Abstract
A hallmark of human papillomavirus (HPV) associated carcinogenesis is the integration of the viral DNA into the cellular genome, usually accompanied by the loss of expression of the viral E2 gene. E2 binds to and represses the viral promoter directing expression of the E6 and E7 oncogenes. The re-introduction and expression of exogenous E2 in HPV-positive cancer cells results in cellular growth arrest, while growth in the context of exogenous E2 can be restored through the expression of exogenous E6 and E7. Here we examine the individual contributions of the viral E6 and E7 genes to this phenotype. E6 alone displays moderate activity, whereas both E7 and adenovirus E1A display high activity in reversing E2-mediated cellular growth suppression. Using defined mutants of E7 and E1A, we show that an intact retinoblastoma interaction domain is required for this function. In addition, we show that the E2-mediated growth arrest of HPV-positive cells results in cellular senescence, and implicate the cyclin/cdk inhibitor p21CIP as a downstream E2 effector in this phenotype.
Keywords: E2/p21CIP/papillomavirus/pRB/senescence
Introduction
Over 90 human papillomavirus (HPV) types have now been identified. A subset of these, termed the ‘high-risk’ types, is strongly associated with anogenital cancers (reviewed in Howley, 1996). Over 90% of cervical cancers express the high-risk E6 and E7 viral oncoproteins (zur Hausen, 1996), which together are sufficient to immortalize primary human keratinocytes (Durst et al., 1987; Schlegel et al., 1988; Woodworth et al., 1988). Mechanistically, the high-risk HPV E6 and E7 proteins interfere with cell cycle control pathways through interaction with a number of specific cellular proteins. The best studied effects of E6 and E7 are the targeted ubiquitylation and degradation of p53 by E6 (Scheffner et al., 1990), and the binding and functional inactivation of the cellular retinoblastoma protein pRb by E7, respectively (Dyson et al., 1989). E6 can also induce telomerase activity in normal human cells, an activity that is thought to be critical for bypassing cellular senescence (Kiyono et al., 1998). E7 has been shown to bind other members of the cellular pocket protein family, p107 and p130, as well as the cyclin/cdk inhibitor p21CIP (Jones et al., 1997).
Although HPV DNA is maintained as an episome in benign, pre-cancerous lesions, it is generally found integrated in cancers and in the cell lines derived from the cancers (Durst et al., 1985; Schwarz et al., 1985). Integration of the viral DNA appears to be a random event with regard to sites of host chromosomal integration, but most often results in the disruption of the viral E2 open reading frame (ORF) and the loss of E2 expression. Since E2 is a negative regulator of the E6/E7 promoter, the loss of E2 results in the deregulated expression of E6 and E7. Several lines of evidence support the notion that the loss of HPV E2 protein expression contributes to carcinogenesis. First, E2 is known to repress directly the viral promoter that drives expression of E6 and E7 (Romanczuk et al., 1990; Thierry and Howley, 1991; Tan et al., 1992; Demeret et al., 1997). Secondly, mutations that disrupt the E2 ORF increase E6/E7-dependent immortalization efficiency of HPV16 genomic DNA (Romanczuk and Howley, 1992). Thirdly, the re-introduction of E2 into HPV-positive, but not HPV-negative cervical cancer cell lines results in a G1 cell cycle arrest (Thierry and Yaniv, 1987; Hwang et al., 1993; Dowhanick et al., 1995; Desaintes et al., 1997; Goodwin et al., 1998). We have recently shown that E2-mediated repression of the E6/E7 promoter is required for the observed cellular growth arrest (Francis et al., 2000).
The papillomavirus E2 proteins have regulatory functions in viral transcription and viral DNA replication. The structure of E2 resembles that of a prototypical transcription factor, with an N-terminal transcriptional activation domain and a C-terminal DNA binding domain, separated by a hinge region. The bovine papillomavirus (BPV) E2 proteins have been studied most extensively. Three different BPV E2 proteins have been detected in BPV-transformed C127 cells. The largest 48 kDa form, E2-TA, represents the product of the complete ORF with the entire transactivation domain. A short internally initiated 30 kDa form of BPV E2, known as E2-TR, is devoid of a transactivation domain, but contains the C-terminal DNA binding/dimerization domain (Howley, 1996). The BPV and HPV full-length E2 proteins can serve either as activators or repressors of transcription, depending upon the context of E2 binding sites within the promoter region. Although the mechanism of promoter repression by viral full-length E2 proteins is not completely resolved, steric interference of bound E2 proteins with the binding of positively acting transcription factors TBP and Sp1 has been suggested (Dostatni et al., 1991; Demeret et al., 1994; Dong et al., 1994). However, recent reports suggest that the E2 transcriptional activation function is required for promoter repression, which is consistent with a more complex scenario. Specific conservative point mutations within the bovine or human E2 transactivation domain that eliminate E2-mediated transcriptional activation also eliminate E6/E7 promoter repression (Goodwin et al., 1998; Nishimura et al., 2000). It remains to be determined whether other functions are impaired by these mutations, such as specific protein–protein interactions between E2 and members of the transcriptional or chromatin remodeling machinery.
We have recently reported that exogenous expression of HPV E6 and E7 from a promoter that is not repressed by E2 can restore cellular growth in the presence of BPV E2-TA, providing evidence that E6/E7 promoter repression is necessary for E2-mediated cellular growth arrest (Francis et al., 2000). To determine the individual roles of the HPV viral oncoproteins in overcoming this growth arrest, we have assayed E6 and E7 individually in transient transfection and colony reduction experiments. Each protein was able, to some extent, to rescue HeLa cells from cellular growth arrest. E7 exhibited a particularly strong phenotype, as did adenovirus (Ad) 12S E1A. A genetic analysis of E7 and of E1A in this assay revealed that the integrity of the retinoblastoma binding domains was critical for this activity, emphasizing the importance of pRb-dependent pathways in E2-mediated cellular growth arrest. We also noted a previously undocumented senescence-like phenotype in cells undergoing E2-mediated growth arrest. Like cellular growth arrest, induction of senescence in HPV-positive cervical cancer cells required a transactivation-competent E2 protein and could be overcome by exogenous expression of HPV16 E7 and Ad 12S E1A. E2-mediated growth arrest was accompanied by the upregulation of a known marker of senescence, the cyclin/cdk inhibitor p21CIP (Dowhanick et al., 1995; Hwang et al., 1996). We show that over expression of p21CIP by itself could induce senescence of HeLa cells. Additionally, microinjection of antisense p21CIP oligonucleotides inhibited senescence in HeLa cells expressing E2. These observations are consistent with a mechanistic model in which E2-mediated growth arrest occurs through cellular senescence, and implicate the cyclin/cdk inhibitor p21CIP as a critical E2 effector.
Results
E6 and E7 can individually rescue HeLa cells from E2-mediated growth arrest
We have previously demonstrated that the E2-mediated growth arrest in HeLa cells requires the repression of the E6/E7 promoter and can be overcome by the exogenous co-expression of HPV16 E6 and E7. In order to assess the individual contributions of these two viral oncoproteins, we performed HeLa cell growth suppression assays in which plasmids expressing HPV16 E6 or E7 were co-transfected with a BPV1 E2-TA-expressing plasmid. The expression of HPV16 E7 and the E7 ΔDLYC mutant, which is defective for binding pRB and p21 (Münger et al., 1989; Jones et al., 1997), was confirmed directly by western blot analysis (data not shown). Expression of HPV16 E6 was assessed indirectly by examining the levels of p53 in transfected cells, in which the proteolysis of p53 is mediated by E6 (data not shown).
Three independent growth suppression experiments are depicted in Table I. HeLa cells were transfected with E2-TA plasmid along with the viral oncoprotein expression vector and neomycin selection plasmid. As controls, cells were transfected either with empty vector or with BPV1 E2-TR and the neomycin selection plasmid. Colony numbers were determined after 20 days of G418 selection. As seen in previous studies, transfection with the full-length E2-TA protein resulted in a dramatic inhibition of colony formation. Colony growth was partially rescued by the co-transfection with plasmids expressing HPV16 E6 and E7, as seen with our previous analysis (Francis et al., 2000). Since both the 12S E1A and E7 proteins induce apoptosis when expressed at high concentrations, the plasmid input ratio of the viral oncogenes relative to E2 was kept low (1:5) in these colony reduction assays. Partial rescue is thus likely to reflect the percentage of cells that were successfully co-transfected with both the E2-TA and the E1A or E7 plasmids. When the individual contributions of E6 and E7 were examined, we found that both E7 and E6 could each partially rescue the E2-mediated HeLa cell growth suppression, although E7 was considerably more effective. In fact, the level of restoration of cellular growth observed with E7 was equal to the level we observed with the combination of E6 and E7. The mutated E7 ΔDLYC protein did not restore colony formation, thus assigning a critical role to the pocket protein interaction domain within E7.
Table I. Effects of HPV16 E6 and E7 proteins on the E2 growth arrest of HeLa cells.
| Colony number |
% colonies | |||
|---|---|---|---|---|
| A | B | C | ||
| Vector alone | 83 | 77 | 102 | 100 |
| E2-TR | 80 | 79 | 98 | 99 (± 4) |
| E2-TA + RSV vector | 1 | 0 | 3 | 2 (± 2) |
| E2-TA + E7 | 10 | 12 | 31 | 19 (± 9) |
| E2-TA + E7 ΔDLYC (pRB–) | 0 | 1 | 2 | 1 (± 1) |
| E2-TA + E6 | 6 | 4 | 9 | 7 (± 2) |
| E2-TA + E6, + E7 | 12 | 15 | 29 | 20 (± 7) |
Colony numbers obtained from three independent experiments are depicted. Vector alone, empty SVE vector; E2-TR, the truncated BPV E2 variant lacking the transactivation domain. The percentage of colonies relative to empty SVE vector was determined for each experiment. The average percentage of colonies is shown in the last column; standard deviations are indicated.
One potential trivial explanation for the rescue of the E2-mediated HeLa cell growth suppression by E6 and/or E7 (as well as Ad 12S E1A, see below) would be the inhibition of E2 expression by these viral oncoproteins. We therefore examined E2 expression in HeLa cells transfected with either vector alone, E2 plus vector, E2 plus E6, E2 plus E7 or E2 plus Ad E1A. RNA was analyzed 48 h post-transfection. No differences were found in the E2 RNA levels from cells transfected with E2 plus vector, and E2 plus any of the viral oncoproteins (data not shown). We therefore concluded that the rescue observed from E2-mediated HeLa cell growth arrest by E6, E7 and Ad E1A was due to effects on signaling pathways governed by the viral oncoproteins.
Adenovirus 12S E1A can rescue HeLa cells from E2-mediated growth arrest
The high-risk HPV E7 proteins share a number of structural and functional attributes with the Ad 12S E1A protein. Through interactions with various cellular factors, E1A promotes cellular immortalization and transformation. Two distinct domains within the N-terminus of E1A are required for its oncogenic activities, one of which interacts with the pRB family of pocket proteins (Whyte et al., 1988; Ewen et al., 1991; Giordano et al., 1991), whereas the other interacts with the p300/CBP family of co-activators (Whyte et al., 1989; Eckner et al., 1994). A third interaction with the cellular co-activator P/CAF has also been recently identified (Reid et al., 1998). This interaction is independent of CBP/p300 binding and maps close to the pRb and p300 interaction domains within conserved region 1. E1A and E7 share the ability to interact with pRb and the related ‘pocket’ proteins p107 and p130 (Dyson et al., 1989; Münger et al., 1989), a property that is reflected in the primary amino acid similarity between conserved regions 1 and 2 within E1A and domains in E7 (Phelps et al., 1988). Both HPV16 E7 and Ad 12S E1A protein also directly bind the cyclin/cdk inhibitor p21CIP in vitro (Funk et al., 1997; Jones et al., 1997; Keblusek et al., 1999).
Since the N-terminus of E1A is in many ways structurally and functionally analogous to high-risk HPV E7, yet also exhibits a unique binding specificity for cellular transcriptional co-activators, we assayed a panel of wild-type and mutant 12S E1A proteins for their ability to protect HeLa cells from E2-mediated cellular growth suppression. These proteins carry N-terminal mutations that abolish pRb, P/CAF and CBP interaction, either individually or together (Reid et al., 1998). As shown in Table II, wild-type 12S E1A, like E7, partially protected HeLa cells from E2-mediated cellular growth arrest. Comparable levels of protein expression could be achieved for the wild-type and mutant E1A proteins (data not shown). The mutant E1A protein TK460, deficient in the CBP/p300 interaction, and the mutant E55 protein, deficient in the P/CAF interaction, exhibited abilities comparable to wild-type E1A in antagonizing E2 growth arrest. In contrast, both the mutant protein TK496, which is deficient only for pocket protein interactions, and the mutant ΔCR1 protein, which does not bind pRB, P/CAF and CBP, were not able to reverse E2-TA-mediated growth suppression of HeLa cells. From these data, we conclude that, as with E7, the E1A rescue does not depend upon interaction with CBP or P/CAF, but does require a functional pRb pocket protein interaction motif.
Table II. Effects of Ad 12S E1A proteins on the E2 growth arrest of HeLa cells.
| Colony number |
% colonies | ||||
|---|---|---|---|---|---|
| A | B | C | D | ||
| Vector alone | 110 | 43 | 109 | 42 | 100 |
| E2-TR | 67 | 44 | 139 | 66 | 112 (± 41) |
| E2-TA + RSV vector | 1 | 1 | 1 | 1 | 2 (± 1) |
| E2-TA + E1A 12 S | 30 | 23 | 53 | 30 | 50 (± 18) |
| E2-TA + E1A TK460 (CBP–) | 26 | 26 | 41 | 18 | 41 (± 15) |
| E2-TA + E1A TK496 (pRb–) | 1 | 2 | 4 | 1 | 3 (± 2) |
| E2-TA + E1A E55 (P/CAF–) | 28 | 10 | 34 | 17 | 30 (± 8) |
| E2-TA + E1A ΔCR1 (CBP–, pRb–, pCAF–) | 6 | 3 | 2 | 2 | 5 (± 2) |
The assays were performed as described for Figure 1A. Colony numbers from four independent experiments are depicted. Characteristics of the individual mutant E1A proteins in their abilities to interact with cellular pRb, CBP or P/CAF are indicated. Vector alone, empty SVE vector; E2-TR, the truncated BPV1 E2 protein lacking the transactivation domain. The average percentage of colonies relative to empty SVE vector is indicated in the last column.
HPV E2 induces cellular senescence in HPV-positive cervical cancer cells
Growth-suppressive effects of E2 on cervical carcinoma cell lines are specific for HPV-positive cells (Dowhanick et al., 1995). In the course of our studies, we have observed that E2-mediated growth suppression of HPV-positive cells is accompanied by morphological changes in transfected cells that are typical of senescent cells. The term cellular or replicative senescence describes the finite replicative capacity of somatic cells in culture, which eventually results in complete cessation of cellular division (reviewed in Smith and Pereira-Smith, 1996). While cell cycle arrest marks the onset of senescence, senescence is not a necessary consequence thereof.
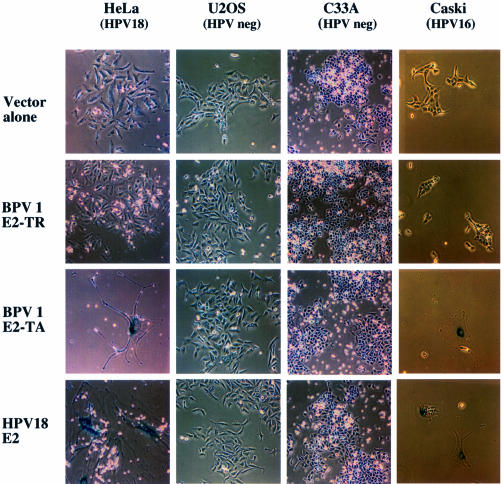
The morphology of HeLa, U2OS, C33A and Caski cells after transfection with vector alone, BPV E2-TR, BPV E2-TA or HPV18 E2 is shown in Figure 1. Cells received the indicated plasmids along with a neoR plasmid and were stained for expression of the senescence-associated β-galactosidase (SA-βGal) senescence-specific marker (Dimri et al., 1995) 2 weeks after G418 selection. We observed dramatic morphological changes in HeLa and Caski cells following expression of E2-TA and HPV18 E2, but not upon expression of the transactivation-defective E2-TR protein or with vector alone. These changes, which were appreciable as early as 3–4 days post-transfection, included an enlarged, flat morphology, resulting in an ∼20- to 50-fold increase in cellular size and the appearance of a vacuolated cytoplasm. Multinucleated cells were also frequently observed. Over 90% of enlarged cells stained positive for the perinuclear β-galactosidase (SA-βGal) activity, a highly specific marker for senescence that is not present in either growth-arrested or differentiated cells in vitro or in vivo (Dimri et al., 1995). A similar phenotype was also observed in the HPV16-positive SiHa cell line (data not shown), but not in the HPV-negative C33A or U2OS cell lines (Figure 1).
Fig. 1. E2-mediated senescence in HPV-positive cell lines. HeLa, U2OS, C33A and Caski cells were co-transfected with expression vectors for the neo resistance gene and either expression vectors for BPV E2-TA, BPV E2-TR or HPV18 E2 or empty SVE vector (vector alone). At 20 days post-selection, the cells were stained for the senescence-specific β-galactosidase marker (SA-βGal) as described in Materials and methods.
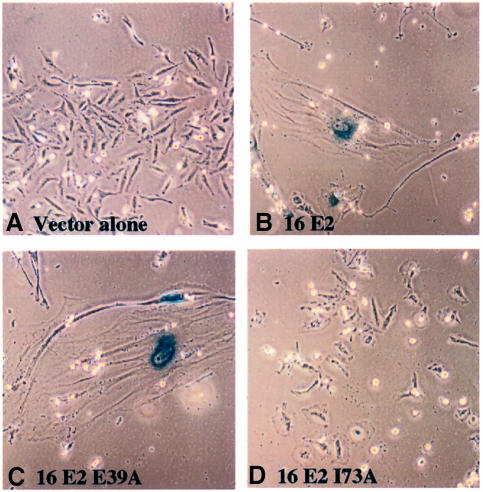
We have previously shown that removal of the BPV E2 transactivation domain resulted in the loss of E2-mediated cellular growth arrest (Dowhanick et al., 1995). Furthermore, the analysis of a panel of mutant BPV1 and HPV16 E2 proteins carrying point mutations within their transactivation domains has established a clear correlation between E2 transcriptional activation activity and growth suppression of HPV-positive cell lines (Goodwin et al., 1998; Nishimura et al., 2000). As shown in Figures 1 and 2, a functional E2 transactivation domain is also required for the induction of senescence. The full-length BPV1 E2-TA protein induced HeLa cell senescence, whereas the E2-TR protein lacking a transactivation domain did not (Figure 1). We also compared the abilities of wild-type HPV16 E2 and two E2 mutants containing single amino acid substitutions within their transactivation domains to induce cellular senescence in HeLa cells. These two HPV16 E2 mutant proteins, E39A and I73A, have been characterized previously and separate the E2 transcriptional activation and replication functions: I73A is defective for transcriptional activation, but functions normally in transient viral DNA replication assays, whereas E39A exhibits the opposite phenotypic pattern (Sakai et al., 1996). As shown in Figure 4C and D, E39A induced cellular senescence in HeLa cells, whereas I73A did not. Therefore, the ability of E2 to induce cellular senescence in HPV-positive cervical carcinoma cells requires an intact transactivation function.
Fig. 2. A functional E2 transactivation function is required for senescence induction. HeLa cells were co-transfected with a neomycin selection plasmid and either empty SVE vector (A) or expression vectors for wild-type HPV16 E2 (B), or the mutant HPV16 E2 proteins E39A or I73A (C and D). Senescence-specific staining was performed as described in the legend to Figure 3.
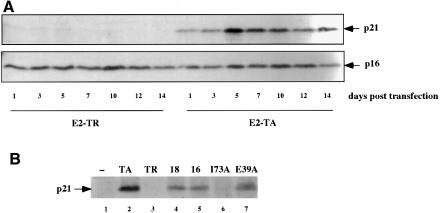
Fig. 4. Induction of the cyclin/cdk inhibitor p21CIP correlates with E2-mediated senescence. (A) HeLa cells were co-transfected with a puromycin selection plasmid and either E2-TA or E2-TR expression vectors. On the next day, the cells were split and placed under puromycin selection (0.4 µg/ml). At days 1–14 post-selection, the cells were harvested for preparation of whole-cell lysates and 50 µg of protein were separated by 12.5% SDS–PAGE. Expression of the p21CIP protein as well as of p16INK4A was detected by western blot analysis. (B) HeLa cells were co-transfected with the puromycin resistance plasmid and expression vectors for either BPV E2-TA, BPV E2-TR, HPV18 E2, HPV16 E2 and the two HPV16 E2 mutant proteins I73A and E39A. p21CIP protein levels were measured on day 3 post-selection as described in (A).
Viral oncoproteins rescue HeLa cells from E2-induced senescence
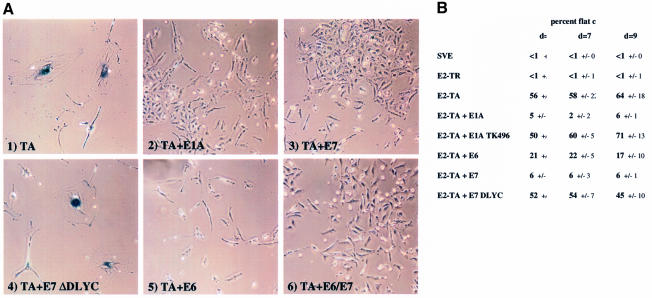
We next examined whether exogenous expression of HPV16 E7 or Ad E1A could rescue HeLa cells from E2-induced senescence. Figure 3A depicts HeLa cells stained for SA-βGal expression 2 weeks post co-transfection with E2-TA and several different viral oncoproteins. Senescence inhibition was observed with all viral oncoproteins (Figure 3A). In order to quantitate the degree of inhibition by the various oncoproteins, we assessed the percentage of enlarged cells relative to the total cell number at days 5, 7 and 9 using the characteristic flat cell phenotype as a marker. Three random fields, each containing >50 cells, were counted. Co-expression of E2 and HPV E7 or Ad 12S E1A resulted in strong inhibition of E2-induced senescence, which is reflected by the dramatic reduction in the number of flat cells earlier in the selection process (Figure 3B). The E1A TK496 and the E7 ΔDLYC mutant proteins, which do not bind pRB, did not reverse the senescent phenotype. HPV16 E6, which exhibited a weak ability to rescue E2-mediated cellular growth suppression (Table I), reduced the number of flat cells observed with E2-TA alone ∼2- to 3-fold compared with the ∼10- to 30-fold reduction observed with either E1A or E7. These data suggest that E2-mediated senescence, like cellular growth arrest, can be overcome by viral oncoproteins and particularly implicate pRb-dependent pathways in this process.
Fig. 3. Viral oncoproteins counteract E2-mediated senescence in HeLa cells. (A) HeLa cells were co-transfected with the neomycin plasmid and BPV E2-TA, either alone (1) or in the presence of wild-type 12S E1A (2), wild-type HPV16 E7 (3), the mutant ΔDLYC E7 (4), wild-type HPV16 E6 (5) or both HPV16 E6 and E7 (6). The cells were selected and stained for SA-βGal activity as described in Figure 1. (B) Cells that assumed the senescence-specific morphology were counted on days (d) 5, 7 and 9 post-selection. Three random fields were counted and averaged for each data point; the standard deviations are as indicated. Each field contained a minimum of 50 cells. The data are expressed as percent enlarged cells relative to total cell number.
The role of p21CIP in E2-mediated senescence
Several laboratories, including our own, have reported an acute rise in p21CIP RNA and protein levels following expression of BPV1 E2-TA in HPV-positive cells (Dowhanick et al., 1995; Hwang et al., 1996; Desaintes et al., 1999). The p21CIP protein is a member of the family of cyclin/cdk inhibitors with well characterized functions in cell cycle control (reviewed in Sherr and Roberts, 1999). Importantly, p21CIP has been described as a marker, in some circumstances even an inducer, of cellular senescence and was indeed originally identified as a senescence-specific protein sdi1 (Noda et al., 1994; Brown et al., 1997; Fang et al., 1999; Kagawa et al., 1999). We explored the role of p21CIP as a potential downstream effector of E2-mediated senescence in HeLa cells. In agreement with the previous studies, Figure 4A demonstrates that the levels of p21 protein were upregulated in HeLa cells following expression of BPV E2-TA. The induction of p21 correlated with the stabilization of p53 (data not shown). The levels of p21CIP remained high over the course of 14 days following expression of E2-TA, but not of E2-TR (Figure 4A) or transfection with the empty SVE vector (data not shown). In contrast, levels of the cyclin/kinase inhibitor p16, which has also been implicated in cellular senescence in some cell systems, did not change upon E2 expression in HeLa cells. Levels of the p21-related p27KIP1 protein were also unaffected by E2 (data not shown).
It has previously been shown in a number of systems that a transcriptional upregulation of p16INK4A correlates with the functional inactivation of pRB, including mutation, deletion or the association of pRB with viral oncoproteins, including E7 (Khleif et al., 1996 and references therein). Although E2-TA expression leads to repression of E7 expression, we did not observe any significant reduction in p16INK4A protein levels over the 14 day time course of our experiment. The lack of a reduction in p16INK4A protein levels could be due to a cell-type associated phenomenon. Alternatively, it is possible that the levels of p16INK4A protein that persist in these cells reflect a prolonged stability of the protein in the absence of p16INK4A transcription. We have not examined p16INK4A mRNA levels in E2-expressing HeLa cells.
To investigate further the correlation between p21CIP induction and cellular senescence in HPV-positive cancer cells, we examined the abilities of the HPV18 and HPV16 E2 proteins to upregulate p21CIP. Expression of HPV18 E2 and HPV16 E2, and the transactivation-competent HPV16 E2 mutant E39A in HeLa cells increased levels of expression of p21CIP protein, whereas the transactivation-defective E2 I73A mutant did not (Figure 4B). From these data, we conclude that the transcriptional induction of p21CIP by E2 proteins correlates with their respective abilities to cause cellular senescence.
Overexpression of p21CIP induces senescence of HeLa cells in the absence of E2
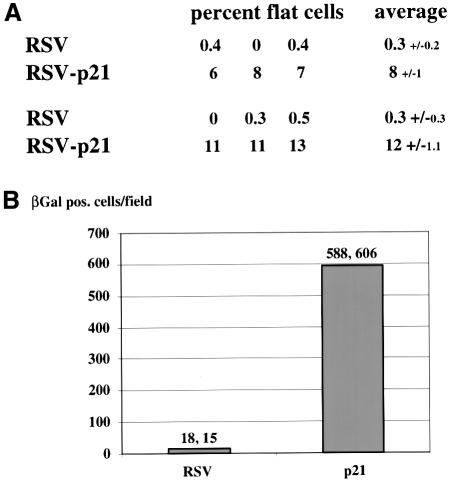
The correlation between E2-mediated senescence and p21CIP expression implicated p21CIP as a potential E2 effector of senescence in HeLa cells. In order to test whether p21CIP expression is sufficient to induce senescence in HeLa cells in the absence of E2, we examined the effect of expression of p21CIP using an RSV-p21 vector that provided efficient overexpression of p21CIP (data not shown). We transfected HeLa cells with either empty vector or the RSV-p21 expression vector along with a neomycin selection plasmid, after which the cells were split and placed under selection. In two independent experiments, the number of cells exhibiting the senescent, flat cell phenotype was quantitated relative to the total number of cells at 7 days from three randomly selected fields (Figure 5A). The background of senescent cells following transfection with empty vector was 0.3% in each of the experiments, whereas transfection with the p21CIP expression vector resulted in a 25- to 40-fold increase to 8–12% of senescent, flat cells at 7 days. At 15 days post-transfection, the cells were fixed and stained for SA-βGal expression, and the number of SA-βGal-positive cells was quantitated over an area of 6 cm2 (Figure 5B). There was an ∼36-fold increase in the number of SA-βGal-positive cells in the p21CIP-transfected cells compared with control transfected cells (Figure 5B). It should be noted, however, that the number of senescent HeLa cells observed following transfection with the p21CIP expression vector was still 5- to 7-fold less than the number induced by E2-TA (compare Figures 3B and 5A). Although this difference could be due to differences in the levels of p21CIP expression, it is also possible that other effectors may contribute to the E2-induced phenotype, either independently or in collaboration with p21CIP.
Fig. 5. Expression of p21CIP causes HeLa cell senescence in the absence of E2. (A) HeLa cells were transfected with the indicated expression vectors using either empty or p21CIP-expressing RSV vector as well as neoR plasmid. Two independent experiments are shown, in which the percentage of cells exhibiting the flat cell, senescent morphology was determined at 7 days. The data are expressed as percent flat cells relative to the total cell number. Three random fields were quantitated and averaged; the standard deviations are indicated. Each field contained a minimum of 100 cells. (B) In the same experiment, the SA-βGal-positive cells were counted over an area of 6 cm2 at 15 days post-transfection. The average from the two independent experiments is shown by the bars, with the individual data indicated at the top.
Antisense p21CIP oligonucleotides inhibit the E2-induced senescent phenotype
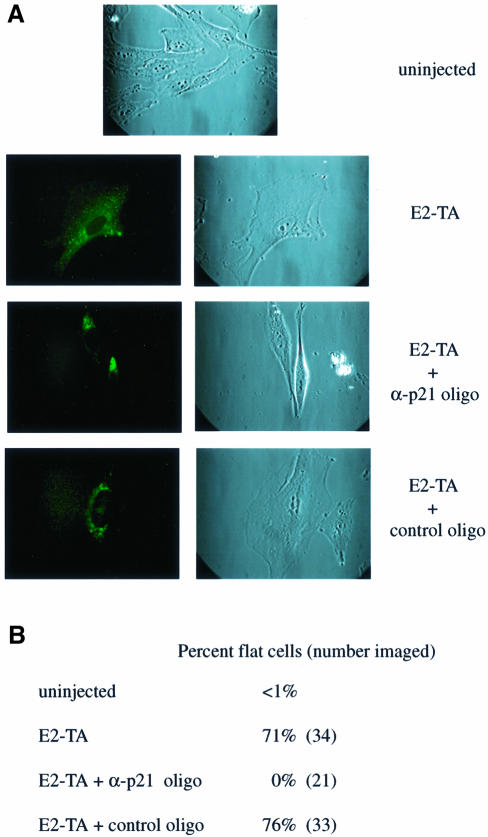
In order to test whether p21CIP is in fact required for E2-mediated senescence, we tested the effect of morpholino antisense p21CIP oligonucleotides using microinjection techniques. HeLa cells were cytoplasmically injected with E2-TA plasmid, either alone or in the presence of a p21CIP antisense oligonucleotide. Cells were examined for the characteristic flat cell morphology 7 days post-injection. Successful injections were assessed by the detection of co-injected fluorescein isothiocyanate (FITC)-labeled dextran as a marker (Figure 6A, left panels). As shown in Figure 6A, injection with E2-TA, either alone or with the random control oligonucleotide, resulted in the typical senescence morphology. Co-injection of E2-TA with the antisense p21 oligonucleotide was sufficient to inhibit this phenotype. Quantitation of these data revealed that >70% of the cells injected with E2-TA alone or together with the control oligonucleotide assumed a senescence-like morphology, whereas the cells co-injected with E2-TA plus the antisense p21 oligonucleotide did not (Figure 6B). A comparison of the cells injected with E2-TA plus the α-p21 oligonucleotide with the uninjected cells (Figure 6A) revealed that the injected cells were not entirely normal. The co-injected cells were more refractile and occasionally displayed cytoplasmic extensions, as depicted in Figure 6B. This altered phenotype could reflect effects of E2-TA that are not mediated by p21. Nonetheless, these data strongly support a critical function for p21CIP in E2-mediated HeLa cell senescence.
Fig. 6. Microinjection of antisense p21CIP oligonucleotides inhibits E2-mediated HeLa cell senescence. (A) Cells were left uninjected or injected cytoplasmically with E2-TA plasmid alone or in the presense of α-p21CIP or control morpholino oligonucleotides. FITC-conjugated dextran served as a co-injection marker (left panel). Imaging was performed on day 7 post-injection. (B) Cells exhibiting the enlarged phenotype were counted on day 8 and are expressed as a percentage of the total imaged cells.
Discussion
Molecular pathways governing E2 growth arrest
E2-mediated growth arrest in cervical cancer cell lines harboring high-risk HPV DNA has been well characterized using short-term cell proliferation studies and longer term colony reduction assays (Hwang et al., 1993, 1996; Dowhanick et al., 1995; Desaintes et al., 1997; Goodwin et al., 1998). E2 represses the viral E6/E7 promoter, which results in the reduction of E6 and E7 protein levels and the release of negative cell cycle regulators such as p53 and pRb. Although it is possible that E2 may have other functions that contribute to growth suppression (Desaintes et al., 1997), these have not yet been identified. For example, E2 may regulate the expression of certain cellular genes. Another consequence of E2 overexpression in HeLa cells is apoptosis, a phenomenon that has been described as p53 independent and occurs early (20–24 h) after E2 transfection (Desaintes et al., 1997, 1999).
We have previously shown that the E2-mediated cellular growth arrest of HPV-positive cervical cancer cells requires repression by E2 of the integrated E6/E7 promoter (Francis et al., 2000). Exogenous co-expression of HPV16 E6 and E7 with E2 can rescue the growth of HeLa cells. In this present study, we have extended our analyses by dissecting the individual contributions of E6 and E7 to the observed rescue. While E6 was able to overcome the growth-suppressive effects of E2, E7 and E1A were more potent in overriding the growth-inhibitory effects of E2 overexpression in HeLa cells. We have also determined that an intact pRb interaction domain is required for this effect by both E1A and E7, emphasizing the apparent role of the retinoblastoma protein (or other members of the pocket protein family) in E2 growth arrest.
One possible mechanism of E2 growth arrest is the downregulation of E7 expression by E2, resulting in increased levels of hypophosphorylated pRb protein, leading to G1 cell cycle arrest. The observed phenotypic reversal of E2 growth suppression by E7 or E1A could be explained by their binding of pRb, thereby releasing the cells from G1 arrest.
E2 induces senescence in HPV-positive cervical carcinoma cells
We report here, as a later consequence of E2 expression, the induction of a cellular state indistinguishable from that of classical replicative senescence in HPV-positive, but not in HPV-negative cervical carcinoma cell lines (Figure 1). We do not know at this point whether there is a relationship between E2-mediated apoptotic and senescent cell death. For example, cells that initially escape from apoptosis may subsequently undergo senescence. Alternatively, the induction of cellular senescence in a subpopulation of cells may protect them from apoptosis, as has been reported for senescent human fibroblasts (Wang, 1995). The fate of an E2-expressing HPV-positive cell—apoptosis or senescence—may also depend on the level of E2 protein expression. In view of a putative role for p21CIP both in E2-induced senescence (see below) and apoptosis of HeLa cells (Tsao et al., 1999), it is intriguing to speculate that both E2-mediated apoptosis and senescence may be related to the function of p21CIP.
We observed that after a period of time, E2-expressing HPV-positive cells assume an enlarged morphology typical of senescent cells and express the specific marker SA-βGal (Dimri et al., 1995). Additionally, these cells had increased expression levels of the cyclin/cdk inhibitor p21CIP, a known marker and under some circumstances even inducer of cellular senescence (Figure 4). As was previously reported for E2-mediated cellular growth arrest, senescence depends upon a transactivation- but not replication-competent E2 protein (Figures 1 and 2). Both the E2-induced growth suppression and senescence phenotypes are inhibited by viral oncoproteins, in particular by HPV16 E7 and Ad E1A (Figure 3), a function that requires an intact pRb interaction domain in each protein. The functions of E2 as a cellular growth suppressor are highly specific for HPV-positive cells, making it an intriguing candidate for use in a gene therapy approach to HPV-associated cancers.
Although the domains of E7 and Ad E1A to which p21 binds have not been clearly mapped, interactions between HPV16 E7 and p21CIP have been shown to involve both the pRb interaction domain and the C-terminus (Funk et al., 1997; Jones et al., 1997). The HPV16 E7 ΔDLYC mutant protein is defective for its interaction with members of the pocket protein family, and for interaction with p21CIP (Jones et al., 1997). Additionally, the CR1 domain of E1A has been implicated in a direct interaction between E1A and p21CIP in vitro (Keblusek et al., 1999). Interaction of E7 and E1A with p21CIP has not yet been separated mutationally from their pRB interaction abilities. It is, therefore, possible that the binding of p21CIP by E7 and E1A also plays an important role in the reversal of E2 growth arrest and senescence.
The p21CIP protein as a mediator of senescence
p21CIP is a member of the cyclin/cdk inhibitor family, which are well characterized cell cycle regulatory proteins (reviewed in Sherr and Roberts, 1999). Several lines of evidence are consistent with the notion that p21CIP functions as a senescence effector in E2-expressing HPV-positive cervical carcinoma cell lines. First, levels of p21CIP are upregulated as early as 1 day post-E2 transfection, peak at day 4, which is when the senescent phenotype first becomes evident, and remain high until day 14 (Figure 4A). Secondly, the ability of various wild-type and mutant E2 proteins to induce senescence correlates with their ability to increase p21CIP expression levels (Figure 4B). p21CIP is also able to cause HeLa cell senescence in the absence of E2 (Figure 5). Moreover, the induction of HeLa cell senescence by E2 is inhibited by antisense p21CIP oligonucleotides (Figure 6).
The mechanistic model that the introduction of E2 into HPV-positive cancer cells suppresses E6 and E7 expression, resulting in the activation of the cell cycle regulatory proteins pRb, p53 and p21CIP, is supported by the data presented in this study. These data are consistent with important roles for pRb and/or other members of the pocket protein family, as well as for p21CIP and p53 as an inducer of p21CIP, in senescence induction. The observed activation of p21CIP is at least in part transcriptional, most likely due to the increase in the levels of p53 following E6 repression. Using luciferase reporter assays with p21 promoter constructs, we have established that E2 induces the p21 promoter in HeLa cells but not a p21 promoter mutant deleted of upstream sequences containing the two p53 binding sites (data not shown).
The induction of p21CIP in other systems is known to result in the activation of pRb through the inhibition of pRb phosphorylation by cyclin/cdk kinases. Overexpres sion of pRb alone in HeLa cells, however, did not result in senescence, although we did note that pRB could stimulate p21CIP-mediated senescence induction ∼2-fold (data not shown). Although the best studied function of p21CIP is the inhibition of pRb phosphorylation by cyclin/cdk kinases, its role in cellular senescence could involve other less well characterized activities.
In this study we observed that the number of senescent cells following p21CIP expression was fewer than that following E2 expression in HeLa cells. The level of colony reduction mediated by p21CIP was also lower than that observed for E2 (data not shown). We also observed that the morphology of HeLa cells co-injected with E2-TA and α-p21 oligonucleotides, while clearly not flat and senescent, was distinct from the morphology of normal, uninjected HeLa cells (Figure 6). While these differences could be explained by variable levels of p21CIP expression in the presence and absence of E2, it is also conceivable that p21CIP may not be the only senescence effector affected by E2. Other molecules could contribute to HeLa cell senescence, either independently or in collaboration with p21CIP. Finally, one cannot rule out the possibility that E2 itself may have direct functions in senescence induction, considering that senescence, much like apoptosis, may involve complex signaling cascades with multiple effector pathways. Nevertheless, since senescence may represent an important natural barrier against tumorigenesis, insights into the mechanisms of senescence induction in tumor cells such as HeLa may ultimately provide novel approaches for the treatment of cancer.
Materials and methods
Recombinant plasmids
Expression plasmids for BPV1 E2-TA (p2450) and E2-TR (p1153) proteins (Spalholz et al., 1991; Winokur and McBride, 1992; Dowhanick et al., 1995), for the HPV16 wild-type (p2091) or mutant I73A (p3670) and E39A (p3667) E2 proteins (Sakai et al., 1996), as well as for HPV 18 E2 (p2092) (Del Vecchio et al., 1992), have been described previously. The numbers indicated for each of these plasmids represent their respective numbers in our laboratory plasmid bank. Expression vectors for the wild-type and mutant Ad 12S E1A proteins were a generous gift from Dr T.Kouzarides (Reid et al., 1998). E1A expression is under the control of an RSV promoter in the context of plasmid pBJ9Ω (RSV empty vector). To construct analogous expression vectors for the HPV oncoproteins, we subcloned coding sequences for the HPV 16 E6 and E7 proteins as well as for the mutant E7 DLYC protein (Münger et al., 1989) into the pCR-Blunt II-TOPO plasmid vector using PCR and the Zero Blunt TOPO PCR Cloning Kit (Invitrogen). The respective sequences were then excised and subcloned into the RSV vector pBJ9Ω using XbaI and HindIII. The resulting plasmids were RSV E6 (p4483), RSV E7 (p4484) and RSV E7 ΔDLYC (p4485). Coding sequences for the human p21CIP gene were subcloned from the pCDNAp21 expression vector, a gift from Dr P.Hinds, into the RSV vector using HindIII–XbaI digestion. The resulting plasmid is RSV p21 (p4486).
Cell lines
The human cervical cancer cell lines HeLa, Caski and C33A, as well as the human osteosarcoma cell line U2OS, were maintained as monolayers in Dulbecco’s modified Eagle’s medium containing 10% fetal calf serum.
Cellular growth suppression assay
Cells were seeded at 1–2 × 105 per 60 mm plate the day before transfection. Transfections were performed using a total of 10 µg of DNA and Fugene™ 6 (Boehringer Mannheim) according to the manufacturer’s instructions. Transfection efficiencies of 70–90% were routinely achieved. Unless indicated otherwise, the cells were transfected with 5 µg of E2 plasmid, 1 µg of neomycin selection plasmid and 1 µg of viral oncogene plasmid. Salmon sperm DNA was used as carrier DNA. At 24 h post-transfection, the cells were split into 3 × 10 cm plates and placed under selection in medium containing 900 µg/ml G418 (Gemini Bio-Products, Inc.) for HeLa and U2OS cells, 400 µg/ml for Caski and 1100 µg/ml for C33A cells. Cells were washed once with phosphate-buffered saline (PBS) and overlayed with fresh selection medium every third day. The number of colonies could easily be determined after 2–3 weeks, after which time they were fixed using 10% formaldehyde in PBS, stained with methylene blue and counted.
Senescence assay
Staining for perinuclear SA-βGal activity was performed as previously described (Dimri et al., 1995).
Immunoblotting and antibodies
For western blot analysis, the cells were washed with PBS and scraped into RIPA buffer (1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 160 mM NaCl, 10 nM Tris pH 7.4, 5 mM EDTA) containing 16 µg/ml benzamidine–HCl, 10 µg/ml phenanthroline, 10 µg/ml aprotinin, 10 µg/ml leupeptin, 10 µg/ml pepstatin A and 1 mM phenylmethylsulfonyl fluoride. The lysates were incubated at 4°C for 30 min, cell debris was pelleted and the protein concentration of the supernatants was determined using Bradford assays (Bio-Rad). Protein lysates were normalized for equal protein concentrations and separated by SDS– PAGE. Proteins were then transferred to polyvinylidene difluoride (PVDF) membranes and probed with the appropriate antibodies. Signals were detected by secondary horseradish peroxidase-conjugated antibody and enhanced chemiluminescence as recommended by the manufacturer (NEN™ Life Science Products, Inc.). The p21CIP monoclonal 6B6 antibody was from PharMingen. The monoclonal antibody to p16INK4A was a generous gift from Dr P.Hinds.
Microinjection
HeLa cells were plated in 35 mm glass bottom dishes (MatTek Corporation, MA) 24 h prior to injection. Cells were injected cytoplasmically with 110 ng/ml E2-TA plasmid alone or with 2 mg/ml morpholino oligonucleotides (GeneTools, LLC). The sequence of the antisense p21CIP oligonucleotide was 5′-CGCCTCCTCTGAGTG CCTCGGTGCC-3′ and that of the standard control oligonucleotide was 5′-CCTCTTACCTCAGTTACAATTTATA-3′. FITC-conjugated dextran was used as a co-injection marker. Microinjections were performed using the CompiC Inject automated system (Luigs & Neumann) at the EMBL (Heidelberg, Germany). Following the injections, cells were cultured in 2% serum at 32°C for 5 days, and then switched to 37°C. Images were taken on day 7 post-injection using a Leica fluorescent microscope equipped with a 63× objective.
Acknowledgments
Acknowledgements
We are grateful to Drs Karl Münger, Philip Hinds, David Thomas (Harvard Medical School) and James Wells (Harvard University) for helpful discussions and for critical readings of this manuscript. We also thank Dr Tony Kouzarides (Wellcome/CRC Institute, Cambridge, UK) for the E1A expression vectors and Dr Philip Hinds for the pCDNAp21 expression vector. We are grateful for the generosity and assistance of Drs Rainer Pepperkok and Rainer Saffrich (European Molecular Biology Laboratories, Heidelberg, Germany) for hosting Alla Karpova and helping her with the microinjection experiments. This research has been supported by a grant (RO1 CA77385) from the National Cancer Institute to P.M.H. S.I.W. has been supported by a Taplin Fellowship and by a fellowship from The Medical Foundation, Inc., Boston, MA. D.A.F. was supported by a Program in Cancer Biology Training grant from the National Cancer Institute (T32 CA72320) to the Department of Pathology. A.Y.K. is a Howard Hughes Medical Institute Predoctoral Fellow.
References
- Brown J.P., Wei,W. and Sedivy,J.M. (1997) Bypass of senescence after disruption of p21Cip1/WAF1 gene in normal diploid human fibroblasts. Science, 277, 831–834. [DOI] [PubMed] [Google Scholar]
- Del Vecchio A.M., Romanczuk,H., Howley,P.M. and Baker,C.C. (1992) Transient replication of human papillomavirus DNAs. J. Virol., 66, 5949–5958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demeret C., Yaniv,M. and Thierry,F. (1994) The E2 transcriptional repressor can compensate for Sp1 activation of the human papillomavirus type 18 early promoter. J. Virol., 68, 7075–7082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demeret C., Desaintes,C., Yaniv,M. and Thierry,F. (1997) Different mechanisms contribute to the E2-mediated transcriptional repression of human papillomavirus type 18 viral oncogenes. J. Virol., 71, 9343–9349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desaintes C., Demeret,C., Goyat,S., Yaniv,M. and Thierry,F. (1997) Expression of the papillomavirus E2 protein in HeLa cells leads to apoptosis. EMBO J., 16, 504–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desaintes C., Goyat,S., Garbay,S., Yaniv,M. and Thierry,F. (1999) Papillomavirus E2 induces p53-independent apoptosis in HeLa cells. Oncogene, 18, 4538–4545. [DOI] [PubMed] [Google Scholar]
- Dimri G.P. et al. (1995) A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl Acad. Sci. USA, 92, 9363–9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong G., Broker,T.R. and Chow,L.T. (1994) Human papillomavirus type 11 E2 proteins repress the homologous E6 promoter by interfering with the binding of host transcription factors to adjacent elements. J. Virol., 68, 1115–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dostatni N., Lambert,P.F., Sousa,R., Ham,J., Howley,P.M. and Yaniv,M. (1991) The functional BPV-1 E2 transactiving protein can act as a repressor by preventing formulation of the initiation complex. Genes Dev., 5, 1657–1671. [DOI] [PubMed] [Google Scholar]
- Dowhanick J.J., McBride,A.A. and Howley,P.M. (1995) Suppression of cellular proliferation by the papillomavirus E2 protein. J. Virol., 69, 7791–7799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durst M., Kleinheinz,A., Hotz,M. and Gissmann,L. (1985) The physical state of human papillomavirus type 16 DNA in benign and malignant genital tumours. J. Gen. Virol., 66, 1515–1522. [DOI] [PubMed] [Google Scholar]
- Durst M., Dzarlieva,P.R., Boukamp,P., Fusenig,N.E. and Gissmann,L. (1987) Molecular and cytogenetic analysis of immortalized human primary keratinocytes obtained after transfection with human papillomavirus type 16 DNA. Oncogene, 1, 251–256. [PubMed] [Google Scholar]
- Dyson N., Howley,P.M., Munger,K. and Harlow,E. (1989) The human papillomavirus-16 E7 oncoprotein is able to bind the retinoblastoma gene product. Science, 243, 934–937. [DOI] [PubMed] [Google Scholar]
- Eckner R., Ewen,M.E., Newsome,D., Gerdes,M., DeCaprio,J.A., Lawrence,J.B. and Livingston,D.M. (1994) Molecular cloning and functional analysis of the adenovirus E1A-associated 300-kD protein (p300) reveals a protein with properties of a transcriptional adaptor. Genes Dev., 8, 869–884. [DOI] [PubMed] [Google Scholar]
- Ewen M.E., Xing,Y., Lawrence,J.B. and Livingston,D.M. (1991) Molecular cloning, chromosomal mapping and expression of the cDNA for p107, a retinoblastoma gene product-related protein. Cell, 66, 1155–1164. [DOI] [PubMed] [Google Scholar]
- Fang L., Igarashi,M., Leung,J., Sugrue,M.M., Lee,S.W. and Aaronson,S.A. (1999) p21Waf1/Cip1/Sdi1 induces permanent growth arrest with markers of replicative senescence in human tumor cells lacking functional p53. Oncogene, 18, 2789–2797. [DOI] [PubMed] [Google Scholar]
- Francis D.A., Schmid,S.I. and Howley,P.M. (2000) Repression of the integrated papillomavirus E6/E7 promoter is required for growth suppression of cervical cancer cells. J. Virol., 74, 2679–2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk J.O., Waga,S., Harry,J.B., Espling,E., Stillman,B. and Galloway,D.A. (1997) Inhibition of CDK activity and PCNA-dependent DNA replication by p21 is blocked by interaction with the HPV-16 E7 oncoprotein. Genes Dev., 11, 2090–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano A., McCall,C., Whyte,P. and Franza,B.R.,Jr (1991) Human cyclin A and the retinoblastoma protein interact with similar but distinguishable sequences in the adenovirus E1A gene product. Oncogene, 6, 481–485. [PubMed] [Google Scholar]
- Goodwin E.C., Naeger,L.K., Breiding,D.E., Androphy,E.J. and DiMaio,D. (1998) Transactivation-competent bovine papillomavirus E2 protein is specifically required for efficient repression of human papillomavirus oncogene expression and for acute growth inhibition of cervical carcinoma cell lines. J. Virol., 72, 3925–3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howley P.M. (1996) Papillomavirinae: the viruses and their replication. In Fields,B.N., Knipe,D.N. and Howley,P.M. (eds), Fields Virology, 3rd edn. Lipppincott-Raven, Philadelphia, PA, pp. 2045–2076. [Google Scholar]
- Hwang E.S., Riese,D.J., Settleman,J., Nilson,L.A., Honig,J., Flynn,S. and DiMaio,D. (1993) Inhibition of cervical carcinoma cell line proliferation by the introduction of a bovine papillomavirus regulatory gene. J. Virol., 67, 3720–3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang E.S., Naeger,L.K. and DiMaio,D. (1996) Activation of the endogenous p53 growth inhibitory pathway in HeLa cervical carcinoma cells by expression of the bovine papillomavirus E2 gene. Oncogene, 12, 795–803. [PubMed] [Google Scholar]
- Jones D.L., Alani,R.M. and Munger,K. (1997) The human papilloma virus E7 oncoprotein can uncouple cellular differentiation and proliferation in human keratinocytes by abrogating p21Cip1-mediated inhibition of cdk2. Genes Dev., 11, 2101–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa S., Fujiwara,T., Kadowaki,Y., Fukazawa,T., Sok-Joo,R., Roth,J.A. and Tanaka,N. (1999) Overexpression of the p21 sdi1 gene induces senescence-like state in human cancer cells: implication for senescence-directed molecular therapy for cancer. Cell Death Differ., 6, 765–772. [DOI] [PubMed] [Google Scholar]
- Keblusek P., Dorsman,J.C., Teunisse,A.F., Teunissen,H., van der Eb,A.J. and Zantema,A. (1999) The adenoviral E1A oncoproteins interfere with the growth-inhibiting effect of the cdk-inhibitor p21CIP1/WAF1. J. Gen. Virol., 80, 381–390. [DOI] [PubMed] [Google Scholar]
- Khleif S.N., DeGregori,J., Yee,C.L., Otterson,G.A., Kaye,F.J., Nevins,J.R. and Howley,P.M. (1996) Inhibition of cyclin D-CDK4/CDK6 activity is associated with an induction of cyclin kinase inhibitor activity. Proc. Natl Acad. Sci. USA, 93, 4350–4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyono T., Foster,S.A., Koop,J.I., McDougall,J.K., Galloway,D.A. and Klingelhutz,A.J. (1998) Both Rb/p16INK4a inactivation and telomerase activity are required to immortalize human epithelial cells. Nature, 396, 84–88. [DOI] [PubMed] [Google Scholar]
- Münger K., Werness,B.A., Dyson,N., Phelps,W.C. and Howley,P.M. (1989) Complex formation of human papillomavirus E7 proteins with the retinoblastoma tumor suppressor gene product. EMBO J., 8, 4099–4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura A., Ono,T., Ishimoto,A., Dowhanick,J.J., Frizzell,M.A., Howley,P.M. and Sakai,H. (2000) Mechanisms of human papilloma virus E2 mediated repression of viral oncogene expression and cervical cancer cell growth inhibition. J. Virol., 74, 3752–3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda A., Ning,Y., Venable,S.F., Pereira-Smith,O.M. and Smith,J.R. (1994) Cloning of senescent cell-derived inhibitors of DNA synthesis using an expression screen. Exp. Cell Res., 211, 90–98. [DOI] [PubMed] [Google Scholar]
- Phelps W.C., Yee,C.L., Munger,K. and Howley,P.M. (1988) The human papillomavirus type 16 E7 gene encodes transactivation and transformation functions similar to those of adenovirus E1A. Cell, 53, 539–547. [DOI] [PubMed] [Google Scholar]
- Reid J.L., Bannister,A.J., Zegerman,P., Martinez-Balbas,M.A. and Kouzarides,T. (1998) E1A directly binds and regulates the P/CAF acetyltransferase. EMBO J., 17, 4469–4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanczuk H. and Howley,P.M. (1992) Disruption of either the E1 or the E2 regulatory gene of human papillomavirus type 16 increases viral immortalization capacity. Proc. Natl Acad. Sci. USA, 89, 3159–3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanczuk H., Thierry,F. and Howley,P.M. (1990) Mutational analysis of cis-elements involved in E2 modulation of human papillomavirus type 16 P97 and type 18 P105 promoters. J. Virol., 64, 2849–2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai H., Yasugi,T., Benson,J.D., Dowhanick,J.J. and Howley,P.M. (1996) Targeted mutagenesis of the human papillomavirus type 16 E2 transactivation domain reveals separable transcriptional activation and DNA replication functions. J. Virol., 70, 1602–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffner M., Werness,B.A., Huibregtse,J.M., Levine,A.J. and Howley,P.M. (1990) The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell, 63, 1129–1136. [DOI] [PubMed] [Google Scholar]
- Schlegel R., Phelps,W.C., Zhang,Y.L. and Barbosa,M. (1988) Quantitative keratinocyte assay detects two biological activities of human papillomavirus DNA and identifies viral types associated with cervical carcinoma. EMBO J., 7, 3181–3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz E., Freese,U.K., Gissman,L., Mayer,W., Roggenbuck,B., Stremlau,A. and zur Hausen,H. (1985) Structure and transcription of human papillomavirus sequences in cervical carcinoma cells. Nature, 314, 111–114. [DOI] [PubMed] [Google Scholar]
- Sherr C.J. and Roberts,J.M. (1999) CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev., 13, 1501–1512. [DOI] [PubMed] [Google Scholar]
- Smith J.R. and Pereira-Smith,O.M. (1996) Replicative senescence: implications for in vivo aging and tumor suppression. Science, 273, 63–67. [DOI] [PubMed] [Google Scholar]
- Spalholz B.A., Vande Pol,S.B. and Howley,P.M. (1991) Characterization of the cis elements involved in the basal and E2 transactivated expression of the bovine papillomavirus P2443 promoter. J. Virol., 65, 743–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan S.-H., Gloss,B. and Bernard,H.-U. (1992) During negative regulation of the human papillomavirus-16 E6 promoter, the viral E2 protein can displace Sp1 from a proximal promoter element. Nucleic Acids Res., 20, 251–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierry F. and Howley,P.M. (1991) Functional analysis of E2 mediated repression of the HPV-18 P105 promoter. New Biol., 3, 90–100. [PubMed] [Google Scholar]
- Thierry F. and Yaniv,M. (1987) The BPV1-E2 trans-acting protein can be either an activator or a repressor of the HPV18 regulatory region. EMBO J., 6, 3391–3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao Y.P., Huang,S.J., Chang,J.L., Hsieh,J.T., Pong,R.C. and Chen,S.L. (1999) Adenovirus-mediated p21WAF1/SDII/CIP1 gene transfer induces apoptosis of human cervical cancer cell lines. J. Virol., 73, 4983–4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E. (1995) Senescent human fibroblasts resist programmed cell death and failure to suppress bcl2 is involved. Cancer Res., 55, 2284–2292. [PubMed] [Google Scholar]
- Whyte P., Buchkovich,K.J., Horowitz,J.M., Friend,S.H., Raybuck,M., Weinberg,R.A. and Harlow,E. (1988) Association between an oncogene and an anti-oncogene: the adenovirus E1A proteins bind to the retinoblastoma gene product. Nature, 334, 124–129. [DOI] [PubMed] [Google Scholar]
- Whyte P., Williamson,N.M. and Harlow,E. (1989) Cellular targets for transformation by the adenovirus E1A proteins. Cell, 56, 67–75. [DOI] [PubMed] [Google Scholar]
- Winokur P.L. and McBride,A.A. (1992) Separation of the transcriptional activation and replication functions of the bovine papillomavirus-1 E2 protein. EMBO J., 11, 4111–4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodworth C.D., Bowden,P.E., Doninger,J., Pirisi,L., Barnes,W., Lancaster,W.D. and DiPaolo,J.A. (1988) Characterization of normal human exocervical epithelial cells immortalized in vitro by papillomavirus types 16 and 18 DNA. Cancer Res., 48, 4620–4628. [PubMed] [Google Scholar]
- zur Hausen H. (1996) Papillomavirus infections—a major cause of human cancers. Biochim. Biophys. Acta, 1288, F55–F78. [DOI] [PubMed] [Google Scholar]