Abstract
DokA, a homolog of bacterial hybrid histidine kinases, is essential for hyperosmotic stress resistance in Dictyostelium. We show that a transient intracellular cAMP signal, dependent on the presence of DokA, is generated in response to an osmotic shock. This variation of cAMP levels contributes to survival under hypertonic conditions. In contrast to the low cAMP levels observed in dokA– strains, overexpression of the receiver domain of DokA causes an increase in cAMP levels, resulting in a rapidly developing phenotype. We present biochemical and cell biological data indicating that the DokA receiver domain is a dominant-negative regulator of a phosphorelay, which controls the intracellular cAMP phosphodiesterase RegA. The activity of the DokA receiver domain depends on a conserved aspartate, mutation of which reverses the developmental phenotype, as well as the deregulation of cAMP metabolism.
Keywords: cAMP/osmotic stress/phosphatases/phosphorelay/signal transduction
Introduction
The second messenger cAMP induces a variety of physiological responses in eukaryotic and prokaryotic cells (Robison et al., 1968; Tang and Gilman, 1992). In the amoeba Dictyostelium discoideum, cAMP acts as a morphoregulatory signal, which controls chemotaxis, gene expression and cell differentiation during development (Parent and Devreotes, 1996; van Haastert, 1997; Verkerke-van Wijk and Schaap, 1997). The synthesis and degradation of cAMP are regulated precisely: during aggregation, cAMP is formed by the adenylyl cyclase ACA (van Haastert, 1997), whereas another adenylyl cyclase, ACG, acts as an osmosensor controlling germination in Dictyostelium spores (van Es et al., 1996). More recently, a novel adenylyl cyclase activity has been detected in cell lysates of rapid developing mutants of Dictyostelium (Kim et al., 1998). It is probably attributed to AcrA, a cyclase expressed throughout development with pivotal function during culmination (Soderbom et al., 1999). Extracellular cAMP, which serves as a chemoattractant during aggregation, is hydrolyzed by a phosphodiesterase (PDE) that is either secreted or anchored to the extracellular face of the plasma membrane (Malchow et al., 1972; Gerisch, 1976). In addition, the intracellular phosphodiesterase RegA has also been described (Shaulsky et al., 1996). In addition to its morphogenic function, cAMP also acts as an intracellular second messenger activating protein kinase A (PKA), which plays an important role in gene expression and cell differentiation during Dictyostelium development (Verkerke-van Wijk and Schaap, 1997 and references therein). It was shown that mutations causing elevated levels of intracellular cAMP or a constitutively active PKA lead to an accelerated development (Coukell and Chan, 1980; Abe and Yanagisawa, 1983; Simon et al., 1992). In contrast to an increased activity of PKA, the prespore-specific inhibition of this cAMP-regulated kinase results in spore heads with a translucent appearance (Hopper et al., 1993), a phenotype resembling Dictyostelium cells deficient in dokA, a gene encoding a hybrid histidine kinase homolog (Schuster et al., 1996). Besides this developmental aberration, dokA– cells were shown to be sensitive to hypertonic stress, indicating that DokA is part of the osmotic response system of Dictyostelium.
Hybrid histidine kinases are proteins that comprise the two central modules of a signal transduction circuitry termed a two-component system. This system acts by transducing information via phosphotransfer reactions (Parkinson, 1993; Stock et al., 1995): a histidine kinase (HK) autophosphorylates on a conserved histidine and subsequently transfers the phosphoryl group to a conserved aspartate on a receiver (RR). Two-component systems are ubiquitous in bacteria and regulate a variety of responses to environmental stimuli. Recently, histidine kinase homologs have also been identified in several eukaryotes including plants, fungi and amoebae (Chang et al., 1993; Ota and Varshavsky, 1993; Alex et al., 1996; Kakimoto, 1996; Schuster et al., 1996; Wang et al., 1996; Zinda and Singleton, 1998). The best characterized eukaryotic two-component system is the yeast Sln1–Ypd1–Ssk1 phosphorelay (Wurgler-Murphy and Saito, 1997). In this more complex version of the classical two-component system, the phosphoryl group is transferred from the conserved histidine (H1) of the kinase Sln1 onto an aspartate (D1) of a receiver domain at the C-terminus of the protein and then via a histidine residue (H2) of the phosphotransfer protein Ypd1 to the conserved aspartate (D2) of a second receiver (Ssk1). Because a phosphorelay offers no signal amplification beyond the initial autophosphorylation of H1, it has been speculated that the components of this pathway provide potential for multiple regulatory checkpoints or the integration of signals that are transduced by different pathways (Hoch, 1995; Appleby et al., 1996).
The yeast Sln1–Ypd1–Ssk1 phosphorelay activates the synthesis of glycerol via a MAP kinase cascade, allowing the cells to adapt to a hyperosmotic environment (Wurgler-Murphy and Saito, 1997). In other microorganisms, two-component systems also play a decisive role in controlling cellular responses in order to adapt to hypertonicity (Burg et al., 1996). These responses include ion transport, accumulation of organic osmolytes and rearrangement of the cytoskeleton (Baumgarten and Feher, 1995; Zischka et al., 1999), e.g. in Escherichia coli, the expression of two porin proteins, OmpF and OmpC, is regulated by the EnvZ–OmpR two-component system (Pratt and Silhavy, 1995).
In Dictyostelium, histidine kinase homologs are involved in osmoregulation, terminal differentiation and spore germination (Schuster et al., 1996; Wang et al., 1996; Singleton et al., 1998; Zinda and Singleton, 1998). Besides the histidine kinase homologs, a histidine phosphotransfer protein (HPt) and a receiver have been discovered in Dictyostelium that seem to be part of a multistep phosphorelay similar to the systems in bacteria and yeast (Shaulsky et al., 1996; Chang et al., 1998; Thomason et al., 1998). The RdeA protein displays a weak homology to Ypd1 and bacterial HPt modules, and the phenotype of rdeA– mutants can be complemented by expression of yeast Ypd1 (Chang et al., 1998). The corresponding receiver RegA consists of an N-terminal receiver domain and a C-terminal phosphodiesterase domain. The C-terminal domain is homologous to mammalian cyclic nucleotide phosphodiesterases and is activated by phosphorylation of the receiver (Thomason et al., 1998). regA– cells resemble previously isolated rdeA mutants in showing accelerated development and aberrant fruiting body formation (Sonneborn et al., 1963; Kessin, 1977; Shaulsky et al., 1998). Furthermore, rdeA– cells have elevated intracellular cAMP levels, which is consistent with a reduced phosphodiesterase activity (Coukell and Chan, 1980; Abe and Yanagisawa, 1983). Because of the similarities in the phenotype of regA– and rdeA– cells, it has been proposed that RdeA phosphorylates RegA in a phosphorelay (Chang et al., 1998; Thomason et al., 1998), as was shown by phosphotransfer in vitro (Thomason et al., 1999).
Here we show that hyperosmotic stress causes a rapid increase in intracellular cAMP concentration, which depends on the presence of the dokA gene. Furthermore, we present evidence that DokA is a negative regulator of the RdeA–RegA pathway by acting as a phosphatase towards the HPt protein RdeA.
Results
Osmotic shock causes a transient intracellular cAMP signal that depends on DokA
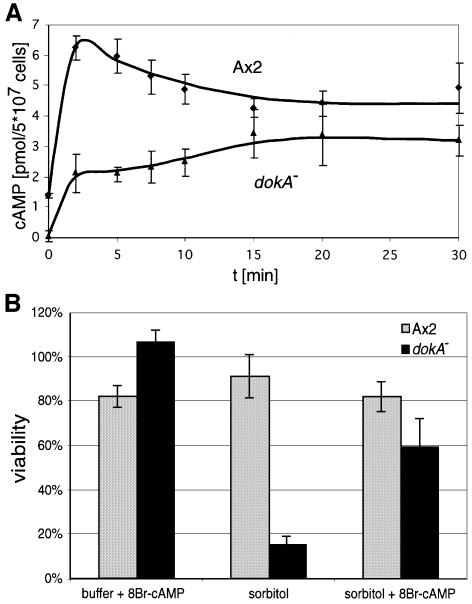
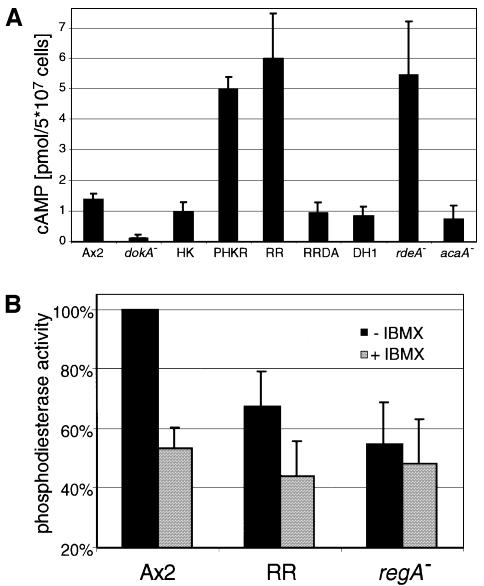
cAMP is the central messenger that regulates aggregation and terminal diffentiation during Dictyostelium development (Verkerke-van Wijk and Schaap, 1997). We have investigated whether the level of intracellular cAMP is also affected by hyperosmotic stress, as mutants in dokA show an osmotic as well as a developmental phenotype (Schuster et al., 1996). Axenically grown wild-type cells of the strain Ax2 were suspended in Soerensen phosphate buffer (SPB; 34 mOsm) for 1 h to synchronize the cells before sorbitol was added to a final concentration of 400 mM (corresponding to 430 mOsm). The amount of cAMP in cell extracts was determined before and after the osmotic shock (Figure 1A). During the incubation in SPB, only low amounts of intracellular cAMP (1.4 pmol/5 × 107 cells) were measured (Figure 1A; t = 0). When sorbitol was added, the amount of intracellular cAMP increased rapidly, reaching a peak after 2 min at 6.5 pmol cAMP/5 × 107 cells, and subsequently decreased to a lower level.
Fig. 1. (A) Concentration of intracellular cAMP in D.discoideum cells in response to osmotic stress. Cells were shaken in SPB and shocked osmotically with 400 mM sorbitol. Ax2 cells (diamonds) accumulate cAMP transiently when exposed to high osmolarity, whereas dokA– cells (triangles) show a smaller increase without a maximum after the shock. Means and standard deviations of three independent experiments are shown. (B) Viability assay of osmotically shocked Ax2 and dokA– cells. Cells were incubated for 10 min in 400 mM sorbitol buffer containing 5 mM 8-Br-cAMP as indicated and for 110 min without 8-Br-cAMP. Cells incubated constantly in SPB correspond to 100%. Means and standard deviations of three (Ax2) and four (dokA–) independent experiments are shown. The simulation of a cAMP peak at the onset of the shock increases the viability rate of dokA– cells ∼4-fold.
In contrast to the wild-type strain, the osmosensitive dokA– cells did not show a peak in intracellular cAMP concentration upon hyperosmotic shock. dokA– cells accumulated only ∼30% of the amount of wild-type cAMP after 2 min in hypertonic buffer. No intracellular cAMP could be measured before the shock, but its content steadily increased during the first 15 min of hyperosmotic stress (Figure 1A). In a control experiment, acaA– cells showed a progression of intracellular cAMP concentration similar to Ax2 cells, with only slightly reduced values (data not shown), indicating that this hyperosmotic shock-dependent cAMP response is not regulated via ACA activity.
A pulse of 8-bromo-cAMP rescues the osmotic phenotype of dokA– cells
dokA– cells have been described to be osmosensitive under hyperosmotic conditions, with a mortality rate reaching 90% after 2 h (Schuster et al., 1996). To investigate whether the difference in cAMP levels between wild-type and dokA– cells is physiologically relevant for survival under hyperosmotic stress, 8-bromo-cAMP (8-Br-cAMP) was added during the shock. 8-Br-cAMP is a membrane-permeable cAMP analog used to raise intracellular cAMP levels in Dictyostelium (Kay et al., 1988). The constant presence of 5–20 mM 8-Br-cAMP during the shock did not increase the viability of dokA– cells (data not shown). To mimic an intracellular cAMP peak within the first minutes of the shock, as observed in Ax2 cells, 5 mM 8-Br-cAMP was added together with the sorbitol, but was removed after 10 min. The cells remained in high tonicity buffer without 8-Br-cAMP for the rest of the experiment. This procedure at the onset of the shock led to an increase in the viability of dokA– cells to ∼60% after 2 h in 400 mM sorbitol (Figure 1B). In the control experiment without addition of 8-Br-cAMP, <15% of the dokA– cells survived. Identically treated wild-type cells showed no significant change in viability.
Overexpression of DokA fragments affects development
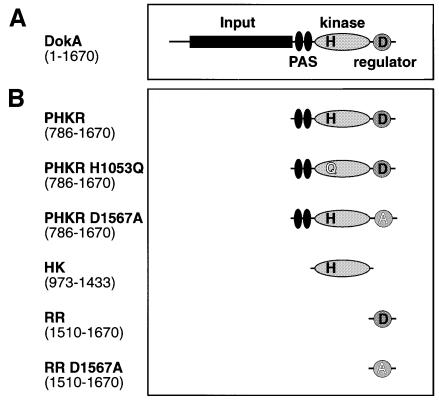
dokA codes for a homolog of a hybrid histidine kinase (Figure 2A) consisting of an N-terminal input, two PAS domains, a kinase domain including the proposed site of histidine phosphorylation (H1053) and a C-terminal receiver domain with the site of aspartyl phosphorylation (D1567). Previous analysis of two-component systems showed that the individual domains can be expressed separately, thereby maintaining their biochemical function (Swanson et al., 1993; Posas et al., 1996). Furthermore, the C-terminal domain of DokA was shown to act as a receiver, as deduced from its reaction with acetyl phosphate (Schuster et al., 1996), which specifically phosphorylates receiver proteins (Lukat et al., 1992). In order to investigate the effect of individual domains of DokA in more detail, we expressed the domains (Figure 2B) in Ax2 cells under control of the constitutively active actin 15 promoter using the plasmid pDEX-RH (Faix et al., 1992). The proteins were expressed in a soluble form under these conditions.
Fig. 2. Construction of cell lines overexpressing DokA fragments. (A) Domain structure of DokA as predicted from sequence analysis. (B) Three truncated forms of DokA (PHKR, HK and RR) were overexpressed in D.discoideum Ax2 cells under the control of the actin 15 promoter using the plasmid pDEX-RH. ‘H’ represents the conserved histidine 1053, ‘D’ represents the conserved aspartate 1567. Mutant forms of DokA fragments were expressed as indicated.
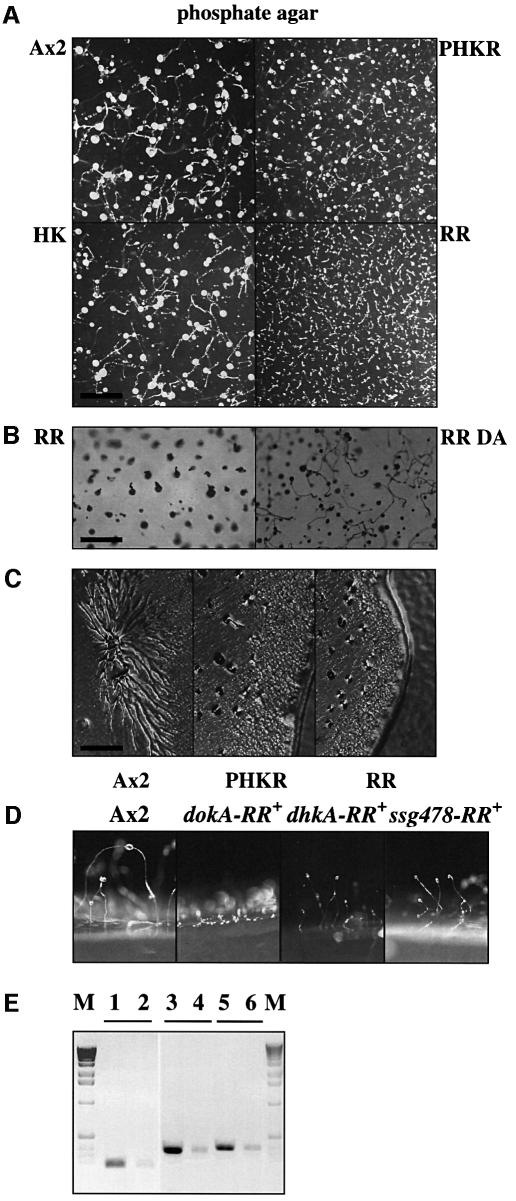
We have examined the effect of overexpression of the DokA fragments on development by spreading Dictyostelium cells carrying the various constructs on phosphate and low-nutrient agar. Cells that overexpress the kinase domain HK developed as wild-type cells, whereas expression of the receiver RR led to a severely altered morphology (Figure 3A and B). Investigating the development by time-lapsed video microscopy, we were able to show that overexpression of RR results in a rapidly developing phenotype with aggregation starting at 4–5 h of starvation. The control cell line Ax2 reached a comparable aggregation state at 7 h. Only small aggregates were formed in the case of the RR overexpression strain without the appearance of distinct streams (Figure 3C). After 10 h, a tip or a small stalk appeared on top of the mound without formation of a spore head. Figure 3B shows the final state of development after 36 h.
Fig. 3. Developmental phenotype of cells overexpressing DokA fragments. (A) Cells were developed on non-nutrient agar at a density of ∼1.5 × 106 cells/cm2. Photographs were taken after 36 h. Wild-type and HK cells develop normally; in contrast, RR-expressing cells form small aggregates and aberrant fruiting bodies. PHKR cells show only a weak developmental phenotype. The bar equals 3 mm. (B) Cells overexpressing the dominant-negative RR and its mutant form RR D1567A were developed as in (A) at 1 × 106 cells/cm2. Photographs were taken after 36 h. Mutation of D1567 reverses the developmental phenotype observed. The bar equals 1.5 mm. (C) Cells were grown on low-nutrient agar together with E.coli B/2. Photographs were taken after 3 days. Ax2 cells form streams during aggregation, PHKR- and RR-overexpressing cell lines do not. The bar equals 0.5 mm. (D) Wild-type cells and cells overexpressing the receiver domains of DokA, DhkA and Ssg478 were developed as in (A). RR cells (dokA-RR+) remain in a mound-like stage, while dhkA-RR+ and ssg478-RR+ cells form normal fruiting bodies slightly smaller than Ax2 cells. (E) Axenically growing wild-type cells and cells expressing the receiver domains of DokA, DhkA and Ssg478 were probed by RT–PCR to demonstrate overexpression in all three strains. M, marker; lanes 1 and 2, dokA-RR+ (RR) and Ax2 cells probed with dokA-RR primer; lanes 3 and 4, dhkA-RR+ and Ax2 cells probed with dhkA-RR primer; lanes 5 and 6, ssg478-RR+ and Ax2 cells probed with ssg478-RR primer.
The rapidly developing phenotype of cells overexpressing RR was strictly dependent on the presence of the conserved residue D1567, which is essential for receiver function, as cells that express the mutant receiver RR D1567A showed normal aggregation and formed normal fruiting bodies (Figure 3B). Western analysis showed no difference in the expression levels of RR and RR D1567A in lysates of both overexpression strains (data not shown). Cells that overexpress the DokA fragment PHKR (consisting of PAS, kinase and receiver domains) exhibited a weaker developmental phenotype. Like cells overexpressing the receiver domain of DokA (RR cells), PHKR-expressing cells developed rapidly, forming small aggregates without streaming (Figure 3C). However, culmination and fruiting body formation were not affected in these cells, which distinguishes them from RR cells (Figure 3A, Table I). It should be noted that rapid aggregation of PHKR cells strictly depends on the presence of the receiver domain with the conserved D1567, as cells expressing the mutant form PHKR D1567A aggregated normally.
Table I. Developmental aberrations of D.discoideum strains.
| Strain | Premature aggregation on phosphate agar | Aberrant fruiting body | Aggregation on cAMP-S agar |
|---|---|---|---|
| Ax2 (wild type) | – | – | – |
| dokA– | – | – | – |
| RR | + | + | + |
| RR D1567A | – | – | – |
| PHKR | + | – | + |
| PHKR H1053Q | + | – | + |
| PHKR D1567A | – | – | – |
| HK | – | – | – |
| regA– | + | + | + |
| WTC10 (DH1:rdeA) | + | + | + |
| FR17 (NC4:rdeA) | + | + | + |
The developmental phenotype of RR cells is shared by rdeA– and regA– mutants
The described developmental phenotype of Dictyostelium cells overexpressing RR resembled that found in rdeA– (Kessin, 1977; Chang et al., 1998) and regA– cells (Thomason et al., 1998). The rdeA gene codes for an HPt protein that acts upstream of the cAMP phosphodiesterase RegA in a two-component phosphorelay system. These rapidly developing or ‘fruity’ (Sonneborn et al., 1963) mutants can be surveyed by either a sporogeneity (Kay, 1982) or a cAMP-S assay (Rossier et al., 1978).
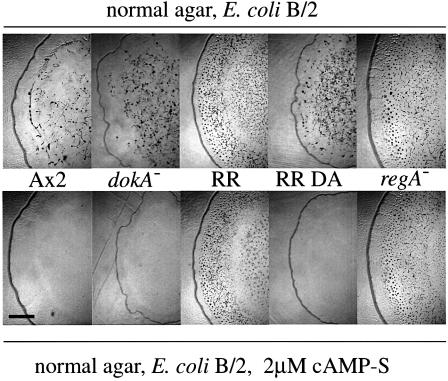
Using the method described by Rossier et al. (1978), we have examined the aggregation of Dictyostelium strains on low-nutrient agar containing 2 µM cAMP-S. cAMP-S is a slowly hydrolyzing analog of cAMP that acts as an agonist for chemotaxis and as an inducer of cAMP pulses. As described, aggregation of wild-type Ax2 cells was completely inhibited on cAMP-S agar, as the cells are not able to detect extracellular cAMP pulses under these conditions (Figure 4; Rossier et al., 1978). In contrast, the isolated mutant FR17 (NC4:rdeA) was shown to be cAMP-S resistant, i.e. able to aggregate and to form minute fruiting bodies (Rossier et al., 1980).
Fig. 4. Development of D.discoideum strains on cAMP-S agar. Cells overexpressing DokA fragments (RR, RR D1567A), null mutants (dokA–, regA–) and Ax2 cells were grown on low-nutrient agar with E.coli B/2. Agar plates were used with or without 2 µM cAMP-S. RR and regA– cells aggregate in the presence of cAMP-S, whereas aggregation of the other strains is completely blocked under these conditions. Photographs were taken after 3 days. The bar equals 2.6 mm.
The aggregates and sporadically forming fruiting bodies on cAMP-S agar of regA– mutants and of cells overexpressing DokA RR were indistinguishable from those formed on agar without cAMP-S (Figure 4). cAMP-S resistance of RR cells was dependent, as was their rapidly developing phenotype, on the presence of the conserved D1567, as cells overexpressing the mutant form RR D1567A were cAMP-S sensitive. As expected, deletion of the dokA gene and overexpression of the kinase domain HK or its mutant form HK H1053Q did not cause cAMP-S resistance (Figure 4; Table I). Cells overexpressing PHKR were also cAMP-S resistant despite their weak developmental phenotype on phosphate agar. In order to examine the role of the conserved amino acids H1053 and D1567, we tested cells overexpressing the mutant forms PHKR H1053Q and PHKR D1567A for cAMP-S resistance. The PHKR D1567A mutant was, like the RR D1567A mutant, cAMP-S sensitive, whereas PHKR H1053Q cells aggregated normally on agar containing cAMP-S (Table I).
In addition, a developmental assay was performed that tests for the cell’s ability to form spores when incubated as submerged monolayers in buffer containing cAMP. It was demonstrated that rdeA– and regA– mutants are sporogenous (Kay, 1989; Thomason et al., 1998). As expected, cells overexpressing the receiver RR were sporogenous as well (data not shown).
As overexpression of a signaling molecule, such as the DokA receiver domain, may be an unphysiological situation, we checked whether these results can be attributed to a specific interaction or whether they are generally caused by overexpression of comparable proteins. Therefore, other receiver domains of Dictyostelium proteins were overexpressed in the Ax2 background. Although the expression level of the receiver domains of DhkA (amino acids L2018–N2150) and Ssg478 (Q1–S148 of the sequenced receiver domain) in dhkA-RR+ and ssg478-RR+ strains, respectively, is increased substantially, as demonstrated by RT–PCR, these strains did not exhibit a rapidly developing phenotype (Figure 3D and E). The effect of DokA RR on the RdeA–RegA phosphorelay system is therefore not due to overexpression of an arbitrary receiver.
Overexpression of the DokA receiver domain causes elevated levels of cAMP
The ‘fruity’ phenotype was attributed to aberrations in PKA activity and cAMP metabolism (Anjard et al., 1992; Thomason et al., 1998). Therefore, we investigated the intracellular cAMP levels of dokA-overexpressing mutants shaken in SPB for 1 h. While in Ax2 cells ∼1.4 pmol of cAMP/5 × 107 cells were measured, the dokA– strain yielded no measurable amount (Figure 5A). Strains overexpressing the kinase domain HK showed wild-type levels. In contrast to this, strains carrying the overexpression construct for RR exhibited cAMP concentrations of ∼6 pmol/5 × 107 cells, thereby reaching levels comparable to those of the strain WTC10, an rdeA– mutant of DH1 (Chang et al., 1998). This elevated cAMP level was not observed in cells overexpressing the mutant form RR D1567A. Elevated cAMP concentrations were also measured in cells overexpressing PHKR, affirming again that an intact receiver domain is essential for the regulatory effect of DokA. acaA– cells showed concentrations almost as high as those of DH1 and Ax2, indicating that this cyclase plays no substantial role at 1 h of starvation.
Fig. 5. (A) Concentration of intracellular cAMP in D.discoideum cells. Cells were synchronized by shaking for 1 h in SPB. (B) Phosphodiesterase activity in lysates of Ax2, RR and regA– cells. Cells overproducing RR show a lower activity compared with wild-type cells. The addition of 0.4 mM IBMX, an inhibitor of RegA, reduces cAMP degradation in all strains to a level similar to the decay measured in regA– lysates. cAMP degradation in Ax2 cell lysates was set as 100%. Means and standard deviations of at least three independent experiments are shown.
RR cells show a reduced intracellular phosphodiesterase activity
To test whether the increased cAMP level in RR cells was due to reduced intracellular phosphodiesterase activity, we examined the degradation of cAMP in lysates of Ax2 and RR cells that were shaken in SPB for 3 h. Dithiothreitol (DTT; 20 mM) was added to the cytosolic fraction of the lysed cells in order to inhibit traces of extracellular PDE. Assuming a constant concentration of phosphodiesterase and consequently pseudo-first order kinetics, a reduction of enzyme activity in RR cells to <70% compared with wild-type cells was observed (Figure 5B). In control experiments, lysates of regA– cells were analyzed and all lysates were incubated with IBMX, a substance reported to inhibit the RegA phosphodiesterase activity (Kim et al., 1998). In these lysates, a decrease in the amount of cAMP corresponding to ∼50% of the wild-type degradation rate was observable, regardless of the examined strain. This might be attributed to incomplete inhibition of the extracellular PDE.
Osmotic stress elevates total cAMP
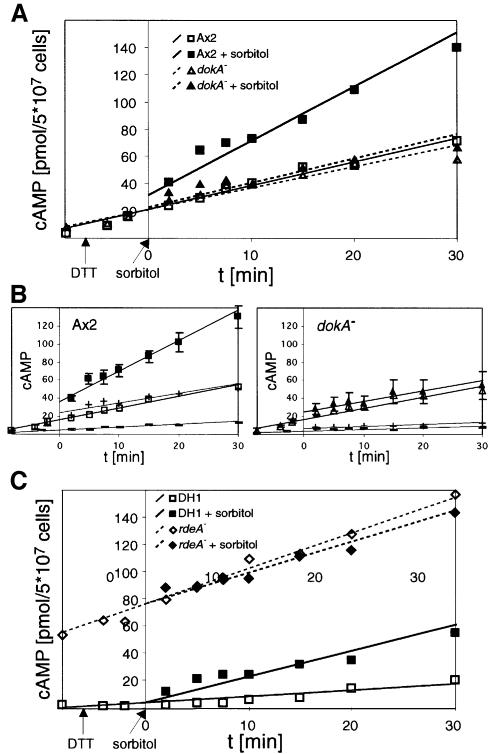
The rise of intracellular cAMP concentration in wild-type cells in response to hyperosmotic stress (Figure 1A) was also paralleled by an increase in the total cAMP concentration, i.e. intra- and extracellular cAMP. To analyze the amount of secreted cAMP, the extracellular PDE was inhibited by DTT (Green and Newell, 1975). As extracellular cAMP triggers ACA activity via the G-protein-coupled cAR receptor and thereby stimulates cAMP production, the addition of DTT caused a constant rise in the monitored total cAMP concentration, which was observed in both wild-type and dokA– cell suspensions (Figure 6A). However, only in the case of Ax2 could an additional increase in the amount of cAMP be observed in response to 400 mM sorbitol. In contrast, the slope of the cAMP curve of dokA– cells remained unaffected under hypertonic conditions, indicating a DokA-dependent net production of cAMP in response to high tonicity.
Fig. 6. (A) Concentration of total cAMP in D.discoideum cells in response to hyperosmotic stress. Ax2 cells show a markedly stronger increase in cAMP when exposed to high tonicity (filled squares) compared with cells in SPB (open squares), whereas the addition of sorbitol has no influence on total cAMP levels in dokA– cells (filled triangles in sorbitol buffer, open triangles in SPB). (B) Total cAMP levels of Ax2 and dokA– cells in SPB (open symbols) and sorbitol buffer (closed symbols) and the fraction of intracellular cAMP (–, in SPB; +, in sorbitol buffer), respectively. The amounts of cAMP in the buffer and in the cell pellets were determined in parallel and normalized on the total cAMP concentration. (C) Comparison of total cAMP concentrations in rdeA– cells and their parent strain DH1 in response to hyperosmotic stress. DH1 cells show an additional increase in 400 mM sorbitol (filled squares) compared with cells in SPB (open squares), while the elevated cAMP levels in rdeA– cells do not increase further (filled diamonds in sorbitol buffer, open diamonds in SPB). In all panels, the means of at least three independent experiments are shown; the lines represent best linear fits. In (B), standard deviations of the total cAMP levels are depicted. Measurements were performed with DTT as inhibitor of the extracellular PDE. Therefore, values cannot be compared with those in Figures 1A and 5A.
In order to exclude influences of cAMP export, we measured intracellular and secreted cAMP in parallel. The difference in cAMP levels of wild-type and dokA– cells is observed in both intra- and extracellular measurements (Figure 6B). Therefore, the export of cAMP seems not to be inhibited by hypertonicity in both strains and cannot account for the different concentrations observed.
The osmotically triggered cAMP increase is RdeA dependent
The previous results suggest a negative regulatory effect of DokA on the two-component signaling modules RdeA and RegA, which control cAMP breakdown in Dictyostelium. Therefore, we also examined total cAMP concentrations of osmotically shocked cells that are blocked in this phosphorelay. rdeA– cells have an elevated cAMP level due to impaired activation of the phosphodiesterase RegA. In accordance with this, we measured markedly higher total cAMP concentrations before as well as after DTT was added (Figure 6C). However, these levels were not affected by hypertonicity. In contrast, the corresponding wild-type strain DH1 exhibited, at a lower level, the additional increase seen in Ax2 cells. This confirms the assumption that the hyperosmotically triggered cAMP increase depends on both DokA and RdeA.
The receiver domain of DokA dephosphorylates RdeA in vitro
The rapidly developing phenotype can also be observed in Ax2 cells overexpressing the N-terminal receiver domain of RegA alone without its effector domain, because it acts as a receiver of phosphoryl groups from RdeA (Thomason et al., 1998), thereby preventing the activation of wild-type RegA. The phosphoryl group transfer between H63 in RdeA and D212 in the receiver domain of RegA was demonstrated in vitro (Thomason et al., 1999). We tested whether the RR domain of DokA could also act as a receiver of phosphoryl groups from RdeA. Purified RR and RRDA protein were obtained from Escherichia coli BL21-CodonPlus(DE3)-RIL strains heterologously expressing the receiver domain of DokA (K1516-G1638) and the correlating mutant DokA RR D1567A, respectively, as glutathione S-transferase (GST) fusion proteins.
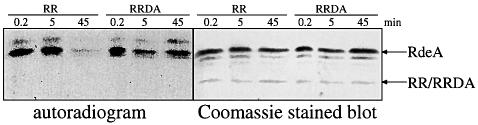
RdeA can be phosphorylated using the catalytic domain of the E.coli histidine kinase CheA and [γ-32P]ATP (Thomason et al., 1999). Freshly phosphorylated GST– RdeA, washed free from nucleotides by gel filtration and dialysis, was added to both GST–RR and GST–RRDA. After 45 min of incubation with the wild-type receiver domain of DokA, the signal corresponding to 32P-labeled RdeA had disappeared almost completely, while the addition of an equal amount of the mutated DokA RR D1567A did not alter the intensity of the signal (Figure 7). This effect was therefore dependent on the presence of the conserved aspartyl residue essential for receiver function. However, a concomitant rise in DokA RR phosphorylation could not be detected. The in vitro dephosphorylation of RdeA in the presence of RR sustains the assumption that the receiver domain of DokA is another phosphotransfer substrate of this HPt protein. RR reduces the phosphorylation level of RegA by acting as a phosphoryl group sink, thereby regulating intracellular phosphodiesterase activity.
Fig. 7. Dephosphorylation of GST–RdeA by DokA RR. The HPt protein RdeA was phosphorylated using the catalytic domain of E.coli CheA and [γ-32P]ATP. Subsequently, either GST–RR or GST–RRDA was added. Proteins were separated by SDS–PAGE, electroblotted, exposed to X-ray film and stained with Coomassie Blue. The addition of RR reduces the phosphorylation level of RdeA, while addition of the D1567A mutant has no influence on RdeA labeling.
Discussion
cAMP is a messenger in the osmotic stress response
DokA is an essential part of the hyperosmotic stress response in Dictyostelium cells (Schuster et al., 1996), but little was known about its mode of action. The measurements of intracellular cAMP levels of Ax2 cells demonstrate that cAMP is regulated in response to hyperosmotic stress. Within 2 min after onset of the stress, the amoebae show a 4- to 5-fold rise in the amount of intracellular cAMP followed by a slower decrease, eventually resulting in an elevated constant level (Figure 1A). Considering that the cells shrink to 50% of their original volume and preserve this size (Zischka et al., 1999), this increase is even more pronounced.
DokA is involved in the regulation of cAMP levels, as dokA– cells showed significantly reduced cAMP concentrations before and during the shock without a distinct peak. The difference during the first 10 min of the shock appears to be decisive for survival of the cells as the viability of dokA– cells is increased markedly by the addition of 8-Br-cAMP in this time slot (Figure 1B). The temporarily elevated cAMP concentrations observed in wild-type cells might contribute to initiate the various responses reported previously (Kuwayama et al., 1996; Zischka et al., 1999).
The cAMP response presumably is not regulated via the adenylyl cyclases known in Dictyostelium. While ACG is only expressed in mature spores (Verkerke-van Wijk and Schaap, 1997), the intracellular cAMP progression of osmotically shocked acaA– cells resembles the signal observed in wild-type cells, indicating that DokA is not a regulator of ACA in the hyperosmotic stress response. Furthermore, both ACA and ACB (AcrA) are inhibited by raised osmolarity (Meima and Schaap, 1999), as found in the cytosol of cells suspended in 400 mM sorbitol.
Effects of overexpression of DokA domains
The examination of mutants overexpressing DokA domains focused the influence of the receiver domain on cAMP levels. Overexpression of this domain causes elevated cAMP levels, an accelerated development with crippled fruiting bodies, sporogeneity and cAMP-S resistance. This phenotype strictly depends on the expression of the intact receiver domain, as cells overexpressing the mutated receiver RR D1567A are indistinguishable from wild type with respect to the properties investigated. According to the two-component paradigm, the kinase domain should be the source of phosphoryl groups transferred to D1567. However, expression of the kinase domain results in a wild-type phenotype. These observations suggest that the receiver domain is sufficient to induce a rise in cAMP concentration.
A first hint on the function of the kinase domain evolved from examination of the effect of PHKR expression on development. Unlike RR cells, these cells form regular fruiting bodies when developing on phosphate agar (Figure 3A), suggesting an inhibitory effect of the kinase domain on the receiver. Apart from that, the developmental phenotype and the elevated cAMP levels resemble RR cells, indicating that these effects depend on the presence of the RR domain. The discrepancy that PHKR cells manage to complete development despite showing a rapidly developing phenotype could be due to a regulatory influence of the kinase domain on the receiver. However, this effect is not dependent on residue H1053, the proposed site of autophosphorylation, indicating that the kinase domain is not directly involved in the phosphotransfer reaction (Table I).
DokA interacts with the RdeA–RegA phosphorelay
The developmental phenotype of RR cells resembles the ‘fruity’ or rapidly developing phenotype found in rdeA– (Sonneborn et al., 1963; Kessin, 1977) and regA– cells (Shaulsky et al., 1998), including attributes such as cAMP-S resistance and sporogeneity. RegA is the only known intracellular cAMP phosphodiesterase in Dictyostelium to date (Shaulsky et al., 1996). It acts downstream of the HPt protein RdeA in a phosphorelay (Chang et al., 1998; Thomason et al., 1998). For DokA, a role as a classical hybrid histidine kinase upstream of the HPt module can be excluded as the dokA– mutant exhibits significantly reduced cAMP levels. The disruption of an initiatory protein in this phosphorelay should result in increased cAMP levels, as seen in rdeA– (Figure 5A) and regA– cells (Thomason et al., 1999), because phosphorylation of the receiver domain of RegA activates its phosphodiesterase moiety (Thomason et al., 1998). The elevated cAMP levels cause an increase in PKA activity, which is assumed to be responsible for rapid development (Abe and Yanagisawa, 1983). In this regard, it is noteworthy that all described cell lines with elevated intracellular cAMP levels were also found to aggregate on cAMP-S agar, which equals chemotaxis-independent aggregation. Therefore, it seems likely that elevated levels enable the cells to proceed in development without sensing explicit extracellular cAMP pulses.
Based on these observations, we tested whether DokA might be a negative regulator of intracellular cAMP degradation. Measurements of the total amount of cAMP showed that the osmotically triggered rise in cAMP levels depends on both DokA and the RegA activator RdeA (Figure 6). The differences observed are caused by an impaired cAMP metabolism, as cAMP export seems not to be affected by hypertonicity or dokA deletion (Figure 6B). Further evidence arises from the in vitro interaction of RdeA and the DokA receiver. The addition of DokA RR reduces RdeA phosphorylation, indicating that the receiver dephosphorylates this HPt protein. In accordance with our notion of two-component signaling, the addition of RR D1567A does not affect RdeA phosphorylation (Figure 7). The failure to detect labeled RR is probably due to a high autodephosphorylation rate, a fact that would be in good agreement with its postulated role as a phosphatase.
The reduction of cAMP phosphodiesterase activity in lysates of RR cells compared with the wild type also supports the idea of DokA as a modulator of RegA activity and overexpressed RR as a dominant-negative form of DokA. The measurements indicate that there is a residual RegA activity in RR cells. This could be due to a minor fraction of phosphoryl groups relayed onto the RegA receiver. Moreover, phosphotransfer from RdeA onto the receiver domain of RegA is not the only way in which this enzyme is regulated. Serine phosphorylation on the C-terminal effector domain by the kinase Erk2 has also been shown to modulate RegA activity (Loomis, 1998).
Model: DokA is a phosphatase regulating a two-component phosphorelay
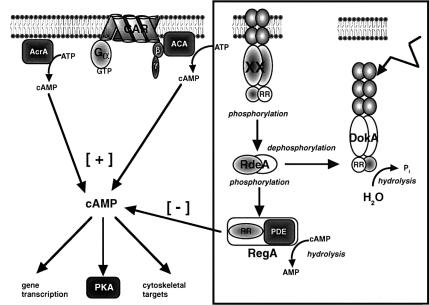
A phosphorelay system provides multiple potential for the integration of various signals and differential regulation by specific phosphatases, as found in the Kin–Spo0 phosphorelay in Bacillus subtilis (Hoch, 1995) or in the quorum-sensing phosphorelay in Vibrio harveyi (Freeman and Bassler, 1999). Moreover, dephosphorylation of the HPt module by the receiver domain of the hybrid histidine kinase ArcB plays an important role in the signal decay of the ArcB–ArcA phosphorelay in E.coli (Georgellis et al., 1998). Our observations suggest that DokA RR acts as a phosphoryl group sink with regard to the RdeA–RegA phosphorelay (Figure 8). This model explains that the dominant-negative effect of the RR fragment depends on the presence of the conserved D1567. Furthermore, overexpression of the receiver domain of RegA, which lacks the PDE domain and thereby constitutes a competing phosphoryl group acceptor, also causes rapid development (Thomason et al., 1998). Since overexpression of either of these receiver domains generates the same effect, while a comparable expression rate of other Dictyostelium receiver domains does not (Figure 3D), it is reasonable to assume that DokA and RegA receivers act specifically. There is no apparent effector domain on DokA, indicating that the central function of this protein is the modulation of a signal: interrupting the RdeA–RegA system and thereby providing an alternative pathway to increase the cAMP concentration inside the cells.
Fig. 8. Model. Nomenclature of gene products: ACA, adenylyl cyclase A; CAR, cAMP receptor; PKA, protein kinase A; XX, unidentified histidine kinase.
According to this model, DokA renders signals from a hyperosmotic environment onto the central second messenger cAMP and its effector PKA. Survival under hypertonic conditions is obviously cAMP dependent; however, only an appropriate temporal progression of the cAMP signal seems to ensure a suitable response. This is corroborated by the observation that the RR cells as well as regA– cells show reduced osmoresistance. Their survival rate is, however, significantly higher compared with dokA– cells (H.Keller, A.Ott and S.C.Schuster, unpublished). Thus, elevated amounts of cAMP are not sufficient for osmotolerance, as reflected by the fact that the presence of 8-Br-cAMP throughout the shock does not rescue dokA– cells.
The regulatory scheme presented here allows the integration of signals from a histidine kinase upstream of RdeA–RegA and from the DokA-mediated osmoregulatory pathway. Such a signaling scheme could be particularly important in the final stages of development, when the maturing spores are exposed to high osmolarity in the sorus (van Es et al., 1996; Cotter et al., 1999).
Materials and methods
Cell culture and development
Amoebae of D.discoideum strain Ax2-214 were grown in Ashworth medium (Watts and Ashworth, 1970) at 22°C, harvested at 3–5 × 106 cells/ml and washed twice with 17 mM SPB pH 6.0. Cells were spread on non-nutrient agar plates (0.9% agar in SPB) and allowed to adhere before buffer was removed.
Strains containing the G418 resistance gene were cultivated in Ashworth medium with 10 µg/ml G418 (Sigma), strain DH1 with 20 µg/ml uracil, and regA– cells with 20 µg/ml blasticidin (ICN). Development was initiated as above.
Construction of Dictyostelium cell lines
To create constructs of DokA fragments, the corresponding regions of the dokA gene were amplified by PCR using the plasmid pDIC3 (Schuster et al., 1996) as template. Site-directed mutagenesis was performed by recombinant PCR (Higuchi, 1990) and confirmed by sequencing with an ABI 377 DNA sequencer. The PCR products were cloned into the EcoRI site of the expression vector pDEX-RH (Faix et al., 1992) and transformed into D.discoideum Ax2 cells by electroporation (Howard et al., 1988). Cells were selected with 10 µg/ml G418 and clones were tested for expression by western analysis. Three independent clones were selected from each transformation for further experiments.
The region encoding a receiver homolog of clone ssg478 from the Dictyostelium cDNA project was sequenced (DDBJ/EMBL/GenBank accession No. AF258796). The dhkA (bp 6052–6450) and ssg478 (bp 1–444 of the receiver domain) fragments coding for the receiver domains of the proteins were amplified from genomic DNA by PCR and cloned into the EcoRI site of pDEX-RH. Transformation into Ax2 and selection were performed as above and confirmed by sequencing.
Osmotic shock viability assay
Axenically grown cells were washed twice with SPB and shaken for 1 h at a cell density of 3 × 107 cells/ml. 8-Br-cAMP (Sigma) was added to a concentration of 5 mM and sorbitol (Merck) to 400 mM. After 10 min, the cells were centrifuged at 750 g, resuspended in 400 mM sorbitol in SPB and shaken for another 110 min. Subsequently, cells were diluted into SPB, plated at ∼150 cells/plate and after 3 days colonies were counted. Control experiments without 8-Br-cAMP and/or without sorbitol were performed accordingly. Plating efficiencies did not vary by >15%.
Developmental assays
Dictyostelium discoideum cells were tested for cAMP-S resistance as described (Wallraff et al., 1984). Cells were examined for aggregation and fruiting body formation after 2–3 days; cAMP-S was from Biolog. To examine sporogeneity, cells were incubated in submerged cultures and sporulation was observed as described previously (Kay, 1989; Anjard et al., 1998).
Assay for intracellular cAMP during osmotic shock
Axenically grown cells were washed twice with SPB and shaken for 1 h at a cell density of 3 × 107 cells/ml. Sorbitol was added to a final concentration of 400 mM and aliquots containing 2.5 × 107 cells were taken at the respective time point (six samples for t = 0; two samples during the shock). Aliquots were centrifuged for 8 s at 4000 g and the supernatant was removed. The cell pellet was resuspended immediately in 200 µl of 3.5% ice-cold HClO4 and, after 30 min, 100 µl of half-saturated KHCO3 solution were added. The suspension was centrifuged at 4°C and 20 000 g. A 200 µl aliquot of the supernatant was assayed for cAMP in duplicate using the Biotrak isotope dilution assay (Amersham).
Assays for cAMP during osmotic shock with inhibited extracellular PDE
Cells were prepared as above and 5 mM DTT was added 6 min before sorbitol addition (400 mM). For determination of total cAMP levels, 500 µl aliquots were mixed with the same volume of 7% ice-cold HClO4 at the respective time point. For extracellular cAMP measurements, cells were centrifuged for 8 s at 4000 g and 400 µl of the supernatant were mixed with 400 µl of 7% HClO4. Intracellular cAMP was determined from cell pellets immediately resuspended in 400 µl of 3.5% HClO4. In each case, duplicate samples of 200 µl were neutralized, centrifuged and 200 µl of the supernatant assayed as above.
Determination of phosphodiesterase activity
Cells were washed twice with SPB and shaken for 3 h at 3 × 107 cells/ml, then washed and resuspended in reaction buffer (25 mM Tris–HCl pH 7.5, 4 mM MgCl2, 1 mM NaN3). Cells were lysed by sonification, the cell debris removed and 20 mM DTT and, if indicated, 0.4 µM IBMX (Sigma) added. After 20 min, 1 µM cAMP (Sigma) was added and, within 20 min of reaction at 22°C, various samples of 150 µl were mixed with 200 µl of methanol/chloroform (3:1) to stop the reaction. After centrifugation at 4°C and 10 000 g, 50 µl of the aqueous phase were assayed for cAMP content in duplicate. Results were standardized on total protein amount.
RT–PCR
Total mRNA of axenically growing cells was prepared with the RNeasy Kit (Qiagen) and equal amounts of RNA were used in a one-step RT–PCR (Promega).
Heterologous expression
The dokA fragments (bp 4589–4957) coding for RR and RR D1567A were amplified by PCR from heterologous expression constructs and cloned into the BamHI and XhoI sites of the E.coli expression vector pGEX-5X-1 (Pharmacia). These plasmids were transformed into BL21-CodonPlus(DE3)-RIL (Stratagene) by electroporation, and GST fusion proteins were purified from lysates of isopropyl-β-d-thiogalactopyranoside (IPTG)-induced cells on glutathione–Sepharose (Pharmacia) columns and, if necessary, on a pre-packed ResourceQ column (Pharmacia). GST–RdeA was prepared accordingly from E.coli BL21 transformed with pPT15 (Thomason et al., 1999).
In vitro radiolabeling
Phosphorylation of GST–RdeA by the CheA catalytic domain was performed in 50 mM Tris–HCl pH 8.3, 50 mM KCl, 10 mM MgCl2, 10% glycerol, 40 µCi of [γ-32P]ATP (ICN), containing 5 µM of each of the two proteins in a volume of 200 µl at 25°C. After 1 h, free nucleotides were removed by gel filtration on G-50 columns (Pharmacia) and a short dialysis step. About 1 µM GST–RR or GST–RRDA in 50 mM Tris–HCl pH 8.3 was added to aliquots of the CheA–RdeA mixture and the reaction was stopped by shock freezing in liquid nitrogen at the indicated time point. Proteins were separated by SDS–PAGE and transferred to a PVDF membrane (PallGelman) using a Trans-Blot SD transfer system (Bio-Rad). Phosphorylation was analyzed by autoradiography and subsequent staining of the membrane with Coomassie Blue.
Acknowledgments
Acknowledgements
We would like to thank J.Stock for a preparation of the CheA catalytic domain and discussions, and G.Gerisch for strains and discussions. We also thank P.Thomason and R.Kay for the regA– strain, J.Gross for WTC10, P.Devreotes for the acaA– strain, P.van Haastert for advice on the cAMP measurements, and R.Kay for the performance of initial sporulation experiments. This work was supported by grants (Schu778/3-1, Schu778/3-2, Schu778/5-1) from the DFG to S.C.S.
References
- Abe K. and Yanagisawa,K. (1983) A new class of rapidly developing mutants in Dictyostelium discoideum: implications for cyclic AMP metabolism and cell differentiation. Dev. Biol., 95, 200–210. [DOI] [PubMed] [Google Scholar]
- Alex L.A., Borkovich,K.A. and Simon,M.I. (1996) Hyphal development in Neurospora crassa: involvement of a two-component histidine kinase. Proc. Natl Acad. Sci. USA, 93, 3416–3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anjard C., Pinaud,S., Kay,R.R. and Reymond,C.D. (1992) Over expression of Dd Pk2 protein kinase causes rapid development and affects the intracellular cAMP pathway of Dictyostelium discoideum. Development, 115, 785–790. [DOI] [PubMed] [Google Scholar]
- Anjard C., Zeng,C., Loomis,W.F. and Nellen,W. (1998) Signal transduction pathways leading to spore differentiation in Dictyostelium discoideum. Dev. Biol., 193, 146–155. [DOI] [PubMed] [Google Scholar]
- Appleby J.L., Parkinson,J.S. and Bourret,R.B. (1996) Signal transduction via the multi-step phosphorelay: not necessarily a road less traveled. Cell, 86, 845–848. [DOI] [PubMed] [Google Scholar]
- Baumgarten C.M. and Feher,J.J. (1995) Osmosis and the regulation of cell volume. In Sperelakis,N. (ed.), Cell Physiology Source Book. Academic Press, San Diego, CA, pp. 180–211. [Google Scholar]
- Burg M.B., Kwon,E.D. and Kultz,D. (1996) Osmotic regulation of gene expression. FASEB J., 10, 1598–1606. [DOI] [PubMed] [Google Scholar]
- Chang C., Kwok,S.F., Bleecker,A.B. and Meyerowitz,E.M. (1993) Arabidopsis ethylene-response gene etr1: similarity of product to two-component regulators. Science, 262, 539–544. [DOI] [PubMed] [Google Scholar]
- Chang W.T., Thomason,P.A., Gross,J.D. and Newell,P.C. (1998) Evidence that the RdeA protein is a component of a multistep phosphorelay modulating rate of development in Dictyostelium. EMBO J., 17, 2809–2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter D.A., Dunbar,A.J., Buconjic,S.D. and Wheldrake,J.F. (1999) Ammonium phosphate in sori of Dictyostelium discoideum promotes spore dormancy through stimulation of the osmosensor ACG. Microbiology, 145, 1891–1901. [DOI] [PubMed] [Google Scholar]
- Coukell M.B. and Chan,F.K. (1980) The precocious appearance and activation of an adenylate cyclase in a rapid developing mutant of Dictyostelium discoideum. FEBS Lett., 110, 39–42. [DOI] [PubMed] [Google Scholar]
- Faix J., Gerisch,G. and Noegel,A.A. (1992) Overexpression of the csA cell adhesion molecule under its own cAMP-regulated promoter impairs morphogenesis in Dictyostelium. J. Cell Sci., 102, 203–214. [DOI] [PubMed] [Google Scholar]
- Freeman J.A. and Bassler,B.L. (1999) Sequence and function of LuxU: a two-component phosphorelay protein that regulates quorum sensing in Vibrio harveyi. J. Bacteriol., 181, 899–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgellis D., Kwon,O., De Wulf,P. and Lin,E.C. (1998) Signal decay through a reverse phosphorelay in the Arc two-component signal transduction system. J. Biol. Chem., 273, 32864–32869. [DOI] [PubMed] [Google Scholar]
- Gerisch G. (1976) Extracellular cyclic AMP phosphodiesterase regulation in agar plate cultures of Dictyostelium discoideum. Cell Differ., 5, 21–25. [DOI] [PubMed] [Google Scholar]
- Green A.A. and Newell,P.C. (1975) Evidence for the existence of two types of cAMP binding sites in aggregating cells of Dictyostelium discoideum. Cell, 6, 129–136. [DOI] [PubMed] [Google Scholar]
- Higuchi R. (1990) Recombinant PCR. In Innis,M.A. and Gelfand,D.H. (eds), PCR Protocols: A Guide to Methods and Applications. Academic Press, San Diego, CA, pp. 177–183. [Google Scholar]
- Hoch J.A. (1995) Control of cellular development in sporulating bacteria by the phosphorelay two-component signal transduction system. In Hoch,J.A. and Silhavy,T.J. (eds), Two-component Signal Transduction. ASM Press, Washington, DC, pp. 129–144. [Google Scholar]
- Hopper N.A., Harwood,A.J., Bouzid,S., Veron,M. and Williams,J.G. (1993) Activation of the prespore and spore cell pathway of Dictyostelium differentiation by cAMP-dependent protein kinase and evidence for its upstream regulation by ammonia. EMBO J., 12, 2459–2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard P.K., Ahern,K.G. and Firtel,R.A. (1988) Establishment of a transient expression system for Dictyostelium discoideum. Nucleic Acids Res., 16, 2613–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakimoto T. (1996) CKI1, a histidine kinase homolog implicated in cytokinin signal transduction. Science, 274, 982–985. [DOI] [PubMed] [Google Scholar]
- Kay R.R. (1982) cAMP and spore differentiation in Dictyostelium discoideum. Proc. Natl Acad. Sci. USA, 79, 3228–3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay R.R. (1989) Evidence that elevated intracellular cyclic AMP triggers spore maturation in Dictyostelium. Development, 105, 753–759. [Google Scholar]
- Kay R.R., Berks,M., Traynor,D., Taylor,G.W., Masento,M.S. and Morris,H.R. (1988) Signals controlling cell differentiation and pattern formation in Dictyostelium. Dev. Genet., 9, 579–587. [DOI] [PubMed] [Google Scholar]
- Kessin R.H. (1977) Mutations causing rapid development of Dictyostelium discoideum. Cell, 10, 703–708. [DOI] [PubMed] [Google Scholar]
- Kim H.J., Chang,W.T., Meima,M., Gross,J.D. and Schaap,P. (1998) A novel adenylyl cyclase detected in rapidly developing mutants of Dictyostelium. J. Biol. Chem., 273, 30859–30862. [DOI] [PubMed] [Google Scholar]
- Kuwayama H., Ecke,M., Gerisch,G. and van Haastert,P.J. (1996) Protection against osmotic stress by cGMP-mediated myosin phosphorylation. Science, 271, 207–209. [DOI] [PubMed] [Google Scholar]
- Loomis W.F. (1998) Role of PKA in the timing of developmental events in Dictyostelium cells. Microbiol. Mol. Biol. Rev., 62, 684–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukat G.S., McCleary,W.R., Stock,A.M. and Stock,J.B. (1992) Phosphorylation of bacterial response regulator proteins by low molecular weight phospho-donors. Proc. Natl Acad. Sci. USA, 89, 718–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malchow D., Nagele,B., Schwarz,H. and Gerisch,G. (1972) Membrane-bound cyclic AMP phosphodiesterase in chemotactically responding cells of Dictyostelium discoideum. Eur. J. Biochem., 28, 136–142. [DOI] [PubMed] [Google Scholar]
- Meima M.E. and Schaap,P. (1999) Fingerprinting of adenylyl cyclase activities during Dictyostelium development indicates a dominant role for adenylyl cyclase B in terminal differentiation. Dev. Biol., 212, 182–190. [DOI] [PubMed] [Google Scholar]
- Ota I.M. and Varshavsky,A. (1993) A yeast protein similar to bacterial two-component regulators. Science, 262, 566–569. [DOI] [PubMed] [Google Scholar]
- Parent C.A. and Devreotes,P.N. (1996) Molecular genetics of signal transduction in Dictyostelium. Annu. Rev. Biochem., 65, 411–440. [DOI] [PubMed] [Google Scholar]
- Parkinson J.S. (1993) Signal transduction schemes of bacteria. Cell, 73, 857–871. [DOI] [PubMed] [Google Scholar]
- Pitt G.S., Milona,N., Borleis,J., Lin,K.C., Reed,R.R. and Devreotes,P.N. (1992) Structurally distinct and stage-specific adenylyl cyclase genes play different roles in Dictyostelium development. Cell, 69, 305–315. [DOI] [PubMed] [Google Scholar]
- Posas F., Wurgler-Murphy,S.M., Maeda,T., Witten,E.A., Thai,T.C. and Saito,H. (1996) Yeast HOG1 MAP kinase cascade is regulated by a multistep phosphorelay mechanism in the SLN1–YPD1–SSK1 ‘two-component’ osmosensor. Cell, 86, 865–875. [DOI] [PubMed] [Google Scholar]
- Pratt L.A. and Silhavy,T.J. (1995) Porin regulon of Escherichia coli. In Hoch,J.A. and Silhavy,T.J. (eds), Two-component Signal Transduction. ASM Press, Washington, DC, pp. 105–127. [Google Scholar]
- Robison G.A., Butcher,R.W. and Sutherland,E.W. (1968) Cyclic AMP. Annu. Rev. Biochem., 37, 149–174. [DOI] [PubMed] [Google Scholar]
- Rossier C., Gerisch,G., Malchow,D. and Eckstein,F. (1978) Action of a slowly hydrolysable cyclic AMP analogue on developing cells of Dictyostelium discoideum. J. Cell Sci., 35, 321–338. [DOI] [PubMed] [Google Scholar]
- Rossier C., Eitle,E., van Driel,R. and Gerisch,G. (1980) Biochemical regulation of cell development and aggregation in Dictyostelium discoideum. In Gooday,G.W., Lloyd,D. and Trinci,A.P.J. (eds), The Eukaryotic Microbial Cell. Cambridge University Press, Cambridge, UK, pp. 405–424. [Google Scholar]
- Schuster S.C., Noegel,A.A., Oehme,F., Gerisch,G. and Simon,M.I. (1996) The hybrid histidine kinase DokA is part of the osmotic response system of Dictyostelium. EMBO J., 15, 3880–3889. [PMC free article] [PubMed] [Google Scholar]
- Shaulsky G., Escalante,R. and Loomis,W.F. (1996) Developmental signal transduction pathways uncovered by genetic suppressors. Proc. Natl Acad. Sci. USA, 93, 15260–15265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaulsky G., Fuller,D. and Loomis,W.F. (1998) A cAMP-phosphodiesterase controls PKA-dependent differentiation. Development, 125, 691–699. [DOI] [PubMed] [Google Scholar]
- Simon M.N., Pelegrini,O., Veron,M. and Kay,R.R. (1992) Mutation of protein kinase A causes heterochronic development of Dictyostelium. Nature, 356, 171–172. [DOI] [PubMed] [Google Scholar]
- Singleton C.K., Zinda,M.J., Mykytka,B. and Yang,P. (1998) The histidine kinase dhkC regulates the choice between migrating slugs and terminal differentiation in Dictyostelium discoideum. Dev. Biol., 203, 345–357. [DOI] [PubMed] [Google Scholar]
- Soderbom F., Anjard,C., Iranfar,N., Fuller,D. and Loomis,W.F. (1999) An adenylyl cyclase that functions during late development of Dictyostelium. Development, 126, 5463–5471. [DOI] [PubMed] [Google Scholar]
- Sonneborn D.R., White,G.J. and Sussman,M. (1963) A mutation affecting both rate and pattern of morphogenesis in Dictyostelium discoideum. Dev. Biol., 7, 79–93. [DOI] [PubMed] [Google Scholar]
- Stock J.B., Surette,M.G., Levit,M. and Park,P. (1995) Two-component signal transduction systems: structure–function relationships and mechanism of catalysis. In Hoch,J.A. and Silhavy,T.J. (eds), Two-component Signal Transduction. ASM Press, Washington, DC, pp. 25–51. [Google Scholar]
- Swanson R.V., Schuster,S.C. and Simon,M.I. (1993) Expression of CheA fragments which define domains encoding kinase, phosphotransfer and CheY binding activities. Biochemistry, 32, 7623–7629. [DOI] [PubMed] [Google Scholar]
- Tang W.-J. and Gilman,A.G. (1992) Adenylyl cyclases. Cell, 70, 869–872. [DOI] [PubMed] [Google Scholar]
- Thomason P.A., Traynor,D., Cavet,G., Chang,W.-T., Harwood,A.J. and Kay,R.R. (1998) An intersection of the cAMP/PKA and two-component signal transduction systems in Dictyostelium. EMBO J., 17, 2838–2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason P.A., Traynor,D., Stock,J.B. and Kay,R.R. (1999) The RdeA–RegA system, a eukaryotic phospho-relay controlling cAMP breakdown. J. Biol. Chem., 274, 27379–27384. [DOI] [PubMed] [Google Scholar]
- van Es S., Virdy,K.J., Pitt,G.S., Meima,M., Sands,T.W., Devreotes,P.N., Cotter,D.A. and Schaap,P. (1996) Adenylyl cyclase G, an osmosensor controlling germination of Dictyostelium spores. J. Biol. Chem., 271, 23623–23625. [DOI] [PubMed] [Google Scholar]
- van Haastert P.J.M. (1997) Transduction of the chemotactic cAMP signal across the plasma membrane. In Maeda,Y., Inouye,K. and Takeuchi,I. (eds), Dictyostelium—A Model System for Cell and Developmental Biology. Universal Academy Press, Tokyo, Japan, pp. 173–191. [Google Scholar]
- Verkerke-van Wijk I. and Schaap,P. (1997) cAMP, a signal for survival. In Maeda,Y., Inouye,K. and Takeuchi,I. (eds), Dictyostelium—A Model System for Cell and Developmental Biology. Universal Academy Press, Tokyo, Japan, pp. 145–162. [Google Scholar]
- Wallraff E., Welker,D.L., Williams,K.L. and Gerisch,G. (1984) Genetic analysis of a Dictyostelium discoideum mutant resistant to adenosine 3′,5′-cyclic phosphorothioate, an inhibitor of wild-type development. J. Gen. Microbiol., 130, 2103–2114. [Google Scholar]
- Wang N., Shaulsky,G., Escalante,R. and Loomis,W.F. (1996) A two-component histidine kinase gene that functions in Dictyostelium development. EMBO J., 15, 3890–3898. [PMC free article] [PubMed] [Google Scholar]
- Watts D.J. and Ashworth,J.M. (1970) Growth of myxamoebae of the cellular slime mould Dictyostelium discoideum. Biochem. J., 119, 171–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurgler-Murphy S.M. and Saito,H. (1997) Two-component signal transducers and MAPK cascades. Trends Biochem. Sci., 22, 172–176. [DOI] [PubMed] [Google Scholar]
- Zinda M.J. and Singleton,C.K. (1998) The hybrid histidine kinase dhkB regulates spore germination in Dictyostelium discoideum. Dev. Biol., 196, 171–183. [DOI] [PubMed] [Google Scholar]
- Zischka H., Oehme,F., Pintsch,T., Ott,A., Keller,H., Kellermann,J. and Schuster,S.C. (1999) Rearrangement of cortex proteins constitutes an osmoprotective mechanism in Dictyostelium. EMBO J., 18, 4241–4249. [DOI] [PMC free article] [PubMed] [Google Scholar]