Abstract
Objective
Celiac Disease (CD) is an autoimmune disease triggered by exposure to gluten containing foods. IgA autoantibodies to tissue transglutaminase (TTG) are elevated in CD, but little is known about the gastrointestinal state prior to the appearance of TTG. Antibodies to wheat storage globulin Glo-3A have been studied in type 1 diabetes (T1D), and may be a marker of altered mucosal barrier and/or immune function. In this study, we investigated antibody responses to Glo-3A in CD.
Methods
In the Diabetes Autoimmunity Study in the Young (DAISY), children are followed prospectively from birth for the appearance of TTG and CD. 50 cases of CD were frequency-matched with 50 controls on age (of TTG seroconversion in the case), gender, ethnicity, presence of a first degree relative with T1D, and HLA-DR3 genotype. In cases and controls, IgG antibodies to Glo-3A were analyzed in a blinded manner in the sample collected at the time of seroconversion to TTG positivity (or the matched sample in controls) and in all previous samples since birth (mean: 4.5 samples). The association between Glo-3A antibody levels and CD case status was explored using t-tests at the TTG positive visit and when Glo-3A levels were highest and mixed modeling to describe Glo-3A over time.
Results
At the time of first elevated TTG (mean 4.9 years), CD cases had higher Glo-3A antibody levels than controls (13.3±17.2 versus 7.6±11.7, p = 0.005). In both cases and controls, Glo-3A antibodies appear to peak at a mean age of 2.9 years, prior to mean age of initial TTG seroconversion. The peak Glo-3A antibody levels were higher in cases than controls a (25.5±21.8 versus 14.9±18.3 p = 0.0007). Using mixed modeling to account for multiple visits per person, cases had higher levels of Glo-3A antibodies than controls at all ages from birth to TTG seroconversion (β = 0.53, p = 0.002).
Conclusion
Compared to controls, CD cases have higher Glo-3A antibody responses beginning years prior to initial detection of TTG.
Keywords: celiac disease, risk factors, transglutaminase autoantibodies, Glo-3A wheat globulin
Introduction
Celiac disease (CD) is a common multi-system autoimmune disease caused by exposure to wheat and related proteins in genetically susceptible individuals(1). Some wheat peptides resist digestion and reach the intestinal mucosa intact. Constitutively expressed tissue transglutaminase in the small intestine alters gliadin peptides by deamidating specific glutamine residues to the negatively charged glutamate(2). These deamidated peptides show enhanced binding to specific pockets in the DQ2 molecule on antigen presenting cells and lead to activation of CD4+ T cells.
Individuals with CD express antibodies to both tissue transglutaminase (TTG) and to deamidated gliadin peptides and these antibodies are specific serologic markers for CD(3). IgA autoantibodies to TTG have become the major biomarker for screening and early diagnosis of CD(4). Patients with CD exhibit altered mucosal immunity(5) and increased intestinal permeability(6). Early or late exposure to gluten-containing cereals in infancy increases the risk for CD(7). This also appears to be the case in other autoimmune conditions, including type 1 diabetes (T1D) (8). In the only animal model of a CD-like syndrome, a gluten-induced increase in gut permeability precedes CD(9). It is not clear whether such an increase precedes CD in humans. Altered mucosal permeability at the time of CD onset and in first-degree relatives as well as the evidence that timing of exposure to gluten containing foods may alter the risk for disease provide evidence that mucosal permeability has an etiologic role in CD.
One of the wheat proteins that may play a role in development of autoimmune diseases is WP5212(10), originally described as a homologue of Glb1. Recent studies identified the gene for this protein and it has been renamed Glo-3A(11). In children with islet autoimmunity or T1D, elevated levels of Glo-3A antibodies were associated with current intake of foods containing gluten, shorter duration of breast-feeding and zonulin, a marker of gut permeability(12). In addition, a patient with both T1D and CD displayed very high levels of antibodies and strong T cell responses to Glo-3A(13) suggesting a possible role for Glo-3A as a candidate protein in the pathogenesis of T1D and/or CD. These findings further suggest that Glo-3A could also be a marker of impaired gut barrier function or impaired oral immune tolerance, as seen in CD.
Antibodies to Glo-3A have not been previously studied in the context of CD or development of TTG. The purpose of this study was to explore antibody responses to Glo-3A in children who develop CD and unaffected controls. We hypothesize that higher Glo-3A antibodies are associated with celiac disease.
Materials and Methods
Study Population
The association between CD and environmental factors is being investigated in an ancillary study to the Diabetes Autoimmunity Study in the Young (DAISY)(14) prospectively following children at increased risk of T1D and CD. DAISY participants are also followed for development of TTG autoantibodies and CD. The details of the newborn screening and follow up have been published elsewhere(8). Briefly, 2281 young high-risk children are being followed: 1056 first-degree relatives of patients with T1D and 1225 infants identified by newborn screening for the HLA-DR,DQ genotypes associated with T1D and CD. Study participants had the initial determination of TTG antibodies during the first year of life, then at 15 and 24 months, and annually thereafter. Written informed consent was obtained from the parents of study participants. The Colorado Multiple Institutional Review Board approved all study protocols.
Antibody analyses
TTG Autoantibodies
IgA TTG autoantibodies were measured using an in-house radioimmunoassay that has a positivity cutoff of 0.05, which is the 99th percentile based on 184 healthy controls with a median age of 15.6 years(15). All assays included positive and negative control sera. The index is calculated as follows: (unknown sample - negative control)/(positive control - negative control). The assay was performed using in vitro transcribed and translated human recombinant transglutaminase. The DNA clone encoding transglutaminase was obtained from human umbilical vein endothelial cells and labeled with 35S. The interassay coefficient of variation, as previously reported, was 12.5%, and intra-assay coefficient of variation was 4.8%. This assay compares favorably to 5 commercially available TTG enzyme-linked immunosorbent assays (ELISA)(16;17). As previously shown(18), the TTG autoantibody index correlates well with intestinal biopsy (Marsh score) in CD with high sensitivity, specificity and positive predictive value, and can identify children before clinical features of CD develop(19).
Glo-3A antibodies
Serum IgG directed against Glo-3A was analyzed in blinded serum samples using a capture ELISA at the Ottawa Hospital Research Institute (OHRI). Details are provided in Simpson et al(20). Briefly, human serum albumin was used to decrease non-specific binding and the secondary antibody was pre-absorbed with purified Glo-3A. Plates were coated with recombinant Glo-3A in coating buffer for 90 min at 37°C. Plates were washed twice with ELISA washing buffer (PBS containing 0.05% Tween20, Sigma), then blocked with blocking buffer (5% human serum albumin in PBS) for 1 hour at 37°C or 4°C overnight. Blocking buffer was removed and samples were diluted 1:20 in dilution buffer (5% human serum albumin in PBS containing 0.05% Tween 20) and added to each duplicate well. ELISA dilution buffer was used in duplicate wells as a blank. Plates were incubated overnight at 4°C. Plates were then washed 6 times with washing buffer with at least three 5–10 min incubation intervals. One hundred μl of pre-absorbed secondary antibody was added to each well and incubated for 90 min at room temperature with slight shaking. The plates were washed and incubated with 100 μl/well 3, 3′, 5, 5′ tetramaethylbenzidine (TMB) peroxidase substrate (BD Opt EIA Cat No. 555214) for 10–20 min at room temperature. The reaction was stopped using 100 μl/well of 1M H2SO4. The plates were evaluated at 450 nm using a Multiscan Ascent plate reader. To standardize the results, arbitrary units were calculated in relation to the serum value of an individual who displayed high levels of Glo-3A antibody. The antibody level in this serum was considered as 100 arbitrary units and used as a standard to calculate the value of other samples in the assay. Two other sera with low and intermediate absorbance were used as internal controls in each assay.
Study Design and Statistical Analysis
To explore our hypothesis, we identified 100 children from the DAISY population. We selected 50 children who met the definition of being a case of CD as having either: 1) a positive TTG test (TTG index >0.05) and a positive intestinal biopsy (Marsh score ≥ 2), or 2) if the parents refused the intestinal biopsy, strongly positive TTG tests (TTG index >0.5) on at least two consecutive tests 3–6 months apart. Previously, we have demonstrated that TTG index >0.5 in our radioimmunoassay predicts positive intestinal biopsy in 96% of asymptomatic cases of CD(19). Fifty children at high risk for T1D and CD who have never been TTG antibody positive served as controls and were frequency matched to the cases on age at the time of TTG seroconversion, gender, ethnicity, presence of a first degree relative with T1D and HLA-DR3 genotype (DR 3/3, 3/x or x/x, where DR3 refers to the DRB1*03,DQB1*0201 HLA genotype).
Antibodies to Glo-3A were measured in the first sample found to be TTG positive (i.e. TTG index >0.05) in cases and in the age-matched sample in controls. In addition, antibodies to Glo-3A were measured in all prior samples collected in both cases and controls. For the 100 study subjects, samples were available as follows: 15 children with 2 visits, 19 with 3 visits, 28 with 4 visits, 13 with 5 visits, 12 with 6 visits, and 13 with 7 or more visits. For cases, the average number of visits was 4.4 (min = 2, max = 10); for controls it was 4.5 (min = 2, max = 11).
Glo-3A antibody levels were natural log transformed to satisfy the assumption of a normal distribution. Initially, we conducted a classic case-control analysis, using a t-test to test whether Glo-3A antibody levels differed between cases and controls at the time of TTG seroconversion in the cases, and at the time of their peak level, which was prior to TTG seroconversion. Secondly, we used mixed modeling approaches to determine whether Glo-3A antibody levels were higher in CD cases than controls throughout the time period prior to TTG seroconversion. For this approach, we modeled the variation of Glo-3A antibodies over all ages prior to the age at conversion to TTG positivity (in cases), accounting for the multiple visits per child. The procedure discussed by Cnaan et al(21) was used to determine best fit polynomials for both the fixed and random effects of age. These models distinguish variability between subjects and variability between repeated measurements over time within subjects. The between subject covariance matrices were restricted to be positive definite. Glo-3A antibody levels were an inverse polynomial function of age for both cases and controls.
All analyses were performed using Statistical Analysis Software version 9.2 (SAS Institute, Cary NC).
Results
Table 1 shows that there were no differences between cases and controls in the frequency-matching variables of age, sex, ethnicity, HLA-DR3 genotype, and having a first degree relative with T1D, suggesting that further adjustment was unnecessary.
Table 1.
Description of Matching Variables in Cases and Controls.
| Characteristic | Cases n = 50 | Controls n = 50 |
|---|---|---|
| Female, n (%) | 27(54) | 27(54) |
| Non-Hispanic White, n (%) | 43(86) | 44(88) |
| HLA DR3+ genotype, n (%) | 43(86) | 43(86) |
| First-degree relative of a T1D patient, n (%) | 17(34) | 17(34) |
| Age at the first appearance of TTG antibodies in cases/age at matched visit in controls, mean (SD) | 4.9(1.9) | 4.9(1.9) |
| Age at the peak of Glo-3A Ab, mean (SD) | 2.9(1.9) | 2.8(2.3) |
Age in years
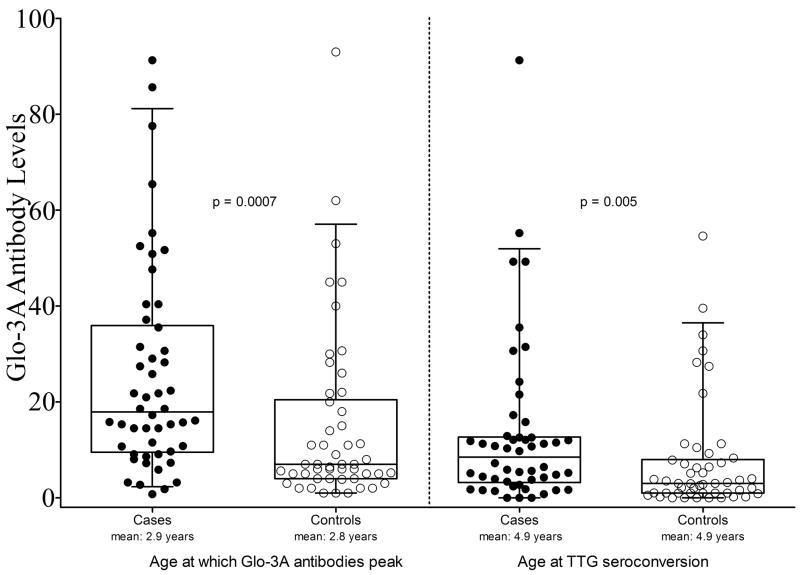
At the time of TTG seroconversion (mean age: 4.9 years), cases had higher Glo-3A antibody levels than controls (13.3±17.2 versus 7.6±11.7, p = 0.005, Figure 1). Visual inspection of the Glo-3A antibody levels by age (Supplemental Figure 1) suggests that the antibody levels appear to peak at an age prior to the age of TTG seroconversion. The mean age at which peak Glo-3A antibody levels were attained did not differ between cases (2.9 years) and controls (2.8 years) (p = 0.77, Table 1). However, at the age of peak Glo-3A antibody levels, cases had higher levels than controls (25.5±21.8 versus 14.9±18.3, p = 0.0007, Figure 1).
Figure 1.
Box and scatter plot of Glo-3A antibody levels at two visits: the age at which Glo-3A antibody levels peak in both cases and controls and the age at which TTG positivity is first identified in patients and age-matched controls. The p-values were generated from the t-tests on natural log transformed Glo-3A levels but the box plots and scatter plots present the actual (untransformed) levels. From bottom to top, the horizontal lines in the box plots represent the 5th percentile, the 25th percentile, the mean, the 75th percentile, and the 95th percentile, respectively. Filled circles (●) represent Glo-3A levels in individual cases, Open circles (○) represent Glo-3A levels in controls.
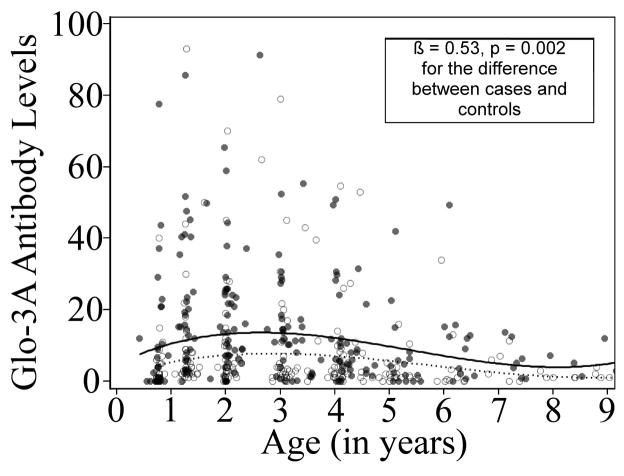
Mixed modeling approaches, accounting for multiple visits per study subject, were used to generate mean predicted curves of Glo-3A antibody levels by age and to test the difference between cases and controls using natural log transformed Glo-3A levels. Cases had higher Glo-3A antibodies at all ages prior to TTG seroconversion (β = 0.53, p = 0.002) than controls (Figure 2).
Figure 2.
Glo-3A antibody levels by age in cases and controls. The Glo-3A data are from samples collected prior to and including the first TTG positive visit in cases or the comparable age-matched visit in controls. The solid circles (●) represent actual Glo-3A levels in cases, the solid line is the mean predicted curve for Glo-3A antibody levels by age in cases. The open circles (○) represent actual Glo-3A levels in controls, the dashed line is the mean predicted curve for Glo-3A levels by age in controls. Mixed modeling approaches were used to generate mean predicted curves of Glo-3A antibody levels by age and to test the difference between cases and controls using natural log transformed Glo-3A levels, which accounts for the multiple visits per study subject. The values for the mean predicted curves were then back-transformed for graphing purposes.
Discussion
In this study of children at increased genetic risk for CD we found that antibodies to Glo-3A are higher in children who go on to develop CD than in matched controls. There was no difference between CD cases and controls in the age when antibodies to Glo-3A peak, but the peak Glo-3A antibody levels as well as Glo-3A levels over time are higher in children who developed CD. While the mean age of seroconversion to TTG positivity in cases was just under 5 years of age, the peak response to Glo-3A was evident, on average, before age 3 years.
The observation of increased levels of antibodies to proteins found in food may be interpreted several ways. The first possible interpretation is that the antibody response is a reflection of increased diversity in the child’s diet. The second is that Glo-3A antibodies are a biomarker of impaired immune tolerance and increased gut permeability. Finally, these results may indicate that the immune pathology and subsequent damage that are characteristic of CD start early in life.
Glo-3A is a salt-soluble globulin, and can remain trapped within the wheat gluten complex upon the processing of wheat for human consumption (10;22). Because storage proteins can become trapped with the gluten complex, the reaction to Glo-3A may be a marker of dietary exposure to gluten and it becomes evident around the time the typical western diet becomes varied. The finding that the mean age at which Glo-3A levels peak does not differ between cases and controls supports this explanation.
It is possible that these higher antibody titers may reflect a higher antigen intake in cases that increased the risk for CD. We do not have a direct measure of wheat intake in these children. We did, though, estimate daily servings of foods containing gluten, obtained from an annual Willett Food Frequency Questionnaire. This questionnaire is a semi-quantitative food survey and therefore does not enable calculation of the weight of wheat proteins ingested. Data are available on approximately 50% of the study visits, so it was not considered as a covariate in these analyses. However, the mean number of daily servings of foods containing gluten for all visits in cases was 4.2, while mean daily servings of foods containing gluten for all visits in controls was 4.8. These results suggest that there is probably no difference in wheat intake between the two groups, but if a difference exists, wheat intake is lower in cases. Therefore, higher antigen intake does not explain higher Glo-3A antibody levels.
Another interpretation may be that the Glo-3A immune response provides an early marker of impaired oral tolerance and increased gut permeability. Glo-3A has also been observed to have sequence homology with the tight junction proteins participating in regulation of intestinal permeability(10) and another food antigen, the highly immunogenic peanut allergen, Ara-h1(10). Intestinal permeability has been implicated in the autoimmune cascade leading to T1D(23;24). Indeed, several studies report that intestinal permeability is increased in CD, and that zonulin, at least in part, is a mediator of this effect(25–27). Zonulin is an intestinal peptide that regulates the opening of gut epithelial cell tight junctions(28). Our finding that CD cases have higher Glo-3A antibody responses throughout childhood could also be interpreted as evidence that impaired oral tolerance and increased gut permeability have an etiologic role in CD. Measuring Glo-3A antibodies along with biomarkers of intestinal permeability over time would help to elucidate how well they are correlated in an individual. Our preliminary data suggest that Glo-3A antibodies in a large proportion of cases peak in the pre-celiac autoimmunity period, a time which has not been previously characterized. (Figs 1, S1)
We interpret the pattern of Glo3A responses, whereby the peak occurs on average at 2.9 years and subsequently wanes, to possibly reflect changes in mucosal exposure to wheat proteins with the evolution of the infant and childhood diet. It is quite possible that the interaction between mucosal host factors (eg. intestinal permeability) and dietary antigen load produce the responses we found, and that this early period in the development of mucosal immunity and tolerance is critical in determining the subsequent risk for celiac disease.
Finally, ongoing exposure to the gluten macromolecular complex in wheat, rye and barley, exposes the patient to a sustained immune insult(4). Classic indicators of this process include elevated TTG autoantibodies and villous atrophy(15;18). Our finding that the Glo-3A antibody response is higher in cases compared to controls prior to the identification of clinical disease may be evidence that these processes are under way long before diagnosis of CD.
Children with diabetes-related autoimmunity have been previously shown to be more reactive to the Glo-3A antigen(12) and in diabetes-prone rats higher Glo-3A reactivity was associated with pancreatic damage(10). CD and T1D share high risk HLA haplotypes(2), and so it is possible that an antigen that provokes an immune response in diabetic individuals could also be involved in the immunopathology of CD.
This study was performed in a group of children at increased genetic risk for CD and therefore the applicability to the general population is unknown. We cannot determine whether Glo-3A antibodies in our control population reflect responses in the population with a lower genetic risk for CD, because no data exist on antibody responses to Glo-3A in the general population. The control population in this study is at increased genetic risk compared to the general population based on the enrollment criteria of the CEDAR study. Further studies are required to examine responses to Glo-3A in a population that reflects the genetic distribution of the general population.
In addition, we cannot exclude the possibility that some of the control subjects will go on to develop celiac disease. Followup of this cohort will enable further analysis of Glo-3A antibodies and celiac disease in this population.
We cannot establish, based on these data, sensitivity, specificity, positive or negative predictive values for Glo-3A antibodies in CD. In order to identify possible diagnostic utility, it is necessary to measure Glo-3A antibodies in a larger sample of children from the general population to establish normal and abnormal levels. In order to rule out the possibility that elevated Glo-3A antibodies in early childhood are a predictor of later development of CD, a larger cohort of children needs to be followed from an early age for development of TTG and CD.
This study shows that higher levels of Glo-3A antibodies are associated with CD both at the time of clinical diagnosis as well as prior to that point. Further studies are warranted exploring this marker of mucosal immunity in relationship to development of CD and T1D.
Supplementary Material
Acknowledgments
This research was supported by NIH grants R37 DK32493 (DAISY) and R01 DK50979, Diabetes Endocrinology Research Center P30 DK57516 (Clinical Investigation Core), and M01RR00069 General Clinical Research Centers Program, NCRR, NIH.
C.E.T. was supported by the NIH grant 5T32 DK63687
Studies in the laboratory of FWS were supported by JDRF and the Canadian Institutes for Health Research (CIHR).
The authors would like to thank the patients and their families who participated in this research and Kim McFann for her assistance with the statistical analysis.
Footnotes
Disclosure: F.W.S and M.M report that the Ottawa Hospital Research Institute may license certain rights to Glo-3A for commercial development that could result in future royalty payment.
Reference List
- 1.Hill ID, Dirks MH, Liptak GS, et al. Guideline for the diagnosis and treatment of celiac disease in children: recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2005;40:1–19. doi: 10.1097/00005176-200501000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Jabri B, Sollid LM. Mechanisms of disease: immunopathogenesis of celiac disease. Nat Clin Pract Gastroenterol Hepatol. 2006;3:516–525. doi: 10.1038/ncpgasthep0582. [DOI] [PubMed] [Google Scholar]
- 3.Liu E, Li M, Emery L, et al. Natural history of antibodies to deamidated gliadin peptides and transglutaminase in early childhood celiac disease. J Pediatr Gastroenterol Nutr. 2007;45:293–300. doi: 10.1097/MPG.0b013e31806c7b34. [DOI] [PubMed] [Google Scholar]
- 4.Dieterich W, Ehnis T, Bauer M, et al. Identification of tissue transglutaminase as the autoantigen of celiac disease. Nat Med. 1997;3:797–801. doi: 10.1038/nm0797-797. [DOI] [PubMed] [Google Scholar]
- 5.Camarca A, Anderson RP, Mamone G, et al. Intestinal T cell responses to gluten peptides are largely heterogeneous: implications for a peptide-based therapy in celiac disease. J Immunol. 2009;182:4158–4166. doi: 10.4049/jimmunol.0803181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lammers KM, Lu R, Brownley J, et al. Gliadin induces an increase in intestinal permeability and zonulin release by binding to the chemokine receptor CXCR3. Gastroenterology. 2008;135:194–204. doi: 10.1053/j.gastro.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Norris JM, Barriga K, Hoffenberg EJ, et al. Risk of celiac disease autoimmunity and timing of gluten introduction in the diet of infants at increased risk of disease. JAMA. 2005;293:2343–2351. doi: 10.1001/jama.293.19.2343. [DOI] [PubMed] [Google Scholar]
- 8.Norris JM, Barriga K, Klingensmith G, et al. Timing of initial cereal exposure in infancy and risk of islet autoimmunity. JAMA. 2003;290:1713–1720. doi: 10.1001/jama.290.13.1713. [DOI] [PubMed] [Google Scholar]
- 9.Hall EJ, Batt RM. Abnormal permeability precedes the development of a gluten sensitive enteropathy in Irish setter dogs. Gut. 1991;32:749–753. doi: 10.1136/gut.32.7.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MacFarlane AJ, Burghardt KM, Kelly J, et al. A type 1 diabetes-related protein from wheat (Triticum aestivum). cDNA clone of a wheat storage globulin, Glb1, linked to islet damage. J Biol Chem. 2003;278:54–63. doi: 10.1074/jbc.M210636200. [DOI] [PubMed] [Google Scholar]
- 11.Loit E, Melnyk CW, MacFarlane AJ, Scott FW, Altosaar I. Identification of three wheat globulin genes by screening a Triticum aestivum BAC genomic library with cDNA from a diabetes-associated globulin. BMC Plant Biol. 2009;9:93. doi: 10.1186/1471-2229-9-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simpson M, Mojibian M, Barriga K, et al. An exploration of Glo-3A antibody levels in children at increased risk for type 1 diabetes mellitus. Pediatr Diabetes. 2009;10:563–572. doi: 10.1111/j.1399-5448.2009.00541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mojibian M, Chakir H, MacFarlane AJ, et al. Immune reactivity to a glb1 homologue in a highly wheat-sensitive patient with type 1 diabetes and celiac disease. Diabetes Care. 2006;29:1108–1110. doi: 10.2337/diacare.2951108. [DOI] [PubMed] [Google Scholar]
- 14.Rewers M, Norris JM, Eisenbarth GS, et al. Beta-cell autoantibodies in infants and toddlers without IDDM relatives: diabetes autoimmunity study in the young (DAISY) J Autoimmun. 1996;9:405–410. doi: 10.1006/jaut.1996.0055. [DOI] [PubMed] [Google Scholar]
- 15.Hoffenberg EJ, Bao F, Eisenbarth GS, et al. Transglutaminase antibodies in children with a genetic risk for celiac disease. J Pediatr. 2000;137:356–360. doi: 10.1067/mpd.2000.107582. [DOI] [PubMed] [Google Scholar]
- 16.Liu E, Li M, Bao F, et al. Need for quantitative assessment of transglutaminase autoantibodies for celiac disease in screening-identified children. J Pediatr. 2005;146:494–499. doi: 10.1016/j.jpeds.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 17.Li M, Yu L, Tiberti C, et al. A report on the International Transglutaminase Autoantibody Workshop for Celiac Disease. American Journal of Gastroenterology. 2009;104:154–163. doi: 10.1038/ajg.2008.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoffenberg EJ, Emery LM, Barriga KJ, et al. Clinical features of children with screening-identified evidence of celiac disease. Pediatrics. 2004;113:1254–1259. doi: 10.1542/peds.113.5.1254. [DOI] [PubMed] [Google Scholar]
- 19.Liu E, Bao F, Barriga K, et al. Fluctuating transglutaminase autoantibodies are related to histologic features of celiac disease. Clin Gastroenterol Hepatol. 2003;1:356–362. doi: 10.1053/s1542-3565(03)00180-0. [DOI] [PubMed] [Google Scholar]
- 20.Simpson M, Mojibian M, Barriga K, et al. An exploration of Glo-3A antibody levels in children at increased risk for type 1 diabetes mellitus. Pediatr Diabetes. 2009;10:563–572. doi: 10.1111/j.1399-5448.2009.00541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cnaan A, Laird NM, Slasor P. Using the general linear mixed model to analyse unbalanced repeated measures and longitudinal data. Stat Med. 1997;16:2349–2380. doi: 10.1002/(sici)1097-0258(19971030)16:20<2349::aid-sim667>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 22.Graveland A, Bongers P, Bosveld P. Extraction and fractionation of wheat flour proteins. Journal of the Science of Food and Agriculture. 1979;30:71–84. [Google Scholar]
- 23.Bosi E, Molteni L, Radaelli MG, et al. Increased intestinal permeability precedes clinical onset of type 1 diabetes. Diabetologia. 2006;49:2824–2827. doi: 10.1007/s00125-006-0465-3. [DOI] [PubMed] [Google Scholar]
- 24.Visser J, Rozing J, Sapone A, et al. Tight junctions, intestinal permeability, and autoimmunity: celiac disease and type 1 diabetes paradigms. Annals of the New York Academy of Sciences. 2009;1165:195–205. doi: 10.1111/j.1749-6632.2009.04037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fasano A, Not T, Wang W, et al. Zonulin, a newly discovered modulator of intestinal permeability, and its expression in coeliac disease. Lancet. 2000;355:1518–1519. doi: 10.1016/S0140-6736(00)02169-3. [DOI] [PubMed] [Google Scholar]
- 26.Madara JL, Trier JS. Structural abnormalities of jejunal epithelial cell membranes in celiac sprue. Lab Invest. 1980;43:254–261. [PubMed] [Google Scholar]
- 27.Schulzke JD, Bentzel CJ, Schulzke I, Riecken EO, Fromm M. Epithelial tight junction structure in the jejunum of children with acute and treated celiac sprue. Pediatr Res. 1998;43:435–441. doi: 10.1203/00006450-199804000-00001. [DOI] [PubMed] [Google Scholar]
- 28.Fasano A. Physiological, pathological, and therapeutic implications of zonulin-mediated intestinal barrier modulation: living life on the edge of the wall. Am J Pathol. 2008;173:1243–1252. doi: 10.2353/ajpath.2008.080192. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.