Abstract
Given the importance of G-protein-coupled receptors as pharmacological targets in medicine, efforts directed at understanding the molecular mechanism by which pharmacological compounds regulate their presence at the cell surface is of paramount importance. In this context, using confocal microscopy and bioluminescence resonance energy transfer, we have investigated internalization and intracellular trafficking of the cholecystokinin-2 receptor (CCK2R) in response to both natural and synthetic ligands with different pharmacological features. We found that CCK and gastrin, which are full agonists on CCK2R-induced inositol phosphate production, rapidly and abundantly stimulate internalization. Internalized CCK2R did not rapidly recycle to plasma membrane but instead was directed to late endosomes/lysosomes. CCK2R endocytosis involves clathrin-coated pits and dynamin and high affinity and prolonged binding of β-arrestin1 or -2. Partial agonists and antagonists on CCK2R-induced inositol phosphate formation and ERK1/2 phosphorylation did not stimulate CCK2R internalization or β-arrestin recruitment to the CCK2R but blocked full agonist-induced internalization and β-arrestin recruitment. The extreme C-terminal region of the CCK2R (and more precisely phosphorylatable residues Ser437-Xaa438-Thr439-Thr440-Xaa441-Ser442-Thr443) were critical for β-arrestin recruitment. However, this region and β-arrestins were dispensable for CCK2R internalization. In conclusion, this study allowed us to classify the human CCK2R as a member of class B G-protein-coupled receptors with regard to its endocytosis features and identified biased agonists of the CCK2R. These new important insights will allow us to investigate the role of internalized CCK2R·β-arrestin complexes in cancers expressing this receptor and to develop new diagnosis and therapeutic strategies targeting this receptor.
Keywords: Cell Surface Receptor, Confocal Microscopy, G-protein-coupled Receptors (GPCR), Peptide Hormones, Receptor Endocytosis, BRET, beta-Arrestin Recruitment, Biased Agonists, Cholecystokinin Receptor, Pharmacology
Introduction
As a general hallmark, GPCRs3 are tightly regulated at the cell surface both acutely and over a long time period. This regulation is thought to be critical to the physiological homeostasis of GPCR signaling, which can be disrupted in pathological states and is strongly affected by acute or repeated administration of clinically relevant drugs. Ligand-induced endocytosis is a general and important mechanism contributing to such GPCR regulation (1). For many GPCRs, the initial step of ligand-induced internalization, also named “desensitization,” involves receptor phosphorylation by GPCR kinases that selectively phosphorylate agonist-activated receptors. Phosphorylation of the receptor and subsequent binding of nonvisual arrestins prevent subsequent interaction of receptors with G-proteins, thus terminating the G-protein-mediated signal. Arrestin-bound receptors are rapidly targeted to the clathrin-coated pits, thereby promoting internalization of receptors (1–4). Alternative mechanisms to those involving recruitment of β-arrestin adaptor and receptor targeting to clathrin-coated pits have been shown to govern internalization of some GPCRs (4, 5). Following internalization, receptors may be sorted to different vesicular traffic routes, such as, for example, rapid recycling to the cell plasma membrane or slow recycling and degradation into lysosomes. Recently, it has been recognized that, in addition to its role in down-regulation of G-protein-mediated signal and cell responsiveness, internalization through β-arrestin recruitment represents a means for GPCRs to trigger signaling pathways independently of G-protein coupling (6, 7). Importantly, pharmacological agents, named “biased ligands” or “functionally selective ligands,” have been discovered, which activate differentially G-protein-dependent and arrestin-dependent signaling pathways (8).
The cholecystokinin-2 receptor (CCK2R), previously named the CCKB/gastrin receptor, belongs to family I of GPCRs, which includes rhodopsin (9–11). Its natural ligands are cholecystokinin and gastrin, two structurally related neuropeptides, which bind the CCK2R with the same high affinity. The CCK2R is expressed in the central nervous system and in the gut, where it represents the predominant CCK receptor subtype. The CCK2R mediates a wide spectrum of agonist-induced biological effects, including anxiety, pain perception, gastric acid secretion, and growth and differentiation of the gastric mucosa (10, 12). Activation of wild-type CCK2R and/or expression of a constitutively active variant may contribute to human diseases (10). These findings have stimulated interest in the identification of antagonists of CCK2R. To date, a large panel of chemically distinct CCK2R antagonists have been discovered and used to assess the functions mediated by CCK2R in animals and humans (13). Furthermore, several of these compounds have reached clinical evaluation stages for indications such as anxiety and panic disorders, sleep disorders, drug dependence, pain, gastroesophagus reflux, and gastric secretion disorders (13). However, some reference molecules believed to be pure antagonists turned out to be endowed with some agonist activity in the stomach and pancreas as well as cells expressing CCK1R or CCK2R (14).
In previous works, we investigated the intrinsic activation mechanism of the CCK2R and the regulation of this activity by different ligands (15–17). This led us to understand how two structurally close nonpeptide ligands display opposite activities (partial agonist and inverse agonist) (16). Moreover, we delineated the role and mechanism of action of RGS-2 (regulator of G-protein signaling-2) in CCK2R-induced inositol phosphate production (17). However, the mechanism and consequences of CCK2R regulation at the cell surface by its natural ligands or by synthetic ligands are not yet precisely known (18, 19).
In the current study, given the importance of cell surface regulation of GPCR by pharmacological agents, we investigated the mechanisms whereby the CCK2R is regulated after stimulation by its natural agonist ligand CCK or after exposure to several synthetic ligands displaying distinct pharmacological activity with respect to CCK2R-mediated production of inositol phosphates. Results from this study led us to classify the CCK2R as a member of the class B GPCRs with respect to its endocytosis features. Furthermore, this study led to identification of biased agonists that are partial agonists on CCK2R-induced inositol phosphate production and antagonists on β-arrestin recruitment and subsequent receptor endocytosis. The C-terminal region of the CCK2R and more precisely phosphorylatable motif Ser437-Xaa438-Thr439-Thr440-Xaa441-Ser442-Thr443 were identified as critical determinants for β-arrestin recruitment. However, this motif and β-arrestins were dispensable for CCK2R internalization. By providing new important insights into the regulation mechanism of the CCK2R by natural agonists and pharmacological agents, this study represents a solid basis to investigate the contribution of internalized CCK2R·β-arrestin complexes to CCK2R-induced intracellular signaling in cancer cells that endogenously express this receptor. It will also allow us to develop new diagnosis and therapeutic strategies based on the targeting of the CCK2R.
EXPERIMENTAL PROCEDURES
Materials
Sulfated [Thr28,Nle31]CCK 25-33 and [Leu15]sulfated gastrin 1–17 were synthesized as described previously (20) and are referred to as CCK and gastrin. 125I-Sodium (2000 Ci/mmol) and myo-[3H]inositol (5 μCi/ml) were from GE Healthcare. CCK was conjugated with Bolton-Hunter reagent, purified, and radioiodinated as described previously (21) and is referred to as 125I-CCK. Alexa Fluor 647-labeled CCK was obtained according to the procedure recently described (22) for glucose insulinotropic polypeptide and is referred to as Alexa F 647-CCK.
Synthetic ligands of the CCK2R (PD135,158 (23), JB93,182, and JB93,242) were used (24). A specific inhibitor of dynamin, dynasore (Calbiochem); an inhibitor of clathrin-dependent uptake, chlorpromazine (Sigma-Aldrich); and an inhibitor of recycling, monensin (Sigma-Aldrich) were also used.
The cDNAs encoding CCK2R, green fluorescent protein (GFP)-tagged CCK2R, and Renilla luciferase (Rluc)-fused CCK2R were generated by subcloning the CCK2R cDNA in pcDNA5/FTR (Invitrogen), pEGFP-N1 (BD Biosciences Clontech), and pRluc-N1(h) (PerkinElmer Life Sciences), respectively. DsRed-tagged Rab5, DsRed-tagged Rab11, and DsRed-tagged Rab7 were obtained from Addgene. GFP-tagged β-arrestin1, yellow fluorescent protein (YFP)-tagged β-arrestin1, and YFP-tagged β-arrestin2 were generous gifts from Marc Caron (Duke University Medical Center, Durham, NC). GFP-tagged β-arrestin2, kindly provided by Robert Lefkowitz (Duke University Medical Center), was subsequently subcloned in pcDNA5/Flp recombination target (FRT). All mutant receptor cDNAs and the GFP-tagged dominant negative V53D-β-arrestin1 and V54D-β-arrestin2 were constructed by an oligonucleotide-directed mutagenesis (QuikChangeTM site-directed mutagenesis kit, Stratagene) using human CCK2R cDNAs cloned in the pcDNA5/FRT vector and GFP-tagged β-arrestin1 and β-arrestin2 as templates, respectively.
Cell Lines
HEK 293 cells stably expressing the CCK2R (Flp-InTM CCK2R-293) and β-arrestin2-GFP (Flp-InTM β-arrestin2-GFP-293) were obtained using the Flp-InTM system (Invitrogen). Flp-InTM 293 cells containing an FRT in their genome were purchased from Invitrogen and cotransfected with pcDNA5/FRT vector carrying CCK2R or β-arrestin2-GFP and the Flp recombinase expression vector pOG44 that mediates integration of the CCK2R-pcDNA5/FRT vector into the genome via FRT sites. Cells that acquired the CCK2R-pcDNA5/FRT vector or β-arrestin2-GFP-pcDNA5/FRT vector were selected using hygromycin B (100 μg/ml; Sigma). MEF cells, wild type and double knock-out for β-arrestins, were generous gifts from Robert Lefkowitz (Duke University Medical Center). Flp-InTM 293 cells, HEK 293 T cells, MEF cells, Flp-InTM β-arrestin2-GFP-293, and Flp-InTM CCK2R-293 cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FBS, in a humidified atmosphere at 95% air and 5% CO2.
Generation of MEF Cells Expressing the CCK2R
Replication-defective, self-inactivating lentiviral vectors were generated in a BSL-3 facility (BiviC core vector production, IFR 150, Toulouse, France) through transient transfection of HEK 293FT cells with packaging and lentiviral vector plasmids using polyethyleneimine. Lentiviral vectors were concentrated by centrifugation using Vivaspin columns and stored at −80 °C. All batches were checked to be replicative virus-free. The viral titers were determined on HT1080 cells and expressed in transduction units/ml. In addition, vector concentrations were quantified by a p24 enzyme-linked immunosorbent assay (ELISA) (Innotest, Ingen, Paris). To generate MEF cells expressing the CCK2R, cells were incubated with the lentiviral vectors (multiplicity of infection = 1) for 24 h and subsequently selected with Zeocin® (Invivogen, Toulouse, France) at 25 μg/ml for 2 weeks. Efficiency of viral transduction and selection was estimated by measuring the percentage of cells expressing GFP by flow cytometry analysis. Expression of CCK2R was assessed by binding Alexa F 647-CCK.
Phospho-ERK and β-Arrestin Immunoblots
Cells were washed with ice-cold buffer A (50 mm Hepes, 150 mm NaCl, 10 mm EDTA, 10 mm Na4P2O7, 100 mm NaF, 2 mm orthovanadate, pH 7.5) and homogenized in 200 μl of lysis buffer (buffer A containing 1% Triton X-100, 0.5 mm phenylmethylsulfonyl fluoride, 20 μm leupeptin, 100 IU/ml Trasylol) for 15 min at 4 °C. The solutes were clarified by centrifugation at 12,000 × g for 10 min at 4 °C. The samples were then washed twice with 30 mm Hepes buffer, pH 7.5, containing 30 mm NaCl and 0.1% Triton X-100, resuspended in SDS sample buffer, and boiled for 5 min. Whole cell lysates were separated by SDS-PAGE. Proteins were transferred to polyvinylidene difluoride membranes (Immobilon, Millipore). Membranes were blocked with saline buffer (1 mm Tris, 14 mm NaCl, pH 7.4) containing 5% nonfat dried milk and incubated overnight with the phospho-ERK1/2 antibody (1:500, Cell Signaling, catalog no. 9106) or β-arrestin antibody (1:300, BD Biosciences, catalog no. 610550). Membranes were washed three times with saline buffer containing 5% nonfat dried milk and incubated with secondary antibody anti-mouse (1:5000 dilution) for 3 h at room temperature. Membranes were washed three times with saline buffer containing 5% nonfat dried milk and incubated with a chemoluminescence HRP Western blotting kit substrate (SuperSignal West Pico Substrate, Pierce, catalog no. 34079) for 5 min before the membrane was unveiled.
Receptor Binding Assay
Cells were plated onto 10-cm culture dishes and grown in DMEM containing 10% FBS in a 5% CO2 atmosphere at 37 °C. After overnight incubation, cells were transfected with 1 μg/plate (excepted when mentioned) of pcDNA5/FRT, pEGFP-N1, and pRluc-N1(h) vectors containing the cDNA for the wild-type or mutated CCK2Rs using a Fugene® 6 transfection reagent (Roche Applied Science). Cells were transferred to 24-well plates 24 h after transfection. HEK 293 cells stably expressing CCK2R (Flp-InTM CCK2R-293) were directly plated onto 24-well plates. Approximately 24 h after transfer to 24-well plates, binding assays were performed using 125I-CCK according to the protocol previously described in detail (16). Receptor density (Bmax) and Kd were calculated from homologous 125I-CCK competition binding experiments using Ligand software (Kell, Cambridge, UK). Ki values for competitors were calculated using the non-linear curve fitting software GraphPad Prism (GraphPad Software, San Diego, CA).
Inositol Phosphate Production Assay
Cells were plated onto 10-cm culture dishes and grown in DMEM containing 10% FBS in a 5% CO2 atmosphere at 37 °C. After overnight incubation, cells were transfected with 1 μg/plate (except when mentioned) of pcDNA5/FRT, pEGFP-N1, and pRluc-N1(h) vectors containing the cDNA for the wild-type and mutated CCK2 receptors or β-arrestin1 and β-arrestin2 using a Fugene® 6 transfection reagent (Roche Applied Science). Cells were transferred to 24-well plates 24 h after transfection. HEK 293 cells stably expressing CCK2R (Flp-InTM CCK2R-293) were directly plated onto 24-well plates. Approximately 24 h after transfer to 24-well plates and after overnight incubation in DMEM containing 2 μCi/ml myo-[2-3H]inositol (specific activity, 10–25 Ci/mmol; PerkinElmer Life Sciences), inositol phosphate production was determined as described previously (16).
Confocal Fluorescence Microscopy
Flp-InTM CCK2R-293, Flp-InTM β-arrestin2-GFP-293, and Flp-InTM 293 cells were plated onto a poly-l-lysine (Sigma-Aldrich)-coated four-well Lab-Tek chambered coverglass (catalog no. 155383, Nunc) and grown in DMEM containing 10% FBS in a 5% CO2 atmosphere at 37 °C. After overnight incubation, cells were transfected with 0.5 μg/well pcDNA5/FRT containing cDNA encoding GFP-tagged CCK2R, mutated CCK2R, β-arrestin1-GFP, β-arrestin2-GFP, K44A dynamin, V53D dominant negative β-arrestin1-GFP, and V54D dominant negative β-arrestin-GFP using a Fugene® 6 transfection reagent (Roche Applied Science). For colocalization experiments, vectors containing cDNA encoding GFP-tagged CCK2R and Rab5-DsRed or Rab11-DsRed or Rab7-DsRed were cotransfected using the same method with 0.5 μg/well for GFP-tagged CCK2R and 1 μg/well for Rabs-DsRed proteins. 24 h after transfection, the DMEM was replaced by phosphate-buffered saline (PBS) calcium- and magnesium-free prior to stimulation. Cells were stimulated with appropriate ligand, and images of GFP, DsRed, or Alexa Fluor 647 fluorescence were collected by using single- or double-line excitation (488, 54, or 633 nm, respectively) on a Zeiss laser-scanning microscope (LSM-510). For β-arrestin1-GFP and β-arrestin2-GFP membrane recruitment experiments, time series over a 5-min period were performed (pictures were taken every 30 s), and the decrease of cytoplasmic fluorescence was measured using the region of interest (ROI) function of LSM-510 software.
Internalization Assay by Flow Cytometry
Flp-InTM CCK2R-293 cells were plated onto poly-l-lysine (Sigma-Aldrich)-coated 6-well plates and grown in DMEM containing 10% FBS in a 5% CO2 atmosphere at 37 °C. After overnight incubation, cells were treated with CCK (0.1 μm) or synthetic ligands (1 μm) in DMEM containing HEPES (20 mm) at various times. Following stimulation, bound ligand was dissociated from the cells three times with DMEM/HEPES (20 mm), and the remaining receptors at the cell membrane were labeled using a saturating concentration of Alexa F 647-CCK (1 μm) for 2.5 h on ice. Cells were washed twice with PBS, 0.1% BSA. Cells were detached from 6-well plates and transferred to FACS tubes. Cell-associated fluorescence was determined using a BD FACSCaliburTM flow cytometer, with Flp-InTM 293 cells that do not express CCK2R as a negative control.
Recycling Assay by Flow Cytometry
Flp-InTM CCK2R-293 cells were plated onto poly-l-lysine (Sigma-Aldrich)-coated 6-well plates and grown in DMEM containing 10% FBS in a 5% CO2 atmosphere at 37 °C. After overnight incubation, cells were treated with 0.1 μm CCK in DMEM/HEPES (20 mm) for 1 h at 37 °C. Following stimulation, cells were washed three times with DMEM/HEPES. The receptors at the cell surface were labeled using Alexa F 647-CCK (1 μm) for 45 min at 37 °C at various times (1, 2, and 3 h) after the end of the CCK stimulation. Binding of Alexa F 647-CCK was carried out in the presence of dynasore (80 μm) in order to block internalization and possible fast recycling during this step of receptor quantification.
BRET Studies
HEK 293 T or Flp-InTM 293 cells were plated onto 10-cm culture dishes and grown in DMEM containing 10% FBS in a 5% CO2 atmosphere at 37 °C. After overnight incubation, cells were transfected with 0.2 μg of Rluc-tagged CCK2R (wild type or mutants) and 10 μg of either β-arrestin1-YFP or β-arrestin2 using polyethyleneimine transfection reagent (Polyplus) or Fugene® 6 transfection reagent (Roche Applied Science). 24 h after transfection, cells were plated in 96-well clear bottom plates (Corning Glass) at a density of 100,000 cells/well in phenol red-free DMEM. After overnight incubation, the phenol red-free medium was removed from HEK293T cells and replaced by PBS. The BRET assay was initiated by adding 10 μl of the cell-permeant substrate specific for Renilla luciferase, coelenterazine H, to the well to yield a final concentration of 5 μm. The agonist/antagonist activity of the compounds was measured by adding them to the well 5 min after the Rluc substrate. Readings started 10 min after the addition of Rluc substrate. BRET experiments were performed at room temperature in a Mithras LB940 instrument (Berthold) that allows the sequential integration of the signals detected in the 465–505 nm and 515–555 nm windows using filters with the appropriate band pass and by using MicroWin 2000 software. For titration experiments, the acceptor/donor ratio was calculated as described previously (25). For time course analysis of the interactions between CCK2R and β-arrestin1/2 interactions, coelenterazine H was added 10 min before the addition of PBS, CCK, or compounds. Readings were then collected at 12.5-s intervals for the next 20 min.
RESULTS
Characterization of Flp-InTM CCK2R-293 Cells
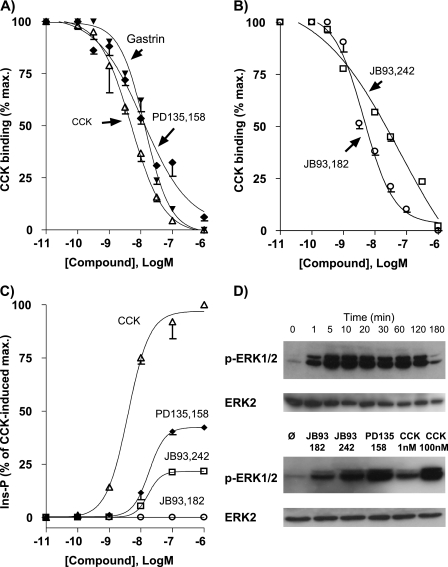
To investigate the regulation of the CCK2R at the cell surface, we established a HEK 293 cell line that permanently expressed the human CCK2R and was named Flp-InTM CCK2R-293. Homologous competition binding studies with these cells indicated that expressed CCK2R bound 125I-CCK with a dissociation constant (Kd) of 4.8 ± 1.0 nm and a maximum binding capacity of 1.98 ± 0.18 pmol/106 cells. The CCK2R displayed a typical CCK2R-like pharmacology, as shown by the high inhibition constant of selective CCK2R ligands (Ki values as follows: gastrin, 13.2 ± 1.5 nm; JB93,242, 40.6 ± 4.7 nm; JB93,182, 4.2 ± 0.3 nm; PD135,158, 11.7 ± 0.8 nm) and the low inhibition constant of the specific CCK1R antagonist SR27,897 (Ki ≥ 10 μm) (Fig. 1, A and B). Furthermore, the CCK2R efficiently coupled with phospholipase C as shown by the dose-response curve for CCK-induced inositol phosphate production (EC50 of 4.0 ± 0.4 nm, maximal production ∼50-fold basal level after 1 h of stimulation). In agreement with our previous results (14, 16), JB93,242 and PD135,158 behaved as partial agonists with respect to inositol phosphate turnover. These two compounds showed efficacies that represented 22 and 42% of that of the full agonists CCK and gastrin (Fig. 1C). However, JB93,182, which acted as a partial inverse agonist on COS-7 cells overexpressing the CCK2R, did not exhibit this activity in Flp-InTM CCK2R-293 due to the lack of detectable basal constitutive activity of the CCK2R in HEK cells (14) (Fig. 1C). Finally, the effect of nonpeptide ligands on ERK1/2 phosphorylation was evaluated because the activation of the CCK2R is recognized to trigger this signaling pathway (10). As shown in Fig. 1D, CCK-stimulated phosphorylation of ERK1/2 was rapid and sustained over time. JB93,182, JB93,242, and PD135,158 also stimulated phosphorylation. Autoradiography scanning indicated that maximal ERK1/2 phosphorylation achieved with JB93,182, JB93,242, and PD135,158 represented ∼15, 30, and 60–70% of the maximum achieved with CCK, respectively (not shown).
FIGURE 1.
Pharmacological features of the CCK2R expressed in Flp-InTM CCK2R-293 cells. Inhibition binding (A and B) was carried out by incubating radioiodinated CCK in the presence of increasing concentrations of competitor as described under “Experimental Procedures.” Calculated dissociation constant (Kd) for CCK was 4.8 ± 1.0 nm and inhibition constants (Ki) were as follows: gastrin, 13.2 ± 1.5 nm; PD135,158, 11.7 ± 0.8 nm; JB93,242, 40.6 ± 4.7 nm; JB93,182, 4.2 ± 0.3 nm. Inositol phosphate production (C) was measured after 60 min of stimulation with CCK or synthetic compounds. Concentrations giving half-maximal production (EC50) were as follows: CCK, 4.0 ± 0.4 nm; PD135,158, 18.0 ± 0.9 nm; JB93342, 17.0 ± 1.2 nm. Results are the mean ± S.E. (error bars) of 3–5 experiments. D, ERK1/2 activation was measured after stimulation with CCK (0.1 μm) for various times (upper gel) or with JB93,182, JB932,42, or PD135,158 (10 μm) or with CCK (1 nm and 100 nm) for 5 min. Autoradiographies of electrophoresis gels are representative of two others.
Characterization of CCK2R Internalization
Endocytosis and trafficking of the CCK2R were traced using fluorescent CCK2R (CCK2R-GFP) and fluorescent CCK (Alexa F 647-CCK). Prior to the use of these tools, biological experiments were carried out in order to check their pharmacological features (supplemental Fig. 1, A and B). When transiently expressed in HEK 293 cells, CCK2R-GFP bound 125I-CCK with a dissociation constant of 1.15 ± 0.20 nm (versus 1.17 ± 0.25 nm for CCK2R) and efficiently stimulated production of inositol phosphates after CCK stimulation (EC50 of 2.00 ± 0.23 nm versus 0.53 ± 0.04 nm for CCK2R). Furthermore, Alexa F 647-CCK competed with 125I-CCK to CCK2R similarly to unmodified CCK (Ki = 2.4 ± 0.1 nm for Alexa F 647-CCK inhibited 1251-CCK binding to CCK2R with the same potency as native CCK.
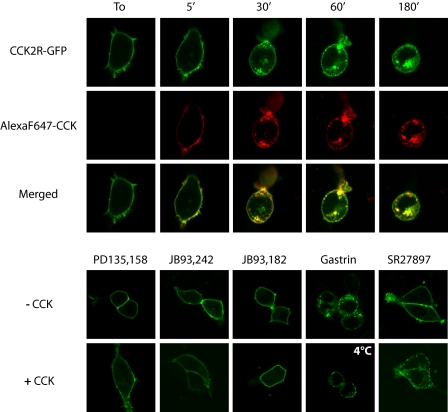
Confocal microscopy observation of living HEK 293 cells transiently expressing CCK2R-GFP showed that CCK2R was expressed uniformly at the cell surface as the unmodified CCK2R immediately after its labeling by Alexa F 647-CCK (Fig. 2). However, soon after the addition of CCK or Alexa F 647-CCK, the membrane fluorescence was relocated in numerous membrane clusters and progressively entered the interior of the cells in punctuate peripheral vesicles. The number of these fluorescent vesicles continuously increased to the detriment of membrane fluorescence, whereas they became more widely distributed within the cytoplasm, including in perinuclear areas, over the time. During CCK2R internalization, the ligand remained trapped together with the receptor in endocytosic vesicles for periods of time as long as 3 h (Fig. 2, merged panels). Gastrin- or CCK-mediated internalization was blocked by incubating the cells at 4 °C. PD135,158, a partial agonist of the CCK2R exhibiting about 40% of maximal CCK-induced inositol phosphate production (Fig. 1C), did not stimulate internalization of CCK2R-GFP, even at supramaximal concentrations, but fully inhibited CCK-induced internalization of CCK2R-GFP. The nonpeptide ligands of the CCK2R, JB93,242 and JB93,182, characterized as a partial agonist and an antagonist/inverse agonist, respectively, were also unable to stimulate CCK2R-GFP internalization and abolished internalization of the receptor induced by CCK (Fig. 2B). The CCK1R antagonist SR27,897 neither induced CCK2R-GFP internalization nor prevented receptor internalization induced by CCK. Last, gastrin stimulated CCK2R-GFP internalization as did CCK.
FIGURE 2.
Confocal microscopy imaging of stimulated CCK2R internalization in HEK cells. HEK cells were transfected with CCK2R-GFP and were incubated at 37 °C with Alexa F 647-CCK for increasing times (upper panels) or with different pharmacological agents for 30 min: partial agonists of the CCK2R on inositol phosphate turnover (PD135,158 and JB93,242, 10 μm), an inverse agonist (JB93,182, 10 μm), the full agonist of the CCK2R (gastrin, 0.1 μm), or an antagonist of the CCK1R (SR27,897, 10 μm) alone or in combination with 0.1 μm CCK (lower panels). The images show internalization of CCK2R-GFP following stimulation by full agonists CCK and gastrin but not other compounds, including potent partial agonists on inositol phosphate turnover that behave as antagonists on CCK2R internalization. Merged images show co-localization of Alexa F 647-CCK with CCK2R-GFP over time. Images are representative of at least three separate experiments.
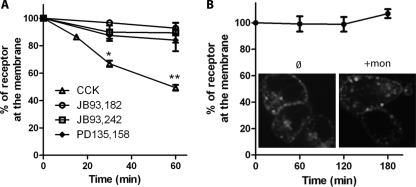
CCK2R internalization was quantified by measuring binding of Alexa F 647-CCK to transfected cells prestimulated with CCK for different times. As shown in Fig. 3A, prestimulation with CCK resulted in a rapid decrease of the amount of fluorescent CCK bound to the cells, indicating that CCK2R progressively internalized during prestimulation with CCK. Half of the CCK2R population was not accessible to Alexa F 647-CCK after 60 min of incubation, a result in satisfactory agreement with confocal microscopy observations (Fig. 2A). In contrast, prestimulations with PD135,158, JB93,242, or JB93,182 did not significantly alter the presence of CCK2R at the cell surface. We further examined the possibility of the population of CCK2R at the cell surface containing a pool of recycled CCK2R in addition to CCK2R that have not yet been internalized. For this purpose, Flp-InTM CCK2R-293 cells stimulated with 0.1 μm CCK at 37 °C for 60 min were then incubated at 37 °C for various times in the absence of CCK, after which CCK2R density was measured using Alexa F 647-CCK. As shown in Fig. 3B, for times up to 3 h, the binding of Alexa F 647-CCK remained stable, which suggested that internalized CCK2R was not significantly recycled at the cell surface within this period of time. This absence of apparent CCK2R recycling within a period shorter than 3 h was confirmed in experiments aimed at quantification of internalization in the presence of the recycling inhibitor, monensin, which had no significant impact on receptor density at the plasma membrane after a challenge with CCK (Fig. 3B). However, as expected, in the presence of monensin, the size of endocytosis vesicles increased (26).
FIGURE 3.
Quantification of internalization and absence of rapid recycling of CCK2R in HEK cells Flp-InTM CCK2R-293. A, quantification of internalization. Cells were preincubated with CCK (0.1 μm) and different pharmacological agents at 37 °C for the times indicated and, after washing out non-internalized ligand, were incubated with Alexa F 647-CCK at a saturating concentration (1 μm) for quantification of membrane CCK2R. B, recycling. Cells were treated with CCK (0.1 μm) for 1 h, washed several times, and incubated with Alexa F 647-CCK (1 μm) for 45 min or 1, 2, or 3 h after the end of CCK stimulation. Confocal microscopy images are inserted to show the lack of effect of the recycling inhibitor monensin (50 μm) on receptor density at the cell surface. Images are representative of at least three separate experiments. Error bars, S.E.
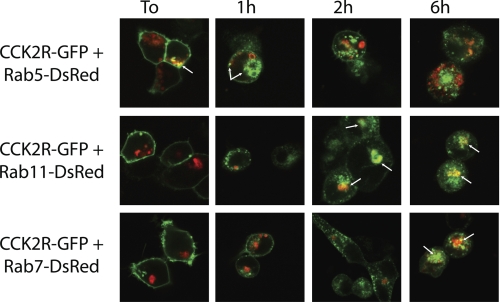
The trafficking and fate of internalized CCK2R under prolonged stimulation was investigated by co-expressing CCK2R-GFP and fluorescent Rab proteins, which are small GTPases regulating intracellular trafficking between functionally distinct membrane compartments within the cell (27). As shown in Fig. 4, following the addition of CCK, CCK2R-GFP immediately and exclusively co-localized in Rab5-illuminated vesicles probably corresponding to early endosomes. Indeed, co-localization with Rab5 was seen in 40% of cells immediately after the addition of CCK; it was maximal (100%) after 1 h and decreased to 70% at 2 h. Localization of CCK2R-GFP in late endosomes or lysosomes containing Rab7 was observed at times longer than 5–6 h poststimulation (80% of cells with co-localization). Localization of CCK2R-GFP to slow recycling endosomes expressing Rab11 was obvious at times longer than 2 h (85% of cells with co-localization) and was maintained for times up to 6 h (100% of cells with co-localization). These co-localization experiments are in line with results indicating that internalized CCK2R was not rapidly recycled to the cell surface but was directed to late endosomes for possible slow recycling or degradation.
FIGURE 4.
Confocal microscopy imaging of intracellular trafficking of internalized CCK2R. HEK cells were transfected with cDNA encoding CCK2R-GFP and Rab5-DsRed, Rab11-DsRed, or Rab7-DsRed. Images were captured at the times indicated after stimulation by CCK (0.1 μm). Arrows show predominant co-localization of CCK2R with Rab5 in early endosomes at initial steps of internalization (time 0 (To) and 1 h), whereas strong co-localization with Rab7 in late endosomes was observed at 5–6 h. Co-localization with Rab11 in recycling endosomes was observed at times longer than 2 h. Each image is representative of 5–7 observations from three separate experiments.
CCK2R Internalization Involves the GTPase Dynamin- and Clathrin-coated Pits
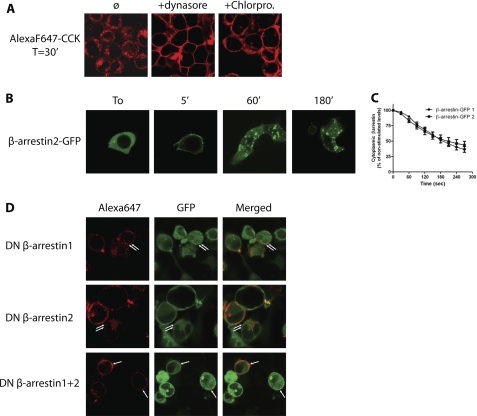
Internalization of many GPCRs following agonist stimulation occurs through clathrin-coated pits and involves recruitment of β-arrestins, the clathrin adaptor AP-2, and the GTPase dynamin (1). We therefore tested whether CCK2R used this major route for internalization. The dependence of CCK2R internalization on dynamin was investigated by using the dynamin inhibitor, dynasore (28), which efficiently abolished internalization, as shown by cell membrane labeling by Alexa F 647-CCK (Fig. 5A). In the presence of chlorpromazine, an inhibitor of clathrin-coated pit formation (29), at a concentration (100 μm) that inhibits Alexa Fluor 488-transferrin endocytosis (not shown), CCK2R labeled by Alexa F 647-CCK failed to internalize (Fig. 5A). Hence, CCK2R internalization in HEK cells is dependent on the membrane GTPase dynamin and most likely occurs through clathrin-coated pits.
FIGURE 5.
Confocal microscopy evidence for an involvement of dynamin, clathrin, and β-arrestins in CCK-stimulated CCK2R internalization. A, HEK cells Flp-InTM CCK2R-293 were incubated with Alexa F 647-CCK (0.1 μm) in the presence of the dynamin inhibitor (dynasore, 80 μm) or the inhibitor of clathrin-coated pit formation (chlorpromazine, 50 μm). B, Flp-InTM CCK2R-293 cells transfected with β-arrestin1-GFP or β-arrestin2-GFP were stimulated with CCK (0.1 μm) for the times indicated. Images show rapid translocation of β-arrestin2-GFP from cytosol to plasma membrane later followed by the appearance of fluorescence-labeled vesicles. C, measurement of the decrease of cytosolic fluorescence showing that β-arrestin1- or β-arrestin2-GFP recruitment to the plasma membrane was rapid and identical (50% of decrease at 200 s). D, Flp-InTM CCK2R-293 cells were transfected with dominant negative β-arrestin1-GFP and/or β-arrestin2-GFP. Confocal microscopy images show that overexpression of dominant negative β-arrestin1 or -2 significantly altered CCK2R internalization, and expression of both β-arrestin1 and -2 blocked internalization in HEK cells. Images are representative of at least three separate experiments.
CCK2R Internalization Involves β-Arrestins
In order to verify whether nonvisual β-arrestins are involved in CCK2R internalization, we first expressed β-arrestin1-GFP or β-arrestin2-GFP in Flp-InTM CCK2R-293 cells. In the absence of stimulation, arrestin2-GFP was localized uniformly in the cytoplasm of the cells (Fig. 5B). β-Arrestin1-GFP was also located in the cytoplasm and slightly in the nucleus (supplementary Fig. 2). The addition of CCK caused rapid illumination (at 5 min) of the cell periphery by the green proteins supporting the translocation of cytosolic β-arrestin2-GFP or β-arrestin1-GFP to the plasma membrane. This recruitment of β-arrestins to the plasma membrane was quantified by measuring the decrease of cytosolic fluorescence. As shown in Fig. 5C, kinetics of β-arrestin2-GFP or β-arrestin1-GFP recruitment to the plasma membrane were similar and so rapid that half of the tagged arrestins initially seen in the cytoplasm were recruited to the plasma membrane within 200 s. After longer times of incubation with CCK, β-arrestin2-GFP and β-arrestin1-GFP were observed as intracellular fluorescent circles resembling endosomes (Fig. 5B and supplemental Fig. 2). It is worth noting that double labeling of endocytosic vesicles by both β-arrestins-GFP and Alexa F 647-CCK lasted for long periods of time (supplemental Fig. 3). Conversely, stimulations by the partial agonists PD135,158 and JB93,242 or by the inverse agonist JB93,182 did not cause translocation of β-arrestin2-GFP or β-arrestin1-GFP to the cell surface (supplemental Fig. 2, shown for time 5 min).
Overexpression of dominant negative of β-arrestin1 or -2 (DN-β-arrestin1 or 2) significantly diminished CCK2R endocytosis (Fig. 5D). Indeed, after 30 min of incubation with Alexa F 647-CCK, in cells overexpressing DN-β-arrestin1 or -2, Alexa Fluor-labeled CCK2R mostly remained trapped in punctuated structures at the plasma membrane (see Fig. 5D and supplemental Fig. 2 for comparison). Some Alexa Fluor-labeled CCK2R were also observed intracellularly, where they co-localized with DN-β-arrestin1-GFP or DN-β-arrestin2-GFP. Strikingly, co-expression of both the dominant negative of β-arrestin1 and 2 efficiently abolished CCK2R internalization (Fig. 5D). Conversely, overexpression of β-arrestin1 and -2 accelerated CCK2R internalization (not shown). Collectively, these experiments suggest that the two nonvisual arrestins, β-arrestin1 and -2, are involved in CCK2R endocytosis.
β-Arrestins Are Directly Recruited by CCK2R upon Stimulation by Full Agonists
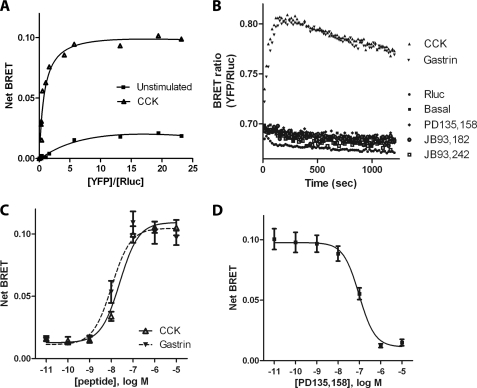
Because β-arrestins were involved in CCK-induced CCK2R internalization and were recruited to the plasma membrane prior to CCK2R internalization, we determined whether recruitment of β-arrestins was due to a direct interaction with the CCK2R and occurred only under full agonist stimulation. For this purpose, a BRET assay was performed using CCK2R-Rluc as the BRET donor and β-arrestin2-YFP or β-arrestin1-YFP as the BRET acceptors co-expressed in HEK 293 cells. Control binding experiments and inositol phosphate assays showed that CCK2R-Rluc displayed pharmacological features identical to those of the untagged CCK2R because the dissociation constant (Kd) of CCK binding was 0.90 ± 0.21 nm, and the EC50 value for production of inositol phosphates was 1.3 ± 0.31 nm (supplemental Fig. 1, C and D). Additionally, stimulation of CCK2R-Rluc by CCK caused recruitment of β-arrestin2-YFP or β-arrestin1-YFP to plasma membrane and underwent rapid internalization, as depicted by fluorescent tracing using Alexa F 647-CCK (data not shown). Together, these results allowed us to use CCK2R-Rluc to monitor β-arrestin recruitment to the CCK2R by BRET.
The BRET titration curve between CCK2R-Rluc and β-arrestin2-YFP or β-arrestin1-YFP (Fig. 6A) indicated that in both unstimulated and CCK-stimulated HEK 293 cells, the BRET donor (CCK2R-Rluc) was progressively associated and finally saturated with BRET acceptor (β-arrestin2-YFP or β-arrestin1-YFP) in the presence of increasing quantities of the latter. In the presence of 0.1 μm CCK, the BRET signal reached 8–10-fold that measured in the absence of CCK. The kinetics of BRET showed that half of maximal recruitment of β-arrestins to the CCK2R occurred 30 s after the addition of CCK or gastrin, and this recruitment remained relatively stable for at least 20 min, which is in line with confocal microscopy observations and quantifications. In contrast, stimulation by PD135,158, JB93,242, or JB93,182 did not cause any significant change in BRET signal (Fig. 6B). Recruitment of β-arrestins to the CCK2R induced by CCK or gastrin was dose-dependent, with EC50 of 26.1 ± 3.1 and 10.5 ± 3.3 nm, respectively (Fig. 6C). These values were slightly higher than the values of the dissociation constants of the peptides determined in binding experiments (4.8 and 13.2 nm). Finally, ligands that did not stimulate β-arrestin recruitment to the CCK2R dose-dependently inhibited CCK-induced BRET signal (for PD135,158, IC50 = 95.9 ± 8.0 nm) (Fig. 6D) (data not shown).
FIGURE 6.
BRET assays showing direct recruitment of β-arrestins to CCK2R stimulated by full agonists but not partial agonists. A, BRET titration curve for the β-arrestin recruitment to CCK2R was measured in HEK 293 cells expressing a constant amount of CCK2R-Rluc and increasing amounts of β-arrestin2-YFP, stimulated by 0.1 μm CCK or not stimulated. Net BRET is expressed as a function of the acceptor/donor ratio and is measured 300 s after the addition of CCK. Specificity of the BRET signal between CCK2R-Rluc and β-arrestin2-YFP was confirmed in experiments showing an absence of BRET signal expressing both CCK2R-Rluc and free YFP (results not shown). B, kinetics of β-arrestin2 recruitment to CCK2R after treatment by full agonists CCK and gastrin (0.1 μm), partial agonists PD135,158 and JB93,242 (10 μm), and inverse agonist JB93,182 (10 μm). C, dose-response curves for CCK and gastrin stimulation of β-arrestin recruitment to CCK2R. D, dose-response curve for inhibition by PD135,158 of CCK-stimulated recruitment of β-arrestin to CCK2R. HEK cells were stimulated for 300 s by 0.1 μm CCK in the absence or in the presence of increasing concentrations of PD135,158 before BRET measurements. Results are expressed as net BRET as described under “Experimental Procedures.” Data are the mean ± S.E. (error bars) of 4–6 independent experiments, each performed in duplicate.
The C-terminal Region of CCK2R Is Critical for β-Arrestin Recruitment but Not for CCK2R Internalization
In many GPCRs, key residues involved in the recruitment of β-arrestins and receptor internalization are phophorylatable amino acids located in the C-terminal region of the receptor (30). We therefore constructed a large series of CCK2Rs in order to identify those residues. Control experiments were conducted to verify that the potency and efficacy of truncated and mutated CCK2R on the G-protein dependent signaling pathway (i.e. inositol phosphate production) were retained. Results depicted in supplemental Table 1 indicate that all of the mutants responded to CCK with potencies similar to that of the wild-type CCK2R, although several truncated and mutated receptors exhibited enhanced maximal responses relative to the wild-type receptor.
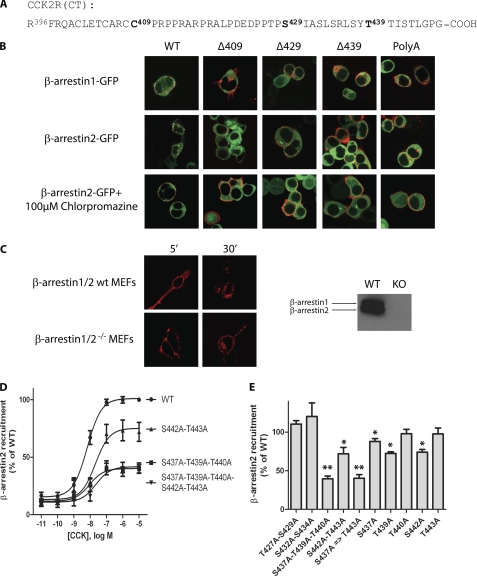
CCK2R truncated at residues 409, 429, and 439 and a CCK2R mutant having all Ser and Thr residues from the C-terminal region substituted by Ala (namely Δ409-CCK2R, Δ429-CCK2R, Δ439-CCK2R, and poly(A)-CCK2R, respectively) (Fig. 7A) were assayed by confocal microscopy for their ability to internalize and recruit GFP-tagged β-arrestins. We observed that Δ409-CCK2R, Δ429-CCK2R, and Δ439-CCK2R as well as poly(A)-CCK2R only slightly recruited β-arrestin2-GFP or β-arrestin1-GFP (Fig. 7B). However, all three truncated and poly(A)-mutated CCK2Rs significantly internalized after stimulation by Alexa F 647-CCK (Fig. 7B). Remarkably, unlike with the wild-type CCK2R, no colocalization could be observed between internalized CCK2R and β-arrestin2-GFP in cells expressing Δ409-CCK2R, Δ429-CCK2R, Δ439-CCK2R, or poly(A)-CCK2R, suggesting that these CCK2R variants would internalize through a β-arrestin-independent mechanism. We next evaluated whether this potential alternative mechanism still involved clathrin-coated pits. Results on Fig. 7B show efficient inhibition of internalization in the presence of the clathrin inhibitor, chlorpromazine. We further verified the possibility of the wild-type CCK2R internalizing in the absence of β-arrestins by expressing the CCK2R in β-arrestin1/2 double knock-out MEF cells. As shown in Fig. 7C, internalization of Alexa Fluor-labeled CCK2R was evident and abundant immediately after the stimulation, supporting the existence of an arrestin-independent mechanism for CCK2R internalization.
FIGURE 7.
Evidence that the C-terminal region of the CCK2R is critical for β-arrestin recruitment to CCK2R and that this interaction is dispensable for CCK2R internalization. A, sequence of the C-terminal region of the CCK2R and positions where CCK2R was truncated (Δ409-CCK2R, Δ429-CCK2R, Δ439-CCK2R) or mutated (all Ser and Thr residues were substituted by Ala (poly(A)-CCK2R). B, Flp-InTM β-arrestin2-GFP-293 cells transfected with CCK2R constructs or Flp-InTM 293 cells co-transfected with β-arrestin1-GFP were stimulated with 0.1 μm Alexa 647 F-CCK. Images were captured at different times (shown for 30 min), showing that although β-arrestin recruitment was almost abolished by C-terminal truncation or mutation of the CCK2R, endocytosis remained abundant and dependent on clathrin-coated pits because it was blocked by chlorpromazine. C, wild-type MEF cells and β-arrestin1/2−/− MEF cells expressing the CCK2R were incubated with Alexa F 647-CCK for the times indicated. Images show intensive internalization of the labeled CCK2R in the two cell lines. Immunoblots show the absence of β-arrestin1 or -2 in β-arrestin1/2−/− MEF cells. D and E, effects of threonine/serine single or multiple mutations on CCK-stimulated β-arrestin recruitment to the CCK2R (D, dose/response curves; E, BRET recruitment induced by 0.1 μm CCK). BRET experiments were performed at least in triplicate according to the protocol described in the legend to Fig. 6. Images are representative of at least three separate experiments. *, p < 0.05; **, p < 0.01 compared with the wild-type CCK2R. Error bars, S.E.
Because the above results indicate that a region downstream of residue 409 in the CCK2R is essential for β-arrestin recruitment, we aimed at identifying more precisely the residues or motifs involved. Results from BRET experiments with CCK2R-Rluc mutated on serine and/or threonine in the C-terminal tail indicated that double mutations of Thr427/Ser429 or Ser432/Ser434 and single mutation of residues Thr440 or Thr443 did not significantly affect recruitment of β-arrestin2-YFP to the receptor (Fig. 7E). In contrast, single mutation of Ser437, Thr439, or Ser442 as well as double mutation of Ser442/Thr443 significantly decreased BRET between CCK2R-Rluc and β-arrestin2-YFP. Simultaneous mutation of Ser437-Thr339-Thr440 or Ser437-Thr339-Thr440-Ser442-Thr443 caused equal maximal decrease in BRET signal (Fig. 7, C and D). This finding highlights the crucial importance of the sequence Ser437-Xaa438-Thr439-Thr440-Xaa441-Ser442-Thr443 for recruitment of β-arrestin2 to the CCK2R.
All together, these data indicate that 1) amino acids crucial for β-arrestin recruitment to the CCK2R and which participate in arrestin-dependent internalization correspond to Ser437-Xaa438-Thr439-Thr440-Xaa441-Ser442-Thr443 located in the C-terminal region of the CCK2R, and 2) truncated and poly(A)-mutated CCK2R and the wild-type CCK2R in cells lacking β-arrestins can efficiently use an alternative mechanism of β-arrestin recruitment for their internalization process; this alternative mechanism most likely involves clathrin-coated pits and amino acids outside of the C-terminal region of the CCK2R.
DISCUSSION
In our effort to gain insight into the functioning and regulation of the CCK2R, which is a GPCR of crucial importance in the central nervous system and in peripheral organs (10, 12), we undertook the current study. We demonstrated that the CCK2R internalizes rapidly and abundantly in response to stimulation by the two equipotent natural agonists of the receptor, CCK and gastrin. The absence of rapid recycling and colocalization of internalized receptors with fluorescent Rab7 and Rab11 indicates targeting of internalized CCK2R to late endosomes or lysosomes for slow recycling or degradation. Although fluorescent CCK or gastrin remained trapped in endocytosic vesicles over times as long as 6 h, we do not know if the peptidic ligands were still intact and bound to internalized CCK2R at these times or if they were degraded by proteases. Other studies have shown that substance P, calcitonin gene-related peptide, and somatostatin are degraded in acidified early endosomes following internalization with their cognate receptors (32). Interestingly, it was suggested that retention of ligand integrity or ligand degradation may impact signaling triggered by internalized GPCR (33).
Internalization of GPCRs can occur through two main membrane structures, caveolae and clathrin-coated pits. Here, we show that chlorpromazine, a chemical inhibitor of clathrin-coated pit formation, strongly affected receptor internalization, supporting recruitment of membrane CCK2R to clathrin-coated pits for their subsequent endocytosis. Furthermore, CCK2R internalization was blocked by dynasore, an inhibitor of the mechanochemical enzyme dynamin, which thus appears to play a key role in the fission of CCK2R-containing endosomes (28). Several lines of evidence show the involvement of β-arrestin1 and -2 in the internalization of the CCK2R. GFP-tagged arrestins were translocated from cytosol to the plasma membrane after CCK or gastrin stimulation; GFP-tagged arrestins co-localized with the CCK2R during endocytosis; overexpression of both β-arrestin1 and -2 dominant negative efficiently abolished CCK2R internalization; and BRET experiments confirmed agonist-stimulated recruitment of β-arrestin1 and -2 to the CCK2R.
Previous study of agonist-mediated translocation of β-arrestins to GPCRs identified two classes of GPCRs (34). Members of class A, which includes β2-adrenergic receptor, μ-opioid receptor, and endothelin type 1 receptor, bind β-arrestin2 with a higher affinity than β-arrestin1, whereas members of class B, comprising angiotensin II type 1A receptor, vasopressin 2 receptor, and substance P receptor, bind both β-arrestin1 and -2 with similar high affinities (34). Subsequently, the affinity with which GPCRs bind arrestins was related to the degree of receptor phosphorylation by GPCR kinases, the intracellular trafficking, and cellular signal triggered by internalized GPCRs (35). In the current study, confocal microscopy analysis of translocation of fluorescent β-arrestin1 or -2 to the plasma membrane indicated that recruitment of both β-arrestin1 and -2 to the CCK2R occurs with rigorously identical kinetics. Moreover, both β-arrestin1 and -2 remained stably associated with endocytosic vesicles throughout the time. This finding was confirmed, at the molecular level, by BRET measurements that account for proximity (≤10 nm) or direct interaction of two partner proteins. In these BRET experiments, binding affinities of the two β-arrestins for the CCK2R as judged by BRET50 were similar, and BRET signal remained almost stable for times longer than 20 min, confirming that the presence of β-arrestins at the periphery of endocytosic vesicles was the result of a stable and direct interaction with the CCK2R. These converging results lead us to classify the CCK2R as belonging to class B of the GPCRs.
The importance of the C-terminal region of the CCK2R (and, more precisely, of phophorylatable Ser/Thr within this region) for β-arrestin recruitment and receptor internalization was further evaluated by analyzing the behavior of C-terminally truncated and mutated CCK2R. We found that determinants for β-arrestin binding to the CCK2R reside in motif Ser437-Tyr438-Thr439-Thr440-Ile441-Ser442-Thr443, which is frequently found in GPCRs (30, 35). It is not known if three or more residues of this motif are phosphorylated upon CCK stimulation because BRET results agree with the view that three phosphates is the threshold number for β-arrestin binding to GPCRs (35).
Strikingly, we observed that in cells co-expressing poly(A)-CCK2R and YFP- or GFP-tagged β-arrestins, a weak translocation of β-arrestins from the cytosol to the plasma membrane was still observed under CCK stimulation, and 35% of maximal signal BRET could be measured between β-arrestin2 and the CCK2R mutant having five Ser/Thr residues substituted by alanine. Internalization of the Alexa Fluor 647-labeled CCK2R was not abolished by C-terminal tail deletion or by exchanging all serine and threonine residues for an alanine, even in conditions of normal endogenous expression of β-arrestins in HEK cells. However, with these CCK2R variants, no colocalization of fluorescent β-arrestins with internalized receptors could be observed. To explain this result, one may suggest that modified CCK2Rs within the C-terminal region form relatively unstable complexes with arrestins that would dissociate at or near the plasma membrane, as observed for GPCRs of group A (34). Alternatively, internalization of the CCK2R could switch from a β-arrestin-dependent to a β-arrestin-independent mechanism when crucial determinants for binding of this adaptor (β-arrestin) are lacking on the receptor. The observation of abundant internalization of the CCK2R in MEF-β-arrestin1/2−/− and its inhibition by chlorpromazine strongly supports this second switch hypothesis.
Precedent exists showing that deletion of the distal carboxyl-terminal region of the A2b adenosine receptor switches internalization from an arrestin- and clathrin-dependent to an arrestin- and clathrin-independent pathway (36). Furthermore, the requirement of β-arrestin for receptor internalization is very dependent on the type of GPCR. For instance, whereas the β2-adrergic receptor does not internalize in MEF-β-arrestin1/2−/− cells, the protease-activated receptor-1, the formyl peptide receptor, and the urotensin-II receptor internalize normally in these cells, and nearly 20% of angiotensin AT1A receptor still internalizes (37–40). Basal constitutive internalization of the ghrelin receptor is arrestin-independent, whereas agonist-stimulated internalization is arrestin-dependent (41).
GPCRs that internalize through clathrin-coated pits independently of β-arrestins can use the heterotetrameric complex AP-2 that connects cargo molecules directly to clathrin. AP-2 is able to recruit molecules through μ2 or α/σ2 subunit interactions with tyrosine-based (YXXΦ) or dileucine ((D/E)XXXL(L/I)) motifs, which are most often located in the C-terminal region of GPCRs. Such mechanisms were reported, for instance, for the P2X4 purinergic receptor, PAR1 protease-activated receptor, and CXCR2 chemokine receptor (42–44). In the case of CCK2R, an analysis of amino acid sequence highlights the presence of two tyrosine-based sorting signals that might be responsible for binding of the CCK2R with the μ2-subunit of the AP-2 complex. These are located in the third intracellular loop at positions 246–249 (YLGL) and 294–297 (YVQL). Future studies will examine if these motifs or other not yet identified mechanisms are responsible for β-arrestin-independent internalization of the CCK2R and if the β-arrestin-independent pathway of internalization is employed in parallel with the β-arrestin-dependent one in the physiological context.
Pioneering work quantified internalization of the rat CCK2R in NIH 3T3 cells and showed the importance of C-terminal tail and serine/threonine residues (18). Although this study did not investigate the role played by arrestins, the results also suggested a contribution to internalization of regions of the CCK2R outside of the C-terminal tail. This view agrees with the general molecular basis whereby β-arrestins bind to agonist-activated receptors through multiple sites of interaction on both the receptor and β-arrestins, which were termed “activation recognition” and “phosphorylation recognition” sites (35, 45, 46).
Results from the current study reinforce the importance of the C-terminal tail of the CCK2R for its downstream signaling and regulation. We previously documented interaction of the C-terminal region of the CCK2R with RGS-2, SHP-2 phosphatase, or phospholipase-C γ1 (17, 47, 48). The motif involved in functional binding of SHP2 phosphatase and phospholipase-C γ1 to the CCK2R is an ITIM motif containing Tyr438, which, once phosphorylated, recruits signaling proteins containing an Src homology 2 domain(s). We suggested that the binding of CCK2R to the Src homology 2 domain of phospholipase-C γ1 in the pancreas of mice overexpressing the CCK2R may participate in the development of preneoplastic lesions and pancreatic cancer (49). Concerning interaction of RGS-2, we demonstrated that it involves Ser434 and Thr439, requires phosphorylation of these residues, and serves for RGS2 recognition by the CCK2R (17).
The current study also provides new important pharmacological data on the CCK2R because it allowed identification of biased ligands of this receptor. Indeed, we show that, unlike CCK and gastrin, which are full agonists with respect to inositol phosphate production, partial agonist PD135,158 or JB93,242 on the G-protein-dependent pathway as well as the antagonist JB93,182 did not promote receptor internalization and were unable to stimulate recruitment of β-arrestins. However, these three nonpeptide ligands acted as antagonists on CCK-induced recruitment of β-arrestins to the CCK2R and its internalization. All three ligands, PD135,158, JB93,242, and, more surprisingly, JB93,182, stimulated ERK1/2 phosphorylation. Delineation of the precise mechanism linking binding of these ligands to the CCK2R with ERK activation will require further investigations. However, based on BRET and confocal microscopy data showing an inability of these ligands to stimulate β-arrestin recruitment and CCK2R internalization, it is unlikely that these two events are involved. Most likely, ERK activation by PD135,158 and JB93,242 would be a consequence of phospholipase-C activation (10). The mechanism leading to ERK1/2 activation by JB93,182 remains unexplained because this compound did not stimulate phospholipase-C or β-arrestin recruitment. Hence, PD135,158 and JB93,242 meet the criteria of biased ligands of the CCK2R because they are capable of stimulating a typical G-dependent signaling pathway but are unable to stimulate β-arrestin recruitment and receptor internalization. JB93182 is another biased ligand capable of stimulating ERK1/2 phosphorylation but unable to stimulate phospholipase C activation, β-arrestin recruitment, and receptor internalization. These findings may have a significant impact on the pharmacology of the CCK2R and cognate strategies to target it. In the recent period, biased ligands for GPCRs, such as, for example, adrenergic, dopamine, and angiotensin II receptors, have been identified and shown to present pharmacological and therapeutic interest (25, 31, 50, 51). CCK2R joins this group of GPCRs. To explain why PD135,158 and JB93,242 stimulate production of inositol phosphates but could not induce β-arrestin recruitment to the CCK2R, we suggest that conformations of the CCK2R stabilized by CCK or by the two partial nonpeptide agonists are distinct and promote phospholipase-C with different efficacies, and only the state stabilized by the full agonist CCK or gastrin is able to promote β-arrestin recruitment. This view agrees with the general concept, now well supported by experimental data, that GPCRs exist in multiple distinct conformational states that can be stabilized by selective ligands and present distinct signaling profiles (8).
Our work represents a solid basis to evaluate the contribution of internalized CCK2R to CCK2R-induced intracellular signaling. The finding that CCK2R expressed in HEK cells remains bound to β-arrestins during intracellular trafficking suggests that part of the CCK2R-induced signaling and effects may be dependent on β-arrestins. Biased ligands identified in the current study should be able to block β-arrestin-dependent signaling and effects downstream of the CCK2R. This is of high importance because the CCK2R is expressed in a large variety of cancers and, according to a previous study (19), undergoes internalization in gastric, pancreatic, and colonic cell lines that endogenously express this receptor.
Supplementary Material
Acknowledgments
We greatly appreciate the gifts of plasmids encoding GFP-tagged β-arrestin1, YFP-tagged β-arrestin1, and YFP-tagged β-arrestin2 from Marc Caron (Duke University Medical Center) as well as GFP-tagged β-arrestin2 and MEF cells, wild type and knock-out for β-arrestins, from Robert Lefkowitz (Duke University Medical Center). We thank Dr. Romina D'Angelo for assistance and advice with imaging (Cellular Imaging Facility IFR150-Rangueil/TRI Platform).
This work was supported by Association pour la Recherche contre le Cancer Grant 4870 and the Ligue Nationale Contre le Cancer (Comité 31).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Table 1 and Figs. 1–3.
- GPCR
- G protein-coupled receptor
- CCK
- cholecystokinin
- CCK1R and CCK2R
- cholecystokinin-1 and -2 receptor, respectively
- Nle
- norleucine
- Rluc
- Renilla luciferase
- FRT
- Flp recombination target
- BRET
- bioluminescence resonance energy transfer
- DN
- dominant negative
- MEF
- mouse embryo fibroblast.
REFERENCES
- 1. Hanyaloglu A. C., von Zastrow M. (2008) Annu. Rev. Pharmacol. Toxicol. 48, 537–568 [DOI] [PubMed] [Google Scholar]
- 2. Traub L. M. (2009) Nat. Rev. Mol. Cell Biol. 10, 583–596 [DOI] [PubMed] [Google Scholar]
- 3. Marchese A., Paing M. M., Temple B. R., Trejo J. (2008) Annu. Rev. Pharmacol. Toxicol. 48, 601–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ferguson S. S. (2001) Pharmacol. Rev. 53, 1–24 [PubMed] [Google Scholar]
- 5. Mayor S., Pagano R. E. (2007) Nat. Rev. Mol. Cell Biol. 8, 603–612 [DOI] [PubMed] [Google Scholar]
- 6. von Zastrow M., Sorkin A. (2007) Curr. Opin. Cell Biol. 19, 436–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sorkin A., Von Zastrow M. (2002) Nat. Rev. Mol. Cell Biol. 3, 600–614 [DOI] [PubMed] [Google Scholar]
- 8. Rajagopal S., Rajagopal K., Lefkowitz R. J. (2010) Nat. Rev. Drug Discov. 9, 373–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wank S. A., Pisegna J. R., de Weerth A. (1992) Proc. Natl. Acad. Sci. U.S.A. 89, 8691–8695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dufresne M., Seva C., Fourmy D. (2006) Physiol. Rev. 86, 805–847 [DOI] [PubMed] [Google Scholar]
- 11. Kopin A. S., Lee Y. M., McBride E. W., Miller L. J., Lu M., Lin H. Y., Kolakowski L. F., Jr., Beinborn M. (1992) Proc. Natl. Acad. Sci. U.S.A. 89, 3605–3609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Noble F., Wank S. A., Crawley J. N., Bradwejn J., Seroogy K. B., Hamon M., Roques B. P. (1999) Pharmacol. Rev. 51, 745–781 [PubMed] [Google Scholar]
- 13. Herranz R. (2003) Med. Res. Rev. 23, 559–605 [DOI] [PubMed] [Google Scholar]
- 14. Foucaud M., Tikhonova I. G., Langer I., Escrieut C., Dufresne M., Seva C., Maigret B., Fourmy D. (2006) Mol. Pharmacol. 69, 680–690 [DOI] [PubMed] [Google Scholar]
- 15. Marco E., Foucaud M., Langer I., Escrieut C., Tikhonova I. G., Fourmy D. (2007) J. Biol. Chem. 282, 28779–28790 [DOI] [PubMed] [Google Scholar]
- 16. Foucaud M., Marco E., Escrieut C., Low C., Kalindjian B., Fourmy D. (2008) J. Biol. Chem. 283, 35860–35868 [DOI] [PubMed] [Google Scholar]
- 17. Langer I., Tikhonova I. G., Boulègue C., Estève J. P., Vatinel S., Ferrand A., Moroder L., Robberecht P., Fourmy D. (2009) Mol. Pharmacol. 75, 502–513 [DOI] [PubMed] [Google Scholar]
- 18. Pohl M., Silvente-Poirot S., Pisegna J. R., Tarasova N. I., Wank S. A. (1997) J. Biol. Chem. 272, 18179–18184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tarasova N. I., Wank S. A., Hudson E. A., Romanov V. I., Czerwinski G., Resau J. H., Michejda C. J. (1997) Cell Tissue Res. 287, 325–333 [DOI] [PubMed] [Google Scholar]
- 20. Moroder L., Wilschowitz L., Gemeiner M., Göhring W., Knof S., Scharf R., Thamm P., Gardner J. D., Solomon T. E., Wünsch E. (1981) Hoppe Seylers Z. Physiol. Chem. 362, 929–942 [PubMed] [Google Scholar]
- 21. Fourmy D., Lopez P., Poirot S., Jimenez J., Dufresne M., Moroder L., Powers S. P., Vaysse N. (1989) Eur. J. Biochem. 185, 397–403 [DOI] [PubMed] [Google Scholar]
- 22. Yaqub T., Tikhonova I. G., Lättig J., Magnan R., Laval M., Escrieut C., Boulègue C., Hewage C., Fourmy D. (2010) Mol. Pharmacol. 77, 547–558 [DOI] [PubMed] [Google Scholar]
- 23. Hughes J., Boden P., Costall B., Domeney A., Kelly E., Horwell D. C., Hunter J. C., Pinnock R. D., Woodruff G. N. (1990) Proc. Natl. Acad. Sci. U.S.A. 87, 6728–6732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kalindjian S. B., Dunstone D. J., Low C. M., Pether M. J., Roberts S. P., Tozer M. J., Watt G. F., Shankley N. P. (2001) J. Med. Chem. 44, 1125–1133 [DOI] [PubMed] [Google Scholar]
- 25. Masri B., Salahpour A., Didriksen M., Ghisi V., Beaulieu J. M., Gainetdinov R. R., Caron M. G. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 13656–13661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mollenhauer H. H., Morré D. J., Rowe L. D. (1990) Biochim. Biophys. Acta 1031, 225–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stenmark H. (2009) Nat. Rev. Mol. Cell Biol. 10, 513–525 [DOI] [PubMed] [Google Scholar]
- 28. Macia E., Ehrlich M., Massol R., Boucrot E., Brunner C., Kirchhausen T. (2006) Dev. Cell 10, 839–850 [DOI] [PubMed] [Google Scholar]
- 29. Wang L. H., Rothberg K. G., Anderson R. G. (1993) J. Cell Biol. 123, 1107–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Oakley R. H., Laporte S. A., Holt J. A., Barak L. S., Caron M. G. (2001) J. Biol. Chem. 276, 19452–19460 [DOI] [PubMed] [Google Scholar]
- 31. Drake M. T., Violin J. D., Whalen E. J., Wisler J. W., Shenoy S. K., Lefkowitz R. J. (2008) J. Biol. Chem. 283, 5669–5676 [DOI] [PubMed] [Google Scholar]
- 32. Cottrell G. S., Padilla B. E., Amadesi S., Poole D. P., Murphy J. E., Hardt M., Roosterman D., Steinhoff M., Bunnett N. W. (2009) J. Biol. Chem. 284, 22411–22425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Roosterman D., Kempkes C., Cottrell G. S., Padilla B. E., Bunnett N. W., Turck C. W., Steinhoff M. (2008) Endocrinology 149, 2200–2207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Oakley R. H., Laporte S. A., Holt J. A., Caron M. G., Barak L. S. (2000) J. Biol. Chem. 275, 17201–17210 [DOI] [PubMed] [Google Scholar]
- 35. Gurevich V. V., Gurevich E. V. (2006) Pharmacol. Ther. 110, 465–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mundell S. J., Matharu A. L., Nisar S., Palmer T. M., Benovic J. L., Kelly E. (2010) Br. J. Pharmacol. 159, 518–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kohout T. A., Lin F. S., Perry S. J., Conner D. A., Lefkowitz R. J. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 1601–1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Paing M. M., Stutts A. B., Kohout T. A., Lefkowitz R. J., Trejo J. (2002) J. Biol. Chem. 277, 1292–1300 [DOI] [PubMed] [Google Scholar]
- 39. Vines C. M., Revankar C. M., Maestas D. C., LaRusch L. L., Cimino D. F., Kohout T. A., Lefkowitz R. J., Prossnitz E. R. (2003) J. Biol. Chem. 278, 41581–41584 [DOI] [PubMed] [Google Scholar]
- 40. Giebing G., Tölle M., Jürgensen J., Eichhorst J., Furkert J., Beyermann M., Neuschäfer-Rube F., Rosenthal W., Zidek W., van der Giet M., Oksche A. (2005) Circ. Res. 97, 707–715 [DOI] [PubMed] [Google Scholar]
- 41. Holliday N. D., Holst B., Rodionova E. A., Schwartz T. W., Cox H. M. (2007) Mol. Endocrinol. 21, 3100–3112 [DOI] [PubMed] [Google Scholar]
- 42. Royle S. J., Bobanović L. K., Murrell-Lagnado R. D. (2002) J. Biol. Chem. 277, 35378–35385 [DOI] [PubMed] [Google Scholar]
- 43. Paing M. M., Johnston C. A., Siderovski D. P., Trejo J. (2006) Mol. Cell. Biol. 26, 3231–3242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fan G. H., Yang W., Wang X. J., Qian Q., Richmond A. (2001) Biochemistry 40, 791–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lan H., Teeter M. M., Gurevich V. V., Neve K. A. (2009) Mol. Pharmacol. 75, 19–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ouedraogo M., Lecat S., Rochdi M. D., Hachet-Haas M., Matthes H., Gicquiaux H., Verrier S., Gaire M., Glasser N., Mély Y., Takeda K., Bouvier M., Galzi J. L., Bucher B. (2008) Traffic 9, 305–324 [DOI] [PubMed] [Google Scholar]
- 47. Arnould M., Tassa A., Ferrand A., Archer E., Estève J. P., Pénalba V., Portolan G., Escherich A., Moroder L., Fourmy D., Seva C., Dufresne M. (2004) FEBS Lett. 568, 89–93 [DOI] [PubMed] [Google Scholar]
- 48. Vatinel S., Ferrand A., Lopez F., Kowalski-Chauvel A., Estève J. P., Fourmy D., Dufresne M., Seva C. (2006) Biochim. Biophys. Acta 1763, 1098–1107 [DOI] [PubMed] [Google Scholar]
- 49. Bierkamp C., Kowalski-Chauvel A., Dehez S., Fourmy D., Pradayrol L., Seva C. (2002) Oncogene 21, 7656–7670 [DOI] [PubMed] [Google Scholar]
- 50. Rajagopal K., Lefkowitz R. J., Rockman H. A. (2005) J. Clin. Invest. 115, 2971–2974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rajagopal K., Whalen E. J., Violin J. D., Stiber J. A., Rosenberg P. B., Premont R. T., Coffman T. M., Rockman H. A., Lefkowitz R. J. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 16284–16289 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.