Abstract
Lycopene can be cleaved by carotene 9′,10′-oxygenase at its 9′,10′ double bond to form apo-10′-lycopenoids, including apo-10′-lycopenal, -lycopenol and -lycopenoic acid. The latter has been recently shown to inhibit lung carcinogenesis both in vivo and in vitro, however, the mechanism(s) underlying this protection is not well defined. In the present study, we report that treatment with apo-10′-lycopenoic acid, in a time- and dose-dependent manner, results in the nuclear accumulation of transcription factor Nrf2 (nuclear factor E2-related factor 2) protein in BEAS-2B human bronchial epithelial cells. The activation of Nrf2 by apo-10′-lycopenoic acid is associated with the induction of phase II detoxifying/antioxidant enzymes including heme oxygenase-1, NAD(P)H:quinone oxidoreductase 1, glutathione S-transferases, and glutamate–cysteine ligases in BEAS-2B cells. Furthermore, apo-10′-lycopenoic acid treatment increased total intracellular glutathione levels and suppressed both endogenous reactive oxygen species generation and H2O2-induced oxidative damage in BEAS-2B cells. In addition, both apo-10′-lycopenol and apo-10′-lycopenal induced heme oxygenase-1 gene expression in BEAS-2B cells. These data strongly suggest that the anti-carcinogenic and antioxidant functions of lycopene may be mediated by apo-10′-lycopenoids via activating Nrf2 and inducing phase II detoxifying/antioxidant enzymes.
Keywords: lycopene, apo-10′-lycopenoic acid, phase II enzymes, Nrf2, GSH, oxidative damage
The chemopreventive effect of lycopene, a carotenoid rich in tomato and tomato-based products, against cancers has been suggested in many epidemiological and animal studies.1–6 One of explanations for the protective effect of lycopene is its ability to induce phase II detoxifying/antioxidant enzymes found in both in vitro and in vivo studies.7–9 The induction of phase II detoxifying/antioxidant enzymes, such as heme oxygenase-1 (HO-1), NAD(P)H:quinone oxidoreductase 1 (NQO1), glutathione S-transferases (GSTs), glutathione reductase (GSR), glutamate–cysteine ligase (catalytic subunit, GCLC; and modifier subunit, GCLM), microsomal epoxide hydrolase 1 (mEH) and UDP glucuronosyltransferase 1 family, polypeptide A6 (UGT1A6), results in the detoxification of carcinogens and the inactivation of reactive oxygen species (ROS), contributing to the protective effect of chemopreventive agents.10 Many of these enzymes are primarily regulated by the nuclear factor-E2 related factor 2 (Nrf2), a transcription factor that binds to the antioxidant response element (ARE) in the 5′-flanking region of target genes.10 Under normal conditions, the majority of the Nrf2 is sequestered in the cytoplasm by Kelch-like erythroid Cap‘n’Collar homologue-associated protein 1 (Keap 1), while only residual nuclear Nrf2 binds to the ARE, driving basal activities. Exposure to certain chemopreventive agents leads to the dissociation of the Nrf2-Keap1 complex in the cytoplasm and the translocation of Nrf2 into the nucleus. The nuclear accumulation of Nrf2 subsequently activates target genes of phase II enzymes.10 Because of its critical roles in the detoxification and antioxidant process during carcinogenesis, Nrf2 has been recognized as a potential molecular target for cancer prevention.10 A wide variety of dietary and synthetic compounds, e.g., sulforaphane,11 curcumin12 and (−)-epigallocatechin-3-gallate,13 have been shown to induce Nrf2-ARE mediated gene expression, which is one of mechanisms for their chemopreventive effects.
Numerous oxidative metabolites of lycopene have been identified in both in vitro14–17 and in vivo18–21 systems, raising the question as to whether the induction of phase II enzymes by lycopene is, at least in part, due to its metabolites.9,22–25 Previously, Ben-Dor et al. showed that an ethanolic extract of lycopene containing unidentified hydrophilic derivatives induced phase II enzymes and activated ARE-driven reporter gene activity at a potency similar to lycopene.9 However, both identification and existence of those chemically induced oxidative derivatives in the mammalian tissues remains unknown. Therefore, the notion that lycopene metabolites contribute to the biological effect of lycopene in terms of the induction of phase II enzymes through ARE-induced expression needs to be demonstrated unambiguously.
The carotene 9′,10′-oxygenase, a specific cleavage enzyme at the 9′,10′ double bonds of carotenoids, has been cloned from humans, rats, mice and ferrets.21,26 We have recently demonstrated that the enzymatic cleavage of lycopene at its 9′,10′-double bond by carotene 9′,10′-oxygenase produces apo-10′-lycopenal, which can be either reduced into apo-10′-lycopenol or oxidized into apo-10′-lycopenoic acid.21 Very recently, we showed that apo-10′-lycopenoic acid treatment for 16 weeks suppresses chemical carcinogen (4-(N-methyl-N-nitrosamino)-1-(3-pyridal)-1-butanone, NNK)-induced lung tumorigenesis in the A/J mouse model.25 Although our in vitro experiments showed that apo-10′-lycopenoic acid inhibits lung cancer cell growth and activates retinoic acid receptor signaling,25 the corresponding effects were not observed in the lungs of the mice after 16 weeks of treatment. These results suggest that, in addition to its growth inhibitory activity, apo-10′-lycopenoic acid may provide protection against the initiation stage of carcinogenesis, e.g., detoxifying NNK or counteracting oxidative insults via its induction of phase II detoxification/antioxidant enzymes.
In the present study, we examined the effects of apo-10′-lycopenoids on the expression of Nrf2-regulated phase II detoxifying/antioxidant enzymes, intracellular levels of glutathione and H2O2-induced oxidative damage in an immortalized human bronchial epithelial cell line, BEAS-2B.
Material and methods
Materials
Apo-10′-lycopenoic acid, apo-10′-lycopenol and apo-10′-lycopenal, with the purity above 99%, were provided by BASF, Germany. Stock solutions of lycopenoids (10 mM) were prepared in tetrahydrofuran (THF) containing 0.025% butylated hydroxytoluene (BHT, from Sigma, St. Louis, MO) and stored at −80°C. Rabbit anti-Nrf2 antibody and mouse anti-NQO1 antibody were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit anti-HO1 antibody was purchased from Assay Designs (Ann Arbor, MI). Mouse anti-β-actin antibody was obtained from Sigma. Horseradish peroxidase (HRP)-conjugated secondary antibodies were purchased from Bio-Rad (Hercules, CA).
Cell culture
BEAS-2B cell, an immortalized human bronchial epithelial cell line, was purchased from American Type Culture Collection (ATCC, Manassas, VA). Cells were grown in a serum free LHC-9 medium (Invitrogen, Carlsbad, CA) in tissue culture plates coated with bovine serum albumin/collagen/fibronectin as previously reported,27 and kept at 37°C in a humidified atmosphere containing 5% CO2. Upon treatment, aliquots from the stock solutions of lycopenoids were added to the cell culture medium to the desired concentrations and stirred vigorously. The control cell culture received THF containing 0.025% BHT only. The final THF concentration in cell culture medium was 0.1%. All procedures were performed under red light.
Western blot
Whole cell protein extraction for measuring HO-1 and NQO1 expression, and nuclear extraction for measuring the nuclear distribution of Nrf2 were prepared as described previously.25,28 Cellular proteins were separated by SDS-PAGE and transferred onto Immobilon-P membranes (Millipore, Bedford, MA). The membranes were blocked with 5% non-fat milk in TBST buffer and incubated with a primary antibody. The protein was detected by a HRP-conjugated secondary antibody and visualized by a Super-Signal West Pico Chemiluminescent Substrate Kit (Pierce, Rockford, IL). The protein levels were analyzed with an imaging densitometer (Bio-Rad, Hercules, CA), using β-actin as a loading control.
Gene expression
The expression of phase II detoxification and antioxidant enzymes was measured by real-time quantitative PCR after the reverse transcription of RNA. Total RNA was extracted using Tri-Pure reagent (Roche Applied Science, Indianapolis, IN). cDNA was generated with M-MLV reverse transcriptase (Invitrogen) as indicated in the manufacturer’s manual.
PCR Primers were designed using Primer Express version 2.0 (Applied Biosystems, Foster City, CA) software. The sequences of the primers specific to each gene are shown as follows: GCLC (NM_001498), 5′-GGAAGGAAGGTGTGTTTCCTGG-3′ and 5′-ACTCCCTCATCCATCTGGCAA-3′; GCLM (NM_002061), 5′-CCAGATGTCTTGGAATGCACTG-3′ and 5′-AGGACTGAACAGGCCATGTCA-3′; GSR (NM_000637), 5′-TGGCACTTG CGTGAATGTTG-3′ and 5′-CACATAGGCATCCCGCTTTTC-3′; GSTP1 (NM_000852), 5′-AGTCCAATACCATCCTGCGTCA-3′ and 5′-CCCGCCTCATAGTTGGTGTAGA-3′; HO-1 (NM_002133), 5′-AGCTCTTTGAGGAGTTGCAGGA-3′ and 5′-AGCTGAGTGTAAGGACCCATCG-3′; mEH (NM_000120), 5′-CCGTAGGCTCTGCTCTGAATGA-3′ and 5′-AACTTCCTTTCCAGGCCTCCA-3′; NAD(P)H:NQO1 (NM_000903), 5′-GTGATATTCCAGTTCCCCCTGC-3′ and 5′-AAGCACTGCCTTCTTACTCCGG-3′; UGT1A6 (NM_205862), 5′-AAACGATCTGCTTGGTCACCC-3′ and 5′-TCCCTTAGTCTCCATGCGCTT-3′; and β-actin (NM_001614), 5′-AAGATCATTGCTCCTCCTGAGC-3′ and 5′-GCTGATCCACATCTGCTGGAA-3′.
Real-time PCR reactions were performed on an Applied Biosystems 7000 sequence detection system, using Platinum SYBR Green qPCR Kit (Invitrogen) according to the manufacture’s instructions. The mRNA levels of the measured genes relative to β-actin mRNA were determined using the 2−ΔΔCT method.29 The mRNA levels were expressed as fold induction, relative to the control.
Measurement of intracellular glutathione
Intracellular glutathione (GSH) levels were measured using the 5,5′-dithiobis-2-nitrobenzoic acid (DTNB)–GSR recycling method.30 In brief, trypsinized cells were rinsed with ice-cold PBS twice, resuspended in 0.5 ml of ice-cold KPE extraction buffer [0.1% Triton X-100, 0.6% sulfosalicylic acid in 0.1 M potassium phosphate buffer with 5 mM EDTA, pH 7.5], followed by sonication (20 sec) and centrifugation (3,000g for 5 min at 4°C). Then 20 μl of the supernatant was added to 120 μl of KPE, containing 0.84 mM DTNB and 1.65 unit/ml GSR. Finally, 60 μl of 0.8 mM NADPH was added and the rate of change in absorbance was measured for 2 min in a 30 sec interval at 410 nm, using uQuant microplate spectrophotometer (BioTek Instruments, Winooski, VT). The protein concentration of cell extracts was determined by Coomassie Protein Assay Reagent (Pierce Biotechnology, Rockford, IL). All measurements were duplicated. The total GSH level was expressed as nM/mg protein.
Lactate dehydrogenase assay
Lactate dehydrogenase (LDH) release, an indicator of cell membrane integrity and cell viability, was used to evaluate H2O2-induced oxidative damage. In brief, BEAS-2B cells were treated with different concentrations of apo-10′-lycopenoic acid or tert-butylhydroquinone (tBHQ) in a 96-well plate for 24 hr, and then switched to a medium containing 100 μM of H2O2 for 30 min. The released and the intracellular LDH activity were then determined with a LDH assay kit (Sigma, St. Louis, MO) as described in the manufacturer’s manual.
Measurement of ROS
Cytosolic ROS levels were measured using 5-(and-6)-carboxy-2′,7′-dichlorofluorescin diacetate (carboxy-H2DCFDA, from Ana-Spec, San Jose, CA) as described previously31 with minor modification. In brief, BEAS-2B cells were treated with the indicated concentration of apo-10′-lycopenoic acid or with 25 μM of tBHQ in a 96-well plate for 24 hr and were incubated with 10 μM carboxy-H2DCFDA at 37°C for 30 min. The carboxy-H2DCFDA was removed, and the cells were incubated with LHC-9 medium for another 5 min. The fluorescence was measured using an FLx800 multidetection microplate reader (BioTek Instruments, Winooski, VT) with the filter set of 485/528 nm (excitation/emission). After the measurement for ROS, the LHC-9 medium was removed and the viable cells in each well were evaluated by measuring total intracellular LDH activity, which was used to normalize cellular ROS level.
Statistical analysis
Results were expressed as means ± SEM unless specifically indicated. Comparison of mean values of control and treatment cells was made using student’s t-test or one-way ANOVA analyses with Fisher’s least significant difference post hoc procedure. A difference between groups was considered significant if p < 0.05.
Results
Apo-10′-lycopenoic acid induces nuclear accumulation of Nrf2
Since Nrf2 is a key transcription factor in regulating the expression of phase II detoxifying/antioxidant enzyme, we first determined whether apo-10′-lycopenoic acid activates Nrf2 by accumulating Nrf2 in the nuclear fraction of cells. Nuclear Nrf2 protein was increased by 50% in BEAS-2B cells treated with 10 μM of apo-10′-lycopenoic acid for 3 hr, as compared to control. After treatment with apo-10′-lycopenoic acid for 6 hr, the accumulation level of the nuclear Nrf2 protein increased by ~1.6-fold, as compared to the control (Fig. 1a). Furthermore, treatment with 3, 5 and 10 μM of apo-10′-lycopenoic acid dose-dependently increased nuclear Nrf2 protein levels by 60, 80, and 180%, respectively (Fig. 1b).
Figure 1.
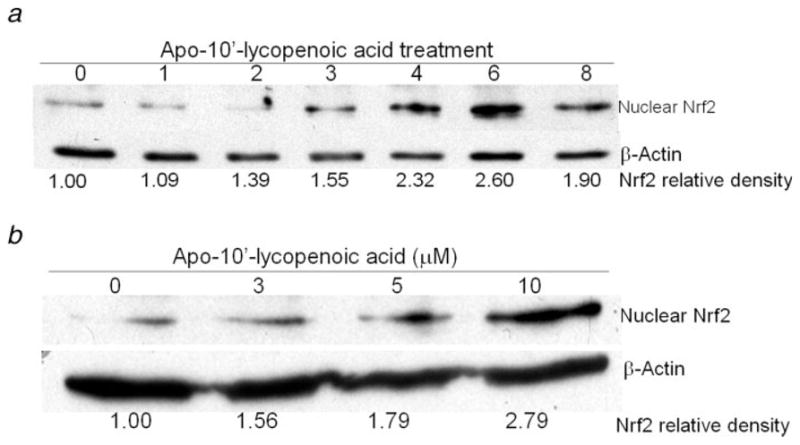
Effect of apo-10′-lycopenoic acid on the nuclear accumulation of Nrf2. (a) BEAS-2B cells were treated with 10 μM of apo-10′-lycopenoic acid for indicated time. (b) BEAS-2B cells were treated with indicated concentration of apo-10′-lycopenoic acid for 6 hr. Nuclear proteins were extracted and nuclear Nrf2 was detected by Western blot and analyzed with an imaging densitometer. The data are representative results of at least 3 independent experiments.
Apo-10′-lycopenoic acid induces the expression of phase II detoxification and antioxidant enzymes
Apo-10′-lycopenoic acid significantly induced the mRNA expressions of phase II enzymes, including HO-1, NQO1, GST, GSR, GCLC, GCLM, mEH and UGT1A6, in BEAS-2B cells, as compared to control cells treated with THF only (Fig. 2). We then examined the dose- and time-dependent responses of apo-10′-lycopenoic acid on the expression of HO-1 gene. Although untreated BEAS-2B cells express very low level of the HO-1 gene, HO-1 mRNA level significantly increased as early as 30 min after treatment with 10 μM of apo-10′-lycopenoic acid and reached a peak after 4 hr of treatment (Fig. 3a). Accordingly, HO-1 protein was detected after 2 hr of apo-10′-lycopenoic acid treatment and steadily increased after 24 hr of the treatment (Fig. 3b). The induction of HO-1 mRNA expression was dose-dependently increased by increasing concentration of apo-10′-lycopenoic acid (p for trend < 0.001) (Fig. 3c). HO-1 protein levels in BEAS-2B are also dose-dependently increased by apo-10′-lycopenoic acid treatment for 24 hr (Fig. 3d).
Figure 2.
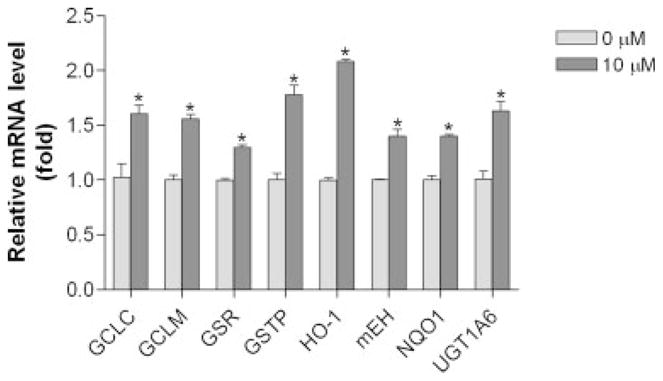
Effect of apo-10′-lycopenoic acid on the expression of phase II detoxifying/antioxidant enzymes. 5 × 105 cells in 6-well plate were treated with 10 μM of apo-10′-lycopenoic acid for 4 hr. Total RNA was extracted, and the mRNA levels of genes were measured by quantitative reverse transcription PCR. Values are means ± SEM of 3 replicate assays. Grey bar: control cells treated with THF only; dark bar: cells treated with 10 μM of apo-10′-lycopenoic acid. *, Statistically significantly different, as compared between control and apo-10′-lycopenoic acid-treated cells, p < 0.05.
Figure 3.
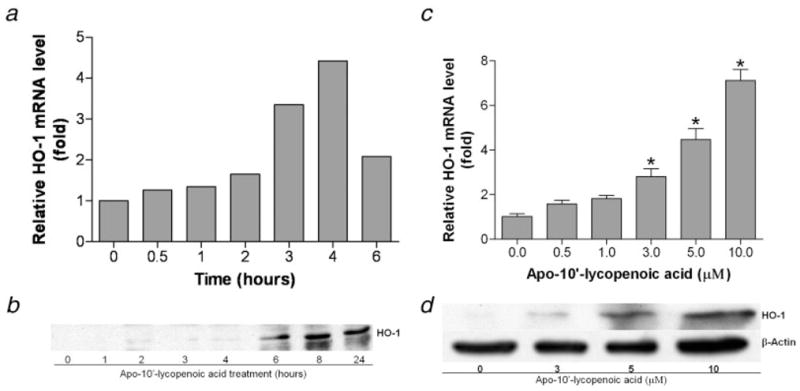
Induction of HO-1 gene expression by apo-10′-lycopenoic acid. Left panel: Time-course of induction of HO-1 mRNA (a) and protein (b). BEAS-2B cells were treated with 10 μM of apo-10′-lycopenoic acid for the indicated time. Right panel: Dose effect of apo-10′-lycopenoic acid on induction of HO-1 mRNA (c) and protein (d). BEAS-2B cells were treated with indicated concentration of apo-10′-lycopenoic acid for 4 hr (c) or 24 hr (d). Transcription level of HO-1 gene was measured by quantitative reverse transcription PCR (a and c) and the protein level was measured by Western blot (b and d). (a), (b) and (d) are representative results of at least 3 repeats. Data in panel (c) are expressed as means ± SEM of 3 replicate assays; *, Statistically significantly different, as compared between control and apo-10′-lycopenoic acid-treated cells, p < 0.05.
We then examined the effect of apo-10′-lycopenoic acid on the expression of NQO1, a key enzyme involved in defending against oxidative insults, maintaining genetic stability, and inhibiting neoplasia.32 The basal expression of NQO1 in BEAS-2B cells is higher as compared to HO-1 expression. We found that the levels of NQO1 mRNA slightly increased (40%) by 10 μM of apo-10′-lycopenoic acid treatment for 4 hr (Fig. 4a). Further induction can still be detected after 24 hr of the treatment (Fig. 4a). Like the induction of HO-1, the induction of NQO1 by apo-10′-lycopenoic acid is also dose-dependent (Figs. 4b and 4c). NQO1 mRNA and protein levels increased by 20–50% after 24 hr treatment with apo-10′-lycopenoic acid, as compared to that of control.
Figure 4.
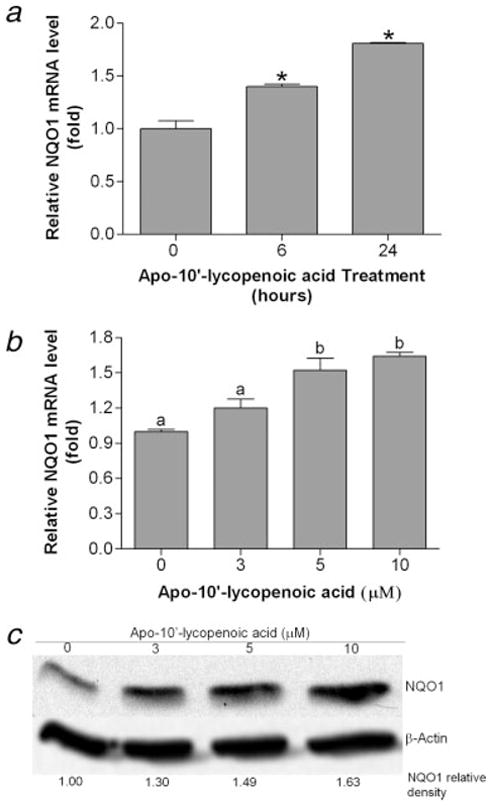
Induction of NQO1 gene expression by apo-10′-lycopenoic acid. (a) Time-course of NQO1 induction. BEAS-2B cells were treated with 10 μM of apo-10′-lycopenoic acid for 6 and 24 hr. (b) and (c), dose effect of apo-10′-lycopenoic acid on NQO1 induction. BEAS-2B cells were treated with the indicated concentration of apo-10′-lycopenoic acid for 6 hr (b) or 24 hr (c). Transcription level of HO-1 gene was measured by quantitative reverse transcription PCR (a and b) and the protein level was measured by western blot (c). Data in panels (a) and (b) are expressed as mean ± SEM of 3 replicate assays, means that do not share a letter differ, p < 0.05. Panel (c) shows a representative result of at least 3 repeats.
We also examined the induction of HO-1 expression by apo-10′-lycopenol and apo-10′-lycopenal, the other 2 metabolites generated by lycopene. We found that, similar to apo-10′-lycopenoic acid, both compounds are able to induce HO-1 expression. Apo-10′-lycopenal shows the strongest potential, with a 12-fold induction of HO-1 gene expression, as compared to the same dose of apo-10′-lycopenol and apo-10′-lycopenoic acid (Fig. 5).
Figure 5.
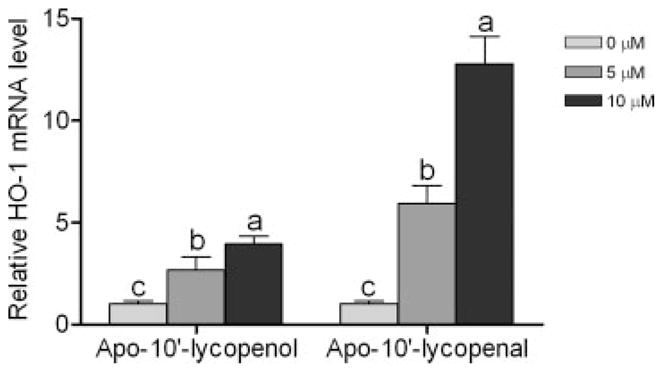
Effect of apo-10′-lycopenol and apo-10′-lycopenal on HO-1 gene expression. BEAS-2B cells were treated with 0, 5 or 10 μM of apo-10′-lycopenol or apo-10′-lycopenal for 4 hr. Total RNA was extract and the transcription level of HO-1 gene was measured by quantitative reverse transcription PCR. Values are means ± SEM of 3 replicate assays. Means that do not share a letter differ in the same group differ, p < 0.05.
Effect of apo-10′-lycopenoic acid on intracellular total GSH, ROS and H2O2-induced oxidative damage
Because of the induction of glutamate–cysteine ligase (GCLC and GCLM, the enzymes are involved in GSH synthesis) by apo-10′-lycopenoic acid, we further examined the effect of apo-10′-lycopenoic acid on the levels of intracellular GSH. As a positive control, treatment with 25 μM of tBHQ, an antioxidant that induces ARE-driven gene expression and GSH synthesis,33 significantly increases total GSH level by 1.2-fold in BEAS-2B cells (p < 0.001) (Fig. 6a). Although treatment with 3 or 5 μM of apo-10′-lycopenoic acid non-significantly increases GSH level by 10 and 15%, respectively, apo-10′-lycopenoic acid treatment at 10 μM significantly increases GSH level by ~70%, as compared to untreated cells (p < 0.001) (Fig. 6a).
Figure 6.
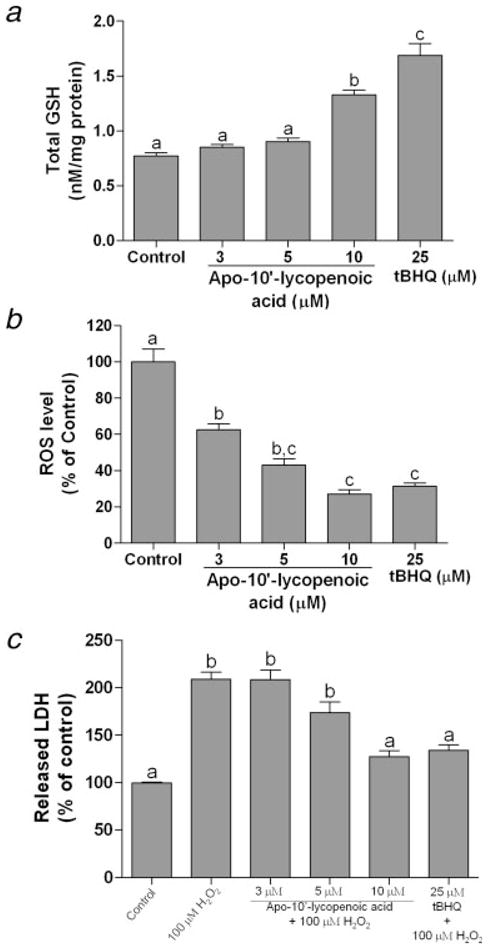
Effect of apo-10′-lycopenoic acid on total intracellular GSH level, endogenous ROS, and H2O2-induced oxidative damage. (a) and (b), BEAS-2B cells were treated with indicated concentration of apo-10′-lycopenoic acid or tBHQ for 24 hr. Total GSH (a) was measured using DTNB-GSR recycling method and ROS (b) was measured using carboxy-H2DCFDA method. (c) BEAS-2B cells were pre-treated with indicated concentration of apo-10′-lycopenoic acid or tBHQ for 24 hr before 100 μM of H2O2 were added and incubated for 30 min. H2O2-induced oxidative damage was measured by LDH releasing assay. Values are means ± SEM of 3 replicate assays. Means that do not share a letter differ, p < 0.05.
To determine whether apo-10′-lycopenoic acid affects intracellular ROS status, we quantified oxidative stress in cells using the dichlorofluorescein assay.31 We found that treatment with apo-10′-lycopenoic acid for 24 hr exerts a dose-dependent inhibition of endogenous ROS generation (Fig. 6b). Treatment with tBHQ at 25 μM for 24 hr decreases intracellular ROS levels by about 70%, as compared to cells treated with THF only (Fig. 6b). The apo-10′-lycopenoic acid treatment at a concentration of 3 μM decrease cellular ROS levels by about 40% (p < 0.01); while 10 μM of apo-10′-lycopenoic acid shows a comparable capability to inhibit ROS production as compared to that of tBHQ treatment at 25 μM (Fig. 6b).
Using LDH release as an indicator of H2O2-induced cytotoxicity, we further evaluated the protective effects of apo-10′-lycopenoic acid against oxidative damage. Treatment with 100 μM of H2O2 for 30 min results in a significant increase in LDH activity in cell culture medium of BEAS-2B cells. This H2O2-induced LDH release was decreased (35% decrease) by pretreatment with 25 μM of tBHQ for 24 hr (Fig. 6c). When we pretreated BEAS-2B cells with 5 and 10 μM of apo-10′-lycopenioc acid for 24 hr, the H2O2-induced LDH release was decreased by 17% (p > 0.05) and 39% (p < 0.01), respectively (Fig. 6c).
Discussion
In the present study, we demonstrate for the first time that an enzymatic metabolite of lycopene, apo-10′-lycopenoic acid induces both the nuclear accumulation of Nrf2 protein and the induction of phase II detoxifying/antioxidant enzymes, including HO-1, NQO1, GCL, GSR, GSTP1, mEH and UGT1A6 in BEAS-2B cells. Apo-10′-lycopenic acid treatment also increased total intracellular GSH level and decreased ROS level in BEAS-2B cells. In addition, other lycopene metabolites (apo-10′-lycopenol and apo-10′-lycopenal) were also shown to induce the HO-1 gene. Since apo-10′-lycopenoids are enzymatic cleavage metabolites of lycopene in mammalian tissues, our results strongly suggest that lycopene metabolites may mediate the activation of Nrf2/ARE signaling and the induction of gene expression by lycopene.9
The induction of phase II detoxifying/antioxidant enzymes has been recognized as one of the mechanisms underlying the anti-carcinogenic activity of carotenoids.10,34 Particularly, lycopene supplementation has been shown to increase the level of GSH and the activity of phase II genes, such as GST and GSR, in hamster buccal pouch mucosa,35 rat liver microsomes7 and red blood cells.7 Increased expression of NQO1 and GST by chemopreventive agents, such as isothiocyanates has been associated with inhibition of NNK bioactivation and enhancement of detoxification.36,37 Furthermore, a recent study reported that the induction of GST by sodium selenite contributed to its chemopreventive effect against NNK induced lung carcinogenesis in the A/J mouse model.38 In the present study, we showed that apo-10′-lycopenoic acid induced the expression of NQO1, GST, as well as other phase II detoxifying/antioxidant enzymes in BEAS-2B cells, indicating a protective effect of lycopene metabolites against lung carcinogenesis in the initiation stage. This hypothesis was supported by the in vivo observation showing that supplementation with apo-10′-lycopenoic acid before carcinogenic initiation suppressed NNK-induced lung tumorigenesis in the A/J mouse model without any significant changes in cell proliferation markers.25 These data provided insights into the mechanisms underlying the beneficial effects of apo-10′-lycopenoids against lung cancer development.
HO-1, the inducible form of heme-oxygenase, plays a critical protective role by reducing oxidative damage and attenuating the inflammatory response in many tissues, particularly in the respiratory system.39,40 Although its roles in carcinogenesis need further clarification, studies showed that a polymorphism of the HO-1 promoter resulting in lower basal and inducible expression of HO-1 is associated with a higher risk of lung41 and oral42 cancer. Therefore, it is believed that HO-1 act as a cytoprotective enzyme in healthy tissues exposed to harmful stimuli, including carcinogens.43 In lung tissues, expression of HO-1 is increased by cigarette smoke exposure, and the expression of HO-1 in BEAS-2B cells has been shown to protect against cigarette smoke extract-induced cell death.40 In the present study, we found that HO-1 expression was induced by apo-10′-lycopenoic acid, -lycopenol and -lycopenal in BEAS-2B cells, suggesting that apo-10′-lycopenoids may protect lung epithelial cells against oxidative stress-related lung carcinogenesis. Interestingly, apo-10′-lycopenol was detected in the lungs of ferrets after lycopene supplementation,21 which provided an additional mechanism for our previous observation that the lycopene supplementation prevented cigarette smoke-induced lung lesions in the ferret model.4
In the present study, all 3 lycopenoids are effective in activating the Nrf2-mediated induction of HO-1. Although the exact mechanisms behind the activating the Nrf2-dependent HO-1 induction by these 3 lycopenoids remain unknown, the chemical properties of these compounds may contribute to the activation of Nrf2, which is controlled through multiple regulatory mechanisms, including Keap1-mediated ubiquitination and degradation, subcellular distribution, and phosphorylation.10,44 Notably, apo-10′-lycopenal showed the strongest induction of HO-1 as compared to apo-10′-lycopenoic acid and apo-10′-lycopenol. This may be due to the highly reactive aldehyde group in the compound, which is capable of Schiff base formation with the amino groups of protein45 and reactive with cellular macromolecules (e.g., directly modifying the reactive cysteine residues in Keap1 and interrupting Keap1-mediated Nrf2 ubiquitination and degradation44). It is also possible that these lycopenoids affect upstream signaling pathways, such as MAPKs (mitogen activated protein kinases), PI3K (phosphoinositol 3-kinase), epidermal growth factor receptor and PKC, which all have been shown to play a role in the regulation of Nrf2-ARE in lung epithelial cells.46,47 Lycopene has been shown to affect certain signaling pathways, e.g., insulin-like growth factor-1 (IGF-1) signaling, which is the upstream of both PI3K and MAPK, by inducing IGF-binding proteins in the lungs of smoke-exposed ferrets.4,23 Clearly, these hypotheses warrant further investigation.
GSH is a key factor regulating the redox environment of a cell. GSH is synthesized from L-glutamate with the help of 2 enzymes, γ-glutamylcysteine synthetase (GCLC and GCLM) and GSH synthetase.48 We have shown that apo-10′-lycopenoic acid treatment for as short a time period as 4 hr induced the expression of GCLC and GCLM. Consistently, treatment with 10 μM of apo-10′-lycopenic acid for 24 hr significantly increased total intracellular GSH levels and markedly decreased ROS levels in BEAS-2B cells. These observations suggest that apo-10′-lycopenoic acid may improve cellular antioxidant capacity, probably through the induction of Phase II enzymes by activating Nrf2/ARE signaling. However, there was a more significant change in the decrease of ROS than in the increase of GSH by apo-10′-lycopenoic acid (Fig. 6b). One explanation may lie in the fact that dichlorofluorescein assay is a method for measuring overall cellular redox status,31 which is affected by many factors, including the levels of total GSH pool, the ratio of reduced to oxidized GSH, and the levels of other antioxidants, such as NADH and NADPH.48 For instance, apo-10′-lycopenoic acid also induces the expression of GSR, which increases the level of reduced GSH in addition to increasing total GSH, thereby further decreasing cellular ROS. Meanwhile, it can not be excluded that apo-10′-lycopenoic acid may act as an antioxidant by itself since carotenoids have been shown to decrease intracellular ROS without activating the ARE.9
H2O2 is a non-radical ROS produced spontaneously as a result of intracellular metabolism. It may directly damage DNA, lipids, and other macromolecules by diffusing throughout the mitochondria and crossing the cell membranes.49,50 It can react with reduced transition metals (ions of Fe, Cu or Co), resulting in generation of much more ROS, such as the hydroxyl radical that can lead to extensive cell oxidative damage.49,50 As an antioxidant cofactor for GSH peroxidase, GSH plays a key role in detoxifying H2O2 by reducing H2O2 to water.51 Various GSH metabolizing enzymes, such as GST,52 GSR,53 and GCL,54 as well as GSH itself55 have been reported to protect against H2O2-induced oxidative damage in different cell lines.56 In the present study, we found that exogenous H2O2 treatment induced significant cell damage in BEAS-2B cells, while pretreatment with apo-10′-lycopenoic acid inhibited H2O2-induced LDH release. This correlated with increased total GSH levels and decreased endogenous ROS generation in apo-10′-lycopenoic acid treated BEAS-2B cells. These data indicate that the protective effect of apo-10′-lycopenoic acid against oxidative stress is via its induction of antioxidant enzymes and intracellular levels of GSH.
Acknowledgments
We are indebted to Dr. Hansgeorg Ernst of BASF, Inc. (Ludwigshafen, Germany) for the gift of apo-10′-lycopenoids. The authors thank Mr. Jonathan Mein and Ms. Sudipta Veeramachaneni for help in the preparation of the article. Any opinions, findings, conclusion or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the views of the U.S. Department of Agriculture, the National Institutes of Health.
Grant sponsor: National Institutes of Health, Bethesda, MD; Grant number: R01CA104932; Grant sponsor: U.S. Department of Agriculture; Grant number: 1950-51000-064.
Abbreviations
- ARE
antioxidant response element
- Carboxy-H2DCFDA
5-(and-6)-carboxy-2′,7′-dichlorofluorescin diacetate
- DTNB
5,5′-dithiobis-2-nitrobenzoic acid
- GCLC
glutamate–cysteine ligase, catalytic subunit
- GCLM
glutamate–cysteine ligase, modifier subunit
- GSH
glutathione
- GSR
glutathione reductase
- GST
glutathione S-transferases
- HO-1
heme oxygenase-1
- LDH
lactate dehydrogenase
- mEH
microsomal epoxide hydrolase
- NQO1
NAD(P)H:quinone oxidoreductase 1
- Nrf2
NF-E2 related factor 2
- ROS
reactive oxygen species
- tBHQ
tert-butylhydroquinone
- THF
tetrahydrofuran
- UGT1A6
UDP glucuronosyltransferase 1 family, polypeptide A6
References
- 1.Clinton SK. Lycopene: chemistry, biology, and implications for human health and disease. Nutr Rev. 1998;56:35–51. doi: 10.1111/j.1753-4887.1998.tb01691.x. [DOI] [PubMed] [Google Scholar]
- 2.Giovannucci E. Tomatoes, tomato-based products, lycopene, and cancer: review of the epidemiologic literature. J Natl Cancer Inst. 1999;91:317–31. doi: 10.1093/jnci/91.4.317. [DOI] [PubMed] [Google Scholar]
- 3.Boileau TW, Liao Z, Kim S, Lemeshow S, Erdman JW, Jr, Clinton SK. Prostate carcinogenesis in N-methyl-N-nitrosourea (NMU)-testosterone-treated rats fed tomato powder, lycopene, or energy-restricted diets. J Natl Cancer Inst. 2003;95:1578–86. doi: 10.1093/jnci/djg081. [DOI] [PubMed] [Google Scholar]
- 4.Liu C, Lian F, Smith DE, Russell RM, Wang XD. Lycopene supplementation inhibits lung squamous metaplasia and induces apoptosis via up-regulating insulin-like growth factor-binding protein 3 in cigarette smoke-exposed ferrets. Cancer Res. 2003;63:3138–44. [PubMed] [Google Scholar]
- 5.Canene-Adams K, Lindshield BL, Wang S, Jeffery EH, Clinton SK, Erdman JW., Jr Combinations of tomato and broccoli enhance antitumor activity in dunning r3327-h prostate adenocarcinomas. Cancer Res. 2007;67:836–43. doi: 10.1158/0008-5472.CAN-06-3462. [DOI] [PubMed] [Google Scholar]
- 6.Siler U, Barella L, Spitzer V, Schnorr J, Lein M, Goralczyk R, Wertz K. Lycopene and vitamin E interfere with autocrine/paracrine loops in the Dunning prostate cancer model. Faseb J. 2004;18:1019–21. doi: 10.1096/fj.03-1116fje. [DOI] [PubMed] [Google Scholar]
- 7.Breinholt V, Lauridsen ST, Daneshvar B, Jakobsen J. Dose-response effects of lycopene on selected drug-metabolizing and antioxidant enzymes in the rat. Cancer Lett. 2000;154:201–10. doi: 10.1016/s0304-3835(00)00401-8. [DOI] [PubMed] [Google Scholar]
- 8.Velmurugan B, Bhuvaneswari V, Burra UK, Nagini S. Prevention of N-methyl-N′-nitro-N-nitrosoguanidine and saturated sodium chloride-induced gastric carcinogenesis in Wistar rats by lycopene. Eur J Cancer Prev. 2002;11:19–26. doi: 10.1097/00008469-200202000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Ben-Dor A, Steiner M, Gheber L, Danilenko M, Dubi N, Linnewiel K, Zick A, Sharoni Y, Levy J. Carotenoids activate the antioxidant response element transcription system. Mol Cancer Ther. 2005;4:177–86. [PubMed] [Google Scholar]
- 10.Giudice A, Montella M. Activation of the Nrf2-ARE signaling pathway: a promising strategy in cancer prevention. Bioessays. 2006;28:169–81. doi: 10.1002/bies.20359. [DOI] [PubMed] [Google Scholar]
- 11.Gao X, Talalay P. Induction of phase 2 genes by sulforaphane protects retinal pigment epithelial cells against photooxidative damage. Proc Natl Acad Sci USA. 2004;101:10446–51. doi: 10.1073/pnas.0403886101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balogun E, Hoque M, Gong P, Killeen E, Green CJ, Foresti R, Alam J, Motterlini R. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem J. 2003;371:887–95. doi: 10.1042/BJ20021619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shen G, Xu C, Hu R, Jain MR, Nair S, Lin W, Yang CS, Chan JY, Kong AN. Comparison of (−)-epigallocatechin-3-gallate elicited liver and small intestine gene expression profiles between C57BL/6J mice and C57BL/6J/Nrf2 (−/−) mice. Pharm Res. 2005;22:1805–20. doi: 10.1007/s11095-005-7546-8. [DOI] [PubMed] [Google Scholar]
- 14.Kim SJ, Nara E, Kobayashi H, Terao J, Nagao A. Formation of cleavage products by autoxidation of lycopene. Lipids. 2001;36:191–9. doi: 10.1007/s11745-001-0706-8. [DOI] [PubMed] [Google Scholar]
- 15.Caris-Veyrat C, Schmid A, Carail M, Bohm V. Cleavage products of lycopene produced by in vitro oxidations: characterization and mechanisms of formation. J Agric Food Chem. 2003;51:7318–25. doi: 10.1021/jf034735+. [DOI] [PubMed] [Google Scholar]
- 16.Aust O, Ale-Agha N, Zhang L, Wollersen H, Sies H, Stahl W. Lycopene oxidation product enhances gap junctional communication. Food Chem Toxicol. 2003;41:1399–407. doi: 10.1016/s0278-6915(03)00148-0. [DOI] [PubMed] [Google Scholar]
- 17.Zhang H, Kotake-Nara E, Ono H, Nagao A. A novel cleavage product formed by autoxidation of lycopene induces apoptosis in HL-60 cells. Free Radic Biol Med. 2003;35:1653–63. doi: 10.1016/j.freeradbiomed.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 18.dos Anjos Ferreira AL, Yeum KJ, Russell RM, Krinsky NI, Tang G. Enzymatic and oxidative metabolites of lycopene. J Nutr Biochem. 2004;15:493–502. doi: 10.1016/j.jnutbio.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 19.Khachik F, Carvalho L, Bernstein PS, Muir GJ, Zhao DY, Katz NB. Chemistry, distribution, and metabolism of tomato carotenoids and their impact on human health. Exp Biol Med (Maywood) 2002;227:845–51. doi: 10.1177/153537020222701002. [DOI] [PubMed] [Google Scholar]
- 20.Gajic M, Zaripheh S, Sun F, Erdman JW., Jr Apo-8′-lycopenal and apo-12′-lycopenal are metabolic products of lycopene in rat liver. J Nutr. 2006;136:1552–7. doi: 10.1093/jn/136.6.1552. [DOI] [PubMed] [Google Scholar]
- 21.Hu KQ, Liu C, Ernst H, Krinsky NI, Russell RM, Wang XD. The biochemical characterization of ferret carotene-9′,10′-monooxygenase catalyzing cleavage of carotenoids in vitro and in vivo. J Biol Chem. 2006;281:19327–38. doi: 10.1074/jbc.M512095200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang XD. Carotenoid oxidative/degradative products and their biological activities. In: Krinsky NI, Mayne ST, Sies H, editors. Carotenoids in health and diseased. New York: Marcel Dekker; 2004. pp. 313–35. [Google Scholar]
- 23.Wang XD. Can smoke-exposed ferrets be utilized to unravel the mechanisms of action of lycopene? J Nutr. 2005;135:S2053–S2056. doi: 10.1093/jn/135.8.2053S. [DOI] [PubMed] [Google Scholar]
- 24.Lindshield BL, Canene-Adams K, Erdman JW., Jr Lycopenoids: are lycopene metabolites bioactive? Arch Biochem Biophys. 2007;458:136–40. doi: 10.1016/j.abb.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 25.Lian F, Smith DE, Ernst H, Russell RM, Wang XD. Apo-10′-lycopenoic acid inhibits lung cancer cell growth in vitro, and suppresses lung tumorigenesis in the A/J mouse model in vivo. Carcinogenesis. 2007;28:1567–74. doi: 10.1093/carcin/bgm076. [DOI] [PubMed] [Google Scholar]
- 26.Kiefer C, Hessel S, Lampert JM, Vogt K, Lederer MO, Breithaupt DE, von Lintig J. Identification and characterization of a mammalian enzyme catalyzing the asymmetric oxidative cleavage of provitamin A. J Biol Chem. 2001;276:14110–6. doi: 10.1074/jbc.M011510200. [DOI] [PubMed] [Google Scholar]
- 27.Reddel RR, Ke Y, Gerwin BI, McMenamin MG, Lechner JF, Su RT, Brash DE, Park JB, Rhim JS, Harris CC. Transformation of human bronchial epithelial cells by infection with SV40 or adenovirus-12 SV40 hybrid virus, or transfection via strontium phosphate coprecipitation with a plasmid containing SV40 early region genes. Cancer Res. 1988;48:1904–9. [PubMed] [Google Scholar]
- 28.Kim Y, Chongviriyaphan N, Liu C, Russell RM, Wang XD. Combined antioxidant ({β}-carotene, {α}-tocopherol and ascorbic acid) supplementation increases the levels of lung retinoic acid and inhibits the activation of mitogen-activated protein kinase in the ferret lung cancer model. Carcinogenesis. 2006;27:1410–9. doi: 10.1093/carcin/bgi340. [DOI] [PubMed] [Google Scholar]
- 29.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 30.Rahman I, Kode A, Biswas SK. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat Protoc. 2006;1:3159–65. doi: 10.1038/nprot.2006.378. [DOI] [PubMed] [Google Scholar]
- 31.Wang H, Joseph JA. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic Biol Med. 1999;27:612–16. doi: 10.1016/s0891-5849(99)00107-0. [DOI] [PubMed] [Google Scholar]
- 32.Nioi P, Hayes JD. Contribution of NAD(P)H:quinone oxidoreductase 1 to protection against carcinogenesis, and regulation of its gene by the Nrf2 basic-region leucine zipper and the arylhydrocarbon receptor basic helix–loop–helix transcription factors. Mutat Res. 2004;555:149–71. doi: 10.1016/j.mrfmmm.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 33.Galloway DC, Blake DG, Shepherd AG, McLellan LI. Regulation of human γ-glutamylcysteine synthetase: co-ordinate induction of the catalytic and regulatory subunits in HepG2 cells. Biochem J. 1997;328 (Part 1):99–104. doi: 10.1042/bj3280099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharoni Y, Danilenko M, Levy J, Stahl W. Anticancer activity of carotenoids: from human studies to cellular processes and gene regulation. In: Krinsky NI, Mayne ST, Sies H, editors. Carotenoids in health and diseased. New York: Marcel Dekker; 2004. pp. 165–96. [Google Scholar]
- 35.Bhuvaneswari V, Velmurugan B, Balasenthil S, Ramachandran CR, Nagini S. Chemopreventive efficacy of lycopene on 7,12-dimethylbenz[a]anthracene-induced hamster buccal pouch carcinogenesis. Fitoterapia. 2001;72:865–74. doi: 10.1016/s0367-326x(01)00321-5. [DOI] [PubMed] [Google Scholar]
- 36.Guo Z, Smith TJ, Wang E, Sadrieh N, Ma Q, Thomas PE, Yang CS. Effects of phenethyl isothiocyanate, a carcinogenesis inhibitor, on xenobiotic-metabolizing enzymes and nitrosamine metabolism in rats. Carcinogenesis. 1992;13:2205–10. doi: 10.1093/carcin/13.12.2205. [DOI] [PubMed] [Google Scholar]
- 37.Guo Z, Smith TJ, Wang E, Eklind KI, Chung FL, Yang CS. Structure-activity relationships of arylalkyl isothiocyanates for the inhibition of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone metabolism and the modulation of xenobiotic-metabolizing enzymes in rats and mice. Carcinogenesis. 1993;14:1167–73. doi: 10.1093/carcin/14.6.1167. [DOI] [PubMed] [Google Scholar]
- 38.Prokopczyk B, Rosa JG, Desai D, Amin S, Sohn OS, Fiala ES, El-Bayoumy K. Chemoprevention of lung tumorigenesis induced by a mixture of benzo(a)pyrene and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone by the organoselenium compound 1,4-phenylenebis(methylene)selenocyanate. Cancer Lett. 2000;161:35–46. doi: 10.1016/s0304-3835(00)00590-5. [DOI] [PubMed] [Google Scholar]
- 39.Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev. 2006;86:583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- 40.Ryter SW, Kim HP, Nakahira K, Zuckerbraun BS, Morse D, Choi AM. Protective functions of heme oxygenase-1 and carbon monoxide in the respiratory system. Antioxidants Redox Signal. 2007;9:2157–73. doi: 10.1089/ars.2007.1811. [DOI] [PubMed] [Google Scholar]
- 41.Kikuchi A, Yamaya M, Suzuki S, Yasuda H, Kubo H, Nakayama K, Handa M, Sasaki T, Shibahara S, Sekizawa K, Sasaki H. Association of susceptibility to the development of lung adenocarcinoma with the heme oxygenase-1 gene promoter polymorphism. Human Genet. 2005;116:354–60. doi: 10.1007/s00439-004-1162-2. [DOI] [PubMed] [Google Scholar]
- 42.Chang KW, Lee TC, Yeh WI, Chung MY, Liu CJ, Chi LY, Lin SC. Polymorphism in heme oxygenase-1 (HO-1) promoter is related to the risk of oral squamous cell carcinoma occurring on male areca chewers. Br J Cancer. 2004;91:1551–5. doi: 10.1038/sj.bjc.6602186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jozkowicz A, Was H, Dulak J. Heme oxygenase-1 in tumors: is it a false friend? Antioxidants Redox Signal. 2007;9:2099–117. doi: 10.1089/ars.2007.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kobayashi M, Yamamoto M. Nrf2-Keap1 regulation of cellular defense mechanisms against electrophiles and reactive oxygen species. Adv Enzyme Regulat. 2006;46:113–40. doi: 10.1016/j.advenzreg.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 45.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 46.Zhang H, Forman HJ. Acrolein induces heme oxygenase-1 through PKC-δ and PI3K in human bronchial epithelial cells. Am J Respir Cell Mol Biol. 2008;38:483–90. doi: 10.1165/rcmb.2007-0260OC. [DOI] [PubMed] [Google Scholar]
- 47.Papaiahgari S, Zhang Q, Kleeberger SR, Cho HY, Reddy SP. Hyperoxia stimulates an Nrf2-ARE transcriptional response via ROS-EGFR-PI3K-Akt/ERK MAP kinase signaling in pulmonary epithelial cells. Antioxidants Redox Signal. 2006;8:43–52. doi: 10.1089/ars.2006.8.43. [DOI] [PubMed] [Google Scholar]
- 48.Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001;30:1191–212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 49.Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 50.Alia M, Ramos S, Mateos R, Bravo L, Goya L. Response of the antioxidant defense system to tert-butyl hydroperoxide and hydrogen peroxide in a human hepatoma cell line (HepG2) J Bioch Mol Toxicol. 2005;19:119–28. doi: 10.1002/jbt.20061. [DOI] [PubMed] [Google Scholar]
- 51.Wu G, Fang YZ, Yang S, Lupton JR, Turner ND. Glutathione metabolism and its implications for health. J Nutr. 2004;134:489–92. doi: 10.1093/jn/134.3.489. [DOI] [PubMed] [Google Scholar]
- 52.Yang Y, Cheng JZ, Singhal SS, Saini M, Pandya U, Awasthi S, Awasthi YC. Role of glutathione S-transferases in protection against lipid peroxidation. Overexpression of hGSTA2-2 in K562 cells protects against hydrogen peroxide-induced apoptosis and inhibits JNK and caspase 3 activation. J Biol Chem. 2001;276:19220–30. doi: 10.1074/jbc.M100551200. [DOI] [PubMed] [Google Scholar]
- 53.Reddan JR, Giblin FJ, Dziedzic DC, McCready JP, Schrimscher L, Reddy VN. Influence of the activity of glutathione reductase on the response of cultured lens epithelial cells from young and old rabbits to hydrogen peroxide. Experiment Eye Res. 1988;46:209–21. doi: 10.1016/s0014-4835(88)80078-2. [DOI] [PubMed] [Google Scholar]
- 54.Shi S, Hudson FN, Botta D, McGrath MB, White CC, Neff-LaFord HD, Dabrowski MJ, Singh NP, Kavanagh TJ. Over expression of glutamate cysteine ligase increases cellular resistance to H2O2-induced DNA single-strand breaks. Cytometry A. 2007;71:686–92. doi: 10.1002/cyto.a.20434. [DOI] [PubMed] [Google Scholar]
- 55.Spitz DR, Kinter MT, Roberts RJ. Contribution of increased glutathione content to mechanisms of oxidative stress resistance in hydrogen peroxide resistant hamster fibroblasts. J Cell Physiol. 1995;165:600–9. doi: 10.1002/jcp.1041650318. [DOI] [PubMed] [Google Scholar]
- 56.Klaunig JE, Kamendulis LM. The role of oxidative stress in carcinogenesis. Annu Rev Pharmacol Toxicol. 2004;44:239–67. doi: 10.1146/annurev.pharmtox.44.101802.121851. [DOI] [PubMed] [Google Scholar]


