shown to reside primarily in the vicinity of the MIDAS and the αC-α6 loops (Fig. 5). In contrast, the residues that directly interact with EV1 and are both necessary and sufficient for binding (amino acids 199–201, 212–216) are located on a side face of α2I (20, 35)(Fig. 5A). Mutation of Asn-289 in the αC helix also inhibits EV1 binding, although Asn-289 might not be sufficient for EV1 binding (48). Asn-289 is located at the edge of the α2I interface with EV1 and is considered likely to exert its effect by conformational means (20). In contrast, Asn-289 mutation did not affect rotavirus binding, even though deletion of the αC helix containing Asn-289 abolished binding. Interestingly, we found that residues where mutations affected both rotavirus and collagen recognition were located to one side of the MIDAS, whereas those inhibiting collagen but not rotavirus clustered separately in an adjacent site (Fig. 5, B and C). Residues where mutation differentially inhibited binding by the various rotavirus strains (Asp-219, Thr-221, and Glu-256) also were located in close mutual proximity within the MIDAS region (Fig. 5C).
TABLE 2.
Comparison of requirements for α2β1 recognition by rotaviruses, type 1 collagen, and EV1
| α2I region | Mutations in α2I residues that affect binding to given liganda: |
||
|---|---|---|---|
| Rotavirus | EV1b | Type 1 collagenc | |
| βA-α1 (DXSXS) | S153, N154, D160, K163 | None affect binding | D151, S153, N154, S155, Y157, D254 |
| βB-βC | None affect binding | None affect binding | None affect binding |
| α3-α4 (surrounds MIDAS) | Q215, D219d, L220, T221d, R231, K232, Y233 | Point mutation did not affect binding | Q215, D219, T221 |
| Hu-mur α2I (Q212-Y216)d | Hu-mur α2I (E205-Y216) | ||
| α4-βD | R242 | None affect binding | None affect binding |
| βd-α5 (surrounds MIDAS) | E256d, G260, S161, D268 | None affect binding | None affect binding |
| αC-α6 (surrounds MIDAS) | T293, N295, K298 | N289 | None affect binding |
| αC deletion | αC deletion not tested | No effect of deletion | |
| αF | E309 | None affect binding | None affect binding |
| Entire α2I | Swap to mouse α2I | Swap to mouse α2I | No effect of swap |
FIGURE 5.
Structural comparison of the α2I locations of amino acids affecting rotavirus, EV1, and type I collagen binding. The sites of mutations inhibiting rotavirus or EV1 binding (A), and rotavirus and/or collagen binding (B, C) are compared individually, with the atoms of residues where mutation affects binding shown as colored-filled spheres. The view in A and B is identical, looking from one side with the MIDAS at the top. In C, the view is from the same side from the top and is restricted to the MIDAS and surrounds. Mutations affecting the binding of rotaviruses (light blue), echovirus (yellow), collagen but not viruses (orange), and both rotaviruses and collagen (olive green) are indicated. In C, amino acids with mutations that affected collagen binding and variably affected rotavirus binding (Asp-219, Thr-221) are shown in pink, and Glu-256 where mutation variably affected rotavirus binding only is shown in purple. In all panels the MIDAS divalent cation is shown in red, the position of a bound model triple-helical collagen peptide is given in green, and the αC loop is shown in dark blue. This is adapted from the x-ray structure of activated α2I bound to triple-helical collagen peptide, Protein DataBase Code 1DZI (21).
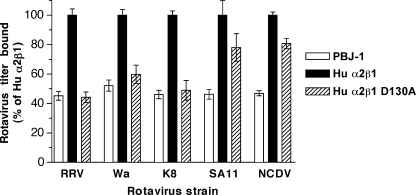
Mutation of β1 (D130A) Abolished α2β1 Binding by RRV and Human Rotaviruses, and Reduced Binding by SA11 and NCDV
The β1 integrin subunit contains an I-like or β I domain that is important for ligand binding (12). Residue Glu-309 is located near the C terminus of α2I and might interact with the β1 I domain MIDAS (49). As the E309A mutation in α2I eliminated rotavirus binding (Figs. 2 and 3), and the D130A mutation within the βI MIDAS of α2β1 abolishes collagen binding (50), the effect of D130A mutation on rotavirus binding was determined. In these experiments, rotavirus binding to CHO cells transfected to express human α2 and human β1 (Hu α2β1) was compared with binding to cells expressing human α2 and human β1 with a D130A mutation (Fig. 6). RRV and SA11 binding to α2β1 on CHO cells is increased by 22 and 27%, respectively, when both integrin subunits are of human origin (17). RRV, Wa, and K8 binding to human α2β1 was eliminated by this mutation, whereas SA11 and NCDV binding was partially reduced (Fig. 6). This differential effect on binding by these rotavirus strains paralleled the effect of T221A mutation and the human-to-mouse swapping mutation at 212–216 in α2I, suggesting a possible functional link between the effects of these mutations in α2I and the D130A mutation in β1.
FIGURE 6.
Effect of D130A mutation in the β1 subunit of human α2β1 on rotavirus binding to cell surface-expressed α2β1. Relative infectious titers of RRV, Wa, K8, SA11, and NCDV bound to CHO cells transfected with human α2β1 (Hu α2β1), or human α2β1 with the point mutation D130A in the β1 subunit (Hu α2β1 D130A) are given. The titer of RRV and SA11 bound to Hu α2β1 cells is increased by 22 and 27%, respectively, over that bound to wt α2 cells (17).
DISCUSSION
Recognition of the α2β1 integrin is part of the process of cell attachment and entry for many rotaviruses. It was found here that the α2 subunit I domain is necessary for α2β1 binding and α2β1-mediated infection by human and bovine rotaviruses. Amino acids in α2β1 that are necessary for binding by several human and animal rotaviruses were identified in our studies. In the α2 subunit I domain, these residues are located mainly in the MIDAS and surrounds, and include amino acids involved in metal ion co-ordination. The residues involved in rotavirus binding to α2β1 were shown to be distinct from those required by EV1, but overlapped with those used by type 1 collagen. This is consistent with the reported competition by type 1 collagen with rotaviruses for α2β1 binding (7). Human rotaviruses exhibited a greater similarity to collagen in the α2β1 residues necessary for binding than did monkey and bovine rotaviruses. For all these rotaviruses, replication mediated through α2β1 was substantially determined by the ability to bind α2β1.
We found that the α6 helix is important for rotavirus recognition, as several α6 mutations abolished binding by all rotavirus strains. This α6 involvement is a novel feature that distinguishes rotavirus binding from that of other α2I ligands, including collagens, EV1, and snake venom toxins jararhagin and EMS16 (20, 21, 34, 35, 44, 48, 51–54). The adjacent αC helix also was necessary for recognition by all rotavirus strains. This also contrasts with binding by type 1 collagen (34, 44, 48, 51), although deletion of amino acids 284–288 in αC weakens type 1 collagen binding (52). The protruding αC helix unwinds and relocates upon α2β1 activation, and is the principal insertion that distinguishes the α subunit I domains of collagen-binding integrins from those of leukocyte integrins (21, 52). It has been proposed that αC might act to inhibit nonspecific collagen recognition (21). The lack of impact of point mutations within αC on rotavirus binding suggests its overall conformation is more important for rotavirus binding than any virus recognition of particular αC residues. Alanine mutagenesis showed that Asp-309 in αF was required for binding by all rotaviruses. In contrast, D309A mutation does not affect collagen binding, cell spreading on collagen, α2β1 avidity for collagen, α2β1 aggregation or EV1 binding (25, 49). The αF site is contained within αC-α7 so is expected to undergo conformational change during activation (12, 21, 49, 55). Similarly, mutation of two residues in the α1 helix that moves inward during activation (56) also affected binding by rotavirus but not collagen or EV1. The importance of these activation-responsive residues in α1, αC-α6, and αF for rotavirus binding might relate to the rotavirus preference for activated α2β1 (30).
Rotavirus binding was affected by mutation of two (Ser-153, Thr-221) of the three residues that directly coordinate the MIDAS metal ion in α2I, one (Glu-256) of the three residues that make water-mediated bonds to the metal ion in α2I and Asp-130 that coordinates the cation in the β1 MIDAS (21, 50). It is likely that loss of virus binding to these mutants is due to the disruption of the metal binding, as for collagen (21). Mutation of these residues differentially affected binding by rotavirus strains, with the exception of S153A. This suggests that RRV and the human rotaviruses have a greater dependence on the α2 and β1 MIDAS than SA11 and NCDV. The rotavirus outer capsid (containing VP5*) and virus infectivity are lost when divalent cations are chelated (57–59) so it has not been feasible to directly evaluate the importance of metal ions for rotavirus recognition of α2β1. Our findings here indicate that a role for divalent cations cannot be ruled out.
As almost half of the α2I point mutations tested reduced rotavirus binding to α2β1, theoretically a substantial portion of the α2I surface could be involved. Most α2I residues where mutation affects EV1 or collagen binding have a direct binding role (20, 21, 34, 35, 44, 51). However, it seems less likely that most of the α2I residues where mutations inhibited rotavirus binding similarly form part of the rotavirus-α2I interface, given their distribution over about 20% of the α2I surface.
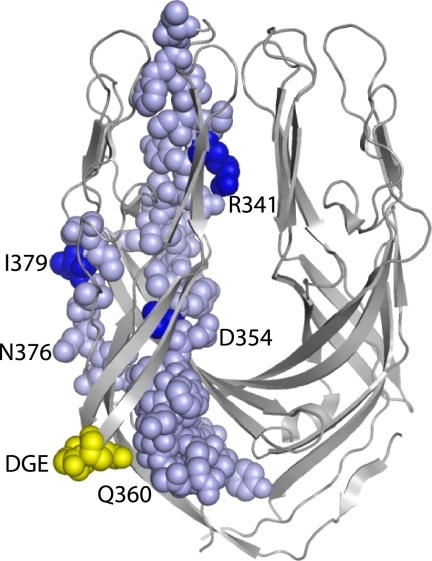
We found that that α2I binding by RRV, Wa, and K8 was strongly affected by several α2I point mutations, swapping of amino acids 212–216 to the mouse sequence and D130A mutation of β1, whereas these mutations had little effect on SA11 and NCDV binding. However, this functional division of these rotavirus strains does not reflect their overall VP4 sequence relatedness. Our VP4 gene segment sequences together with data from the National Center for Biotechnology Information5 show that RRV, SA11, and NCDV share >84% VP4 aa sequence identity, whereas RRV shares 69–71% identity with Wa and K8. As truncated RRV VP5* protein (amino acids 247–474) is sufficient for α2I protein binding (7), the relatedness of this VP5* sequence among these rotaviruses was determined. RRV VP5* amino acids 247–474 showed 82 and 83% identity with Wa and K8, and 86 and 87% with SA11 and NCDV, respectively. In contrast, a smaller region of RRV VP5* (amino acids 335–380) showed 93 and 91% identity with Wa and K8, respectively, and 84% with SA11 and NCDV. In the RRV VP5* structure that represents the protease-primed form, segments of the amino acids 335–380 region are surface-located and near the DGE sequence (Fig. 7). No structure of VP5* from any other rotavirus strain is available for comparison. These findings indicate that sequence variation in VP5* amino acids 335–380 parallels rotavirus strain-specific differences in the effects of α2I mutation on virus binding. It is proposed that VP5* amino acids 335–380 could influence rotavirus recognition of α2β1 in addition to the DGE site.
FIGURE 7.
Location of DGE sequence (yellow) and residues 335 to 380 (pale blue) in the truncated RRV dimeric VP5* structure considered to represent the protease-activated form. Residues differing between the RRV/Wa/K8 and SA11/NCDV rotavirus clusters are indicated in dark blue. The VP5* dimer is oriented with the hydrophobic loops required for cell membrane disruption at the top of the image. This is adapted from the x-ray structure of dimeric RRV VP5*, Protein DataBase Code 2B4H (63).
It is intriguing that RRV and human rotaviruses could not bind to α2β1 when α2I residues 212 to 216, or the entire α2I domain, were swapped to the mouse sequence. As SA11 and NCDV binding was unaffected by the small swapping mutation, this effect is not a general rotavirus property. Although our data could be interpreted as suggesting that Wa and RRV might not recognize mouse α2β1, indirect evidence favoring RRV recognition of mouse α2β1 on immortalized cholangiocytes has been obtained (11). We have been unable to determine if mouse α2β1 is a rotavirus receptor, as a mouse cell line that supports rotavirus replication, expresses α2β1 in our hands and is suitable for virus binding assays could not be identified despite extensive efforts. Alternatively, insertion of the mouse α2I domain or residues 212–216 might render the partnership of human α2 with hamster β1 as non-functional for binding particular rotaviruses. However, recognition of this heterologous α2β1 by antibody Ha1/29 indicated that it is expressed on the cell surface and retains a functional epitope. Also, insertion of human α2I regions into mouse α2 and association with hamster β1 does not impair EV1 binding (35).
In response to type 1 collagen, α2β1 induces cell signaling through the p38α mitogen-activated protein kinase pathway (60). On CHO cells expressing human α2, collagen- or antibody-induced α2β1 clustering produces transient p38 kinase activation 15 min later (25). This p38 activation can be considered as an indicator of the α2I Glu-336-dependent conformational change in α2β1 to the open form (25, 49). As RRV does not activate p38 in the first hour after infection when using α2β1 (61), it unlikely that rotavirus binding induces the open α2β1 conformation. Rotavirus activation of p38 and phosphatidylinositol 3-kinase (PI3K) later in the replicative cycle is independent of integrin recognition as it is also induced by integrin-independent rotavirus strains including CRW-8 (40, 61). PI3K is a possible upstream effector of the p38 signaling induced via collagen-stimulated α2β1 (60). These data provide evidence that rotavirus does not induce α2β1 activation despite preferentially binding to activated α2β1. Similarly to rotavirus, EV1 activation of p38 is unrelated to α2β1 binding and occurs at a post-entry stage (25). Consistent with this, EV1 induces α2β1 clustering but not conformational change, and preferentially recognizes inactive α2β1 (25, 62). The distinct mechanisms of α2β1 recognition by rotavirus and EV1 are shown through the differing α2I residues required for binding and opposite conformational preferences. Rotavirus is distinguished from collagen by α2I binding site requirements and the likely inability to activate α2β1 or p38.
Our studies have delineated regions of α2β1 that are involved in rotavirus recognition, including sites distinct from those used by other α2β1 ligands. These include the α1, αC-α6, and αF helices in α2I. A greater dependence on MIDAS residues of human over animal rotaviruses also was identified. Structural studies of complexes of α2I with VP5* or virions of human and animal rotaviruses are needed to provide a detailed understanding of how rotaviruses interface with α2β1. Overall, the information obtained in the present study indicates the feasibility of developing compounds that block rotavirus but not collagen binding to α2β1. This is important because blocking collagen binding to α2β1 is expected to affect wound healing and thrombosis. Such compounds could lead to an effective drug for rotavirus disease.
Acknowledgments
We thank Gavan Holloway for assistance with nucleotide sequencing. The generous gifts of monoclonal antibodies by Dale Godfrey and Fiona Watt are much appreciated.
This work was supported, in whole or in part, by National Institutes of Health Grant 1R01CA131015-01A2 (to Y. T.) and Grants 350252, 350253, 509006, and 628319 (to B. S. C.) from the National Health and Medical Research Council of Australia (NHMRC).
NCBI Reference Sequence: XM_001095246.1.
- α2I
- integrin α2 subunit-inserted domain
- EV1
- human echovirus 1
- MIDAS
- metal ion-dependent adhesion site
- PI3K
- phosphatidylinositol 3-kinase
- RLMFI
- relative linear median fluorescence intensity.
REFERENCES
- 1. Hynes R. O. (2002) Cell 110, 673–687 [DOI] [PubMed] [Google Scholar]
- 2. Stewart P. L., Nemerow G. R. (2007) Trends Microbiol. 15, 500–507 [DOI] [PubMed] [Google Scholar]
- 3. Triantafilou K., Takada Y., Triantafilou M. (2001) Crit. Rev. Immunol. 21, 311–322 [PubMed] [Google Scholar]
- 4. Greenberg H. B., Estes M. K. (2009) Gastroenterology 136, 1939–1951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ciarlet M., Crawford S. E., Cheng E., Blutt S. E., Rice D. A., Bergelson J. M., Estes M. K. (2002) J. Virol. 76, 1109–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Coulson B. S., Londrigan S. L., Lee D. J. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 5389–5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Graham K. L., Halasz P., Tan Y., Hewish M. J., Takada Y., Mackow E. R., Robinson M. K., Coulson B. S. (2003) J. Virol. 77, 9969–9978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hewish M. J., Takada Y., Coulson B. S. (2000) J. Virol. 74, 228–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zárate S., Espinosa R., Romero P., Guerrero C. A., Arias C. F., López S. (2000) Virology 278, 50–54 [DOI] [PubMed] [Google Scholar]
- 10. Seo N. S., Zeng C. Q., Hyser J. M., Utama B., Crawford S. E., Kim K. J., Höök M., Estes M. K. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 8811–8818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jafri M., Donnelly B., Allen S., Bondoc A., McNeal M., Rennert P. D., Weinreb P. H., Ward R., Tiao G. (2008) Am. J. Physiol. Gastrointest. Liver Physiol. 295, G16–G26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Luo B. H., Carman C. V., Springer T. A. (2007) Annu. Rev. Immunol. 25, 619–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Blanchard H., Yu X., Coulson B. S., von Itzstein M. (2007) J. Mol. Biol. 367, 1215–1226 [DOI] [PubMed] [Google Scholar]
- 14. Graham K. L., Fleming F. E., Halasz P., Hewish M. J., Nagesha H. S., Holmes I. H., Takada Y., Coulson B. S. (2005) J. Gen. Virol. 86, 3397–3408 [DOI] [PubMed] [Google Scholar]
- 15. Guerrero C. A., Méndez E., Zárate S., Isa P., López S., Arias C. F. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 14644–14649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Haselhorst T., Fleming F. E., Dyason J. C., Hartnell R. D., Yu X., Holloway G., Santegoets K., Kiefel M. J., Blanchard H., Coulson B. S., von Itzstein M. (2009) Nat. Chem. Biol. 5, 91–93 [DOI] [PubMed] [Google Scholar]
- 17. Londrigan S. L., Graham K. L., Takada Y., Halasz P., Coulson B. S. (2003) J. Virol. 77, 9486–9501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Coulson B. S. (1997) in Leukocyte Typing VI (Kishimoto T., Kikutani H., von dem Borne A. E. G., Kr., et al. ed) Garland Publishing, Inc., New York [Google Scholar]
- 19. Bergelson J. M., St John N. F., Kawaguchi S., Pasqualini R., Berdichevsky F., Hemler M. E., Finberg R. W. (1994) Cell Adhes. Commun. 2, 455–464 [DOI] [PubMed] [Google Scholar]
- 20. Xing L., Huhtala M., Pietiäinen V., Käpylä J., Vuorinen K., Marjomäki V., Heino J., Johnson M. S., Hyypiä T., Cheng R. H. (2004) J. Biol. Chem. 279, 11632–11638 [DOI] [PubMed] [Google Scholar]
- 21. Emsley J., Knight C. G., Farndale R. W., Barnes M. J., Liddington R. C. (2000) Cell 101, 47–56 [DOI] [PubMed] [Google Scholar]
- 22. Jin M., Andricioaei I., Springer T. A. (2004) Structure 12, 2137–2147 [DOI] [PubMed] [Google Scholar]
- 23. Aquilina A., Korda M., Bergelson J. M., Humphries M. J., Farndale R. W., Tuckwell D. (2002) Eur. J. Biochem. 269, 1136–1144 [DOI] [PubMed] [Google Scholar]
- 24. Tulla M., Pentikäinen O. T., Viitasalo T., Käpylä J., Impola U., Nykvist P., Nissinen L., Johnson M. S., Heino J. (2001) J. Biol. Chem. 276, 48206–48212 [DOI] [PubMed] [Google Scholar]
- 25. Jokinen J., White D. J., Salmela M., Huhtala M., Kapyla J., Sipila K., Puranen J. S., Nissinen L., Kankaanpaa P., Marjomaki V., Hyypia T., Johnson M. S., Heino J. (2010) EMBO J. 29, 196–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Coulson B. S. (1993) J. Clin. Microbiol. 31, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ludert J. E., Feng N., Yu J. H., Broome R. L., Hoshino Y., Greenberg H. B. (1996) J. Virol. 70, 487–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bass D. M., Mackow E. R., Greenberg H. B. (1991) Virology 183, 602–610 [DOI] [PubMed] [Google Scholar]
- 29. Kirkwood C. D., Bishop R. F., Coulson B. S. (1998) J. Virol. 72, 9348–9352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Graham K. L., Takada Y., Coulson B. S. (2006) J. Gen. Virol. 87, 1275–1283 [DOI] [PubMed] [Google Scholar]
- 31. Graham K. L., Zeng W., Takada Y., Jackson D. C., Coulson B. S. (2004) J. Virol. 78, 11786–11797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Trask S. D., Kim I. S., Harrison S. C., Dormitzer P. R. J. Virol. 84, 1764–1770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dormitzer P. R., Nason E. B., Prasad B. V., Harrison S. C. (2004) Nature 430, 1053–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kamata T., Liddington R. C., Takada Y. (1999) J. Biol. Chem. 274, 32108–32111 [DOI] [PubMed] [Google Scholar]
- 35. King S. L., Kamata T., Cunningham J. A., Emsley J., Liddington R. C., Takada Y., Bergelson J. M. (1997) J. Biol. Chem. 272, 28518–28522 [DOI] [PubMed] [Google Scholar]
- 36. Takada Y., Hemler M. E. (1989) J. Cell Biol. 109, 397–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gamble J. R., Matthias L. J., Meyer G., Kaur P., Russ G., Faull R., Berndt M. C., Vadas M. A. (1993) J. Cell Biol. 121, 931–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. O'Connell P. J., Faull R., Russ G. R., D'Apice A. J. (1991) Immunol. Cell Biol. 69, 103–110 [DOI] [PubMed] [Google Scholar]
- 39. Tenchini M. L., Adams J. C., Gilberty C., Steel J., Hudson D. L., Malcovati M., Watt F. M. (1993) Cell Adhes. Commun. 1, 55–66 [DOI] [PubMed] [Google Scholar]
- 40. Halasz P., Holloway G., Turner S. J., Coulson B. S. (2008) J. Virol. 82, 148–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pellicci D. G., Hammond K. J., Coquet J., Kyparissoudis K., Brooks A. G., Kedzierska K., Keating R., Turner S., Berzins S., Smyth M. J., Godfrey D. I. (2005) J. Immunol. 175, 4416–4425 [DOI] [PubMed] [Google Scholar]
- 42. Coulson B. S., Kirkwood C. (1991) J. Virol. 65, 5968–5974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kaljot K. T., Shaw R. D., Rubin D. H., Greenberg H. B. (1988) J. Virol. 62, 1136–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kamata T., Takada Y. (1994) J. Biol. Chem. 269, 26006–26010 [PubMed] [Google Scholar]
- 45. Edelman J. M., Chan B. M., Uniyal S., Onodera H., Wang D. Z., St John N. F., Damjanovich L., Latzer D. B., Finberg R. W., Bergelson J. M. (1994) Cell Adhes. Commun. 2, 131–143 [DOI] [PubMed] [Google Scholar]
- 46. Tuckwell D. S., Smith L., Korda M., Askari J. A., Santoso S., Barnes M. J., Farndale R. W., Humphries M. J. (2000) Biochem. J. 350, 485–493 [PMC free article] [PubMed] [Google Scholar]
- 47. Vuoriluoto K., Jokinen J., Kallio K., Salmivirta M., Heino J., Ivaska J. (2008) Exp. Cell Res. 314, 3369–3381 [DOI] [PubMed] [Google Scholar]
- 48. Dickeson S. K., Mathis N. L., Rahman M., Bergelson J. M., Santoro S. A. (1999) J. Biol. Chem. 274, 32182–32191 [DOI] [PubMed] [Google Scholar]
- 49. Connors W. L., Jokinen J., White D. J., Puranen J. S., Kankaanpää P., Upla P., Tulla M., Johnson M. S., Heino J. (2007) J. Biol. Chem. 282, 14675–14683 [DOI] [PubMed] [Google Scholar]
- 50. Valdramidou D., Humphries M. J., Mould A. P. (2008) J. Biol. Chem. 283, 32704–32714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kamata T., Puzon W., Takada Y. (1994) J. Biol. Chem. 269, 9659–9663 [PubMed] [Google Scholar]
- 52. Käpylä J., Ivaska J., Riikonen R., Nykvist P., Pentikäinen O., Johnson M., Heino J. (2000) J. Biol. Chem. 275, 3348–3354 [DOI] [PubMed] [Google Scholar]
- 53. Lambert L. J., Bobkov A. A., Smith J. W., Marassi F. M. (2008) J. Biol. Chem. 283, 16665–16672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Horii K., Okuda D., Morita T., Mizuno H. (2004) J. Mol. Biol. 341, 519–527 [DOI] [PubMed] [Google Scholar]
- 55. Lee J. O., Bankston L. A., Arnaout M. A., Liddington R. C. (1995) Structure 3, 1333–1340 [DOI] [PubMed] [Google Scholar]
- 56. Shimaoka M., Xiao T., Liu J. H., Yang Y., Dong Y., Jun C. D., McCormack A., Zhang R., Joachimiak A., Takagi J., Wang J. H., Springer T. A. (2003) Cell 112, 99–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Prasad B. V., Wang G. J., Clerx J. P., Chiu W. (1988) J. Mol. Biol. 199, 269–275 [DOI] [PubMed] [Google Scholar]
- 58. Yeager M., Dryden K. A., Olson N. H., Greenberg H. B., Baker T. S. (1990) J. Cell Biol. 110, 2133–2144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cohen J., Laporte J., Charpilienne A., Scherrer R. (1979) Arch. Virol. 60, 177–186 [DOI] [PubMed] [Google Scholar]
- 60. Ivaska J., Reunanen H., Westermarck J., Koivisto L., Kähäri V. M., Heino J. (1999) J. Cell Biol. 147, 401–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Holloway G., Coulson B. S. (2006) J. Virol. 80, 10624–10633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bergelson J. M., Chan B. M., Finberg R. W., Hemler M. E. (1993) J. Clin. Invest. 92, 232–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yoder J. D., Dormitzer P. R. (2006) EMBO J. 25, 1559–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]