Abstract
Several studies have shown the protective effects of dietary enrichment of various lipids in several late-onset animal models of Alzheimer Disease (AD); however, none of the studies has determined which structure within a lipid determines its detrimental or beneficial effects on AD. High-sensitivity enzyme-linked immunosorbent assay (ELISA) shows that saturated fatty acids (SFAs), upstream omega-3 FAs, and arachidonic acid (AA) resulted in significantly higher secretion of both Aβ 40 and 42 peptides compared with long chain downstream omega-3 and monounsaturated FAs (MUFA). Their distinct detrimental action is believed to be due to a structural template found in their fatty acyl chains that lack SFAs, upstream omega-3 FAs, and AA. Immunoblotting experiments and use of APP-C99-transfected COS-7 cells suggest that FA-driven altered production of Aβ is mediated through γ-secretase cleavage of APP. An early-onset AD transgenic mouse model expressing the double-mutant form of human amyloid precursor protein (APP); Swedish (K670N/M671L) and Indiana (V717F), corroborated in vitro findings by showing lower levels of Aβ and amyloid plaques in the brain, when they were fed a low fat diet enriched in DHA. Our work contributes to the clarification of aspects of structure-activity relationships.
Keywords: Aging, Alzheimer Disease, Fatty Acid, Lipid, Lipid Structure, Micfatty Acid, Amyloid, Transgenic e, COS-7 Cells, Plaques
Introduction
Alzheimer Disease (AD)2 is a currently incurable progressive neurological disease that affects higher brain functions, ultimately leading to dementia and death (1). The molecular key event triggering the harmful cascade in AD is generally considered to be increased soluble oligomeric Aβ, or more specifically Aβ42 levels (2–4). Whereas Aβ generation depends on several factors, the prime event in Aβ generation is cleavage of APP-C99 by γ-secretase. This is because it is the last essential step in Aβ40 versus Aβ42 generation, and it is also this cleavage for which most mechanistic information linking lipid homeostasis with Aβ production is available (5, 6).
Several studies have shown the protective effects of various lipids on several parameters of AD; however, none of the studies has determined which structure within a lipid decides its detrimental or beneficial effects on Aβ production or the Aβ40/42 ratio. Using an AD-cell culture model we identified the parameters, which may define whether a lipid or FA will affect Aβ production. A simple structure-activity relationship (SAR) study was performed in an attempt to examine the following factors: chain length, saturation, position of unsaturation (either at omega-3, -6, -7, or -9 carbon), and orientation (cis, trans) of double bonds as well as the effects of natural and synthetic/conjugated lipids. Based on our in vitro data, we designed a diet high in DHA but low in all deleterious fats and cholesterol, and showed for the first time in an early-onset robust mouse model of AD (which represents individuals who are at high risk of developing early-onset AD due to genetic mutations), that the protective structural template built-in the acyl chain of DHA indeed improves AD-type neuropathology, provided other deleterious fats are eliminated from the diet. Though, there is a substantial amount of data that conferred a protective effect of DHA dietary enrichment beginning at 6 months of age and onwards in several animal models of AD (7–11) (with little or no information about other lipids present in the diet). However, to date none of the studies has shown the effects of the initiation of DHA dietary enrichment prior to plaque formation.
Finally, understanding the rules that govern the interaction between lipids and Aβ production will ultimately help to understand the mechanistic implications of γ-secretase regulation and may result in improved dietary advice and food additives aimed at the prevention and treatment of early-onset AD.
EXPERIMENTAL PROCEDURES
Mice, Treatment, and Tissue Preparation
All experimental procedures were performed according to the animal care guidelines of the University of Western Ontario (UWO; UCRE; approval ID; 2004-065-06). All studies were performed on 5-month-old congenic C57/129Sv male mice heterozygous for TgCRND8, which express a double-mutant form of human APP695; Swedish (K670N/M671L) and Indiana (V717F) (12). Mice used in these studies were housed on 12 h light/dark cycles. At 3 weeks of age, TgCRND8 mice were provided ad libitum access to water and one of three diets (n = 5 for each diet) until 23–24 weeks of age at which time they were sacrificed by cervical decapitation. Brains were rapidly removed and sectioned sagitally into hemibrains. One hemibrain was used for ELISA. The other hemibrain was fixed in 10% neutral buffered formalin and 0.1 m PBS for 48 h and then stored at 4 °C in PBS and 1% sodium azide until used for immunohistochemistry.
Dietary Manipulations
The experimental diet involved the following three groups: (Diet 1) conventional mouse chow (chow; PicoLab Mouse Chow Diet 20; Purina Mills, St. Louis, MO); (Diet 2) Soybean oil (1%), high protein, cholesterol-free control diet (low fat/-DHA; Research Diets Product L10047, New Brunswick); (Diet 3) Soybean oil (1%), high protein, cholesterol free diet supplemented with 2% DHA (low fat/+DHA; Nu-Chek-Prep, Inc). Composition of the diets 2 and 3 was as follows: alcohol-extracted casein (304.7 g/kg), dl-methionine (4.6 g/kg), sucrose (279.3 g/kg), maltodextrin (Fro-Dex) (284.4 g/kg), vitamin Mix, V10001 (AIN-76A) (10.2 g/kg), mineral mix, S10001 (AIN-76A) (35.0 g/kg), cellulose (40.6 g/kg), xanthan gum (8.6 g/kg), soybean oil (10.2 g/kg) with and without DHA (20.0 g/kg). The experimental diets (2) and (3) were supplemented separately by the recommended amount of essential linoleic (LA) and linolenic acids (LNA). Chow diet was used to compare the effects of low fat/-DHA diet alone on AD parameters.
Immunohistochemistry and Image Analysis
Plaque density was measured using immunohistochemistry with a biotinylated-4G8 monoclonal antibody (Signet Laboratories, Dedham, MA), directed against amino acids 17–24 of the β-amyloid peptide. A mean area of the field 0.307 mm2 was examined in three regions of neocortex, hippocampus and amygdala: 0.5–0.9 mm, 0.9–1.35 mm, and 1.35–1.8 mm mediolateral from the bregma (Mouse Brain Atlas, Franklin and Paxinos 2007) on 40 μm thick rostral (cross) sections of each hemisphere. Plaque density was calculated by dividing total area of Aβ-positive structures by total area of region analyzed (in square micrometers).
Cell Culture
COS-7 cells were stably transfected with pCEP-SP-C99 (encoding the C-terminal fragment of human APP; APP-C99) plasmids, maintained in DMEM (Sigma) with 10% fetal calf serum (FCS, PAN), and 200 μg/ml hygromycin (PAN). APP-C99 is a truncated APP form that is fully capable of forming Aβ. APP-C99 contains the signal peptide (SP) of APP, two additional amino acids (Asp and Ala), the Aβ domain and the complete C terminus of APP. APP-C99 only requires γ-secretase activity for Aβ generation (13). Cells were cultured in 10-cm dishes, washed three times with FCS-free medium (to get rid of the lipids present in the FCS) and incubated for 12 h with lipid-free DMEM containing 0.01 to 125 μm of lipid-BSA complexes. Lipid containing DMEM were renewed every 4 h to maintain a steady concentration of lipid. The 8–12 h-conditioned medium was analyzed. Cells grew equally well and maintained normal morphology at all concentrations of lipids used.
Fatty Acid Preparations
FAs (Nu-Chek-Prep, Inc.), organic solvents, and BSA solutions were purged with argon extensively, prior to use. The protocols to form fresh FA-BSA complex (2:1) is based on established procedures (14).
Sandwich ELISAs
Quantification of Aβ40 and 42 production in conditioned medium (15) and in brain (Signet Laboratories, Dedham, MA) was done by standard ELISA methods.
Immunoprecipitation and Quantitative Western Blotting
A high-sensitive Western blot to determine total in vitro Aβ (quantitative immunoprecipitation) and APP-C99 using W0–2 monoclonal antibody (1 μg/ml) directed against amino acids 4–10 of human Aβ precipitates established in our laboratory has been described previously (16). Band intensities were scanned and quantified with Image Gauge software (Bio-Rad), and ratios of the background subtracted protein bands normalized to β-actin were analyzed.
Statistics
All values were presented as means ± S.E. of the mean (S.E.). Protein densitometric, Aβ40 and 42 levels, and plaque area were compared by Student's unpaired two-tailed t tests. C99, total Aβ levels and ratios of Aβ40/42 to DHA concentration were analyzed using one-way ANOVA followed by post Tukey test. The significance level was chosen to be 0.05 (p ≤ 0.05). Correlations between parameters were tested by linear regression analysis.
RESULTS
Instead of doing a blinded, full-blown animal study, we initially screened FAs with no or multiple double bonds on in vitro Aβ levels in the cell culture AD model to generate the pilot data as well as to set up the direction for future in vivo experiments and to do the power analysis. It is important to mention that transfected COS-7 cells are well established as a model for examining the effects of AD proteins/genes on a number of parameters (13, 17). Our results also demonstrate that APP protein could be effectively expressed in COS-7 cells as has already been shown (13, 17–19) and that the transfected COS-7 cells could be used as a model of APP protein overexpression to examine the function of APP in mammalian cells (13, 17). In addition, our recent reports (14, 20–21) demonstrate that transfected COS-7 cells not only can be used for lipid studies but the results of these experiments can also be reproduced in AD animal models.
Effects of Fatty Acids with No or Single Double Bond on in Vitro Aβ Levels
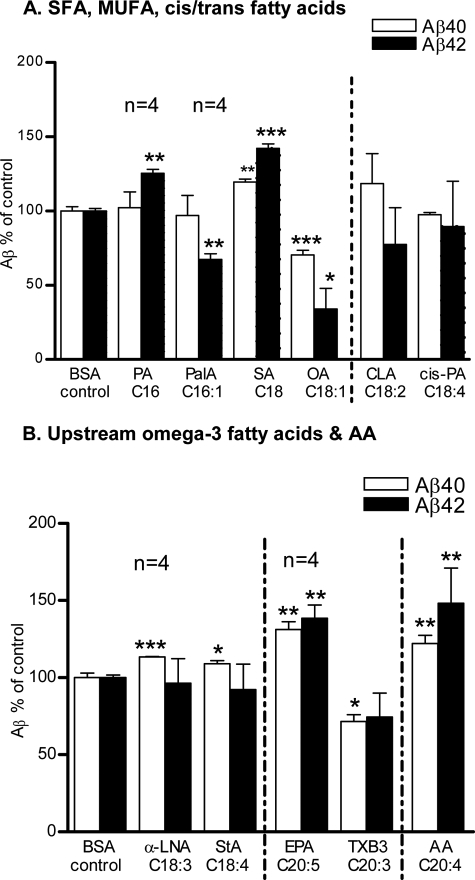
Supplementing the COS-7 cells with long chain saturated fatty acids such as palmitic acid (PA; C16:0) and stearic acid (SA; C18:0) resulted in a substantial increase in the secretion of both Aβ peptides (Aβ40 = 7%, Aβ42 = 26%; p < 0.01 by PA, Aβ40 = 22%; p < 0.01, Aβ42 = 48%; p < 0.001 by SA) into the conditioned medium compared with the BSA control. To determine the effects of the incorporation of a single double bond in the acyl chains of PA and SA, we next checked the effects of monounsaturated FAs (MUFAs) of the ω-9 pathway. Interestingly, palmitoleic acid (PalA; C16:1 Δ9, w-9) selectively reduced the levels of Aβ42 peptide up to 30% (p < 0.01). However, an almost similar reduction (Aβ40 = 30%; p < 0.001, Aβ42 = 44%; p < 0.05) was achieved by OA at 0.1 μm compared with the BSA control (Fig. 1A). To investigate the effects of conjugated cis and trans double bonds on Aβ levels, we next evaluated conjugated linoleic acid (CLA; C18:2 Δ9Z,11E, omega-7) and cis-parinaric acid (cis-PA; C18:4 Δ9Z,11E,13E,15Z, omega-3). The term CLA refers to a collection of positional and geometrical isomers of octadecadienoic acid with conjugated double bonds at 9Z,11E (22). cis-PA is a naturally occurring fluorescent PUFA and contains an unusual conjugated tetraene. CLA and cis-PA decreased Aβ42 levels (24% by CLA and 10% by cis-PA) compared with control; however, their effects were not significant (Fig. 1A).
FIGURE 1.
Effects of fatty acids with no or single double bond on in vitro Aβ levels. The BSA control normalized Aβ levels in the conditioned medium after 12 h of incubation with 25 μm (stated otherwise in results) of: A, saturated & monounsaturated FAs: PA, PalA, SA, OA, cis- and trans-conjugated double bonds containing FAs: CLA and cis-PA. B, upstream omega-3 FAs: αLNA, StA, EPA, TXB3, and omega-6 FA: AA. The mean ± S.E. are shown for all plots (n = 3, except where noted). ***, p < 0.001; **, p < 0.01; *, p < 0.05.
Effects of Upstream Omega-3 Fatty Acids, and Arachidonic Acid on in Vitro Aβ Levels
Next, we checked the effects of upstream omega-3 FAs comprised of 3–5 double bonds and 18–20 carbons in their acyl chains. Surprisingly, as the number of double bonds increases in the acyl chains of α-linolenic acid (α-LNA; C18:3 Δ9,12,15, cis), stearidonic acid (StA; C18:4 Δ6,9,12,15, cis), and eicosapentaenoic acid (EPA; C20:5, Δ5,8,11,14,17, cis) it gradually increases the levels of both Aβ40 (15%; p < 0.001 by α-LNA, 10%; p < 0.05 by StA, 31%; p < 0.001 by EPA), and Aβ42 peptides (40%; p < 0.05 by EPA) compared with the BSA controls. Thromboxane B3 (TXB3, 9α,11,15S-trihydroxythromba-5Z,13E, 172-trien-1-oic acid, C20:3 Δ5Z,13E,172) is a stable hydrolysis product of TXA3, synthesized from EPA by cyclooxygenase (COX) and thromboxane synthase (23, 24) and was included to draw some light on the action mechanism of EPA. In contrast to EPA, TXB3 decreased the secretion of both Aβ peptides (Aβ40 = 26%, p < 0.05) at 0.01 μm compared with the BSA control; however, the decrease in Aβ42 was not significant. Arachidonic acid (AA; C20:4 Δ5,8,11,14, cis) is composed of 20 carbons like EPA; in addition AA is a typical omega-6 FA. As expected, supplementing the COS-7 cells with AA resulted in >20% and >40% increase (p < 0.01) in the secretions of Aβ40 and 42 peptides, respectively in the conditioned medium (Fig. 1B).
Effects of Downstream Omega-3 Fatty Acids on in Vitro Aβ Levels
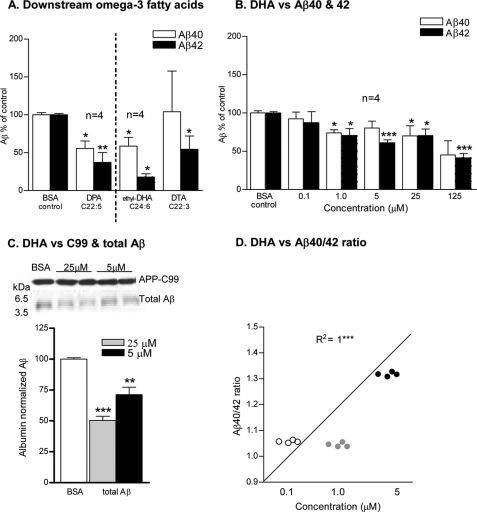
We next checked the effects of downstream omega-3 FAs bearing 3–6 double bonds and 22–24 carbons in their acyl chains on Aβ levels. Docosapentaenoic acid (DPA; C22:5 Δ7,10,13,16,19, cis); the immediate precursor of DHA showed a significant decline in the levels of both Aβ peptides (Aβ40 = >40%; p < 0.05, Aβ42 = >60%; p < 0.01) compared with the BSA control. Similarly, ethyl 4,7,10,13,16,19-docosahexaenic acid (C2H4-DHA) at 1.0 μm and docosatrienoic acid (DTA; C22:3 Δ13,16,19, cis) though rare omega-3 FAs but profoundly decreased the secretion of Aβ species (Aβ40 = 40%; p < 0.05, Aβ42 = 80%; p < 0.05 by ethyl-DHA and Aβ42 = >40%; p < 0.05 by DTA) compared with the BSA controls (Fig. 2A).
FIGURE 2.
Effects of downstream omega-3 fatty acids on in vitro Aβ levels. The BSA control normalized Aβ levels in the conditioned medium after 12 h of incubation with 25 μm: A, downstream omega-3 FAs: DPA, ethyl-DHA, DTA, and B, DHA. C, Western blot of APP-C99 and total Aβ levels in the extracts of APP-C99-transfected COS-7 cells after 12 h of incubation with DHA. Respective molecular masses (kDa) are shown on the left. Plot shows densitometric analysis of the Western blot. D, plot shows in vitro Aβ40/42 ratios versus DHA concentration, symbols show individual replicates at each concentration of DHA used. The mean ± S.E. are shown, (n = 3, except where noted). ***, p < 0.001; **, p < 0.01; *, p < 0.05.
Effects of DHA on in Vitro Aβ Levels
These results also provided a mechanistic insight into the structure-activity relationship of DHA. DHA (C22:6 Δ4,7,10,13,16,19, cis) supplementation resulted in >30% decrease in the levels of both Aβ peptides starting at 1.0 μm, (p < 0.05) onwards into the conditioned medium (Fig. 2B) and the corresponding one-fold decrease in the total Aβ and APP-C99 protein levels in the cell extracts of APP-C99-transfected cells in a concentration-dependent manner compared with the BSA-treated cells (Fig. 2C). Interestingly, the decrease in Aβ42 is more pronounced than Aβ40 at all concentrations used except at 25 μm.
Effects of DHA on in Vitro Aβ40/42 Ratio
Significant and direct correlation was found between increasing ratios of Aβ40/42 and increasing concentrations of DHA (R2 = 1; p < 0.001) up to 5 μm (Fig. 2D). Together, these results suggest that there is an association between DHA concentration and γ-secretase activity.
Effects of Low Fat/+DHA Diet on Plaque Pathology
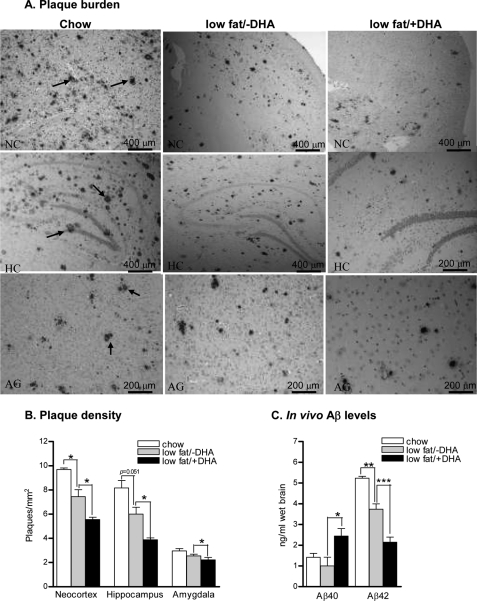
As DHA stood out to be the most potent FA from in vitro studies for its inhibitory effects on in vitro Aβ levels. We therefore designed a diet high in protein and DHA and low in all deleterious fats and cholesterol (based on an ∼18% reduction of fat intake). In agreement with the in vitro DHA data, brain sections of low fat/+DHA mice showed a significant decrease in the plaque density/mm2 of frontal cortex: 5.5 ± 0.19, 7.5 ± 0.57, 9.7 ± 0.12; p < 0.05, hippocampus: 3.8 ± 0.14, 6.0 ± 0.56, 8.1 ± 0.61; p = 0.051, and amygdala: 2.2 ± 0.19, 2.5 ± 0.14, 2.9 ± 0.18; p < 0.05 compared with low fat/-DHA and chow mice, respectively (Fig. 3, A and B).
FIGURE 3.
Effects of low fat/+DHA diet on plaque pathology and in vivo Aβ levels. A, photomicrographs of Aβ stained mice brains in the neocortex (NC), hippocampus (HC), and amygdala (AG) of chow, low fat/−DHA and low fat/+DHA mice. Arrows indicate Aβ-stained plaques. Plots show quantitative analyses of plaque density (B) and Aβ40 and Aβ42 levels in the hippocampi of chow, low fat/−DHA and low fat/+DHA mice (C). The mean ± S.E. are shown, (n = 5 for each group). ***, p < 0.001; **, p < 0.01; *, p < 0.05.
Effects of Low Fat/+DHA Diet on in Vivo Aβ Levels
Similarly, hippocampus and adjacent cortices of low fat/+DHA animals demonstrated a >100% increase in Aβ40 (2.64 ± 0.18, 1.00 ± 0.41, 1.4 ± 0.19; ng/ml, p < 0.05) and ∼40% decrease in the levels of Aβ42 peptides (2.1 ± 0.25, 3.7 ± 0.26, 5.2 ± 0.08; ng/ml; p < 0.01) compared with low fat/−DHA and chow mice, respectively (Fig. 3C). Noticeably, deleterious fat/cholesterol depletion from the diet (low fat/−DHA) alone imparted significant protective effects on amyloid plaque burden and Aβ levels compared with chow mice.
DISCUSSION
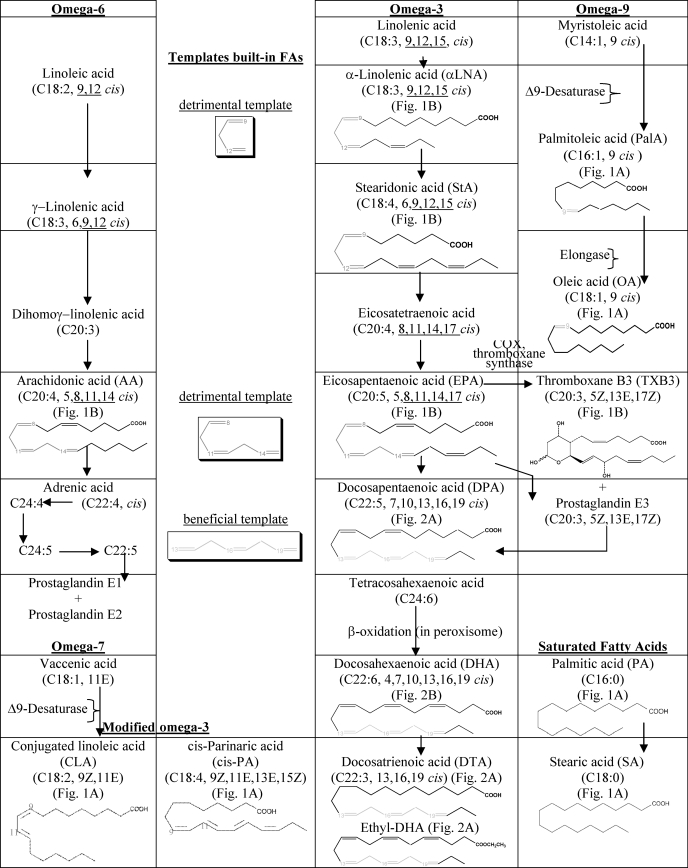
In this study we examined the potential effects of SFAs, MUFA, and PUFA of omega-3, -6, -7, and -9 pathways to explore the structural templates built-in the fatty acyl chains of these FAs for their likely ability to enhance or suppress AD. We identified that double bonds in an all cis configuration strictly at positions Δ13,16,19 (built-in DPA, DHA, DTA, and C2H4-DHA chains) are a beneficial combination for decreasing Aβ production. In contrast, double bonds with an all cis configuration either at positions Δ9,12 (such as linolenic acid (LNA) (data not shown), αLNA, StA), or at Δ8,11,14 (such as EPA and AA) (Table 1, insets) are expected to be detrimental, because they tend to increase Aβ levels. Especially, double bonds at positions Δ9,12 and Δ8,11,14 within 18C and 20C long FAs, respectively appear to be such detrimental templates that even introducing one more double bond either at C15 or C17 of omega-3 FAs offered no advantage to alleviate their harmful effects on Aβ levels.
TABLE 1.
Fatty acid pathways
This latter distinct structural template also explains why OA with only one double bond, lowers the Aβ levels more significantly than AA with four double bonds. Similarly, OA with one double bond and DPA with five double bonds are equally potent for lowering Aβ levels, which indicates possibly identical roles of OA and DPA either on membrane fluidity or Aβ generation/metabolism. This finding also indicates that the extent of (un)saturation and the acyl chain length might be of relevance to affect Aβ production. OA was the only FA among the C18 linear chain FAs, investigated in the present study, to have lowering effects on Aβ levels, probably due to the molecular width (12.80 Å) and length (8.50 Å (where 1 Å = 0.1 nm)) of OA that allows OA to adopt an U-shaped curve (25). This finding also provides an explanation why LNA (data not shown), α-linolenic acid (α-LNA), and stearidonic acid (StA) with chain lengths identical to OA either have no effects or increase Aβ levels. Possibly, the folding of the acyl chains of LNA, α-LNA, and StA at the positions of three double bonds decreased their molecular width than OA.
The most important finding of the present investigation is that it is not the position of the first double bond exclusively (for instance omega-3 or omega-6) that determines the role of PUFA on Aβ production, contrary to general belief (26–31). Rather the overall three-dimensional architecture and thermodynamic properties of each double bond in the lipid acyl chain play an important role in determining the physiological roles of FAs on different parameters of AD. In addition, our finding of the active structural template built in PUFA further provides the basis for some of the already published differential roles of omega-3 and omega-6 PUFAs. For instance, the role of linoleic acid (LA) and AA as the precursors of pro-inflammatory prostaglandins E1 (PGE1) and E2 (PGE2) and the role of omega-3 PUFAs as inhibitor of PGE2 formation (32) and precursors of anti-inflammatory PGE3.
As expected, SFAs (PA and SA) increased Aβ levels in the conditioned medium compared with MUFA and downstream FAs of the omega-3 pathway, in line with previous findings where a direct causal role of PA and SA has been shown in the amyloidogenic processing of APP and in the hyperphosphorylation of tau in neurons mediated by astroglia (10, 11). A few other putative explanations for the increase in Aβ levels by SFAs, apart from their three-dimensional architecture, might be based on either in the lower membrane fluidity of the transfected cells due to the SFA-enriched phospholipids (PL) (33) or due to the ceramide formation by SFA (34). The beneficial effects of PalA on Aβ levels demonstrates that the incorporation of a single double bond in the acyl chain of PA does have a positive consequence probably either on membrane fluidity or Aβ generation/clearance.
The detrimental influence of EPA on Aβ levels is consistent with the harmful effects of EPA reported in literature (35–37). Interestingly, both AA and EPA are the typical stereotypes of omega-6 and omega-3 types of FAs, respectively but in the present investigation, their effects on Aβ levels are comparable. The 3-dimensional configuration these FA adopt in solution might explain this similarity in their action. For instance, EPA binds in the cycloxygenase (COX) active site in a configuration generally similar to that observed for AA (38). Although EPA binds in a manner similar to AA, the additional C17-C18 double bond at the omega end of EPA decreases the flexibility of EPA-substrate in the COX active site resulting in a strained binding orientation. The position of C13 in EPA differs by 1.4 Å with respect to C13 of AA. Therefore, it is not surprising that C13 in both FAs are in correspondingly similar positions (39). This also explains why EPA instead being precursor of DHA does not decrease Aβ levels (35–37). Clearly, the preferred acyl configurations are very different for EPA and DHA, which further justifies that specific geometrical aspects must be invoked to explain the effects of PUFA (chain) on Aβ levels.
During an inflammatory response both AA and EPA are metabolized by COX and lipoxygenase (LOX) enzymes to form corresponding eicosanoids. In general, eicosanoids derived from EPA (3-series prostanoids) are inducers of inflammation, blood vessel constriction, and clotting though less potent than eicosanoids (2-series prostanoids) derived from AA (29, 40). We included one EPA-derived 3-series prostanoid, i.e. thromboxane B3 (TXB3) in our studies to draw some light on the action mechanism of EPA. Surprisingly, TXB3 decreased the level of both Aβ peptides in the conditioned medium. Structure-activity relationship revealed that TXB3 possesses an entirely different structural template than EPA with two cis and one trans double bonds (Δ5Z,13E,17Z), which may explain the differences in the effects of EPA and TXB3 on Aβ levels.
The lowering effects of DHA (C22:6 D4,7,10,13,16,19, cis) on Aβ levels is consistent with its classical neuroprotective role (reviewed in Ref. 28) and could be caused by its special structural template. Primarily, this template may perhaps exert influence by altering membrane fluidity or trafficking of COS-7 cells. Secondly, DHA might have lowered Aβ levels by down-regulating AA incorporation into membrane phospholipids (PL) of COS-7 cells (41). The polyallylic chains of DHA have a tendency to adopt back-folded (hairpin-like) structures due to the double bent conformations, which increase the interfacial area per lipid (42) that may be important for solvating the hydrophobic surfaces of integral membrane proteins, such as APP. Further, the differences in conformational freedom and dynamics between DHA (43), EPA, and AA (35) would likely expect to have an impact on protein function and thus may explain their differential effects on Aβ levels. DHA, DPA, docosatriene (DTA), and ethyl-DHA with identical structural templates were equally effective in decreasing the levels of both Aβ species in the conditioned medium. Given the protective actions of DTA in stroke (39), in inflammation (44, 45) and animal models of AD (46) the lowering of Aβ levels by DTA in our in vitro AD model was not surprising. Because the levels of APP-C99 in the cell extract of DHA supplemented cells was unchanged, the decrease in Aβ levels in the cell extract as well as in conditioned medium is most likely the result of either decreased Aβ production or due to the overactive Aβ clearance mechanism(s).
Though, as a member of omega-3 FAs, the inability of α-LNA to reduce Aβ levels may also be explained by its inability to be converted into DHA (47, 48), a situation most likely to be present in COS-7 cells. In addition, in solution α-LNA behaves like a SFA and enhances the adverse effects of cholesterol (49).
Based on the in vitro data, we designed a diet high in DHA but low in all deleterious fats and cholesterol, and showed that DHA indeed improve AD-type neuropathology in the brains of TgCRND8 mice that demonstrate severe AD-like amyloid pathogenesis, including Aβ brain deposits as early as 3 months of age (12). First, the hippocampal levels of Aβ42 peptides decreased significantly leading to significant decreases in cortical, hippocampal as well as amygdala amyloid plaque burden (both size and number of plaques). The discrepancy in the levels of in vivo and in vitro Aβ40 might result due to the differential effects of DHA on β- versus γ-secretase activity. Because APP-C99 (expressed by COS-7 cells) only requires γ-secretase activity for Aβ generation, in contrast full-length APP expressed by TgCRND mice requires both β- and γ-secretase activities for Aβ generation. The increase in in vivo Aβ40 levels is most likely due to an overall increase in the non-amyloidogenic α-secretase (sAPPα, CTFα, Aβ40) and decrease in amyloidogenic β-secretase (CTFβ, Aβ42) proteolytic processing of APP.
In conclusion, our results provide a structural basis for studies of the interactions of FAs with membrane-associated proteins, such as APP as well as Aβ production and degradation. This simple pharmacophoric/SAR approach would naturally be a very useful tool for virtual drug discovery once these templates are suitably refined to account for some of the predictions presently observed. Further structural studies of these biologically important molecules are vital to provide critical insights into the mechanism(s) that mediate their effects on Aβ metabolism as well as rationale for the use of specific naturally occurring lipids as novel AD therapeutics. More importantly, beneficial effects of protective FAs cannot be achieved perhaps without eliminating the deleterious fats from the diet and yet the amount of protective FAs in the diet needs to be regulated.
Grants from the FP5 framework LipiDiet project QLK-2002-172, the FP7 project LipiDiDiet 211696, London and Middlesex Alzheimer Association (Marion and Chester Fish Alzheimer Disease Research Grant), the Canadian Institutes of Health Research (MOP49546) (to R. F. R.), and the Alexander von Humboldt and Ontario Mental Health Foundation Awards (to Z. A.) funded this research.
- AD
- Alzheimer Disease
- AA
- arachidonic acid
- FA
- fatty acid
- PUFA
- polyunsaturated FA
- DHA
- 4,7,10,13,16,19-docosahexaenic acid
- DTA
- docosatrienoic acid
- SFA
- saturated fatty acid
- MUFA
- monounsaturated FA
- EPA
- eicosapentaenoic acid
- LNA
- linolenic acid
- DPA
- docosapentaenoic acid.
REFERENCES
- 1. Mattson M. P. (2004) Nature 430, 631–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lambert M. P., Barlow A. K., Chromy B. A., Edwards C., Freed R., Liosatos M., Morgan T. E., Rozovsky I., Trommer B., Viola K. L., Wals P., Zhang C., Finch C. E., Krafft G. A., Klein W. L. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 6448–6453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McLean C. A., Cherny R. A., Fraser F. W., Fuller S. J., Smith M. J., Beyreuther K., Bush A. I., Masters C. L. (1999) Ann. Neurol. 46, 860–866 [DOI] [PubMed] [Google Scholar]
- 4. St George-Hyslop P. H. (2000) Sci. Am. 283, 76–83 [DOI] [PubMed] [Google Scholar]
- 5. Grimm M. O., Grimm H. S., Pätzold A. J., Zinser E. G., Halonen R., Duering M., Tschäpe J. A., De Strooper B., Müller U., Shen J., Hartmann T. (2005) Nat. Cell Biol. 7, 1118–1123 [DOI] [PubMed] [Google Scholar]
- 6. Simons M., Keller P., De Strooper B., Beyreuther K., Dotti C. G., Simons K. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 6460–6464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hashimoto M., Tanabe Y., Fujii Y., Kikuta T., Shibata H., Shido O. (2005) J. Nutr. 135, 549–555 [DOI] [PubMed] [Google Scholar]
- 8. Hashimoto M., Hossain S., Agdul H., Shido O. (2005) Biochim. Biophys. Acta 1738, 91–98 [DOI] [PubMed] [Google Scholar]
- 9. Lim G. P., Calon F., Morihara T., Yang F., Teter B., Ubeda O., Salem N., Jr., Frautschy S. A., Cole G. M. (2005) J. Neurosci. 25, 3032–3040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Patil S., Chan C. (2005) Neurosci. Lett. 384, 288–293 [DOI] [PubMed] [Google Scholar]
- 11. Patil S., Sheng L., Masserang A., Chan C. (2006) Neurosci. Lett. 406, 55–59 [DOI] [PubMed] [Google Scholar]
- 12. Chishti M. A., Yang D. S., Janus C., Phinney A. L., Horne P., Pearson J., Strome R., Zuker N., Loukides J., French J., Turner S., Lozza G., Grilli M., Kunicki S., Morissette C., Paquette J., Gervais F., Bergeron C., Fraser P. E., Carlson G. A., George-Hyslop P. S., Westaway D. (2001) J. Biol. Chem. 276, 21562–21570 [DOI] [PubMed] [Google Scholar]
- 13. Lichtenthaler S. F., Beher D., Grimm H. S., Wang R., Shearman M. S., Masters C. L., Beyreuther K. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 1365–1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Amtul Z., Uhrig M., Supino R., Beyreuther K. (2010) Neurosci. Lett. 481, 73–77 [DOI] [PubMed] [Google Scholar]
- 15. Jensen M., Hartmann T., Engvall B., Wang R., Uljon S. N., Sennvik K., Näslund J., Muehlhauser F., Nordstedt C., Beyreuther K., Lannfelt L. (2000) Mol. Med. 6, 291–302 [PMC free article] [PubMed] [Google Scholar]
- 16. Ida N., Hartmann T., Pantel J., Schröder J., Zerfass R., Förstl H., Sandbrink R., Masters C. L., Beyreuther K. (1996) J. Biol. Chem. 271, 22908–22914 [DOI] [PubMed] [Google Scholar]
- 17. Tagawa K., Maruyama K., Ishiura S. (1992) Ann. N.Y. Acad. Sci. 674, 129–137 [DOI] [PubMed] [Google Scholar]
- 18. Hartmann T., Bergsdorf C., Sandbrink R., Tienari P. J., Multhaup G., Ida N., Bieger S., Dyrks T., Weidemann A., Masters C. L., Beyreuther K. (1996) J. Biol. Chem. 271, 13208–13214 [DOI] [PubMed] [Google Scholar]
- 19. Iizuka T., Shoji M., Kawarabayashi T., Sato M., Kobayashi T., Tada N., Kasai K., Matsubara E., Watanabe M., Tomidokoro Y., Hirai S. (1996) Biochem. Biophys. Res. Commun. 218, 238–242 [DOI] [PubMed] [Google Scholar]
- 20. Amtul Z., Westaway D., Cechetto D., Rozmahel R. F. (2010) Oleic Acid Ameliorates Amyloidosis in Cellular and Mouse Models of Alzheimer's Disease (in press), 2010. October 5 doi:10.1111/j.1750-3639.2010.00449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Oksman M., Iivonen H., Hogyes E., Amtul Z., Penke B., Leenders I., Broersen L., Lütjohann D., Hartmann T., Tanila H. (2006) Neurobiol. Dis. 23, 563–572 [DOI] [PubMed] [Google Scholar]
- 22. Carta G., Angioni E., Murru E., Melis M. P., Spada S., Banni S. (2002) Prostaglandins Leukot. Essent. Fatty Acids 67, 187–191 [DOI] [PubMed] [Google Scholar]
- 23. Kulkarni P. S., Srinivasan B. D. (1986) Prostaglandins 31, 1159–1164 [DOI] [PubMed] [Google Scholar]
- 24. Kulkarni P. S., Kaufman P. L., Srinivasan B. D. (1987) J. Ocul. Pharmacol. 3, 349–356 [DOI] [PubMed] [Google Scholar]
- 25. Oda M., Ueno T., Kasai N., Takahashi H., Yoshida H., Sugawara F., Sakaguchi K., Hayashi H., Mizushina Y. (2002) Biochem. J. 367, 329–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Birch E. E., Birch D. G., Hoffman D. R., Uauy R. (1992) Invest. Ophthalmol. Vis. Sci. 33, 3242–3253 [PubMed] [Google Scholar]
- 27. Davis P. J. (1992) Biochem. Cell Biol. 70, 1313–1318 [DOI] [PubMed] [Google Scholar]
- 28. Jicha G. A., Markesbery W. R. (2010) Clin. Interv. Aging 5, 45–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kris-Etherton P. M., Harris W. S., Appel L. J. (2002) Circulation 106, 2747–2757 [DOI] [PubMed] [Google Scholar]
- 30. Price P. T., Nelson C. M., Clarke S. D. (2000) Curr. Opin. Lipidol. 11, 3–7 [DOI] [PubMed] [Google Scholar]
- 31. Watanabe S., Doshi M., Hamazaki T. (2003) Prostaglandins Leukot. Essent. Fatty Acids 69, 51–59 [DOI] [PubMed] [Google Scholar]
- 32. Colin A., Reggers J., Castronovo V., Ansseau M. (2003) Encephale 29, 49–58 [PubMed] [Google Scholar]
- 33. Stubbs C. D., Smith A. D. (1990) Biochem. Soc. Trans. 18, 779–781 [DOI] [PubMed] [Google Scholar]
- 34. Mathias S., Peña L. A., Kolesnick R. N. (1998) Biochem. J. 335, 465–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Horrobin D. F., Jenkins K., Bennett C. N., Christie W. W. (2002) Prostaglandins Leukot Essent Fatty Acids 66, 83–90 [DOI] [PubMed] [Google Scholar]
- 36. Homan R., Grossman J. E., Pownall H. J. (1991) J. Lipid Res. 32, 231–241 [PubMed] [Google Scholar]
- 37. Chiu L. C., Wan J. M., Ooi V. E. (2000) Int. J. Oncol. 17, 789–796 [DOI] [PubMed] [Google Scholar]
- 38. Malkowski M. G., Ginell S. L., Smith W. L., Garavito R. M. (2000) Science 289, 1933–1937 [DOI] [PubMed] [Google Scholar]
- 39. Marcheselli V. L., Hong S., Lukiw W. J., Tian X. H., Gronert K., Musto A., Hardy M., Gimenez J. M., Chiang N., Serhan C. N., Bazan N. G. (2003) J. Biol. Chem. 278, 43807–43817 [DOI] [PubMed] [Google Scholar]
- 40. Calder P. C. (2002) Proc. Nutr. Soc. 61, 345–358 [DOI] [PubMed] [Google Scholar]
- 41. Kuehl F. A., Jr., Egan R. W. (1980) Science 210, 978–984 [DOI] [PubMed] [Google Scholar]
- 42. Huber T., Rajamoorthi K., Kurze V. F., Beyer K., Brown M. F. (2002) J. Am. Chem. Soc. 124, 298–309 [DOI] [PubMed] [Google Scholar]
- 43. Eldho N. V., Feller S. E., Tristram-Nagle S., Polozov I. V., Gawrisch K. (2003) J. Am. Chem. Soc. 125, 6409–6421 [DOI] [PubMed] [Google Scholar]
- 44. Serhan C. N., Hong S., Gronert K., Colgan S. P., Devchand P. R., Mirick G., Moussignac R. L. (2002) J. Exp. Med. 196, 1025–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yagaloff K. A., Franco L., Simko B., Burghardt B. (1995) Prostaglandins Leukot Essent Fatty Acids 52, 293–297 [DOI] [PubMed] [Google Scholar]
- 46. Lukiw W. J., Cui J. G., Marcheselli V. L., Bodker M., Botkjaer A., Gotlinger K., Serhan C. N., Bazan N. G. (2005) J. Clin. Invest. 115, 2774–2783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Agren J. J., Törmälä M. L., Nenonen M. T., Hänninen O. O. (1995) Lipids 30, 365–369 [DOI] [PubMed] [Google Scholar]
- 48. Sanders T. A., Younger K. M. (1981) Br. J. Nutr. 45, 613–616 [DOI] [PubMed] [Google Scholar]
- 49. Pitman M. C., Suits F., Mackerell A. D., Jr., Feller S. E. (2004) Biochemistry 43, 15318–15328 [DOI] [PubMed] [Google Scholar]